Abstract
We report a novel case of an infant with neurofibromatosis type 1 (NF1) who presented with new onset presumed focal impaired awareness seizures with motor onset followed by rapid progression to infantile spasms (IS). Electroencephalography (EEG) captured evolution from focal epileptiform discharges to multifocal and generalized discharges, then to hypsarrhythmia over three days. Development of IS within days of focal seizure onset is rapid, and to our knowledge, has not been demonstrated electrographically. The pattern of rapid ictal transition to hypsarrhythmia is essential for neurologists to be able to recognize as it can help lead to early treatment, which is necessary for improved outcomes in IS.
Keywords: Infantile spasms, Hypsarrhythmia, Neurofibromatosis 1, Focal epilepsy
Highlights
-
•
Patients with a non-lesional MRI in NF1 are at risk for epilepsy and infantile spasms.
-
•
The dynamic period between focal seizures and infantile spasms can be rapid.
-
•
The pattern of rapid ictal transition to hypsarrhythmia is important for early treatment.
1. Introduction
Infantile spasms (IS) reflect an age-specific epilepsy syndrome defined by the presence of epileptic spasms often in conjunction with hypsarrhythmia recorded with electroencephalography (EEG). If IS progress to an epileptic encephalopathy, along with psychomotor delay and developmental regression, it is known as West syndrome. EEG patterns in IS can include hypsarrhythmia or modified hypsarrhythmia, with variations such as asymmetry, or even no associated hypsarrhythmia with a similar response to treatment [1]. Focal seizures preceding IS have been reported in the literature, but the rapidity of clinical and electrographic transition over a few days has not previously been described to our knowledge. In our case of IS, EEG captured rapid evolution from focal discharges to multifocal discharges, then to hypsarrhythmia over three days.
2. Materials and methods
2.1. Patient case
The patient was an 11-month-old female with neurofibromatosis type 1 (NF1) and developmental delay presented to the emergency department following a first-time seizure described as generalized shaking lasting 5 min. Family history was notable for unclassified epilepsy in two second-degree paternal relatives. Her initial 2-hour EEG captured frequent left hemispheric multifocal epileptiform discharges with left-sided focal slowing in the context of normal sleep architecture (Fig. 1). Due to the clinical description and left-hemispheric EEG findings, this seizure was classified as a focal to bilateral tonic–clonic seizure [2].
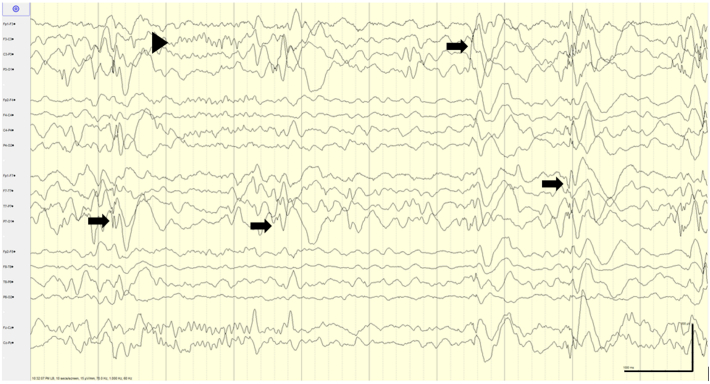
Fig. 1.
EEG with left-sided focal epileptiform discharges. At presentation, EEG demonstrated abundant (5 in 10 s) high-amplitude left temporo-parietal, occipital and frontal spike and slow wave discharges in the setting of left-sided cerebral dysfunction in the context of normal sleep architecture (arrowhead). Sensitivity: 15 μV/mm, Timebase: 10 s/screen, HFF (high-frequency filter): 70 Hz, LFF (low-frequency filter): 1 Hz.
Legend key: Arrows: epileptiform discharges; Arrowheads: sleep architecture.
Despite the initiation of levetiracetam, the next day, the patient had multiple focal impaired awareness seizures with motor onset characterized by left-sided stiffening, jerking, and gaze deviation lasting up to 10–15 min. Due to change in semiology that was witnessed by the family and our team, a second EEG was obtained, which demonstrated multifocal and bisynchronous spike-and-wave discharges, again with a background containing normal sleep structures (Fig. 2).
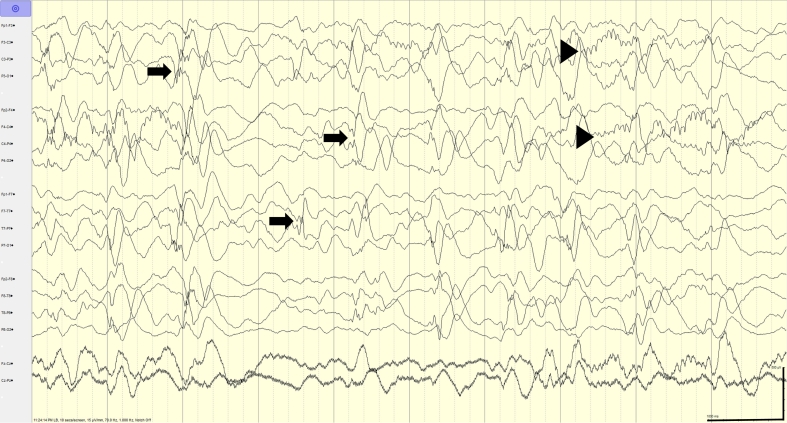
Fig. 2.
Multifocal discharges. One day later, EEG demonstrated high-amplitude abundant (> 5 in 10 s) bilateral independent temporo-parietal discharges and bisynchronous spike-and-wave (arrows) in the context of normal sleep architecture (arrowheads). Sensitivity: 15 μV/mm, Timebase: 10 s/screen, HFF (high-frequency filter): 70 Hz, LFF (low-frequency filter): 1 Hz.
Legend key: Arrows: epileptiform discharges; Arrowheads: sleep architecture.
The following morning, a brain magnetic resonance imaging (MRI) study was performed demonstrating abnormal focal areas of signal intensity (FASI), consistent with her known NF1 diagnosis. No focal MRI abnormalities that would explain focal seizures were seen. After the MRI, a new seizure type described as clusters of bilateral arm extension at the shoulder to 45–90° was observed. Multiple events were captured on EEG, demonstrating epileptic spasms with hypsarrhythmia (Fig. 3, Fig. 4), consistent with IS. Subsequently, treatment with high dose steroids was initiated.
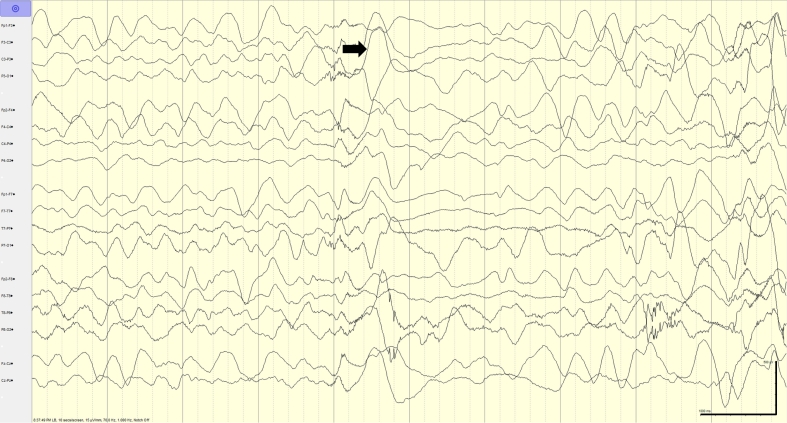
Fig. 3.
Epileptic spasm. High voltage generalized delta followed by an electrodecrement clinically associated with arm extension. Sensitivity: 15 μV/mm, Timebase: 10 s/screen, HFF (high-frequency filter): 70 Hz, LFF (low-frequency filter): 1 Hz.
Legend key: Arrows: epileptiform discharges
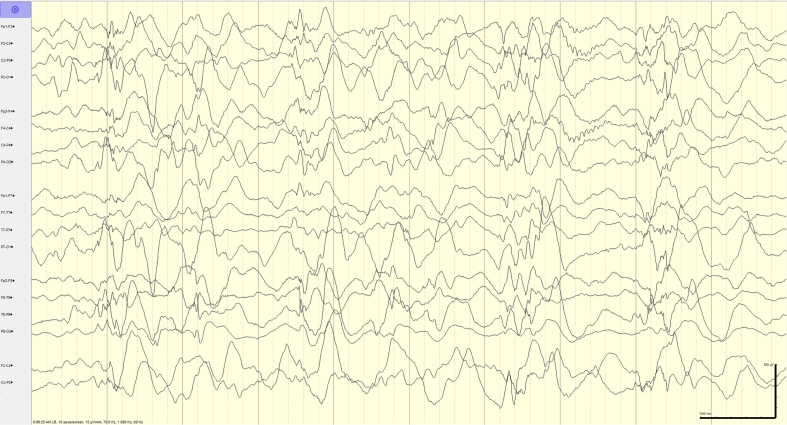
Fig. 4.
Hypsarrhythmia. 72 h after presentation, EEG evolved to hypsarrhythmia. Sensitivity: 15 μV/mm, Timebase: 10 s/screen, HFF (high-frequency filter): 70 Hz, LFF (low-frequency filter): 1 Hz.
IS resolved after one-time course of high-dose steroids. Follow-up EEG at 2 months continued to demonstrate multifocal potential epileptogenicity but showed no evidence of hypsarrhythmia or clinical spasms. She was continued on levetiracetam. At a one-year follow-up, no further seizures of any type have occurred.
3. Results and discussion
Epilepsy in individuals with NF1 is uncommon, with the rate in recent studies around 5.8–6.5%. Focal epilepsy is the most common type of epilepsy in NF1, often secondary to a structural abnormality such as a tumor [3]. In our patient's case, no structural lesion was identified, highlighting that individuals with NF1 without structural lesions are also at risk for both focal epilepsy and IS.
The specific prevalence of IS in NF1 is 0.76%, which is higher than in the general population (0.02–0.05%). Ruggieri et al. in 2009, reported ten patients with both NF1 and IS [4]. No patient had epilepsy prior to the onset of IS. Out of these ten patients, six developed further seizure types including generalized tonic–clonic seizures, focal impaired awareness seizures, and myoclonic seizures; with two of the six meeting criteria for Lennox–Gastaut syndrome [4]. At one-year follow-up, our patient continues to remain seizure-free on levetiracetam monotherapy. Based on this, her infantile spasms were classified as symptomatic IS with NF1 as the etiology.
This case demonstrates the rapid evolution of focal hemispheric dysfunction and epileptiform discharges to a pattern of hypsarrhythmia on EEG. There are previous descriptions of focal seizures evolving to IS [[5], [6], [7], [8]]. In 1999, Kubota et al. discussed that there can be a temporal link between focal seizures and epileptic spasms based on observations in 8 of 45 patients (17%). However, seven out of the eight patients had severe “organic brain lesions” [5]. Ohtsuka et al. reported a transition from focal seizures to hypsarrhythmia in patients with IS, but the time course was unclear [6]. Philippi et al. performed a retrospective study reviewing EEGs of patients with IS obtained prior to diagnosis and identified a highly dynamic period on EEG, with an evolution from focal discharges to multifocal and generalized discharges and then to hypsarrhythmia. This dynamic period evolved over 3 to 6 weeks [7]. In our case, development of IS within three days of focal seizure onset was captured on EEG, demonstrating that the dynamic period between pre-hypsarrhythmia to hypsarhythmia may be as short as three days, rather than over the relatively prolonged period of 3–6 weeks. This pattern of rapid evolution can be a unique EEG signature that allows for further monitoring and early treatment of IS, if suspected.
Donat et al. proposed a mechanism by which focal seizures lowered the threshold for generating IS and hypsarrhythmia [8]. Our case explores the theory that the dynamic period characterized by multifocal and generalized interictal discharges preceding hypsarrhythmia could represent activation of multiple neuronal networks, including cortical, thalamic, and brainstem activation [9]. Based on prior literature, we postulate that the multifocal discharges may lower the threshold for the synchronous activation of cortical and subcortical units and thus facilitate the development of hypsarrhythmia. The other consideration is that this rapid ictal transition could be a manifestation of the evolving phenotype from pre-hypsarrhythmia to clinically apparent epileptic spasms with hypsarrhythmia.
This case demonstrates the clinical importance of seizure semiology and comparison with EEG findings, as the discordant findings of focal impaired awareness seizures with motor onset with left-sided clinical features in the setting of prior left-sided hemispheric discharges and dysfunction, led to re-evaluation. Due to this re-evaluation, we were able to obtain further EEG recordings that showed evolution of multifocal discharges to hypsarrhythmia. The pattern of rapid evolution to hypsarrhythmia is important for trainees and pediatric neurologists to be able to recognize as it may lead to early treatment. Early diagnosis and effective treatment of IS are vital for improving long-term outcomes [10]. This case illustrates how the combination of clinical and EEG findings, identification of those at high risk for evolution to IS, close follow-up, and clear counseling of families regarding infantile spasms can lead to early treatment.
Ethical statement
All coauthors (Lindsay Pagano, MD, Robert P Carson, MD, PhD and Lori C Jordan, MD, PhD) have read and agreed to the content of this case report.
Consent was obtained from family regarding this case report. No pictures or identifying information was used.
There is no commercial involvement in the manuscript or other conflicts of interest by any of the authors. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT statement
Shital H. Patel: Conceptualization, Methodology, Writing - original draft preparation, reviewing and editing; Robert P. Carson: Visualization, Writing - reviewing and editing; Lori C. Jordan: Methodology, Writing - reviewing and editing; Lindsay M. Pagano: Supervision; Conceptualization, Writing - reviewing and editing
Declaration of competing interest
None.
References
- 1.Demarest Scott T., Shellhaas Renée A., Gaillard William D., Keator Cynthia, Nickels Katherine C., Hussain Shaun A., Pediatric Epilepsy Research Consortium The impact of hypsarrhythmia on infantile spasms treatment response: observational cohort study from the National Infantile Spasms Consortium. Epilepsia. 2017;58(12):2098–2103. doi: 10.1111/epi.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher Robert S., Cross J. Helen, French Jacqueline A., Higurashi Norimichi, Hirsch Edouard, Jansen Floor E. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 3.Ostendorf Adam P., Gutmann David H., Weisenberg Judith L.Z. Epilepsy in individuals with neurofibromatosis type 1. Epilepsia. 2013;54(10):1810–1814. doi: 10.1111/epi.12348. [DOI] [PubMed] [Google Scholar]
- 4.Ruggieri Martino, Iannetti Paola, Clementi Maurizio, Polizzi Agata, Incorpora Gemma, Spalice Alberto. Neurofibromatosis type 1 and infantile spasms. Childs Nerv Syst. 2009;25(2):211–216. doi: 10.1007/s00381-008-0706-5. [DOI] [PubMed] [Google Scholar]
- 5.Kubota Toshiko, Aso Kosaburo, Negoro Tamiko, Okumura Akihisa, Natsume Jun, Takada Hiroyuki. Epileptic spasms preceded by partial seizures with a close temporal association. Epilepsia. 1999;40(11):1572–1579. doi: 10.1111/j.1528-1157.1999.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsuka Yoko. West syndrome and its related epileptic syndromes. Epilepsia. 1998;39(S5):30–37. doi: 10.1111/j.1528-1157.1998.tb05147.x. [DOI] [PubMed] [Google Scholar]
- 7.Philippi Heike, Wohlrab Gabriele, Bettendorf Uli, Borusiak Peter, Kluger Gerhard, Strobl Karl. Electroencephalographic evolution of hypsarrhythmia: toward an early treatment option. Epilepsia. 2008;49(11):1859–1864. doi: 10.1111/j.1528-1167.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- 8.Donat Jane F., Wright Francis S. Simultaneous infantile spasms and partial seizures. J Child Neurol. 1991;6(3):246–250. doi: 10.1177/088307389100600308. [DOI] [PubMed] [Google Scholar]
- 9.Siniatchkin Michael, Baalen Andreas Van, Jacobs Julia, Moeller Friederike, Moehring Jan, Boor Rainer. Epilepsia; 2007. Different neuronal networks are associated with spikes and slow activity in hypsarrhythmia. [DOI] [PubMed] [Google Scholar]
- 10.Wilmshurst Jo M., Gaillard William D., Vinayan Kollencheri P., Tsuchida Tammy N., Plouin Perrine, Bogaert Patrick V. Summary of recommendations for the management of infantile seizures: Task Force Report for the ILAE Commission of Pediatrics. Epilepsia. 2015;56(8):1185–1197. doi: 10.1111/epi.13057. [DOI] [PubMed] [Google Scholar]