Abstract
Background/objective
Irisin is suggested to be an exercise beneficial effects mediator. This study aimed to examine the effects of the aerobic exercise (AE), resistance exercise (RE), and combined exercise (CE) on the plasma levels of irisin and some metabolic and anthropometric indices.
Methods
Sixty overweight women with metabolic syndrome were assigned equally into four groups: AE, RE, CE, and control. The study variables were measured before and 24 h after the intervention period.
Results
None of the study groups showed statistically significant changes in the serum irisin. However, muscle mass significantly increased in the RE and CE groups. Also, a significant decrease was observed in the body fat percentage in all groups. In addition, compared with the control group, the homeostatic model assessment of insulin resistance in the AE (p = 0.021), RE (p = 0.039), and in the CE (p = 0.003) groups reduced significantly. According to the analysis of indices’ changes, serum irisin was significantly correlated with the body fat percentage (r = 0.532) and HOMA-IR (r = 0.424).
Conclusions
The systematic exercise program for 8-weeks did not change circulating irisin and no statistically significant difference was observed between the exercise methods. Also, serum irisin seemed to be associated with the glycemic status, body fat and weight independent of exercise activity.
RCT registration code
IRCT20180806040721N2.
Registry name
Iranian Registry of Clinical Trials.
Keywords: Metabolic syndrome, Irisin, HOMA-IR, Exercise program, Insulin, Aerobic, Resistance
Introduction
Despite the unquestionable health beneficial effects of regular exercise, little is known about underlying molecular pathways that regulate these effects.1 There are several signal peptides termed myokines, which have been introduced in recent years as key regulators.2 Myokines are secreted from skeletal contracting muscles, the largest organ in the human body, and are known to have endocrine effects on other organs. Irisin is one of the newest myokines described by Boström et al. in 2012.3 Irisin is an exercise-induced myokine and has been suggested to increase energy expenditure through the browning of adipose tissue.2 It has been suggested that the browning process of adipose tissue via irisin may be a body response to the cold environment and physical activity.4 The upregulation of the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), as a transcript activator, due to physical activity enhances the expression of fibronectin type III domain-containing protein 5 (FNDC5) gene. FNDC5 gene encodes the precursor protein for irisin production.3
Previous research on irisin secretion has shown that muscle tissue plays an important role in raising the blood level of irisin following exercise.5,6 Serum irisin levels rise and peak immediately and last shortly after the exercise session.7 It has been reported that elevated irisin levels in response to exercise may reduce weight in obese individuals and insulin resistance in patients with diabetes.8, 9, 10 In addition, serum irisin has been found to have an inverse correlation with glucose intolerance.11 However, it is not clear whether exercise can increase the baseline serum irisin. As shown in population studies, high serum irisin levels reduce the risk of BMI enhancement and coronary atherosclerosis.12,13 Moreover, a short-term increase in serum irisin due to exercise is responsible for some beneficial metabolic changes.14,15 This becomes more complicated when we find that the lipid tissue is responsible for the baseline level of irisin in the blood among obese individuals.16 Furthermore, the serum irisin level is positively correlated with higher waist circumference, body fat mass, and unfavorable lipid profile.17,18
Putting aside the conflicting results, physical activity is regularly prescribed in patients with different metabolic diseases including diabetes,1 dyslipidemia and metabolic syndrome (MetS). MetS, as a cluster of health-related conditions including glucose intolerance, hypertension, dyslipidemia and central obesity, is an overwhelming health problem across the globe.19 Regardless of the molecular pathways, regular exercise, especially a program with a combination of aerobic and resistance training can improve MetS indices.20
To the best of our knowledge, the effects of a variant exercise program on serum irisin using a well-controlled clinical trial have not been studied yet. Therefore, this study aimed to examine two hypotheses: (i) whether different exercise regimens including aerobic, resistance, and a combination of aerobic and resistance, could increase the serum irisin and, (ii) whether changes in the serum irisin levels are associated with changes in other metabolic parameters in women with MetS.
Materials and methods
Participants
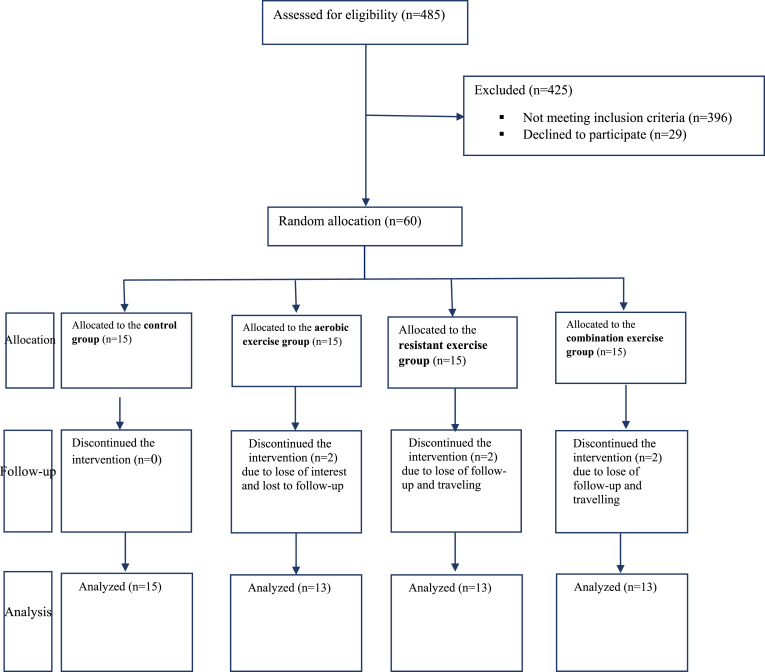
This randomized controlled trial was performed on the basis of the CONSORT 2010 flow diagram (Fig. 1) to examine the effects of aerobic exercise (AE), resistant exercise (RE), and combined exercise (CE) on the levels of plasma irisin, fasting blood sugar (FBS), insulin, insulin resistance (HOMA-IR), lipid profile, and body composition in obese women with MetS. The primary hypothesis of this study was that a significant change in the irisin level would be observed after the exercise program compared to that at the baseline measurements. The second hypothesis was that statistically significant changes would be observed in the plasma levels of HOMA-IR and lipid profiles. MetS was confirmed when at least three of the following conditions were met: abdominal obesity (waist circumference greater than 88 cm in women and 102 in men), triglyceride ≥ 150 mg/dL, HDL-cholesterol ≤ 40 mg/dL in men and ≤50 mg/dL in women, hypertension (systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg), and increased FBS (≥100 mg/dL).21 Inclusion criteria for subjects were as follows: having at least three diagnostic criteria of MetS, aged between 46 and 60 years and being at the pre-menopausal period, non-smoker, no history of cancer, no history of cardiovascular and musculoskeletal disorders, no use of dietary or ergogenic supplements, and willingness to take part in the study. Exclusion criteria were unwillingness to continue with the intervention, missing more than 10% of the exercise sessions, pregnancy and starting a specific dietary regimen. The Ethics Committee affiliated with Shiraz University of Medical Sciences approved the research proposal and corroborated its ethical considerations throughout the study (decree code: IR.SUMS.REC. 1397.279).
Fig. 1.
Study flow diagram.
Study design
The study protocol was registered on the Iranian Registry of Clinical Trials (IRCT) under registration code of IRCT20180806040721N2. The study was conducted from February to April 2018 on 60 overweight/obese women with MetS selected from women visiting a public clinic affiliated with Shiraz University of Medical Sciences, Iran. Block randomization with the block size of four was used for assigning the subjects into four groups: (I) AE, (II) RE, (III) CE, and (IV) control group. Each group consisted of 15 women who were not blind to group assignments due to the nature of interventions. However, neither the laboratory staff nor the data collectors or the biostatistician were aware of group assignments. The eligible women were contacted by a nurse to participate in the study. The women were informed of the study method and objectives and were asked to sign the informed consent form before the initiation of the study. They were examined by a general practitioner before commencing the study to ensure that the interventions would not jeopardize their health condition.
Intervention: the exercises program
The control group were advised not to change their physical activity during the study. Also, their compliance with the interventions was monitored every other week via phone calls.
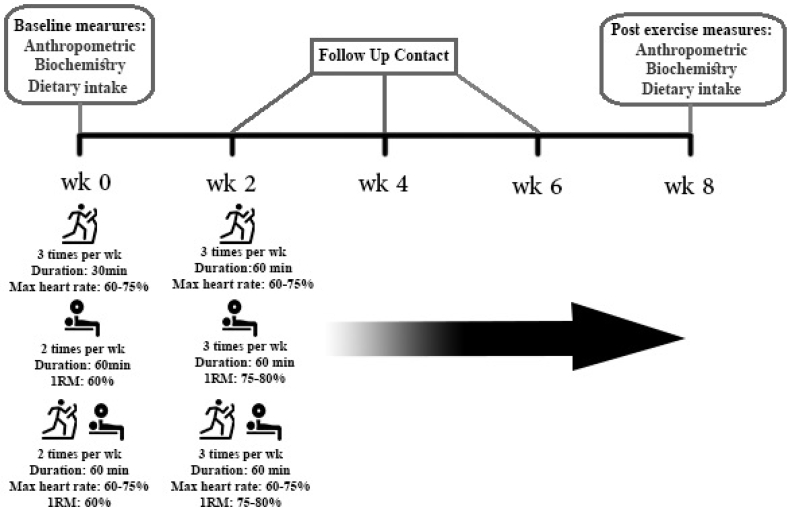
Aerobic exercise (AE) consisted of running on the treadmill (Cosmuse/hp model Saturn,® Germany) three times per week on non-consecutive days. In the group-based training sessions, the exercise time was increased gradually from 30 min to 60 min in each session after two weeks. Each 60-minute session consisted of stretching exercises (10 min), treadmill walking and stationary cycling (40 min), and balance exercises (10 min). They were allowed to have a 5- to 10-minute rest during each session. The exercise intensity was based on the maximum heart rate; it started at 60% and was gradually elevated to 75% during the exercise. The heart rate was monitored using the wristband heart rate monitor. The accuracy of the wristband heart rate monitor was approved through comparing the results with those collected via electrocardiography. The target heart rate was calculated using the Karvonen formula: (HR Target = HR Rest + [(%Desired intensity) QUOTE QUOTE ×(HR Max - HR Rest)]).22
Resistance training (RE): The exercise techniques were instructed to the women and their performance was supervised by professional trainers in group-based training sessions. The exercise was performed for 2 sessions per week (each session 60 min) during the first two weeks and was increased to 3 sessions on non-consecutive days per week. Similar to the aerobic exercise, each resistance session was started with 10 min of stretching exercises followed by 40 min of strength exercises and 10 min of balance exercises. Strength training consisted of 2 sets of 10 different exercises, including bench press, seated row, shoulder press, chest press, lateral pull-down, abdominal crunches, leg press, leg extension, triceps pushdown, and seated bicep curls, for upper and lower parts of the body.23 The subjects had 8-10 repetitions for each exercise and 5–10 min of rest between each set. For the first two weeks of training, the intensity of the exercises was 60% one repetition maximum (1RM) and was elevated to 75–80% 1RM from the 3rd week on. One repetition maximum was calculated based on the Brzycki formula:
Combined exercise (CE): This group also participated in group-based training sessions, so that they performed both AE and RE simultaneously in one session. The CE group performed exercise two sessions a week for the first two weeks and three sessions for the rest of the intervention period. Each session was started with 10 min stretching exercises and 20 min walking on a treadmill, followed by 5 min rest and one set of strength training, consisting of 10 different exercises similar to the RE exercise program. The sessions were ended with 10 min balance exercises. The intensities of the aerobic and strength exercises were gradually increased according to the AE and RE protocols, respectively.
Nutrition and supplements
Dietary intake was evaluated using a 24-hour dietary recall before and after the intervention. The subjects were also asked not to change their dietary habits during the intervention. They were contacted every other week by a nutritionist to ensure that no dietary changes occurred. A 24-hour dietary recall questionnaire was used to collect data on dietary energy and macronutrient content. Data were entered into Nutritionist IV software (First Databank® Inc., Hearst Corp., San Bruno, CA) for analysis.
Measurement
Demographic data were collected before the intervention. The anthropometric parameters, including body weight, height circumference, waist circumference, and hip circumference, were measured in the morning without shoes and with minimal clothing according to the WHO guideline.24 The body mass index (BMI) and waist-to-hip ratio (WHR) were calculated. Body composition parameters, including body fat percentage (%BF) and skeletal muscle mass (SMM), were measured using the bioelectric impedance analysis (InBody 720; BioSpace®, Co., Korea) with patients in the standing position, in the normal hydration status, and no vigorous exercise session for at least 6 h before the measurement. Blood pressure was measured in the seated position after 5 min rest, and the average of the two consecutive measurements was recorded.
Before the intervention, 5 mL of venous blood was taken from each participant in the morning after 12 h of fasting. The samples were incubated for 15 min in the room temperature for clotting and then centrifuged for 10 min in 4000 rpm to separate serum. The serum was frozen at −70 °C until the time of laboratory analysis. In the second stage, the blood samples were collected 24 h after the last exercise bout of the eighth week of the intervention and all processes on blood samples were repeated.
Fasting serum glucose, total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglyceride were measured by commercial kits (Pars Azmoon®, Tehran, Iran) and using the photometric and enzymatic methods and an autoanalyzer (Biotecnica instrument®, Rome, Italy). The serum fasting insulin and serum irisin levels were assessed by the enzyme-linked immunosorbent assay (ELISA) method and using the human insulin kit (ZellBio®, Ulm, Germany) and human irisin kit (Phoenix Pharmaceuticals®, Burlingame, USA). HOMA-IR was calculated using the following formula: HOMA-IR = [fasting glucose (mg/dL) × fasting insulin (mU/L)/405]. An overview of the study protocol is presented in Fig. 2.
Fig. 2.
An overview of study protocol.
Statistical analysis
The sample size was calculated to detect at least a 1.5 ng/ml (SD = 1.3) difference between the groups in terms of the plasma irisin level after the interventions. In addition, the alpha and power were set at 0.05 and 80%, respectively. Accordingly, the sample size was estimated as 15 participants per group.25,26 The extracted data were entered into the SPSS software for analysis. The Kolmogorov-Smirnov test was used to assess the normal distribution of data. The baseline status of the individuals was compared using the ANOVA test. The paired sample t-test and the Mann-Whitney U test were used to compare differences in the means of parametric and non-parametric variables, respectively, before and after the intervention period. The effectiveness of exercise programs was examined through comparing the mean difference of changes in each variable using the ANCOVA test, adjusted for the baseline body weight as a covariate, followed by the Bonferroni post hoc test. The significance level was set as p < 0.05, and the data analysis was performed using the IBM SPSS Statistics 25 (Armonk, NY: IBM Corp).
Results
Participant characteristics
The data were collected from 54 women (control group: n = 15; AE group: n = 13; RE group: n = 13; CE group: n = 13). Six women (AE group: n = 2; RE group: n = 2; CE group: n = 2) were excluded because of failure to complete more than 90% of the exercise sessions or traveling (full analysis set; Fig. 1). The participants’ mean age was 53.47 (SD = 6.53) years, and 40 (60%) participants had minimal or no formal education. Age and education showed no statistically significant difference between the study groups.
Baseline in terms of anthropometric, dietary recall, and biochemical characteristics
As shown in Table 1, except body weight, no statistically significant differences were observed between the groups at the baseline in terms of anthropometric and biochemical characteristics. About 23% of the participants were diagnosed with diabetes. According to the dietary recall, as summarized in Table 2, energy, macronutrient, and fiber intakes at the end of the study had no differences significantly with those at the baseline.
Table 1.
The baseline characteristics of the women in the groups.
| Characteristic | Control (n = 15) |
Aerobic (n = 15) |
Resistance (n = 15) |
Combination (n = 15) |
P-valuea |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Height, cm | 157.69 ± 8.25 | 160.00 ± 10.29 | 159.08 ± 7.39 | 156.83 ± 7.82 | 0.802 |
| Weight, kg | 75.61 ± 9.36 | 73.25 ± 12.28 | 74.75 ± 11.68 | 76.83 ± 8.96 | 0.036 |
| BMI, kg.cm2 | 30.41 ± 3.83 | 28.61 ± 3.46 | 29.54 ± 2.95 | 31.23 ± 3.40 | 0.051 |
| Body fat, % | 35.15 ± 7.77 | 34.86 ± 4.15 | 35.40 ± 9.95 | 33.84 ± 7.09 | 0. 239 |
| Muscle, Kg | 39.07 ± 8.41 | 42.83 ± 11.76 | 41.83 ± 7.76 | 42.08 ± 6.84 | 0.775 |
| SBP, mmHg | 133.08 ± 11.12 | 136.40 ± 15.21 | 131.17 ± 9.68 | 131.68 ± 11.47 | 0.184 |
| DBP mmHg | 87.31 ± 9.14 | 91.02 ± 10.11 | 93.55 ± 8.99 | 89.21 ± 10.19 | 0.323 |
| FBS, mg. dL | 119.07 ± 8.94 | 128.91 ± 31.65 | 115.50 ± 12.76 | 130.66 ± 9.49 | 0.186 |
| Insulin, μIU. mL | 10.15 ± 1.60 | 11.57 ± 1.04 | 10.58 ± 1.55 | 10.72 ± 1.38 | 0.894 |
| HOMA-IR | 2.98 ± 0.35 | 3.37 ± 0.49 | 3.02 ± 0.65 | 3.46 ± 0.48 | 0.604 |
| TG, mg/dl | 160.53 ± 11.70 | 154.41 ± 12.08 | 155.41 ± 11.27 | 155.41 ± 12.68 | 0.567 |
| LDL, mg/dl | 136.61 ± 12.37 | 131.58 ± 11.96 | 129.58 ± 11.98 | 122.41 ± 11.03 | 0.098 |
| HDL, mg/dl | 51.84 ± 9.59 | 51.91 ± 9.26 | 58.16 ± 13.24 | 47.25 ± 8.40 | 0.092 |
| Chol, mg/dl | 152.23 ± 8.47 | 161.25 ± 10.12 | 169.25 ± 14.55 | 153.41 ± 13.05 | 0.531 |
| Irisin, ng/mL | 9.14 ± 1.32 | 8.81 ± 1.07 | 9.42 ± 0.81 | 8.64 ± 0.98 | 0.304 |
Values are presented as mean ± standard deviation.
BMI: Body mass index; FBS: Fasting blood sugar; HOMIIR: Homeostatic Model Assessment for Insulin Resistance; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; TG: Triglyceride; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure.
Analysis of variance (ANOVA).
Table 2.
Dietary intake at baseline and at the end of the interventions in the groups.
| Marker | Control |
Aerobic |
Resistance |
Combination |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p-valuea | Pre | Post | p-valuea | Pre | Post | p-valuea | Pre | Post | p-valuea | |
| Energy (kcal) | 2245 ± 183 | 2298 ± 93 | 0.651 | 2214 ± 209 | 2301 ± 216 | 0.391 | 2188 ± 119 | 2243 ± 232 | 0.083 | 2147 ± 154 | 2287 ± 127 | 0.411 |
| Protein (g) | 84.74 ± 11.9 | 87.31 ± 9.3 | 0.401 | 81.91 ± 17.5 | 82.66 ± 13.3 | 0.683 | 79.92 ± 10.1 | 82.47 ± 8.1 | 0.432 | 85.80 ± 9.3 | 85.66 ± 12 | 0.762 |
| Total fat (g) | 67.35 ± 8.2 | 66.34 ± 9.2 | 0.514 | 65.43 ± 8.5 | 62.88 ± 10.1 | 0.184 | 67.58 ± 5.9 | 64.19 ± 5.5 | 0.214 | 68.22 ± 11.6 | 67.08 ± 7.5 | 0.452 |
| Saturated fat (g) | 14.2 ± 3.1 | 12.9 ± 5.2 | 0.213 | 19.06 ± 6.9 | 18.74 ± 8.3 | 0.444 | 16.09 ± 4.4 | 16.24 ± 5.2 | 0.782 | 15.4 ± 6.8 | 15.9 ± 4.4 | 0.691 |
| Monounsaturated fat (g) | 26.18 ± 6.5 | 29.03 ± 5.3 | 0.153 | 23.57 ± 6.7 | 24.10 ± 8.3 | 0.383 | 26.26 ± 11.3 | 27.88 ± 10.6 | 0.611 | 27.44 ± 12.7 | 26.85 ± 10.8 | 0.473 |
| Polyunsaturated fat (g) | 26.96 ± 7.0 | 24.42 ± 8.2 | 0.282 | 22.81 ± 9.1 | 20.04 ± 7.2 | 0.172 | 25.23 ± 9.1 | 20.07 ± 6.0 | 0.072 | 25.38 ± 7.4 | 24.33 ± 8.8 | 0.492 |
| Carbohydrate (g) | 324.96 ± 35.0 | 337.94 ± 43.2 | 0.424 | 346.12 ± 61.2 | 351.11 ± 38.29 | 0.272 | 315.02 ± 59.8 | 333.85 ± 41.1 | 0.063 | 297.45 ± 58.5 | 335.16 ± 48.3 | 0.051 |
| Dietary fiber (g) | 19.0 ± 70 | 19.3 ± 50 | 0.491 | 15.3 ± 40 | 16.10 | 0.141 | 15.8 ± 61 | 16.20 | 0.333 | 17.1 ± 40 | 17.90 | 0.361 |
p-value from the paired sample t-test comparing pre- and post-intervention dietary intakes in each group.
Intergroup comparison
After the interventions, a significant reduction was observed in the women’s weight, body fat percentage, and BMI in the AE and CE groups (Table 3). In addition, resistance and combined exercise groups gained significant skeletal muscle mass (1.67 Kg and 1.33 Kg, respectively) after the exercise period. All the intervention groups exhibited improvements in the insulin resistance index compared to the baseline. However, the RE group did not reach a significant level. Fasting serum triglyceride levels in the intervention groups declined significantly. However, changes in other lipid profiles were not significant, except for the total cholesterol in the AE (p = 0.033) and CE (p = 0.022) groups, in which a decline from the baseline values was observed. The serum level of irisin after eight weeks of the intervention did not change significantly in the AE (p = 0.221), RE (p = 0.152) and CE (p = 0.303) groups from the baseline measurements (7% increase, 3% decrease, and 3% increase, respectively).
Table 3.
Laboratory values and anthropometric measures in the groups at baseline and after 8 weeks of the interventions.
| Marker | Control (n = 15) |
Aerobic (n = 13) |
Resistance (n = 13) |
Combination (n = 13) |
P Valueb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | P valuea | Pre | Post | P value1 | Pre | Post | P valuea | Pre | Post | P valuea | ||
| Weight (Kg) | 75.61 ± 9.36 | 75.84 ± 9.50 | 0.193 | 73.25 ± 12.28 | 71.73 ± 6.91 | 0.031 | 74.75 ± 11.68 | 74.21 ± 9.74 | 0.112 | 76.83 ± 8.96 | 74.02 ± 9.09 | 0.011 | 0.001 |
| BMI (kg/cmb) | 30.41 ± 3.83 | 30.51 ± 3.85 | 0.182 | 28.61 ± 3.46 | 28.01 ± 3.61 | 0.033 | 29.54 ± 2.95 | 29.33 ± 2.98 | 0.104 | 31.23 ± 3.40 | 30.08 ± 3.04 | 0.014 | 0.001 |
| Body fat (%) | 35.15 ± 7.77 | 34.92 ± 8.06 | 0.642 | 34.86 ± 4.15 | 30.82 ± 5.38 | 0.014 | 35.40 ± 9.95 | 33.93 ± 9.46 | 0.083 | 33.84 ± 7.09 | 31.50 ± 8.26 | 0.013 | 0.011 |
| Muscle (Kg) | 39.07 ± 8.41 | 39.15 ± 7.72 | 0.101 | 42.83 ± 11.76 | 43.16 ± 10.70 | 0.102 | 41.83 ± 7.76 | 43.50 ± 10.75 | 0.012 | 42.08 ± 6.84 | 43.41 ± 8.69 | 0.033 | 0.013 |
| SBP (mmHg) | 133.08 ± 11.12 | 135.12 + 11.09 | 0.893 | 136.40 ± 15.21 | 131.22 + 10.30 | 0.214 | 131.17 ± 9.68 | 131.78 + 13.59 | 0.781 | 131.68 + 11.47 | 127.65 + 10.05 | 0.141 | 0.234 |
| DBP (mmHg) | 87.31 ± 9.14 | 88.06 + 7.5 | 0.452 | 91.02 ± 10.11 | 88.50 + 6.48 | 0.191 | 93.55 ± 8.99 | 90.04 + 10.10 | 0.243 | 89.21 + 10.19 | 86.87 + 8.92 | 0.172 | 0.411 |
| Glucose (mg/dL) | 119.07 ± 8.94 | 122.38 ± 9.05 | 0.102 | 128.91 ± 31.65 | 105.08 ± 28.13 | 0.011 | 115.50 ± 12.76 | 113.25 ± 12.96 | 0.111 | 130.66 ± 9.49 | 111.14 ± 9.93 | 0.022 | 0.010 |
| Insulin (μIU/mL) | 10.15 ± 1.60 | 9.98 ± 1.61 | 0.283 | 11.57 ± 1.04 | 10.50 ± 1.09 | 0.084 | 10.58 ± 1.55 | 9.32 ± 1.52 | 0.042 | 10.72 ± 1.38 | 8.45 ± 1.62 | 0.011 | 0.007 |
| HOMA-IR | 2.98 ± 0.35 | 3.01 ± 0.54 | 0.451 | 3.37 ± 0.49 | 2.72 ± 0.51 | 0.035 | 3.02 ± 0.65 | 2.60 ± 0.64 | 0.050 | 3.46 ± 0.48 | 2.31 ± 0.55 | 0.001 | 0.001 |
| TG (mg/dL) | 160.53 ± 11.70 | 160.00 ± 13.44 | 0.593 | 154.41 ± 12.08 | 140.75 ± 12.67 | 0.011 | 155.41 ± 11.27 | 148.66 ± 10.94 | 0.022 | 155.41 ± 12.68 | 142.75 ± 8.33 | 0.011 | 0.020 |
| LDL (mg/dL) | 136.61 ± 12.37 | 137.30 ± 11.75 | 0.121 | 131.58 ± 11.96 | 129.50 ± 12.74 | 0.232 | 129.58 ± 11.98 | 126.33 ± 12.04 | 0.173 | 122.41 ± 11.03 | 117.25 ± 11.29 | 0.061 | 0.060 |
| HDL (mg/dL) | 51.84 ± 9.59 | 52.00 ± 10.97 | 0.781 | 51.91 ± 9.26 | 51.08 ± 9.03 | 0.222 | 58.16 ± 13.24 | 58.83 ± 14.07 | 0.121 | 47.25 ± 8.40 | 50.08 ± 8.45 | 0.050 | 0.264 |
| Cholesterol (mg/dL) | 152.23 ± 8.47 | 151.11 ± 9.85 | 0.762 | 161.25 ± 10.12 | 157.00 ± 9.12 | 0.033 | 169.25 ± 14.55 | 167.33 ± 12.85 | 0.101 | 153.41 ± 13.05 | 147.83 ± 13.20 | 0.022 | 0.284 |
| Irisin (ng/mL) | 9.14 ± 1.32 | 9.02 ± 1.33 | 0.249 | 8.81 ± 1.07 | 9.04 ± 1.15 | 0.221 | 9.42 ± 0.81 | 9.31 ± 0.97 | 0.152 | 8.64 ± 0.98 | 8.78 ± 1.33 | 0.303 | 0.117 |
Values are presented as mean ± standard deviation.
BMI: Body mass index; FBS: Fasting blood sugar; HOMIIR: Homeostatic Model Assessment for Insulin Resistance; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; TG: Triglyceride; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure.Paired sample t-test is based on the analysis of variance, comparing the groups for exact changes (pre- and post-intervention measures); P < 0.05 for all comparisons.
Paired sample t-test.
Based on the ANCOVA for comparing the groups (pre- and post-intervention measures).
Intragroup comparison
Fig. 3 shows changes in each parameter and the intragroup comparisons after the interventions in the study groups. Body weight decreased significantly in the AE and CE groups in comparison with that of the control group (p = 0.026 and p = 0.019, respectively). In addition, body fat percentage decreased significantly in all intervention groups. However, only the RE and CE groups developed greater skeletal muscle masses.
Fig. 3.
Comparison of changes in anthropometric and biochemical indices between the groups.
∗: Significant difference in comparison with the control group (p < 0.05).
&: Significant difference in comparison with the AE group (p < 0.05).
#: Significant difference in comparison with the RE group (p < 0.05).
On the other hand, irrespective of the type of exercise, the interventions induced a significant reduction in fasting glucose (p = 0.013), HOMA-IR (p < 0.001), and triglyceride (p = 0.020) in comparison to those of the control group. At the eighth week of follow-up, comparing the changes in serum irisin showed no statistically significant differences between the groups (p = 0.117).
Table 4 represents correlations between the changes in the irisin level and other variables. The changes in serum irisin were statistically correlated with the changes in the body weight (r = −0.357, p = 0.01), body fat percentage (r = 0.532, p = 0.01), and HOMA-IR (r = 0.424, p = 0.02). Also, Irisin was inversely correlated with cholesterol (r = −0.637, p = 0.01) in the AE group.
Table 4.
Correlations between the variables.
| Group: Irisin/Markers | Weight | BMI | Glucose | Insulin | HOMI-IR | Body fat | Muscle | HDL | LDL | TG | Cholesterol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All: Irisin | −0.357a | 0.307 | 0.381 | 0.319 | 0.424a | 0.532a | −0.191 | −0.064 | 0.089 | 0.266 | 0.178 |
| Combination: Irisin | 0.073 | 0.057 | 0.022 | 0.558 | 0.570 | 0.370 | 0.095 | 0.100 | 0.159 | −0.413 | 0.066 |
| Resistance: Irisin | −0.548 | −0.535 | 0.058 | −0.423 | −0.308 | 0.016 | 0.326 | −0.229 | -.0551 | −0.111 | −0.637a |
| Aerobic: Irisin | 0.208 | 0.201 | −0.243 | 0.247 | 0.044 | 0.076 | −0.286 | 0.172 | 0.447 | −0.343 | 0.137 |
BMI: Body mass index; HOMIIR: Homeostatic Model Assessment for Insulin Resistance; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; TG: Triglyceride.
Indicates that the correlation is statistically significant at the 0.05 level (2-tailed).
Discussion
This study aimed to compare the effects of eight weeks of aerobic, resistance, and a combination of aerobic and resistance exercises on the serum levels of irisin, glycemic indices, and lipid profiles in women with MetS. The main finding of this randomized controlled trial was that serum irisin levels did not change after exercise compared with those before the intervention. In addition, none of the AE, RE or CE groups exhibited a different pattern of irisin secretion due to the exercise interventions compared with that of the control group. The effectiveness of the exercise regimens was confirmed based on the significant reductions of body fat in the AE, RE, and CE groups and greater skeletal muscle masses in the RE and CE groups.
Several studies have explored the effects of different exercise regimens on serum irisin. Nonetheless, their results have not been consistent enough to warrant a firm conclusion. This study found no significant change in circulating irisin due to the exercise intervention, but Huh et al.25 showed that regardless of the participants’ age, fitness level, or health status, different exercise regimens, including high-intensity swimming, treadmill, and resistance exercise, increased the level of circulating irisin. Similarly, Daskalopoulou et al.27 showed that three different exercise protocols significantly increased serum irisin, especially after a maximal exercise workload. These discrepant results could be due to differences in study designs. Huh et al. measured serum irisin during and minutes after an acute exercise session, but the subjects in the present study were evaluated after several weeks of training and in the resting state. Norheim et al. reported that the plasma levels of irisin increased right after 45 min of ergometer cycling and dropped 2 h post exercise. Since the production of irisin is dependent on PGC-1α activity and FNDC5 transcription, during acute exercise, the sharp increase in serum irisin could be due to FNDC5 mRNA translation rather than FNDC5 transcription.4 The study of Norheim et al. showed that PGC-1α transcription and FNDC5 mRNA expression increased significantly after 12 weeks of systematic exercise, but these increases did not translate to higher concentrations of serum irisin, which was consistent with our results. Likewise, Hecksteden et al.28 reported that 26 weeks of aerobic or strength training did not induce an increase in irisin production while it significantly improved maximal performance.
In addition to acute or chronic exercise intervention, patients’ anthropometric and health status may also affect the results. In this study, the subjects were overweight/obese and were diagnosed with MetS. Similarly, the participants in Norheim et al.’s study were obese and prediabetic volunteers, but the Pekkala1 et al.’s29 study focused on healthy man, and their findings did not confirm the effects of AE, RE, and CE in inducing PGC-1α and FNDC5 expression or irisin in healthy men. Timmons et al. (2012) studied the effects of endurance and resistance exercise on 205 healthy individuals and those with type II diabetes for 6 weeks. The results showed a greater expression of fibronectin type III domain-containing protein 5 (FNDC5) in the intervention group compared to that of the control group.5
Recently, Osella et al.30 evaluated the effects of different diets on the serum irisin concentration and found that vegetable proteins and saturated fatty acids were positively associated with serum irisin. Unlike the studies reviewed above, the current study used a dietary regimen based on the pre-intervention dietary intake to adjust the effect of dietary components on serum irisin.
As expected, exercise interventions induced a positive change in glycemic indices, and the change was more significant in the CE group. Our results were similar to those of a previous study that evaluated the efficacy of different exercise regimens on serum omentin-1 in diabetic women.20 The Pearson correlation test showed that changes in the HOMA-IR, body weight and body fat percentage were correlated positively with serum irisin in all participants. A positive relationship between serum irisin and metabolic disorders, such as obesity, diabetes, and cardiovascular diseases, has been reported in previous studies.31,32 Stengel et al.33 showed that obese patients had a higher level of circulating irisin than normal-weight subjects. In addition, serum irisin was positively correlated with fat mass and insulin resistance. Our study showed that a reduction in insulin resistance or body fat percentage was associated with lower serum irisin. Contrariwise, Crujeiras et al.34 reported that the circulating irisin increased proportionally to the weight regain after that patients underwent a calorie-restricted weight reduction diet.
There are two hypotheses to explain the underlying mechanism behind the association of serum irisin and weight gain. In the first hypothesis, an increase in serum irisin is an adaptive response to weight gain and an increase in body fat. In recent years, the interaction between adipose tissue and muscle mass has been emphasized as it plays an important role in regulating body weight and improving metabolic risk factors.35 Accordingly, the conversion of white fat tissue to brown adipose tissue can increase thermogenesis and energy consumption and, eventually, lead to weight loss through myokine irisin.8 Irisin stimulates the expression and activity of uncoupling protein1 (UCP1) and causes the browning of the white adipose tissue. Therefore, it increases thermogenesis and total energy expenditure, which, in turn, can reduce obesity.36,37 This hypothesis was supported by the results of an animal study, in which irisin improved hyperinsulinemia and glucose tolerance in mice with a high-fat diet.38 In the second hypothesis, an increase in serum irisin in adiposity suggests potential irisin resistance. Irisin is supposed to improve UCP1 production and promote the browning of white adipose tissues. However, it is contradictory that normal weight or anorexic patients have lower levels of circulating irisin than obese individuals. While adipose tissue itself secretes irisin, an increase in the body fat and irisin secretion does not lead to expected beneficial effects; for instance, it may lead to leptin resistance.39 Leptin is an adipocyte hormone secreted in response to fat deposits and influences the central nervous system to suppress the appetite. Despite the anorexigenic effects of leptin and its high level in adiposity, obese patients often do not experience reduced appetite.40
The results of this study also showed a significant reduction of fasting triglyceride in all groups and total cholesterol in the CE group compared to those of the control group. Nonetheless, no significant correlation was found between the changes in the lipid profile and serum irisin. Previous studies have shown a positive correlation between lipid profile and irisin concentration in adolescents and adults.41,42 Contrary to recent studies that used a cross-sectional design, in the present study, we conducted a randomized controlled trial to examine the correlation between the changes in irisin concentration and serum lipids. To the best of our knowledge, no other RCTs have investigated the relationship between irisin and lipid profile. Also, it must be added that changes in total cholesterol or triglycerides and irisin level were independent.
Study strengths and limitations
As a limitation, the inclusion of the expression of FND5 gene in skeletal muscle and fat mass could reveal the source of circulating irisin in the body. The process of evaluating the dietary intake before the intervention and using a dietary regimen based on the pre-intervention intake improved the quality of data collection and can be considered a strength of this study. Lastly, we recruited women aged 46–60 years that may influence the generalizability of our results to the whole population, so our results should be interpreted with caution when other age groups are considered.
Conclusions
All exercise groups experienced a significant positive change in anthropometric indices after eight weeks of training. In addition, fasting serum glucose and insulin resistance index decreased in the exercise groups in comparison with those of the control group. Despite no significant changes in serum irisin in the study groups, the changes in circulating irisin were positively correlated with the changes in the body weight, body fat percentage and HOMA-IR. Aerobic, resistance, and combined exercises reduced serum triglyceride. Also, a combination of aerobic and resistance exercises effectively reduced LDL-cholesterol. Finally, no significant changes were observed in systolic and diastolic blood pressure after the interventions. Different exercise regimens did not lead to different results, but combined exercises had more beneficial effects on MetS risk factors. The contradictory results on the effect of exercise in serum irisin levels highlight the need for larger-scale RCTs on healthy and non-healthy participants.
Ethics approval and consent to participate
The research proposal was approved by the Ethics Committee affiliated with Shiraz University of Medical Sciences (decree code: 4500, IR.SUMS.REC. 1397.279). Also, the research protocol was registered on the Iranian clinical trial registration website under the code of IRCT20180806040721N2.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable requests.
Funding
This research was supported under a research grant (grant no.: 97-01-04-17589) from Shiraz University of Medical Sciences, Iran. The study funder had no role in study design, data collection, writing of the manuscript, and submission.
CRediT authorship contribution statement
Aria Dianatinasab: Conceptualization, Investigation, Methodology, Writing - original draft. Roghayeh Koroni: Data curation, Project administration, Software. Mehrdad Bahramian: Data curation, Visualization. Mojtaba Vaismoradi: Supervision, Validation, Writing - review & editing. Mohammad Fararouei: Formal analysis, Funding acquisition, Project administration, Validation, Writing - review & editing. Sasan Amanat: Conceptualization, Formal analysis, Resources.
Declaration of competing interest
The authors stated no conflict of interest.
Acknowledgments
The authors would like to thank the participation of the women in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jesf.2020.06.004.
Contributor Information
Aria Dianatinasab, Email: dinati.aria@gmail.com.
Roghayeh Koroni, Email: r.koroni1371@gmail.com.
Mehrdad Bahramian, Email: m.bahramian69@gmail.com.
Mojtaba Vaismoradi, Email: mojtaba.vaismoradi@nord.no.
Mohammad Fararouei, Email: fararouei@sums.ac.ir.
Sasan Amanat, Email: sasan.amanat@gmail.com.
Abbreviations used
- MetS
Metabolic Syndrome
- AE
Aerobic Exercise
- RE
Resistant Exercise
- CE
Combined Exercise
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- FBS
Fasting Blood Sugar
- SD
Standard Deviation
- BMI
Body Mass Index
- WHR
Waist-to-Hip Ratio
- HDL
High-Density Lipoprotein
- LDL
Low-Density Lipoprotein
- TG
Triglyceride
- UCP1
Uncoupling Protein-1
- FNDC5
Fibronectin Type III Domain-Containing Protein 5
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Amanat S., Ghahri S., Dianatinasab A., Fararouei M., Dianatinasab M. Exercise and type 2 diabetes. Adv Exp Med Biol. 2020;1228:91–105. doi: 10.1007/978-981-15-1792-1_6. [DOI] [PubMed] [Google Scholar]
- 2.Huh J.Y., Siopi A., Mougios V., Park K.H., Mantzoros C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J Clin Endocrinol Metab. 2015;100:E453–E457. doi: 10.1210/jc.2014-2416. [DOI] [PubMed] [Google Scholar]
- 3.Boström P., Wu J., Jedrychowski M.P. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norheim F., Langleite T.M., Hjorth M. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–749. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 5.Timmons J.A., Baar K., Davidsen P.K., Atherton P.J. Is irisin a human exercise gene? Nature. 2012;488:E9–E10. doi: 10.1038/nature11364. discussion E10-11. [DOI] [PubMed] [Google Scholar]
- 6.Stengel A., Hofmann T., Goebel-Stengel M., Elbelt U., Kobelt P., Klapp B.F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity--correlation with body mass index. Peptides. 2013;39:125–130. doi: 10.1016/j.peptides.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Fox J., Rioux B.V., Goulet E.D.B. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: a meta-analysis. Scand J Med Sci Sports. 2018;28:16–28. doi: 10.1111/sms.12904. [DOI] [PubMed] [Google Scholar]
- 8.Bostrom P., Wu J., Jedrychowski M.P. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H.-J., Lee H.-J., So B., Son J.S., Yoon D., Song W. Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: a pilot study. Physiol Res. 2016;65:271. doi: 10.33549/physiolres.932997. [DOI] [PubMed] [Google Scholar]
- 10.Whillier S. Exercise and insulin resistance. Adv Exp Med Biol. 2020;1228:137–150. doi: 10.1007/978-981-15-1792-1_9. [DOI] [PubMed] [Google Scholar]
- 11.Choi Y.K., Kim M.K., Bae K.H. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract. 2013;100:96–101. doi: 10.1016/j.diabres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Liu R., Shi L., Peng N., Zhang Q., Li H. Higher baseline serum irisin decreases risk for body mass index increment in Chinese populations: a 3.2-year cohort study. Diabetes Ther. 2019;10:713–723. doi: 10.1007/s13300-019-0588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hisamatsu T., Miura K., Arima H. Relationship of serum irisin levels to prevalence and progression of coronary artery calcification: a prospective, population-based study. Int J Cardiol. 2018;267:177–182. doi: 10.1016/j.ijcard.2018.05.075. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z., Chen K., Han Y. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J Cardiovasc Pharmacol. 2018;72:259. doi: 10.1097/FJC.0000000000000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Zhao Y.T., Zhang S. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J Cell Physiol. 2017;232:3775–3785. doi: 10.1002/jcp.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huh J.Y., Panagiotou G., Mougios V. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crujeiras A.B., Zulet M.A., Lopez-Legarrea P. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism. 2014;63:520–531. doi: 10.1016/j.metabol.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Polyzos S.A., Anastasilakis A.D., Efstathiadou Z.A. Irisin in metabolic diseases. Endocrine. 2018;59:260–274. doi: 10.1007/s12020-017-1476-1. [DOI] [PubMed] [Google Scholar]
- 19.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.AminiLari Z., Fararouei M., Amanat S. The effect of 12 Weeks aerobic, resistance, and combined exercises on omentin-1 levels and insulin resistance among type 2 diabetic middle-aged women. Diabetes Metab J. 2017;41:205–212. doi: 10.4093/dmj.2017.41.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Expert Panel on Detection E. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Jama. 2001;285:2486. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Yavari A., Najafipoor F., Aliasgarzadeh A., Niafar M., Mobasseri M. Effect of aerobic exercise, resistance training or combined training on glycaemic control and cardiovascular risk factors in patients with type 2 diabetes. Biol Sport. 2012;29:135. [Google Scholar]
- 23.Yavari A., Najafipoor F., Aliasgarzadeh A., Niafar M., Mobasseri M. Effect of aerobic exercise, resistance training or combined training on glycaemic control and cardiovascular risk factors in patients with type 2 diabetes. Biol Sport. 2012;29:135. [Google Scholar]
- 24.Organization W.H. Report of a WHO Expert Committee; 1995. Physical Status: The Use of and Interpretation of Anthropometry. [PubMed] [Google Scholar]
- 25.Huh J.Y., Siopi A., Mougios V., Park K.H., Mantzoros C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J Clin Endocrinol Metab. 2015;100:E453–E457. doi: 10.1210/jc.2014-2416. [DOI] [PubMed] [Google Scholar]
- 26.Shabani R., Izaddoust F. Effects of aerobic training, resistance training, or both on circulating irisin and myostatin in untrained women. Acta Gymnica. 2018;48:47–55. [Google Scholar]
- 27.Daskalopoulou S.S., Cooke A.B., Gomez Y.H. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur J Endocrinol. 2014;171:343–352. doi: 10.1530/EJE-14-0204. [DOI] [PubMed] [Google Scholar]
- 28.Hecksteden A., Wegmann M., Steffen A. Irisin and exercise training in humans - results from a randomized controlled training trial. BMC Med. 2013;11:235. doi: 10.1186/1741-7015-11-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pekkala S., Wiklund P.K., Hulmi J.J. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol. 2013;591:5393–5400. doi: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osella A.R., Colaianni G., Correale M. Irisin serum levels in metabolic syndrome patients treated with three different diets: a post-hoc analysis from a randomized controlled clinical trial. Nutrients. 2018;10:844. doi: 10.3390/nu10070844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sesti G., Andreozzi F., Fiorentino T.V. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 2014;51:705–713. doi: 10.1007/s00592-014-0576-0. [DOI] [PubMed] [Google Scholar]
- 32.Shoukry A., Shalaby S.M., El-Arabi Bdeer S., Mahmoud A.A., Mousa M.M., Khalifa A. Circulating serum irisin levels in obesity and type 2 diabetes mellitus. IUBMB Life. 2016;68:544–556. doi: 10.1002/iub.1511. [DOI] [PubMed] [Google Scholar]
- 33.Stengel A., Hofmann T., Goebel-Stengel M., Elbelt U., Kobelt P., Klapp B.F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity–correlation with body mass index. Peptides. 2013;39:125–130. doi: 10.1016/j.peptides.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Crujeiras A.B., Zulet M.A., Lopez-Legarrea P. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism. 2014;63:520–531. doi: 10.1016/j.metabol.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Moreno-Navarrete J.M., Ortega F., Serrano M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98:E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 36.O’Neill S., O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y., Xie C., Wang H. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am J Physiol Endocrinol Metab. 2016;311:E530–E541. doi: 10.1152/ajpendo.00094.2016. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Li R., Meng Y. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514–525. doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- 39.Sahin-Efe A., Upadhyay J., Ko B.J. Irisin and leptin concentrations in relation to obesity, and developing type 2 diabetes: a cross sectional and a prospective case-control study nested in the Normative Aging Study. Metabolism. 2018;79:24–32. doi: 10.1016/j.metabol.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Crujeiras A.B., Carreira M.C., Cabia B., Andrade S., Amil M., Casanueva F.F. Leptin resistance in obesity: an epigenetic landscape. Life Sci. 2015;140:57–63. doi: 10.1016/j.lfs.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Jang H.B., Kim H.J., Kang J.H., Park S.I., Park K.H., Lee H.J. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metabolism. 2017;73:100–108. doi: 10.1016/j.metabol.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Shoukry A., Shalaby S.M., El-Arabi Bdeer S., Mahmoud A.A., Mousa M.M., Khalifa A. Circulating serum irisin levels in obesity and type 2 diabetes mellitus. IUBMB Life. 2016;68:544–556. doi: 10.1002/iub.1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable requests.