Abstract
Pandemic coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and poses an unprecedented challenge to healthcare systems due to the lack of a vaccine and specific treatment options. Accordingly, there is an urgent need to understand precisely the pathogenic mechanisms underlying this multifaceted disease. There is increasing evidence that the immune system reacts insufficiently to SARS-CoV-2 and thus contributes to organ damage and to lethality. In this review, we suggest that the overwhelming production of reactive oxygen species (ROS) resulting in oxidative stress is a major cause of local or systemic tissue damage that leads to severe COVID-19. It increases the formation of neutrophil extracellular traps (NETs) and suppresses the adaptive arm of the immune system, i.e. T cells that are necessary to kill virus-infected cells. This creates a vicious cycle that prevents a specific immune response against SARS-CoV-2. The key role of oxidative stress in the pathogenesis of severe COVID-19 implies that therapeutic counterbalancing of ROS by antioxidants such as vitamin C or NAC and/or by antagonizing ROS production by cells of the mononuclear phagocyte system (MPS) and neutrophil granulocytes and/or by blocking of TNF-α can prevent COVID-19 from becoming severe. Controlled clinical trials and preclinical models of COVID-19 are needed to evaluate this hypothesis.
Keywords: COVID-19, Oxidative stress, Immune system, Lymphopenia, T cells, Neutrophil extracellular traps (NETs)
1. Introduction
The present pandemic represents the third known outbreak of a coronavirus (CoV)-associated severe human disease due to spillover of an animal coronavirus to humans (Wu et al., 2020). The first time this group of viruses entered the public spotlight was in 2002 during the Severe Acute Respiratory Syndrome (SARS) epidemic caused by SARS-CoV (Drosten et al., 2003). Subsequently, in 2012 the Middle East respiratory syndrome (MERS)-CoV was identified in patients (Zaki et al., 2012). Finally, SARS-CoV-2 was discovered and associated with severe coronavirus disease 2019 (COVID-19) (Gorbalenya et al., 2020; Wu et al., 2020; Zhou et al., 2020b).
SARS-CoV, MERS-CoV, and SARS-CoV-2 belong to a family of enveloped positive-strand RNA viruses which infect vertebrates. There are also four endemic coronaviruses that are known to cause the common cold, a mild human disease of the upper-respiratory tract (Corman et al., 2018; Fung and Liu, 2019). SARS-CoV and SARS-CoV-2 are closely related with 79% genome sequence identity (Coronaviridae Study Group of the International Committee on Taxonomy of, 2020). Cellular entry of coronaviruses is accomplished by the spike (S) protein in the viral envelope. This molecule confers upon the viral particle its crown-like shape, from which the name “coronavirus” is derived. After biosynthesis, the S protein is cleaved into a S1 and S2 subunit by a cellular furin protease (Walls et al., 2020). This step strongly enhances the cell tropism and transmissibility of the virus. Interestingly, the furin cleavage site of SARS-CoV-2 S-protein is different from SARS-CoV and other related bat viruses (Coutard et al., 2020). The S1 subunit binds to Angiotensin-Converting Enzyme 2 (ACE2), which functions as a receptor for both SARS-CoV (Li et al., 2003) and SARS-CoV-2 (Hoffmann et al., 2020; Zhou et al., 2020b). The high affinity-binding of the S protein of SARS-CoV-2 to human ACE2 is in all likelihood due to natural selection and not the result of intentional manipulation (Andersen et al., 2020). If the S2 subunit is primed on the cell surface by a protease such as the type II transmembrane serine protease (TMPRSS2) (Hoffmann et al., 2020), it triggers fusion between viral and host cell membranes facilitating direct entry of SARS-CoV-2 into the host cell. It is likely, however, that SARS-CoV-2 not only binds to ACE2, a metallopeptidase, but also to lectins such as C-type lectin DC-SIGN similar to SARS-CoV (Li et al., 2003; Jeffers et al., 2004; Yang et al., 2004). Recent studies discovered that neuropilin-1, which is strongly expressed by endothelial cells and epithelial cells facing the nasal cavity, promotes SARS-CoV-2 cell entry (Cantuti-Castelvetri et al., 2020; Daly et al., 2020).
Importantly, SARS-CoV, which caused the 2002–2004 SARS outbreak, is associated with immune dysregulation resulting in immunopathogenesis (Perlman and Dandekar, 2005). Although SARS-CoV-2 is cytopathic during replication in cell culture (Park et al., 2020) and the immune response often eliminates SARS-CoV-2 without causing symptoms, there is accumulating evidence that immunopathogenesis is also a key piece in the puzzle of COVID-19 pathogenesis.
In this review, we discuss not only important features of SARS-CoV-2 shaping the current pandemic but also the accumulating evidence for the pathogenic role of the immune system in COVID-19 and its therapeutic implications.
2. Transmission of SARS-CoV-2 in humans
Originating in a market in Wuhan, China, the virus managed to travel around the globe within a remarkably short period of time (Dong et al., 2020). The transmission characteristics of SARS-CoV-2 are key for understanding the dynamic development of the current pandemic. The pathogen enters the body mostly through inhalation of droplets and aerosols containing infectious viral particles (Lu et al., 2020; Qian et al., 2020; Van Doremalen et al., 2020). First, infection is established in the upper respiratory tract (nasal cavity, pharynx, and larynx). The goblet secretory cells of the nasal mucosa express ACE2 (Ziegler et al., 2020) making this anatomical site a main portal for initial infection and transmission (Sungnak et al., 2020). Similar to influenza A virus (IAV), high levels of virus shedding in the upper respiratory tract is observed in individuals infected with SARS-CoV-2 (Zou et al., 2020). This results in early virus transmission before the onset of symptoms (Li et al., 2020a; Wolfel et al., 2020). Transmission of SARS-CoV-2 by infected individuals that stay asymptomatic or only have mild symptoms also takes place (Jiang et al., 2020). In fact, it has been estimated that undocumented infections, which are often asymptomatic or oligosymptomatic, are the driving force behind the pandemic (Li et al., 2020c). In stark contrast, SARS-CoV replicates only in the lower respiratory tract (trachea, bronchi, bronchioles, and alveoli) resulting in early onset of more severe symptoms and virus transmission after debut of symptoms (Cheng et al., 2004).
Supporting these clinical observations, infection experiments with non-human primates demonstrated SARS-CoV-2 antigen in ciliated epithelial cells of nasal mucosae (Rockx et al., 2020) whereas this has not been reported for SARS-CoV (Kuiken et al., 2003) or MERS (Rockx et al., 2020). IAV, however, shows tropism for the mucosa of the upper respiratory tract in an animal model (Richard et al., 2020) similar to SARS-CoV-2. Due to the restriction of transmission to the symptomatic phase, quarantine measures during the SARS outbreak in 2002–2004 were successful and could stop the virus from spreading further. In contrast, transmission of SARS-CoV-2 in the asymptomatic, oligosymptomatic, or presymptomatic phase is the Achilles’ heel of Covid-19 pandemic control because it makes symptom-based isolation of SARS-CoV-2 infected individuals inefficient (Gandhi et al., 2020).
3. Clinical course of COVID-19
The clinical course of SARS-CoV-2 infection is highly variable and ranges from asymptomatic or oligosymptomatic to severe organ dysfunction and death. In the incubation period and the early phase of COVID-19, SARS-CoV-2 replication in the upper respiratory tract triggers only a limited innate response. At this point, symptoms may be absent and viral spread may be terminated. The exact proportion of subclinical infections has to be determined in future serological studies. In the first week, however, non-productive cough, aching limbs, general fatigue, sore throat, loss of smell/taste, and headache may occur. Subsequently, SARS-CoV-2 can reach the lower respiratory tract and the lung alveoli (Li et al., 2020d). Whereas severe IAV infection is usually observed within a few days, severe COVID-19 occurs in the second symptomatic week and patients are often hospitalized for 3–4 days before being transferred to the intensive care unit (ICU). A characteristic feature found in chest computed tomography (CT) of acute lung injury (ALI) in severe COVID-19 are bilateral patchy infiltrates and “ground glass” opacities (Ye et al., 2020). Shortness of breath and fever may be a harbinger of severe COVID-19, which is complicated in 15%–30% of cases by an acute respiratory distress syndrome (ARDS) (Chen et al., 2020b; Guan et al., 2020; Huang et al., 2020; Wang et al., 2020a; Zhou et al., 2020a). A plethora of studies have shown that SARS-CoV-2 is not restricted to lung tissue but spreads systemically thereby causing an extensive immune response and considerable damage in other organs including brain, heart and blood vessels, liver, kidneys, and intestine (Li et al., 2020d; Shi et al., 2020). ARDS requires admission to the ICU, and is often accompanied by microthrombosis resulting in shock and multiorgan failure. In fact, acute cardiovascular and kidney damage are frequently observed in patients with severe COVID-19 and contribute to the case fatality rate (Akhmerov and Marban, 2020; Bonow et al., 2020; Madjid et al., 2020; Ronco and Reis, 2020). The severity of COVID-19 correlates with increased age, comorbidity factors such as diabetes, obesity, chronic lung disease, and cardiovascular disease, and male sex, although severe disease is not limited to these risk groups (Guo et al., 2020; Chen et al., 2020b; Garg et al., 2020; Yang et al., 2020a).
4. Pathogenesis of COVID-19
4.1. SARS-CoV-2 infection
The epithelium in the respiratory tract functions as a physical barrier and also contributes to the elimination of invading pathogens. The alveoli in the lower respiratory tract are lined with type I alveolar epithelial cells (AEC I) which cover 90–95% of the alveolar surface and regulate gas exchange. In contrast, type II alveolar epithelial cells (AEC II) represent only about 7% of the alveolar surface and produce antimicrobial products such as complement, lysozyme, and surfactant proteins (SP). Although lung inflammation causes the most prominent symptoms of COVID-19, ACE2 mRNA expression in the lung is only moderate and concentrated in AEC II cells (Li et al., 2020b; Zhao et al., 2020). Similar to SARS-CoV, the novel coronavirus may also nonproductively infect immune cells such as T cells (Gu et al., 2005), macrophages (Cheung et al., 2005; Yilla et al., 2005), monocytes (Yilla et al., 2005) and dendritic cells (DCs) (Law et al., 2005). SARS-CoV-2 may induce phenotypic and functional maturation of cells of the mononuclear phagocyte system (MPS) as SARS-CoV has been shown to upregulate MHC class II and costimulatory molecules on monocyte-derived DCs (Tseng et al., 2005). The MPS comprises not only DCs but also macrophages and monocytes. These immune cells are similar with regard to ontogeny, location, function and phenotype (Guilliams et al., 2014). Intriguingly, MPS cells account for the majority of immune cells in the inflamed lung microenvironment of severe COVID-19 (Liao et al., 2020). SARS-CoV-2 may use MPS cells as a Trojan horse in order to disseminate within the human organism and establish systemic infection (Park, 2020) similar to other emerging pathogens such as hantaviruses (Raftery et al., 2002; Raftery et al., 2020).
Although SARS-CoV-2 primarily replicates in the respiratory tract, autopsies demonstrate that it can infect cells in multiple organs, including the lungs, pharynx, heart, liver, brain, and kidneys (Puelles et al., 2020). Expression level and tissue distribution of ACE2 and TMPRSS2 mark the route of viral spread throughout the body (Sungnak et al., 2020). Besides lung epithelial cells other cells in the kidney, intestine, and heart express also ACE2 and can be infected (Zou et al., 2020; Hamming et al., 2004; Farkash et al., 2020; Hikmet et al., 2020; Zhao et al., 2020; Ziegler et al., 2020). Importantly, endothelial cells express ACE2 (Hamming et al., 2004; Ferrario et al., 2005) and are susceptible to infection with SARS-CoV-2 (Varga et al., 2020). In accordance, infection of human blood vessel organoids with SARS-CoV-2 can be blocked by soluble ACE2 molecules (Monteil et al., 2020). Sinus endothelial cells in lymph nodes were also positive (Hamming et al., 2004). Virus infection of endothelial cells causes vascular injury, inflammation of the vessel walls, and vascular dysfunction.
4.2. Megakaryocytes and platelets
It is becoming apparent that clotting at multiple sites including the brain plays an important role in COVID-19 pathogenesis (Willyard, 2020; Wise, 2020). In most of the terminally ill COVID-19 patients disseminated intravascular coagulation (DIC) is found (Tang et al., 2020) and may play a major role in the development of multi organ failure (Gando et al., 2020). In accordance, high blood levels of D-dimers, which are produced when protective mechanisms try to dissolve clots, can predict a severe course of COVID-19 (Zhang et al., 2020b). In fact, the extent of thrombosis is remarkably high affecting 20–40% of COVID-19 patients that are critical ill (Klok et al., 2020; Middeldorp et al., 2020; Poissy et al., 2020). The underlying mechanisms are unclear but most likely involve virus-induced inflammation of vessels (Wise, 2020). In addition, a drop in platelet numbers occurs and is associated with a severe course of COVID-19 and higher lethality (Lippi et al., 2020). Altered thrombopoiesis and increased destruction, consumption and sequestration of platelets may all contribute to thrombocytopenia in severe COVID-19 (Xu et al., 2020).
Platelet-releasing megakaryocytes reside not only in the bone marrow but also in the vasculature of the lung (Lefrancais et al., 2017). It is estimated that up to 60% of total platelet production in humans is due to platelet biogenesis in the lung (Lefrancais and Looney, 2019; Washington et al., 2020). Intriguingly, histopathologic analyses of SARS-CoV-2-related death revealed platelet–fibrin thrombosis and intravascular megakaryocytes in many organs, including the heart, lungs, kidneys, and liver (Fox et al., 2020). The pathogenic role of these broadly distributed extramedullary megakaryocytes in COVID-19 is unclear at the moment. It is likely, however, that the observed thrombosis in microvessels of COVID-19 patients is due to immunothrombosis. This form of thrombosis, which is triggered by inflammatory stimuli, aims at immobilizing invading pathogens and may represent an evolutionarily ancient component of the innate immune system in mammals (Engelmann and Massberg, 2013; Gaertner and Massberg, 2016). Thus, an inadequate innate immune response plays a crucial role in the pathogenesis of COVID-19.
4.3. Innate immune response
The innate immune response in the lung is a well ordered process in which several tiers of defense are involved (Iwasaki et al., 2017). At the site of infection in the respiratory tract, invading pathogens are detected by specialized cells such as airway epithelial cells (AEC), mast cells and MPS cells . These sensor cells are well equipped with pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I) and other RIG-I-like receptors (RLRs) (Chow et al., 2018). Pathogen-associated molecular patterns (PAMPs) derived from viruses trigger a specific combination of PRRs and adaptor molecules resulting ideally in an immune response tailor-made for the pathogen. For example, double-stranded RNA (dsRNA) represents a prototypical virus-derived PAMP that is also generated during coronavirus replication and detected by RIG-I (Kindler et al., 2016).
In addition, replication of cytopathic viruses such as coronaviruses cause multiple changes in cellular homeostasis such as accumulation of misfolded protein aggregates, lysosomal disruption, mitochondria damage and imbalanced ion concentrations (Zhao and Zhao, 2020). This may trigger pyroptosis, a necrotic form of programmed cell death, which ushers into secretion of pro-inflammatory molecules of the IL-1 family (Chen et al., 2019; Tsuchiya, 2020). Virus-induced cell death also releases intracellular damage-associated molecular patterns (DAMPs) such as high-mobility group box 1 (HMGB1) and histones, which are normally hidden from recognition by PRRs (Schaefer, 2014). However, upon release DAMPs trigger classical PRRs in neighboring epithelial and endothelial cells to generate additional pro-inflammatory cytokines and chemokines including IL-6, IP-10, macrophage inflammatory protein 1α (MIP1α), MIP1β and MCP1 (Tay et al., 2020). These then attract more effector cells (MPS cells and T cells) thereby driving the inflammatory process.
Sensing of coronaviruses by RIG-I and other PRRs starts an innate immune response that - if not blocked by the virus - would efficiently restrict viral replication (Tay et al., 2020). First-order cytokines such as IFN-I (IFN-α and IFN-β) and IFN-III are released and help to control and eliminate virus infections (Lazear et al., 2019). They may directly clear virus from infected cells by activating IFN-stimulated genes (ISGs) that exert direct antiviral activities or recruit antiviral effector immune cells such as macrophages. On the other hand, the antiviral immune response is a double-edged sword: It may be harmful for the organism if the timing, vigor, and target tissue of the immune response is not adequate as often observed during infection with zoonotic viruses (Supramaniam et al., 2018).
4.3.1. Interferons and proinflammatory cytokines
IFN-I/IFN-III are important antiviral cytokines, which induce a similar but not identical expression pattern of ISGs (Lazear et al., 2019). Due to different receptor usage, nearly every nucleated cell responds to type I IFN, whereas the response to type III IFNs is restricted to barrier tissues such as mucosal surfaces in the respiratory tract (Wack et al., 2015). A plethora of studies have reported an emerging role of IFN-III in the first line defenses against respiratory viral infections (Andreakos et al., 2017; Galani et al., 2017) making IFN-III a candidate for prevention and treatment of COVID-19 (Prokunina-Olsson et al., 2020). Supporting this notion, prophylactic and therapeutic treatment with IFN-III in a mouse model of COVID-19 protected mice from the deleterious consequences of infection (Dinnon et al., 2020). It has to be taken into account, however, that in mouse models IFN-III can also have detrimental effects during long-term treatment and if considerable lung damage already exists before IFN-III application (Broggi et al., 2020).
To study the innate response to SARS-CoV-2 near the site of infection, transcriptomic profiling of cells in bronchoalveolar lavages (BAL) from COVID-19 patients was performed (Zhou et al., 2020c). Bronchoscopic BAL is a useful technique for collecting lower respiratory tract specimens at the alveolar level from different areas of the lung and can predict the etiology of pneumonia in critical ill patients admitted to the ICU (Choi et al., 2014). A recent study reported elevated levels of both pro-inflammatory cytokines and IFN-I/III in BAL fluid from severe COVID-19 cases (Broggi et al., 2020). In contrast, Zhou et al. found no significant upregulation of IFN-I in BAL cells although robust ISG induction was detected (Zhou et al., 2020c). In accordance, compared to other respiratory viruses, SARS-CoV-2 replication induces unusually low levels of interferon IFN-I and IFN-III both in cell culture and in ferrets, which serve as an animal model. A similar finding has been reported in post mortem lung samples from COVID-19 patients (Blanco-Melo et al., 2020). In ex vivo human lung tissue explants, SARS-CoV-2 did not significantly induce IFN-I despite more efficient replication in comparison to SARS-CoV (Chu et al., 2020). Finally, an impaired IFN-I response in association with a high virus load was observed in the blood of severe and critical COVID-19 patients (Hadjadj et al., 2020).
These clinical and experimental observations strongly suggest that SARS-CoV-2 can efficiently subvert induction of IFN-I/III in infected cells as demonstrated for SARS-CoV (Chow et al., 2018). A recent report demonstrates that the nonstructural protein 1 (Nsp1) of SARS-CoV-2 interferes with RIG-I dependent innate immune responses that would otherwise facilitate production of IFN-I/III and virus elimination (Thoms et al., 2020). Furthermore, replication of genomic and subgenomic coronavirus RNA takes place in a special double membrane compartment that separates viral PAMPs from important PRRs such as RIG-I (Frieman and Baric, 2008). This compartmentalization also decreases detection of viral replication by cytoplasmic sensors. Finally, activation of NF-κB impairs IFN-I signaling thereby facilitating viral replication (Wei et al., 2006; Pauli et al., 2008) suggesting that cross-regulation between IFN-I/III and NF-κB signaling cascades exists (Smits et al., 2010).
Low levels of IFN-I/III allow prolonged viral replication that in turn facilitates oxidative stress. The latter is often induced by respiratory viruses (Khomich et al., 2018) and oxidatively modified proteins are found in BAL derived from ARDS patients or patients at risk of ARDS (Lenz et al., 1999). It reflects an imbalance between generation of reactive oxygen species (ROS) by enzymes such as NADPH oxidases, and scavenging of ROS by endogenous antioxidants (Chatterjee, 2016). This imbalance can divert from virus-specific, IFN-I/III driven innate immune responses and result in activation of compensatory but less-specific antiviral responses driven by the redox-sensitive transcription factor NF-κB (Schreck et al., 1991). Moreover, high ROS levels trigger oxidation of proteins, lipids and DNA, which subsequently may act as DAMPs that foster inflammation and tissue injury (Imai et al., 2005; Shimada et al., 2012). Virus-induced oxidative stress in conjunction with necrosis of virus-infected cells leads to the generation and release of oxidized endogenous ligands that function as strong DAMPs and are sensed by TLRs (Gill et al., 2010). In a mouse model of virus-induced ALI, oxidative stress triggers lung injury by upregulating production of NF-κB driven proinflammatory cytokines such as TNF-α, IL-1β, or IL-8 and adhesion molecules (Imai et al., 2008). Diversion of antiviral innate immunity by oxidative stress can foster pathological inflammation and unleash a “cytokine storm”, in which MPS cells play a crucial role (Merad and Martin, 2020). Indeed, levels of NF-κB-driven proinflammatory cytokines such as TNF-α, IL-6, IL-8 (CXCL8), GM-CSF and G-CSF as well as chemokines such as MCP1, IP10 and MIP1-α are also strongly enhanced in human individuals and animal models after infection with SARS-CoV-2 (Blanco-Melo et al., 2020; Chen et al., 2020, Chen et al., 2020c; Hadjadj et al., 2020; Huang et al., 2020; Liu et al., 2020a; Qin et al., 2020; Yang et al., 2020b; Zhou et al., 2020c). In uninfected MPS cells, the NF-κB pathway may be triggered by interaction of the viral S-protein with receptor molecules on the cell surface (Dosch et al., 2009). High amounts of anti-inflammatory cytokines such as the IL-10 family cytokines are also detected in severe COVID-19 (Chen et al., 2020a) and are induced as part of a negative feed-back loop (Ouyang and O'garra, 2019).
In mouse models of coronavirus-induced ALI, it has been shown that the timing of the IFN-I response relative to the kinetics of virus replication is critical in determining the outcome of infection with MERS-CoV (Channappanavar et al., 2019). Early IFN-I administration was protective. In contrast, late administration enhanced lethality by facilitating recruitment of neutrophils and inflammatory MPS cells, thereby increasing the release of proinflammatory cytokines, and impairing virus-specific T cell responses (Channappanavar et al., 2019). Similarly, in a mouse model of SARS-CoV infection the endogenous IFN-I response was delayed and lagged behind peak virus replication resulting in a deleterious effect of IFN-I (Channappanavar et al., 2016). Supporting a crucial role for NF-κB-driven inflammation, inhibition of NF-κB signaling in mice infected with SARS-CoV reduced inflammatory lung pathology and significantly enhanced survival (Dediego et al., 2014).
In aged experimental animals, SARS-CoV triggers a much stronger NF-kB driven innate response than in younger animals resulting in exacerbated ALI despite similar levels of viral replication (Baas et al., 2008; Rockx et al., 2009; Smits et al., 2010). This could explain why the severity of COVID-19 increases with age (Chen et al., 2020b) if SARS-CoV-2 does the same in humans. This effect is best explained by an age-related increase in ROS levels that reflect a compensatory homeostatic response to maintain physiological intracellular signaling pathways during aging (Lopez-Otin et al., 2013). It is well described that ROS have not only tissue damaging effects but also play an important physiological role in regulating various cellular processes including differentiation, autophagy, metabolic adaptation, and immune cell activation (Sena and Chandel, 2012). During infection with pathogenic respiratory viruses such as SARS-CoV-2, however, ROS levels in elder people may further increase and reach a certain threshold resulting in overactivation of NF-kB and inflammatory tissue damage (Chung et al., 2006). Similarly, the higher susceptibility of men to oxidative stress (Kander et al., 2017) may contribute to the fact that men are more prone to severe COVID-19 than women (Grasselli et al., 2020; Richardson et al., 2020).
Altogether, it is likely that the delayed and insufficient IFN-I response to SARS-CoV-2 allows prolonged virus replication and increased oxidative stress, which triggers a NF-κB-driven cytokine storm resulting in excessive inflammation.
4.3.2. Neutrophils and neutrophil extracellular traps (NETs)
Intriguingly, transcriptome analysis of human lung epithelial cells infected with SARS-CoV-2 in vitro and analysis of cytokines/chemokines in sera from COVID-19 patients revealed overexpression of several NF-κB-driven chemokines that attract neutrophils, e.g. CXCL2 and CXCL8 (Blanco-Melo et al., 2020). Increased levels of neutrophil attractant proteins such as CXCL8 are also detected in patients severely infected with SARS-CoV (Wong et al., 2004), MERS-CoV (Min et al., 2016), or SARS-CoV-2 (Chen et al., 2020, Chen et al., 2020c; Hadjadj et al., 2020; Huang et al., 2020; Liu et al., 2020a; Qin et al., 2020; Yang et al., 2020b; Zhou et al., 2020c). Neutrophils are the most abundant immune cells and represent about 50–70% of all circulating leukocytes (Ley et al., 2018). Under physiological conditions approximately 1011 neutrophils are generated each day in the human organism (Dancey et al., 1976). They are short-lived and act as first line of defense against invading pathogens (Bardoel et al., 2014; Nemeth et al., 2020). A body of evidence now exists showing that neutrophils also play an important role in combating viral infections (Naumenko et al., 2018). Large numbers of neutrophils have been shown to accumulate within the pulmonary microcirculation in the steady state suggesting that respiratory viruses interact with this important cell type at a very early stage of infection (Devi et al., 2013; Casanova-Acebes et al., 2018). Finally, activated neutrophils are of central importance to the pathogenesis of ALI, e.g. by releasing proteolytic enzymes or ROS (Lee and Downey, 2001; Grommes and Soehnlein, 2011).
Patients with severe COVID-19 are often lymphopenic whereas the number of neutrophils is increased (Fox et al., 2020; Wang et al., 2020a). There is ample evidence that an increased neutrophil-to-lymphocyte ratio is an independent risk factor precipitating a severe course of COVID-19 (Liu et al., 2020b; Qin et al., 2020; Song et al., 2020; Zhang et al., 2020a). Neutrophils and platelets closely interact to fulfill their functions in innate immune responses (Kim and Jenne, 2016). The formation of neutrophil-platelet complexes facilitates recruitment of neutrophils to inflamed tissue (Sreeramkumar et al., 2014). Moreover, neutrophils show a more vigorous defense against pathogens and release higher amounts of neutrophil mediators after binding to platelets. Finally, platelets can induce neutrophils to produce neutrophil extracellular traps (NETs) that protect from viral infection (Jenne et al., 2013). NETs are formed when neutrophils undergo a form of programmed cell death (Takei et al., 1996; Brinkmann et al., 2004) that has also been referred to as NETosis. However, the demarcation of NETosis from other forms of cell death is a matter of debate (Boeltz et al., 2019). Thus, in this review the term “NET formation” is used in preference to NETosis. NET formation may also occur without disruption of cellular membranes preserving neutrophil functions such as chemotaxis and phagocytosis (Clark et al., 2007; Yipp et al., 2012). NETs are net-like structures composed of chromatin festooned with neutrophil granule proteins. Several enzymes are involved in the process of NET formation including neutrophil elastase (NE), which enters the nucleus, degrades nuclear proteins and initiates nuclear disintegration (Papayannopoulos, 2018).
Analysis of autopsy samples from the lungs of COVID-19 patients revealed neutrophil infiltration in pulmonary capillaries, acute capillaritis with fibrin deposition, extravasation of neutrophils into the alveolar space, and neutrophilic mucositis (Barnes et al., 2020). A prominent feature of COVID-19 is multiorgan inflammation with vessel walls containing neutrophils (Varga et al., 2020). Within alveolar capillaries of patients with COVID-19, neutrophils and platelets get entrapped in fibrin meshworks (Fox et al., 2020). These neutrophils are partially degenerated suggesting that NETs are generated (Fox et al., 2020). Indeed, high serum levels of cell-free DNA, DNA-myeloperoxidase (MPO) complexes, and citrullinated histone H3 (Cit-H3) have been detected in COVID-19 and correlate with disease severity (Zuo et al., 2020a). Viable SARS-CoV-2 can directly induce NET formation in healthy neutrophils by an unknown mechanism (Veras et al., 2020). MPO-DNA and Cit-H3 are regarded as specific markers for NETs suggesting that NETs may drive cytokine release, respiratory failure, and microvascular injury in COVID-19. Several viruses including respiratory viruses reportedly induce NETs, which subsequently bind, entrap and neutralize virions, while contributing to immune activation via pattern recognition receptors (PPR) (Saitoh et al., 2012; Raftery et al., 2014; Schönrich and Raftery, 2016; Muraro et al., 2018). Importantly, ROS such as highly diffusible hydrogen peroxide (H2O2) stimulate not only NF-κB-driven production of proinflammatory cytokines like TNF-α, IL-1β, or IL-8 (Schreck et al., 1991) but also NET formation (Fuchs et al., 2007; Tripathi et al., 2018).
Virus-induced overflow of NETs can cause local or systemic damage leading to severe immunopathology and disease (Narasaraju et al., 2011; Schönrich and Raftery, 2016; Schulz et al., 2020). NETs contain high amounts of granule-derived enzymes such as cathelicidins, defensins, NE or myeloperoxidase (MPO) and nuclear proteins (e.g. histones) with strong cationic properties. In particular, histone H4 possesses a high density of cationic residues, which confers the ability to bind to negatively charged plasma membranes. This can result in cell lysis through pore formation ultimately causing tissue damage and inflammation (Silvestre-Roig et al., 2019). Such toxicity is not solely associated to histone H4 but also to other members of the histone family and readily links to detrimental outcomes in multiple diseases. In this way, NETs are cytotoxic for lung epithelium and vascular endothelium. Supporting a pathogenic role of histones, blockade of extracellular histone toxicity using activated protein C (Xu et al., 2009), modified forms of heparin (Wildhagen et al., 2014; Rasmuson et al., 2019), anti-histone antibodies (Silvestre-Roig et al., 2019; Chirivi et al., 2020), C1 esterase inhibitor (C1INH) (Wygrecka et al., 2016), or blocking peptides (Silvestre-Roig et al., 2019) reduces inflammation, tissue injury and death.
Excessive NET production has been observed in many respiratory diseases including COPD, asthma, and cystic fibrosis as well as in ALI and sepsis (Twaddell et al., 2019). Analysis of mouse models revealed a pathogenic role of unbalanced NET formation in ALI (Narasaraju et al., 2011; Caudrillier et al., 2012; Liu et al., 2016; Lefrançais et al., 2018). The loss of granule content in neutrophils and a reduced capacity for NET formation protects mice from ALI (Adrover et al., 2020). In human ARDS increased levels of NETs in the plasma are associated with a more severe and lethal course (Lefrançais et al., 2018; Bendib et al., 2019; Adrover et al., 2020). Moreover, histones accumulate in the plasma of patients with sepsis (Xu et al., 2009) contributing to cell toxicity, organ damage and death. Intriguingly, NETs prime macrophages to increase production of the IL-1β precursor molecule (Nahrendorf and Swirski, 2015). Subsequently, DAMPs generated during lytic viral replication signal through the NOD-like receptor protein 3 (NLRP3) inflammasome resulting in processing of the Il-1β precursor molecule, which is then secreted by macrophages. Active IL-1β, in turn, has been shown to induce NET formation in vitro and in vivo (Keshari et al., 2012; Meher et al., 2018). Thus, there may be a NET-IL-1β loop that enhances production of NETs and active IL-1β during SARS-CoV-2 replication thereby exacerbating tissue damage (Barnes et al., 2020; Yaqinuddin and Kashir, 2020). In addition, experiments in mouse models revealed that histone-DNA complexes bind to C-type lectins via the histone moiety and activate resident immune cells (i.e. macrophages) through endosomal TLR-9 dependent responses (Lai et al., 2020). This results in secretion of pro-inflammatory cytokines such as IL-6 or TNF-α (Lai et al., 2020), together with IL-1β important drivers of the cytokine storm observed during later stages of COVID-19 (Huang et al., 2020; Mehta et al., 2020).
Both venous thromboembolism and arterial thrombotic complications occur in COVID-19 (Klok et al., 2020; Zhou et al., 2020a). In COVID-19 patients with thrombosis, higher levels of circulating NETs are found as compared to controls (Zuo et al., 2020b). Microvascular injury, coagulopathy, thrombosis and antiphospholipid antibodies drive the pathology of severe COVID-19 (Magro et al., 2020; Zhang et al., 2020c). Supporting this notion the blood levels of D-dimer, a marker of fibrin degradation and hyperactive coagulation, strongly increase in severe COVID-19 (Lippi and Favaloro, 2020; Zhang et al., 2020a). Numerous papers have implicated NET formation in conditions associated with venous/arterial thrombosis (Fuchs et al., 2010; Brill et al., 2012; Borissoff et al., 2013; Van Avondt et al., 2019) or vasculitis (Martinod and Wagner, 2014; Doring et al., 2020; Silvestre-Roig et al., 2020). NETs support immune-mediated blood coagulation, fibrin formation, and microvascular clotting by several mechanisms including recruitment and activation of platelets (Gaertner and Massberg, 2016; Laridan et al., 2019). Platelets that have been activated (e.g. via TLR4), interact with neutrophils via P-selectin and its ligand (PSGL-1) thereby facilitating NET formation (Sreeramkumar et al., 2014; Etulain et al., 2015). It is well described that activated platelets trigger NET formation (Clark et al., 2007; Massberg et al., 2010). If physiological immunothrombosis gets out of control, however, it may foster shock-associated intravascular coagulation in multiple organs. Supporting the crucial role of NETs in thrombotic events, depletion of neutrophils prevents thrombus formation (Von Brühl et al., 2012). In the circulation, the two nucleases DNase-1 and DNase-1L3 are responsible for the disintegration of NETs and for the control of NET-driven thromboembolic events (Jimenez-Alcazar et al., 2017). Pathological immunothrombosis is prone to affect pulmonary, cardiovascular, and renal function (Noubouossie et al., 2019) and can be dispersed by DNase treatment in animal models (Kolaczkowska et al., 2015; Wang et al., 2018). This strategy, however, is only partially successful because toxic histones remain bound to the vessel wall (Kolaczkowska et al., 2015) and the timing of DNase application seems to be important (Mai et al., 2015).
Although most SARS-CoV infections in children remain asymptomatic or show only mild symptoms (Team, 2020), recent reports from the United Kingdom, Italy and New York City suggest that COVID-19 in infants and children may be associated with Kawasaki-like inflammatory symptoms (Jones et al., 2020). Kawasaki disease (KD) represents an acute febrile illness of infants and children that is associated with multisystemic vasculitis and coronary artery lesions (Kawasaki et al., 1974). Intriguingly, spontaneous NET formation in KD patients has been reported suggesting that NETs contribute to the pathogenesis of KD (Yoshida et al., 2020) and possibly to KD-like inflammatory symptoms in COVID-19 of infants and children (Thierry, 2020).
Taken together, a slew of recent papers has indicated the crucial contribution of neutrophils and NETs to the multiple facets of COVID-19 disease. Beyond their pathogenic role, neutrophils and NETs are important markers for severe COVID-19 and crucial targets for novel therapeutic strategies.
4.4. Antigen specific immune defense by the adaptive immune system
While the activation of the innate immune system as a first line of defense starts within minutes to hours, an effective immune response of the adaptive immune system only occurs within several days. An efficient long lasting and antigen specific immune reaction, however, is essential to control viral infections in the long term and to prevent virus persistence. In viral infections, effector CD8+ T cells play an important role in killing virus-infected cells, whereas CD4+ T-cells provide help to effector CD8+ T cells and B cells. The latter produce neutralizing antibodies that are important for viral clearance and long-lasting antiviral immunity. Both CD4+ and CD8+ T cells produce IFN-γ, which exerts its antiviral effects not only by inducing ISGs but also by regulating the antiviral immune response (Boehm et al., 1997). A third type of T cells, the regulatory T cells (Treg), counteracts these activities when necessary, for example to limit the time of activation (Li et al., 2018). Mostly, Treg belong to the CD4+ T cell population and express FoxP3 (forkhead box P3), however, some of them are also CD8+ T cells. Expression of the anti-inflammatory cytokine IL-10 enhances the activity of Treg cells leading to immunosuppression and T cell anergy. Treg also counteract inflammatory processes that are induced by cells of the innate immune system. They inhibit IL-6 production of neutrophils and dictate that neutrophils produce anti-inflammatory molecules (IL-10, TGF-β and indoleamine 2,3-dioxygenase (IDO)) (Lewkowicz et al., 2013). Through the release of TGF-β, Treg induce neutrophil apoptosis. In this way, they can contribute to the resolution of an acute lung injury (D'alessio et al., 2009). Treg can also inhibit neutrophil infiltration into injured tissue. This improved the healing of myocardial infarction and kidney ischemia-reperfusion injury (Kinsey et al., 2009; Weirather et al., 2014). It can therefore be assumed that Treg also play an important role in tissue regeneration in COVID-19.
Sustained stimulation by viral antigens can lead to exhaustion of the T cells, which are responsible for eliminating the viruses (Schönrich and Raftery, 2019). Exhausted T cells (Tex) express programmed cell death protein 1 (PD-1) and/or T cell immunoglobulin and mucin domain-3 (TIM-3) and are finally depleted by apoptosis. Blockade of IL-10 or checkpoint inhibitors that block the PD-1 pathway can at least in part revert T cell exhaustion.
4.4.1. Lymphopenia and failure of antiviral T cell responses in severe COVID-19
Systemic viral infections are usually accompanied by lymphocytosis, an increase in lymphocytes in the blood. This is due to the fact that the pool of antigen specific CD8+ T lymphocytes needs to be enlarged. In COVID-19, however, decreased numbers of CD4+ and CD8+ T cells and low IFN-γ production by CD4+ T cells are observed (Chen et al., 2020a; Liu et al., 2020a; Wang et al., 2020a). The numbers of CD8+ T cells appear as independent predictor for COVID-19 severity and treatment efficacy (Wang et al., 2020b). In fact, the subacute progression of COVID-19 implicates that both T cell depletion and T cell exhaustion contribute to the failure of the adaptive immune system (Vardhana and Wolchok, 2020). In accordance, T cells from COVID-19 patients show strongly upregulated PD-1 and TIM-3 (Diao et al., 2020). The resulting weak adaptive immune response permits sustained virus replication, which further drives immunopathogenesis through the excessive inflammatory activity of the innate immune cells, a vicious cycle. In line with this assumption, the severity of the disease correlates with an increase in the neutrophil to lymphocyte ratio (Liu et al., 2020b; Qin et al., 2020; Song et al., 2020; Zhang et al., 2020a). At the moment, however, the precise mechanisms contributing to the failure of the antiviral T cell response in COVID-19 are enigmatic.
4.4.2. Oxidative stress as potential cause of lymphopenia and T cell suppression in COVID-19
One potential mechanism for COVID-19-associated reduction in T cell numbers is apoptosis. Chu et al. showed that MERS-CoV, but not SARS-CoV, can efficiently infect human T cells and trigger their apoptosis via both extrinsic and intrinsic apoptosis pathways (Chu et al., 2016). SARS-CoV-2 can induce lymphocyte apoptosis via enhancing Fas signaling (Chen et al., 2020d). In addition to Fas-induced apoptosis by the virus, a reduction in T cell numbers can also be due to oxidative stress (Klemke et al., 2008; Wabnitz et al., 2010), which is typically present in COVID-19 and ARDS (Imai et al., 2008; Kellner et al., 2017). A pro-oxidative milieu leads to oxidation of essential regulatory proteins in T cells, such as cofilin (Klemke et al., 2008; Wabnitz et al., 2010; Samstag et al., 2013) or L-plastin (Balta et al., 2019). Consequently, T cells become hypo-responsive or even die. As described above, activated neutrophils and MPS cells are to a large extent responsible for the massive release of ROS into the lung tissue. In addition, the massive TNF-α release during the cytokine storm described above could exacerbate ROS production via a positive feedback loop by activating NADPH oxidases (Blaser et al., 2016). TNF-α induced ROS production could also contribute to the extension of COVID-19 symptoms to distant tissues such as the brain (Sandoval et al., 2018).
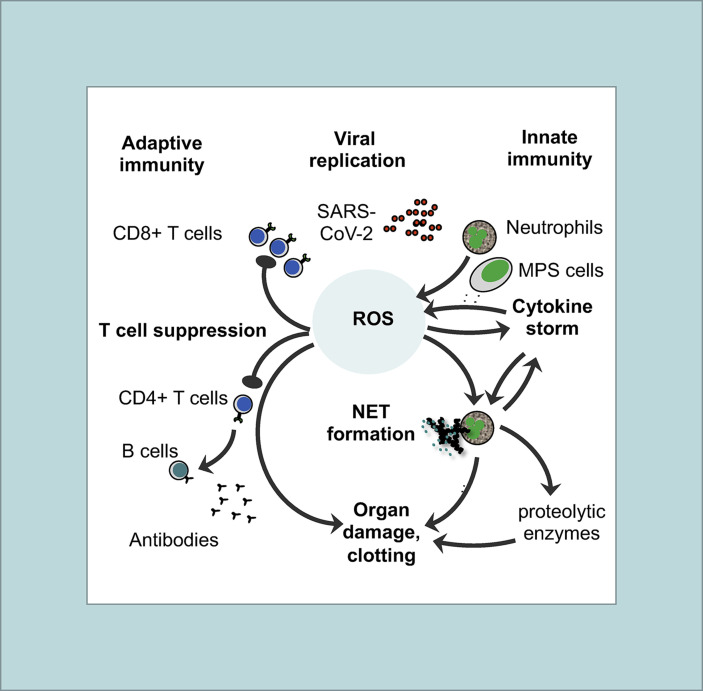
It is estimated that more than 1,7 billion people (more than 20% of the world's population) have a health problem that increases the risk for severe COVID-19 such as cardiovascular disease or diabetes mellitus (Clark et al., 2020). In the pathogenesis of these comorbidities imbalanced ROS production is also key (Shah and Brownlee, 2016), supporting the importance of oxidative stress for severe COVID-19. High glucose and also hypoxia/reperfusion occurring upon ventilation of COVID-19 patients promote ROS production. This can induce NLRP3 inflammasome-mediated pyroptosis, which is further enhanced in the presence of the bacterial component LPS (Shah and Brownlee, 2016). Moreover, SARS-CoV papain like protein significantly triggered a ROS/p38 MAPK/STAT3 pathway, leading to activation of the TGF-β1 promoter in lung epithelial cells. This effect correlated with up-regulation of pro-fibrotic responses in vitro and in vivo (Li et al., 2016). A ROS mediated release of TGF-β could also contribute to the observed lymphopenia in COVID-19, since TGF-β is a potent immunosuppressive substance acting on T cells. Finally, a pro-oxidative state in T cells (increase in ROS and decrease in GSH) was shown to promote the development of Treg cells (Liang et al., 2020). A relative increase in Treg would also counteract the T cell defense of SARS-CoV-2. Consistent with this assumption, it was reported that, unlike other T cells, the Treg population in COVID-19 patients was not decreased (Shi et al., 2020b). Overall, oxidative stress likely plays a central role in the pathogenesis of severe COVID-19 (Fig. 1 ).
Fig. 1.
Basic features of the immunopathogenesis in COVID-19with central role of ROS: Both innate (right site) and adaptive immune responses (left site) are involved in immunopathogenesis of COVID-19. The pathogenic cascade starts with prolonged and extensive replication of SARS-CoV-2 in lung epithelial cells and in endothelial cells of the vessels due to viral evasion of the IFN-I/III response. As a consequence, neutrophils and MPS cells (e.g. macrophages, monocytes, and immature DCs) are massively recruited to the inflammatory tissue. Activated neutrophils and MPS cells produce large amounts of ROS thereby creating an imbalanced oxidative stress response. Oxidation of endogenous molecules (e.g. DNA and lipids) results in DAMPs that trigger pro-inflammatory cytokine secretion (cytokine storm) through TLR signaling thereby activating the redox-sensitive transcription factor NF-κB. ROS and TLR signaling also induce an overflow of NETs. There may be several positive feedback loops between cytokines (TNF-α, IL-1β) and ROS production as well as between cytokines (TNF-α, IL-1β) and NET formation. ROS, NETs and proteolytic enzymes released by activated neutrophils also contribute to organ damage and clotting in vessels. On the other side, imbalanced ROS production also suppresses the T cell response thereby contributing to lymphopenia in COVID-19. As a result, less activated antiviral CD8 T cells are available to kill virus-infected cells and to eliminate the virus. Moreover, CD4+ T cells are less efficient in helping B cells to produce neutralizing antibodies and establish long-term immunity.
5. Treatment options for COVID-19 associatedimmunopathogenesis
The precise understanding of COVID-19 associated immunopathogenesis as summarized in Fig. 1 paves the way for developing novel treatment options. Approaches that interfere with neutrophil recruitment, neutrophil activation, and NET formation have been discussed in detail elsewhere (Barnes et al., 2020; Narasaraju et al., 2020). In addition, ROS production, cytokine storm, and T cell suppression can all be targeted specifically to ameliorate the course of SARS-CoV-2 infection. For reasons of space, we will concentrate in this overview on the antagonization of ROS and proinflammatory cytokines, which represent key targets to interrupt the vicious cycle that drives severe COVID-19.
5.1. Antioxidative substances could improve the survival and function of T cells in COVID-19 patients and prevent NET formation
5.1.1. Vitamin C
Recently, it has been hypothesized that vitamin C, also known as ascorbic acid, could have a protective effect that prevents ALI in sepsis (Li, 2018). In mouse models, vitamin C infusion indeed protected from the deleterious consequences of sepsis (Fisher et al., 2011). It enhanced the epithelial barrier function, increased alveolar fluid clearance, attenuated the proinflammatory response, and prevented sepsis-associated coagulation abnormalities (Fisher et al., 2012). Importantly, vitamin C prevented the activation of NF-kB and neutrophil sequestration (Fisher et al., 2011). In line with these findings, antioxidant vitamins including vitamin C protected against ARDS in a rat model (Erol et al., 2019). A clinical trial in sepsis patients showed that a high dose of vitamin C infusion is well tolerated in sepsis patients (Fowler et al., 2014). However, vitamin C infusion in a randomized study in patients with sepsis and ARDS did not improve organ dysfunction or the markers for inflammation and vascular injury (Fowler et al., 2019). Yet, in contrast to the mouse experiments described above, the vitamin C infusion in the patients was only carried out at a very late stage of the disease, in which the harmful effects of oxidative stress had probably already occurred.
For COVID-19, treatment with vitamin C still appears to be an attractive option. In this context, it is interesting to note that patients with infections often have a reduced vitamin C level due to metabolic changes (Hemilä, 2017). Vitamin C has multiple effects on the development, proliferation and function of lymphocytes. It has been shown to promote differentiation and proliferation of T cells (Manning et al., 2013; Ang et al., 2018; Van Gorkom et al., 2018; Kouakanou et al., 2020). When melanoma primed DCs were preincubated with vitamin C before co-culture with T cells, an enhanced generation of melanoma-specific effector and effector memory CD8+ T cells was found. This was accompanied by an increased killing of melanoma cells (Jeong et al., 2014). Such an effect would be helpful in COVID-19, since the decrease in CD8+ T cells was a predictor for COVID-19 severity and treatment efficacy (Wang et al., 2020b). Besides acting as an antioxidant, parts of the effects of vitamin C were due to its influence on epigenetic regulation (e.g. enhancement of histone demethylation). In addition, it inhibited the activation of NLRP3 inflammasomes in a macrophage cell line by blocking the intracellular shuttling of thioredoxin-interacting protein (TXNIP), a key molecule of cellular redox regulation (Choe and Kim, 2017). It was also found that the presence of vitamin C is necessary for the apoptosis and clearance of dead neutrophils from infection sites by macrophages (Carr and Maggini, 2017). Furthermore, vitamin C significantly weakened NET formation in healthy people (Bozonet and Carr, 2019). Infusion of vitamin C, therefore, appears to be a promising option to counteract the oxidative stress that leads to NF-kB driven cytokine storm and NET formation and promotes ALI/ARDS.
In three controlled studies vitamin C reduced the incidence of pneumonia in common cold and an improvement of the pneumonia by the vitamin C treatment was reported in two controlled studies (Hemila and Louhiala, 2013). A prospective randomized placebo controlled triple masked clinical trial (https://clinicaltrials.gov/ct2/show/NCT04264533) launched in February 2020 in Wuhan, China, will show how vitamin C infusion affects severe COVID-19 (Carr, 2020). Additional clinical trials are on the way, e.g. in the USA (https://clinicaltrials.gov/ct2/show/NCT04344184) and in Italy (https://clinicaltrials.gov/ct2/show/NCT04323514). Contrary to what is planned in these clinical studies, vitamin C treatment should, however, be started earlier (between days 1 and 7, and especially before an intensive care unit is required), in order to prevent ROS-induced suppression of antiviral T cells and maintain a normal neutrophil to lymphocyte ratio. This may prevent SARS-CoV-2 infection from spreading and worsening towards ARDS.
5.1.2. N-acetylcysteine (NAC)
Likewise, another antioxidant may be an attractive treatment option for COVID-19, namely N-acetylcysteine (NAC). It is a well-known drug used for mucolysis and treatment of bronchitis and also inhibits NET formation by human neutrophils in vitro (Kirchner et al., 2013). Importantly, NAC was shown to prevent immunosuppression of T cells in a pro-oxidative environment (Liang et al., 2018) and may, therefore, be able to reverse lymphopenia in COVID-19.
NAC is a precursor of glutathione (GSH), a very important cellular antioxidant. Infection of differentiated normal human bronchial epithelial cell (NHBEC) cultures with respiratory syncytial virus (RSV) significantly decreased the intracellular levels of GSH. Supplementation with NAC increased GSH and inhibited RSV infection, thereby restoring normal ciliary activity and inhibiting mucin release (Mata et al., 2012). NAC has also been tested in IAV infection suggesting that it attenuates symptoms and reduces the incidence of clinically apparent disease (De Flora et al., 1997; Lai et al., 2010). In a randomized controlled trial (RCT) with 39 patients in China suffering from community-acquired pneumonia (CAP), which is usually caused by bacteria or viruses, high dose NAC improved oxidative stress parameters (reduced malondialdehyde (MDA) and increased total antioxidant capacity (TAOC)) and diminished inflammatory factors (TNF-α). NAC-related adverse effects did not occur. Yet, the CT score of pneumonia did not change (Zhang et al., 2018). A phase II clinical trial has started at the beginning of May to treat severe or critically ill COVID-19 patients with NAC. This will be a non-randomized and non-masked single-institution study at Memorial Sloan Kettering Cancer Center, USA (https://clinicaltrials.gov/ct2/show/NCT04374461). An additional study that is planned by the Cambridge Health Alliance, USA, aims to determine whether NAC treatment can prevent those with mild or moderate COVID-19 from progressing to severe disease (https://clinicaltrials.gov/ct2/show/record/NCT04419025).
5.2. Blockade of proinflammatory cytokines
Finally, one further option to prevent imbalanced ROS production and a pathological cytokine storm of COVID-19 is by blocking the effect of TNF-α (Feldmann et al., 2020) or other proinflammatory cytokines such as IL-6. The respective biologicals such as the TNF-α antibodies infliximab and adalimumab have a good safety profile and are already frequently used to treat chronic inflammatory diseases (Silva et al., 2010) without impairing clearance of respiratory viruses (Schett et al., 2020). In RSV infected mice, anti-TNF-a treatment actually diminished the perivascular and peribronchial infiltrates and reduced the destruction of the lung tissue (Hussell et al., 2001). It does not seem unlikely that the inhibition of TNF mediated ROS induction contributed to this beneficial effect, besides blocking other known TNF functions. Therefore, anti-TNF treatment should probably be started early in the course of COVID-19 to interrupt the pathogenesis.
The RECOVERY trial (https://clinicaltrials.gov/ct2/show/NCT04381936) recently reported as a major breakthrough that dexamethasone (DXM) saves lives of severe COVID-19 patients (Ledford, 2020). DXM has a broad range of inhibitory effects on the immune system including inhibition of proinflammatory cytokine production (Tobler et al., 1992) by shifting TLR4 signaling away from NF-kB driven pro-inflammatory responses (Mogensen et al., 2008; Broering et al., 2011; Chuang et al., 2017; Curtale et al., 2017). This mechanism could be important in COVID-19 as TLR4 has been identified as a key pathway in a mouse model of virus-induced ALI/ARDS (Imai et al., 2008). In accordance, DXM impairs NET formation induced by TLR4 signaling in vitro (Wan et al., 2017), downregulates NETs levels in an animal model of asthma (Vargas et al., 2017), and is associated with reduced circulating NETs in patients with asthma (Gál et al., 2020). However, the precise mechanism underlying the beneficial effects of dexamethasone in severe COVID-19 has to be elucidated in future studies.
6. Concluding remarks
A severe course of COVID-19 is a result of both the direct cytolytic effects of SARS-CoV-2 and the deleterious consequences of the immune response. The latter comprises a virus-induced cytokine storm as well as imbalanced ROS production by cells of the MPS and neutrophils resulting in extensive NET formation, inflammation and tissue destruction. Moreover, excess ROS contribute to a suppression of the adaptive immune system due to paralysis or elimination of CD8+ cytotoxic T cells and CD4+ helper T cells. As a result, the killing of virus infected cells and an efficient production of antiviral antibodies needed for the development of a long-lasting antiviral immunity are disturbed. Given the central role of oxidative stress and formation of pro-inflammatory NETs for the pathogenesis of severe COVID-19, treatment strategies should aim at decreasing the net ROS levels and NET formation, e.g. by antioxidants such as vitamin C or NAC and/or by inhibiting ROS production, e.g. by antagonizing the activation of cells of the MPS and neutrophil granulocytes and/or by inhibiting TNF-α. This oxidative stress-based concept of COVID-19 pathogenesis and treatment should be validated in randomized controlled clinical studies. As preclinical animal models for SARS-CoV-2-associated disease mice can be used that express human ACE2 as a transgene (Bao et al., 2020) or after adenovirus-mediated transfer to lung cells (Hassan et al., 2020; Sun et al., 2020). In the future, it will be important to carry out more research into the influence of SARS-CoV-2 on various human immune cell populations in order to gain a deeper insight into the cellular and molecular mechanisms that promote the progression from mild to severe COVID-19. The detailed elucidation of immunopathogenic mechanisms induced by SARS-CoV-2 will pave the way for novel treatment options for COVID-19.
Declaration of competing interest
The authors declare no financial or commercial conflict of interest.
Acknowledgement and funding
The authors apologize that many research articles with relevance to the field were not included in this review due to space limitations. This study was supported by the Ministry of Social Affairs and Integration Baden-Württemberg, Germany, to Y.S. (KIG BaWü).
References
- Adrover J.M., Aroca-Crevillen A., Crainiciuc G., Ostos F., Rojas-Vega Y., Rubio-Ponce A., Cilloniz C., Bonzon-Kulichenko E., Calvo E., Rico D., Moro M.A., Weber C., Lizasoain I., Torres A., Ruiz-Cabello J., Vazquez J., Hidalgo A. Programmed 'disarming' of the neutrophil proteome reduces the magnitude of inflammation. Nat. Immunol. 2020;21:135–144. doi: 10.1038/s41590-019-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmerov A., Marban E. COVID-19 and the heart. Circ. Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreakos E., Salagianni M., Galani I.E., Koltsida O. Interferon-lambdas: front-line guardians of immunity and homeostasis in the respiratory tract. Front. Immunol. 2017;8:1232. doi: 10.3389/fimmu.2017.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang A., Pullar J.M., Currie M.J., Vissers M.C.M. Vitamin C and immune cell function in inflammation and cancer. Biochem. Soc. Trans. 2018;46:1147–1159. doi: 10.1042/BST20180169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas T., Roberts A., Teal T.H., Vogel L., Chen J., Tumpey T.M., Katze M.G., Subbarao K. Genomic analysis reveals age-dependent innate immune responses to severe acute respiratory syndrome coronavirus. J. Virol. 2008;82:9465–9476. doi: 10.1128/JVI.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balta E., Hardt R., Liang J., Kirchgessner H., Orlik C., Jahraus B., Hillmer S., Meuer S., Hübner K., Wabnitz G.H., Samstag Y. Spatial oxidation of L-plastin downmodulates actin-based functions of tumor cells. Nat. Commun. 2019;10:4073. doi: 10.1038/s41467-019-11909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., Qu Y., Li F., Lv Q., Wang W., Xue J., Gong S., Liu M., Wang G., Wang S., Song Z., Zhao L., Liu P., Zhao L., Ye F., Wang H., Zhou W., Zhu N., Zhen W., Yu H., Zhang X., Guo L., Chen L., Wang C., Wang Y., Wang X., Xiao Y., Sun Q., Liu H., Zhu F., Ma C., Yan L., Yang M., Han J., Xu W., Tan W., Peng X., Jin Q., Wu G., Qin C. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020 doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- Bardoel B.W., Kenny E.F., Sollberger G., Zychlinsky A. The balancing act of neutrophils. Cell Host Microbe. 2014;15:526–536. doi: 10.1016/j.chom.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Dassler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., Mcallister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D., Yost C.C., Weber A., Zuo Y., Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217:e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendib I., De Chaisemartin L., Granger V., Schlemmer F., Maitre B., Hüe S., Surenaud M., Beldi-Ferchiou A., Carteaux G., Razazi K., Chollet-Martin S., Mekontso Dessap A., De Prost N. Neutrophil extracellular traps are elevated in patients with pneumonia-related acute respiratory distress syndrome. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2019;130:581–591. doi: 10.1097/ALN.0000000000002619. [DOI] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., Tenoever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045 e1039. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser H., Dostert C., Mak T.W., Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26:249–261. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Boehm U., Klamp T., Groot M., Howard J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Boeltz S., Amini P., Anders H.-J., Andrade F., Bilyy R., Chatfield S., Cichon I., Clancy D.M., Desai J., Dumych T., Dwivedi N., Gordon R.A., Hahn J., Hidalgo A., Hoffmann M.H., Kaplan M.J., Knight J.S., Kolaczkowska E., Kubes P., Leppkes M., Manfredi A.A., Martin S.J., Maueröder C., Maugeri N., Mitroulis I., Munoz L.E., Nakazawa D., Neeli I., Nizet V., Pieterse E., Radic M.Z., Reinwald C., Ritis K., Rovere-Querini P., Santocki M., Schauer C., Schett G., Shlomchik M.J., Simon H.-U., Skendros P., Stojkov D., Vandenabeele P., Berghe T.V., Van Der Vlag J., Vitkov L., Von Köckritz-Blickwede M., Yousefi S., Zarbock A., Herrmann M. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26:395–408. doi: 10.1038/s41418-018-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonow R.O., Fonarow G.C., O’gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- Borissoff J.I., Joosen I.A., Versteylen M.O., Brill A., Fuchs T.A., Savchenko A.S., Gallant M., Martinod K., Ten Cate H., Hofstra L., Crijns H.J., Wagner D.D., Kietselaer B. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler. Thromb. Vasc. Biol. 2013;33:2032–2040. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozonet S.M., Carr A.C. The role of physiological vitamin C concentrations on key functions of neutrophils isolated from healthy individuals. Nutrients. 2019;11:1363. doi: 10.3390/nu11061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill A., Fuchs T.A., Savchenko A.S., Thomas G.M., Martinod K., De Meyer S.F., Bhandari A.A., Wagner D.D. Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemostas. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Broering R., Montag M., Jiang M., Lu M., Sowa J.-P., Kleinehr K., Gerken G., Schlaak J.F. Corticosteroids shift the Toll-like receptor response pattern of primary-isolated murine liver cells from an inflammatory to an anti-inflammatory state. Int. Immunol. 2011;23:537–544. doi: 10.1093/intimm/dxr048. [DOI] [PubMed] [Google Scholar]
- Broggi A., Ghosh S., Sposito B., Spreafico R., Balzarini F., Lo Cascio A., Clementi N., De Santis M., Mancini N., Granucci F., Zanoni I. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science. 2020 doi: 10.1126/science.abc3545. eabc3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Ohja R., Pedro L., Djannatian M., Franz J., Kuivanen S., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Osterlund P., Joensuu M., Meunier F., Butcher S., Winkler M., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and provides a possible pathway into the central nervous system. bioRxiv. 2020:137802. doi: 10.1126/science.abd2985. 2020.2006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit. Care. 2020;24:133. doi: 10.1186/s13054-020-02851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova-Acebes M., Nicolás-Ávila J.A., Li J.L., García-Silva S., Balachander A., Rubio-Ponce A., Weiss L.A., Adrover J.M., Burrows K., N A.G., Ballesteros I., Devi S., Quintana J.A., Crainiciuc G., Leiva M., Gunzer M., Weber C., Nagasawa T., Soehnlein O., Merad M., Mortha A., Ng L.G., Peinado H., Hidalgo A. Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 2018;215:2778–2795. doi: 10.1084/jem.20181468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudrillier A., Kessenbrock K., Gilliss B.M., Nguyen J.X., Marques M.B., Monestier M., Toy P., Werb Z., Looney M.R. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Zheng J., Wohlford-Lenane C., Abrahante J.E., Mack M., Sompallae R., Mccray P.B., Jr., Meyerholz D.K., Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Invest. 2019;130:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S. In: Oxidative Stress and Biomaterials. Dziubla T., Butterfield D.A., editors. Academic Press; 2016. Chapter two - oxidative stress, inflammation, and disease; pp. 35–58. [Google Scholar]
- Chen Y., Feng Z., Diao B., Wang R., Wang G., Wang C., Tan Y., Liu L., Wang C., Liu Y., Liu Y., Yuan Z., Ren L., Wu Y. medRxiv; 2020. The Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Directly Decimates Human Spleens and Lymph Nodes. 2020.2003.2027.20045427. [Google Scholar]
- Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J., Li F. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.K., Wong D.A., Tong L.K., Ip S.M., Lo A.C., Lau C.S., Yeung E.Y., Lim W.W. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C.Y., Poon L.L., Ng I.H., Luk W., Sia S.F., Wu M.H., Chan K.H., Yuen K.Y., Gordon S., Guan Y., Peiris J.S. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirivi R.G.S., Van Rosmalen J.W.G., Van Der Linden M., Euler M., Schmets G., Bogatkevich G., Kambas K., Hahn J., Braster Q., Soehnlein O., Hoffmann M.H., Es H., Raats J.M.H. Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J.-Y., Kim S.-K. Quercetin and ascorbic acid suppress fructose-induced NLRP3 inflammasome activation by blocking intracellular shuttling of TXNIP in human macrophage cell lines. Inflammation. 2017;40:980–994. doi: 10.1007/s10753-017-0542-4. [DOI] [PubMed] [Google Scholar]
- Choi S.H., Hong S.B., Hong H.L., Kim S.H., Huh J.W., Sung H., Lee S.O., Kim M.N., Jeong J.Y., Lim C.M., Kim Y.S., Woo J.H., Koh Y. Usefulness of cellular analysis of bronchoalveolar lavage fluid for predicting the etiology of pneumonia in critically ill patients. PloS One. 2014;9 doi: 10.1371/journal.pone.0097346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K.T., Gale M., Jr., Loo Y.M. RIG-I and other RNA sensors in antiviral immunity. Annu. Rev. Immunol. 2018;36:667–694. doi: 10.1146/annurev-immunol-042617-053309. [DOI] [PubMed] [Google Scholar]
- Chu H., Chan J.F., Wang Y., Yuen T.T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X., Zhang X., Cai J.P., Zhou J., Yuan S., Kok K.H., To K.K., Chan I.H., Zhang A.J., Sit K.Y., Au W.K., Yuen K.Y. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., Zhou J., Wong B.H., Li C., Chan J.F., Cheng Z.S., Yang D., Wang D., Lee A.C., Li C., Yeung M.L., Cai J.P., Chan I.H., Ho W.K., To K.K., Zheng B.J., Yao Y., Qin C., Yuen K.Y. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 2016;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang T.-Y., Cheng A.-J., Chen I.-T., Lan T.-Y., Huang I.-H., Shiau C.-W., Hsu C.-L., Liu Y.-W., Chang Z.-F., Tseng P.-H., Kuo J.-C. Suppression of LPS-induced inflammatory responses by the hydroxyl groups of dexamethasone. Oncotarget. 2017;8 doi: 10.18632/oncotarget.17683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.Y., Sung B., Jung K.J., Zou Y., Yu B.P. The molecular inflammatory process in aging. Antioxidants Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- Clark A., Jit M., Warren-Gash C., Guthrie B., Wang H.H.X., Mercer S.W., Sanderson C., Mckee M., Troeger C., Ong K.L., Checchi F., Perel P., Joseph S., Gibbs H.P., Banerjee A., Eggo R.M., Nightingale E.S., O’reilly K., Jombart T., Edmunds W.J., Rosello A., Sun F.Y., Atkins K.E., Bosse N.I., Clifford S., Russell T.W., Deol A.K., Liu Y., Procter S.R., Leclerc Q.J., Medley G., Knight G., Munday J.D., Kucharski A.J., Pearson C.a.B., Klepac P., Prem K., Houben R.M.G.J., Endo A., Flasche S., Davies N.G., Diamond C., Van Zandvoort K., Funk S., Auzenbergs M., Rees E.M., Tully D.C., Emery J.C., Quilty B.J., Abbott S., Villabona-Arenas C.J., Hué S., Hellewell J., Gimma A., Jarvis C.I. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. The Lancet Global Health. 2020 doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S.R., Ma A.C., Tavener S.A., Mcdonald B., Goodarzi Z., Kelly M.M., Patel K.D., Chakrabarti S., Mcavoy E., Sinclair G.D., Keys E.M., Allen-Vercoe E., Devinney R., Doig C.J., Green F.H., Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- Corman V.M., Muth D., Niemeyer D., Drosten C. In: Advances in Virus Research. Kielian M., Mettenleiter T.C., Roossinck M.J., editors. Academic Press; 2018. Chapter eight - hosts and sources of endemic human coronaviruses; pp. 163–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy Of, V The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B., Valle C., De Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtale G., Renzi T.A., Drufuca L., Rubino M., Locati M. Glucocorticoids downregulate TLR4 signaling activity via its direct targeting by miR-511-5p. Eur. J. Immunol. 2017;47:2080–2089. doi: 10.1002/eji.201747044. [DOI] [PubMed] [Google Scholar]
- D'alessio F.R., Tsushima K., Aggarwal N.R., West E.E., Willett M.H., Britos M.F., Pipeling M.R., Brower R.G., Tuder R.M., Mcdyer J.F., King L.S. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.L., Simonetti B., Antón-Plágaro C., Kavanagh Williamson M., Shoemark D.K., Simón-Gracia L., Klein K., Bauer M., Hollandi R., Greber U.F., Horvath P., Sessions R.B., Helenius A., Hiscox J.A., Teesalu T., Matthews D.A., Davidson A.D., Cullen P.J., Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1126/science.abd3072. 2020.2006.2005.134114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancey J.T., Deubelbeiss K.A., Harker L.A., Finch C.A. Neutrophil kinetics in man. J. Clin. Invest. 1976;58:705–715. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora S., Grassi C., Carati L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur. Respir. J. 1997;10:1535–1541. doi: 10.1183/09031936.97.10071535. [DOI] [PubMed] [Google Scholar]
- Dediego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeno J.M., Fernandez-Delgado R., Fett C., Castano-Rodriguez C., Perlman S., Enjuanes L. Inhibition of NF-kappaB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi S., Wang Y., Chew W.K., Lima R., N A.G., Mattar C.N., Chong S.Z., Schlitzer A., Bakocevic N., Chew S., Keeble J.L., Goh C.C., Li J.L., Evrard M., Malleret B., Larbi A., Renia L., Haniffa M., Tan S.M., Chan J.K., Balabanian K., Nagasawa T., Bachelerie F., Hidalgo A., Ginhoux F., Kubes P., Ng L.G. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J. Exp. Med. 2013;210:2321–2336. doi: 10.1084/jem.20130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnon K.H., Leist S.R., Schäfer A., Edwards C.E., Martinez D.R., Montgomery S.A., West A., Yount B.L., Hou Y.J., Adams L.E., Gully K.L., Brown A.J., Huang E., Bryant M.D., Choong I.C., Glenn J.S., Gralinski L.E., Sheahan T.P., Baric R.S. A mouse-adapted SARS-CoV-2 model for the evaluation of COVID-19 medical countermeasures. bioRxiv. 2020 2020.2005.2006.081497. [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring Y., Libby P., Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ. Res. 2020;126:1228–1241. doi: 10.1161/CIRCRESAHA.120.315931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142:19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., Van Der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- Erol N., Saglam L., Saglam Y.S., Erol H.S., Altun S., Aktas M.S., Halici M.B. The protection potential of antioxidant vitamins against acute respiratory distress syndrome: a rat trial. Inflammation. 2019;42:1585–1594. doi: 10.1007/s10753-019-01020-2. [DOI] [PubMed] [Google Scholar]
- Etulain J., Martinod K., Wong S.L., Cifuni S.M., Schattner M., Wagner D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126:242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkash E.A., Wilson A.M., Jentzen J.M. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J. Am. Soc. Nephrol. 2020 doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., Richards D., Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Fisher B.J., Kraskauskas D., Martin E.J., Farkas D., Wegelin J.A., Brophy D., Ward K.R., Voelkel N.F., Fowler A.A., 3rd, Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;303:L20–L32. doi: 10.1152/ajplung.00300.2011. [DOI] [PubMed] [Google Scholar]
- Fisher B.J., Seropian I.M., Kraskauskas D., Thakkar J.N., Voelkel N.F., Fowler A.a.I., Natarajan R. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury*. Crit. Care Med. 2011;39:1454–1460. doi: 10.1097/CCM.0b013e3182120cb8. [DOI] [PubMed] [Google Scholar]
- Fowler A.A., 3rd, Truwit J.D., Hite R.D., Morris P.E., Dewilde C., Priday A., Fisher B., Thacker L.R., 2nd, Natarajan R., Brophy D.F., Sculthorpe R., Nanchal R., Syed A., Sturgill J., Martin G.S., Sevransky J., Kashiouris M., Hamman S., Egan K.F., Hastings A., Spencer W., Tench S., Mehkri O., Bindas J., Duggal A., Graf J., Zellner S., Yanny L., Mcpolin C., Hollrith T., Kramer D., Ojielo C., Damm T., Cassity E., Wieliczko A., Halquist M. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. Jama. 2019;322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A.A., Syed A.A., Knowlson S., Sculthorpe R., Farthing D., Dewilde C., Farthing C.A., Larus T.L., Martin E., Brophy D.F., Gupta S., Fisher B.J., Natarajan R., Medical Respiratory Intensive Care Unit, N Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. medRxiv; 2020. Pulmonary and Cardiac Pathology in Covid-19: the First Autopsy Series from New Orleans. 2020.2004.2006.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M., Baric R. Mechanisms of severe acute respiratory syndrome pathogenesis and innate immunomodulation. Microbiol. Mol. Biol. Rev. 2008;72:672–685. doi: 10.1128/MMBR.00015-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Jr., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- Gaertner F., Massberg S. Blood coagulation in immunothrombosis—at the frontline of intravascular immunity. Semin. Immunol. 2016;28:561–569. doi: 10.1016/j.smim.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Gál Z., Gézsi A., Pállinger É., Visnovitz T., Nagy A., Kiss A., Sultész M., Csoma Z., Tamási L., Gálffy G., Szalai C. Plasma neutrophil extracellular trap level is modified by disease severity and inhaled corticosteroids in chronic inflammatory lung diseases. Sci. Rep. 2020;10:4320. doi: 10.1038/s41598-020-61253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani I.E., Triantafyllia V., Eleminiadou E.E., Koltsida O., Stavropoulos A., Manioudaki M., Thanos D., Doyle S.E., Kotenko S.V., Thanopoulou K., Andreakos E. Interferon-lambda mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity. 2017;46:875–890. doi: 10.1016/j.immuni.2017.04.025. [DOI] [PubMed] [Google Scholar]
- Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control covid-19. N. Engl. J. Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gando S., Fujishima S., Saitoh D., Shiraishi A., Yamakawa K., Kushimoto S., Ogura H., Abe T., Mayumi T., Sasaki J., Kotani J., Takeyama N., Tsuruta R., Takuma K., Yamashita N., Shiraishi S.I., Ikeda H., Shiino Y., Tarui T., Nakada T.A., Hifumi T., Otomo Y., Okamoto K., Sakamoto Y., Hagiwara A., Masuno T., Ueyama M., Fujimi S., Umemura Y., Japanese Association for Acute Medicine Focused Outcomes Research in Emergency Care in Acute Respiratory Distress Syndrome, S. Trauma Study, G The significance of disseminated intravascular coagulation on multiple organ dysfunction during the early stage of acute respiratory distress syndrome. Thromb. Res. 2020;191:15–21. doi: 10.1016/j.thromres.2020.03.023. [DOI] [PubMed] [Google Scholar]
- Garg S., Kim L., Whitaker M., O'halloran A., Cummings C., Holstein R., Prill M., Chai S.J., Kirley P.D., Alden N.B., Kawasaki B., Yousey-Hindes K., Niccolai L., Anderson E.J., Openo K.P., Weigel A., Monroe M.L., Ryan P., Henderson J., Kim S., Como-Sabetti K., Lynfield R., Sosin D., Torres S., Muse A., Bennett N.M., Billing L., Sutton M., West N., Schaffner W., Talbot H.K., Aquino C., George A., Budd A., Brammer L., Langley G., Hall A.J., Fry A. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, march 1-30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R., Tsung A., Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., De Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., Coronaviridae Study Group of the International Committee on Taxonomy Of, V The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., Iotti G., Latronico N., Lorini L., Merler S., Natalini G., Piatti A., Ranieri M.V., Scandroglio A.M., Storti E., Cecconi M., Pesenti A., For The, C.-L.I.C.U.N Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. J. Am. Med. Assoc. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes J., Soehnlein O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group For C. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M., Ginhoux F., Jakubzick C., Naik S.H., Onai N., Schraml B.U., Segura E., Tussiwand R., Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., Qin R., Wang H., Shen Y., Du K., Zhao L., Fan H., Luo S., Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19 (2020) Diabetes Metabol. Res. Rev. 2020;3319 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Pere H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pene F., Marin N., Roche N., Szwebel T.-A., Smith N., Merkling S., Treluyer J.-M., Veyer D., Mouthon L., Blanc C., Tharaux P.-L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kerneis S., Terrier B. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. medRxiv. 2020 doi: 10.1126/science.abc6027. 2020.2004.2019.20068015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., Van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.O., Case J.B., Winkler E.S., Thackray L., Kafai N.M., Bailey A.L., Mccune B.T., Fox J.M., Chen R.E., Al Soussi W.B., Turner J.S., Schmitz A.J., Lei T., Shrihari S., Keeler S.P., Fremont D.H., Greco S., Mccray P.B., Perlman S., Holtzman M.J., Ellebedy A.H., Diamond M.S. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell. 2020 doi: 10.1016/j.cell.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H. Vitamin C and infections. Nutrients. 2017;9 doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemila H., Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD005532.pub2. CD005532. [DOI] [PubMed] [Google Scholar]
- Hikmet F., Méar L., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. bioRxiv. 2020 doi: 10.15252/msb.20209610. 2020.2003.2031.016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T., Pennycook A., Openshaw P.J. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., Van Loo G., Ermolaeva M., Veldhuizen R., Leung Y.H., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J.S., Slutsky A.S., Akira S., Hultqvist M., Holmdahl R., Nicholls J., Jiang C., Binder C.J., Penninger J.M. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Foxman E.F., Molony R.D. Early local immune defences in the respiratory tract. Nat. Rev. Immunol. 2017;17:7–20. doi: 10.1038/nri.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E., Babcock G.J., Thomas W.D., Thackray L.B., Young M.D., Mason R.J., Ambrosino D.M., Wentworth D.E., Demartini J.C., Holmes K.V. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne C.N., Wong C.H., Zemp F.J., Mcdonald B., Rahman M.M., Forsyth P.A., Mcfadden G., Kubes P. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13:169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Jeong Y.J., Kim J.H., Hong J.M., Kang J.S., Kim H.R., Lee W.J., Hwang Y.I. Vitamin C treatment of mouse bone marrow-derived dendritic cells enhanced CD8(+) memory T cell production capacity of these cells in vivo. Immunobiology. 2014;219:554–564. doi: 10.1016/j.imbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Jiang X.L., Zhang X.L., Zhao X.N., Li C.B., Lei J., Kou Z.Q., Sun W.K., Hang Y., Gao F., Ji S.X., Lin C.F., Pang B., Yao M.X., Anderson B.D., Wang G.L., Yao L., Duan L.J., Kang D.M., Ma M.J. Transmission potential of asymptomatic and paucisymptomatic SARS-CoV-2 infections: a three-family cluster study in China. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Alcazar M., Rangaswamy C., Panda R., Bitterling J., Simsek Y.J., Long A.T., Bilyy R., Krenn V., Renne C., Renne T., Kluge S., Panzer U., Mizuta R., Mannherz H.G., Kitamura D., Herrmann M., Napirei M., Fuchs T.A. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358:1202–1206. doi: 10.1126/science.aam8897. [DOI] [PubMed] [Google Scholar]
- Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Bradley Segal J., Nguyen E.L., Barsh G.R., Maskatia S., Mathew R. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp. Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- Kander M.C., Cui Y., Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J. Cell Mol. Med. 2017;21:1024–1032. doi: 10.1111/jcmm.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Kosaki F., Okawa S., Shigematsu I., Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–276. [PubMed] [Google Scholar]
- Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., Black S.M. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) Adv. Exp. Med. Biol. 2017;967:105–137. doi: 10.1007/978-3-319-63245-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshari R.S., Jyoti A., Dubey M., Kothari N., Kohli M., Bogra J., Barthwal M.K., Dikshit M. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PloS One. 2012;7 doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomich O.A., Kochetkov S.N., Bartosch B., Ivanov A.V. Redox biology of respiratory viral infections. Viruses. 2018;10:392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Jenne C.N. Role of platelets in neutrophil extracellular trap (NET) production and tissue injury. Semin. Immunol. 2016;28:546–554. doi: 10.1016/j.smim.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Kindler E., Thiel V., Weber F. Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv. Virus Res. 2016;96:219–243. doi: 10.1016/bs.aivir.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey G.R., Sharma R., Huang L., Li L., Vergis A.L., Ye H., Ju S.T., Okusa M.D. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner T., Hermann E., Moller S., Klinger M., Solbach W., Laskay T., Behnen M. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediat. Inflamm. 2013;2013:710239. doi: 10.1155/2013/710239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke M., Wabnitz G.H., Funke F., Funk B., Kirchgessner H., Samstag Y. Oxidation of cofilin mediates T cell hyporesponsiveness under oxidative stress conditions. Immunity. 2008;29:404–413. doi: 10.1016/j.immuni.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Klok F.A., Kruip M., Van Der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., Van Paassen J., Stals M.a.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E., Jenne C.N., Surewaard B.G.J., Thanabalasuriar A., Lee W.-Y., Sanz M.-J., Mowen K., Opdenakker G., Kubes P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 2015;6:6673. doi: 10.1038/ncomms7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouakanou Léonce, Xu Yan, Peters Christian, He Junyi, Wu Yangzhe, Yin Zhinan, Kabelitz Dieter. Vitamin C promotes the proliferation and effector functions of γδ T cells. Cellular and Molecular Immunology. 2020;17:462–473. doi: 10.1038/s41423-019-0247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., Van Amerongen G., Van Riel D., Laman J.D., De Jong T., Van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., Van Der Werf S., Escriou N., Manuguerra J.C., Stohr K., Peiris J.S., Osterhaus A.D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.J., Cruz F.M., Rock K.L. Immune sensing of cell death through recognition of histone sequences by C-type lectin-receptor-2d causes inflammation and tissue injury. Immunity. 2020;52:123–135. doi: 10.1016/j.immuni.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K.Y., Ng W.Y., Osburga Chan P.K., Wong K.F., Cheng F. High-dose N-acetylcysteine therapy for novel H1N1 influenza pneumonia. Ann. Intern. Med. 2010;152:687–688. doi: 10.7326/0003-4819-152-10-201005180-00017. [DOI] [PubMed] [Google Scholar]
- Laridan E., Martinod K., De Meyer S.F. Neutrophil extracellular traps in arterial and venous thrombosis. Semin. Thromb. Hemost. 2019;45:86–93. doi: 10.1055/s-0038-1677040. [DOI] [PubMed] [Google Scholar]
- Law H.K., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W., Nicholls J.M., Peiris J.S., Lau Y.L. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Schoggins J.W., Diamond M.S. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50:907–923. doi: 10.1016/j.immuni.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- Lee W.L., Downey G.P. Neutrophil activation and acute lung injury. Curr. Opin. Crit. Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Lefrancais E., Looney M.R. Platelet biogenesis in the lung circulation. Physiology. 2019;34:392–401. doi: 10.1152/physiol.00017.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançais E., Mallavia B., Zhuo H., Calfee C.S., Looney M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI insight. 2018;3 doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancais E., Ortiz-Munoz G., Caudrillier A., Mallavia B., Liu F., Sayah D.M., Thornton E.E., Headley M.B., David T., Coughlin S.R., Krummel M.F., Leavitt A.D., Passegue E., Looney M.R. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz A., Jorens P., Meyer B., De Backer W., Van Overveld F., Bossaert L., Maier K. Oxidatively modified proteins in bronchoalveolar lavage fluid of patients with ARDS and patients at-risk for ARDS. Eur. Respir. J. 1999;13:169–174. doi: 10.1034/j.1399-3003.1999.13a31.x. [DOI] [PubMed] [Google Scholar]
- Lewkowicz N., Klink M., Mycko M.P., Lewkowicz P. Neutrophil--CD4+CD25+ T regulatory cell interactions: a possible new mechanism of infectious tolerance. Immunobiology. 2013;218:455–464. doi: 10.1016/j.imbio.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Ley K., Hoffman H.M., Kubes P., Cassatella M.A., Zychlinsky A., Hedrick C.C., Catz S.D. Neutrophils: new insights and open questions. Sci Immunol. 2018;3:eaat4579. doi: 10.1126/sciimmunol.aat4579. [DOI] [PubMed] [Google Scholar]
- Li J. Evidence is stronger than you think: a meta-analysis of vitamin C use in patients with sepsis. Crit. Care. 2018;22:258. doi: 10.1186/s13054-018-2191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Tan J., Martino M.M., Lui K.O. Regulatory T-cells: potential regulator of tissue repair and regeneration. Front. Immunol. 2018;9:585. doi: 10.3389/fimmu.2018.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ji F., Wang L., Wang L., Hao J., Dai M., Liu Y., Pan X., Fu J., Li L., Yang G., Yang J., Yan X., Gu B. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, xuzhou, China. Emerg. Infect. Dis. 2020;26:1626–1628. doi: 10.3201/eid2607.200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.W., Wang C.Y., Jou Y.J., Yang T.C., Huang S.H., Wan L., Lin Y.J., Lin C.W. SARS coronavirus papain-like protease induces Egr-1-dependent up-regulation of TGF-beta1 via ROS/p38 MAPK/STAT3 pathway. Sci. Rep. 2016;6:25754. doi: 10.1038/srep25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.-Y., Li L., Zhang Y., Wang X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious Diseases of Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg. Microb. Infect. 2020;9:687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Jahraus B., Balta E., Ziegler J.D., Hübner K., Blank N., Niesler B., Wabnitz G.H., Samstag Y. Sulforaphane inhibits inflammatory responses of primary human T-cells by increasing ROS and depleting glutathione. Front. Immunol. 2018;9:2584. doi: 10.3389/fimmu.2018.02584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Ziegler J.D., Jahraus B., Orlik C., Blatnik R., Blank N., Niesler B., Wabnitz G., Ruppert T., Hübner K., Balta E., Samstag Y. Piperlongumine acts as an immunosuppressant by exerting prooxidative effects in human T cells resulting in diminished TH17 but enhanced Treg differentiation. Front. Immunol. 2020;11:1172. doi: 10.3389/fimmu.2020.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Chen L., Li J., Wang X., Wang F., Liu L., Zhang S., Zhang Z. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. medRxiv. 2020 2020.2002.2023.20026690. [Google Scholar]
- Lippi G., Favaloro E.J. D-Dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb. Haemostasis. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X., Luo M., Chen L., Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., Wang L., Zhou W., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Su X., Pan P., Zhang L., Hu Y., Tan H., Wu D., Liu B., Li H., Li H., Li Y., Dai M., Li Y., Hu C., Tsung A. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci. Rep. 2016;6:37252. doi: 10.1038/srep37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Gu J., Li K., Xu C., Su W., Lai Z., Zhou D., Yu C., Xu B., Yang Z. COVID-19 outbreak associated with air conditioning in restaurant, guangzhou, China, 2020. Emerg. Infect. Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai S.H.C., Khan M., Dwivedi D.J., Ross C.A., Zhou J., Gould T.J., Gross P.L., Weitz J.I., Fox-Robichaud A.E., Liaw P.C., Group, F.T.C.C.C.T.B Delayed but not early treatment with DNase reduces organ damage and improves outcome in a murine model of sepsis. Shock. 2015;44:166–172. doi: 10.1097/SHK.0000000000000396. [DOI] [PubMed] [Google Scholar]
- Manning J., Mitchell B., Appadurai D.A., Shakya A., Pierce L.J., Wang H., Nganga V., Swanson P.C., May J.M., Tantin D., Spangrude G.J. Vitamin C promotes maturation of T-cells. Antioxidants Redox Signal. 2013;19:2054–2067. doi: 10.1089/ars.2012.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinod K., Wagner D.D. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S., Grahl L., Von Bruehl M.-L., Manukyan D., Pfeiler S., Goosmann C., Brinkmann V., Lorenz M., Bidzhekov K., Khandagale A.B., Konrad I., Kennerknecht E., Reges K., Holdenrieder S., Braun S., Reinhardt C., Spannagl M., Preissner K.T., Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- Mata M., Sarrion I., Armengot M., Carda C., Martinez I., Melero J.A., Cortijo J. Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: effectiveness of N-acetylcysteine. PloS One. 2012;7 doi: 10.1371/journal.pone.0048037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher A.K., Spinosa M., Davis J.P., Pope N., Laubach V.E., Su G., Serbulea V., Leitinger N., Ailawadi G., Upchurch G.R., Jr. Novel role of IL (Interleukin)-1beta in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2018;38:843–853. doi: 10.1161/ATVBAHA.117.309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., Mcauley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Hlh across Speciality Collaboration, U.K COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp S., Coppens M., Van Haaps T.F., Foppen M., Vlaar A.P., Muller M.C.A., Bouman C.C.S., Beenen L.F.M., Kootte R.S., Heijmans J., Smits L.P., Bonta P.I., Van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemostasis. 2020 doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min C.K., Cheon S., Ha N.Y., Sohn K.M., Kim Y., Aigerim A., Shin H.M., Choi J.Y., Inn K.S., Kim J.H., Moon J.Y., Choi M.S., Cho N.H., Kim Y.S. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci. Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen T.H., Berg R.S., Paludan S.R., Ostergaard L. Mechanisms of dexamethasone-mediated inhibition of Toll-like receptor signaling induced by Neisseria meningitidis and Streptococcus pneumoniae. Infect. Immun. 2008;76:189–197. doi: 10.1128/IAI.00856-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado Del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913 e907. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro S.P., De Souza G.F., Gallo S.W., Da Silva B.K., De Oliveira S.D., Vinolo M.a.R., Saraiva E.M., Porto B.N. Respiratory Syncytial Virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci. Rep. 2018;8:14166. doi: 10.1038/s41598-018-32576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M., Swirski F.K. Neutrophil-macrophage communication in inflammation and atherosclerosis. Science. 2015;349:237–238. doi: 10.1126/science.aac7801. [DOI] [PubMed] [Google Scholar]
- Narasaraju T., Tang B.M., Herrmann M., Muller S., Chow V.T.K., Radic M. Neutrophilia and NETopathy as key pathologic drivers of progressive lung impairment in patients with COVID-19. Front. Pharmacol. 2020;11:870. doi: 10.3389/fphar.2020.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A., Phoon M.C., Van Rooijen N., Chow V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumenko V., Turk M., Jenne C.N., Kim S.J. Neutrophils in viral infection. Cell Tissue Res. 2018;371:505–516. doi: 10.1007/s00441-017-2763-0. [DOI] [PubMed] [Google Scholar]
- Nemeth T., Sperandio M., Mocsai A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020;19:253–275. doi: 10.1038/s41573-019-0054-z. [DOI] [PubMed] [Google Scholar]
- Noubouossie D.F., Reeves B.N., Strahl B.D., Key N.S. Neutrophils: back in the thrombosis spotlight. Blood. 2019;133:2186–2197. doi: 10.1182/blood-2018-10-862243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., O'garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50:871–891. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- Park M.D. Macrophages: a trojan horse in COVID-19? Nat. Rev. Immunol. 2020;20:351. doi: 10.1038/s41577-020-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W.B., Kwon N.-J., Choi S.-J., Kang C.K., Choe P.G., Kim J.Y., Yun J., Lee G.-W., Seong M.-W., Kim N.J., Seo J.-S., Oh M.-D. Virus isolation from the first patient with SARS-CoV-2 in Korea. J. Kor. Med. Sci. 2020;35:e84. doi: 10.3346/jkms.2020.35.e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli E.K., Schmolke M., Wolff T., Viemann D., Roth J., Bode J.G., Ludwig S. Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Jeanpierre E., Rauch A., Labreuche J., Susen S., Lille, I.C.U.H.C.-G. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- Prokunina-Olsson L., Alphonse N., Dickenson R.E., Durbin J.E., Glenn J.S., Hartmann R., Kotenko S.V., Lazear H.M., O’brien T.R., Odendall C., Onabajo O.O., Piontkivska H., Santer D.M., Reich N.C., Wack A., Zanoni I. COVID-19 and emerging viral infections: the case for interferon lambda. J. Exp. Med. 2020;217:e20200653. doi: 10.1084/jem.20200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., Braun F., Lu S., Pfefferle S., Schröder A.S., Edler C., Gross O., Glatzel M., Wichmann D., Wiech T., Kluge S., Pueschel K., Aepfelbacher M., Huber T.B. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2911400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Miao T., Liu L., Zheng X., Luo D., Li Y. medRxiv; 2020. Indoor Transmission of SARS-CoV-2. 2020.2004.2004.20053058. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M.J., Kraus A.A., Ulrich R., Kruger D.H., Schonrich G. Hantavirus infection of dendritic cells. J. Virol. 2002;76:10724–10733. doi: 10.1128/JVI.76.21.10724-10733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M.J., Lalwani P., Krautkrmer E., Peters T., Scharffetter-Kochanek K., Kruger R., Hofmann J., Seeger K., Kruger D.H., Schonrich G. beta2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J. Exp. Med. 2014;211:1485–1497. doi: 10.1084/jem.20131092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M.J., Lalwani P., Lütteke N., Kobak L., Giese T., Ulrich R.G., Radosa L., Krüger D.H., Schönrich G. Replication in the mononuclear phagocyte system (MPS) as a determinant of hantavirus pathogenicity. Frontiers in Cellular and Infection Microbiology. 2020;10 doi: 10.3389/fcimb.2020.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmuson J., Kenne E., Wahlgren M., Soehnlein O., Lindbom L. Heparinoid sevuparin inhibits Streptococcus-induced vascular leak through neutralizing neutrophil-derived proteins. Faseb. J. 2019;33:10443–10452. doi: 10.1096/fj.201900627R. [DOI] [PubMed] [Google Scholar]
- Richard M., Van Den Brand J.M.A., Bestebroer T.M., Lexmond P., De Meulder D., Fouchier R.a.M., Lowen A.C., Herfst S. Influenza A viruses are transmitted via the air from the nasal respiratory epithelium of ferrets. Nat. Commun. 2020;11:766. doi: 10.1038/s41467-020-14626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., Mcginn T., Davidson K.W., the Northwell C.-R.C. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J. Am. Med. Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Baas T., Zornetzer G.A., Haagmans B., Sheahan T., Frieman M., Dyer M.D., Teal T.H., Proll S., Van Den Brand J., Baric R., Katze M.G. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 2009;83:7062–7074. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., Oude Munnink B.B., De Meulder D., Van Amerongen G., Van Den Brand J., Okba N.M.A., Schipper D., Van Run P., Leijten L., Sikkema R., Verschoor E., Verstrepen B., Bogers W., Langermans J., Drosten C., Fentener Van Vlissingen M., Fouchier R., De Swart R., Koopmans M., Haagmans B.L. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C., Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat. Rev. Nephrol. 2020;16:308–310. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., Uehata T., Iwasaki H., Omori H., Yamaoka S., Yamamoto N., Akira S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Samstag Y., John I., Wabnitz G.H. Cofilin: a redox sensitive mediator of actin dynamics during T-cell activation and migration. Immunol. Rev. 2013;256:30–47. doi: 10.1111/imr.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval R., Lazcano P., Ferrari F., Pinto-Pardo N., González-Billault C., Utreras E. TNF-α increases production of reactive oxygen species through Cdk5 activation in nociceptive neurons. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.00065. 65-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G., Sticherling M., Neurath M.F. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat. Rev. Immunol. 2020;20:271–272. doi: 10.1038/s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönrich G., Raftery M.J. Neutrophil extracellular traps go viral. Front. Immunol. 2016;7:366. doi: 10.3389/fimmu.2016.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönrich G., Raftery M.J. The PD-1/PD-L1 Axis and virus infections: a delicate balance. Frontiers in Cellular and Infection Microbiology. 2019;9:207. doi: 10.3389/fcimb.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C., Gabriel G., Von Kockritz-Blickwede M. Detrimental role of neutrophil extracellular traps during dengue virus infection. Trends Immunol. 2020;41:3–6. doi: 10.1016/j.it.2019.11.010. [DOI] [PubMed] [Google Scholar]
- Sena Laura a., Chandel Navdeep s. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M.S., Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ. Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Tan M., Chen X., Liu Y., Huang J., Ou J., Deng X. medRxiv; China: 2020. Immunopathological Characteristics of Coronavirus Disease 2019 Cases in Guangzhou. 2020.2003.2012.20034736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Crother Timothy r., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf Andrea j., Vergnes L., Ojcius David m., Rentsendorj A., Vargas M., Guerrero C., Wang Y., Fitzgerald Katherine a., Underhill David m., Town T., Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L.C., Ortigosa L.C., Benard G. Anti-TNF-alpha agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy. 2010;2:817–833. doi: 10.2217/imt.10.67. [DOI] [PubMed] [Google Scholar]
- Silvestre-Roig C., Braster Q., Ortega-Gomez A., Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020;17:327–340. doi: 10.1038/s41569-019-0326-7. [DOI] [PubMed] [Google Scholar]
- Silvestre-Roig C., Braster Q., Wichapong K., Lee E.Y., Teulon J.M., Berrebeh N., Winter J., Adrover J.M., Santos G.S., Froese A., Lemnitzer P., Ortega-Gomez A., Chevre R., Marschner J., Schumski A., Winter C., Perez-Olivares L., Pan C., Paulin N., Schoufour T., Hartwig H., Gonzalez-Ramos S., Kamp F., Megens R.T.A., Mowen K.A., Gunzer M., Maegdefessel L., Hackeng T., Lutgens E., Daemen M., Von Blume J., Anders H.J., Nikolaev V.O., Pellequer J.L., Weber C., Hidalgo A., Nicolaes G.a.F., Wong G.C.L., Soehnlein O. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 2019;569:236–240. doi: 10.1038/s41586-019-1167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits S.L., De Lang A., Van Den Brand J.M., Leijten L.M., Van I.W.F., Eijkemans M.J., Van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C.-Y., Xu J., He J.-Q., Lu Y.-Q. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv. 2020 2020.2003.2005.20031906. [Google Scholar]
- Sreeramkumar V., Adrover J.M., Ballesteros I., Cuartero M.I., Rossaint J., Bilbao I., Nácher M., Pitaval C., Radovanovic I., Fukui Y., Mcever R.P., Filippi M.-D., Lizasoain I., Ruiz-Cabello J., Zarbock A., Moro M.A., Hidalgo A. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhuang Z., Zheng J., Li K., Lok-Yin Wong R., Liu D., Huang J., He J., Zhu A., Zhao J., Li X., Xi Y., Chen R., Alshukairi A.N., Chen Z., Zhang Z., Chen C., Huang X., Li F., Lai X., Chen D., Wen L., Zhuo J., Zhang Y., Wang Y., Huang S., Dai J., Shi Y., Zheng K., Leidinger M.R., Chen J., Li Y., Zhong N., Meyerholz D.K., Mccray P.B., Perlman S., Zhao J. Generation of a broadly useful model for COVID-19 pathogenesis vaccination, and treatment. Cell. 2020 doi: 10.1016/j.cell.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M., Talavera-Lopez C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L., Network H.C.a.L.B. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supramaniam A., Lui H., Bellette B.M., Rudd P.A., Herrero L.J. How myeloid cells contribute to the pathogenesis of prominent emerging zoonotic diseases. J. Gen. Virol. 2018;99:953–969. doi: 10.1099/jgv.0.001024. [DOI] [PubMed] [Google Scholar]
- Takei H., Araki A., Watanabe H., Ichinose A., Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996;59:229–240. doi: 10.1002/jlb.59.2.229. [DOI] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Renia L., Macary P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team C.C.-R. Coronavirus disease 2019 in children - United States, february 12-april 2, 2020. MMWR. Morbidity and mortality weekly report. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry A.R. Does the newly observed inflammatory syndrome in children demonstrate a link between uncontrolled neutrophil extracellular traps formation and COVID-19? Pediatr. Res. 2020 doi: 10.1038/s4139-020-0996-1. [DOI] [PubMed] [Google Scholar]
- Thoms M., Buschauer R., Ameismeier M., Koepke L., Denk T., Hirschenberger M., Kratzat H., Hayn M., Mackens-Kiani T., Cheng J., Stuerzel C.M., Froehlich T., Berninghausen O., Becker T., Kirchhoff F., Sparrer K.M.J., Beckmann R. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. bioRxiv. 2020 doi: 10.1126/science.abc8665. 2020.2005.2018.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler A., Meier R., Seitz M., Dewald B., Baggiolini M., Fey M.F. Glucocorticoids downregulate gene expression of GM-CSF, NAP-1/IL-8, and IL-6, but not of M-CSF in human fibroblasts. Blood. 1992;79:45–51. [PubMed] [Google Scholar]
- Tripathi J.K., Sharma A., Sukumaran P., Sun Y., Mishra B.B., Singh B.B., Sharma J. Oxidant sensor cation channel TRPM2 regulates neutrophil extracellular trap formation and protects against pneumoseptic bacterial infection. Faseb. J. 2018 doi: 10.1096/fj.201800605. fj201800605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.T., Perrone L.A., Zhu H., Makino S., Peters C.J. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J. Immunol. 2005;174:7977–7985. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K. Inflammasome-associated cell death: pyroptosis, apoptosis, and physiological implications. Microbiol. Immunol. 2020;64:252–269. doi: 10.1111/1348-0421.12771. [DOI] [PubMed] [Google Scholar]
- Twaddell S.H., Baines K.J., Grainge C., Gibson P.G. The emerging role of neutrophil extracellular traps in respiratory disease. Chest. 2019;156:774–782. doi: 10.1016/j.chest.2019.06.012. [DOI] [PubMed] [Google Scholar]
- Van Avondt K., Maegdefessel L., Soehnlein O. Therapeutic targeting of neutrophil extracellular traps in atherogenic inflammation. Thromb. Haemostasis. 2019;119:542–552. doi: 10.1055/s-0039-1678664. [DOI] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., De Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorkom G.N.Y., Klein Wolterink R.G.J., Van Elssen C., Wieten L., Germeraad W.T.V., Bos G.M.J. Influence of vitamin C on lymphocytes: an overview. Antioxidants. 2018;7:41. doi: 10.3390/antiox7030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhana S.A., Wolchok J.D. The many faces of the anti-COVID immune response. J. Exp. Med. 2020;217:e20200678. doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas A., Boivin R., Cano P., Murcia Y., Bazin I., Lavoie J.P. Neutrophil extracellular traps are downregulated by glucocorticosteroids in lungs in an equine model of asthma. Respir. Res. 2017;18:207. doi: 10.1186/s12931-017-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras F.P., Pontelli M., Silva C., Toller-Kawahisa J., De Lima M., Nascimento D., Schneider A., Caetite D., Rosales R., Colon D., Martins R., Castro I., Almeida G., Lopes M.I., Benatti M., Bonjorno L., Giannini M., Luppino-Assad R., Almeida S., Vilar F., Santana R., Bollela V., Martins M., Miranda C., Borges M., Pazin-Filho A., Cunha L., Zamboni D., Dal-Pizzol F., Leiria L., Siyuan L., Batah S., Fabro A., Mauad T., Dolhnikoff M., Duarte-Neto A., Saldiva P., Cunha T., Alves-Filho J.C., Arruda E., Louzada-Junior P., Oliveira R., Cunha F. SARS-CoV-2 triggered neutrophil extracellular traps (NETs) mediate COVID-19 pathology. medRxiv. 2020 doi: 10.1084/jem.20201129. 2020.2006.2008.20125823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Brühl M.-L., Stark K., Steinhart A., Chandraratne S., Konrad I., Lorenz M., Khandoga A., Tirniceriu A., Coletti R., Köllnberger M., Byrne R.A., Laitinen I., Walch A., Brill A., Pfeiler S., Manukyan D., Braun S., Lange P., Riegger J., Ware J., Eckart A., Haidari S., Rudelius M., Schulz C., Echtler K., Brinkmann V., Schwaiger M., Preissner K.T., Wagner D.D., Mackman N., Engelmann B., Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabnitz G.H., Goursot C., Jahraus B., Kirchgessner H., Hellwig A., Klemke M., Konstandin M.H., Samstag Y. Mitochondrial translocation of oxidized cofilin induces caspase-independent necrotic-like programmed cell death of T cells. Cell Death Dis. 2010;1:e58. doi: 10.1038/cddis.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wack A., Terczynska-Dyla E., Hartmann R. Guarding the frontiers: the biology of type III interferons. Nat. Immunol. 2015;16:802–809. doi: 10.1038/ni.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., Mcguire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292 e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan T., Zhao Y., Fan F., Hu R., Jin X. Dexamethasone inhibits S. Aureus-induced neutrophil extracellular pathogen-killing mechanism, possibly through toll-like receptor regulation. Front. Immunol. 2017;8:60. doi: 10.3389/fimmu.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Xie T., Sun S., Wang K., Liu B., Wu X., Ding W. DNase-1 treatment exerts protective effects in a rat model of intestinal ischemia-reperfusion injury. Sci. Rep. 2018;8:17788. doi: 10.1038/s41598-018-36198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington A.V., Esponda O., Gibson A. Platelet biology of the rapidly failing lung. Br. J. Haematol. 2020;188:641–651. doi: 10.1111/bjh.16315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Sandbulte M.R., Thomas P.G., Webby R.J., Homayouni R., Pfeffer L.M. NFkappaB negatively regulates interferon-induced gene expression and anti-influenza activity. J. Biol. Chem. 2006;281:11678–11684. doi: 10.1074/jbc.M513286200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirather J., Hofmann U.D., Beyersdorf N., Ramos G.C., Vogel B., Frey A., Ertl G., Kerkau T., Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- Wildhagen K.C., Garcia De Frutos P., Reutelingsperger C.P., Schrijver R., Areste C., Ortega-Gomez A., Deckers N.M., Hemker H.C., Soehnlein O., Nicolaes G.A. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2014;123:1098–1101. doi: 10.1182/blood-2013-07-514984. [DOI] [PubMed] [Google Scholar]
- Willyard C. Coronavirus blood-clot mystery intensifies. Nature. 2020;581:250. doi: 10.1038/d41586-020-01403-8. [DOI] [PubMed] [Google Scholar]
- Wise J. Covid-19 and thrombosis: what do we know about the risks and treatment? BMJ. 2020;369 doi: 10.1136/bmj.m2058. [DOI] [PubMed] [Google Scholar]
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brunink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;58:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H., Lit L.C., Hui D.S., Chan M.H., Chung S.S., Sung J.J. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wygrecka M., Kosanovic D., Wujak L., Reppe K., Henneke I., Frey H., Didiasova M., Kwapiszewska G., Marsh L.M., Baal N., Hackstein H., Zakrzewicz D., Müller-Redetzky H.C., De Maat S., Maas C., Nolte M.W., Panousis C., Schermuly R.T., Seeger W., Witzenrath M., Schaefer L., Markart P. Antihistone properties of C1 esterase inhibitor protect against lung injury. Am. J. Respir. Crit. Care Med. 2016;196:186–199. doi: 10.1164/rccm.201604-0712OC. [DOI] [PubMed] [Google Scholar]
- Xu J., Zhang X., Pelayo R., Monestier M., Ammollo C.T., Semeraro F., Taylor F.B., Esmon N.L., Lupu F., Esmon C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020;99:1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.-Y., Huang Y., Ganesh L., Leung K., Kong W.-P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Shen C., Li J., Yuan J., Yang M., Wang F., Li G., Li Y., Xing L., Peng L., Wei J., Cao M., Zheng H., Wu W., Zou R., Li D., Xu Z., Wang H., Zhang M., Zhang Z., Liu L., Liu Y. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. 2020 2020.2003.2002.20029975. [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqinuddin A., Kashir J. Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: targeting a potential IL-1β/neutrophil extracellular traps feedback loop. Med. Hypotheses. 2020;143:109906. doi: 10.1016/j.mehy.2020.109906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur. Radiol. 2020;295:202–207. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilla M., Harcourt B.H., Hickman C.J., Mcgrew M., Tamin A., Goldsmith C.S., Bellini W.J., Anderson L.J. SARS-coronavirus replication in human peripheral monocytes/macrophages. Virus Res. 2005;107:93–101. doi: 10.1016/j.virusres.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yipp B.G., Petri B., Salina D., Jenne C.N., Scott B.N., Zbytnuik L.D., Pittman K., Asaduzzaman M., Wu K., Meijndert H.C., Malawista S.E., De Boisfleury Chevance A., Zhang K., Conly J., Kubes P. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Takeshita S., Kawamura Y., Kanai T., Tsujita Y., Nonoyama S. Enhanced formation of neutrophil extracellular traps in Kawasaki disease. Pediatr. Res. 2020;87:998–1004. doi: 10.1038/s41390-019-0710-3. [DOI] [PubMed] [Google Scholar]
- Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Qin X., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N. Engl. J. Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemostasis. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Ju Y., Ma Y., Wang T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: a randomized controlled trial. Medicine. 2018;97 doi: 10.1097/MD.0000000000013087. e13087-e13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhou X., Zhu C., Feng F., Qiu Y., Feng J., Jia Q., Song Q., Zhu B., Wang J. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. medRxiv. 2020 doi: 10.3389/fmolb.2020.00157. 2020.2003.2012.20035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Zhao W. NLRP3 inflammasome-A key player in antiviral responses. Front. Immunol. 2020;11:211. doi: 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. bioRxiv; 2020. Single-cell RNA Expression Profiling of ACE2, the Putative Receptor of Wuhan 2019-nCov. 2020.2001.2026.919985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., Guo L., Yang J., Wang C., Jiang S., Yang D., Zhang G., Li H., Chen F., Xu Y., Chen M., Gao Z., Yang J., Dong J., Liu B., Zhang X., Wang W., He K., Jin Q., Li M., Wang J. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27:883–890. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., 2nd, Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.E., Barbry P., Leslie A., Kiem H.P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J., Lung-Network@HumancellatlasOrg, H.C.a.L.B.N.E.A. Network, H.C.a.L.B. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Yalavarthi S., Shi H., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11) doi: 10.1172/jci.insight.138999. Published 2020 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Zuo M., Yalavarthi S., Gockman K., Madison J.A., Shi H., Knight J.S., Kanthi Y. medRxiv; 2020. Neutrophil Extracellular Traps and Thrombosis in COVID-19. 2020.2004.2030.20086736. [DOI] [PMC free article] [PubMed] [Google Scholar]