1. Introduction
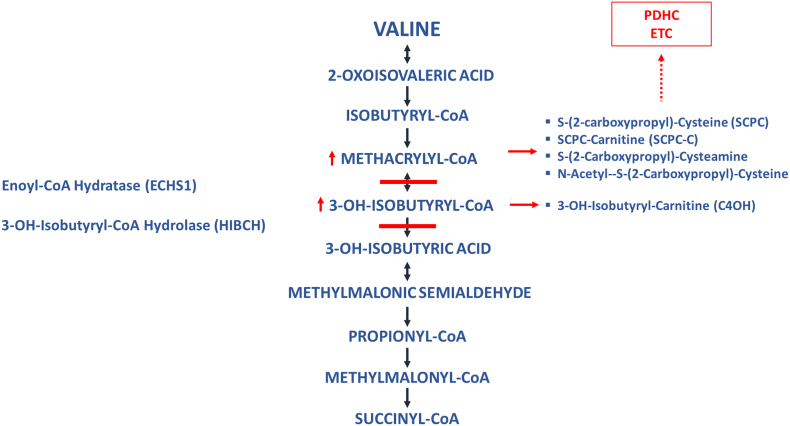
3-hydroxyisobutyryl-CoA hydrolase (HIBCH) and short-chain enoyl-CoA hydratase (ECHS1 or crotonase) deficiencies are rare disorders in valine catabolism. HIBCH is responsible for the conversion of 3-OH-isobutyryl-CoA to 3-OH-isobutyric acid and its deficiency results in the buildup of 3-OH-isobutyric acid, which conjugates with free carnitine to 3-OH-isobutyryl carnitine (C4OH), which can be detected in acylcarnitine analysis. The ECHS1 enzyme is reversible which results in the buildup of methacrylyl-CoA, a highly reactive compound which conjugates with thiol groups and results in the accumulation of several toxic intermediates, including S-(2-carboxypropyl)-cysteamine, S-(2-carboxypropyl)-cysteine (SCPC) and its carnitine ester (SCPC-C) (Fig. 1) [1].
Fig. 1.
Pathway of valine catabolism. Solid lines denote the metabolic blocks. Solid arrows highlight accumulated metabolites. Dotted arrow shows inhibition of secondary pathways.
HIBCH deficiency was initially reported in 1982 [2] with the gene defect identified in 2007 [1] . To date 22 patients in 16 families have been described. Hallmark findings of the condition include rapidly progressive neurological decline with early death and Leigh's-like findings on brain MRI. An attenuated form of the disease has been reported with late-onset presentation and a milder clinical course presenting with ataxia, movement disorder and optic atrophy, widening the clinical spectrum [3,4] .
ECHS1 deficiency, caused by defects in the ECHS1 gene, appears to be a more common disorder. ECHS1 is the enzyme upstream of HIBCH in the valine degradation pathway. This enzyme not only plays an important role in valine catabolism by degradation of methacrylyl-CoA, but is also active in the catabolism of 3-methylcrotonyl-CoA (leucine pathway), tiglyl-CoA (isoleucine pathway) as well as crotonyl-CoA (fatty acid oxidation) [5,6]. The defect was initially reported by Peters et al. in 2014 [7] with at least 46 additional patients published to date. The clinical, biochemical and genetic characteristics of 42 patients in 33 families have been summarized by Sharpe et al. in 2018 [6], with 4 single new cases reported afterwards [[8], [9], [10], [11]]. Clinical presentation is heterogeneous, ranging from a severe neonatal fatal form to a milder disease characterized by exercise induced movement disorder. Several mutations have been described in the ECHS1 gene including two founder mutations, one of Pakistani [12] and one of French-Canadian origin [13].
Most patients with either HIBCH or ECHS1 deficiency are diagnosed after severe neurological damage has already occurred and are frequently mistaken as mitochondrial defects. This may be due to the fact that the buildup of methacrylyl metabolites cause secondary inhibitions of key enzymes in energy production, which contributes to the clinical and biochemical presentations [5,14]. With the increasing availability of biochemical, enzymatic and molecular testing, these disorders can now be diagnosed earlier, which highlights the importance of therapeutic interventions to avoid or minimize neurological damage. Other disorders affecting valine catabolic pathway, such as maple syrup urine disease (MSUD), propionic acidemia (PA) and methylmalonic acidemia (MMA), are routinely treated with dietary interventions with the aim of reducing the accumulation of toxic metabolites, therefore Luopatty et al. suggested that limiting valine intake could be a potential therapeutic intervention in HIBCH deficiency [1]. To date there have been a few single-case reports describing the clinical outcome of HIBCH patients on a valine restricted diet [4,15] and similar principles have been applied for the dietary treatment of one ECHS1 patient [10]. While results are encouraging, the information is limited by the short-term follow-up of these single cases, as well as the paucity of information regarding diet composition and biochemical markers while on treatment. Here we present the results of a prospective treatment intervention with a valine restricted diet in five patients, two with HIBCH and three with ECHS1 deficiencies, and report on the long-term effects on the clinical, neuroimaging and biochemical outcomes.
2. Patients
Patients 1 (male) and 2 (female), a sibling pair with HIBCH deficiency, have been described in detail previously [16]. Briefly, they presented in infancy with a clinical and MRI phenotype consistent with Leigh syndrome (Fig. 2). Diagnosis was established via whole exome sequencing (WES) which revealed a homozygous mutation in the HIBCH gene (c.196C > T; p.Arg66Trp). Enzyme activity in fibroblasts confirmed the disorder at 12 and 13 years of age, respectively, and a valine restricted diet was started one year later. At that time, both patients were wheelchair bound, nonverbal and had minimal interaction with their surroundings. They had microcephaly, poor vision, abnormal eye movements, optic nerve atrophy, severe dystonia, scoliosis, gastroesophageal reflux and dysphagia and they were exclusively fed via gastrostomy tube. Patients 3 and 4 are male siblings with ECHS1 deficiency. They were born to nonconsanguineous parents of Caucasian and Samoan decent. Briefly, patient 3 was first evaluated at 13 m of age due to a family history of a brother who died at age 9 y of a suspected mitochondrial disease. Physical exam revealed hypertonicity and a brain MRI was consistent with Leigh syndrome (Fig. 3). He had delayed development but was ambulatory and fed orally until 3 y, when he was admitted with a severe metabolic crisis with acidosis and ketosis. As a result, he developed severe truncal hypotonia with hypertonia / rigidity in extremities, becoming non-ambulatory. He was also noted to have nystagmus and dysphagia requiring a gastrostomy tube for nutritional support. Feedings were complicated due to frequent emesis leading to malnutrition. He had several subsequent admissions due to intercurrent illnesses and progressively became less interactive. At age 6 y the diagnosis of ECHS1 deficiency was confirmed at the enzymatic and molecular level and he was started on a low valine diet. At the time he was encephalopathic, nonverbal, with minimal interaction with his surroundings. He had nystagmus, no response to light and severe truncal hypotonia, appendicular hypertonia and dystonic posturing. He remained GT fed, with poor feeding tolerance and malnutrition. Patient 4 is the older sibling of patient 3. He had normal developmental milestones until 6 m, when he began to show signs of regression. He was first evaluated at age 2.1 y due to an intercurrent illness that triggered an acute metabolic decompensation with lactic acidosis and ketosis. MRI findings were consistent with Leigh's disease (Fig. 3). Over the years, patient 4 had a milder clinical course than his brother, as he had no feeding intolerance or frequent admissions after the initial episode. The diagnosis of ECHS1 deficiency was confirmed at 13.8 y of age, when dietary treatment was initiated. At the time his physical exam demonstrated decreased vision and intermittent nystagmus. He had slurred speech and drooling and was able to eat by mouth with some assistance. He was interactive but slow to respond to stimuli or verbal commands. He attended school with a modified curriculum and had increasing learning difficulties. His hypertonia had progressed to the point that he was non-ambulatory and began to develop joint contractures despite treatment with oral baclofen. Both patients 3 and 4, had decreased ECHS1 enzymatic activity in fibroblasts and they were found to have novel heterozygous variants in the ECHS1 gene. The c.832G > A (p.Ala278Thr) variant was inherited from the father and the c.489G > A (p.Pro163=) variant was inherited from the mother. Further details on the biochemical, enzymatic and molecular studies for this family are being submitted in a separate paper.
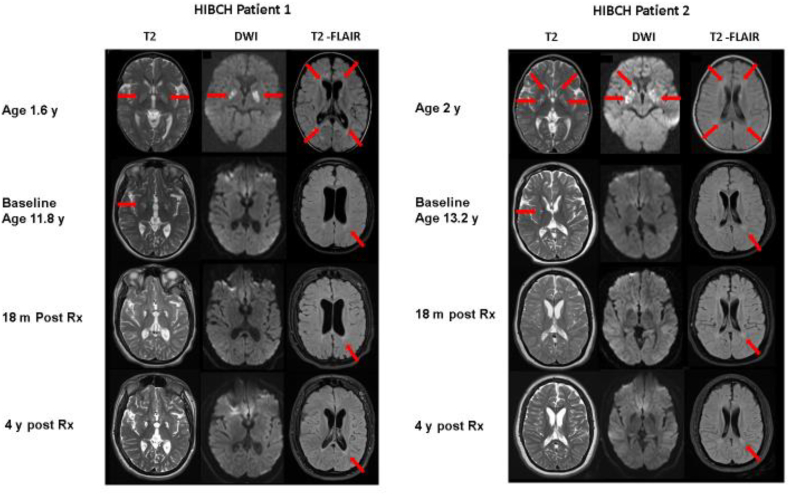
Fig. 2.
MRI of the brain in patients 1 and 2 with HIBCH deficiency at different ages and stages (pre, post treatment). Axial cuts at similar levels in T2, diffusion weighted imaging (DWI) and T2-FLAIR modalities are shown. Detailed explanation in the text. For patient 1, see arrows for hyperintensities (in T2) and restricted diffusion (in DWI) in bilateral basal ganglia, as well as periventricular white matter hyperintensity in T2-FLAIR. For patient 2, see arrows for hyperintensities and cystic lesions (in T2) and restricted diffusion (in DWI) in bilateral basal ganglia, as well as periventricular white matter hyperintensity in T2-FLAIR.
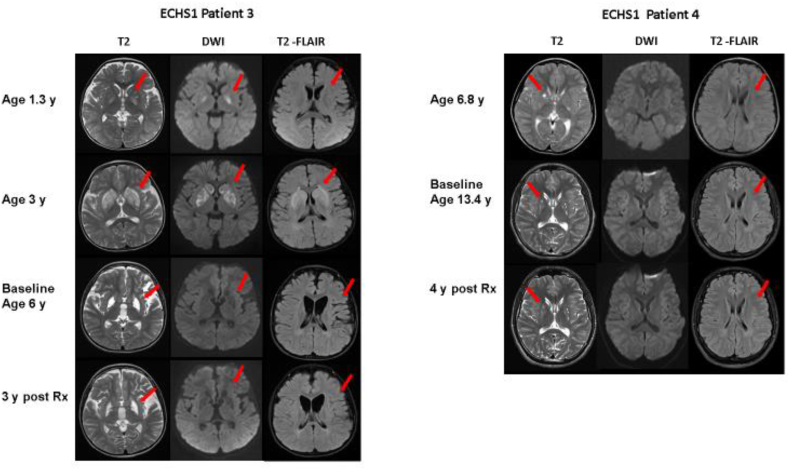
Fig. 3.
MRI of the brain in patients 3 and 4 with ECHS1 deficiency at different ages and stages (pre, post treatment). Axial cuts at similar levels in T2, diffusion weighted imaging (DWI) and T2-FLAIR modalities are shown. Detailed explanation in the text. For patient 3, see arrows for hyperintensities (in T2) and restricted diffusion (in DWI) in bilateral basal ganglia, as well as periventricular white matter hyperintensity in T2-FLAIR at 1.3 and 3 years of age. Images prior to treatment initiation (baseline) and 3 years post-treatment show residual cystic changes in the basal ganglia (T2 arrow), with no acute changes in DWI. Persistent white matter signal abnormalities with stable diffuse brain atrophy are also shown (T2-FLAIR arrow). For patient 4, mild cystic lesions in bilateral basal ganglia (T2 arrow) and mild periventricular white matter hyperintensities (T2-FLAIR arrow) were noted, with no acute changes in DWI.
Patient 5 is the second child of a nonconsanguineous Mexican couple, his older sister is not affected. He was born at 38 weeks via vaginal delivery and had an uneventful post-natal course. At 11 m, mother noted that he was not eating well, had lost weight, had decreased muscle tone and appeared tired. Around the same time, he stopped babbling and turning over, and was less responsive to stimuli. He was admitted to an outside hospital and had an MRI of the brain showing abnormal T2 hyperintensity involving bilateral globi pallidi and mild prominence of the bifrontal subarachnoid spaces (Fig. 4). A mitochondrial disease was suspected, and he was started on treatment with Coenzyme Q10 and carnitine. Work up included a mitochondrial dual gene panel that included full sequence of mitochondrial DNA and 164 nuclear genes, which was unrevealing. The patient progressively developed severe dystonia and agitation that was difficult to control and at 13 m he required GT placement for nutritional support due to dysphagia and persistent emesis. He was first evaluated at CHOC Children's at 15 m. Physical exam revealed irritability, stridor, truncal hypotonia and severe hypertonicity of extremities with hyperreflexia and dystonic posturing. Laboratory testing revealed elevated CK (peak value >10,000 units/L, normal <374), mild elevation in lactic acid at 2.7 mmol/L (normal <2.2), and normal carnitine, acylcarnitines, ammonia and urine organic acids. A muscle biopsy showed normal histology, mtDNA content and ETC activities and WES was negative. He was treated with IV fluids, baclofen and trihexyphenidyl. One month later he developed seizures that responded to phenobarbital and soon thereafter he was diagnosed with auditory neuropathy. He continued with symptomatic care without significant improvement, CK normalized, lactic acid remained intermittently elevated. At 33 m, sequencing of ECHS1 gene was ordered and revealed a novel frameshift variant expected to be deleterious, c.849-852del (p.Lys284Profs*31) and a known pathogenic mutation, c.713C > T (p.Ala238Val). He was started on low valine diet at 3 years.
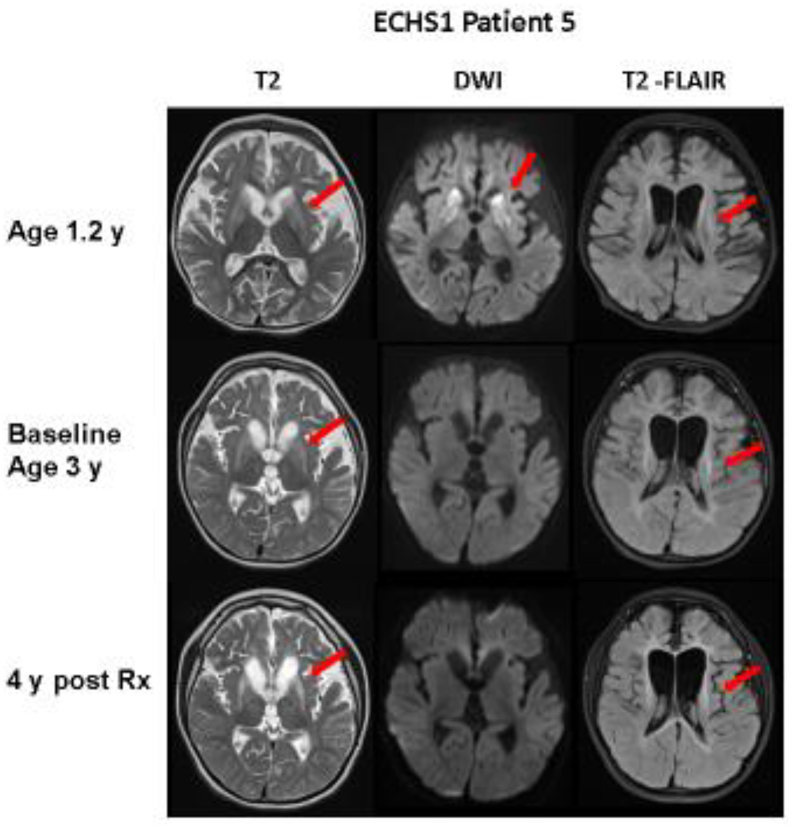
Fig. 4.
MRI of the brain in patient 5 with ECHS1 deficiency at different ages and stages (pre, post treatment). Axial cuts at similar levels in T2, diffusion weighted imaging (DWI) and T2-FLAIR modalities are shown. Detailed explanation in the text. At 1.2 year of age, hyperintense lesions (in T2) and restricted diffusion (in DWI) in basal ganglia with mild diffuse white matter hyperintensities are shown (arrows). At 3 y, as well as 4 years post treatment, cystic changes in basal ganglia and mild white matter signal abnormalities persisted (see arrows) with no acute changes seen in DWI.
A summary of all the patients' initial characteristics is presented in Table 1.
Table 1.
Initial presentation.
| Patient | Sex | Mutations | Age of Onset | Age at Dx | Brain MRI | Biochemical Investigations | Enzyme Activity (Fibroblasts) | Developmental Delay | Regression | Seizures | Dystonia | Visual Impairment | Hearing Loss | Cardiomyopathy | GT Feedings | Clinical Course | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIBCH | 1 | M | c.196C > T p.(Arg66Trp) c.196C > T p.(Arg66Trp) | 5 m | 13 y | Leigh's | Normal lactate and PDHC ↑ C4OH | ↓ | Yes | Yes | No | Yes | Yes | No | No | Yes | Severe, progressive |
| 2 | F | c.196C > T p.(Arg66Trp) c.196C > T p.(Arg66Trp) | 6 m | 12 y | Leigh's | Normal lactate, ↑ C4OH | ↓ | Yes | Yes | No | Yes | Yes | No | No | Yes | Severe, progressive | |
| ECHS1 | 3 | M | c.832G > A p.(Ala278Thr) c.489G > A p.(Pro163=) | 15 m | 6 y | Leigh's | Intermittent lactic acidosis & ketosis | ↓ | Yes | Yes | No | Yes | Yes | No | No | Yes | Severe, progressive |
| 4 | M | c.832G > A p.(Ala278Thr) c.489G > A p.(Pro163=) | 6 m | 13 y | Leigh's | Intermittent lactic acidosis & ketosis | ↓ | Yes | Yes | No | Yes | Yes | No | No | No | Moderate, progressive | |
| 5 | M | c.849_852del p.(Lys284Profs*31) c.713C > T p.(Ala238Val) | 11 m | 3 y | Leigh's | Intermittent lactic acidosis & ketosis | ND | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Severe, progressive |
PDHC: Pyruvate dehydrogenase complex, ND: Not done
Methods: All patients were followed by the same team of metabolic physicians, pediatric neurologists and dietitians with routine clinical evaluations at the Neurometabolic clinic. Baseline brain MRI imaging was obtained prior to initiation of the diet and repeated 18 m – 4 y thereafter. To determine the starting valine restriction in our patients, we reviewed the recommendations for valine intake in two other conditions where valine restriction is the standard of care, MSUD and PA (Nutrition Support Protocols, Ross, 2001). In addition, we considered the patients' baseline valine intake. Given the location of the enzyme defects in the valine degradation pathway, our valine intake aim was mid-range of the two known conditions. Our goal for all patients was to restrict intact (natural) protein to keep valine in the lower normal range, while avoiding any amino acid deficiencies. Changes in our patients' valine intake was easily managed in four of our five patients (1, 2, 3 and 5) as they were fed via gastrostomy tube. Patient 4 was an oral feeder and his dietary valine (protein) intake and adjustments were more difficult to implement. The initial intact protein decrease was ~30% in patients 1–4, with subsequent adjustments based on amino acid profiles. Patient 5 was diagnosed and began treatment one year after the other four patients; therefore, we were able to titrate his diet more rapidly. In the four-gastrostomy tube fed patients, we provided additional branched-chain amino acid (BCAA) free medical food (Ketonex-1®/Ketonex-2®) to meet total protein needs and used protein-free medical food products (Pro-Puree® and Polycal®) to meet energy needs. See Table 2 for details of the diet intervention. No other changes were made in their treatment plans. All patients were receiving carnitine prior to initiation of the diet, and they remained on the same carnitine dose during treatment. Plasma amino acids, lactic acid and chemistry labs, obtained after overnight fast, were monitored every 2–6 m throughout the treatment. Additionally, over the first 19–24 months, C4OH (3-OH-Isobutyryl-3-OH-butyrylcarnitine) was monitored in HIBCH patients and SCPC levels were measured in all patients in urine blotters via LC/MS-MS using the +320 > 119 m/z transition as previously described [17]. Results are reported as percentage of decrease for each metabolite over the study period. Data was also analyzed using Wilcoxon non-parametric t-test via GraphPad Prism 7.03. Urine organic acids were also measured during this period, via GC–MS.
Table 2.
Summary of the nutritional treatment.
| Total Energy (kcal/d) | Total Protein (g/day) | Intact Protein (g/day) | Valine Intake (mg/day) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age (years) | Weight (kg) | Baseline | Last Rx | Baseline | Last Rx | Baseline | Last Rx | Decrease (%) | Baseline | Last Rx | Decrease (%) |
| 1 | 13.0 | 39 | 1250 | 1055 | 50 | 44.3 | 50 | 11.3 | 77 | 2254 | 653 | 71 |
| 2 | 14.5 | 44 | 1250 | 1055 | 50 | 44.3 | 50 | 11.3 | 77 | 2254 | 653 | 71 |
| 3 | 6.3 | 13 | 1630 | 2117 | 30 | 19.5 | 30 | 19.5 | 35 | 1795 | 1167 | 35 |
| 4 | 13.8 | 45 | 1725 | 1190 | 65 | 37.0 | 65 | 37.0 | 43 | NA | NA | NA |
| 5 | 3.8 | 15 | 932 | 880 | 39 | 20.1 | 39 | 9.6 | 75 | 2452 | 605 | 75 |
3. Results
3.1. Diet Composition (Valine Intake)
In all gastrostomy fed patients valine intake was reduced significantly (35–75%). In patient 4, who did not have a gastrostomy tube, intact protein decreased from 65 g/d to 37 g/d, approximately 43%. See Table 3 and (Fig. 5) for results of the diet intervention.
Table 3.
Response to treatment.
| Patient |
Gastrointestinal symptoms |
Awareness and Interaction |
Muscle tone |
Spontaneous Movements |
Expressive language |
Vision |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | ||
| HIBCH | 1 | Frequent Emesis | Improved | Minimal | Improved | Truncal hypotonia, dystonia | Improved | No | Yes | No | Makes sounds | No visual response | No changes |
| 2 | Frequent Emesis | Resolved | Minimal | Improved | Truncal hypotonia, dystonia | Improved | No | Yes | No | Makes sounds | No visual response | Able to track | |
| ECHS1 | 3 | Frequent Emesis | Significantly improved | Minimal | Improved | Truncal hypotonia, dystonia | Improved | No | Yes | No | Makes sounds | Blinking to light | Able to track |
| 4 | None | None | Decreased | Improved | Hypertonia in extremities | Improved | Wheel chair bound | Able to ambulate with help | Slow, difficult to complete sentences | Improved speech. Talks in full sentences. DC speech therapy | Moderate impairment | Improved | |
| 5 | Frequent Emesis | Improved | Minimal | Improved | Truncal hypotonia, dystonia | Improved | Minimal | Improved | Cooing, makes sounds | Babbling, word approximations | Moderate impairment | No changes | |
Fig. 5.
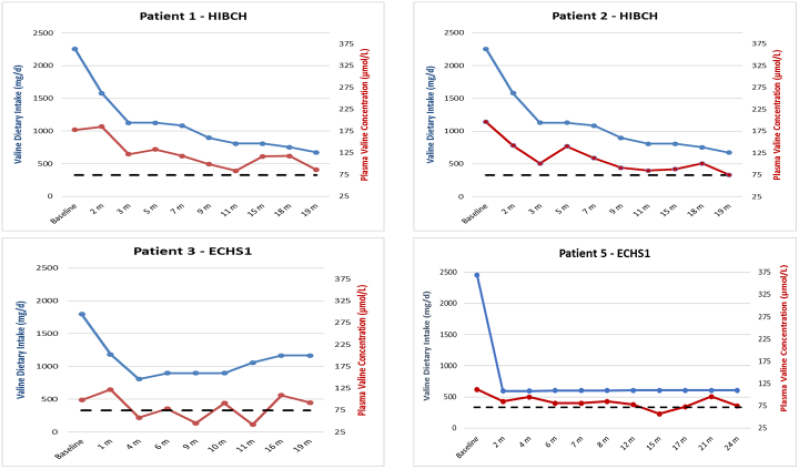
Comparison of valine dietary intake (blue line) with plasma valine concentration (red line). Normal valine values 74–321 umol/L. Dotted lines denote the low-normal level (74 umol/L).
3.2. Biochemical markers
Plasma valine levels decreased as expected. The target range (74–90 μmol/L) was reached within the first 11 months in patients 1 and 2, and within the first month in patient 5, whose valine intake was lowered more rapidly. Patients required minimal adjustments to the diet once they reached the target range, except for patient 3 who required frequent changes to maintain desired levels (Fig. 5). In patient 4 we were unable to fully implement protein restriction. Accordingly, his baseline valine level (255 μmol/L) decreased only modestly to an average of 196 μmol/L (n = 6) and did not reach the target range. Isoleucine and leucine levels were maintained within the normal range and did not require supplementation.
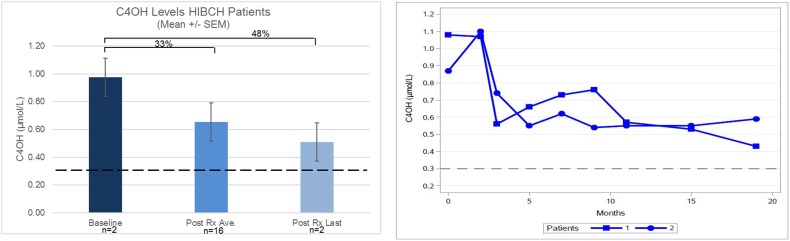
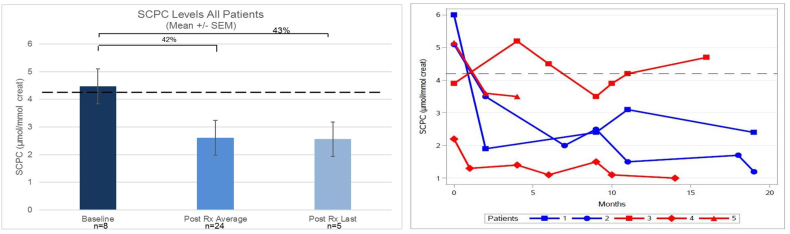
In both HIBCH patients C4OH decreased an average of 33% over the 19 months treatment period, or 48% when comparing the baseline and last value (Fig. 6 A and B). In all patients, SCPC decreased 42% on average and 43% when comparing baseline and the last measurement (Fig. 7 A). However, changes varied from patient to patient, being more significant for patients 1, 2 and 5. No changes in SCPC were seen in patient 3, while in patient 4 levels were always within normal limits (Fig. 7 B).
Fig. 6.
Changes in C4OH levels with diet intervention.
A: Percentage of decrease in C4OH levels from baseline, compared to average post treatment levels as well as last value post treatment. Dotted lines denote upper C4OH normal levels. B: C4OH levels in patients 1 and 2 with HIBCH defect.
Fig. 7.
Changes in SCPC levels with diet intervention.
A: Percentage of decrease SCPC levels from baseline, compared to average post treatment levels as well as last value post treatment. Dotted lines denote upper SCPC normal levels. B: SCPC levels in each individual patient, documented during the study.
Blood levels of C4OH and SCPC correlated significantly with the changes in dietary valine intake (Fig. 8 A and B). Only trace levels of 2,3-dihydroxy-2-methyl-butyric acid [7,17] found in the urine organic acids of all patients at baseline and continued at trace or undetectable levels during the study. Similarly, no significant elevations were detected in lactic acid levels before or during the treatment. No other significant biochemical abnormalities were observed in the patients during the study.
3.3. Clinical response
Clinical outcomes are presented in Table 3. Four out of the five patients had frequent emesis at baseline, and they all showed improvement or resolution of those symptoms under treatment. Prior to the dietary interventions, most patients (4/5) were encephalopathic, showing minimal awareness of surroundings. After treatment, all patients showed improvement in the level of consciousness and increased social interaction. They were able to smile, laugh, responded to music and made sounds or cried demanding attention. All these changes were documented during clinic visits as well as reported by parents, caretakers and teachers.
At baseline patients 1, 2, 3 and 5 had severe truncal hypotonia and dystonia, while after treatment they had much better head control and decreased severity and frequency of dystonic posturing. Patient 4 had severe spasticity in all extremities prior to treatment, which decreased significantly over time. Of note, no changes in any of the medications used to control muscle tone or movement disorders were made during the study.
All patients also experienced improvement in spontaneous movements, being able to raise their hands and move their heads. Patient 4, who was spastic, and wheelchair bound was able to regain ambulation with assistance as his hypertonicity improved. Expressive language was minimal or not present in patients 1, 2, 3 and 5 and improved slightly during the study, as patients were able to make more sounds and have some word approximations. The most significant improvement was documented in patient 4, the least affected patient, whose improvement allowed for discontinuation of speech therapy. Minimal improvements were also noted in vision in patients 2, 3 and 4. Patients 1 and 2, had a total of 3 emergency room evaluations due to fever / UTI; patient 3 had a 16 day admission for fever secondary to a respiratory infection (solitary nodular lesion in lung, that resolved with ATB treatment); patient 4 had no ED or inpatient visits and patient 5 underwent a fundoplication during the treatment period. None of the patients had a metabolic crisis during the above-mentioned intercurrent events.
MRI of the brain in all patients showed stabilization or improvement of the images over time. For patient 1 (Fig. 2), initial MRI was obtained at 1.6 y and showed hyperintensities (T2, T2-FLAIR) and restricted diffusion (DWI) in bilateral basal ganglia, as well as periventricular white matter hyperintensity evident in T2-FLAIR (Fig. 2, patient 1). A new MRI was obtained as baseline at 11.8 y of age, prior to the initiation of treatment. It demonstrated cystic encephalomalacia in bilateral basal ganglia, persistent diffuse hyperintensity in white matter and mild diffuse brain atrophy; consistent with the progression of the disease. DWI modality did not show signs of acute disease. Follow up MRI's were obtained after 18 m and 4 y on dietary treatment. There was no further progression of the disease and a decrease in the level of atrophy was noted (shown in T2-FLAIR).
Patient 2's initial MRI was obtained at 2 y and showed similar T2 hyperintensities and restricted diffusion in bilateral basal ganglia, as well as periventricular white matter hyperintensity (Fig. 2, patient 2). MRI obtained prior to treatment initiation, at 13.2 y of age, showed residual cystic changes in basal ganglia with no restricted diffusion in DWI, persistent white matter signal changes and increased diffuse brain atrophy. Follow up MRI's were obtained 18 m and 4 y after treatment. Similar to patient 1, there was no further progression of the disease and there was minimal decrease in brain atrophy.
Patient 3 had initial MRI at age 1.3 y (Fig. 3, patient 3) and a repeat MRI was done at age 3 y, when he was readmitted with another metabolic crisis. Both MRI's, in all modalities, show involvement of the basal ganglia, affecting bilateral globus pallidi in the initial one with extended lesions in bilateral caudate and putamen in the second MRI. Diffuse periventricular white matter intensities in T2 were also visualized in both MRI's. Baseline images obtained prior to diet initiation at 6 y, show residual cystic changes in the basal ganglia, with no acute changes in DWI and persistent white matter signal abnormalities with increased diffuse brain atrophy. A follow up MRI was obtained 3 y after treatment and showed no further progression of the basal ganglia involvement and stabilization / improvement in brain atrophy.
Patient 4, with a milder ECHS1 phenotype, had initial MRI at 6.8 y (Fig. 3, patient 4), which showed mild cystic lesions in bilateral basal ganglia and mild periventricular white matter hyperintensities, without atrophy. No acute changes in DWI were present. His pre-treatment MRI at 13.4 y was stable and last MRI obtained 4 years post diet initiation, shows no progression of the disease.
Patient 5, with severe ECHS1 phenotype, had initial MRI at 1.2 y, showing hyperintense lesions in basal ganglia with mild disuse white matter hyperintensities. Moderate diffuse brain atrophy was already evident (Fig. 4).
Pretreatment MRI at 3 y, showed cystic changes in basal ganglia, no acute changes in DWI. Mild white matter signal abnormalities persisted, and brain atrophy was increased. After 4 years on treatment, his MRI was essentially unchanged.
4. Discussion
HIBCH and ECHS1 deficiency can be devastating neurological disorders. Due to the similar presentation to mitochondrial disease, many patients remain undiagnosed or are diagnosed late. Although some evidence suggests that HIBCH deficiency could be detected at birth, the disease is not currently included in newborn screening programs [16]. Availability of WES and/or whole genome sequence (WGS) has increased the detection of these defects highlighting the need for therapies. Other disorders affecting valine catabolism (MSUD, PA and MMA), are treated with restriction of the respective offending amino acids. With the same rationale, case reports have outlined improvements on a valine restricted diet in 2 patients with HIBCH and 1 patient with ECHS1 deficiency [4,10,15]. While these reports are encouraging, minimal details about the dietary interventions and biochemical follow-up are given in these publications. Due to the paucity of information we were cautious about the implementation of valine restriction and closely monitored patients' valine levels. In patients 1 and 2, with HIBCH deficiency, a valine restriction of 653 mg/d was needed to maintain plasma valine levels at the target range. The above valine intake is ~30% higher than the upper recommended range for MSUD patients of same age range. Similarly, patient 5, who has ECHS1 deficiency, was able to maintain target valine levels with an intake of 605 mg/day, ~ 50% above the MSUD recommendations for his age range. The implemented valine restriction resulted in a significant reduction in intact protein, requiring the use of a valine-restricted medical food. As such a product is not available; a BCAA free medical food was used, which is commonly prescribed for treatment of MSUD patients. The same approach was used with the previously reported case with ECHS1 (10) and a patient with HIBCH deficiency (15) while a medical food with no valine, methionine and threonine, commonly prescribed for patients with PA and MMA, was used in another patient with HIBCH deficiency [4]. Although both treatments will result in valine restriction, the use of a BCAA restricted medical food will also result in restriction of leucine and isoleucine. ECHS1 is also involved in the catabolism of these two amino acids [14] but to date there is no evidence of the accumulation of toxic metabolites from these pathways. Furthermore, leucine and isoleucine levels in our patients remained within normal limits and therefore no dietary manipulation of these amino acids was needed. Patient 3 was severely malnourished at the beginning of the study. He required a higher valine intake (1167 mg/day) to maintain valine levels in the desired range. It is possible that his requirements were higher due to protein accretion and anabolism. However, the patient had poor weight gain during the study despite receiving more than 2000 cal per day. Work up for malabsorption was negative and the nutritional profile of his formula as well as biochemical markers did not detect any deficiencies. Therefore, we do not have a clear explanation for his malnutrition and lack of weight gain. Interestingly, two other patients with poor weight gain despite the support of gastrostomy feedings have been described, so it is possible that malnutrition could be part of the phenotype in some patients with ECHS1 deficiency [12,14]. Patient 4, who was an oral feeder, was unable to follow the diet strictly. His plasma valine levels decreased ~23% on average but remained above the desired target. Of note, he still showed clinical improvements.
Dietary treatment had the expected effect on the biochemical profile as demonstrated by the decrease in C4OH and SCPC levels (Fig. 6, Fig. 7). This effect was more pronounced for patients 1, 2 and 5. Despite the documented reductions, due to the small sample size, the results were not statistically significantly different. Additionally, there was a statistically significant correlation between valine intake and C4OH and SCPC levels, supporting the fact that the reduction in the above-mentioned markers was a consequence of the decrease in valine load (Fig. 8 A and B).
In contrast to the expected progression or worsening of the disease, which has been widely documented for HIBCH [1,2,5,18,19] and ECHS1 patients [7,9,12,14,[20], [21], [22]], our patients demonstrated sustained clinical improvements. As shown in Table 3, positive changes were noted in gastrointestinal symptoms, alertness, interaction, muscle tone, spontaneous movements and expressive language. Due to pre-existent severe damage, improvements were small, but had a positive impact in quality of life for patients and families. No side effects or nutritional deficiencies were noted and, none of the patients sustained new metabolic decompensations after treatment initiation, despite having intercurrent illnesses and procedures.
Consistent with the documented clinical changes, brain MRI's showed stabilization or improvement 18 m – 4 y after initiation of treatment. This is in contrast with the progression of the MRI abnormalities reported in other patients with HIBCH [17,19,23] and ECHS1 deficiency [7,9,12,14,20,21] and represent a remarkable change in the progressive course of the MRI changes that were documented in our own patients before dietary treatment was initiated (Fig. 2, Fig. 3, Fig. 4). Interestingly, improvements in basal ganglia abnormalities were reported in an HIBCH patient after implementation of frequent carbohydrate-rich meals [18]. Although details on the diet composition in that patient were not reported, it is possible that the increased carbohydrate intake resulted in a low protein diet.
Our study results are in agreement with the clinical improvement noted in previously published case reports (15,4,10), significantly increase the total number of cases who benefited from dietary treatment and add detailed information about diet implementation, biochemical response and MRI follow up. Although these results are encouraging, these observational studies are limited by the low number of patients included, and the advanced disease at treatment initiation, a frequent problem when dealing with rare and progressive diseases.
5. Conclusion
A valine restricted diet appears to be beneficial for patients with HIBCH and ECHS1 defects, improving the biochemical and clinical manifestations of these disorders. Based on our experience, we suggest that the valine intake should be greater than recommended for MSUD and lower than what is recommended PA and MMA. Clinicians can reduce the valine content of the diet safely by limiting intact protein and using an appropriate amount valine-free and other protein-free medical foods to meet energy, valine, total protein and micronutrient needs. Plasma amino acid levels should be followed periodically, and diet adjusted accordingly, as done in other organic acidemias. Although improvements in our patients were limited due to advanced disease, it is possible that dietary treatment will have a greater impact in patients with early diagnosis and/or mild clinical presentations.
Authors contributions
Abdenur JE: Conceptualization, Methodology, Investigation, Formal analysis, Supervision, Funding acquisition, Writing original draft, Writing-review editing. Sowa M: Conceptualization, Methodology, Investigation, Formal analysis, Writing original draft, Writing-review editing. Simon M: Investigation, Methodology, Data curation, Writing-review editing. Steenari M: Investigation, Methodology, Visualization, Writing-review editing. Skaar J: Investigation, Data curation. Eftekharian S: Formal analysis, Visualization. Chang R: Investigation, Data curation. Ferdinandusse S: Investigation, Writing-review editing. Pitt J: Investigation, Writing-review editing.
Acknowledgements
The authors thank the families for their commitment to participation in these studies and all the providers who cared for the patients and families. This work was supported by the National Institutes of Health [5T32GM008243-32] to MSi; the Fry Family Foundation [40031028] to MSi, SE
References
- 1.Loupatty F.J., Clayton P.T., Ruiter J.P.N., Ofman R., Ijlst L. Mutations in the gene encoding 3-hydroxyisobutyryl-CoA hydrolase results in progressive infantile neurodegeneration. Am. J. Hum. Genet. 2007;80(1):195–199. doi: 10.1086/510725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown G.K., Hunt S.M., Scholem R. beta-hydroxyisobutyryl coenzyme A deacylase deficiency: a defect in valine metabolism associated with physical malformations. Pediatrics. 1982;70(4):532–538. [PubMed] [Google Scholar]
- 3.Schottmann G., Sarpong A., Lorenz C., Weinhold N., Gill E. A movement disorder with dystonia and ataxia caused by a mutation in the HIBCH gene. Mov. Disord. 2016;31(11):1733–1739. doi: 10.1002/mds.26704. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y., Zhang J., Yu K., Feng F., Sun X. A therapeutic regimen for 3-hydroxyisobutyryl-CoA hydrolase deficiency with exercise-induced dystonia. Eur. J. Paediatr. Neurol. 2019;23(5):755–759. doi: 10.1016/j.ejpn.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Ferdinandusse S., Waterham H.R., Heales S.J.R., Brown G.K., Hargreaves I.P. HIBCH mutations can cause Leigh-like disease with combined deficiency of multiple mitochondrial respiratory chain enzymes and pyruvate dehydrogenase. Orphanet J. Rare Dis. 2013;8:188. doi: 10.1186/1750-1172-8-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharpe A.J., McKenzie M. Mitochondrial fatty acid oxidation disorders associated with short-chain Enoyl-CoA hydratase (ECHS1) deficiency. Cells. 2018;7:6. doi: 10.3390/cells7060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters H., Buck N., Wanders R., Ruiter J., Waterham H. ECHS1 mutations in Leigh disease. A new inborn error of metabolism affecting valine metabolism. Brain. 2014;137:2903–2908. doi: 10.1093/brain/awu216. Pt 11. [DOI] [PubMed] [Google Scholar]
- 8.Carlston C.M., Ferdinandusse S., Hobert J.A., Mao R., Longo N. Extrapolation of variant phase in mitochondrial short-chain Enoyl-CoA hydratase (ECHS1) deficiency. JIMD Rep. 2019;43:103–109. doi: 10.1007/8904_2018_111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aretini P., Mazzanti C.M., La Ferla M., Franceschi S., Lessi F. Next generation sequencing technologies for a successful diagnosis in a cold case of Leigh syndrome. BMC Neurol. 2018;18(1):99. doi: 10.1186/s12883-018-1103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shayota B.J., Soler-Alfonso C., Bekheirnia M.R., Mizerik E., Boyer S.W. Case report and novel treatment of an autosomal recessive Leigh syndrome caused by short-chain enoyl-CoA hydratase deficiency. Am. J. Med. Genet. A. 2019;179(5):803–807. doi: 10.1002/ajmg.a.61074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchino S., Iida A., Sato A., Ishikawa K., Mimaki M. A novel compound heterozygous variant of ECHS1 identified in a Japanese patient with Leigh syndrome. Human Genom. Variation. 2019;6:19. doi: 10.1038/s41439-019-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haack T.B., Jackson C.B., Murayama K., Kremer L.S., Schaller A. Deficiency of ECHS1 causes mitochondrial encephalopathy with cardiac involvement. Ann. Clin. Transl. Neurol. 2015;2(5):492–509. doi: 10.1002/acn3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tetreault M., Fahiminiya S., Antonicka H., Mitchell G.A., Geraghty M.T. Whole-exome sequencing identifies novel ECHS1 mutations in Leigh syndrome. Hum. Genet. 2015;134(9):981–991. doi: 10.1007/s00439-015-1577-y. [DOI] [PubMed] [Google Scholar]
- 14.Ferdinandusse S., Friederich M.W., Burlina A., Ruiter J.P.N., Coughlin C.R. Clinical and biochemical characterization of four patients with mutations in ECHS1. Orphanet J. Rare Dis. 2015;10(1):542. doi: 10.1186/s13023-015-0290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soler-Alfonso C., Enns G.M., Koenig M.K., Saavedra H., Bonfante-Mejia E. Identification of HIBCH gene mutations causing autosomal recessive Leigh syndrome. A gene involved in valine metabolism. Pediatr. Neurol. 2015;52(3):361–365. doi: 10.1016/j.pediatrneurol.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Stiles A.R., Ferdinandusse S., Besse A., Appadurai V., Leydiker K.B. Successful diagnosis of HIBCH deficiency from exome sequencing and positive retrospective analysis of newborn screening cards in two siblings presenting with Leigh’s disease. Mol. Genet. Metab. 2015;115(4):161–167. doi: 10.1016/j.ymgme.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters H., Ferdinandusse S., Ruiter J.P., Wanders R.J.A., Boneh A. Metabolite studies in HIBCH and ECHS1 defects. Implications for screening. Mol. Genet. Metab. 2015;115(4):168–173. doi: 10.1016/j.ymgme.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Yamada K., Naiki M., Hoshino S., Kitaura Y., Kondo Y. Clinical and biochemical characterization of 3-hydroxyisobutyryl-CoA hydrolase (HIBCH) deficiency that causes Leigh-like disease and ketoacidosis. Mol. Genet. and Metab. Rep. 2014;1:455–460. doi: 10.1016/j.ymgmr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan H., Chen X., Lv W., Linpeng S., Liang D. Truncating mutations of HIBCH tend to cause severe phenotypes in cases with HIBCH deficiency. A case report and brief literature review. J. Hum. Genet. 2018;63(7):851–855. doi: 10.1038/s10038-018-0461-8. [DOI] [PubMed] [Google Scholar]
- 20.Huffnagel I.C., Redeker E.J.W., Reneman L., Vaz F.M., Ferdinandusse S. Mitochondrial encephalopathy and transient 3-Methylglutaconic aciduria in ECHS1 deficiency. Long-term follow-up. JIMD Rep. 2018;39:83–87. doi: 10.1007/8904_2017_48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzsimons P.E., Alston C.L., Bonnen P.E., Hughes J., Crushell E. Clinical, biochemical, and genetic features of four patients with short-chain enoyl-CoA hydratase (ECHS1) deficiency. Am. J. Med. Genet. A. 2018;176(5):1115–1127. doi: 10.1002/ajmg.a.38658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada K., Aiba K., Kitaura Y., Kondo Y., Nomura N. Clinical, biochemical and metabolic characterisation of a mild form of human short-chain enoyl-CoA hydratase deficiency. Significance of increased N-acetyl-S-(2-carboxypropyl)cysteine excretion. J. Med. Genet. 2015;52(10):691–698. doi: 10.1136/jmedgenet-2015-103231. [DOI] [PubMed] [Google Scholar]
- 23.Reuter M.S., Sass J.O., Leis T., Köhler J., Mayr J.A. HIBCH deficiency in a patient with phenotypic characteristics of mitochondrial disorders. Am. J. Med. Genet. A. 2014:3162–3169. doi: 10.1002/ajmg.a.36766. 164A (12. [DOI] [PubMed] [Google Scholar]