Abstract
Background
Precision medicine approaches aim to tackle diseases on an individual level through molecular profiling. Despite the growing knowledge about diseases and the reported diversity of molecular phenotypes, the descriptions of human health on an individual level have been far less elaborate.
Methods
To provide insights into the longitudinal protein signatures of well-being, we profiled blood plasma collected over one year from 101 clinically healthy individuals using multiplexed antibody assays. After applying an antibody validation scheme, we utilized > 700 protein profiles for in-depth analyses of the individuals’ short-term health trajectories.
Findings
We found signatures of circulating proteomes to be highly individual-specific. Considering technical and longitudinal variability, we observed that 49% of the protein profiles were stable over one year. We also identified eight networks of proteins in which 11–242 proteins covaried over time. For each participant, there were unique protein profiles of which some could be explained by associations to genetic variants.
Interpretation
This observational and non-interventional study identifyed noticeable diversity among clinically healthy subjects, and facets of individual-specific signatures emerged by monitoring the variability of the circulating proteomes over time. To enable more personal hence precise assessments of health states, longitudinal profiling of circulating proteomes can provide a valuable component for precision medicine approaches.
Funding
This work was supported by the Erling Persson Foundation, the Swedish Heart and Lung Foundation, the Knut and Alice Wallenberg Foundation, Science for Life Laboratory, and the Swedish Research Council.
Keywords: Affinity proteomics, Longitudinal profiling, Plasma proteomics, pQTLs, Precision medicine
Research in Context.
Evidence before this study
Proteins circulating the human blood can provide important information about health or disease states of an individual. Today, many studies focus on finding proteins related to diseases or specific conditions even though our knowledge about if and why proteins differ between individuals, which protein levels vary over time, and how protein profiles appear in clinically-healthy persons is still limited.
Added value of this study
Here, we used multiplexed immunoassays to study a large number of proteins circulating in plasma of 101 clinically healthy and well characterized individuals over one year. We found a substantial individuality in the protein profiles between the participants, which for some of the proteins could be explained by genetic variants. Our analysis also showed that protein profiles varied among the participants over time, which indicated that a variety of short-term as well as continuous changes can occur even in healthy people.
Implications of all the available evidence.
Our findings add to the understanding of molecular signatures of human health and provide important information for studies aiming at finding common protein biomarkers for diseases. Together with evidence from other studies, it appears necessary to consider the diversity, individuality, and variability over time as critical aspects of molecular signatures that aid to advance precision medicine.
Alt-text: Unlabelled box
1. Introduction
Human blood serves as a minimally-invasive source to gain insights about different physiological processes by studying the transcriptome, proteome, or metabolome. Just recently multi-omics studies have emerged to also determine longitudinal profiles of human health and disease [1, 2–3]. Regular monitoring of molecular markers holds the promise to identify perturbations affecting an individual's baseline levels and follow these changes as a healthy system transitions into a disease state [4]. However, longitudinal studies of clinically healthy subjects remain sparse and limited to certain technologies and analytes.
The blood proteome, consisting of both cellular and soluble proteins, has received a revived interest due to advances in protein technologies. This includes mass cytometry [5] to study immune systems, as well as mass spectrometry [6] and affinity assays [7] for profiling serum or plasma. For the circulating plasma proteome, nearly 5,000 proteins have this far been detected when combing discoveries from all assays and technologies [8]. Surprisingly, only 730 proteins are predicted to be actively secreted into the circulation,[9] attributing many of the currently detected proteins to cellular leakage that occur naturally due to cell apoptosis or renewal, or may possibly appear during sample preparation [10]. Even though highly multiplexed assays have enabled large scale assessment of pre-symptomatic health states,[3] additional investigations will complement our molecular description and understanding of the facets of health in healthy individuals.
Here, we used an affinity-based proteomics approach [11] to explore the longitudinal profiles of circulating proteins from 101 clinically healthy individuals selected from the Swedish SCAPIS cohort,[12] who donated blood four times during one year. The objective of this study was to capture the signatures and variability of personal plasma proteomes at baseline and follow these proteins over one year.
2. Materials and methods
2.1. Wellness samples
The Swedish SciLifeLab SCAPIS Wellness Profiling (S3WP) program consists of 101 individuals recruited from the Swedish CArdioPulmonary bioImage Study (SCAPIS), an ongoing prospective observational study [12]. SCAPIS includes 30,154 individuals between 50-65 years that have been randomly selected from the general Swedish population and invited to join the study. All individuals are extensively phenotyped prior to entering the S3WP program. The S3WP study is non-interventional and observational with the aim of collecting longitudinal clinical traits and molecular omics data for all 101 participants. Primary exclusion criteria in SW3P are; (1) previously received health care for myocardial infarction, stroke, peripheral artery disease or diabetes, (2) presence of any clinically significant disease that may interfere with the results or the subject´s ability to participate in the study, (3) any major surgical procedure or trauma within four weeks of the first study visit, or (4) medication for hypertension or hyperlipidaemia. During 2015–2016, the 101 subjects in SW3P visited the clinic every three months, four times in total. In 2016–2018, 97 subjects continued to visit the clinic two additional times with a gap of six months between appointments. Each visit included the measurement of body weight, waist and hip circumference, body fat using bioimpedance (Tanita MC-780MA), and blood pressure. Changes in health and lifestyle was recorded at each visit with a questionnaire covering factors such as diseases, infections, medication, exercise level, and personal perception of health. All 101 subjects were instructed to fast overnight (at least 8 hours) before the collection of blood, urine and feces. Human EDTA plasma samples were transferred on dry ice to SciLifeLab and stored at -80°C upon arrival. The experimental design is described in the Supplementary Information. Clinical and demographic characteristics are presented in Table S1 and Table S2. Genome analysis is described in the Supplementary Materials and Methods. Informed consent was obtained for all participants. The study was performed in accordance with the declaration of Helsinki and the study protocol was approved by the Ethical Review Board of Göteborg, Sweden (Regionala etikprövnignsnämnden, Gothenburg, Dnr 407-15, 2015-06-25).
2.2. TwinGene samples
In the present study, serum samples from a set of 3,000 individuals from the TwinGene study [13] were used for validation purposes. The details about the study, the sample selection criteria and randomization, the plasma protein profiling and genome analysis of the samples are described in the Supplementary Materials and Methods, as well as by Hong et al. [14] The TwinGene study was approved by the Ethical Review Board (Regionala Etikprövningsnämnden, Stockholm, Dnr 2007/644-31) and informed consent was received from all participants.
2.3. Sample preparation
Crude EDTA plasma was stored at -80°C. Prior to aliquoting, samples were transferred to -20°C overnight, thawed at 4°C and then vortexed and centrifuged at 3000 rpm for 2 minutes (Allegra X-12, Beckman Coulter). Using a liquid handling robot (EVO150, TECAN), samples were randomized across 96-well microtiter plates (Supplementary Information). Protein labelling was performed with biotin, as previously described by Drobin et al. [11] Briefly, EDTA plasma was diluted ~1:10 in Phosphate buffered saline (PBS) (09-9400-100, Medicago) and biotinylated with EZ-Link-NHS-PEG4-Biotin (21330, Thermo Scientific) dissolved in dimethyl sulfoxide (DMSO) (276855, Sigma-Aldrich). Following 2 h incubation at 4°C, the reaction was quenched with Tris-HCl 0.5 M, pH 8.0. Prior to analysis, a test was performed to confirm successful biotinylation: 24 randomly selected samples were diluted, incubated with the antibody array, and analyzed using the protocol detailed below (see Antibody suspension bead array assays). Further, a test measuring reactivity of human IgM to rabbit IgGs was performed as described in the Supplementary Information and Materials and Methods (Fig. S11). Labelled plasma was stored at -20°C until analysis.
2.4. Antibody suspension bead array assays
We used a total of 1,450 antibodies raised against 896 unique protein targets, including 1,285 antibodies from the Human Protein Atlas (HPA) project,[15] 72 mouse monoclonal antibodies (BioSystems International Kft) and 93 antibodies from other commercial vendor (Supplementary Data file S1). Each suspension bead array (SBA) was assembled by covalently coupling antibodies to magnetic and color-coded MagPlex beads (Luminex Corp.) and mixing these beads to create the arrays. The procedures for antibody coupling and bead mixing can be found in the Supplementary Materials and Methods, and together with the following protocol for plasma profiling described by Drobin et al. [11] Briefly, 5 µl of the beads from one SBA was aliquoted into each of the wells of 384-well microtiter plates. Biotinylated plasma was diluted 1:50 in PVXC buffer (0.1% casein, 0.5% (w/v) polyvinylalcohol, 0.8% (w/v) polyvinylpyrrolidone (P8136 and PVP360, Sigma-Aldrich), supplemented with 0.5 mg/ml purified rabbit IgG (P120-301, RRID: AB_479829, Bethyl laboratories) and then heated at 56°C for 30 minutes. Forty-five microliters plasma was transferred to the bead plate and incubated with the beads overnight, during which time the antibodies bind their corresponding antigen in the sample. Low-affinity complexes and unbound proteins were removed in consecutive washing steps with PBS-T 0.05% (1xPBS with 0.05% Tween20 (P9416, Sigma-Aldrich)) (EL406 washer, BioTek). Beads were incubated for 10 minutes with 0.4% paraformaldehyde (16% W/V, 43368.9M, Alfa Aesar), washed, and then incubated for 20 minutes with R-phycoerythrin-labelled streptavidin 1:750 (SA10044, Invitrogen). Lastly, the beads were washed and fluorescent signal from binding events were detected with a FlexMap 3D instrument (Luminex Corp.). Signal intensities reported as Median Fluorescent Intensity (MFI) were exported from the software xPONENT (Luminex Corp.) and at least 32 events per bead ID were used for data processing. As described in Table S3, Table S4, Table S5, and Supplementary Materials and Methods, a subset of antibodies was selected for statistical analyses. The antibody selection was based on performance in the SBA assay, GWAS, and orthogonal proteomics approaches including sandwich assays, mass spectrometry and proximity extension assays (Table S6, Table S7, Supplementary Data file S2, and Supplementary Materials and Methods).
2.5. Statistical analysis
Data analysis and visualizations were performed using the statistical software R version 3.6.0 [16] as described below, with details provided in the Supplement. All statistical and technical evaluations were performed using log-transformed MFI unless otherwise stated. To account for plate and batch effects, AbsPQN with Multi-MA normalization was applied by 96-well microtiter plate (Supplementary Statistical Analysis and Fig. S12). Associations between protein profiles and clinical traits were tested by linear mixed effects models using the R package lmerTest (v3.1-1)[17] (Supplementary Data file S3) and visualized with the circlize package (v0.4.8) [18]. The ranking of P-values from the association tests was confirmed in a sensitivity analysis by linear mixed effects models applied on the complete data set from 101 individuals. Spearman's rho statistic was used for estimating the correlation between variables, unless otherwise specified. Inter-class correlations were calculated with the R function ICC() from the psych package (v1.9.12.31) and out of six possible ICC forms we selected ICC(3, 1) levels as output. The arbitrary level of ICC ≥ 0.8 was selected for indicating stability, based on suggested guidelines on the interpretation of ICC values [19]. For the seasonal association analysis, a model for regular cyclic movements across time was fitted to each protein profile (Supplementary Statistical Analysis). UMAP analysis was performed on centered and scaled SBA data using the R package umap (v0.2.4.1). As UMAP has several hyperparameters that can influence the resulting embedding, we compared if results were conserved with Euclidian distance while varying the set seed, n_neighbors and min_dist parameters, as exemplified in Fig. 4 with n_neighbors = 10 and min_dist = 0.25. Protein modules were defined using the WGCNA (v1.66)[20,21] as described in further detail in the Supplementary Statistical Analysis. Protein profiles were standardized to z-scores and applying a linear model, three variables; intercept, slope, and sum of residuals (absolute value) were calculated over time for each individual and protein (Supplementary Statistical Analysis).
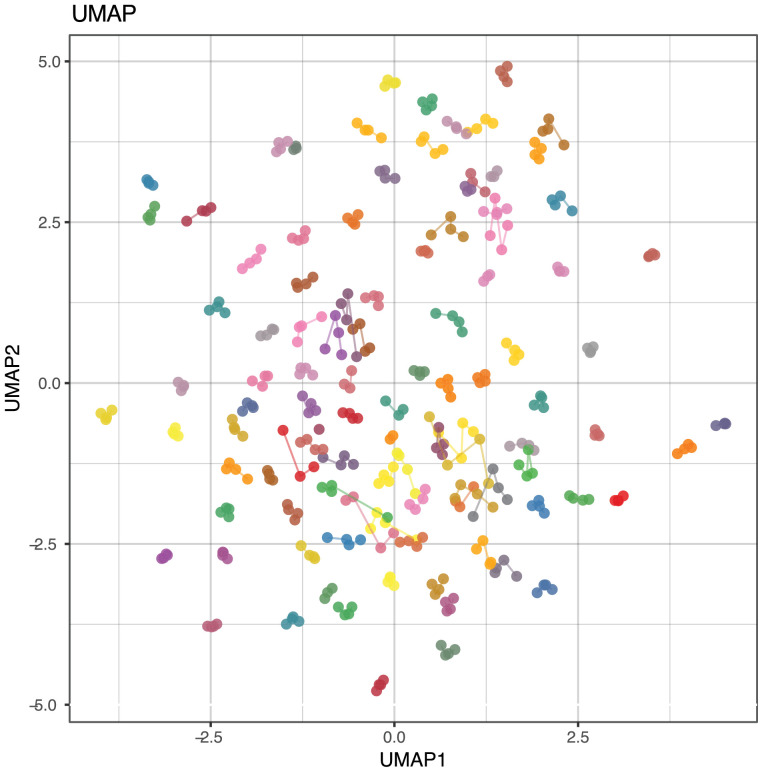
Fig. 4.
Diversity of individual-specific protein profiles. UMAP analysis of 734 protein features and samples from four visits, coloured by subject (N = 101). Coloured lines indicate which samples belong to the same individual. UMAP, Uniform Manifold Approximation and Projection.
3. Results
3.1. Study overview
We delineated the longitudinal characteristics of proteome profiles in a Wellness profiling cohort (denoted S3WP) of 101 individuals who donated plasma samples at four visits during one year (Fig. 1). With our antibody bead array data, we performed a series of data analyses on clinical, longitudinal, network and genetic aspects in order to capture the inter-individual diversity and longitudinal variability in the circulating proteomes. Further details about the demographic characteristics and experimental design can be found in Table S1, Table S2, and in the Supplementary Information.
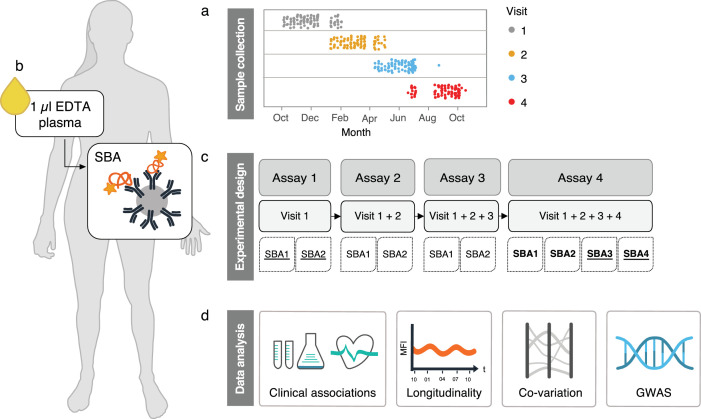
Fig. 1.
Experimental design and data analysis pipeline. (A) Over the course of one year, samples from 101 individuals were collected at four different visits to the clinic. (B) Plasma proteins were measured from 1 µl EDTA plasma with antibodies conjugated to beads. (C) Following each completed visit, samples were randomized within an assay and analysed together with all previously collected visits. In total, four SBAs were created and incubated with the samples as indicted in the flowchart. (D) Protein profiles were tested for associations to clinical traits, longitudinal stability, networks of co-regulation and GWAS.
The underlined labels correspond to assays where the complete set of samples were analysed in duplicate. Labels in bold correspond to assays where the SBA was incubated with 96 replicated samples for technical validation. SBA, suspension bead array; GWAS, genome wide association study
3.2. Annotation of antibody-derived protein profiles
First, we selected the most reliable antibodies from the initial set of 1,450 antibodies targeting nearly 900 unique proteins (Supplementary Data file S1, Fig. S1). As described in the Supplementary Information, we applied a combination of antibody validation criteria that resulted in the measurement of 734 proteins (Table S3, Table S4, and Table S5). This assessment included the use of genome wide association studies (GWASs) to identify single nucleotide polymorphisms (SNPs) in the protein-encoding regions of the target genes (Table 1 and Fig. S2). Further, sandwich immunoassays, proximity ligation assays and targeted proteomics assays (Supplementary Data file S2, Table S7) were used for antibody scoring (for details see Supplement). In summary, we annotated the 1,450 antibodies included in the assays and selected 734 unique protein features for further investigations.
Table 1.
Circulating proteins with cis-pQTLs.
| Protein | Antibody† | P‡ | Top SNP‡ | Variant* | TwinGene P§ |
|---|---|---|---|---|---|
| CLEC3B | HPA034794 | 5.31 × 10−38 | rs4683026 | Gly - Ser | n.a. |
| HRG | Bsi0137 | 3.65 × 10−25 | rs12493926 | Asn - Ile | < 1 × 10−300 |
| C1R | HPA001551 | 2.18 × 10−22 | rs1801046 | Leu - Ser | n.a. |
| GC | Bsi0185 | 8.13 × 10−20 | rs843005 | Asp - Glu | 1.17 × 10−95 |
| CFH | MAB4779 | 7.60 × 10−17 | rs61818923 | n.a. | |
| CFH | Bsi0885 | 1.81 × 10−16 | rs1048663 | Glu - Asp | 8.96 × 10−269 |
| AGT | HPA001557 | 3.26 × 10−16 | rs4762 | Thr - Met | n.a. |
| F9 | HPA000254 | 5.16 × 10−16 | rs422187 | Thr - Ala | n.a. |
| F12 | Bsi0849 | 1.25 × 10−14 | rs1801020 | 7.62 × 10−126 | |
| C4A | OASA01015 | 5.15 × 10−14 | rs386480 | n.a. | |
| LRG1 | Bsi3134 | 8.50 × 10−10 | rs10426311 | n.a. | |
| C6 | Bsi0731 | 3.88 × 10−9 | rs7443604 | Ala - Glu | 1.67 × 10−71 |
| AHSG | Bsi0907 | 4.58 × 10−9 | rs13073106 | Ser - Thr | 1.24 × 10−16 |
| FGL1 | HPA049320 | 6.83 × 10−9 | rs10093134 | n.a. | |
| HP | Bsi1809 | 1.18 × 10−8 | rs811053 | 4.86 × 10−88 |
The ID of the antibodies used in SBA assays.
Top associated SNP by ranking and the nominal P-value for the association (linear regression).
Non-synonymous SNPs in almost perfect linkage disequilibrium (LD) with the top SNP (R2 ≥ 0.8) in genomic data of Utah residents from north and West Europe (CEU) in the 1000 Genome project. The amino-acid variants were shown after the SNP ID. Additional details are provided in Table S6.
GWAS analysis conducted with the TwinGene cohort also revealed significant associations when matching SNPs with those identified in the S3WP study.
3.3. Clinical associations of circulating proteins
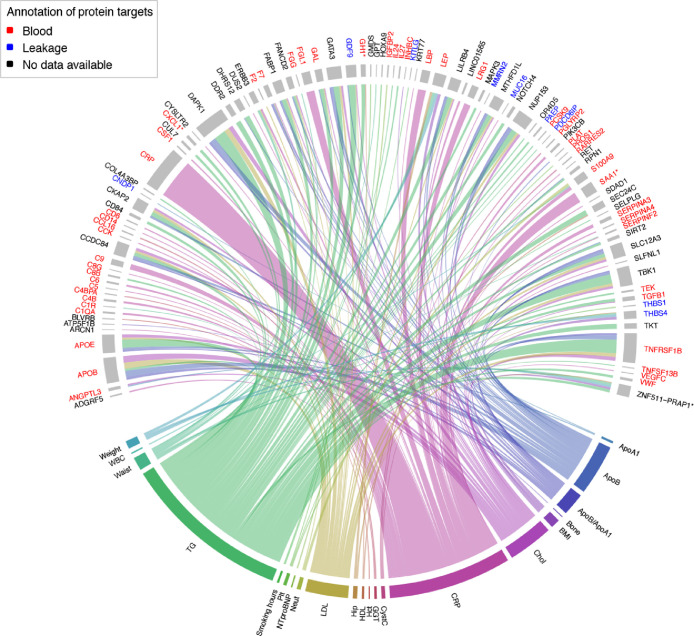
First, we referenced the 734 protein profiles to the clinical traits measured with standardized clinical tests, BMI and smoking habits. Applying linear-mixed effect models, we identified statistically significant protein-trait associations (FDR P ≤ 0•001). As shown in Fig. 2 and Fig. S3, these associations were enriched for traits like triglycerides (TG) (n = 42), CRP (n = 32), apolipoprotein B (ApoB) (n = 21), total cholesterol (Chol) (n = 19), low-density lipoprotein (LDL) (n = 28), and the ratio of ApoB/apolipoprotein A1 (ApoB/ApoA1) (n = 13). As expected, correlations were observed between clinical and proteomic data for CRP (r=0.93) and ApoB (r=0.71). In the linear-mixed effect models, this translated to strong associations for CRP (FDR P = 3•47 × 10−160) and ApoB (FDR P = 3•90 × 10−25). We discovered other noticeable associations for TNFRSF1B and DAPK1 with TG (FDR P = 3•01 × 10−55 and P = 5•20 × 10−48) (Supplementary Data file 3). Other top-ranked associations for the clinical traits (FDR P < 1 × 10−3) were for BLVRB to hematocrit (Hct); THBS1 to platelet count (Plt); S100A9 to the count of white blood cells (WBC) and neutrophils (Neut); SAA and FGL1 to CRP; LEP to BMI; ANGPTL3 to ApoA1; RARRES2 to cystatin C (CystC); CCL16 to of gamma-glutamyl transferase (GGT); and IGFBP2 to levels of N-terminal pro B-type natriuretic peptide (NTproBNP). This showed that a variety of expected associations were replicated in our proteomics approach, many related to secreted proteins, and that associations to the traits related to inflammation and lipid metabolism were most prominent.
Fig. 2.
Association map of proteomics and clinical traits. Chord diagram of associations
(FDR P < 0•001) between protein profiles and clinical traits obtained from linear mixed effect models. Line thickness is proportional to -log10(P-value) and coloured by clinical trait. Protein features that represent a family of several proteins are denoted with one gene name followed by “*”. Feature names are coloured red if predicted to be actively secreted into blood, or blue if they appear in blood due to cell leakage [9,40] (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
3.4. Assessment of longitudinal protein profiles
To capture longitudinal changes across the four consecutive visits, we investigated the reproducibility of protein measurements across repeated assays (Fig. 1). We performed inter-assay correlations of the protein levels (“technical variability”) and compared these to inter-visit correlations (“longitudinal variability”)(Fig. S4A). Intraclass correlations (ICC) were computed for both measures and allowed us to consider the technical variability when judging the longitudinal variability. Protein profiles with ICC ≥ 0.8 were defined as technically consistent and/or longitudinally stable. Out of all protein targets, 61% (447/734) revealed a high technical stability and 58% (428/734) were stable longitudinally. Reassuringly, the distribution of ICCs obtained from the proteomics approach was similar to the values obtained from the clinical tests (Fig. S4A). A total of 49% (359/734) of all proteins could be measured with a high precision when including both the inter-visit and inter-assay ICC ≥ 0.8. Both inter-assay ICCs and inter-visit ICCs of these proteins were not influenced by the mean levels of the obtained profiles (r= -0.19 and r = -0.03).
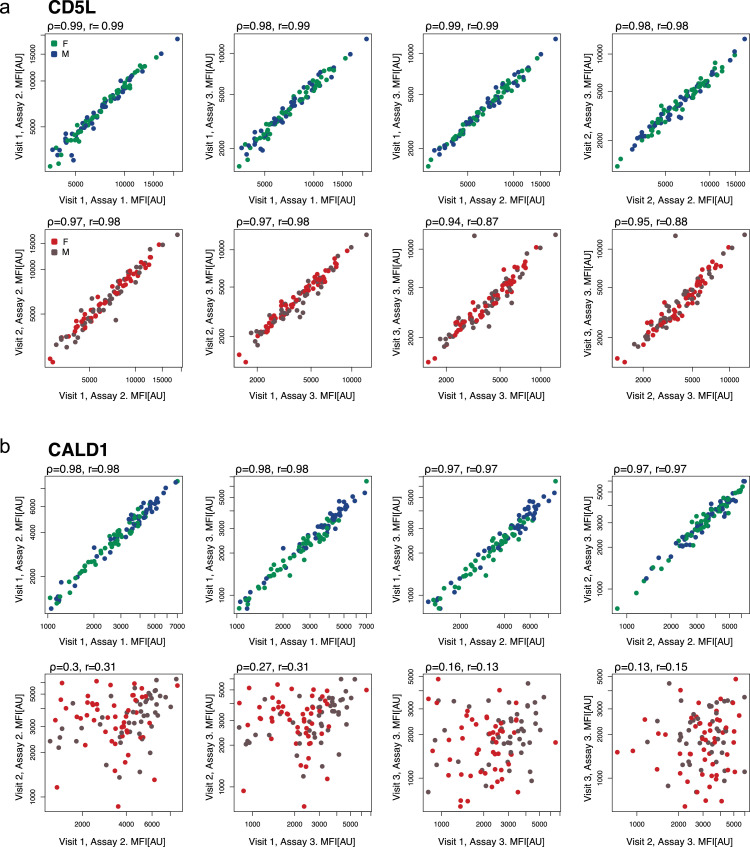
The most consistently measured and longitudinally least variable protein was CD5 antigen-like (CD5L, inter-visit ICC = 0.97) as exemplified by its technical and longitudinal profiles shown in Fig. 3A and Fig. S4B. On the other end, Caldesmon 1 (CALD1) was one of the proteins with high technical precision (inter-assay ICC = 0.88) but also a high variation between consecutively collected samples (inter-visit ICC = 0.32), as shown in Fig. 3B and Fig. S4C. This aligns with our previous findings of CALD1 being susceptible to conditions related to plasma preparation [22]. In summary, we found that ~50% of the proteins were measured with high precision and low longitudinal variability in blood plasma throughout one year. An additional analysis of seasonal effects on the plasma proteome only found levels of FLNA and BLVRB to fluctuate with season (FDR P < 0•01) (Fig. S5 and Supplementary Information)
Fig. 3.
Inter-assay and inter-visit variability. Shown are correlations of technical (inter-assay, upper panel) and longitudinal (inter-visit, lower panel) profiles. (A) CD5 antigen-like (CD5L) represents a both technically and longitudinally stable protein, while levels of (B) Caldesmon 1 (CALD1) vary between visit but not repeated assays. Each dot represents one individual, coloured by sex (F, female; M, male), MFI relates to median fluorescent intensity and AU are arbitrary units. Correlations are indicated by ρ (Spearman's Rho) and r (Pearson correlation coefficient).
3.5. Global analysis of protein profiles
Next, we investigated if the combination of protein profiles contributed to personal plasma proteome signatures. We used Uniform Manifold Approximation and Projection (UMAP)[23] to compress the data from all 101 samples and 734 proteins into two dimensions (Fig. 4). Each subject clustered predominantly with itself across all four visits. This implied that the plasma proteome signatures were diverse and composed of unique combinations of protein profiles for each individual participant. In Fig. S6, we provide an example of contrasting the technical and biological variability per individual, and experiments performed closest in time had the lowest technical variability. Computing the individual longitudinal variability per participants revealed ICCs = 0.99 ± 0.005 (mean ± SD). This highlights the existence of a stable and person-specific proteome signature, but also suggests that there is a considerable diversity in the circulating proteomes between clinically healthy subjects.
3.6. Longitudinal co-regulation of plasma proteins
In addition to investigating each protein individually, we explored if there were longitudinal networks of co-varying protein profiles. We used weighted gene co-expression network analysis (WGCNA) to define, annotate and analyse modules of co-regulated and interconnected protein profiles (for details see Supplementary Information).
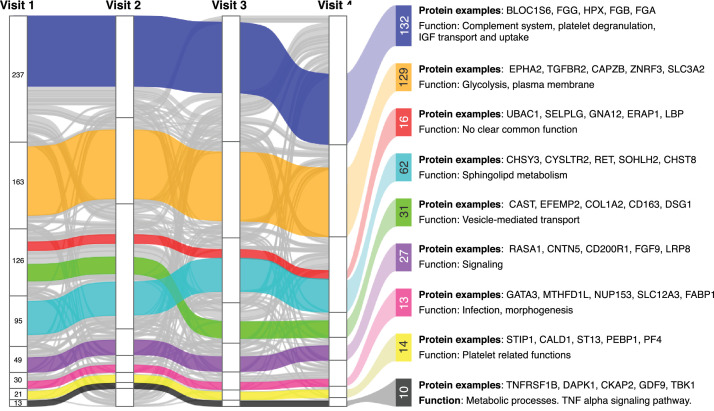
Computed for each visit, WGCNA resulted in eight mega modules (Fig. S7) and each mega module contained 11–242 proteins. We then tracked the mega module membership across all visits to create a map of the longitudinally patterns of conserved “core modules” (Fig. S8). From the eight mega modules per visit, we created core modules by matching all possible combinations of sequential overlaps between the mega modules across the visit (Fig. S9). A protein was ultimately assigned to one of the core modules if it was part of a particular pattern across all visits. Eight out of nine core modules contained at least one protein, and 59% (434/734) of the proteins could be assigned to one of these eight core modules (Fig. 5).
Fig. 5.
Networks of co-varying proteins. WGCNA was used to determine co-varying proteins per visit (stacked groups) and across visits (horizontal bands). Each vertical line represents one protein and its mega module membership in each visit. Proteins are coloured according to the core pattern they belong to. Proteins that do not belong to any core pattern are grey. Each core pattern is annotated to the right with the number of proteins it contains, a summary of associated pathways and GO terms, and examples of proteins following the given pattern. The examples of proteins given are the five proteins with the highest correlation to the core pattern eigengene.
The eight core modules were then annotated for their biological functions and their relation to clinical traits, as it is expected that proteins within the same core module could share biological functions and interactions, or can be controlled by common mechanisms (Fig. 5, Supplementary Data file S4). This revealed associated pathways and annotations related to biological functions like complement system, vesicle transportation, platelets and metabolic processes. Next, we explored links between groups of co-varying proteins with the available clinical traits. We identified a number of statistically significant associations (FDR P < 0•01, linear regression or analysis of variance) for the blue and black WGCNA core modules that correlated with lipid related traits, but in opposite directions. The blue pattern was negatively correlated with the levels of triglycerides and the fraction of ApoB and ApoA1, and conversely the black pattern was positively correlated with these traits as well as levels of ApoB and LDL. Additionally, the blue pattern was negatively correlated with CRP. Thus, these two different sets of co-regulated proteins likely have opposite functions within lipid metabolism by being linked to LDL and HDL respectively. This is consistent with the fact that the LDL associated protein ApoB follows the black core pattern and the HDL associated proteins ApoA1 and ApoA4 follow the blue pattern. None of the other core modules had significant associations to the available clinical traits, even though the effects were mostly consistent across visits. None of the core modules had significant associations to sex or age.
In summary, we found longitudinally conserved modules of protein co-expression networks with associations to biological functions and clinical traits.
3.7. Genetic effects on the measurement of the plasma proteome
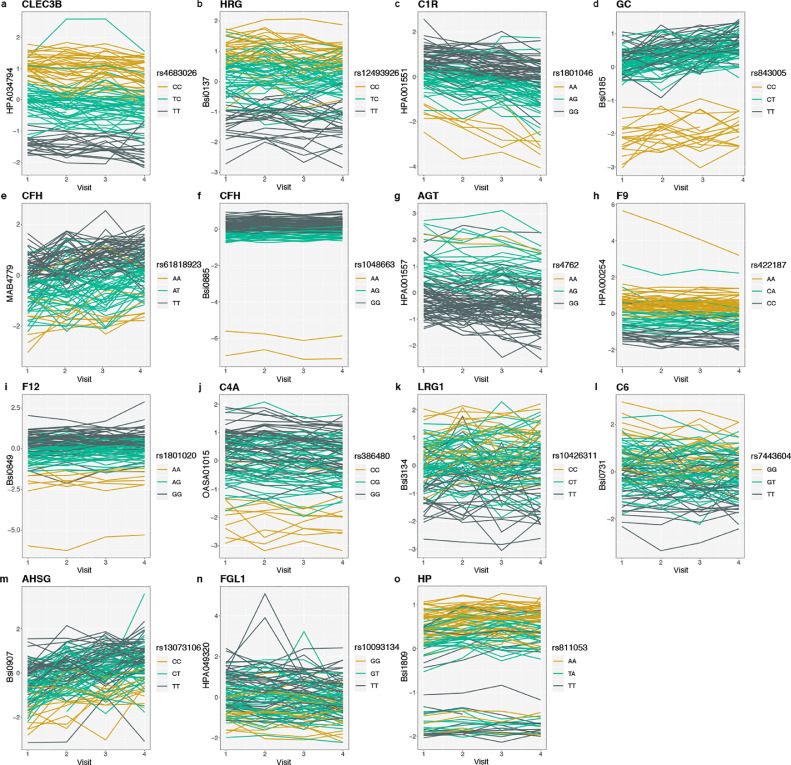
As introduced above, we used genetic data obtained by whole genome sequencing (details in Supplementary Materials and Methods). Using linear regression, we found 15 cis-protein quantitative trait loci (pQTLs) for 14 proteins’ profiles (P < 1•35 × 10−8, Bonferroni P < 0•05) from the association tests with non-redundant ~3.7M SNPs (see Table 1 and Fig. S2). All 14 unique proteins were annotated to be secreted into blood and primarily expressed by the liver [9] and the longitudinal profiles stratified by genotypes are shown in Fig. 6A-O. Following our previous observations [14] and recent insights connecting the circulating proteome with genetic variation,[24] we investigated if differences in detected proteins can be linked to protein polymorphisms (Supplementary Information). Even though non-synonymous SNPs are rare (< 0.3%),[25] we found these to be overrepresented among the identified cis-pQTLs. Indeed, out of the 15 identified pQTLs, nine of the loci (60%) contained variations that induce a change of amino acids in the protein sequence. Because these genetic associations were obtained in the relatively small S3WP cohort of 101 subjects, we corroborated the results by checking for the same associations between genetic variation and circulating proteins in an independent set of 3,000 individuals from the TwinGene study [13].
Fig. 6.
Longitudinal characteristics of plasma protein genotypes. The line plots show plasma proteins associated to genetic variants where z-scores were used to represent protein levels. Each line represented one individual and colour codes the genotypes. Only individuals with data from all four visits were included for visualization.
Among the most significant association between genetic variation and circulating proteins were Tetranectin (C-type lectin domain family 3 member B, CLEC3B) and the SNP rs4683026 (P = 5•31 × 10−38). One of the perfect linkage disequilibrium (LD) proxys (R2 = 1) of that SNP is rs13963, which can lead to two proteoforms with either a serine at the 106th position or a glycine. For CLEC3B, a protein secreted by the lung, muscle, spleen, and adipose tissue, the profile levels were highest for the CC genotype and decreased as the number of C alleles decreased (Fig. 6A). This indicated that the assay preferred the Gly106 isoform produced by the C allele over the Ser106 isoform produced by the T allele.
Similarly, proteoforms of liver secreted vitamin-D-binding protein (GC) could be linked to non-synonymous SNPs, hence reporting an isoform-specific affinity rather than differences in abundance [26]. We indeed found that specific alleles had major effects on the profiles of this circulating protein (Fig. 6D). We concluded that the profiles of GC detected by the assays were strongly determined by the genetic variants rs222047, rs843005 and rs7041. Reassuringly, rs7041 had previously been described as a cis-pQTL of GC when using an even larger study set and another type of quantitative immunoassay [27].
As shown in Fig. 6I, we also found a cis-pQTL SNP rs1801020 (P = 1•25 × 10−14) corresponding to the 5’ untranslated region of the coagulation factor XII (F12) gene,[28] and we replicated this association in the TwinGene cohort (P = 7•61 × 10−126). The common genetic variant rs1801020 modulates F12 liver expression,[29] and thus provides additional evidence that the detected SNP modulates gene expression, which in turn impacts the F12 protein abundance rather than the protein sequence. Out of all 101 subjects, we found that among seven participants with the F12 genotype, one individual had substantially lower secreted levels of F12. Subsequent tests of the participant in the clinic confirmed a delayed activated partial thromboplastin time (aPTT), which has also previously been described for this F12 polymorphism [29].
Besides GC and F12, we identified subgroups of individuals linked to differences in plasma protein levels for the secreted liver proteins haptoglobin (HP) and complement factor H (CFH). Lower levels of HP were determined in 22% (21/93) of the plasma samples from the S3WP study participants and in 15% (447/2,974) of the sera from TwinGene study (Fig. S10A). For one of the anti-CFH antibodies (Bsi0885), the detected protein levels were lower in plasma of 2% (2/93) of the S3WP individuals, and equally in 2% (62/2974) of the TwinGene participants (Fig. S10B). Compared to the second anti-CFH antibody (MAB4779), which was not included when analysing the TwinGene samples, the main SNP for Bsi0885 did induce a missense mutation affecting the protein's sequence. This effect could explain the differences in binding properties of the antibodies towards the variants of CFH. No distinct population subgroups with either lower GC or F12 protein levels were detected in the serum samples of the TwinGene study (Fig. S10C-D). As further described in the Supplement, we also compared pQTLs with eQTLs and other RNA expression data to pinpoint the source of expression regulation of proteins with cis-pQTLs (Table S6). There were no significant associations between the genetic and clinical data.
In summary, distinct differences in plasma protein levels can be explained by genetic variants. These insights are valuable when comparing the protein levels between individuals as they can provide another motivation for why a more precise and personalized assessment of health in circulation requires both longitudinal monitoring and the influence of genetics.
3.8. Facets of individual and longitudinal protein profiles
UMAP analysis revealed that overall, person-specific profiles remained stable over time. To identify inter-individual differences on a protein level, we z-scored the data and determined the inter-quartile range (IQR) as a measure of diversity between individuals. Only minor differences between the participants of the study where seen for proteins such as DSC3, GFAP, and GDF15 (IQRs ≤ 0.15), as compared to more prominent inter-individual diversity in levels for the liver proteins LEPR, IGFBP2, FCN2 or SERPINA1 (IQRs ≥ 1.5).
To further illustrate changes occurring in the plasma proteomes, we queried the data for representative examples among the participant's protein profiles (Fig. 7). We asked which protein might vary due to distinct events, remain different during the study, or gradually change over time in any of the individuals. We again used protein z-scores with all participants serving as a reference population. To this end, we selected the 359 most stable protein profiles (ICC ≥ 0.8) in order to focus on capturing individual rather than common patterns. We applied an annotation scheme that was based on the parameters we obtained from fitting linear models to every protein profile (Supplementary Statistical Analysis). We scored each of the protein profiles for every individual based on three criteria:
-
(i)
baseline = individual deviation of protein levels from the population,
-
(ii)
trend = a person's changes in (increasing or decreasing) protein levels,
-
(iii)
fluctuation = fluctuation of protein levels as deviation from linear changes.
In total, 33,028 profiles were derived from 359 proteins and 92 individuals. We classified each profile to each criterion as deviating if the obtained values were >±3xSD of the population average. As shown in Fig 7, 3,7% (1,223/33,028) of all possible participant-protein measurements revealed a variation at the individual level on one or several of the categories. This frequency is ten times higher than observing these variations by chance (0,27%). We then summarized these scores and evaluated the outcome per protein (including all individuals) and per participants (including all profiles), as reported in Supplementary Data file S5.
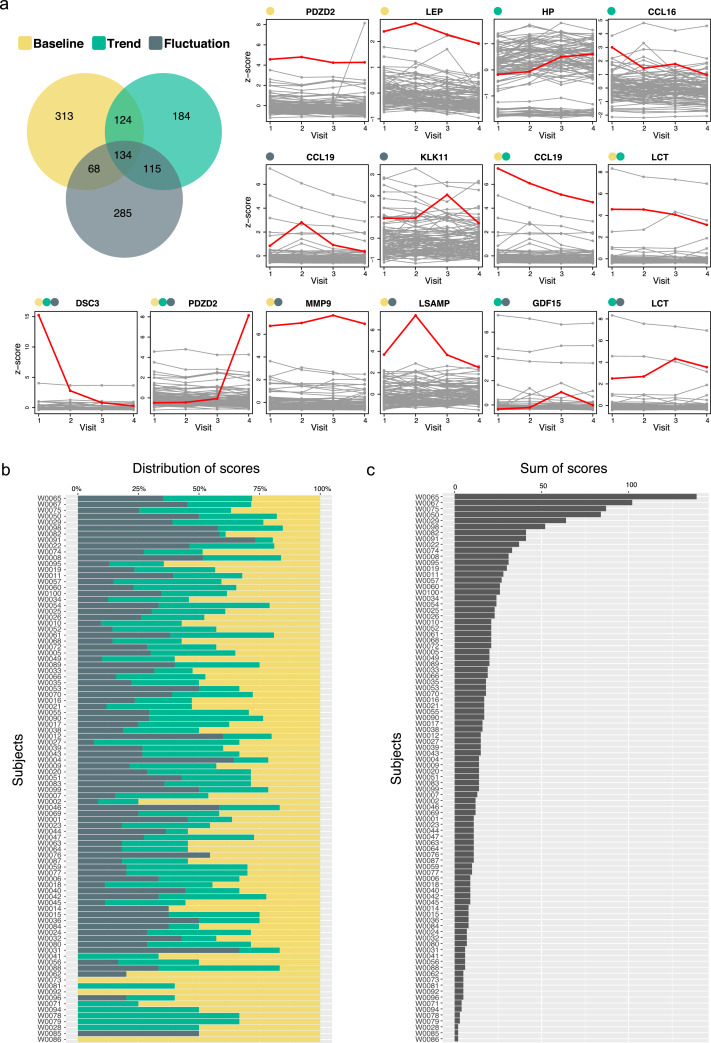
Fig. 7.
Facets of longitudinal protein variability. Protein profiles were stratified by their longitudinal profiles. (A) Venn Diagram indicates the number of observations (protein per individual) that was deviating (± 3xSD) from the population mean in terms of protein baseline, trend and fluctuations. Here, we selected 14 protein profile examples. Each grey line represents one individual. One selected individual with a particular protein profile is highlighted in red, and the category of the red profile is marked on the left side of the protein name. (B) Distribution of the three annotation criteria per subject, and (C) the sum of the three annotation criteria per individual (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Concerning the proteins, levels of CAPZB, RPSA, FGFR1, BPGM, and PECAM1 were longitudinally preserved and least unique to any participant as these were neither elevated, changed nor fluctuated over time. Interestingly, none of these proteins were annotated to be secreted into bloodstream, hence likely represent leakage products. In contrast, we found that secreted blood proteins GDF15 and MMP9, as well as LCT, were variable proteins along the longitudinal axis when considering the total number of individuals with changes to any of the three annotation criteria. As exemplified by GDF15, aspects of inter-individual diversity and longitudinal variability can present as independent characteristics of circulating proteins.
Among the protein profiles of each participant, there was at least one protein with elevated baseline or trends of fluctuating levels (Fig. 7B and C). The most variable profiles were found for participant W0065, as there were 39 proteins being different in term of baseline levels, 51 increased or decreased, and 49 proteins fluctuated over time. In contrast, participant W0086 was ranked as most stable (accumulated score = 2, for calculation see Supplementary Statistical Analysis) and the two deviating profiles corresponded to elevated baseline for the two proteins PDZD2 and KLK11.
This analysis illustrated that there is diversity in terms of how longitudinally stable or variable an individual's plasma proteome can be. It is likely that many of the unique and stable trajectories that deviate from the average population baseline might be due to genetic effects, lifestyle factors or medication, however our study was underpowered to extract other associations of weaker effect sizes.
4. Discussion
We profiled 101 individuals using a multiplexed affinity proteomic assay and found that the plasma proteome signatures were highly individual-specific. To address concerns about antibody validation, our exploratory multiplexed approach applied a scoring scheme to identify binders with consistent performance across assays and longitudinally collected samples. We highlighted findings related to individual protein variability, found interesting links to genetic components and networks of co-regulatory proteins, and lastly demonstrated the potential benefits of individual-level, longitudinal protein profiling. Our observations can have substantial impact on studies searching for common disease proteins across a population, because both the inter-individual diversity and longitudinal variability can have influence on the composition of the circulating proteome.
Affinity binders are important tools frequently used in research and as diagnostic reagents. The current concern surrounding the reproducibility of data derived from such research has raised awareness and increased the efforts in developing strategies for antibody validation [30]. The utility of antibodies is context dependent and the performance may vary depending on technological method or sample composition. Therefore, it is necessary to annotate and validate the specificity of each binder in its intended application, preferably by using orthogonal methods as applied here. The selected antibodies have passed several validation steps in the generation pipeline,[31] but validation needs to be tailored for plasma analysis [32,33]. The multiplexed assay applied here was an exploratory effort allowing the analysis of large numbers of samples and analytes. The method relied on a single binding event between an antibody and its target protein, hence, the inherent risk of the method is off-target binding, non-specific binding, or capturing of protein complexes [34]. Notably, several proteins measured with our assays were identified with a cis-pQTL, providing inferred evidence for on-target binding. By combining information for each antibody regarding technical reproducibility and supportive data including other antibody-free assays,[35] we developed a transparent annotation strategy to limit our analysis from the initial 1450 antibodies to a set of ~700 high confidence protein profiles of which almost 50% were very stable over time. Indeed, many proteins in the latter selection can be expected to be detectable in the circulation because these were either secreted from solid tissues into blood or leaked from blood cells.
Considering the intra-individual diversity, we found that ~50% of the studied protein profiles were stable across the four sampling occasions over one calendar year. We acknowledge though that some proteins can appear to be more dynamic depending on the studied timespan, or if any other perturbation, disease or intervention occur among the participants. For the majority of the proteins, variability was low for both the technical replicates and between time points. Hence, the fluctuations observed between two time points could also likely be due to technical variability rather than lifestyle related perturbations. Nonetheless, changes that occurred on a continuous scale for a subset of the study group could serve as supportive evidence for physiological rather than technical changes. Clearly, our observations are restricted to the proteins targeted by our assays and may be affected by limitations in terms of the sensitivity to the effects from protein interactions as well as co-enrichment of other proteins [34]. Nonetheless, we observed a high consistency when profiling circulating proteins in the longitudinal sample collection, which encompasses the process of drawing blood, processing the collections and analysing different samples from the same subject. Hence, a longitudinal assessment reflected a combination of different variables such as sampling, biobanking, assays and physiological changes. However, we found and focused on protein profiles with low longitudinal variability. This indicated that the applied concept, sampling schemes and method had the precision to define personal baseline values and capture individual changes.
We applied multivariate analysis to cluster 734 protein features from four time points and found individual-specific profiles that were retained throughout one year. This observation was further supported by the fact that the majority of proteomic and clinical profiles showed ICC > 0.9 between the visits for each individual. Comparing the technical and biological variation within each patient, we observed a supportive consistency between repeated assays and visits. However, and subject to further investigations, we found that reprocessing of the samples, time between experiments, and sample freezing/thawing cycles were factors that can affect the consistency of some of the protein profiles.
It is worth noting that our study included a small number of subjects (n=101) followed during a relativity short time span (one year). Further, the participants were deemed clinically healthy with a balanced (and possibly more deliberately healthy) lifestyle during this period. Both the intra-individual diversity and the longitudinal variability are important observations because these can lay the foundation for a next-generation of studies aiming to more accurately assess diseased individuals or those in treatment. It will, however, require even larger study populations with defined interventions such as common disease incidence, drug treatment, or surgery, to determine which subset of proteins are informative for a particular disease phenotype.
We also applied a network approach to study any coordinated change of several proteins over time. Although proteins within the same WGCNA-defined module were covarying, we did not collect evidence about their physical interactions. These modules rather suggest that there is an interconnection that can coordinate protein expression. Hence, and as previously observed by others on single time point measurements,[36] there are possible processes that co-regulate protein levels via common mechanisms. Indeed, most of the identified core patterns were significantly enriched by proteins related to a particular biological function such as lipid metabolism (LDL, HDL and triglycerides). An added value of longitudinal profiling was further illustrated by WGCNA because not all proteins that co-varied within a single time point also continued to do so across all visits. Our findings suggested that coordination of other disease related networks and processes exists, but these may require a more dedicated study design and include pre-selected proteins. Nonetheless, we demonstrated in a study of clinically healthy individuals that processes related to metabolism, coagulation and inflammation were among the major coordinated functions of the plasma proteome and that these should be considered in any assessment of human health states.
Profiling the plasma proteomes identified groups of participants that presented with distinct differences in circulating protein levels. These subgroups could be linked to cis-pQTLs such as for the proteins GC, F12, CFH and HP. Investigating the identified SNPs, we found that the variation at the loci for GC and CFH coded for a missense mutation. This implied that the assay measured the relative abundance of specific proteoforms rather than detecting different concentration levels. We explained this by changes inducted to the sequence, structure or even post-translational modifications that will make the antibodies bind to each of the proteoforms with a different affinity. Reassuringly, we observed concordant associations even in serum samples of the TwinGene cohort, which we used as a validation set and that consisted mainly of elderly individuals.
One striking observation from a precision profiling perspective was to find a single participant with deficiency in F12 among all 101 individuals, and being able to use proteomic and genetic information to pinpoint a possible mechanism of lower levels of circulating F12. A deficiency in F12 is rare and generally non-symptomatic, however, in vitro F12 deficiency results in prolonged activated partial thromboplastin time (aPTT) [37]. Indeed, aPPT is a common screening test for hemostatic function. An underlying unknown F12 deficiency can have clinical consequences for the patient through inhibited or delayed invasive procedures or surgery.Furthermore, common variants of F12, not resulting in deficiency, have been correlated with aPTT, presumably through modulating F12 levels [38]. A patient would therefore benefit from knowing about such a deficiency prior to surgery, in order to avoid extensive diagnostic workup aiming to exclude clinically relevant hemostatic disorders. The case of F12 illustrates how our proteomics data from continuous monitoring of a particular parameter can be combined with genetic data to generate information with direct clinical utility.
Collecting the pQTLs also allowed us to annotate whether differences in protein profiles were due to missense variants in protein coding regions or rather affecting gene expression. We used eQTL data accessible on the GTEx portal,[39] accepting that the data is derived from tissues and cells from other individuals than the ones included in this study. Ideally, transcriptomic data from the same individuals should be incorporated in future analysis. Nonetheless, we found eQTLs in the liver (LRG1; F12), the artery (C6), pancreas (FGL1) or thyroid (C4A). Similarly, the relation of the pQTLs and splicing QTLs (sQTLs) were studied to annotate circulating proteins levels in relation to alternative splicing. We found sQTLs in the liver (AHSG; CFH), adipose tissue (CLEB3B), spleen (CFH), and thyroid (C4A). Connecting information from the pQTLs provides a useful approach to further annotate the levels of circulating proteins, even in 101 individuals. Nonetheless, the ~10 proteins we have discussed point at the value gained when connecting proteomic with genetic data, such as for defining patient-specific cut-offs for disease classifications. This awareness will further assist our understanding of assay specific data in the context of precision phenotyping.
Lastly, we investigated the longitudinal variability of each protein across time at the level of each individual. We dissected this into three distinct categories of longitudinal variation that occurred among the participants. First, we identified individuals with increased or decreased baseline abundance of proteins that are consistent throughout one year. It can be hypothesized that baseline levels above or below a relative population mean might be due to genotype, and that other factors such as medication can further influence these. Next, we found proteins with gradually increasing or decreasing abundance across time. Monitoring the progressive changes across the consecutive sampling can highlight which proteins play a role in pre-symptomatic manifestation of a condition, or they reveal how effective a treatment of chronic conditions has been. Lastly, we found proteins that increased or decreased during shorter terms as these were captured only during specific visits. It remained a challenge to link many of these perturbations to reported changes in health, lifestyle or behaviour of an individual. Here a more detailed integration of the data on an individual level as well as decomposing the aspects related to sampling, shipment and analysis might be required. However, this demonstrated the importance to follow plasma proteomes over time in order to assess where on the spectrum of inter-individual diversity and longitudinal variability a specific individual resides. The distinction between time-resolved events and consistently changing or deviating baselines will consequently be important aspects to consider when implementing blood-based protein measurement to assess health in a clinic setting. It is by multiple layers of interconnected data, longitudinal sampling and individual-specific assessment over time that we can start predicting protein trajectories in time. Hence, utilizing such collected information will enable to distinguish between lifestyle related and short-lasting events (e.g. stress) over physiological processes that point at the onset, progression or manifestation of a disease or condition.
In conclusion, we profiled longitudinal plasma samples from 101 subjects using exploratory affinity assays and found that proteome profiles of clinically healthy individuals were diverse and highly individual-specific. While there were proteins varying over time in some individuals, many of the circulating proteins as well as their co-regulated networks were predominantly stable in this study population. Our work highlights the facets of individual-specific proteomes and the need to consider both inter-individual diversity and longitudinal variability when assessing health or disease states.
5. Data and material availability
The S3WP datasets used for this report have been deposited with the Swedish National Data Service (www.snd.gu.se, a data repository certified by Core Trust Seal). The dataset can be made available for validation purposes by contacting snd@snd.gu.se. Data access will be evaluated according to Swedish legislation. Data access for research related questions in the S3WP program can be made available by contacting the corresponding author. Researchers interested in using Swedish Twin Registry data must obtain approval from a Swedish Ethical Review Board and from the Steering Committee of the Swedish Twin Registry. Researchers using the data are required to follow the terms of an agreement containing a number of clauses designed to ensure protection of privacy and compliance with relevant laws. For further information, contact Patrik Magnusson (Patrik.Magnusson@ki.se).
Funding sources
This work was primarily supported by the Erling Persson Foundation for the KTH Centre for Precision Medicine (MU), and the Swedish Heart and Lung Foundation (GB). We also acknowledge the Knut and Alice Wallenberg Foundation for funding the Human Protein Atlas project (MU), and Science for Life Laboratory for Plasma Profiling Facility (JS). The Swedish Twin Registry is managed by Karolinska Institutet and receives funding through the Swedish Research Council under the grant no 2017-00641. The funders had no role in writing of the manuscript nor in the decision to publish. The authors have not been paid to write this article by any agency.
Declaration of Competing Interest
The other authors declare no competing interests.
Author contributions
TDC, RSH, AB, MD, FE, BF performed experiments. TDC, MGH, CET, FE and JMS performed data analysis. PKEM provided TwinGene samples and supervised related analyses. LF and ISK managed the S3WP project samples and data. JO provided clinical expertise. AG and GB coordinated the collection of S3WP samples and clinical data. GB and MU provided funding and supervised the S3WP study. TDC and JMS conceived the experiments and wrote the manuscript with input from all co-authors. All co-authors approved the final version of the manuscript.
Acknowledgments
We like to thank all participants of the S3WP study and the staff nurses collecting the samples. We acknowledge The Swedish Twin Registry for access to samples and data. We also thank every current or former member of the Affinity Proteomics Division at SciLifeLab, especially Elin Birgersson, Philippa Pettingill, Sofia Bergström, and Cecilia Mattson for technical support. We thank Helian Vunk for targeted proteomics data and Valtteri Wirta's team for their sequencing work. We also thank the entire staff of the Human Protein Atlas for their tremendous efforts.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102854.
Appendix. Supplementary materials
References
- 1.Piening BD, Zhou W, Contrepois K. Integrative personal omics profiles during periods of weight gain and loss. Cell Syst. 2018;6(2):157–170. doi: 10.1016/j.cels.2017.12.013. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price ND, Magis AT, Earls JC. A wellness study of 108 individuals using personal, dense, dynamic data clouds. Nat Biotechnol. 2017;35(8):747–756. doi: 10.1038/nbt.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams SA, Kivimaki M, Langenberg C. Plasma protein patterns as comprehensive indicators of health. Nat Med. 2019;25(12):1851–1857. doi: 10.1038/s41591-019-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu JX, Thomas CE, Brunak S. Network biology concepts in complex disease comorbidities. Nat Rev Genet. 2016;17(10):615–629. doi: 10.1038/nrg.2016.87. [DOI] [PubMed] [Google Scholar]
- 5.Spitzer MH, Nolan GP. Mass cytometry: single cells, many features. Cell. 2016;165(4):780–791. doi: 10.1016/j.cell.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ignjatovic V, Geyer PE, Palaniappan KK. Mass spectrometry-based plasma proteomics: considerations from sample collection to achieving translational data. J Proteome Res. 2019 doi: 10.1021/acs.jproteome.9b00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JG, Gerszten RE. Emerging affinity-based proteomic technologies for large-scale plasma profiling in cardiovascular disease. Circulation. 2017;135(17):1651–1664. doi: 10.1161/CIRCULATIONAHA.116.025446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwenk JM, Omenn GS, Sun Z. The human plasma proteome draft of 2017: building on the human plasma peptideatlas from mass spectrometry and complementary assays. J Proteome Res. 2017;16(12):4299–4310. doi: 10.1021/acs.jproteome.7b00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhlen M, Karlsson MJ, Hober A. The human secretome. Sci Signal. 2019;12(609) doi: 10.1126/scisignal.aaz0274. [DOI] [PubMed] [Google Scholar]
- 10.Geyer PE, Voytik E, Treit PV, et al. Plasma Proteome Profiling to detect and avoid sample-related biases in biomarker studies. EMBO Mol Med2019: e10427. [DOI] [PMC free article] [PubMed]
- 11.Drobin K, Nilsson P, Schwenk JM. Highly multiplexed antibody suspension bead arrays for plasma protein profiling. Methods Mol Biol. 2013;1023:137–145. doi: 10.1007/978-1-4614-7209-4_8. [DOI] [PubMed] [Google Scholar]
- 12.Bergstrom G, Berglund G, Blomberg A. The Swedish CArdioPulmonary BioImage Study: objectives and design. J Intern Med. 2015;278(6):645–659. doi: 10.1111/joim.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnusson PK, Almqvist C, Rahman I. The Swedish twin registry: establishment of a biobank and other recent developments. Twin Res Hum Genet. 2013;16(1):317–329. doi: 10.1017/thg.2012.104. [DOI] [PubMed] [Google Scholar]
- 14.Hong M-G, Dodig-Crnković T, Chen X. Levels of histidine-rich glycoprotein variants in human blood are associated to chronological age and predict mortality. bioRxiv. 2019 [Google Scholar]
- 15.Uhlen M, Fagerberg L, Hallstrom BM. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220) doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 16.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Gr Stat. 1996;5(3):299–314. [Google Scholar]
- 17.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82(13):1–26. [Google Scholar]
- 18.Gu ZG, Gu L, Eils R, Schlesner M, Brors B. circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30(19):2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 19.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langfelder P, Zhang B, Horvath S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics. 2008;24(5):719–720. doi: 10.1093/bioinformatics/btm563. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mo B. 2005;4 doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- 22.Qundos U, Hong MG, Tybring G. Profiling post-centrifugation delay of serum and plasma with antibody bead arrays. J Proteomics. 2013;95:46–54. doi: 10.1016/j.jprot.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 23.McInnes L, Healy J, Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. 2018.
- 24.Sun BB, Maranville JC, Peters JE. Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73–79. doi: 10.1038/s41586-018-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JI, Ju YS, Park H. A highly annotated whole-genome sequence of a Korean individual. Nature. 2009;460(7258):1011–1U96. doi: 10.1038/nature08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson CM, Lutsey PL, Misialek JR. Measurement by a Novel LC-MS/MS methodology reveals similar serum concentrations of vitamin d-binding protein in blacks and whites. Clin Chem. 2016;62(1):179–187. doi: 10.1373/clinchem.2015.244541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun W, Kechris K, Jacobson S. Common genetic polymorphisms influence blood biomarker measurements in COPD. PLoS Genet. 2016;12(8) doi: 10.1371/journal.pgen.1006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanaji T, Okamura T, Osaki K. A common genetic polymorphism (46 C to T substitution) in the 5′-untranslated region of the coagulation factor XII gene is associated with low translation efficiency and decrease in plasma factor XII level. Blood. 1998;91(6):2010–2014. [PubMed] [Google Scholar]
- 29.Corral J, Antón AI, Quiroga T. Influence of the F12 -4 C>T polymorphism on hemostatic tests. Blood Coagul Fibrinol. 2010;21(7):632–639. doi: 10.1097/MBC.0b013e32833a9048. [DOI] [PubMed] [Google Scholar]
- 30.Uhlen M, Bandrowski A, Carr S. A proposal for validation of antibodies. Nat Methods. 2016;13(10) doi: 10.1038/nmeth.3995. 823-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson P, Paavilainen L, Larsson K. Towards a human proteome atlas: high-throughput generation of mono-specific antibodies for tissue profiling. Proteomics. 2005;5(17):4327–4337. doi: 10.1002/pmic.200500072. [DOI] [PubMed] [Google Scholar]
- 32.Neiman M, Fredolini C, Johansson H. Selectivity analysis of single binder assays used in plasma protein profiling. Proteomics. 2013;13(23-24):3406. doi: 10.1002/pmic.201300030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haeussler RS, Bendes A, Iglesias M. Systematic Development Of Sandwich Immunoassays For The Plasma Secretome. bioRxiv. 2019 doi: 10.1002/pmic.201900008. [DOI] [PubMed] [Google Scholar]
- 34.Fredolini C, Bystrom S, Sanchez-Rivera L. Systematic assessment of antibody selectivity in plasma based on a resource of enrichment profiles. Sci Rep. 2019;9(1):8324. doi: 10.1038/s41598-019-43552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edfors F, Forsstrom B, Vunk H. Screening a Resource of Recombinant Protein Fragments for Targeted Proteomics. J Proteome Res. 2019;18(7):2706–2718. doi: 10.1021/acs.jproteome.8b00924. [DOI] [PubMed] [Google Scholar]
- 36.Emilsson V, Ilkov M, Lamb JR. Co-regulatory networks of human serum proteins link genetics to disease. Science. 2018;361(6404):769–773. doi: 10.1126/science.aaq1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachler M, Niederwanger C, Hell T. Influence of factor XII deficiency on activated partial thromboplastin time (aPTT) in critically ill patients. J Thromb Thrombol. 2019;48(3):466–474. doi: 10.1007/s11239-019-01879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houlihan LM, Davies G, Tenesa A. Common variants of large effect in F12, KNG1, and HRG are associated with activated partial thromboplastin time. Am J Hum Genet. 2010;86(4):626–631. doi: 10.1016/j.ajhg.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45(6):580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhlen M, Karlsson MJ, Zhong W. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science. 2019;366(6472) doi: 10.1126/science.aax9198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.