Abstract
Background
Leprosy, cutaneous leishmaniasis (CL) and Chagas disease (CD) are neglected tropical diseases with a high psychosocial burden (PSB). These conditions are endemic in Norte de Santander and Arauca in Colombia, but data on the related PSB are scarce. Therefore, we assessed mental distress, participation restriction and stigma among CD, CL and leprosy patients.
Methods
In 2018, 305 leprosy, CD or CL patients were interviewed using a self-report questionnaire to assess mental distress, participation scale for participation restriction and explanatory model interview catalogue (EMIC) for stigma. Descriptive statistics and the significance of median score differences were compared.
Results
Fifty percent of CD patients and 49% of leprosy patients exhibited mental distress, percentages which were significantly higher than that of CL (26%). Twenty-seven percent of leprosy patients experienced participation restriction, which was lower for CL (6%) and CD (12%). Median EMIC scores were significantly higher for leprosy patients than for CD (27%) and CL (17%) patients.
Conclusions
We found high levels of PSB among leprosy, CD and CL patients. Mental distress was highest among CD patients. Participation restriction and stigma were more prevalent in leprosy patients. Rural residence or lower educational status may impact PSB. Further investigation is needed to formulate evidence-based, holistic interventions.
Keywords: Chagas disease, cutaneous leishmaniasis, leprosy, mental health, social participation, stigmatization
Introduction
Neglected tropical diseases (NTDs) are estimated to affect over 1 billion people. They exist primarily in countries with (sub)tropical climates, disproportionally affecting people living in poverty, with poor sanitation and restricted healthcare access. Norte de Santander and Arauca are regions of Colombia that exhibit an extremely high endemicity of three NTDs: Chagas disease (CD), cutaneous leishmaniasis (CL) and leprosy.1–3 Colombia achieved a prevalence of leprosy below 1/10 000 over a decade ago.4 However, the slow decline and the relatively high percentage of cases with disabilities upon diagnosis are indicative of a significant remaining disease burden.3 Between 1990 and 2016, 249 745 cases of leishmaniasis were registered in the endemic areas in Colombia, with a median incidence rate of 81/100 000 inhabitants.5 Between 2008 and 2016, 7172 cases of CD were reported in Colombia, with a median incidence rate of 11/100 000 inhabitants.5 High detection rates for leprosy are found in Arauca (4.73/10 000) and Norte de Santander (3.86/10 000).4 The prevalence of CD in both departments ranges from 0.1 to >15% at subdistrict level.6 The incidence of CL in Arauca is relatively low, 0–0.31/100.000, but is much higher in Norte de Santander at 2.33–18.13/100.000.3
NTDs cause physical impairments, which may lead to irreversible impairments where there is a lack of access to healthcare and delays in detection.7 These impairments often result in social disadvantage and potentially prevent affected persons from participating in key aspects of life. This further increases the disease burden.8 The full burden of NTDs is therefore not merely physical, but the result of multiple morbidogenic factors, such as stigma, social exclusion and mental distress. These factors are associated with a lower quality of life and can contribute to aggravation of the disease itself, sustaining a highly complex chronic morbidity.9
Both leprosy and CL may lead to permanent skin lesions, disfigurement and physical disability, and are strongly associated with social stigma, decreased self-esteem, decreased social participation and mental distress.10–12 While CD does not lead to visible impairment, it is also highly stigmatized. Because cardiac complications occur in a subfraction of patients in the chronic stage of the disease, CD is widely believed to reduce one's productivity and well-being. This causes a societal perception of affected people as being weak and worthless.11 Obligatory serological testing is often demanded by employers, which results in exclusion from the labor market, causing mental distress and lower social participation.1
The total disability adjusted life years associated with NTDs equal those of malaria or HIV/AIDS, and there is considerable evidence that their impact is significantly underestimated because disease consequences, such as stigma and mental distress, are routinely not taken into account.13,14 Until now, no data have been available on the levels of mental distress, participation restriction and stigma among these patients in Colombia. This information is urgently needed to better understand the true disease burden and to support advocacy for scaling up funding and implementation of available disease management, disability prevention and inclusion interventions.13
Materials and methods
Study design
This pilot study used a descriptive approach to determine levels of stigma, mental distress and participation restriction among people affected by CD, CL or leprosy in two regions of Colombia.
Study area
The study was conducted in Norte de Santander and Arauca between April and June 2018, where CD, CL and leprosy are endemic.1–3,15 Life expectancy at birth in both areas is 70 y. Sixty-four percent of the population in Arauca and 77% in Norte de Santander live in urban areas.
Study population
Adults with a diagnosis of leprosy, CL or CD that was reported to the national NTD program and who were living in Norte de Santander or Arauca were eligible for this study. The sample was based on the estimated total number of affected people living in these endemic areas. Accurate estimations for leprosy and CL were possible, but the number of persons seropositive for CD was unknown. Sample size calculations required a minimum of 92 participants per disease. People who agreed to participate in the study were confidentially interviewed individually face-to-face.
All patient information was derived from the national NTD program registration system. Clinical information such as treatment status, disease progression and disability grading were not registered in this system and no access to additional medical files was granted. Each interview contained four questionnaires: a sociodemographic questionnaire, the self-reporting questionnaire (SRQ), the participation scale (P-Scale) and the explanatory model interview catalogue (EMIC). The questionnaires have been culturally validated for Colombia and for their applicability to people affected by CL and leprosy.16 The SRQ is a screening instrument for psychiatric disturbances and includes the main consequences of mental health problems.17 A high total score (maximum 20) indicates mental distress. The P-Scale measures restrictions to social participation based on the conceptual framework of the WHO International Classification of Functioning, Disability and Health (ICF).18 The participation score is the sum of all ratings, ranging from 0 (no restriction) to 90 (severe restriction). The EMIC measures stigma resulting from health conditions.19 The total score ranges from 0 (no stigma) to 45 (high stigma level). Participants responded verbally to the questionnaires. Each participant was interviewed by two interviewers: one asked the questions and the other filled in the questionnaires based on each participant's answers. The researchers received instructions on administering the questionnaires from experienced colleagues.
Ethical considerations
This study was approved by the Ethics Committee of the Francisco de Paula Santander University in the city of Cúcuta, by means of Act No. 2 dated 21 November 2017. Every participant signed an informed consent form.
Statistical analysis
Cut-off points were adopted from previous studies that used the SRQ and P-scale to investigate leprosy in Brazil.20,21 For the SRQ, ≥8 is equal to probable mental health impairment.22 For the P-scale, the cut-off point is >12.21 For the EMIC, no cut-off point can be objectively measured. However, in line with the approach taken by Sermrittirong et al., the cut-off point of 12 was chosen.23
To test the internal consistency of the three questionnaires, Cronbach's Alpha was used. Univariate analysis was performed through the Kruskal Wallis test, which tested the significance of median score differences between the diseases. The pair-wise Mann-Whitney test was used to determine the differences between each pair of NTDs or other grouping variables. Multivariate analysis was conducted to control for the (joint) confounding effects of covariates that had an independent effect on the various outcome variables. Bootstrapped stepwise multivariate regression with backward elimination was used to find associations between mental distress, participation restriction, stigma and the demographics of the population in the dataset. The multivariate analysis was carried out using a model with all demographic variables potentially associated with the outcome, with a p-value of <0.2 identified through univariate analysis. Variables with p-values ≥0.05 were eliminated one by one until all variables that remained in the model were statistically significant (p<0.05).
In order to assess the psychosocial burden (PSB) of each disease more comprehensively, a composite categorical variable was constructed. This represented the sum of all three questionnaires, with presence of perceived stigma, mental distress and participation restriction corresponding to a score of 1 and an absence of these factors to a score of 0. The total score ranges from 0 to 3, with 3 representing high PSB.
Data analysis was performed with software packages EpiInfo 7.2.2.2 (Centers for Disease Control and Prevention) and SPSS Statistics 25 (IBM Corp.).
Results
Of the 430 potential study participants identified, 305 were successfully contacted and agreed to participate in the study. One person was excluded for presenting concomitantly with leprosy and CD. The final sample comprised 304 people: 106 people diagnosed with leprosy, 98 diagnosed with CL and 100 people diagnosed with CD. Table 1 shows sociodemographic data per disease. Leprosy patients were on average older than participants in the other disease categories, with 71% aged >50 y. In addition, leprosy patients were typically diagnosed ≥5 y earlier and lived predominantly in urban areas.
Table 1.
Sociodemographic characteristics of the participants per disease
| Variable | Chagas disease (n=100) | Leprosy (n=106) | Cutaneous leishmaniasis (n=98) | Total (n=304) | ||||
|---|---|---|---|---|---|---|---|---|
| Frequency | (%) | Frequency | (%) | Frequency | (%) | Frequency | (%) | |
| Gender | ||||||||
| Male | 57 | 57 | 56 | 52.8 | 59 | 60.2 | 172 | 56.6 |
| Female | 43 | 43 | 50 | 47.2 | 39 | 39.8 | 132 | 43.4 |
| Age group, y | ||||||||
| 18–35 | 24 | 24 | 4 | 3.8 | 24 | 24.5 | 52 | 17.1 |
| 36–50 | 37 | 37 | 27 | 25.5 | 31 | 31.6 | 95 | 31.2 |
| 51–65 | 31 | 31 | 45 | 42.5 | 26 | 26.5 | 102 | 33.6 |
| 66–80 | 8 | 8 | 30 | 28.2 | 17 | 17.4 | 55 | 18.1 |
| Residence area | ||||||||
| Urban | 53 | 53 | 101 | 95.3 | 57 | 58.2 | 211 | 69.4 |
| Rural | 47 | 47 | 5 | 4.7 | 41 | 41.8 | 93 | 30.6 |
| Level of education | ||||||||
| None | 16 | 16 | 14 | 13.2 | 8 | 8.1 | 38 | 12.5 |
| Primary school | 47 | 47 | 62 | 58.5 | 43 | 43.9 | 152 | 50.0 |
| Secondary school | 26 | 26 | 25 | 23.6 | 29 | 29.6 | 80 | 26.3 |
| Tertiary education | 11 | 11 | 5 | 4.7 | 18 | 18.4 | 34 | 11.2 |
| Marital status | ||||||||
| Single | 13 | 13 | 28 | 26.4 | 21 | 21.4 | 62 | 20.4 |
| Married/cohabitation | 73 | 73 | 58 | 54.7 | 68 | 69.4 | 199 | 65.4 |
| Separated | 10 | 10 | 9 | 8.5 | 4 | 4.1 | 23 | 7.6 |
| Widow | 4 | 4 | 11 | 10.4 | 5 | 5.1 | 20 | 6.6 |
| Years since diagnosis | ||||||||
| 0–1 | 11 | 11 | 1 | 1.0 | 11 | 11.2 | 23 | 7.6 |
| 1–5 | 50 | 50 | 31 | 29.2 | 80 | 81.6 | 161 | 52.9 |
| Over 5 | 39 | 39 | 74 | 69.8 | 7 | 7.2 | 120 | 39.5 |
| Health insurance | ||||||||
| Contributory | 38 | 38 | 26 | 24.5 | 46 | 46.9 | 110 | 36.2 |
| Subsidized | 62 | 62 | 80 | 75.5 | 52 | 53.1 | 194 | 63.8 |
Levels of mental distress
Internal validity of the SRQ was good for the total population (α=0.88) and for the disease samples separately (α=0.85–0.90). Based on the SRQ cut-off value, 41.8% of the total sample, 50% of people affected by CD, 25.5% of people affected by CL and 49.1% of people affected by leprosy were classified as experiencing mental distress. Mean and median outcomes on the questionnaires are shown in Table 2. The level of mental distress, represented by the total SRQ score, differed significantly among the disease groups (p=0.001). The median for CD was 7.5, 5 for CL and 7 for leprosy. These differences were significant between people affected by CL or leprosy (p=0.003) and between people affected by CL or CD (p=0.001). No significant difference was found between people affected by leprosy or CD (p=0.853).
Table 2.
Mean and median scores per questionnaires and disease type
| Total | Chagas disease | Cutaneous leishmaniasis | Leprosy | Kruskal-Wallis between diseases | ||
|---|---|---|---|---|---|---|
| SRQ | Mean | 6.9 (6.4 to 7.5) | 7.7 (6.7 to 8.6) | 5.4 (4.5 to 6.3) | 7.7 (6.7 to 8.8) | |
| Median | 6 (3 to 11) | 7.5 (4 to 11) | 5 (1 to 8) | 7 (3 to 12) | ||
| % above cut-off point | 41.8% | 50% | 25.5% | 49.1% | p=0.001 | |
| P-Scale | Mean | 6.3 (5.2 to 7.5) | 5.0 (3.3 to 6.8) | 3.7 (2.5 to 4.9) | 10.0 (7.5 to 12.5) | |
| Median | 2 (0 to 8) | 1 (0 to 6) | 2 (0 to 4) | 6 (1–14) | ||
| % above cut-off point | 15.5% | 12% | 6.1% | 27.4% | p<0.001 | |
| EMIC | Mean | 9.3 (8.2 to 10.4) | 7.9 (6.3 to 9.5) | 5.4 (3.9 to 6.9) | 14.2 (12.2 to 16.3) | |
| Median | 6.0 (3.0 to 12.0) | 6.0 (2 to 12) | 3.0 (0 to 8) | 12.0 (6 to 21) | ||
| % above cut-off point | 32.2% | 27.0% | 17.3% | 51.9% | p<0.001 |
Abbreviations: EMIC, explanatory model interview catalogue; P-Scale, participation scale; SRQ, self-reporting questionnaire
Means are followed by the 95% confidence interval.
Medians are followed by the interquartile range.
Levels of participation restriction
Internal consistency was good (α=0.86), including for the disease populations separately (α=0.85–0.87). Our results show that 15.5% of the total sample—12% of people affected by CD, 6.1% of people affected by CL and 27.4% of people affected by leprosy—experienced participation restrictions. P-Scale outcomes, which are provided in Table 2, had a median of 1 in the CD group, 2 in the CL group and 6 in the leprosy group. These differences were significant between CL and leprosy (p<0.001) and between CD and leprosy (p<0.001), but not between CL and CD (p=0.678).
Levels of stigma
Internal consistency of the EMIC was good for the total sample (α=0.85) and for the disease samples separately (α=0.80–0.85). EMIC outcomes were significantly different among the three diseases (p<0.001). The median EMIC outcome for the leprosy group was 12, with 67.9% experiencing significant stigma. The medians for the other NTDs were lower: 3 for CL and 6 for CD; however, 25.5% and 39.0%, respectively, of the affected persons perceived significant stigma. Table 2 shows EMIC outcomes per disease. The Mann Whitney-U test showed significantly higher EMIC scores in leprosy than in CL (p=0.001) and CD (p=0.001). Furthermore, the EMIC scores in CD were significantly higher than in CL (p=0.001).
Sociodemographic factors associated with mental distress
A significant difference in SRQ scores was found between people living in rural and urban areas for the total population (p=0.033), with those people living in rural areas exhibiting higher levels of mental distress. This association was confirmed in the CD group (p=0.026) and CL group (p=0.008). For the total sample, an association was found between educational levels and SRQ scores (p=0.001 – p=0.010), with tertiary education associated with less mental distress compared with primary or no education.
Sociodemographic factors associated with participation restriction
A significant difference in P-Scale scores was found between people affected by CL living in rural and urban areas (p=0.037), indicating that those living in rural areas experience higher participation restrictions. For the total sample, an association was found between P-Scale scores and education. People with a tertiary education level felt less restricted compared with those with no, primary or secondary education. Finally, people diagnosed ≥5 y earlier experienced greater participation restrictions than people diagnosed <5 y earlier (p=0.003).
Sociodemographic factors associated with stigma
A significant difference in EMIC scores was found between people living in rural and urban areas for CD (p<0.001) and CL (p<0.001). People affected by CD who live in rural areas had a median EMIC score of 10, whereas their counterparts in urban areas had a median EMIC score of 3. For people affected by CL, their median EMIC scores were 6 and 0, respectively. This indicated that people affected by CD or CL in rural areas anticipated or perceived a higher level of stigma than those in urban areas.
For the total population, educational levels were found to significantly impact experience of stigma. Secondary and tertiary education alumni reported lower stigma levels compared with people without education (p<0.001 – p=0.049) and people with tertiary education also reported less stigma than those with only primary education (p<0.001).
In addition, people diagnosed <5 y earlier had a higher stigma score than those diagnosed in the previous year (p=0.001) or in the previous 4 y (p=0.001).
Multivariate analysis
The significant bootstrapped models for the influence of multiple factors on the determinants for PSB are shown in Table 3. The SRQ results showed that participants with a lower education level had higher mean levels of mental distress. This model explained 10% of the variability of mental distress in the total population (R2=0.10). For the three individual diseases, a similar outcome was found: in CD, level of education explained 11% of the variability, 4% in leprosy and 13% in CL.
Table 3.
Significant bootstrapped multivariate regression models
| SRQ | P-scale | EMIC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | p-value | R2 | Model | p-value | R2 | Model | p-value | R2 | |
| Total | Education | 0.001 | 0.118 | Education Years since diagnosis |
0.001 0.002 |
0.074 | Years since diagnosis Education Living area |
0.001 0.002 0.029 |
0.083 |
| Chagas disease | Education | 0.001 | 0.113 | NS | NS | NS | Living area | 0.002 | 0.16 |
| Cutaneous leishmaniasis | Education | 0.001 | 0.131 | Living area | 0.027 | 0.085 | Living area | 0.001 | 0.163 |
| Leprosy | Education | 0.029 | 0.038 | Gender Years since diagnosis |
0.036 0.037 |
0.068 | NS | NS | NS |
Abbreviations: EMIC, explanatory model interview catalogue; P-Scale, participation scale; SRQ, self-reporting questionnaire
Participants with lower levels of education and a longer existing diagnosis had significantly higher levels of participation restriction. For people affected by CD disease, no associations were found on the P-scale. For people affected by leprosy, multivariate analysis showed that women and people with a longer existing diagnosis experienced restriction more often. For people affected by CL in rural areas, the level of participation restriction was significantly higher and this explained 8% of the variability.
Significantly higher levels of stigma were found through multivariate analysis for participants with lower levels of education, living in a rural area and diagnosed a longer time before. For CD and CL, living in a rural area corresponded to significantly higher EMIC scores. In the models for both diseases, it explained 16% of stigma variability.
PSB
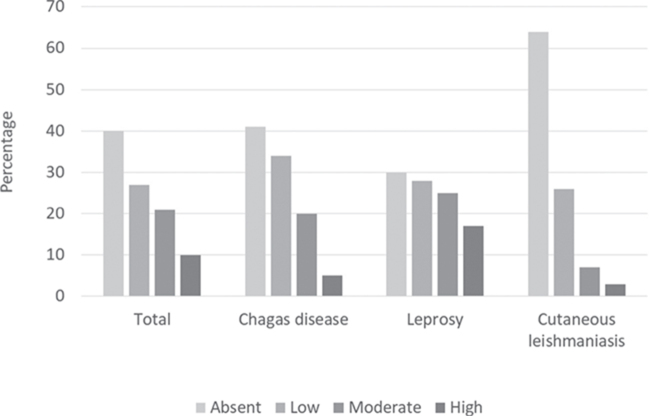
In total, 31.6% (77/165) of the patients exhibited a moderate or high PSB. The highest percentages were found in the leprosy group (41.5%, 42/70) followed by the CD group (25%, 25/59) (Figure 1).
Figure 1.
Psychosocial burden of the total study participants and per disease.
Discussion
In this cross-sectional study, we investigated the PSB (levels of mental distress, participation restriction and stigma) among patients affected by three NTDs in Colombia. The questionnaires used exhibited high internal consistency when applied to all disease groups. Mental distress was evident in a substantial part of our population, while participation restriction was primarily encountered in leprosy patients. Stigma varied significantly among the disease groups, but was encountered in every third participant in the total study population. Interestingly, the percentage of participants exhibiting scores above the cut-off value in at least two of the three questionnaires, depending on the disease category, ranged between 10% and 42%. These findings suggest the existence of a substantial PSB among our study participants. Certain sociodemographic factors (low educational level, living in rural settings) seemed to be associated with higher scores. Similar studies have also found that low levels of education contribute to less health knowledge.24 Increased health education could therefore be an important intervention to reduce PSB in persons affected by NTDs.25
Our findings are partly in agreement with existing evidence, although extensive comparison is limited because of the scarcity of similar studies. Mental distress and participation restriction levels of CD patients have rarely been investigated. Using different instruments, Ozaki et al. found similar levels of mental impairment and stigma in CD patients in Brazil.11 It is unclear whether these results are generalizable. As disease-related perceived stigma and mental distress are largely subjective, varying according to the sociocultural background in the respective area/country, context-specific variation is likely.26 Animal model data, however, are supportive of a causal relation between CD and mental distress. In a 2010 review, da Silva et al. found that chronic CD leads to sleep dysfunction and memory impairment in rats.27 Furthermore, this review provides evidence of memory impairment, quality of life reduction and depression, as well as direct central nervous system effects in infected humans. However, national programs and operational research still focus primarily on vector control, neglecting the potential psychosocial aspects of CD. In this light, stigma and, in particular, mental distress in CD patients, urgently merit further investigation.
In previous studies from Brazil and Ethiopia using the SRQ, mental distress was also found to be significant, yet lower levels were observed among leprosy patients.20,28 The discrepancy observed might reflect differences in cultural backgrounds and disease perceptions, influencing the mental health of people affected by leprosy. It could also be the result of significant differences in social determinants, such as socioeconomic status. With respect to participation restriction in leprosy patients, our findings seem to reflect current prevalence research, while with respect to stigma and the mean EMIC score, our results are similar to prior studies conducted in Nepal and West Bengal, but substantially lower than findings in African settings.21,23 Again, cultural, sociodemographic and clinical characteristics of the study populations may account for these discrepancies.
Regarding CL, we found that mental distress and participation restriction levels in our study participants were comparable with the pilot findings of van ‘t Noordende et al. and significantly lower when compared with their leprosy and CD findings.29 However, the exact location of CL-related lesions was not recorded in the national registers. Also, the vast majority of study participants affected by CL stated that they had successfully concluded treatment at least 1 y earlier and did not exhibit any apparent facial scars or other visible disfigurement. A 2018 review reported that the impact on mental distress was often hard to measure because of its time dependence in relation to treatment.30 Furthermore, a 2014 Colombian study stated that perceived severity of CL was associated with lesion type, body location and the length of time someone had lived with a manifestation.31 The latter factor might partly explain the relatively low scores observed in both questionnaires. Perceived stigma was encountered in 18% of our CL patients. These findings suggest rather limited stigmatization of people affected by CL in Colombia, in contrast to literature from other endemic countries, particularly the Middle East.32,33
To our knowledge, this is the first study to assess mental distress, participation restriction and stigma in patients with CD, CL and leprosy, not only in Colombia, but also in Spanish-speaking Latin America in general. The study addresses a significant knowledge gap, allowing deeper insights into the PBS of prominent NTDs of the South American subcontinent that are urgently needed for the development of comprehensive and inclusive NTD interventions. The research used questionnaires previously culturally validated for Colombia, a strength that allows comparisons between countries for leprosy.16 However, the use of these questionnaires in CD and CL is relatively new. Additionally, validation of the questionnaires by Fischer et al. did not include validation of the cut-off points.16
Our study has some limitations. First, it does not allow causal inference, as it has a merely descriptive character. In the absence of a healthy control group, it cannot be assumed that findings are solely disease-related and not confounded by other factors. However, previous studies in Colombia using the SQR instrument in the general population have yielded substantially lower mental distress scores, suggesting that our results at least partially reflect disease-specific PSB.34 With respect to participation restriction and stigma, the instruments used in our study are generally designed for people affected by disability and/or a stigmatizing disease, thus the meaningfulness of using them in a healthy control group would be questionable. A further limitation is the lack of data on severity of the participants' disease manifestations, as this information is not routinely captured in the respective national program registries that served as our clinical information source. Data on disease severity would allow more detailed insight into the PSB of these diseases, drawing more valid conclusions when comparing the results across the three NTDs.
Conclusions
This study describes the levels of mental distress, participation restriction and stigma found in people affected by leprosy, CL and CD in two co-endemic areas of Colombia. Our findings indicate that these diseases have a significant PSB in our study setting. Further investigation and analysis on a larger scale are urgently needed to better understand the full dimensions of the burden of NTDs, and to inform health policymakers of the need for holistic and more inclusive approaches within their respective control programs.
Acknowledgments
The authors wish it to be known that LJG and RvW should be regarded as joint first authors. The authors acknowledge the valuable contribution of the patients interviewed.
Contributor Information
Libardo J Gómez, German Leprosy and TB Relief Association, DAHW América del Sur, Calle 128 B No. 56 C 05, Bogotá, Colombia.
Robin van Wijk, NLR, Wibautstraat 137k, 1097 DN Amsterdam, the Netherlands.
Lena van Selm, NLR, Wibautstraat 137k, 1097 DN Amsterdam, the Netherlands.
Alberto Rivera, German Leprosy and TB Relief Association, DAHW América del Sur, Calle 128 B No. 56 C 05, Bogotá, Colombia.
Martha C Barbosa, German Leprosy and TB Relief Association, DAHW América del Sur, Calle 128 B No. 56 C 05, Bogotá, Colombia.
Sandra Parisi, German Leprosy and TB Relief Association, DAHW América del Sur, Calle 128 B No. 56 C 05, Bogotá, Colombia.
Wim H van Brakel, NLR, Wibautstraat 137k, 1097 DN Amsterdam, the Netherlands.
Jofren Arevalo, Francisco de Paula Santander University, 12E96 Avenida Gran Colombia, Cúcuta, Colombia.
William Quintero, Francisco de Paula Santander University, 12E96 Avenida Gran Colombia, Cúcuta, Colombia.
Mitzi Waltz, Vrije Universiteit Amsterdam, De Boelelaan 1085, 1081 HV Amsterdam, the Netherlands.
Karl Philipp Puchner, German Leprosy and TB Relief Association, Raiffeisenstraße 3, 97080 Würzburg, Germany; Global Health–Disaster Medicine, Medical School, National and Kapodistrian University of Athens, Mikras Asias 17, Athens, 115 27, Greece.
Authors' contributions
Data collection for this research was carried out by RvW, LvS, JA and WQ. LJG, AR, MCB, SP, MW, WHvB and KPP supervised the research for the universities and Non-Governmental Organisations (NGOs) involved. All authors contributed to data analysis and writing this article.
Funding
This study was supported by the German Leprosy and TB Relief Association, Würzburg, Germany.
Competing interests
None declared.
Ethical approval
Ethical approval for this study was obtained from the Universidad Francisco de Paula Santander in Cúcuta, Colombia.
References
- 1. Stanaway JD, Roth G. The burden of Chagas disease: Estimates and challenges. Glob Heart. 2015;10:139–44. [DOI] [PubMed] [Google Scholar]
- 2. Cardona-Castro NM. Restrepo-Jaramillo S, Gil De La Ossa M, et al. Infection by Mycobacterium leprae of household contacts of lepromatous leprosy patients from a post-elimination leprosy region of Colombia. Mem Inst Oswaldo Cruz. 2005;100:703–7. [DOI] [PubMed] [Google Scholar]
- 3. PAHO and WHO Leishmaniasis In: Informe Epidemiológico de Las Américas, Washington DC: Pan-American Health Organisation, 2018. [Google Scholar]
- 4. Cardona-Castro N. Leprosy in Colombia: Post elimination stage. Lepr Rev. 2013;84:238–247. [PubMed] [Google Scholar]
- 5. Padilla JC, Lizarazo FE, Murillo OL et al. Epidemiología de las principales enfermedades transmitidas por vectores en Colombia, 1990-2016. Biomedica. 2017;37:27–40. [DOI] [PubMed] [Google Scholar]
- 6. Olivera MJ, Fory JA, Porras JF et al. Prevalence of Chagas disease in Colombia: A systematic review and meta-analysis. PLoS One. 2019;14:e0210156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mackey TK, Liang BA, Cuomo R et al. Emerging and reemerging neglected tropical diseases: A review of key characteristics, risk factors, and the policy and innovation environment. Clin Microbiol Rev. 2014;27:949–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mountford-Zimdars A, Harrison N. Access to Higher Education: Theoretical Perspectives and Contemporary Challenges. London: Taylor and Francis, 2016. [Google Scholar]
- 9. Hofstraat K, Brakel WH. Social stigma towards neglected tropical diseases: A systematic review. Int Health. 2016;8:i53–70. [DOI] [PubMed] [Google Scholar]
- 10. Al-Kamel MA, Cardona-Castro N, Alonso LM et al. Stigmata in cutaneous leishmaniasis: Historical and new evidence-based concepts. PLoS Negl Trop Dis. 2017;23:238–247. [Google Scholar]
- 11. Ozaki Y, Guariento ME, Almeida EA. Quality of life and depressive symptoms in Chagas disease patients. Qual Life Res. 2011;20:133–138. [DOI] [PubMed] [Google Scholar]
- 12. Van Brakel W. Stigma in leprosy: Concepts, causes and determinants. Lepr Rev. 2014;85:36–47. [PubMed] [Google Scholar]
- 13. Mieras LF, Anand S, Brakel WH et al. Neglected tropical diseases, cross-cutting issues workshop, 4–6 February 2015, Utrecht, the Netherlands: Meeting report. Int Health. 2016;8:i7–11. [DOI] [PubMed] [Google Scholar]
- 14. Mitra AK, Mawson AR. Neglected tropical diseases: Epidemiology and global burden. Trop Med Infect Dis. 2017;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romero-Montoya IM, Beltrán-Alzate JC, Ortiz-Marín DC et al. Leprosy in Colombian children and adolescents. Pediatr Infect Dis J. 2014;33:321–2. [DOI] [PubMed] [Google Scholar]
- 16. Fischer J, Jansen B, Rivera A et al. Validation of a cross-NTD toolkit for assessment of NTD-related morbidity and disability. PLoS Negl Trop Dis. 10.1371/journal.pone.0223042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beusenberg M, Orley JH, Organization WH . A User's Guide to the Self Reporting Questionnaire (SRQ). Geneva: World Health Organization, 1994. [Google Scholar]
- 18. Van Brakel WH, Anderson AM, Mutatkar RK et al. The participation scale: Measuring a key concept in public health. Disabil Rehabil. 2006;28:193–203. [DOI] [PubMed] [Google Scholar]
- 19. Van Brakel WH. Measuring health-related stigma—A literature review. Psychol Health Med. 2006;11:307–34. [DOI] [PubMed] [Google Scholar]
- 20. Cunha MADS, Antunes DE, Da Silveira RWM et al. Application of the SRQ20 and the protocol of psychological assessment in patients with leprosy in a reference centre in Brazil. Lepr Rev. 2015;86:229–39. [PubMed] [Google Scholar]
- 21. Lesshafft H, Heukelbach J, Barbosa JC et al. Perceived social restriction in leprosy-affected inhabitants of a former leprosy colony in Northeast Brazil. Lepr Rev. 2010;81:69–78. [PubMed] [Google Scholar]
- 22. De Souza VTC, Junior WMS, De Jesus AMR et al. Is the WHO disability grading system for leprosy related to the level of functional activity and social participation? Lepr Rev. 2016;8:191–200. [PubMed] [Google Scholar]
- 23. Sermrittirong S, Van Brakel WH, Kraipui N et al. Comparing the perception of community members towards leprosy and tuberculosis stigmatisation. Lepr Rev. 2015;86:54–62. [PubMed] [Google Scholar]
- 24. Adhikari B, Kaehler N, Chapman RS et al. Factors affecting perceived stigma in leprosy affected persons in Western Nepal. PLoS Negl Trop Dis. 2014;8:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaehler N, Adhikar B, Raut S et al. Perceived stigma towards leprosy among community members living close to Nonsomboon leprosy Colony in Thailand. PLoS One. 2015;10:e0129086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ventura-Garcia L, Roura M, Pell C et al. Socio-cultural aspects of Chagas disease: A systematic review of qualitative research. PLoS Negl Trop Dis. 2013;7:e2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silva AS, Pimentel VC, Fiorenza AM et al. Activity of cholinesterases and adenosine deaminase in blood and serum of rats experimentally infected with Trypanosoma cruzi. Ann Trop Med Parasitol. 2011;105:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gelaye B, Lemma S, Deyassa N et al. Prevalence and correlates of mental distress among working adults in Ethiopia. Clin Pract Epidemiol Ment Heal CP EMH. 2012;8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van ‘t Noordende AT, Kuiper H, Ramos AN et al. Towards a toolkit for cross-neglected tropical disease morbidity and disability assessment. Int Health. 2016;8:i71–i81. [DOI] [PubMed] [Google Scholar]
- 30. Bennis I, De Brouwere V, Belrhiti Z et al. Psychosocial burden of localised cutaneous Leishmaniasis: A scoping review. BMC Public Health. 2018;18:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carrillo-Bonilla LM, Trujillo JJ, Álvarez-Salas L et al. Study of knowledge, attitudes, and practices related to leishmaniasis: Evidence of government neglect in the Colombian Darién. Cad Saude Publica. 2014;30:2134–44. [DOI] [PubMed] [Google Scholar]
- 32. Kassi M, Kassi M, Afghan AK et al. Marring leishmaniasis: The stigmatization and the impact of cutaneous leishmaniasis in Pakistan and Afghanistan. PLoS Negl Trop Dis. 2008;2:e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reithinger R, Aadil K, Kolaczinski J et al. Social impact of leishmaniasis Afghanistan. Emerg Infect Dis. 2005;11:634–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tamayo Martínez N, Rincón Rodríguez CJ, Santacruz C et al. Mental problems, mood and anxiety disorders in the population displaced by violence in Colombia; results of the National Mental Health Survey 2015. Rev Colomb Psiquiatr. 2016;45:113–8. [DOI] [PubMed] [Google Scholar]