Abstract
Background
Bacteria are sources of numerous molecules used in treatment of infectious diseases. We investigated effects of molecules produced by 26 Pseudomonas aeruginosa strains against infection of mammalian cell cultures with Trypanosoma cruzi, the aetiological agent of Chagas disease.
Methods
Vero cells were infected with T. cruzi in the presence of wild-type P. aeruginosa supernatants or supernatants of mutants with defects in the production of various virulence, quorum sensing and iron acquisition factors. Quantification of T. cruzi infection (percentage of infected cells) and multiplication (number of amastigotes per infected cell) was performed and cell viability was determined.
Results
Wild-type P. aeruginosa products negatively affected T. cruzi infection and multiplication in a dose-dependent manner, without evident toxicity for mammalian cells. PvdD/pchE mutation (loss of the P. aeruginosa siderophores pyoverdine and pyochelin) had the greatest impact on anti–T. cruzi activity. Negative effects on T. cruzi infection by pure pyochelin, but not pyoverdine, or other P. aeruginosa exoproducts studied, were quantitatively similar to the effects of benznidazole, the current standard therapy against T. cruzi.
Conclusions
The P. aeruginosa product pyochelin showed promising activity against T. cruzi and might become a new lead molecule for therapy development.
Keywords: Trypanosoma cruzi, Chagas disease, Pseudomonas aeruginosa, drug discovery, therapy, pyochelin
Introduction
Chagas disease is a worldwide systemic and often chronic disease caused by the protozoan Trypanosoma cruzi. In Latin America, which is regarded as the endemic area for T. cruzi infection, at least 6 million people are estimated to be affected,1,2 There is also an increasing incidence of Chagas disease in the USA and other non-endemic countries.3,4 It is estimated that more than 300 000 individuals in the USA are infected, with up to 45 000 developing cardiomyopathies.1–4 Due to severe side effects and limited efficacy, the current standards of therapy (benznidazole or 5-nitrofuran nifurtimox) are mainly recommended for treatment of the acute phase and early chronic infections.5,6
Antimicrobials are weapons microorganisms produce in order to stake out their claims in ecosystems with limited resources and a multitude of microbial inhabitants.7 The bacterium Pseudomonas aeruginosa and the fungus Aspergillus fumigatus form such an ecosystem in the lungs of cystic fibrosis patients.8,9P. aeruginosa has been shown to produce molecules that interfere with A. fumigatus biofilm metabolism and growth.10 Using supernatants from P. aeruginosa mutants comparison with wild-type, we recently described that the siderophore pyoverdine is the main antifungal molecule in a low-iron environment.10 Here we used supernatants from the same P. aeruginosa wild-type and mutants and investigated the effects on T. cruzi infection and multiplication in search of new molecules that would ultimately help in treating Chagas disease.
Materials and methods
Materials
Benznidazole (BNZ), amphotericin B (AmB), pyoverdine (PYOV), Pseudomonas Quinolone Signal (PQS), Giemsa solution, Bouin’s fixative solution, 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide inner salt (XTT) and menadione were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pyochelin (PYOC) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Foetal calf serum (FCS; catalogue number 16140071) and RPMI 1640 were obtained from Gibco (New York, NY, USA). The homoserine lactones 3-oxo-C12-HSL and C4-HSL were synthesized as described previously.11,12
Strains
PA14, a widely studied P. aeruginosa strain,13 is the parental strain of all PA14 mutants studied here. The T. cruzi strain Y (TcY, TcII, ATCC 50832) was used.
PA14 mutants
All PA14 mutants investigated here, with the exception of the lasI and pscC mutants, were described previously. The lasI mutant is lacking 3-oxo-C12-HSL production,14 while the pscC mutant is defective in all three secretion systems.15
P. aeruginosa culture filtrate production
PA14 wild-type and mutant planktonic culture filtrate (Pasup) were prepared as detailed previously.10 In brief, RPMI 1640 medium (Lonza, Walkersville, MD, USA) was used and quantitated P. aeruginosa suspensions were grown for 24 h at 37°C. Culture supernatants were filtered (0.22 μm) after the growth period.
Isolation of trypanomastigotes
Culture-derived trypanomastigotes of the Y strain (TcII) were obtained from monolayers of Vero cells (cell line based on kidney epithelial cells from African green monkey, CCL-81; ATCC, Manassas, VA, USA) that had been infected at a ratio of 5:1 (trypanomastigotes:Vero cells). Vero cells were incubated at 37°C in RPMI 1640, enriched with 5% inactivated foetal bovine serum (FBS), supplemented with antibiotics (penicillin 500 μ/mL and streptomycin 0.5 mg/mL). Parasites were collected from the culture supernatants by centrifugation at 1000 g for 10 min and the sediment was suspended in RPMI with 5% FBS. Parasites were counted using a Neubauer chamber and the number was adjusted according to assay needs.
Infection with T. cruzi
Monolayers of Vero cells were prepared on Matrigel-coated eight-well chamber slides at a density of 2 × 105 cells/well and cultivated for 24 h at 37°C in a 5% carbon dioxide (CO2) atmosphere. Infection was carried out at a target effector ratio of 1:5 (cell:parasite), with 24 h interaction time in the presence of drugs (AmB, BNZ), pure substances (PYOV, PYOC, PQS, oxo-C12-HSL or C4-HSL) or P. aeruginosa supernatants before replacing the medium with RPMI with 5% FBS without drugs, supernatants or parasites. Forty-eight hours after infection, cells were washed with phosphate-buffered saline, fixed with Boudin’s solution and Giemsa stained. The number of infected cells (defined as at least one 1 amastigote per cell) as well as the number of amastigotes per infected cells (a measure of multiplication) was determined for each vision field by microscopy. About 25–40% of all cells in the control wells were found to be infected by TcY. For comparison with treated wells, control infections were regarded as 100%.
Cell metabolism
The viability of uninfected cells was determined by XTT metabolic assay.16 Cells (105) were seeded into a 96-well plate and allowed to settle for 24 h at 37°C in 5% CO2 and 80% humidity. After medium change, drugs (AmB, BNZ), pure substances (PYOV, PYOC, PQS, oxo-C12-HSL or C4-HSL) or P. aeruginosa supernatants were added and cells were incubated for 24 h at 37°C in 5% CO2 and 80% humidity. XTT/menadione (200 μg/mL and 40 μM, respectively) in RPMI with 5% FBS was added to each well and incubated at 37°C. Tests were evaluated using a plate reader (Opsys MR, DYNEX Technologies, Chantilly, VA, USA).
Statistical analysis
Results were analysed using Student’s t test if two equal-size groups were compared or by Student’s t test with Welch modification if the two groups showed unequal sample sizes.
Results
P. aeruginosa produces molecules that affect T. cruzi Y infection and multiplication
P. aeruginosa exports a wide range of molecules that can be investigated using its culture supernatants. We used cell-free supernatants produced by planktonically growing P. aeruginosa PA14 cells (PA14sup) as well as dilutions of these supernatants against infection of Vero cells with the T. cruzi strain TcY. AmB, a known inhibitor of T. cruzi, was used as a comparator.17 Our results show that PA14sup in dilutions of up to 1:16 significantly interfered with T. cruzi infection (Figure 1A), as well as multiplication (Figure 1B), without affecting cell metabolism (Figure 1C). Vero cell metabolism was even modestly enhanced by PA14sup (Figure 1C).
Figure 1.
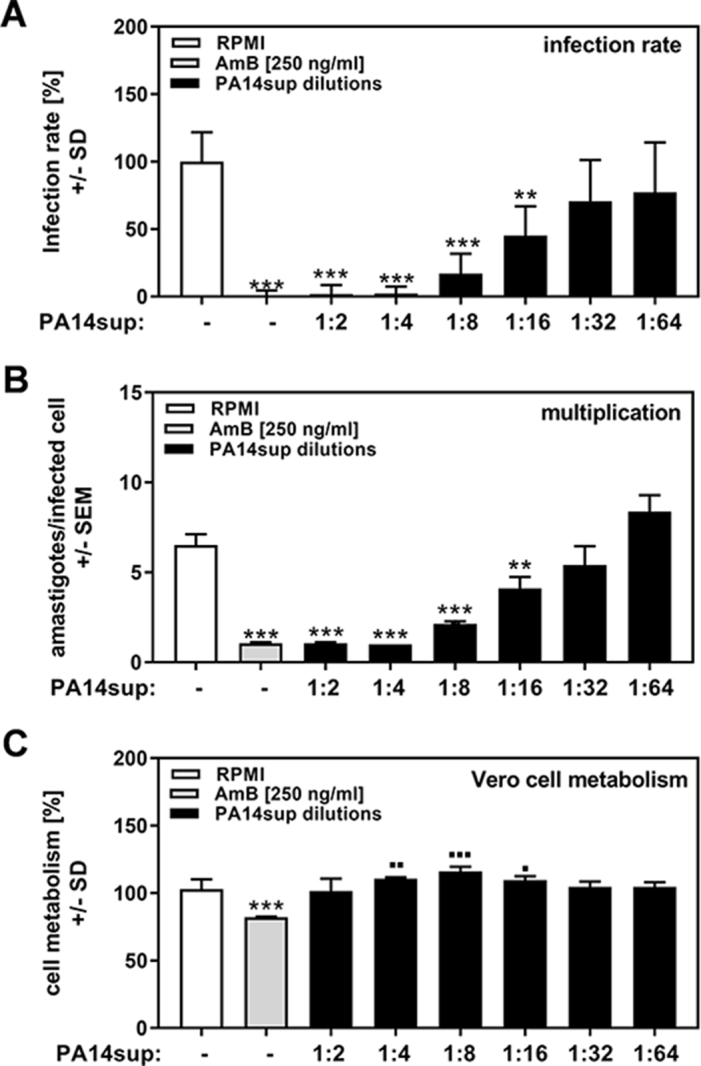
P. aeruginosa molecules affect T. cruzi Y strain infection and multiplication. Vero cells (2 × 105/well) were infected with TcY at a ratio of 5 trypanosoma per cell for 24 h at 37°C in 5% CO2 and 80% humidity. Infections took place in the presence of pure RPMI, AmB (250 ng/mL) or 1:2 dilutions of planktonic PA14 wild-type cell-free supernatants (1:2 to 1:64). Twenty-four hours after infection, cells were washed and fresh medium without PA14 supernatants or TcY was added. (A) Infection rates and (B) multiplication were determined 48 h after infection. (C) Cell viability was determined by XTT assay. Statistics: Welch t test; comparison, RPMI vs all other bars. Asterisks represent significant decreases, squares represent significant increases. One, two or three asterisks or squares: p≤0.05, p≤0.01 or p≤0.001, respectively.
Determination of P. aeruginosa products involved in T. cruzi Y inhibition
In order to define the P. aeruginosa product responsible for anti-trypanosoma activity, we used a set of 26 PA14 mutants with single or double gene defects in virulence factors, upstream quorum sensing factors that induce virulence factor production or molecules responsible for iron uptake.10 We found that several PA14 mutants showed defects in anti-trypanosoma infection (Figure 2A) and multiplication (Figure 2B). For T. cruzi infection, mutants in pvdD/pchE (mutant 1), rsmA (mutant 8), pchE (mutant 16) and hcnA (mutant 24) showed the highest loss of activity (PA14 vs PA14 mutants, all p≤0.001). Next highest were mutants in pqsA (mutant 4), lasR (mutant 7) and phzC1/C2 (mutant 15) (PA14 vs PA14 mutants, all p≤0.01) and HSI 1/2 (mutant 12), chiC (mutant 22) and lecA (mutant 23) (PA14 vs PA14 mutants, all p≤0.05). For T. cruzi multiplication, mutants pvdD/pchE (mutant 1), pqsA (mutant 4), rsmA (mutant 8), pchE (mutant 16) and hcnA (mutant 24) showed the largest loss of activity (PA14 vs PA14 mutants, all p≤0.001). Next highest were phzC1/C2 (mutant 15 vs PA14, p≤0.01), lasR (mutant 7), pqsA/pqsH polar (mutant 21), lecA (mutant 23) and lasI (mutant 26) (PA14 vs PA14 mutants, all p≤0.05). All mutants showing significant losses in anti-trypanosoma activity are detailed in Table 1. The combined loss of both pyoverdine and pyochelin (pvdD/pchE mutant 1) seemed to increase T. cruzi multiplication over measurements obtained in control wells (Figure 2B). AmB was toxic for Vero cells, but none of the supernatants showed such toxicity (Figure 2C). In fact, most supernatants, with the exception of supernatants for rsmYZ (mutant 18), increased Vero cell metabolism (Figure 2C).
Figure 2.
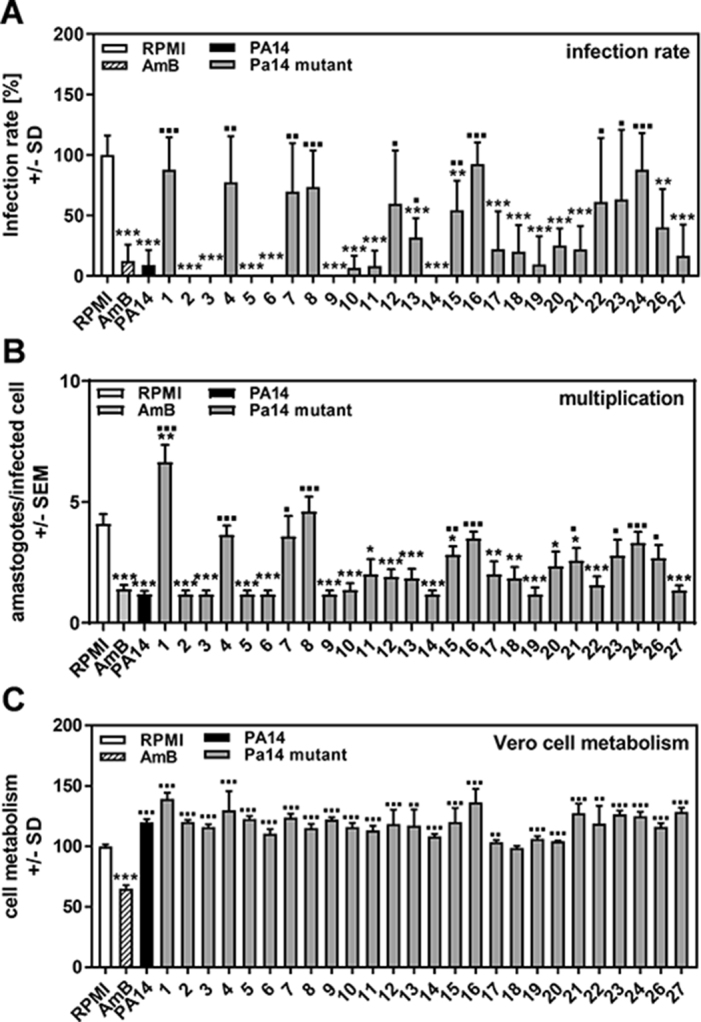
Determination of P. aeruginosa products involved in T. cruzi Y strain inhibition. Vero cells (2 × 105/well) were infected with TcY at a ratio of 5 trypanosoma per cell for 24 h at 37°C in 5% CO2 and 80% humidity. Infections took place in the presence of pure RPMI, AmB (250 ng/mL) or 1:2 dilutions of planktonic PA14 wild-type or mutant cell-free supernatants. Twenty-four hours after infection, cells were washed and fresh medium without supernatants or TcY was added. (A) Infection rates and (B) multiplication were determined 48 h after infection. (C) Cell viability was determined by XTT assay. Statistics: Welch t test; comparisons (A and B): asterisks: RPMI vs all other bars; squares: PA14 wild-type supernatant (black bar) vs all other bars; (C): asterisks represent significant decreases, squares represent significant increases. One, two or three asterisks or squares: p≤0.05, p≤0.01 or p≤0.001, respectively. Mutants: 1: pvdD-pchE-; 2: pqsE-; 3: mvfR-; 4: pqsA-; 5: pqsH-; 6: lasR-rhlR-; 7: lasR-; 8: rsmA-; 9: pqsA-pqsH- (not polar); 10: pvdD-; 11: rhlR-; 12: HSI-1/2-; 13: pvcA-; 14: rhlA-; 15: phzC1-phzC2-; 16: pchE-; 17: exoU-; 18: rsmY-rsmZ-; 19: HSI-2/3-; 20: HSI-1/3-; 21: pqsA-pqsH- (polar); 22: chiC-; 23: lecA-; 24: hcnA-; 26: lasI-, 27: pscC-.
Table 1.
PA14 mutants with significant loss of anti–T. cruzi activity
| No. | Mutant | Mutation result | Reference |
|---|---|---|---|
| 1 | pvdD-/pchE- | Pyoverdine–pyochelin double siderophore mutant | 10 |
| 4 | pqsA- | Gene for anthranilate-CoA ligase lost; loss of extracellular quinolones, HAQ biosynthesis, including HHQ, PQS and DHQ, thus absent or decreased activity of MvfR | 34 |
| 7 | lasR- | Lacks several QS-regulated factors, including proteases, 3-oxo-C12-HSL; delayed activation of RhlR QS pathway, less pyoverdine | 17 |
| 8 | rsmA- | Global post-transcriptional regulator mutant | 35 |
| 12 | HSI 1/2 | Double mutant defective in two of three type VI secretion systems | 36 |
| 15 | phzC1-/phzC2- | Double phenazine mutant (completely abrogated), no pyocyanin | –a |
| 16 | pchE- | Loss of pyochelin (siderophore) mutant | 18 |
| 21 | pqsA-pqsH- polar | ED2, pqsA::TnPhoA, pqsH::Gm, the mutation in pqsA is polar, kan and gm resistant | –a |
| 22 | chiC- | Loss of chitinase | 18 |
| 23 | lecA- | Loss of galactose-specific lectin A | 18 |
| 24 | hcnA- | No hydrogen cyanide production | 18 |
| 26 | lasI- | No 3-oxo-C12-HSL production | 14 |
aUnpublished lab strain (available from E. Deziel).
Pyochelin affects T. cruzi Y infection and multiplication
As detailed in Table 1, a variety of P. aeruginosa–produced molecules could be responsible for anti–T. cruzi effects. To examine the effects of P. aeruginosa–produced molecules on T. cruzi directly and compare them to the currently licensed anti-trypanosome drug BNZ,5 we investigated the anti–T. cruzi effects of pure pyochelin, pyoverdine, PQS, C4-HSL and 3-oxo-C12-HSL. Our results show that at 10 μM, only pyochelin reduced T. cruzi infection to levels that were achievable by the in vitro equivalent to the therapeutic serum range of benznidazole (11.5–23 μM) (Figure 3A). Lesser concentrations of pyochelin did not affect T. cruzi infection (data not shown). Pyochelin also reduced T. cruzi multiplication, but not to levels achievable by benznidazole (Figure 3B). When T. cruzi was incubated with pyochelin prior to infection for 2 h, neither infection nor multiplication was affected (data not shown), suggesting that pyochelin is not directly toxic for T. cruzi. Pyoverdine did not affect T. cruzi infection (Figure 3A), but increased T. cruzi multiplication above levels observed in control wells (Figure 3B). The quorum-sensing signals PQS, C4-HSL and 3-oxo-C12-HSL did not affect T. cruzi infection or its multiplication (Figures 3A and B). Vero cell metabolism was slightly affected by pyoverdine, and to a greater degree by PQS, but not by pyochelin (Figure 3C). C4-HSL and 3-oxo-C12-HSL stimulated Vero cell metabolism (Figure 3C).
Figure 3.
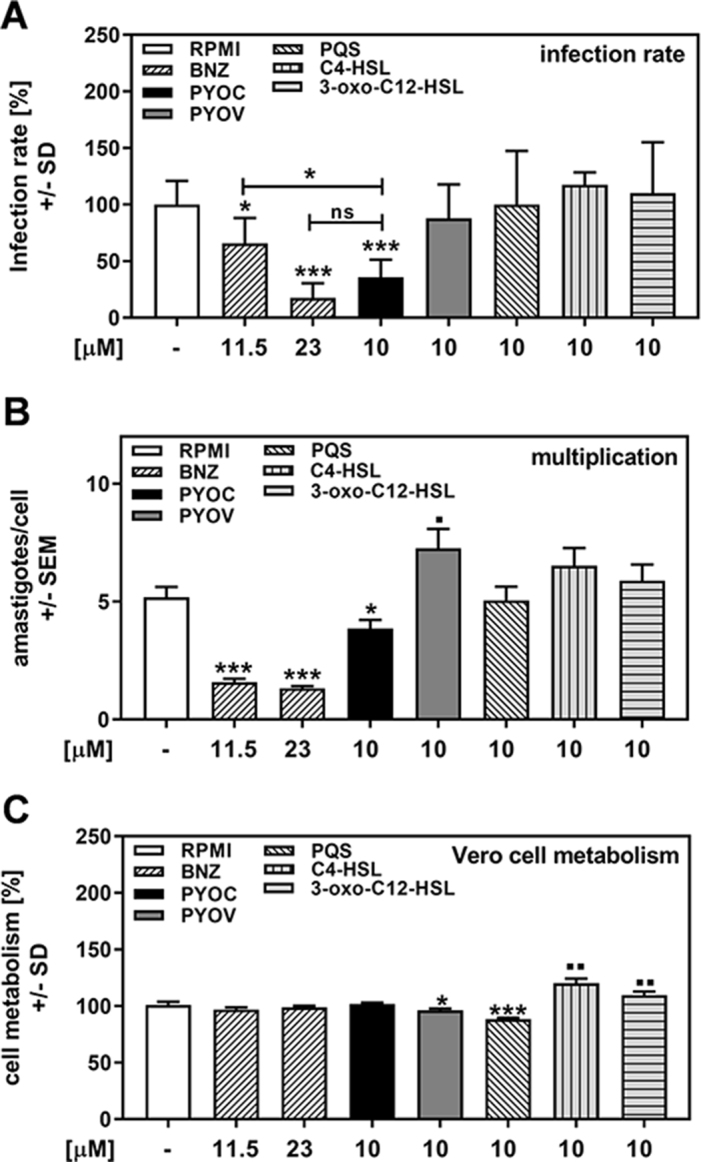
Pyochelin affects T. cruzi Y strain infection and multiplication. Vero cells (2 × 105/well) were infected with TcY at a ratio of 5 trypanosoma per cell for 24 h at 37°C in 5% CO2 and 80% humidity. Infections took place in the presence of pure RPMI, BNZ (11.5 or 23 μM), pyochelin (PYOC, 10 μM), pyoverdine (PYOV, 10 μM), PQS (10 μM), C4-HSL (10 μM) or 3-oxo-C12-HSL (10 μM). Twenty-four hours after infection, cells were washed and fresh medium without drugs or TcY was added. (A) Infection rates and (B) multiplication were determined 48 h after infection. (C) Cell viability was determined by XTT assay. Statistics: Welch t test. Comparisons: RPMI vs all other bars. Asterisks represent significant decreases, squares represent significant increases. One, two or three asterisks or squares: p≤0.05, p≤0.01 or p≤0.001, respectively. ns: not significant.
Discussion
For a long time, chemotherapy for T. cruzi infection, and the resulting Chagas disease, has been limited to benznidazole and nifurtimox. Both are predominantly used in the treatment of acute and early chronic-phase Chagas disease, owing to severe side effects with high doses and long-term application.5,6 New potential treatments under investigation, either alone or in combination, include inhibitors of ergosterol biosynthesis, calcium homeostasis, trypanothione metabolism, cysteine proteases, pyrophosphate metabolism, protein and purine synthesis, as well as lysophospholipid analogues (LPAs) and natural substances.18 Certain azoles, such as posaconazole and itraconazole, were promising candidates for anti-trypanosoma therapy in clinical studies, but appear not to be curative,6 whereas amphotericin B prolonged life in mice and rapidly cleared the blood of all parasites but did not clear tissue infection.17
Most antibiotics that are used in humans are produced by microbes.19P. aeruginosa is less well-known as a source for antibiotics but produces a multitude of antimicrobials in order to survive in microenvironments with competition for limited resources.7 An example of such a competitive ecosystem is the lungs of cystic fibrosis patients, where the bacterium P. aeruginosa and the fungus A. fumigatus fight for resources.8,9,20P. aeruginosa has been shown to produce molecules that interfere with A. fumigatus biofilm metabolism and growth, of which the siderophore pyoverdine is a main antifungal molecule.10
In the present study, we found that exported products from P. aeruginosa also have a negative impact on T. cruzi infections, without affecting mammalian cells. In contrast to PA14 wild-type, several PA14 mutants were unable to inhibit T. cruzi infection and multiplication, suggesting that they lacked production of the active compounds. The loss of pvdD/pchE, rsmA, pchE and hcnA showed the greatest impact on anti–T. cruzi activity, followed by the loss of pqsA, lasR, phzC1/C2, pqsA/pqsH (polar) and lasI.
Iron is a crucial nutrient for P. aeruginosa, as well as for many other organisms, including A. fumigatus and T. cruzi.10,21,22 Pyoverdine and pyochelin are the major siderophores of P. aeruginosa. It has been shown that pyoverdine-bound iron is not available for A. fumigatus,10 while it is not yet known if pyochelin acts in the same way or if T. cruzi can use or is affected by pyoverdine- or pyochelin-bound iron. The loss of both the siderophores pyochelin and pyoverdine, or pyochelin alone, significantly interfered with T. cruzi infection and multiplication, while the loss of pyoverdine alone had no effect. Anti–T. cruzi activity could also be replicated by treatment with pyochelin, while pyoverdine addition showed no inhibition. Consequently, although both pyochelin and pyoverdine are P. aeruginosa siderophores and involved in iron acquisition, only pyochelin was active against T. cruzi infection. Pyoverdine and pyochelin bind ferric iron, but pyoverdine binds with greater affinity.23 Earlier reports showed that iron is a crucial factor for intracellular growth of T. cruzi in macrophages21 and for infection in mice.22 Therefore one possible mechanism of pyochelin action is limiting iron availability for T. cruzi by binding available iron in the medium. However, since pyoverdine, the better iron chelator, has no effect, this hypothesis is unlikely. Pyochelin also binds other metals, like zinc and copper.23,24 Our current experiments cannot rule out that binding of these metals affects T. cruzi infection.
Pyochelin-bound iron causes oxidative damage and inflammation, especially in the presence of pyocyanin, another compound exported by P. aeruginosa.25,26 For example, pyochelin-bound iron enhances the production of intracellular reactive oxygen species (ROS) in Enterococcus faecalis over time, causing a significant increase in lipid peroxidation and cell death by disrupting the integrity of the bacterial membrane.27 It is possible that pyochelin might induce oxidative stress to inhibit T. cruzi. The phzC1/C2 mutant cannot produce phenazines, including the redox-active pyocyanin.28 The phzC1/C2 mutant lost considerable anti–T. cruzi activity, supporting a potential mechanism of pyochelin and pyocyanin inducing oxidative stress in T. cruzi. Whereas excessive oxidative stress is toxic for T. cruzi, it can be mitigated by the T. cruzi aspartic proteinase TcAP1.29 Moderate oxidative stress is beneficial for T. cruzi multiplication,30 however, pyochelin demonstrated inhibition of multiplication as well. Further studies will be required to support an oxidative stress mode of action.
Other mutations also led to a loss in the anti–T. cruzi activity of PA14 supernatants, suggesting that additional P. aeruginosa compounds may also be effective inhibitors. PqsA is an enzyme involved in the synthesis of 4-hydroxy-2-alkylquinolines (HAQs), including PQS. Mutation of pqsA prevents HAQ production, leading to decreased activity of MvfR, a key transcriptional regulator in the quorum sensing pathway that is required for full P. aeruginosa virulence.31 While both the loss of pqsA and pqsA/pqsH (polar) also resulted in a loss of anti–T. cruzi activity, the mvfR- mutant did not result in reduced anti–T. cruzi activity of P. aeruginosa supernatants, nor did the addition of pure PQS affect T. cruzi. It would be worthwhile testing other HAQs produced by the pqsA pathway for anti–T. cruzi activity.
The other key autoinducers of the quorum sensing pathway are 3-oxo-C12-HSL and C4-HSL, where detection by LasR and RhlR, respectively, leads to downstream production of virulence factors like toxins and proteases in addition to regulating swarming and biofilm activity.32 LasI is a synthase that produces 3-oxo-C12-HSL. While the lasI mutant showed a reduction of anti–T. cruzi activity, the addition of 3-oxo-C12-HSL or C4-HSL had no effect. The lasR mutant suffered a greater loss in anti–T. cruzi activity, suggesting that one of the downstream virulence factors produced by the pathway may have additional anti–T. cruzi effects.
Mutations in rsmA, hcnA, chiC, lec and HSI 1/2 also had an impact on P. aeruginosa activity against T. cruzi and may reflect other factors that could participate in anti–T. cruzi action.
Almost all the P. aeruginosa supernatants, whether from wild-type or a mutant, increased the metabolism of Vero cells. The reason for this might be traces of Pseudomonas-produced lipopolysaccharide (LPS) in the bacterial supernatants.33 LPS is a well-known stimulator of mammalian cell proliferation, but it does not stimulate T. cruzi multiplication.34
In our study, infection and treatment took place at the same time. Although preliminary results indicate that 2 h of pre-incubating T. cruzi Y strain with pyochelin does not have significant effects on infection, it might be possible that prolonged incubation affects the parasite. Another possibility would be that pyochelin inhibits T. cruzi growth at the stage of the epimastigote proliferative form.
Although the results presented here are preliminary, the P. aeruginosa product pyochelin has significant anti–T. cruzi Y strain activity, partially in the same range as therapeutic concentrations of benznidazole. Whether pyochelin could become a future lead substance for therapeutic drug development against Chagas disease will also depend on its performance in in vivo studies that determine its selectivity index.35 Our results also suggest that there are other P. aeruginosa molecules with anti–T. cruzi activity that are yet to be defined.
Acknowledgments
The authors thank Prof. Eric Déziel for providing all P. aeruginosa mutants used in this work. Technical assistance by Marife Martinez is gratefully acknowledged.
Contributor Information
Gabriele Sass, California Institute for Medical Research, San Jose, CA 95128, USA.
Laura C Miller Conrad, Department of Chemistry, San Jose State University, San Jose, CA 95112, USA.
Terrence-Thang H Nguyen, Department of Chemistry, San Jose State University, San Jose, CA 95112, USA.
David A Stevens, California Institute for Medical Research, San Jose, CA 95128, USA; Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, CA 94305, USA.
Authors’ contributions
Funding
This work was supported by the Foundation for Research in Infectious Diseases (grant CIMR3424 to DAS) and the National Institutes of Health (grant 5SC3GM118199 to LCMC. TTHN was supported by a National Science Foundation S-STEM grant (DUE 1258366).
Competing interests
None declared.
Ethical approval
Not required.
References
- 1. World Health Organization . Chagas disease (American trypanosomiasis). Available from: https://www.who.int/en/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis).
- 2. Lidani KCF, Andrade FA, Bavia Let al. Chagas disease: from discovery to a worldwide health problem. Front Public Health. 2019;7:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coura JR, Viñas PA. Chagas disease: a new worldwide challenge. Nature 2010;465(7301):S6–S7. [DOI] [PubMed] [Google Scholar]
- 4. Manne-Goehler J, Umeh CA, Montgomery SP, Wirtz VJ. Estimating the burden of Chagas disease in the United States. PLoS Negl Trop Dis. 2016;10(11): e0005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urbina JA. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 2010;115(1–2):55–68. [DOI] [PubMed] [Google Scholar]
- 6. Sales Junior PA, Molina I, Fonseca Murta SMet al. Experimental and clinical treatment of Chagas disease: a review. Am J Trop Med Hyg. 2017;97(5):1289–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gionco B, Tavares ER, Oliveira AGet al. New insights about antibiotic production by Pseudomonas aeruginosa: a gene expression analysis. Front Chem. 2017;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Folkesson A, Jelsbak L, Yang Let al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012;10(12):841–851. [DOI] [PubMed] [Google Scholar]
- 9. Sabino R, Ferreira JA, Moss RBet al. Molecular epidemiology of Aspergillus collected from cystic fibrosis patients. J Cyst Fibros. 2015;14(4):474–481. [DOI] [PubMed] [Google Scholar]
- 10. Sass G, Nazik H, Penner Jet al. Studies of Pseudomonas aeruginosa mutants indicate pyoverdine as the central factor in inhibition of Aspergillus fumigatus biofilm. J Bacteriol. 2017;200(1):e00345–e00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chhabra SR, Harty C, Hooi DSet al. Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-L-homoserine lactone as immune modulators. J Med Chem. 2003;46(1):97–104. [DOI] [PubMed] [Google Scholar]
- 12. Hodgkinson JT, Galloway WRJD, Casoli M, Keane Het al. Robust routes for the synthesis of N-acylated-L-homoserine lactone (AHL) quorum sensing molecules with high levels of enantiomeric purity. Tetrahedron Lett. 2011;52(26):3291–3294. [Google Scholar]
- 13. Lee DG, Urbach JM, Wu Get al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006;7(10):R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maisuria VB, Los Santos YL, Tufenkji N, Déziel E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci Rep. 2016;6: 30169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee VT, Smith RS, Tümmler B, Lory S. Activities of Pseudomonas aeruginosa effectors secreted by the type III secretion system in vitro and during infection. Infect Immun. 2005;73(3):1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scudiero DA, Shoemaker RH, Paull KDet al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48(17):4827–4833. [PubMed] [Google Scholar]
- 17. Clemons KV, Sobel RA, Martinez Met al. Lack of efficacy of liposomal amphotericin B against acute and chronic Trypanosoma cruzi infection in mice. Am J Trop Med Hyg. 2017;97(4):1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Apt W. Current and developing therapeutic agents in the treatment of Chagas disease. Drug Des Devel Ther. 2010;4:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clardy J, Fischbach MA, Currie CR. The natural history of antibiotics. Curr Biol 2009;19(11):R437–R441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams HD, Davies JC. Basic science for the chest physician: Pseudomonas aeruginosa and the cystic fibrosis airway. Thorax. 2012;67(5):465–467. [DOI] [PubMed] [Google Scholar]
- 21. Loo VG, Lalonde RG. Role of iron in intracellular growth of Trypanosoma cruzi. Infect Immun. 1984;45(3):726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lalonde RG, Holbein BE. Role of iron in Trypanosoma cruzi infection of mice. J Clin Invest. 1984;73(2):470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brandel J, Humbert N, Elhabiri Met al. Pyochelin, a siderophore of Pseudomonas aeruginosa: physicochemical characterization of the iron(III), copper(II) and zinc(II) complexes. Dalton Trans. 2012;41(9):2820–2834. [DOI] [PubMed] [Google Scholar]
- 24. Braud A, Hannauer M, Mislin GLA, Schalk IJ. The Pseudomonas aeruginosa pyochelin-iron uptake pathway and its metal specificity. J Bacteriol. 2009;191(11):3517–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Britigan BE, Roeder TL, Rasmussen GTet al. Interaction of the Pseudomonas aeruginosa secretory products pyocyanin and pyochelin generates hydroxyl radical and causes synergistic damage to endothelial cells. Implications for Pseudomonas-associated tissue injury. J Clin Invest. 1992;90(6):2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Britigan BE, Rasmussen GT, Cox CD. Augmentation of oxidant injury to human pulmonary epithelial cells by the Pseudomonas aeruginosa siderophore pyochelin. Infect Immun. 1997;65(3):1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ong KS, Cheow YL, Lee SM. The role of reactive oxygen species in the antimicrobial activity of pyochelin. J Adv Res. 2017;8(4):393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hassan HM, Fridovich I. Mechanism of the antibiotic action pyocyanine. J Bacteriol. 1980;141(1):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valenzuela L, Sepúlveda S, Ponce Iet al. The overexpression of TcAP1 endonuclease confers resistance to infective Trypanosoma cruzi trypomastigotes against oxidative DNA damage. J Cell Biochem. 2018;119(7):5985–5995. [DOI] [PubMed] [Google Scholar]
- 30. Finzi JK, Chiavegatto CW, Corat KFet al. Trypanosoma cruzi response to the oxidative stress generated by hydrogen peroxide. Mol Biochem Parasitol. 2004;133(1):37–43. [DOI] [PubMed] [Google Scholar]
- 31. Déziel E, Gopalan S, Tampakaki APet al. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol Microbiol. 2005;55(4):998–1014. [DOI] [PubMed] [Google Scholar]
- 32. Smith RS. Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. 2003;6(1):56–60. [DOI] [PubMed] [Google Scholar]
- 33. Pier GB. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int J Med Microbiol. 2007;297(5):277–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Villalta F, Kierszenbaum F. Enhanced multiplication of intracellular (amastigote) stages of Trypanosoma cruzi in vitro. J Protozool. 1984;31(3):487–489. [DOI] [PubMed] [Google Scholar]
- 35. Nwaka S, Hudson A. Innovative lead discovery strategies for tropical diseases. Nat Rev Drug Discov. 2006;5(11):941–955. [DOI] [PubMed] [Google Scholar]


