Abstract
The Human Immunodeficiency Virus (HIV-1) infection remains a persistent predicament for the State of Texas, ranking seventh among the most documented HIV cases in the United States. In this regard, the Rio Grande Valley (RGV) in South Texas is considered as one of the least investigated areas of the state with respect to HIV infection and HIV associated comorbidities. Considering the 115% increase in average HIV incidence rates per 100,000 within the RGV from 2007-2015, it is worth characterizing this population with respect to their HIV-1 infection, HIV-1 Associated Neurocognitive Disorders (HAND), and the association of treatment with combined antiretroviral therapy (cART). Moreover, the increased rate of Type-2 Diabetes (T2D) in the RGV population is intertwined with that of HIV-1 infection facing challenges due to the lack of knowledge about prevention to inadequate access to healthcare. Hence, the role of T2D in the development of HAND among the people living with HIV (PLWH) in the RGV will be reviewed to establish a closer link between T2D and HAND in cART-treated patients of the RGV.
Keywords: HIV-1, T2D, Hispanics, cART, HAND, diabetes, incidence rate, prevalence rate, CNS, Brain, MoCA, MMSE
1. INTRODUCTION
1.1. Prevalence and Incidence Rates of HIV-1 in the RGV
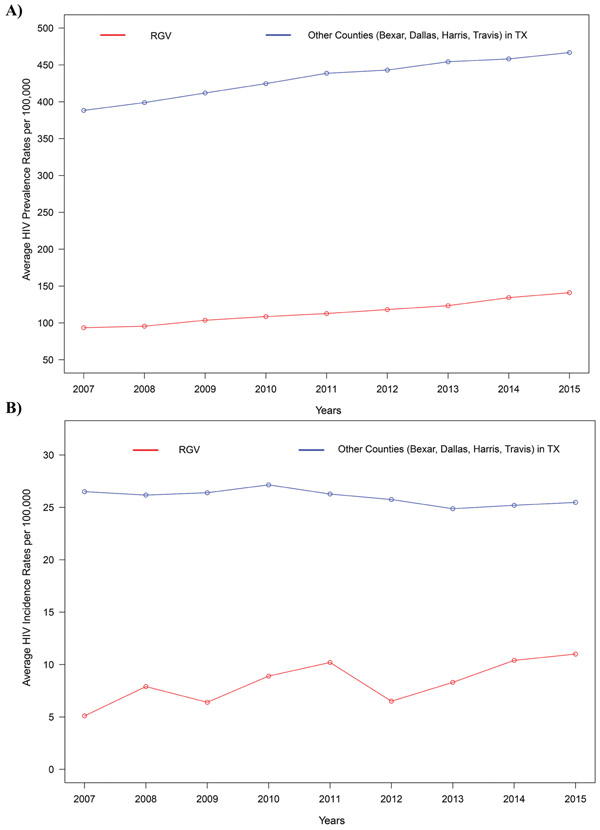
HIV-1 infection has been a concern for Texas, now ranking seventh in the US with the most HIV documented cases [1]. Geographically, the RGV consists of four major counties (Starr, Hidalgo, Willacy, and Cameron) in the southeastern part of Texas on the Texas and Mexico border area. It has gained attention because of its exponentially increasing Hispanic population that now exceeds 1.4 million. Moreover, one-third of RGV’s population live under the federal poverty line, which limits their access to adequate health care service [2]. We have investigated the incidence rate of HIV-1 infection (2007-2015) and T2D change (2004-2013) in the RGV compared to four other highly populated counties in Texas, namely Bexar, Dallas, Harris, and Travis (Fig. 1). Our independent analysis from the Texas Department of State Health Services (TDSHS) and Texas State Library and Archives Commission records indicated that there is a significant increase in the average HIV-1 prevalence rate per 100,000 residents in the RGV over the years from 2007 to 2015 (p-value 0.0003) (Fig. 2A). In addition, there was a significant increase in the average HIV-1 incidence rates per 100,000 residents in the RGV over the years from 2007 to 2015 (p-value 0.0165) compared to other populated counties in Texas (Fig. 2B) [3-5]. Though the HIV-1 incidence rates are higher in the other four counties in Texas compared to the RGV, there was a clear upward trend in the HIV-1 incidence rates in the RGV in the last nine years. Although the RGV is less populous, the rate of HIV-1 infection is increasing at a faster rate in the RGV than the other counties [5].
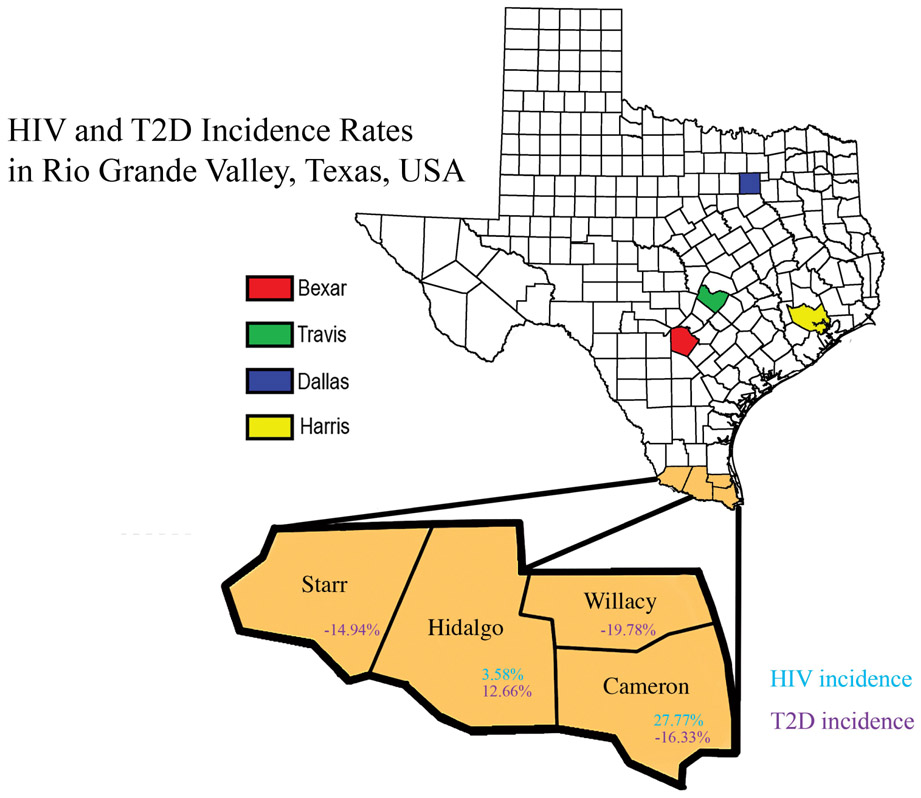
Fig. (1).
The map illustrates the different counties of the RGV’s HIV and T2D incidence rates. The percentage difference was calculated to analyze the increase in HIV incidence rates from 2007-2015 in the RGV. Hidalgo county had a 3.58% increase while Cameron County had 27.77%. In 2007 TDSHS HIV census reported zero new cases in Starr and Willacy County; therefore, no percentage difference was calculated. According to our statistical analysis, T2D incidence rate from 2004 to 2013 in Hidalgo County were increased by 12.66% whereas the incidence rates in Cameron, Willacy, and Starr counties were decreased.
Fig. (2).
(A) A trend analysis using the Mann-Kendall test was performed in order to investigate the change in the average HIV-1 prevalence rates per 100,000 in the RGV compared to four other highly populated counties in Texas namely Bexar, Dallas, Harris, and Travis during the period from 2007 to 2015. Both, average HIV-1 prevalence rates in the RGV and Other areas of Texas depict significantly increasing trends (p-value=0.0003) at a 5% level of significance. (B). A trend analysis using the Mann-Kendall test was performed in order to investigate the change in the average HIV-1 incidence rates per 100,000 in the RGV compared to four other highly populated counties in Texas namely Bexar, Dallas, Harris, and Travis during the period from 2007 to 2015. Average HIV-1 incidence rates in the RGV depicted a significantly increasing trend (p-value=0.0165) whereas other areas of Texas resulted in a significantly decreasing trend (p-value=0.0476) at a 5% level of significance. Data for this analysis was obtained from the Texas Department of State Health Services (https://www.dshs.texas.gov/records) and Texas State Library and Archives Commission (https://www.tsl.texas.gov/ref/abouttx/popcnty2010-11.html).
Considering the low socioeconomic status of the RGV, the poverty-stricken areas along the Texas-Mexico border known as colonias, and remote access to healthcare, make it very difficult to provide quality care to these patients [6]. Since HIV-1 infection causes a progressive decline of the human immune system, especially without combined antiretroviral treatment (cART), timely detection and adhering to the treatment are a necessity to avoid progression to fully developed AIDS and any further complications [7]. Therefore, understanding the HIV infection/AIDS in the RGV is important to provide access to HIV testing, HIV-disease prevention and early intervention services, pre- and post-exposure prophylaxis, as part of ending the HIV/AIDS epidemic in this region.
2. HIV ASSOCIATED NEUROCOGNITIVE DISORDER (HAND)
People living with HIV (PLWH) can now live an extended period provided they are optimally treated with cART. In spite of this remarkable success in the treatment outcome, mild and moderate forms of cognitive impairment have been observed in the patients who are progressively evolving towards a nuerobehavioral manifestation also known as HAND [8]. HAND can be subdivided into three subtypes: asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND) and HIV associated dementia (HAD) [9]. Various neurological symptoms have been identified in conjunction with HAND, early neurocognitive decline with impaired thinking, attention, concentration, memory, speed of information processing and judgment and associated extrapyramidal motor dysfunction can also be found [10-12]. The immunopathogenesis of HAND is associated with HIV-1 infected monocytes and T-cells infecting microglia of the brain resulting in a cascade of proinflammatory cytokines such as Tumor Necrosis Factor-alpha (TNF) and interleukins (ILs) that eventually activate other resident microglia and astrocytes [13].
Neuroimaging of HAND patients reveal generalized atrophy consisting of enlarged ventricles and widened sulci. On autopsy, pathological features included a reduction in the number of neurons, abnormal dendritic arborization, and neuronal apoptosis [14, 15]. Toxins implicated in this process include quinolinic acid, arachidonic acid metabolites, and others. Quinolinic acid has been of special interest because it interacts with HIV protein gp120 to bind with the NMDA receptor, which results in receptor neurotoxicity [16-18]. While it has been established that HIV Tat and Rev protein are neurotoxic, actual studies linking HIV viral load to HAND are lacking [19, 20] TNF-alpha may play a pivotal role in the production of neurotoxic metabolites as it has been shown to stimulate gliosis as well as independently altering neuronal function by direct toxicity to oligodendrocytes in vitro [21-23]. Besides, it stimulates mononuclear phagocytes and astrocytes to produce several neurotoxins, which include quinolinic acid and nitric oxide. It also potentially increases HIV viral load which would indirectly further compromise the remaining Central Nervous System (CNS) function [24, 25].
3. ASSOCIATED COMORBID NEUROCOGNITIVE CHANGES
The effects of the added burden in the form of diabetes are of great interest with HIV infection since they likely impact overlapping neuropathogenesis and impact CNS pathologies and disease progression for both disease states [26, 27]. Inflammatory cytokines linked either directly or indirectly to HIV neurotoxicity include TNF-alpha, Interleukin - 1 (IL-1), interferongamma (IFN-gamma), and IL-6. These cytokines act to stimulate predominately mononuclear phagocytes and astrocytes as well as to generate other cytokines and inflammatory precursors such as eicosanoids, nitric oxide, quinolinic acid, and superoxides [28]. TNF-alpha, IL-1, and IL6 potentiate HIV replication, while HIV stimulates the production of these cytokines [29, 30].
When diabetes is present along with features of HAND, neuropsychological testing has given support to the theory that diabetes may hasten or worsen HIV related cognitive dysfunction [31]. It has been established that many of the described pathological abnormalities associated with HAND are also associated with diabetes [32, 33]. Therefore, HAND and dementia have also been frequently attributed to diabetes [34, 35]. A fully developed dementia due to the complications of diabetes reveals neurocognitive deficits and frontal lobe dysfunction overlapping with those found in HAND [36-39]. Comparative imaging reveals similar degrees of generalized atrophy predominately in the frontal lobes, and cerebral functional imaging reveals gray matter abnormalities in frontal regions [40]. Finally, pathological studies indicated a similar pattern of neuronal loss, dendritic arborization abnormalities, and frontal cortex neuronal dropout. More recently, insulin deficiency and insulin resistance have been associated with pathological abnormalities akin to Alzheimer’s disease type neurodegeneration [41]. This is similar to the neurotoxic cytokine cascade reported for HAND and has led to the speculation that Alzheimer’s disease is “type 3 diabetes” [42-46].
While cytokines have been implicated in the neuropathogenesis of both diabetes and HAND, relatively little work has been done regarding the interactive effects of both on the immune system of the CNS. Evidence that diabetes dysregulates the immune system is quite convincing; however, diabetes can also alter the native cytokine levels, making them presumably more susceptible to neurocognitive impairment [47, 48]. The estimated prevalence of T2D in the USA is 12.3%, while in the RGV, it is 30.7 %. Considering the fact that the RGV population is disproportionately affected, we hypothesize that HIV infection in the RGV is prone to develop or progress to T2D much faster or earlier during their lifetime [49]. Therefore, the root cause of this incidence needs to be investigated for proposing a better treatment option.
4. DEVELOPMENT OF HAND THROUGH cART THERAPY
Early intervention with cART has improved the lifespan of infected patients by suppressing their viral load [50]. Yet, HIV remains latent in the CNS, causing neurobehavioral impairment even with adequate cART prophylaxis [51, 52]. The pathogenesis of cognitive impairment during HIV infection is still not completely understood [53]. Previous studies have indicated that certain classes of anti-HIV drugs cannot cross the Blood-Brain Barrier (BBB) therefore, facilitating a viral reservoir for neuroinflammatory changes that can potentially lead to HAND [54-56]. Viral reservoir formation and related neuroinflammation are a significant impediment to treating HAND [57]. Moreover, some of the existing drug combinations like Efavirenz / Emtricitabine / Tenofovir have shown brain and other organ-related toxicities in patients [58, 59]. To overcome this issue, new drug combinations have been modeled like Emtricitabine / Rilpivirine / Tenofovir and have proven to be better tolerated [60, 61]. Nonetheless, it has not been investigated how far the new class of drugs can help in eliminating the residual viral reservoir in the brain and improve the neurocognitive dysfunction in treated patients when compared to other classes of cART drugs [62].
Over 50% of HIV infected patients, including those with well-controlled systemic infection, commonly experience memory problems, depression, psychomotor slowing, difficulties in concentration, planning, and multitasking are clinically consistent with HAND [63]. The deposition of Aβ plaques is also a common pathological feature of HIV infection [64]. However, it is not clear whether this accumulation is due to AD-like processes, HIV-associated immunosuppression, Tat protein-induced Aβ elevations, and/or the effects of cART. Published studies suggest that cART has played a significant role in cART induced neurological disorders that, in turn, developed into HAND [65-67]. cART formularies have a wide range of toxic effects on neurons, a study comparing two cART formularies showed a significant degeneration of MAP-2 neurons, after being treated with Efavirenz and Atazanavir [68].
5. TESTING FOR HAND
Neuropsychological testing helps delineate the early stages of HIV-related neurocognitive impairment and/or cART regimens. However, the various screening tools that have been developed for detecting HAND lack sensitivity and specificity, making them unreliable measures of HIV-CNS disease [69]. A recent study in the USA and South Africa compared the Montreal Cognitive Assessment (MoCA) sensitivity and specificity to the Mini-Mental state examination (MMSE) in patients already diagnosed with HAND. The MMSE was originally developed to diagnose cortical dementia and is the most widely used in cognitive impairment screening instrument [70]. Results showed the MMSE with a sensitivity of 23.81% and a specificity of 97.96%, while MoCA had a sensitivity of 89.32 % and specificity of 22.45% [71]. MMSE poses a clinically challenging task as it requires several other factors including level education, as well as age that moderately affect the MMSE score in older adults. While others have mentioned adjusting scores for age and education fails to identify people in the early stages of HAND through MMSE [72, 73]. MoCA is a validated cognitive screening test that was developed in response to the poor sensitivity and high rate of producing false negatives of the MMSE [69, 74]. The MoCA test is advantageous when compared to other neurocognitive screening tools because it assesses attention, motor skills, language, orientation, and short-term memory with high sensitivity. Moreover, it is available in Spanish and is less of a test burden on patients making it more acceptable. Advantageously, the MoCA is also more promising for populations with low literacy rates [71, 75]. The Spanish version of MoCA has maintained adequate psychometric properties for its target population <6 years of education level [76]. On the contrary, MoCA has also shown no significant correlation between cognitive scores with lower educational levels among Hispanics in a separate study [77]. In addition, ethnicity and cultural differences in cognitive scores have been observed among HIV+African-American, Hispanic, Asian, and Caucasian (non-Hispanic) populations within the USA, respectively [78]. In this regard, MoCA has been tested on the Hispanic population with Alzheimer’s disease but is still not a widely accepted method for testing cognitive status in minority populations [79].
6. PREVALENCE AND INCIDENCE RATES OF T2D IN THE RIO GRANDE VALLEY
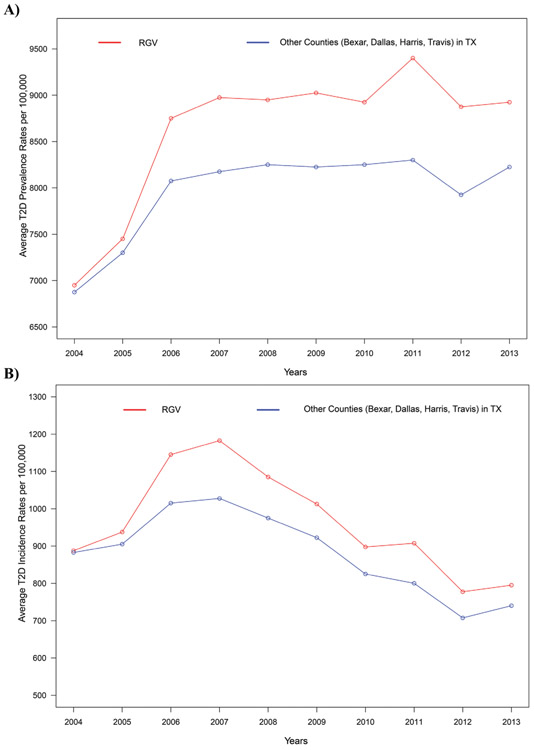
T2D is a metabolic disease in which blood glucose levels are abnormally high (hyperglycemia) due to an inadequate secretion of insulin made by the beta cells (β) [80]. The overall prevalence of diagnosed diabetes in the RGV has increased up to 30.7%, which is much higher than the US national average of 12.3% [49]. In our study, we have performed trend analyses using the Mann-Kendall (MK) test [81-83]. MK test statistically assesses whether there is a consistent increase (or decrease) over time. However, MK test is not well suited when there are alternative increasing and decreasing trends. Our independent analysis of average T2D prevalence rate in other areas of Texas does not show any significant trend for the period of 2007-2013 (p-value 1.0000) (The timeline was selected from the year 2007 to 2013 to be consistent with the trend analysis for the T2D incidence rate due to the above limitation in the MK test). Although the prevalence of T2D is significantly higher in the RGV than the other populated counties of Texas, no such monotonic changes were observed in average T2D cases (p-value: 0.4475, Fig. 3A) from 2007 to 2013. Nonetheless, from the year 2010 to 2011, there was a 13% increase in the T2D prevalence rate in the Hidalgo County in the RGV, creating a significant increase in the average T2D prevalence rate in the RGV as observed in Fig. (3A). According to our analysis, we can see that even though the population in the RGV is less than the four major counties of Texas [84], the rate of T2D is significantly higher than the rest of the states. As per Fig. (3B), we can see increasing trends in average T2D incidence rates from the year 2004 to 2007 and decreasing trends afterwards, both in the RGV and other areas of Texas. Our independent analysis of T2D incidence rates for other areas of Texas supports the claim that there is a decreasing trend (p-value:0.0069) in T2D incidence rates from the year 2007 to 2013. We also observe a decreasing trend in the average T2D incidence rates in the RGV (p-value:0.0163) from 2007 to 2013. However, the average T2D incidence rate in the RGV has decreased only by 10% from 2004 to 2013.
Fig. (3).
(A) Shows the average T2D prevalence rates per 100,000 in the RGV compared to four other counties in Texas namely Bexar, Dallas, Harris, and Travis for 2004 to 2013. The MK trend analysis did not show a significant trend in other areas of Texas (p-value: 1.0000) within the period of 2007-2013. Please note that that trend analysis was performed for a shorter time period than the graph depicts (from 2007 to 2013), as the MK test is not well suited when there was an alternating increasing and decreasing trend (as T2D incidence rates of RGV and other areas of Texas both increase from 2004-2006 and then decrease). Although the prevalence of T2D is significantly higher in the RGV than the other populated counties of Texas, a monotonic change was not observed in the average T2D rate (p-value: 0.4475, Fig. 3A) from 2007 to 2013. Nonetheless, from the year 2010 to 2011, there was a 13% increase in the T2D prevalence rate in the Hidalgo county in the RGV, creating a significant increase in the average T2D prevalence rate in the RGV as observed in Fig. (3A). (B), T2D incidence rates for other areas of Texas supports the claim that there is a decreasing trend (p-value: 0.0069) in T2D incidence rates from the year 2007 to 2013. A decreasing trend in the average T2D incidence rates in the RGV (p-value: 0.0163) was observed from 2007 to 2013. However, the average T2D incidence rate in the RGV has decreased only by 10% from 2004 to 2013.. Data for this analysis was obtained from the Centers for Disease Control and Prevention. (https://www.cdc.gov/diabetes/data/countydata/countydataindicators.html).
The connection between T2D diabetes and neurological disorders arises from various disease manifestations across different populations [85-89]. In addition, there is a strong correlation between HIV infection and T2D with respect to chronic immune activation, endothelial dysfunction, inflammation, and associated neurological disorders [90-93]. Therefore, it has been observed that there is a growing concern among patients with T2D to find the right line of treatment when they find themselves as HIV positive. Since T2D is associated with many other complications, treating these patients with the right combination of cART needs more collaborative approaches among clinicians in order to avoid multi-organs related complications [94, 95].
7. DEVELOPMENT OF T2D THROUGH cART THERAPY IN OTHER STUDIES
Recent studies suggest that cART regimens and an ongoing infection of HIV are highly associated with the development of metabolic disorders like T2D, dyslipidemia, and lipodystrophy. [96, 97]. Despite the sophistication in cART regimens, the prevalence of T2D has significantly increased compared to other HIV-1 associated co-morbidities. A cross-sectional study conducted by The Data Collection on Adverse events of HIV studied over 130,000 HIV-infected patients and the development of new-onset diabetes. The study concluded that 744 patients out of the population pool developed diabetes during the study (an incidence rate of 5.72 per 1,000) was attributed to the significant exposure of cART regimens for long periods of time [92, 94, 95, 98]. The mechanism of cART-induced hyperglycemia is still unknown. It is suggested by some studies that certain cART regimens are peptidomimetic inhibitors, therefore, these regimens are able to alter the production of pro-insulin to insulin leading to hyperglycemia [99]. It has also been observed that cART regimens have a direct impact on the GLUT-4 transporter. The inhibitory effects of the transporter protein lead to a decrease in insulin secretion [100]. Considering that a greater risk of developing T2D among PLWH from the RGV, developing a treatment model will be very crucial than any other population studied so far.
8. METHODOLOGY FOR INCIDENCE AND PREVALENCE RATE CALCULATION
The prevalence rates of HIV-1 infection: The number of people living with HIV for the years 2007 to 2015 was obtained from the Texas HIV Surveillance Report 2017 [5]. The county population per each year was obtained by the Texas State Library and Archives Commission [3, 4]. The prevalence rates for HIV-1 were calculated per each county for each year; (Number of people living with HIV-1/County population) 100,000. The average HIV-1 prevalence rates for the four counties: Cameron, Hidalgo, Starr, and Willacy were considered as the average HIV-1 prevalence rate of the RGV per each year. Similarly, the average HIV-1 prevalence rate of the four other populated counties: Bexar, Dallas, Harris, and Travis were considered as the average HIV-1 prevalence rate of these counties in Texas per each year. Fig. (2A) represents these two averages HIV-1 prevalence rates (named as the RGV and Other counties) per each year.
The incidence rates of HIV-1 infection: The HIV-1 incidence rates per 100,000 were obtained from the Texas HIV Surveillance Report 2017 [5]. The average HIV-1 incidence rate for the four counties: Cameron, Hidalgo, Starr, and Willacy were considered as the average HIV-1 incidence rate of the RGV per each year. Similarly, the average HIV-1 incidence rate of the four other populated counties: Bexar, Dallas, Harris, and Travis were considered as the average HIV-1 incidence rate of these Counties in Texas per each year. Fig. (2B) represents these two averages HIV-1 incidence rates (named as RGV and Other counties) per each year.
The prevalence rates of T2D: The T2D prevalence rates per 1000 were obtained from the Center for Disease Control and Prevention Data for the year of 2004-2013 [101]. The average T2D prevalence rate for the four counties: Cameron, Hidalgo, Starr, and Willacy were considered as the Average T2D prevalence rate of the RGV per each year. Similarly, the average T2D prevalence rate of the four other populated counties: Bexar, Dallas, Harris, and Travis were considered as the average of T2D prevalence rates of these Counties in Texas per each year. Finally, those values were multiplied by 100 to convert it to the rates per 100,000. Fig. (3A) represents these two average T2D prevalence rates (named as RGV and Other counties) per each year.
The incidence rates of T2D: The T2D incidence rates per 1000 were obtained from the Center for Disease Control and Prevention Data [102]. The average T2D incidence rate for the four counties: Cameron, Hidalgo, Starr, and Willacy were considered as the average T2D incidence rate of the RGV per each year. Similarly, the average of T2D incidence rate of the four other populated counties: Bexar, Dallas, Harris, and Travis was considered as the average T2D incidence rate of these Counties in Texas per each year. Finally, those values were multiplied by 100 to convert it to the rates per 100,000. Fig. (3B) represents these two average T2D incidence rates (named RGV and Other counties) per each year.
9. SUMMARY
In the early 2000s, the Hispanic community in the United States accounted for 12.5% of the population [103]. In 2005, the Hispanic population grew to 14.4%. In the same year, Hispanics comprised 18% of HIV cases [104, 105]. The HIV+ Hispanic patient population in the RGV presents significant challenges with regard to optimizing health status in triply and quadruply diagnosed patients. There are many factors that have been considered based on education and socioeconomic status. In this regard, it is worth to mention that as per American Community Survey Data obtained from 2013- 2017, the Rio Grande Valley has an average of 33.4% people living below the poverty line, and 61% of residents have obtained a high school education or higher [106]. Consequently, the low socioeconomic status of this community and remote access to healthcare make it very difficult to provide quality care to these patients. These are the other related factors in the development of both HAND and T2D including the use of cART treatment of these patients. Accessing proper healthcare is a significant issue among the RGV population. There are obvious delays in making the diagnosis of any major illness especially HIV/AIDS. Therefore, most of the time when the patients are detected with HIV, they are at the advanced stages of the disease with several other HIV associated comorbidities including T2D and HAND. Thus, having a robust HIV-testing and early intervention program providing effective treatment strategies is critical for this community. A focus on integrated, team collaborative care with wrap-around services for HIV/AIDS and T2D is essential.
For all persons in an HIV/AIDS treatment program, the benefits of integrated care are in combined comorbidities like cognitive changes, psychiatric disorders and diabetes. Ideally, HIV/AIDS and diabetes treatment programs would offer neurobehavioral assessments and early neuropsychiatric interventions for HAND and diabetes. Drug-drug interactions between ARV drug and hypoglycemic agents and prescribed psychotropics can increase or decrease the action of prescribed regimens. Thus, special attention must be given to potential drug-drug interactions in the triply-quadruply diagnosed patient. Whenever possible, ARV drug with high penetrance to the CNS may be used with the co-morbid neurocognitive disorder to improve neurocognitive impairment.
CONCLUSION
The majority of the RGV population are Mexican Americans with a significantly higher risk of developing T2D compared to any other studied population. However, even though T2D does affect HIV-1 infection and neurocognitive disorders, no separate cART treatment strategy has been designed for such patient populations in the RGV. Therefore, it's crucial to address the development of a treatment strategy for their HIV infection and other treatment-emergent comorbidities primarily. Such strategies will aid them to tackle the disease more effectively or will minimize the adverse effects of their cART regimen. Detecting and managing the treatment of HIV associated co-morbidities would increase the patient’s lifespan while mitigating any therapy complications.
ACKNOWLEDGEMENTS
The authors acknowledge Deepa Roy for carefully editing the manuscript. The authors also appreciate the publicly available data from the Texas Department of State Health Services record to formulate this review.
FUNDING
NIH/NINDS, (Grant No. R15NS108815-01(UR)) and authors also acknowledge the research support from the College of Health Professions at UTRGV.
LIST OF ABBREVIATIONS
- ARV
Anti-Retroviral
- ANI
Asymptomatic Neurocognitive Impairment
- BBB
Blood-Brain Barrier
- cART
Combined Antiretroviral Therapy
- CDC
Centers for Disease Control and Prevention
- CNS
Central Nervous System
- HAD
HIV Associated Dementia
- HAND
HIV Associated Neurocognitive Disorders
- HIV-1
Human Immunodeficiency Virus
- MMSE
Mini-Mental State Examination
- MND
Mild Neurocognitive Disorder
- MoCA
Montreal Cognitive Assessment
- PLWH
People Living with HIV
- RGV
Rio Grande Valley
- T2D
Type-2-Diabetes
- TDSHS
Texas Department of State Health Services
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Center for Disease Prevention. HIV Surveillance Report, 2016. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- [2].United States Census Bureau. Community Facts: Texas. 2016. https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml?src=bkmk
- [3].Texas State Library and Archive Commission. Population Estimates of Texas Counties, 2010-2017: Arranged in Alphabetical Order. 2018. https://www.tsl.texas.gov/ref/abouttx/popcnty201011.html
- [4].Texas State Library and Archive Commission. Population Estimates of Texas Counties, 2000-2009: Arranged in Alphabetical Order. 2011. https://www.tsl.texas.gov/ref/abouttx/popcnty201011.html
- [5].Texas Health and Human Services. Texas HIV Surveillance Report. 2016. 2016. https://www.dshs.texas.gov/hivstd/reports/HIVSurveillanceReport.pdf
- [6].Manusov EG, Diego VP, Smith J, et al. UniMóvil: A Mobile Health Clinic Providing Primary Care to the Colonias of the Rio Grande Valley, South Texas. Front Public Health 2019; 7: 215 [ 10.3389/fpubh.2019.00215] [PMID: 31497586] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Balasubramaniam M, Pandhare J, Dash C. Immune Control of HIV. J Life Sci (Westlake Village) 2019; 1(1): 4–37. [ 10.36069/JoLS/20190603] [PMID: 31468033] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumar S, Himanshu D, Tandon R, Atam V, Sawlani KK, Verma SK. Prevalence of HIV Associated Neurocognitive Disorder using Modified Mini Mental State Examination and its Correlation with CD4 Counts and Anti-retroviral Therapy. J Assoc Physicians India 2019; 67(4): 47–51. [PMID: 31299839] [PubMed] [Google Scholar]
- [9].Elbirt D, Mahlab-Guri K, Bezalel-Rosenberg S, Gill H, Attali M, Asher I. HIV-associated neurocognitive disorders (HAND). Isr Med Assoc J 2015; 17(1): 54–9. [PMID: 25739180] [PubMed] [Google Scholar]
- [10].Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis 2013; 13(11): 976–86. [ 10.1016/S1473-3099(13)70269-X] [PMID: 24156898] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Olivier IS, Cacabelos R, Naidoo V. Risk Factors and Pathogenesis of HIV-Associated Neurocognitive Disorder: The Role of Host Genetics. Int J Mol Sci 2018; 19(11): E3594 [ 10.3390/ijms19113594] [PMID: 30441796] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tierney S, Woods SP, Verduzco M, Beltran J, Massman PJ, Hasbun R. Semantic Memory in HIV-associated Neurocognitive Disorders: An Evaluation of the “Cortical” Versus “Subcortical” Hypothesis. Arch Clin Neuropsychol 2018; 33(4): 406–16. [ 10.1093/arclin/acx083] [PMID: 29028880] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 2015; 45: 1–12. [ 10.1016/j.bbi.2014.10.008] [PMID: 25449672] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adle-Biassette H, Levy Y, Colombel M, et al. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol 1995; 21(3): 218–27. [ 10.1111/j.1365-2990.1995.tb01053.x] [PMID: 7477730] [DOI] [PubMed] [Google Scholar]
- [15].Garden GA, Budd SL, Tsai E, et al. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci 2002; 22(10): 4015–24. [ 10.1523/JNEUROSCI.22-10-04015.2002] [PMID: 12019321] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Epstein LG. HIV neuropathogenesis and therapeutic strategies. Acta Paediatr Jpn 1998; 40(2): 107–11. [ 10.1111/j.1442-200X.1998.tb01892.x] [PMID: 9581298] [DOI] [PubMed] [Google Scholar]
- [17].Lipton SA. HIV-related neurotoxicity. Brain Pathol 1991; 1(3): 193–9. [ 10.1111/j.1750-3639.1991.tb00659.x] [PMID: 1669708] [DOI] [PubMed] [Google Scholar]
- [18].Pittaluga A, Pattarini R, Severi P, Raiteri M. Human brain N-methyl-D-aspartate receptors regulating noradrenaline release are positively modulated by HIV-1 coat protein gp120. AIDS 1996; 10(5): 463–8. [ 10.1097/00002030-199605000-00003] [PMID: 8724036] [DOI] [PubMed] [Google Scholar]
- [19].Eggers C, Arendt G, Hahn K, et al. HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J Neurol 2017; 264(8): 1715–27. [ 10.1007/s00415-017-8503-2] [PMID: 28567537] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schrack JA, Jacobson LP, Althoff KN, et al. Effect of HIV-infection and cumulative viral load on age-related decline in grip strength. AIDS 2016; 30(17): 2645–52. [ 10.1097/QAD.0000000000001245] [PMID: 27603294] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Planès R, Serrero M, Leghmari K, BenMohamed L, Bahraoui E. HIV-1 Envelope Glycoproteins Induce the Production of TNF-α and IL-10 in Human Monocytes by Activating Calcium Pathway. Sci Rep 2018; 8(1): 17215 [ 10.1038/s41598-018-35478-1] [PMID: 30464243] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pozniak PD, Darbinyan A, Khalili K. TNF-α/TNFR2 Regulatory Axis Stimulates EphB2-Mediated Neuroregeneration Via Activation of NF-κB. J Cell Physiol 2016; 231(6): 1237–48. [ 10.1002/jcp.25219] [PMID: 26492598] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rawat P, Teodorof-Diedrich C, Spector SA. Human immunodeficiency virus Type-1 single-stranded RNA activates the NLRP3 inflammasome and impairs autophagic clearance of damaged mitochondria in human microglia. Glia, 2019; 67(5): 802–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guha D, Wagner MCE, and Ayyavoo V, Human immunodeficiency virus type 1 (HIV1)-mediated neuroinflammation dysregulates neurogranin and induces synaptodendritic injury. J Neuroinflammation 2018; 15(1): 126. [PMID: 29703241] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Marks WD, Paris JJ, Schier CJ, et al. HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J Neurovirol 2016; 22(6): 747–62. [ 10.1007/s13365-016-0447-2] [PMID: 27178324] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim BH, Kelschenbach J, Borjabad A, et al. Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HIV-associated neurocognitive disorders in EcoHIV-infected mice. AIDS 2019; 33(6): 973–84. [ 10.1097/QAD.0000000000002150] [PMID: 30946151] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wu M, Fatukasi O, Yang S, et al. HIV disease and diabetes interact to affect brain white matter hyperintensities and cognition. AIDS 2018; 32(13): 1803–10. [ 10.1097/QAD.0000000000001891] [PMID: 29794829] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lovelace MD, et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017; 112(Pt B): 373–88. [ 10.1016/j.neuropharm.2016.03.024] [DOI] [PubMed] [Google Scholar]
- [29].Falasca F, Di Carlo D, De Vito C, et al. Evaluation of HIV-DNA and inflammatory markers in HIV-infected individuals with different viral load patterns. BMC Infect Dis 2017; 17(1): 581 [ 10.1186/s12879-017-2676-2] [PMID: 28830393] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hoel H, Ueland T, Knudsen A, et al. Soluble markers of IL-1 activation as predictors of first-time myocardial infarction in HIV-infected individuals. J Infect Dis 2019. [Epub ahead of print]. [ 10.1093/infdis/jiz253] [PMID: 31077280] [DOI] [PubMed] [Google Scholar]
- [31].Vance DE, Fazeli PL, Dodson JE, Ackerman M, Talley M, Appel SJ. The synergistic effects of HIV, diabetes, and aging on cognition: implications for practice and research. J Neurosci Nurs 2014; 46(5): 292–305. [ 10.1097/JNN.0000000000000074] [PMID: 25099061] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dufouil C, Richert L, Thiébaut R, et al. Diabetes and cognitive decline in a French cohort of patients infected with HIV-1. Neurology 2015; 85(12): 1065–73. [ 10.1212/WNL.0000000000001815] [PMID: 26156515] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012; 78(7): 485–92. [ 10.1212/WNL.0b013e3182478d64] [PMID: 22330412] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Roberts RO, Geda YE, Knopman DS, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol 2008; 65(8): 1066–73. [ 10.1001/archneur.65.8.1066] [PMID: 18695056] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Valcour VG, Shikuma CM, Shiramizu BT, et al. Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J Acquir Immune Defic Syndr 2005; 38(1): 31–6. [ 10.1097/00126334-200501010-00006] [PMID: 15608521] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Velayudhan L, Poppe M, Archer N, Proitsi P, Brown RG, Lovestone S. Risk of developing dementia in people with diabetes and mild cognitive impairment. Br J Psychiatry 2010; 196(1): 36–40. [ 10.1192/bjp.bp.109.067942] [PMID: 20044657] [DOI] [PubMed] [Google Scholar]
- [37].Danna SM, Graham E, Burns RJ, Deschênes SS, Schmitz N. Association between Depressive Symptoms and Cognitive Function in Persons with Diabetes Mellitus: A Systematic Review. PLoS One 2016; 11(8): e0160809 [ 10.1371/journal.pone.0160809] [PMID: 27526176] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci 2015; 1353: 60–71. [ 10.1111/nyas.12807] [PMID: 26132277] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Palta P, Carlson MC, Crum RM, et al. Diabetes and Cognitive Decline in Older Adults: The Ginkgo Evaluation of Memory Study. J Gerontol A Biol Sci Med Sci 2017; 73(1): 123–30. [ 10.1093/gerona/glx076] [PMID: 28510619] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Risacher SL, Saykin AJ. Neuroimaging biomarkers of neurodegenerative diseases and dementia. Semin Neurol 2013; 33(4): 386–416. [ 10.1055/s-0033-1359312] [PMID: 24234359] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol 2008; 2(6): 1101–13. [ 10.1177/193229680800200619] [PMID: 19885299] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Canet G, Dias C, Gabelle A, et al. HIV Neuroinfection and Alzheimer’s Disease: Similarities and Potential Links? Front Cell Neurosci 2018; 12: 307 [ 10.3389/fncel.2018.00307] [PMID: 30254568] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kandimalla R, Thirumala V, Reddy PH. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim Biophys Acta Mol Basis Dis 2017; 1863(5): 1078–89. [ 10.1016/j.bbadis.2016.08.018] [PMID: 27567931] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Leszek J, Trypka E, Tarasov VV, Ashraf GM, Aliev G. Type 3 Diabetes Mellitus: A Novel Implication of Alzheimers Disease. Curr Top Med Chem 2017; 17(12): 1331–5. [ 10.2174/1568026617666170103163403] [PMID: 28049395] [DOI] [PubMed] [Google Scholar]
- [45].Milanini B, Valcour V, Differentiating H. Differentiating HIV-Associated Neurocognitive Disorders From Alzheimer’s Disease: an Emerging Issue in Geriatric NeuroHIV. Curr HIV/AIDS Rep 2017; 14(4): 123–32. [ 10.1007/s11904-017-0361-0] [PMID: 28779301] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Salminen A, Kaarniranta K, Kauppinen A, et al. Impaired autophagy and APP processing in Alzheimer’s disease: The potential role of Beclin 1 interactome. Prog Neurobiol 2013; 106-107: 33–54. [ 10.1016/j.pneurobio.2013.06.002] [PMID: 23827971] [DOI] [PubMed] [Google Scholar]
- [47].Saedi E, Gheini MR, Faiz F, Arami MA. Diabetes mellitus and cognitive impairments. World J Diabetes 2016; 7(17): 412–22. [ 10.4239/wjd.v7.i17.412] [PMID: 27660698] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Umegaki H Type 2 diabetes as a risk factor for cognitive impairment: current insights. Clin Interv Aging 2014; 9: 1011–9. [ 10.2147/CIA.S48926] [PMID: 25061284] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Millard AV, Graham MA, Mier N, et al. Diabetes Screening and Prevention in a High-Risk, Medically Isolated Border Community. Front Public Health 2017; 5: 135 [ 10.3389/fpubh.2017.00135] [PMID: 28660184] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8(12): e81355 [ 10.1371/journal.pone.0081355] [PMID: 24367482] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Marban C, Forouzanfar F, Ait-Ammar A, et al. Targeting the Brain Reservoirs: Toward an HIV Cure. Front Immunol 2016; 7: 397 [ 10.3389/fimmu.2016.00397] [PMID: 27746784] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Roy U, Bulot C, Honer zu Bentrup K, Mondal D. Specific increase in MDR1 mediated drug-efflux in human brain endothelial cells following co-exposure to HIV-1 and saquinavir. PLoS One 2013; 8(10): e75374 [ 10.1371/journal.pone.0075374] [PMID: 24098380] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kanmogne GD, Singh S, Roy U, et al. Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nano-formulated antiretroviral drugs to human brain endothelial cells. Int J Nanomedicine 2012; 7: 2373–88. [ 10.2147/IJN.S29454] [PMID: 22661891] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Grant I Neurocognitive disturbances in HIV. Int Rev Psychiatry 2008; 20(1): 33–47. [ 10.1080/09540260701877894] [PMID: 18240061] [DOI] [PubMed] [Google Scholar]
- [55].Robinson-Papp J, Byrd D, Mindt MR, Oden NL, Simpson DM, Morgello S. Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Arch Neurol 2008; 65(8): 1096–101. [ 10.1001/archneur.65.8.1096] [PMID: 18695060] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS 2011; 25(3): 357–65. [ 10.1097/QAD.0b013e32834171f8] [PMID: 21124201] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Herskovitz J, Gendelman HE. HIV and the Macrophage: From Cell Reservoirs to Drug Delivery to Viral Eradication. J Neuroimmune Pharmacol 2019; 14(1): 52–67. [ 10.1007/s11481-018-9785-6] [PMID: 29572681] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ibarra-Barrueta O, Palacios-Zabalza I, Mora-Atorrasagasti O, Mayo-Suarez J. Effect of concomitant use of montelukast and efavirenz on neuropsychiatric adverse events. Ann Pharmacother 2014; 48(1): 145–8. [ 10.1177/1060028013510396] [PMID: 24259633] [DOI] [PubMed] [Google Scholar]
- [59].Scourfield A, Zheng J, Chinthapalli S, et al. Discontinuation of Atripla as first-line therapy in HIV-1 infected individuals. AIDS 2012; 26(11): 1399–401. [ 10.1097/QAD.0b013e328353b047] [PMID: 22441251] [DOI] [PubMed] [Google Scholar]
- [60].De Clercq E Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem Pharmacol 2016; 119: 1–7. [ 10.1016/j.bcp.2016.04.015] [PMID: 27133890] [DOI] [PubMed] [Google Scholar]
- [61].Lamorde M, Byakika-Kibwika P, Tamale WS, et al. Effect of Food on the Steady-State Pharmacokinetics of Tenofovir and Emtricitabine plus Efavirenz in Ugandan Adults. Aids Res Treat 2012; 2012: 105980 [ 10.1155/2012/105980] [PMID: 22454762] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rilpivirine Ogbuagu O., emtricitabine and tenofovir alafenamide: single-tablet combination for the treatment of HIV-1 infection in selected patients. Expert Rev Anti Infect Ther 2016; 14(12): 1113–26. [ 10.1080/14787210.2016.1255551] [PMID: 27797606] [DOI] [PubMed] [Google Scholar]
- [63].Goodkin K, Fletcher MA, Cohen N. Clinical aspects of psychoneuroimmunology. Lancet 1995; 345(8943): 183–4. [ 10.1016/S0140-6736(95)90180-9] [PMID: 7823677] [DOI] [PubMed] [Google Scholar]
- [64].Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry 1998; 65(1): 29–33. [ 10.1136/jnnp.65.1.29] [PMID: 9667557] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hui L, Ye Y, Soliman ML, et al. Antiretroviral Drugs Promote Amyloidogenesis by De-Acidifying Endolysosomes. J Neuroimmune Pharmacol 2019. [ 10.1007/s11481-019-09862-1] [PMID: 31338753] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiatry 2000; 69(3): 376–80. [ 10.1136/jnnp.69.3.376] [PMID: 10945813] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P. HIV-1 infection and cognitive impairment in the cART era: a review. AIDS 2011; 25(5): 561–75. [ 10.1097/QAD.0b013e3283437f9a] [PMID: 21160410] [DOI] [PubMed] [Google Scholar]
- [68].Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol 2012; 18(5): 388–99. [ 10.1007/s13365-012-0120-3] [PMID: 22811264] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Valcour VG. Evaluating cognitive impairment in the clinical setting: practical screening and assessment tools. Top Antivir Med 2011; 19(5): 175–80. [PMID: 22298886] [PMC free article] [PubMed] [Google Scholar]
- [70].Oshinaike OO, Akinbami AA, Ojo OO, Ojini IF, Okubadejo UN, Danesi AM. Comparison of the Minimental State Examination Scale and the International HIV Dementia Scale in Assessing Cognitive Function in Nigerian HIV Patients on Antiretroviral Therapy. Aids Res Treat 2012; 2012; 581531 [ 10.1155/2012/581531] [PMID: 23050130] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Joska JA, Witten J, Thomas KG, et al. A Comparison of Five Brief Screening Tools for HIV-Associated Neurocognitive Disorders in the USA and South Africa. AIDS Behav 2016; 20(8): 1621–31. [ 10.1007/s10461-016-1316-y] [PMID: 26860536] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Monroe T, Carter M. Using the Folstein Mini Mental State Exam (MMSE) to explore methodological issues in cognitive aging research. Eur J Ageing 2012; 9(3): 265–74. [ 10.1007/s10433-012-0234-8] [PMID: 28804426] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yoelin AB, Saunders NW. Score Disparity Between the MMSE and the SLUMS. Am J Alzheimers Dis Other Demen 2017; 32(5): 282–8. [ 10.1177/1533317517705222] [PMID: 28503934] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kang IW, Beom IG, Cho JY, Son HR. Accuracy of Korean-Mini-Mental Status Examination Based on Seoul Neuro-Psychological Screening Battery II Results. Korean J Fam Med 2016; 37(3): 177–81. [ 10.4082/kjfm.2016.37.3.177] [PMID: 27274389] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53(4): 695–9. [ 10.1111/j.1532-5415.2005.53221.x] [PMID: 15817019] [DOI] [PubMed] [Google Scholar]
- [76].Zhou Y, Ortiz F, Nuñez C, et al. Use of the MoCA in Detecting Early Alzheimer’s Disease in a Spanish-Speaking Population with Varied Levels of Education. Dement Geriatr Cogn Disord Extra 2015; 5(1): 85–95. [ 10.1159/000365506] [PMID: 25873930] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mondragón JD, Celada-Borja C, Barinagarrementeria-Aldatz F, Burgos-Jaramillo M, Barragán-Campos HM. Hippocampal Volumetry as a Biomarker for Dementia in People with Low Education. Dement Geriatr Cogn Disord Extra 2016; 6(3): 486–99. [ 10.1159/000449424] [PMID: 27920792] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Boone KB, Victor TL, Wen J, Razani J, Pontón M. The association between neuropsychological scores and ethnicity, language, and acculturation variables in a large patient population. Arch Clin Neuropsychol 2007; 22(3): 355–65. [ 10.1016/j.acn.2007.01.010] [PMID: 17320344] [DOI] [PubMed] [Google Scholar]
- [79].Milani SA, Marsiske M, Striley CW. Discriminative Ability of Montreal Cognitive Assessment Subtests and Items in Racial and Ethnic Minority Groups. Alzheimer Dis Assoc Disord 2019; 33(3): 226–32. [ 10.1097/WAD.0000000000000310] [PMID: 31058685] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005; 365(9467): 1333–46. [ 10.1016/S0140-6736(05)61032-X] [PMID: 15823385] [DOI] [PubMed] [Google Scholar]
- [81].Gibert RO. Statistical Methods for Environmental Pollution Monitoring. Wiley, NY, 1987. [Google Scholar]
- [82].Kendall MG. Rank Correlation Methods. Charles Griffin, London, 1975. [Google Scholar]
- [83].Mann HB. Non-parametric tests against trend. Econometrica 1945; 13: 163–71. [ 10.2307/1907187] [DOI] [Google Scholar]
- [84].Center for Disease Control and Prevention. County Data Indicators. Diagnosed Diabetes Incidence. 2013. https://www.cdc.gov/diabetes/data/countydata/countydataindicators.html
- [85].Grigolon RB, Brietzke E, Mansur RB, et al. Association between diabetes and mood disorders and the potential use of anti-hyperglycemic agents as antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 2019; 95: 109720 [ 10.1016/j.pnpbp.2019.109720] [PMID: 31352032] [DOI] [PubMed] [Google Scholar]
- [86].Hajek T, Calkin C, Blagdon R, Slaney C, Alda M. Type 2 diabetes mellitus: a potentially modifiable risk factor for neurochemical brain changes in bipolar disorders. Biol Psychiatry 2015; 77(3): 295–303. [ 10.1016/j.biopsych.2013.11.007] [PMID: 24331546] [DOI] [PubMed] [Google Scholar]
- [87].Khan NM, Ahmad A, Tiwari RK, Kamal MA, Mushtaq G, Ashraf GM. Current challenges to overcome in the management of type 2 diabetes mellitus and associated neurological disorders. CNS Neurol Disord Drug Targets 2014; 13(8): 1440–57. [ 10.2174/1871527313666141023160448] [PMID: 25345504] [DOI] [PubMed] [Google Scholar]
- [88].Zhang D, Shi L, Song X, et al. Neuroimaging endophenotypes of type 2 diabetes mellitus: a discordant sibling pair study. Quant Imaging Med Surg 2019; 9(6): 1000–13. [ 10.21037/qims.2019.05.18] [PMID: 31367554] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhou Z, Zhu Y, Liu Y, Yin Y. Comprehensive transcriptomic analysis indicates brain regional specific alterations in type 2 diabetes. Aging (Albany NY) 2019; 11(16): 6398–421. [ 10.18632/aging.102196] [PMID: 31449493] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Harrison ML, Wolfe AS, Fordyce J, Rock J, García AA, Zuñiga JA. The additive effect of type 2 diabetes on fibrinogen, von Willebrand factor, tryptophan and threonine in people living with HIV. Amino Acids 2019; 51(5): 783–93. [ 10.1007/s00726-019-02715-4] [PMID: 30868261] [DOI] [PubMed] [Google Scholar]
- [91].Hove-Skovsgaard M, Gaardbo JC, Kolte L, et al. HIV-infected persons with type 2 diabetes show evidence of endothelial dysfunction and increased inflammation. BMC Infect Dis 2017; 17(1): 234 [ 10.1186/s12879-017-2334-8] [PMID: 28356058] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Masenga SK, Toloka P, Chiyenu K, et al. Type 2 diabetes mellitus prevalence and risk scores in treated PLWHIV: a cross-sectional preliminary study. BMC Res Notes 2019; 12(1): 145 [ 10.1186/s13104-019-4183-6] [PMID: 30876484] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Prioreschi A, Munthali RJ, Soepnel L, et al. Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: a systematic review and meta-analysis. BMJ Open 2017; 7(3): e013953 [ 10.1136/bmjopen-2016-013953] [PMID: 28360243] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ahmed D, Roy D, Cassol E. Examining Relationships between Metabolism and Persistent Inflammation in HIV Patients on Antiretroviral Therapy. Mediators Inflamm 2018; 2018: 6238978 [ 10.1155/2018/6238978] [PMID: 30363715] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lin SP, Wu CY, Wang CB, Li TC, Ko NY, Shi ZY. Risk of diabetes mellitus in HIV-infected patients receiving highly active antiretroviral therapy: A nationwide population-based study. Medicine (Baltimore) 2018; 97(36): e12268 [ 10.1097/MD.0000000000012268] [PMID: 30200166] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Aaron E, Alvare T, Gracely EJ, Riviello R, Althoff A. Predictors of Linkage to Care for Newly Diagnosed HIV-Positive Adults. West J Emerg Med 2015; 16(4): 535–42. [ 10.5811/westjem.2015.4.25345] [PMID: 26265965] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Paul R Neurocognitive Phenotyping of HIV in the Era of Antiretroviral Therapy. Curr HIV/AIDS Rep 2019; 16(3): 230–5. [ 10.1007/s11904-019-00426-9] [PMID: 31168712] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 2008; 31(6): 1224–9. [ 10.2337/dc07-2013] [PMID: 18268071] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yarasheski KE, Tebas P, Sigmund C, et al. Insulin resistance in HIV protease inhibitor-associated diabetes. J Acquir Immune Defic Syndr 1999; 21(3): 209–16. [ 10.1097/00126334-199907010-00005] [PMID: 10421244] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hruz PW. Molecular mechanisms for insulin resistance in treated HIV-infection. Best Pract Res Clin Endocrinol Metab 2011; 25(3): 459–68. [ 10.1016/j.beem.2010.10.017] [PMID: 21663839] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Center for Disease Prevention. Diagnosed Diabetes Prevelance. 2014. https://www.cdc.gov/diabetes/data/countydata/county-dataindicators.
- [102].Center for Disease Prevention. Diagnosed Diabetes Incidence. 2014: https://www.cdc.gov/diabetes/data/countydata/countydataindicators.html.
- [103].United States Census Bureau. Resident Population of the United States. 2002.
- [104].United States Census Bureau. Annual estimates of the population by sex, race and Hispanic or Latino origin for the United States: April 1, 2000. to July 1, 2006.
- [105].Center for Disease Prevention . HIV/AIDS Surveillance Report. 2005. http://www.cdc.gov/hiv/topics/surveillance/resources/reports
- [106].United States Census Bureau. Community Facts: Texas. 2017. https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml?src=bkmk