Abstract
Dried blood spots (DBS) are widely utilized as part of universal newborn screening and as a means of transporting samples from field sites. We use DBS from African field sites to assess for rare maternal-fetal cell exchange during pregnancy known as microchimerism. We aimed to develop a protocol to maximize the quantity of high-quality genomic DNA (gDNA) extracted from DBS. The total gDNA yield obtained from control DBS utilizing a Qiagen-based protocol and a Chelex® 100 resin-based protocol was first compared. Variations of the Chelex® protocol were subsequently tested to develop an optimized protocol. The gDNA was quantified by qPCR targeting the human beta-globin gene. DNA yield for a given experimental condition was normalized to a Chelex® control performed on the same day, and the total yields were compared using a Student’s t-test. The control Chelex® protocol yielded 590% more DNA than the QIAamp® DNA Blood Mini Kit . The absolute efficiency of the control Chelex® protocol was 54%, compared to an absolute efficiency of 9% for the QIAamp® DNA Blood Mini Kit. Modification of the Chelex® protocol to include a second heat precipitation from the same DBS increased the gDNA yield by 29% (P < 0.001). Our optimized protocol including this modification increased the absolute efficiency of extraction to 68%. The gDNA extracted using the Chelex® protocol was stable through repeated freeze–thaw cycles. In a mock microchimerism experiment, rare donor alleles at a frequency of 10 in 100 000 could be identified in gDNA from DBS extracted using the optimized Chelex® protocol. Our findings may be of significance for a diverse range of applications that utilize DBS and require high-quality DNA, including newborn screening programs, pathogen and drug resistance screening from remote field sites, forensics, and rare allele detection.
Introduction
Whole blood spotted onto filter paper provides a convenient method for collecting, transporting, and storing blood samples [1]. This approach is widely utilized as a part of universal newborn screening as well as a means of transporting samples from field sites [2]. Dried blood spots (DBS) are particularly common as a means of preserving whole-blood samples for study of pathogens, such as malaria and HIV, and drug resistance [2–6]. A variety of different filter papers are used for this purpose, including Whatman Grade 3 (GE Healthcare Lifesciences, Marlborough, USA) [4, 5] and S&S 903 (formerly Schleicher & Schuell; GE Healthcare Lifesciences, Marlborough, USA) [7], based on their availability and relative low cost, with recent papers designed specifically for the preservation of nucleic acids (e.g. FTA cards, GE Healthcare Lifesciences, Marlborough, USA) [6]). Whatman Grade 3 and S&S 903 filter papers passively absorb blood, whereas FTA paper relies on a matrix for nucleic acid capture and preservation.
Our lab investigates very rare maternal cells that traffic into a fetus during pregnancy, known as maternal microchimerism and the implication for offspring immunity and susceptibility to infectious disease [8]. These cells are generally present at levels between 1 in 100 000 to 10 in 100 000 genomic equivalents (gEq). Several methods have been utilized to extract genomic DNA (gDNA) from DBS, including standard kit-based protocols (e.g. QIAamp® DNA Blood Mini Kit and QIAamp® DNA Micro Kit) [9–12] and Chelex® 100 resin-based protocols [2, 3, 9–13], however, the sensitivity of these methods to detect very rare alleles has not been investigated. In order to utilize historical DBS collected from African field sites in the study of maternal microchimerism, we aimed to develop an optimized protocol for the extraction of the maximum quantity of high-quality gDNA from DBS.
Materials and methods
Human subjects
This study was approved by the Seattle Children’s IRB and written informed consent was obtained from all participants.
DBS generation
Blood from two anonymous donors (Donor A and Donor B) was collected into ethylenediaminetetraacetic acid (EDTA) tubes (BD, Franklin Lakes, USA) and a complete blood count was obtained for each donor. For the DBS used in protocol optimization, 20 µl of fresh whole blood from Donor A was spotted onto Whatman Grade 3 Filter Paper (GE Healthcare Life Sciences Catalogue Number 1003-090). DBS were dried for a minimum of 20 days at room temperature prior to extraction. The entire 20 µl DBS were subsequently cut from filter paper using sterile scissors and placed into 1.5 ml LoBind Eppendorf tubes.
DBS extraction
Commercial kit-based extraction
DBS were extracted using a QIAamp® DNA Blood Mini Kit (QIAGEN Catalogue Number 51104) according to the manufacturer’s recommended instructions (‘DNA Purification from Dried Blood Spots’, 2016) with slight modification (hereafter referred to as ‘Qiagen protocol’): 50 µl of molecular-grade water was added to the column to elute gDNA and allowed to incubate for 5 min. The column was then placed in a 1.5 ml LoBind Eppendorf tube (‘1st elution’) and centrifuged to collect the eluate. A second 50 µl elution of molecular-grade water was then added to the column, and the process was repeated (‘2nd elution’). To determine whether any additional gDNA could be recovered from the processed DBS, samples were taken through the Qiagen protocol a second time, again with a double elution.
Chelex-resin based extraction
We developed a control Chelex® 100 resin-based protocol similar to prior work (hereafter referred to as ‘Chelex® control protocol’) [2], against which the Qiagen protocol and our Chelex® 100 resin-based protocol modifications were compared. Because saponin, a plant-based detergent, may have batch to batch variability and is not widely available in molecular grade form (particularly important for microchimerism studies), we chose to use Tween® 20 as the detergent in our control condition.
One ml of freshly made 0.5% Tween® 20 detergent diluted in PBS was added to each tube containing a DBS, inverted three times and incubated at 4°C overnight. The next morning the supernatant was removed, 1 ml of fresh PBS was added and the tubes were inverted three times. Tubes were then incubated at 4°C for 30 min. During this time, a solution of 5% (m/v) 50–100 mesh Chelex® resin in molecular grade water was heated to 95°C. The PBS wash was fully removed and 200 µL of the pre-heated 5% Chelex solution was added to the samples. The samples were vortexed for 30 s, then incubated at 95°C for 15 min with gentle vortexing every 5 min. The tubes were then centrifuged for 3 min at 21 130 g to pellet the Chelex® beads and any degraded paper. The supernatant (approximately 180 µl) containing the eluted gDNA was transferred to a new 1.5 ml LoBind Eppendorf tube. The eluate was then centrifuged again for 3 min as above and 150 µl was removed and transferred to a final 1.5 ml LoBind Eppendorf tube for use or storage. These two centrifugation steps are essential to ensure that there is no carryover of Chelex® into the final eluate; residual Chelex® may bind Mg2+ required for polymerase function and therefore inhibit downstream PCR applications. The control condition was independently run for each experiment detailed below to control for variability in age of the DBS and reagent preparation on any given day. Five replicates were tested for each condition, and all conditions for a given protocol variation were run on the same day.
Detergent
Saponin has been traditionally used in many DBS extraction protocols and is a biologically derived detergent which selectively lyses the cell membrane via cholesterol interactions [14]. As potential alternatives, both Tween® 20 and Triton™ X-100 are widely available, molecular grade, nonionic, non-denaturing detergents. However, Triton™ X-100 may lead to a higher degree of permeabilization [14]. We compared the effect of 0.5% Saponin (Acros Organics Catalog No. AC419231000), 0.5% Tween® 20 (Fisher Bioreagents Catalog No. BP337-500) and 0.5% Triton™ X-100 (Fisher Bioreagents Catalog No. BP337-500) during the overnight incubation.
Chelex® 100 size
Chelex® resin comes in a variety of ‘mesh’ sizes, in which the mesh size is inversely proportional to the size of the resin beads. Small size (larger mesh) beads are easier to handle without clogging pipette tips; however, they are difficult to visualize to ensure no carryover in the final elution. We have found that the use of wide-bore pipette tips (Rainin Catalog No. 30389241) significantly improves the ease of use of the large size Chelex® resin. In order to test the effect of Chelex® resin size, we compared 200–400 mesh Chelex® (Bio Rad Catalog No. 142-1253) with 50–100 mesh Chelex® (Bio Rad Catalog No. 142-2822).
56°C incubation
Some protocols include a 56–60°C incubation, with [12] or without [15, 16] the addition of Proteinase K, prior to the heat precipitation at 95°C. We therefore tested the effect of a 56°C incubation for 20 min with vortexing every 5 min before continuing with the 95°C precipitation outlined in the control protocol.
Elution buffer
Genomic DNA is most often eluted into molecular grade water, Tris, or Tris–EDTA. Molecular grade water is inexpensive, but Tris has the advantage of acting as a pH buffer, and Tris–EDTA has the advantage of acting as both a pH buffer and DNase inhibitor, although EDTA in high concentrations may have inhibitory effects on PCR reactions [17]. We therefore compared the effects of eluting into molecular grade water, 10 mM Tris–Cl (pH 8.5), heron referred to as ‘Tris’ (Buffer EB, Qiagen, Valencia, USA), or 10 mM Tris–Cl, 0.5 EDTA (pH 9.0), heron referred to as ‘Tris EDTA’ (Buffer AE, Qiagen, Valencia, USA).
Second lysis
To test the efficiency of our initial extraction, we evaluated the effect of re-processing a DBS that had already been taken through the extraction protocol, beginning with a second overnight lysis.
Second heat precipitation
To evaluate whether gDNA was effectively precipitated from the DBS, we subjected DBS that had already gone through one complete extraction protocol to an additional round of heat precipitation. After a sample was processed once, we added an additional 200 µl of 5% Chelex® solution heated to 95°C to the 1.5 mL LoBind tube containing the DBS. Samples were incubated at 95°C for 15 min with vortexing every 5 min and processed as above.
Optimized protocol
We subsequently combined elution into Tris–EDTA and a second heat precipitation step into an ‘optimized protocol’ to test against the control protocol with regard to absolute efficiency. Tris–EDTA was chosen as it may protect gDNA from degradation during long term storage.
Freeze–thaw stability
Chelex® resin-based extraction generates single-stranded DNA, which may be more susceptible to shearing from ice crystal formation during repeated freezing and thawing [18]. To test for stability of our eluted gDNA, we took control samples through 20 freeze–thaw cycles, in which the sample was frozen at 80°C, then thawed at room temperature, and an aliquot was removed. Prior to beginning the freeze thaws, we removed an aliquot and stored it at 4°C, denoted as ‘0 freeze–thaws’.
DNA quantification
The gDNA yield was quantitated by qPCR targeting the human beta-globin gene as previously published [19]. Quantification was performed after a single freeze–thaw for all samples, except the optimized experiment in which the ‘0 freeze–thaws’ samples were quantified after storage at 4°C.
Mock microchimerism experiment
Donor A and Donor B were HLA Class II genotyped (Scisco Genetics, Seattle, WA, USA) in order to identify a non-shared heterozygote allele marking Donor B in the background of Donor A (DQB1*06). Blood from Donor B was mixed into blood from Donor A at an expected ratio of 10 in 100 000 based upon the white blood cell count of each sample. A total of 20 µl of this admixture was used to make DBS which were dried and three DBS were extracted using our optimized protocol.
Quantitative PCR was performed targeting DQB1*06 utilizing the complete volume of eluate from these DBS (150 µl from first precipitation and 150 µl from second precipitation) in order to capture the sensitivity of the assay relative to the full amount of gDNA recovered; 28 wells in total were run for each sample. The DQB1*06 assay is part of a panel of previously validated qPCR assays developed for the detection of microchimerism [19, 20]. The microchimerism level presented is calculated as: (sum of DQB61*06 amplifications across all wells)/(betaglobin per well * number of wells), after adjustment for the relative DQB1*06 and betaglobin standard curves [21].
Statistical methods
Initial comparison between the Chelex® resin-based method and the QIAamp® DNA Blood Mini Kit considered the absolute efficiency of gDNA extraction, based on the white blood count for Donor A, expressed as gEq recovered/gEq expected. This calculation was based on a conservative estimate of the final eluate volume for the Chelex® resin-based approach of 150 µl when 200 µl is initially added; in many cases, it is possible to recover more eluate and thus the efficiency we present likely underestimates the true efficiency of the protocol. With regard to the QIAamp® DNA Blood Mini Kit, we considered a final eluate volume of 45 µl for each elution step when 50 µl is applied to the column. Total gEq recovered for all conditions was calculated as the concentration of the sample by beta-globin qPCR multiplied by the final elution volume. All experimental conditions were run in five replicates. All samples were within two standard deviations of the mean and therefore no samples were removed from analysis.
We compared the mean efficiency of the Chelex® control method to the QIAamp® DNA Blood Mini Kit using a two-tailed Student’s t-test. When considering experimental variations to optimize the Chelex® extraction, we normalized the experimental condition to the control condition conducted on that day and present the fold change, to account for changes in the DBS age over time. Standard error for the relative DNA yield was generated using bootstrapping (repeated sampling with replacement from experimental data), and the P-value was generated using a two-tailed Student’s t-test.
All statistics were conducted in R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Chelex® resin-based extraction versus QIAamp® DNA blood mini kit
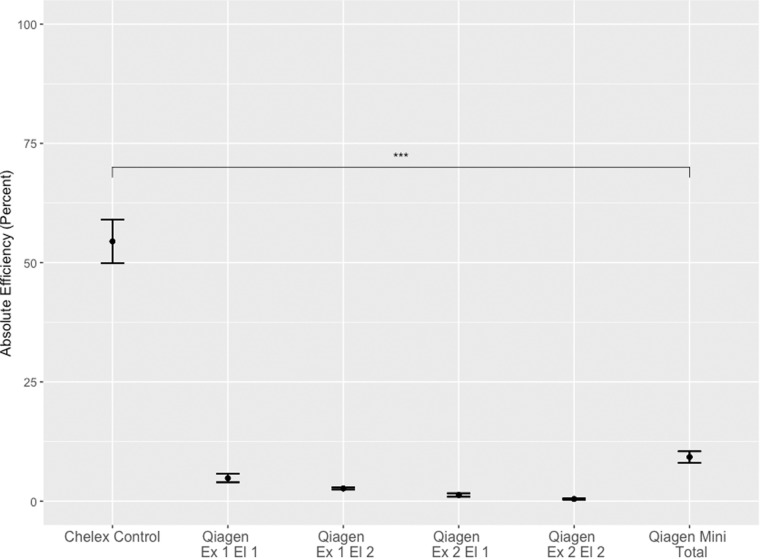
In comparisons of DNA extraction by our control Chelex® method or QIAamp® DNA Blood Mini Kit, DNA extraction by the control Chelex® resin-based extraction method yielded significantly more gDNA than the QIAamp® DNA Blood Mini Kit, with a total efficiency from the Chelex® control protocol of 54.5% and a total efficiency of the QIAamp® DNA Blood Mini Kit of 9.2% (P < 0.001) (Fig. 1). Within the QIAamp® DNA Blood Mini Kit condition, a second elution off the column increased the total efficiency from 5% to 7%, whereas ‘re-extracting’ the DBS had very little effect.
Figure 1:
Control Chelex® 100 resin-based protocol versus QIAamp DNA Blood Mini Kit for the extraction of gDNA from DBS. QIAamp DNA Blood Mini Kit was performed with a first (Ex 1, El 1) and second elution (Ex 1, El 2). Following this, the DBS was processed through the kit a second time (Ex 2, El 1 and Ex 2, El 2). The total Qiagen yield was calculated by adding together the DNA yield from both extractions and both elutions. Replicates of five were conducted for each condition in a single experiment. Data are represented as mean efficiency ± standard error.
Optimization of Chelex resin-based extraction
After finding the Chelex® control protocol to be superior to the QIAamp® DNA Blood Mini Kit, we aimed to further optimize the Chelex® protocol through (i) variation of the detergent, (ii) variation in the size of the Chelex® resin, (iii) addition of a 56°C incubation step, (iv) variation in elution buffer, (v) re-processing the DBS through the entire protocol, and (vi) re-precipitating the DBS.
There was no significant difference between Tween® 20 (control) and saponin (fold change: 1.14, P = 0.13) or Triton™ X-100 (fold change: 1.14, P = 0.2); between 50–100 mesh Chelex® (control) and 200–400 mesh Chelex® (fold change: 1.0, P = 0.99); with absence (control) or addition of a 56°C incubation (fold change: 0.95, P = 0.47); or with elution into molecular grade water (control), Tris (fold change: 0.99, P = 0.99), or Tris EDTA (fold change 1.05, P = 0.3).
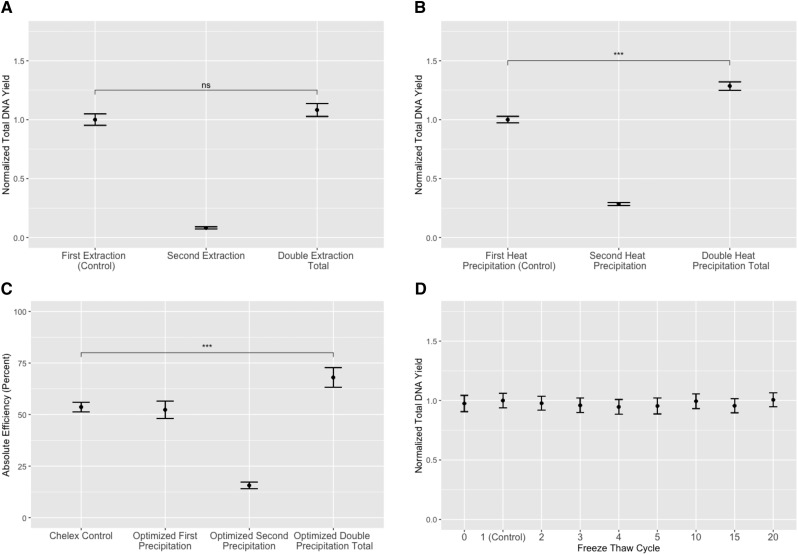
Re-extracting previously extracted DBS beginning at the lysis step resulted in an 8% increase in total DNA yield (Fig. 2a), whereas simply re-precipitating the DBS with fresh Chelex and a second 95°C incubation significantly increased the total yield by 29% (P < 0.001) (Fig. 2b). Based on the results of these studies, we generated an ‘optimized’ protocol which utilized Tween® 20 for lysis (as molecular grade, widely available, and easy to use), 50–100 mesh Chelex® (as easier to use), and elution into Tris–EDTA for DNA stability, as well as the addition of a second heat-precipitation step. This optimized protocol demonstrated the consistent effect of the second heat precipitation and yielded an absolute efficiency of 68% (Fig. 2c). We additionally tested for DNA stability using a Chelex®-based extraction by taking the control protocol through 20 freeze thaw cycles and did not find evidence of significant degradation (Fig. 2d).
Figure 2:
Optimization of Chelex® 100 resin-based extraction. Replicates of five were conducted for each condition in a single experiment. (A) Second overnight lysis does not increase the total gDNA yield. The eluant for each lysis was collected separately. The total double lysis yield was calculated by adding together the DNA yield from both elutions. The total DNA from each elution and the total is represented normalized to the control ± standard error. (B) Second 95°C heat precipitation significantly increases the total gDNA yield. The eluant for each heat precipitation was collected separately. The total double heat precipitation yield was calculated by adding together the DNA yield from both elutions. The DNA from each elution and the total is represented normalized to the control ± standard error. (C) Absolute efficiency of control versus optimized protocols. For the optimized protocol, each eluant from the heat precipitation was collected separately. The total optimized double precipitation was calculated by adding together the DNA yield from both elutions. Data from each elution and the total is represented as mean DNA yield ± standard error. (D) Control protocol extracted gDNA is stable through multiple freeze–thaw cycles. Each freeze–thaw eluant was collected separately and is represented as the mean DNA yield normalized to one freeze–thaw (control) ± standard error.
Detection of mock microchimerism
We evaluated mock microchimerism in three DBS replicates of Donor B spiked into Donor A at a ratio of 10 per 100 000 with extraction utilizing our optimized Chelex protocol. We evaluated the ability to detect microchimerism separately in the first and second heat precipitation in order to assess for loss of signal associated with extended heat exposure and potential resultant DNA degradation, as well as for each sample overall. We were able to detect mock microchimerism in both precipitations in all three DBS (Table 1), at a level that varied between 1 in 100 000 and 12 in 100 000.
Table 1:
Detection of mock microchimerism at a level of 10 per 100,000 from DBS using optimized Chelex® 100 protocol
| Sample | Condition | # Wells | # Positive Wells | Mock microchimerism per 100,000 |
|---|---|---|---|---|
| Sample 1 | 1st Precipitation | 14 | 2 | 1 |
| 2nd Precipitation | 14 | 1 | 3 | |
| Total | 28 | 3 | 2 | |
| Sample 2 | 1st Precipitation | 14 | 7 | 5 |
| 2nd Precipitation | 14 | 6 | 12 | |
| Total | 28 | 13 | 7 | |
| Sample 3 | 1st Precipitation | 14 | 7 | 6 |
| 2nd Precipitation | 14 | 5 | 10 | |
| Total | 28 | 12 | 7 |
Discussion
DBS are a quick and convenient way to collect whole blood that requires no additional processing at the time of collection and allows for sample storage at room temperature. This has made them attractive for newborn screening programs and for sample collection and transport from field sites. DBS are widely used in both the malaria and HIV fields to detect pathogen and drug resistance as gDNA extracted from DBS is amenable to downstream molecular applications. Although new forms of paper (e.g. FTA paper) are now available that are intended to aid in the preservation and utilization of DNA from DBS, many historic samples already exist on common, inexpensive paper such as Whatman Grade 3 or S&S 903.
Our lab studies maternal cells that traffic into the fetus, known as maternal microchimerism, and their effect on immunity in the infant. These maternal cells are often very rare (1 to 10 in 100 000) and thus difficult to detect. Because detection requires significant amounts of gDNA suitable for qPCR testing, we were motivated to develop an optimized protocol to extract the maximum quantity of high quality gDNA from DBS. For the purposes of our experiments, we selected DBS on Whatman Grade 3 for testing as it is the most common paper used by our collaborators to generate field samples. Column-based kits such as QIAamp® Blood Mini kit yield pure, double-stranded DNA but may be subject to significant DNA loss, while the Chelex® extraction method yields samples with unpurified single-stranded DNA but increased recovery [11, 22]. Our initial approach compared a basic Chelex® resin-based approach to the commonly used QIAamp® Blood DNA Mini kit. Earlier work in our lab had directly compared the QIAamp® DNA Mini kit to the QIAamp® DNA Micro kit and found that the Mini kit consistently outperformed the Micro kit (data not shown).
Similar to what some [11, 22] but not all others [5, 12] report, we found that our Chelex® control yielded more DNA than the Qiagen-based approach; however, what was most striking was the degree of difference between the two approaches, where the Chelex® control approach yielded 590% more DNA and achieved an overall efficiency of 54%. Based on this, we next sought to optimize the Chelex®-based extraction so as to maximize yield, while confirming that the ssDNA was stable and performed well in downstream qPCR assays.
Prior reports utilizing Chelex®-based extraction primarily use saponin for cell lysis [2, 11, 22, 23], while other papers report success with Tween® 20 [12, 15, 16]. We compared saponin, Tween® 20, and Triton™ X-100 and found that the three detergents achieved similar total DNA yields but that Tween® 20 was preferable because it is molecular grade, widely available, and easier to prepare. Similarly, there was not a difference in DNA yield by Chelex® size but we found that 50–100 mesh Chelex® (large size) was easier to visualize to ensure no carryover into the final eluate. No difference in DNA yield was found between elution in molecular grade water, Tris, or Tris–EDTA, although our optimized protocol utilized Tris–EDTA for DNA stability. These results suggest that variations in each of these conditions according to reagent availability and preference should not affect ultimate DNA yield. Some publications utilize an additional 56–60°C incubation prior to the 95–100°C degree precipitation, with [12] or without [15, 16] the addition of Proteinase K. We tested the addition of this incubation step without Proteinase K and did not find any benefit associated with the inclusion of this step. We speculate that the additional pre-heating step may improve yield in the setting of less efficient lysis, and that the absence of improvement we experienced both with this modification and with variation of detergent reflected highly efficiency lysis with our overnight incubation.
In order to understand the efficiency of our extraction, we attempted to ‘re-extract’ a DBS that had already been processed, beginning at the lysis step. We found only a small, non-significant increase in the total yield with this approach. We did observe that the DBS started to physically degrade when taken through a complete re-extraction and hypothesize that the wood pulp may inhibit downstream qPCR, as tannic acids present in the wood pulp contain electronegative groups that can chelate magnesium ions, inhibiting Taq polymerase function [24, 25].
In contrast, when we took the DBS through a second heat precipitation at 95°C, we recovered 29% more DNA. Of note, this double precipitation yields two separate aliquots with a volume of 150 µl each, where the second is more dilute and may not be appropriate for all applications. We tried combining the two 15 min incubations into a single 30 min incubation at 95°C, but this did not improve total yield (data not shown), suggesting that the addition of fresh hot Chelex® is required. This may reflect more efficient capture of gDNA that was already precipitated but remained at the bottom of the tube during the first extraction or alternatively the additional benefit of ‘fresh’ Chelex®, if the Mg2+ binding capacity of the first Chelex® is saturated. To the best of our knowledge, this is the first report of such a protocol modification with resultant significant increase in total gDNA yield.
The two major concerns with Chelex® extracted DNA are that there is no purification step to remove hemoglobin, proteins, or other contaminants that might inhibit downstream applications and that the DNA is single stranded and therefore may be susceptible to hydrodynamic or enzymatic degradation. In particular, hemoglobin can directly inhibit polymerase activity, whereas immunoglobulin G binds single stranded DNA hindering DNA polymerization [26]. Previous studies have found that PCR assays may be inhibited by the impurities found in Chelex® extraction products [9, 13]; however, we have not found inhibition at up to 20% sample to total qPCR volume (data not shown), which may reflect efficient washing of the DBS after lysis or precipitation of these proteins during the final centrifuge steps to eliminate Chelex® carry over. Previous studies have reported no significant DNA degradation associated with repeated freeze thawing of Chelex®-extracted samples [22]. Similarly, we found that our Chelex®-extracted DNA was stable through 20 freeze thaw cycles. In addition, if Chelex® beads are carried over into the final eluate they may chelate Mg2+ and inhibit downstream polymerase-based applications. Using the 50–100 mesh Chelex®, we have found that the beads can be easily visualized to ensure no contamination of the final eluate.
When we applied this technique to our specific application of the detection of microchimerism we found that the optimized Chelex®-based method consistently allowed detection of low level mock microchimerism in all samples in gDNA from both the first and second precipitation. This suggests that DNA that has undergone a second 95°C heat incubation is still amplifiable by qPCR and remains useful for detecting the presence of rare alleles such as microchimerism.
Here we describe an optimized protocol for the extraction of high quality gDNA from DBS. We present a novel second heat precipitation step that allowed us to recover 29% more gDNA, bringing our total efficiency to 68%. Despite being single stranded and unpurified, our recovered gDNA was stable through freeze thaw cycles, did not inhibit downstream qPCR, and could be used to reliably detect rare donor alleles. This technique will allow us to identify rare maternal microchimerism in historic DBS from field sites. In addition, our technique is more broadly relevant to newborn screening, in mother to child transmission of infection (e.g. CMV or HIV), as well as pathogen detection and drug resistance testing from field sites.
Funding
Funding for this work was provided by NIH/NIAID (K08 AI135072 to W.E.H.) and the Burroughs Wellcome Fund (CAMS 1017213 to W.E.H.).
References
- 1. Freeman JD, Rosman LM, Ratcliff JD. et al. State of the science in dried blood spots. Clin Chem 2018; 64: 656–79. [DOI] [PubMed] [Google Scholar]
- 2. Plowe CV, Djimde A, Bouare M. et al. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg 1995; 52: 565–8. [DOI] [PubMed] [Google Scholar]
- 3. Fernandez I, Wirtz RA, Lanar DE. et al. Serologic and genetic characterization of Plasmodium vivax from whole blood-impregnated filter paper discs. Am J Trop Med Hyg 1992; 46: 473–9. [DOI] [PubMed] [Google Scholar]
- 4. Baidjoe A, Stone W, Ploemen I. et al. Combined DNA extraction and antibody elution from filter papers for the assessment of malaria transmission intensity in epidemiological studies. Malar J 2013; 12:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwang J, Jaroensuk J, Leimanis ML. et al. Long-term storage limits PCR-based analyses of malaria parasites in archival dried blood spots. Malar J 2012; 11:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smit PW, Elliott I, Peeling RW. et al. An overview of the clinical use of filter paper in the diagnosis of tropical diseases. Am J Trop Med Hyg 2014; 90: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rich SM, Leendertz FH, Xu G. et al. The origin of malignant malaria. Proc Natl Acad Sci USA 2009; 106: 14902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harrington WE, Kanaan SB, Muehlenbachs A. et al. Maternal microchimerism predicts increased infection but decreased disease due to Plasmodium falciparum during early childhood. J Infect Dis 2017; 215: 1445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. SjöHolm MIL, Dillner J, Carlson J.. Assessing quality and functionality of DNA from fresh and archival dried blood spots and recommendations for quality control guidelines. Clin Chem 2007;53:1401–7. [DOI] [PubMed] [Google Scholar]
- 10. Baidjoe A, Rosenthal PJ, Bousema T. et al. The effect of storage and extraction methods on amplification of Plasmodium falciparum DNA from dried blood spots. Am J Trop Med Hyg 2015; 92: 922–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsiang MS, Lin M, Dokomajilar C. et al. PCR-based pooling of dried blood spots for detection of malaria parasites: optimization and application to a cohort of Ugandan children. J Clin Microbiol 2010; 48: 3539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McMichael GLSouth Australian Cerebral Palsy Research GroupHighet AR, Gibson CS. et al. Comparison of DNA extraction methods from small samples of newborn screening cards suitable for retrospective perinatal viral research. J Biomol Tech 2011; 22: 5–9. [PMC free article] [PubMed] [Google Scholar]
- 13. Singh UA, Kumari M, Iyengar S.. Method for improving the quality of genomic DNA obtained from minute quantities of tissue and blood samples using Chelex 100 resin. Biol Proced Online 2018; 20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amidzadeh Z, Behbahani AB, Erfani N. et al. Assessment of different permeabilization methods of minimizing damage to the adherent cells for detection of intracellular RNA by flow cytometry. Avicenna J Med Biotechnol 2014; 6: 38–46. [PMC free article] [PubMed] [Google Scholar]
- 15. Fischer A, Lejczak C, Lambert C. et al. Simple DNA extraction method for dried blood spots and comparison of two PCR assays for diagnosis of vertical human immunodeficiency virus type 1 transmission in Rwanda. J Clin Microbiol 2004; 42: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollegaard MV, Thorsen P, Norgaard-Pedersen B. et al. Genotyping whole-genome-amplified DNA from 3- to 25-year-old neonatal dried blood spot samples with reference to fresh genomic DNA. Electrophoresis 2009; 30: 2532–5. [DOI] [PubMed] [Google Scholar]
- 17. Huggett JF, Novak T, Garson JA. et al. Differential susceptibility of PCR reactions to inhibitors: an important and unrecognised phenomenon. BMC Res Notes 2008; 1:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roder B, Fruhwirth K, Vogl C. et al. Impact of long-term storage on stability of standard DNA for nucleic acid-based methods. J Clin Microbiol 2010; 48: 4260–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lambert NC, Erickson TD, Yan Z. et al. Quantification of maternal microchimerism by HLA-specific real-time polymerase chain reaction: studies of healthy women and women with scleroderma. Arthritis Rheum 2004; 50: 906–14. [DOI] [PubMed] [Google Scholar]
- 20. Gammill HS, Guthrie KA, Aydelotte TM. et al. Effect of parity on fetal and maternal microchimerism: interaction of grafts within a host? Blood 2010; 116: 2706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loubiere LS. et al. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab Invest 2006; 86: 1185–92. [DOI] [PubMed] [Google Scholar]
- 22. Baidjoe A, Rosenthal PJ, Bousema T. et al. The effect of storage and extraction methods on amplification of Plasmodium falciparum DNA from dried blood spots. Am J Trop Med Hyg 2015; 92: 922–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Färnert A, Mårtensson A, Gil JP. et al. Short report: rapid DNA extraction from archive blood spots on filter paper for genotyping of Plasmodium falciparum. Am J Trop Med Hyg 2005; 72: 249–51. [PubMed] [Google Scholar]
- 24. Kontanis EJ, Reed FA.. Evaluation of real-time PCR amplification efficiencies to detect PCR inhibitors. J Forensic Sci 2006; 51: 795–804. [DOI] [PubMed] [Google Scholar]
- 25. McCord BR, Pionzio AM, Thompson R.. Conference Proceedings: Analysis of the effect of a Variety of PCR Inhibitors on the Amplification of DNA using Real Time PCR, Melt Curves and STR Analysis National Criminal Justice Reference Service, 2014.
- 26. Sidstedt M, Hedman J, Romsos EL. et al. Inhibition mechanisms of hemoglobin, immunoglobulin G, and whole blood in digital and real-time PCR. Anal Bioanal Chem 2018; 410: 2569–83. [DOI] [PMC free article] [PubMed] [Google Scholar]