To the Editor,
Since its first description in December 2019 in Wuhan City (Hubei, China), a novel type of mutated coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected over 3.6 million people and caused more than 257 000 deaths worldwide (as of May 5, 2020). There is growing concern that acute respiratory disease occurring in coronavirus disease (COVID-19) is strongly associated with cardiovascular damage. Patients with COVID-19 are at risk of cardiac arrhythmias, acute coronary syndromes, heart failure-related events, and fulminant myocarditis.1 Myocardial injury may occur at different phases of COVID-19 disease (ie, viral, pulmonary, inflammatory, and recovery phase), even late after the onset of symptoms.2 The mechanisms of cardiovascular injury from SARS-CoV-2 have not yet been fully elucidated and are likely to be multifactorial. SARS-CoV-2 viral particles have been identified by real-time polymerase chain reaction (PCR) testing in cardiac tissue, providing evidence that direct cardiotoxicity might occur.1 In addition, SARS-CoV-2 has been shown to establish a receptor binding domain with angiotensin-converting enzyme 2 (ACE2) before entering the host cell via endocytosis. Since more than 7.5% of myocardial cells have positive ACE2 expression, this could mediate SARS-CoV-2 entry into cardiomyocytes and cause direct cardiotoxicity.3 Furthermore, hyperinflammation due to cytokine release mediated by the virus may lead to myocardial and vascular inflammation, plaque instability, a hypercoagulable state, and endothelial cell dysfunction. Finally, cardiac injury may also be mediated by other systemic consequences of COVID-19 infection, including sepsis and disseminated intravascular coagulation. According to postmortem biopsies, the pathological features in cardiac tissue range from minimal changes to interstitial inflammatory infiltration and myocyte necrosis.1
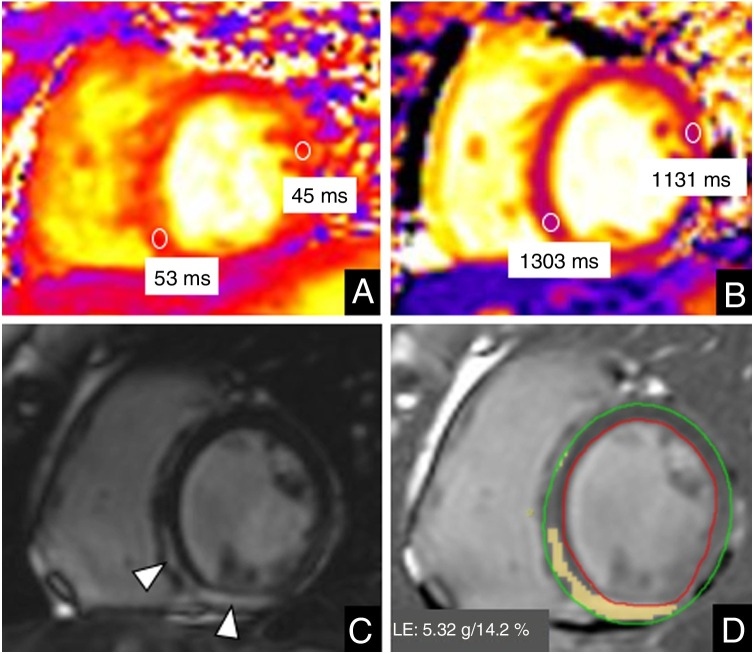
We describe 2 different presentations of myocarditis. The first patient was an asymptomatic 26-year-old-pregnant woman diagnosed with gestational diabetes who was admitted for delivery. She required a cesarean section. As part of the preoperative protocol a PCR test was performed, which was positive. The procedure was uneventful and the patient gave birth to a healthy neonate. No abnormalities were observed on a chest X-ray performed the day after surgery and the patient was discharged after 2 days of hospitalization. A week later, she was seen in the emergency department for chest pain radiating to her left arm and was prescribed nonsteroidal anti-inflammatory drugs and colchicine. Due to persistent symptoms and tachycardia, she was admitted to hospital 1 week later. She had no fever or respiratory symptoms. The results of chest X-ray and an electrocardiogram were normal. Echocardiography showed normal systolic function. Troponin T levels were high (319.4 ng/L). The patient underwent cardiac magnetic resonance (CMR) on a 3T system (Magnetom VIDA, Siemens Healthineers, Erlangen, Germany). A conventional CMR protocol to rule out myocarditis was performed. Cine images revealed normal systolic function (left ventricular ejection fraction 59%), with no regional wall motion abnormalities. High signal intensity on T2 maps (53 ms, normal value < 48 ms) and prolonged native T1 values were observed in basal and mid-inferoseptal and inferior myocardial segments (1303 ms, normal value < 1200 ms).4 Late gadolinium enhanced (LGE) images showed mesocardial and subepicardial enhancement of those segments, representing 14.2% of the total ventricular mass (figure 1 ). Based on CMR findings and the clinical and epidemiological context, a diagnosis of myocarditis due to SARS-CoV-2 infection was established. No myocardial biopsy was performed.
Figure 1.
Cardiac magnetic resonance imaging in a 26-year-old woman with COVID-19 myocarditis. Mid-ventricular short axis view. A: T2 map. B: native T1 map. C: late gadolinium enhancement (LGE). D: quantification of late gadolinium enhancement. The study revealed slightly increased values on T2 maps (53 ms vs 45 ms of remote myocardium) and prolonged native T1 values (1303 ms vs 1131 ms of remote myocardium) in basal and mid-inferoseptal and inferior myocardial segments. These segments showed mesocardial and subepicardial enhancement on LGE sequences (arrowhead in C). The extent of LGE corresponded to 14.2% of the total ventricular mass.
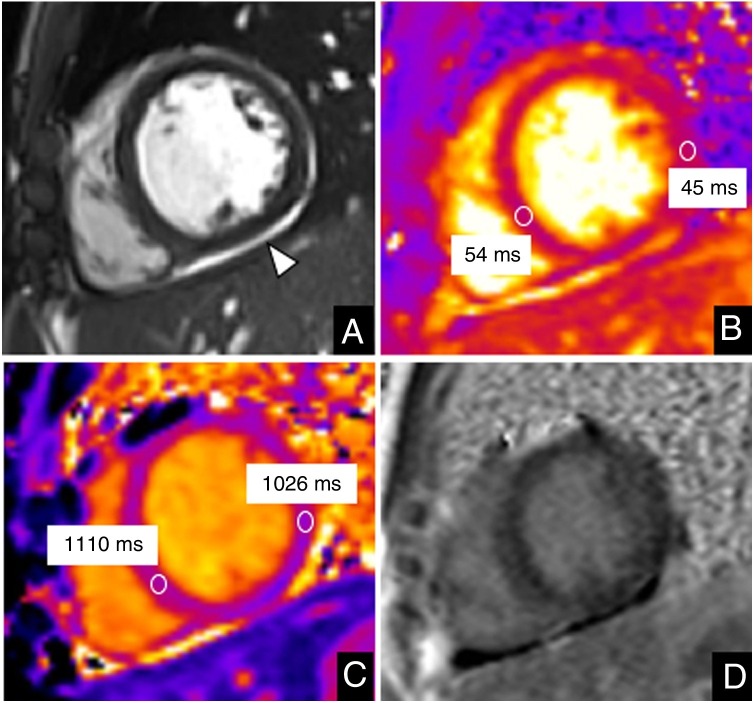
The second patient was a 13-year-old boy who was admitted after 2 days of fever (40 °C). He reported mild cough, odynophagia, abdominal pain, and vomiting in the past few days. Laboratory tests showed mild elevation of C-reactive protein, D-dimer, ferritin, brain natriuretic peptide, and troponin I (190 pg/mL) levels. His relatives were PCR-positive for SARS-CoV-2 and he also had a positive PCR-test. The CMR study performed on a 1.5T system (Magnetom Aera, Siemens Healthineers, Erlangen, Germany) showed normal biventricular function, no regional wall motion abnormalities, slightly increased T2 (54 ms, normal < 52 ms) and native T1 values (1110 ms, normal < 985 ms)5 in the ventricular septum reflecting myocardial edema, and no LGE (figure 2 ). A small pericardial effusion was noted. He was diagnosed with myocarditis.
Figure 2.
Cardiac magnetic resonance imaging in a 13-year-old boy with COVID-19 and myocarditis. Mid-ventricular short axis view. A: cine image. B: T2 map. C: native T1 map. D: late gadolinium enhancement (LGE). The study showed mild pericardial effusion (arrowhead in A). Slightly increased values in the ventricular septum were demonstrated on T2 maps (54 ms vs 45 ms of remote myocardium) and native T1 values were high, particularly in the ventricular septum (1110 ms vs 1026 ms of remote myocardium) in keeping with myocardial edema. No LGE was observed.
CMR allows targeting of several features of myocarditis, such as contractile dysfunction, inflammatory edema, and necrosis, and has become the gold standard for the noninvasive assessment of the disease. The cornerstone of diagnosis are conventional cine images, T2-weighted sequences, T1 and T2 parametric maps, and LGE images. Furthermore, the degree of myocardial necrosis determined by LGE is a strong predictor of prognosis.
In the setting of the COVID-19 pandemic, approaches to CMR need to be adapted to allow for safe practices for urgent and semiurgent studies and appropriate deferral of elective examinations.6 Many patients with confirmed active COVID-19 may present with clinical suspicion of inflammatory or ischemic cardiac events and CMR may offer an efficient diagnostic imaging choice to obtain critical information for clinical decision-making.
References
- 1.Kang Y., Chen T., Mui D. Cardiovascular manifestations and treatment considerations in covid-19. Heart. 2020 doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosyns B., Lochy S., Luchian M.L. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur Heart J Cardiovasc Imaging. 2020;21:709–714. doi: 10.1093/ehjci/jeaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Knobelsdorff-Brenkenhoff F., Prothmann M., Dieringer M.A. Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson. 2013;15:53. doi: 10.1186/1532-429X-15-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottbrecht M., Kramer C.M., Salerno M. Native T1 and Extracellular Volume Measurements by Cardiac MRI in Healthy Adults: A Meta-Analysis. Radiology. 2019;290:317–326. doi: 10.1148/radiol.2018180226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y., Chen T., Bryant J. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson. 2020;22:26. doi: 10.1186/s12968-020-00628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]