Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Infection, Clinical trials, Challenges
Abstract
Since the first outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Wuhan, Hubei, China in December 2019, it is now recognized as a pandemic by the World Health Organization (WHO) as more than 200 countries and territories worldwide are affected with an increasing incidence. The SARS-CoV-2 infection results in a spectrum of non-specific signs and symptoms, ranging from asymptomatic infection, to flu-like illness such as fever, cough, dry cough and fatigue, to pneumonia, acute respiratory distress syndrome, and even multi-organ failures with high morbidity and mortality. SARS-CoV-2 is mainly transmitted through respiratory droplets that infected people exhale during incubation and onset period. By 12 June 2020, over 7.5 million confirmed cases of Coronavirus disease 2019 (COVID-19) with more than 421,000 deaths in the world have been reported to the WHO. No specific medication is approved to treat COVID-19, raising the urgent need for antiviral drug development. By 12 June 2020, there are over 1000 clinical trials registered in clinicaltrials.gov for treatment of COVID-19. This review summarizes the epidemiology, virology, clinical presentation, pathophysiology, diagnosis, and particularly the antiviral drugs currently under clinical trials for treatment of SARS-CoV-2 infection, together with the challenges and perspectives of this disease are also discussed.
1. Introduction
Coronavirus disease 2019 (COVID-19) is an acute respiratory infection disease caused by severe acute respiratory syndrome coronavirus 2 (SARS coronavirus 2 or SARS-CoV-2) that was first identified in December 2019 in Wuhan, Hubei, China. The incidence of COVID-19 has grown dramatically in China and the virus has rapidly spread to more than 200 countries of the world since the late February 2020. On 28 Feb 2020, World Health Organization raised the global COVID-19 risk to highest level. COVID-19 outbreak has become a potential threat to the global public health. The impact of economic burden imposed by COVID-19 is substantial and considerable, in medical resources, quarantine measures, restriction of trade, disruption of production, loss of job, deteriorated finance, etc. Indeed, this pandemic highlights the need for effective therapeutics for SARS-CoV-2 infections that can improve patients’ clinical conditions. Although mountains of novel findings on COVID-19 are accumulating, the pathogenesis of COVID-19 remains to be fully elucidated for development of better intervention strategies. In this review, we will give a general overview of the existing studies on the epidemiology, virology, clinical presentation, pathophysiology, diagnosis, and treatment of SARS-CoV-2 infection.
2. Epidemiology
2.1. Geographic distribution
COVID-19 cases were firstly reported in Wuhan city, Hubei province, China, in September 2019. From then on, cases have been distributed globally except for Antarctica. By June 12th 2020, more than 7,5 00,000 confirmed cases of COVID-19 have been reported. Approximately 3,500,000 cases were cured and more than 421,000 infected people were died with global death-to-cases ratio 5.61 %. Updated cases throughout the world can be found in the following websites: https://coronavirus.jhu.edu/map.html and https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
2.2. Incubation period and viral shedding duration
Estimation of the incubation period and viral shedding duration is helpful to assess the risk of transmission, make measures of isolation and quarantine, and provide rational antiviral interventions for treating patients. Based on the current epidemiological investigations, the incubation period of COVID-19 is about 1–14 days, with a median time of 3–7 days, and can be up to 24 days in a tiny minority of cases from exposure to symptoms onset [[1], [2], [3]]. Viral shedding may begin 2–3 days before the presence of the first symptom [4]. Median duration of viral shedding has found to be 20.0 days and the longest duration could be up to 37 days in survivors, while the viral RNA in non-survivors could be detected until death [5]. In the cases of severe COVID-19 patients, the median duration of viral shedding has shown to be 31.0 days from illness onset.
2.3. Transmission
Novel coronavirus is mainly transmitted through respiratory droplet, aerosol, direct or indirect contact. Several studies have provided epidemiological evidences that human-to-human transmission of novel coronavirus in family, hospitals, and even across cities were occurred [[6], [7], [8]]. Guan et al. found that patterns of human-to-human transmission included familial aggregation [6], asymptomatic virus carrier [9], and the three-phase outbreak [10], which were in accordance with other findings. The “super-spreaders” may occur in this pandemic [1]. Prominently, infected persons without symptoms during the incubation period could be sources of infection, suggesting the asymptomatic transmission of SARS-CoV-2 [9,[11], [12], [13], [14], [15]]. The rapid and uncontrollable spread of this virus may ascribe to the “silent transmission” by some patients with atypical symptoms.
More and more evidences have proved that this virus can spread through multiple routes as well. To et al. detected the self-collected saliva, a non-invasive specimen, by one-step real-time RT-qPCR assay targeting the S protein [6]. They obtained the positive results that were consistent with those detected through nasopharyngeal or sputum specimens, which may be due to the infection of epithelial cells in saliva gland ducts or the secretions from nasopharynx and lung in the saliva. Therefore, virus in saliva may allow transmission via speaking or coughing [16].
Zhang et al. firstly reported the presence of viral nucleotide in anal swabs and blood. During the later stage of infection, the level of viral RNA was higher in anal swab than in blood, suggesting the possibility of multiple routes transmission of virus including oral-fecal or body fluid route [17]. Studies of Xiao et al. have shown that the viral RNA tested in feces was positive even in gastrointestinal tracts was negative [18]. Another two findings emphasized that the diarrhea as onset symptom should not be ignored and the fecal-oral transmission route should be taken seriously [19,20]. Chen et al. analyzed the viral RNA levels in pharyngeal, swab, blood, and the anal swab by real-time PCR. The concentrations of viral RNA in blood and anal swabs were positively correlated with the severity of COVID-19. Since the spike protein of novel coronavirus interacts with the host ACE2 receptor for viral attachment and entry to establish infection, the presence of viral RNA in digestive tract in which ACE2 is abundantly expressed confirmed the viral replication in extrapulmonary sites. Early detection of viral RNA in the blood and digestive tract may be helpful in predicting the progression of COVID-19 [21].
A clinician who wore an N95 mask but not safety goggles was infected with SARS-CoV-2 while working with COVID-19 patients in Wuhan, suggesting the potential transmission through mucous membranes in the eyes [22]. Despite no detectable viral RNA was in the conjunctival swabs samples from infected patients reported in the further studies [23], Deng et al. have confirmed that SARS-CoV-2 could be transmitted through ocular conjunctival route in a rhesus macaques model, cautioning us the necessity of eye protection to prevent the spread of SARS-CoV-2 [24].
Chen et al. reported potential vertical transmission in nine pregnant women with laboratory confirmed COVID-19 infection. When the newborns were delivered by caesarean section, amniotic fluid, cord blood, throat swabs from newborns, and breastmilk were collected for testing and all of them were negative for the novel coronavirus. Currently there is no conclusive evidence supporting that women with COVID-19 in the late trimester of pregnancy could cause intrauterine infection due to vertical transmission. However, this investigation is restricted to the small number of cases and the pregnant women in the third trimester [25,26]. It should be noted that infants and pregnant women remain susceptible to COVID-19 infection. Special care should be taken to prevent neonatal infections from mothers with COVID-19.
Overall, the above studies warn the virus transmission through multiple routes including respiratory tract, alimentary tract, nasolacrimal system, oral-fecal, and body fluid route, and that the potential maternal-fetal vertical transmission should not be ignored. The properties of viral shedding before symptoms appear and via multiple routes indicate the strong infectiousness of SARS-CoV-2 and pose a huge challenge in controlling the spread of disease.
3. Virology of SARS-CoV-2
SARS-CoV-2 belongs to the family coronaviruses and subgenus sarbecovirus (beta-CoV lineage B) and is a new strain virus distinct from Severe Acute Respiratory Syndrome (SARS-CoV) and Middle East Respiratory Syndrome (MERS-CoV). The genome sequence of SARS-CoV-2 shows more than 85 % identity to a bat SARS-like CoV (bat-SL-CoVZC45, MG772933.1) [27]. Its genome is a positive-sense single-stranded RNA of about 30 kb with structured untranslated region (UTR) on the 5′-end, poly (A) on the 3′-end, and 11 open reading frames encoding 27 proteins. The gene order is as follows: 5′ UTR (nt 1–265)-replicase ORF1ab (nt 266−21,555)-Spike (nt 21,563-25,384)-ORF3a (nt 25,393-26220)-Envelope (nt 26,245-26,472)-Membrane (nt 26,523-27,191)-ORF6 (nt 27,202-27,387)-ORF7a (nt 27,394-27,759)-ORF7b (nt 27,756-27887)-ORF8 (nt 27,894-28,259)-Nucleocapsid (nt 28,274-29533)-ORF10 (nt 29,558-29,674)-3’UTR (nt 29,675-29,903). The ORF1ab gene accounts for two-thirds of the genome and encodes 16 non-structural proteins (NSP1-NSP16) while the remaining genome encodes 4 structural proteins (S, M, E, N) and 6 accessory proteins (ORF3a, ORF6, ORF7a, ORF7b, ORF8, ORF10) [[28], [29], [30], [31]]. Currently more than 40,000 SARS-CoV-2 genomes publicly available (https://www.gisaid.org/ https://bigd.big.ac.cn/ncov/release_genome#) represent the genomic diversity of the virus in the world. Though all sequenced SARS-CoV-2 genomes share more than 99% similarity, it has been found that there are at least two hypervariable genomic hotspots. One is a silent mutation in the ORF1ab, the other one is an amino acid polymorphism (Serine/Leucine) in ORF8 which is predicted to induce structural disorder of the protein in the C-terminal portion [30]. 149 mutations were identified in 103 SARS-CoV-2 genome sequences and through population genetic analyses, investigators uncovered two major types of SARS-CoV-2 in circulation (L and S type) based on two tightly linked SNPs at position 8,782 and 28,144. The S type (∼30 %) is the ancestral version while L type (∼70 %) is derived from S type, although L type is more prevalent and more aggressive in the outbreak. Based upon the evolution of novel coronavirus, there may be great distinctions in transmissibility, pathogenicity, and virulence between S and L type [32]. Forster et al. analyzed 160 complete SARS-CoV-2 genomes by phylogenetic network and found three central variants named A, B, and C. The genome of type A is the most closely related to the bat coronavirus, which is supposed to be the “root of the outbreak”. Type A is mainly found in United States and Australia. Type B is distinguished from type A by two mutations T8782C and C28144 T, and is prevalent in Wuhan and East Asia. It seems that type B is resistant outside Ease Asia populations, since type B is not intended to spread outside East Asia without further mutated. Type C is derived from type B by the mutation G26144 T and mainly found in Europeans, and also found in Singapore, Hong Kong, Taiwan, and South Korea but absent in mainland China [33].
In addition to the viral mutations mentioned above, the human genetic variation may partly contribute to the geographical variations in the prevalence and mortality of COVID-19 pandemic.
Delanghe et al. have investigated the role of the D/I polymorphism in intron 16 of host’s angiotensin-converting enzyme-1 (ACE1) in the epidemiology of COVID-19 infections. Prevalence and mortality data of the COVID-19 infections from European, African, Mediterranean, Middle East and Asian countries were included in the study. They found that the frequency of ACE1 D-allele was negatively correlated with prevalence of COVID-19, suggesting the confounded role of ACE1 D/I polymorphism in the circulation of SARS-CoV-2 and the outcome of the infection [[34], [35], [36], [37]]. While complement component 3 (C3) polymorphism, a central component of the innate immune system, has been found to be a principal component of gene frequencies among European populations and a crucial determinant for COVID-19 prevalence and mortality [37]. Dai’s paper suggested infected patients carrying A allele of ABO blood group type especially those with cardiovascular diseases in particular hypertension, tend to develop severe COVID-19 [38]. SARS-CoV-2 virion particles are enveloped, roughly spherical or moderately polymorphic with diameter ranging from 80−160 nm [39]. SARS-CoV-2 has four structural proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins necessary for virion particle formation, and four highly conserved nonstructural proteins including papain-like protease (PLpro, nsp3), 3CL-protease (3CLpro, nsp5), RNA-dependent RNA polymerase (RdRp, nsp12), and helicase (nsp 13) [40] that are needed for viral RNA replication [41,42]. The N protein forms a ribonucleoprotein complex with viral RNA. The S protein is responsible for virus entry to the host cell by binding to the cellular receptor angiotensin-converting enzyme 2 (ACE2). The width of the S protein is about 7 nm, and the length is about 23 nm [39]. S protein has unique insertion of four amino acids (PRRA), which is a furin-like or TMPRSS2 cleavage site [43,44]. S protein can be cleaved into S1 and S2 subunits. When the S1 subunit is dissociated, S2 undergoes a conformational change, extending itself from a compressed form to a nail shape. S1 is the receptor binding domain that helps the virus attach to the surface of the host cell, then the cellular proteases prime the S protein and cleave it at specific site, thereby promoting the S2 mediated fusion process of virus with host cell membrane. The incorporation of PRRA resulting in the cleavage of S protein and triggering fusion, distinguished from other beta-coronaviruses, may greatly affect the regulation of the pathogenicity and transmissibility of SARS-CoV-2 [45,46].
Understanding how SARS-CoV-2 hijacks the host cells during infection is crucial for developing therapeutic strategies. A global collaboration have identified 332 high-confidence interactions between SARS-CoV-2 proteins and human proteins. These protein interactions participate in several complexes and biological processes including DNA replication, epigenetic and gene expression regulators, vesicle trafficking, lipid modification, RNA processing and regulation, ubiquitin ligases, signaling, nuclear transport machinery, cytoskeleton, mitochondria, and extracellular matrix. Around 40 % of these interacting proteins were bound up with endomembrane compartments or vesicle trafficking pathways. The SARS-CoV-2 main protease, Nsp5 may affect the transport of endoplasmic reticulum and mitochondria. Some viral proteins also showed interaction with natural immune signaling proteins involved in IFN pathway, NF-κB pathway, etc, which may take part in cytokine storm. 69 compounds were found to target these protein interactions including FDA approved drugs, compounds in clinical trials, and preclinical compounds. Based on screening assays, two broad sets of drugs and compounds: mRNA translation inhibitors and Sigma1 and Sigma2 receptor modulators, were showed to exert antiviral activity [47].
4. Clinical and laboratory manifestations
4.1. Clinical manifestations
The clinical manifestations of COVID-19 range from asymptomatic infection, to flu-like illness such as fever, cough, dry cough and fatigue, to pneumonia, acute respiratory distress syndrome, and even multi-organ failures with high morbidity and mortality. The clinical diversity of non-specific signs and symptoms is poorly understood and seems to be correlated to the age, underlying comorbidities, and immunity status of the individual.
Wang et al. reported 138 hospitalized cases of 2019-nCoV infected pneumonia in which most patients had fever, fatigue, dry cough, lymphopenia, prolonged prothrombin time, and higher level of lactate dehydrogenase [14]. Specifically, abnormal coagulation features including higher D-dimer and fibrin degradation product levels, longer prothrombin time and activated partial thromboplastin time, are significantly associated with poor prognosis [48]. Chest CT scans in the lungs of all patients showed ground glass opacity or bilateral patchy shadows [14]. But a larger scale of research on 1,099 laboratory-confirmed COVID-19 patients from 552 hospitals in 31 provinces throughout China showed that a certain amount of patients did not have fever or abnormalities on chest CT, making the diagnosis more complicating [1]. Severe patients who received intensive care unit (ICU) care were more likely to have two or more underlying comorbidities that usually associated with poor clinical outcomes. Hypertension, cardiovascular disease, and diabetes were the most common comorbidities, while chronic obstructive pulmonary disease (COPD) was less common. Other comorbidities include pharyngeal pain, dizziness, abdominal pain, dyspnea, anorexia, cerebrovascular diseases, hepatitis B infections, chronic kidney diseases, and malignancy, and complications include acute respiratory distress syndrome [47], arrhythmia, and shock [14,49].
The fact that SARS-CoV could attack the nervous system was confirmed by the viral genome sequence detected in the infected patients’ cerebrospinal fluid. In the findings of Mao et al., more than 30 % patients showed the neurological manifestations including central nervous system symptoms (headache, dizziness, disturbance of consciousness, epilepsy, etc), peripheral nervous system symptoms (decreased taste, decreased smell, decreased appetite, neuralgi, etc), and skeletal muscle injury. The neurological symptoms were more frequent in severe patients than in non-severe patients. It is worth noting that the first symptoms of some patients occurred in the nervous system, such as facial paralysis, hallucinations, etc. The virus circulation in the blood and crossing the blood-brain barrier, as well as the highly expression levels of ACE2 in vascular endothelial cells of nervous system and in smooth muscle cells of skeletal muscle, may contribute to the viral infection in nervous system [50]. On March 4, a 56-year-old COVID-19 patient in China was firstly reported to present with viral encephalitis (https://www.caixinglobal.com/2020-03-06/new-research-sparks-debate-over-whether-coronavirus-can-infect-the-nervous-system-101524986.html). Though this is a rare case, in terms of clinical treatment, it provides some important indications. When the central nervous system is infected, cerebral edema or even hernia may occur, which is life-threatening. It is necessary to monitor the neurological indicators to avoid the death. Since the primary damage of SARS-CoV-2 infection is mainly in the lungs, whether viral infection leading to pathological damage in other tissues and organs is primary or secondary remains to be further investigated.
4.2. Laboratory manifestations
Laboratory abnormalities provide some vital implications that may serve as predictors for severity of disease. Older age, higher sequential organ failure assessment score, and levels of D-dimer greater than 1 μg/mL were three potential risk factors related to poor prognosis, as reported by Zhou et al. [5]. White blood cells count, neutrophil count, and levels of D-dimer, creatine kinase, and creatine were higher in ICU patients compared to non-ICU patients [14]. Liu et al. found that the viral load of SARS-CoV-2 from patients’ respiratory tracts was positively correlated to the lung disease severity. Albumin, percentage of lymphocytes and neutrophils, lactate dehydrogenase, and C-reactive protein were highly associated to the acute lung injury and may be useful to predict the disease severity. Notably, the angiotensin II levels in the plasma of the infected patients were markedly higher than that in healthy persons and strongly related to viral load and pulmonary injury, suggesting the potential treatment with angiotensin receptor blockers for COVID-19 [51].
5. Pathophysiology
One’s own immunity serves as a powerful defense against pathogens. Imbalance of immune function occurs if the immune system of the individual to the infection is less active or hyperactive than normal.
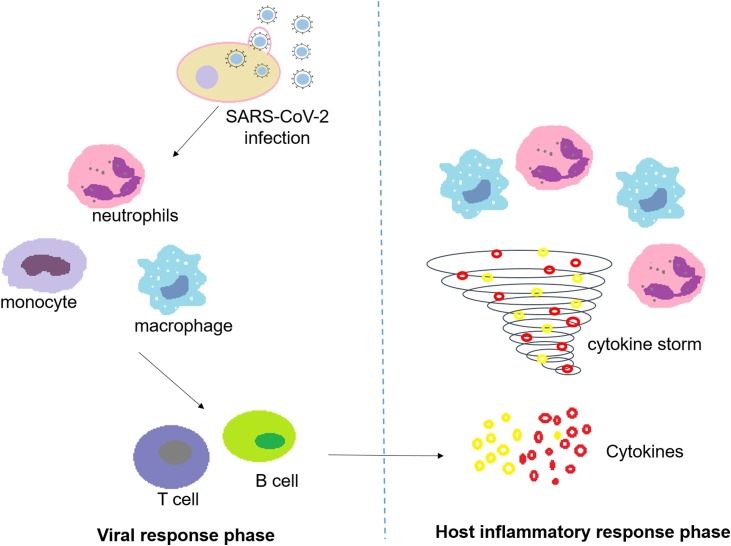
During the SARS-CoV-2 infection, the induced responses are two phased (Fig. 1 ). In the viral response phase, virus infected cells activate an innate immune response, recruiting neutrophils, macrophages and monocytes. To clear the virus, the specific immune response priming the adaptive T and B cell subsequently is required to promote. In most cases of incubation and mild to moderate stages, normally this process is able to resolve the infection. However, in some cases, virus can inhibit the inherent immunity and thus evade the acquired immune response. This imbalance of immune function occurs resulting in propagation of virus and damage of tissues. Xu et al. reported that the counts of peripheral CD4 and CD8 T cells of a patient with severe immune injury caused by COVID-19 were reduced but their status was hyperactivated [52]. Zhang et al. reported a couple cases and found that the levels of immune cells CD3, CD4, CD8, CD19 and CD16+56 were declined during the onset period of infection. CD19+ and CD16+56+ cells, the cytotoxic natural killer cells exerting antiviral activity, were increased when the patient’s condition obtained improvement, suggesting the predictor role of CD19+ and CD16+56+ as progression of infection [53].
Fig. 1.
The viral response phase and host inflammatory response phase during SARS-CoV-2 infection.
In the host inflammatory response phase, damaged cells caused by SARS-CoV-2 infection in the lungs induce inflammation that is mainly regulated by pro-inflammatory macrophages and granulocytes. Hyperactive immune response of the individual to the infection may trigger a cytokine storm, resulting in life-threatening widespread pulmonary inflammation. Huang et al. reported that cytokine storm was closely related to the disease severity in SARS-CoV-2 infection. Initial concentrations of IL1B, IL1RA, IL7, IL8, IL9, IL10, basic FGF, GCSF, GMCSF, IFNγ, IP10, MCP1, MIP1A, MIP1B, PDGF, TNFα, and VEGF in plasma were higher in infected patients than in healthy persons. Among the infected patients, levels of IL2, IL7, IL10, GCSF, IP10, MCP1, MIP1A, and TNFα in plasma were higher in ICU patients as compared to non-ICU patients [54]. However, the questions that remains unanswered are: How does the body perceive the virus and release cytokines? Why and how higher levels of cytokines cause the deterioration of clinical conditions and even mortality? Which cytokines and their pathways can be potential therapeutic targets? In response to viral infections, the host needs to effectively mobilize the immune system to clear virus and avoid the cytokine storm triggered by over activation. Our understanding of virus-induced cytokine storms and the balance between immune system and virus is still limited. To address these problems, we still have a long way to go.
6. Detection and diagnosis
6.1. Nucleic acid testing
Currently, nucleic acid testing kits are commercial available and still the gold standard to confirm the presence of the SARS-CoV-2. Chu’s group has established two one-step quantitative reverse transcription-PCR assays. Primers binding to the ORF1b and N genes of subgenus sarbecoviruses respectively were used to amplify the viral genome. But these developed assays couldn’t distinguish SARS-CoV, SARS-CoV-2, and other sarbecoviruses, further sequence analyses of amplicons that are positive in RT-PCR results are required to confirm the species [55]. Shirato et al. have developed two nested RT-PCR assays with primers bounding to the ORF1 and S protein, and two real-time RT-PCR assays with primers binding to the sequence encoding N protein, successfully detecting 25 SARS-CoV-2 positive infected patients in Japan [56]. Corman et al. have designed a workflow for diagnosis of novel coronavirus without the virus isolates available. Making use of the genetically similarity to 2003 SARS-CoV and synthetic nucleic acid technology, they have developed three RT-PCR assays as follows, primers binding to E gene can detect bat-associated SARS-related coronaviruses; specific probe hybridizing to RNA-dependent RNA polymerase (RdRp) was used to make a distinction between SARS-CoV-2 and SARS-CoV; the RT-PCR assay designed to amplify the region of N gene was less sensitive so that it was not further validated [57].
Normally the throat and nasal swab in the upper respiratory tract of the patients are sampled for nucleic acid testing, which is fast and convenient for physicians to operate and causes no trauma to the patients. Nonetheless, novel coronavirus mainly infects the lower respiratory tract and the viral load in the upper respiratory tract may not be high enough for detection, resulting in false negatives [58]. In addition, given the viral replicative activity is influenced by the host immune system, it is possible that one has clinical symptoms but the quantity of virus is not sufficient for detection, leading to the occurrence of false negative results as well. What’s more, the progression and severity of pneumonia cannot be concluded based on the real-time RT PCR results. Infected patients with false negative RNA results bring enormous challenges for medical workers to provide appropriate treatment and take isolation measures in time. Diagnosing the confirmed COVID-19 cases merely by nucleic acid testing is obviously not accurate enough.
6.2. Chest CT images
The most common manifestations of chest CT in patients with novel coronavirus pneumonia are ground glass opactities and patchy consolidation in bilateral lungs. According to the distributions and types of lesions, chest CT manifestations can be divided into three stages: at the early stage, only a single ground-glass opacity or irregular solid nodules of varying sizes can be seen. As the infection progresses, ground-glass opactities with a rounded morphology, reticulation, or crazy paving pattern and patchy, parenchyma or mixed consolidation distributed in the middle and peripheral area of bilateral lungs may present. Nodules involving multiple lobes are increased and enlarged. A few patients may have small pleural effusions. When the disease progresses to a critical stage, more extensive pulmonary consolidation and ground-glass opactities, diffuse pulmonary lesions, increased density of bilateral lungs and even “white lung” may appear. Pleural effusions, lymphadenopathy and an air bronchogram sign may present in a small number of patients [[59], [60], [61], [62], [63], [64], [65]]. A clinical trial involving in using CT scores to predict the mortality of patients with SARS-CoV-2 pneumonia have been completed (NCT04284046).
It is not surprising that patients have false negative results in nucleic acid testing but the CT images show some features of novel coronavirus infection [65]. Therefore, in the latest edition of China’s diagnosis and treatment guidelines for new coronavirus pneumonia, chest CT scan is one of the clinical diagnosis standards of suspected cases and the nucleic acid testing kit is then used to make a clinical diagnosis of confirmed cases of novel coronavirus infection.
6.3. Antibody detection
Not as nucleic acid testing or Chest CT images that can detect acute infection, antibody tests detects the person ever infected, especially those with asymptomatic infection. IgG and IgM antibodies in serum of SARS-CoV-2 infected patients arise simultaneously or sequentially within around 1–3 weeks after illness onset [66]. N and different forms of S protein are the major antigens for detecting the antibodies induced by SARS-CoV-2 infection. Different types of assays for antibody testing can be applied to assess different aspects of immunological response and function of antibodies induced by virus infection.
Zhao et al. have developed a serology ELISA kit using CHO cell expressing fully glycosylated, full length SARS-CoV-2 S1 protein for surveillance of the infection at the early stage. Glycosylation of S1 protein is important to facilitate the protein folding properly and increase the protein’s affinity to the receptor, ensuring the great sensitivity and high specificity of the ELISA kit [67]. Padoan et al. have developed a chemiluminescence immunoassay using MAGLUMITM 2000 Plus for simultaneously detecting SARS-CoV-2 IgG/IgM in sera. Using this system, serum tubes with a gel separator can be the primary samples for direct viral inactivation, which is safe for the operators. They measured the kinetics of immunoglobulin time and showed the rapid increase of IgM and IgG on 6–7 days since the symptom onset [68]. Perera et al. have developed an ELISA assay using recombinant receptor-binding domain of the S protein for screening the SARS-CoV-2 infection and plaque reduction neutralization tests using live virus for confirming the induced protective immunity. This strategy could be used to evaluate infection attack rates in the population and estimate the severity of illness and herd immunity [69]. Nie et al. have developed a pseudovirus-based neutralization assay using a vesicular stomatitis virus pseudovirus production system for determining neutralizing antibodies against SARS-CoV-2. Full-length spike protein of SARS-CoV-2 was pseudotyped in the virus, mimicking the viral attachment and entry during infectious process. This assay is much safe and can be performed in BSL-2 laboratories [70].
6.4. Other diagnosis methods
Except for the methods mentioned above, other diagnosis methods are expected to increase the detection rates of infection. A clinical trial on evaluation of rapid diagnostic kit detecting IgM/IgG for novel coronavirus pneumonia is ongoing in China (ChiCTR2000029870). CRISPR-Cas system applied as a diagnostic tool is characterized by fast speed, highly specificity and sensitivity. Hou et al. have developed a rapid diagnostic assay based on CRISPR-Cas13a with a near single-copy sensitivity for SARS-CoV-2 detection [71]. Zhang et al. have published a detailed protocol using the CRISPR-based SHERLOCK system for detection of COVID-19. Without PCR amplification, the results can be read out in less than an hour (https://www.broadinstitute.org/files/publications/special/COVID19%20detection%20(updated). pdf).
Lamb et al. have developed a rapid diagnostic test for COVID-19 virus using Revers Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) that can be applied to rapidly detect the virus in serum, urine, saliva, oropharyngeal swabs, and nasopharyngeal swabs in 30 min [72]. Yu et al. have established an isothermal LAMP based system coupled with a pH indicator allowing the colorimetric detection for COVID-19 coronavirus (iLACO). This iLACO is rather sensitive that can detect as low as 10 copies of the targeted ORF1ab gene in 15−40 min. Compared to RT-PCR, these two approaches are more rapid, accurate, and can be conducted with limited facilities [73].
7. Treatments
Currently no specific antiviral medication or vaccine is approved for treating patients with suspected or confirmed COVID-19. Chinese National Health Commission recommends the use of interferon-α, lopinavir/ritonavir, ribavirin, chloroquine, and arbidol for confirmed SARS-CoV-2 infections. Inappropriate use of antibacterial agent, especially the broad spectrum antibiotic, is not suggested. Plasma from cured patient is suitable for the severe and critically ill patients. Blood purification therapy can remove inflammatory factors and block cytokine storms, thus relieving the damage of inflammation to the body, which is useful for the severe and critically ill patients having cytokine-release syndromes in the early and middle stages. Tocilizumab can be used for the severe patients with elevated IL-6 levels. Traditional Chinese medicines (TCMs) have a long history of application in human disease in China. Chinese health authorities also recommend dialectical treatments for patients in different conditions, eg, jinhuaqinggan granule, lianhuaqingwen capsule, and shufengjiedu capsule for the people in medical observation period; lung-clearing and detoxification decoction for moderate or severe patients, etc.
As no licensed drug for COVID-19 at present, efforts to discover antivirals targeting virus at various stages during infection and host factors involved in the virus life cycle, regulation of immune system or other cellular processes, are on the fast track. We search the ClinicalTrials.gov and give a brief introduction of some representative drugs and therapies as follows (Table 1 ).
Table 1.
Representative treatments under clinical trials for COVID-19.
| classification | Conditions | drug /cocktail/intervention | mechanism | Phases | NCT Number |
|---|---|---|---|---|---|
| direct-acting antiviral | Severe COVID-19 | Remdesivir | RdRp inhibitor for Ebola virus disease and Marburg virus infections | Phase 3 | NCT04292899 |
| Moderate COVID-19 | Phase 3 | NCT04292730 | |||
| Mild/Moderate COVID-19 | Phase 3 | NCT04252664 | |||
| COVID-19 | Phase 2 | NCT04280705 | |||
| Severe COVID-19 | Phase 3 | NCT04257656 | |||
| moderate COVID-19 | Clevudine | inhibition of HBV DNA polymerase and reverse transcriptase. | Phase 2 | NCT04347915 | |
| COVID-19 | Carrimycin | Macrolides antibiotic for systemic bacterial infections | Phase 4 | NCT04286503 | |
| Suspected/Mild/common COVID-19 | combination of Bromhexine Hydrochloride Tablets, Arbidol Hydrochloride Granules, Recombinant Human Interferon-α2β Spray, Favipiravir Tablets | Bromhexine Hydrochloride: clearing mucus from the respiratory tract; Arbidol Hydrochloride: membrane fusion inhibitor for influenza, Favipiravir:RdRp inhibitor for influenza; Oseltamivir: neuraminidase inhibitor for influenza A and B, Lopinavir/ritonavir: protease inhibitor; ASC09: Protease inhibitor for HIV; Ganovo: protease inhibitor for Hepatitis C virus; Darunavir/Cobicistat: protease inhibitor for HIV; Ribavirin: nucleoside inhibitor for RSV infection, hepatitis C and some viral hemorrhagic fevers. |
Not Applicable | NCT04273763 | |
| COVID-19 | Abidol hydrochloride, Oseltamivir, Lopinavir/ritonavir | Phase 4 | NCT04255017 | ||
| COVID-19 | ASC09/oseltamivir, ritonavir/oseltamivir, oseltamivir | Phase 3 | NCT04261270 | ||
| COVID-19 | Arbidol | Phase 4 | NCT04260594 | ||
| COVID-19 | Ganovo + ritonavir+/-Interferon atomization, Long acting interferon, Recombinant cytokine gene-derived protein, Lopinavir + ritonavir,Chinese medicines + interferon atomization | Phase 4 | NCT04291729 | ||
| direct-acting antiviral | COVID-19 | ASC09/ritonavir, lopinavir/ritonavir | Not Applicable | NCT04261907 | |
| COVID-19 | Darunavir/Cobicistat | Phase 3 | NCT04252274 | ||
| COVID-19 | combination of Lopinavir/ Ritonavir, Ribavirin and IFN-beta |
Phase 2 | NCT04276688 | ||
| COVID-19 | Abidol Hydrochloride combined with Interferon atomization | Phase 4 | NCT04254874 | ||
| host target agent | COVID-19 | Duvelisib | PI3K inhibition | Phase 2 | NCT04372602 |
| COVID-19 | Recombinant human angiotensin-converting enzyme 2 (rhACE2) | receptor of SARS-CoV-2 | Not Applicable | NCT04287686 | |
| COVID-19 | Isotretinoin | inhibition of ACE 2 receptor | Phase 3 | NCT04361422 | |
| Severe/Critical COVID-19 | Bevacizumab Injection | anti-VEGF drug for cancers | Phase 2|Phase 3 | NCT04275414 | |
| COVID-19 | Hydroxychloroquine | antimalarial drug | Phase 3 |
NCT04261517 NCT04329611 |
|
| COVID-19 | Anti-CD147 Humanized Meplazumab for Injection | CD147: receptor of SARS-CoV-2 | Phase 1 |Phase 2 |
NCT04275245 | |
| COVID-19 | Lactoferrin | non-specific host defense, inhibition of virus entry, reduce IL-6, TNF a, and downregulate ferritin |
Phase 2 Phase 3 |
NCT04421534 | |
| COVID-19 Requiring Hospitalization | Losartan | AT1 inhibitor | Phase 2 | NCT04312009 | |
| COVID-19 Not Requiring Hospitalization | Losartan | Phase 2 | NCT04311177 | ||
| COVID-19 | Losartan | Phase 1 | NCT04335123 | ||
| host target agent | COVID-19 Associated Acute Respiratory Distress | Aviptadil | prevents NMDA-induced caspase-3 activation in the lung, inhibits cytokines production, protects against pulmonary edema | Phase 2 | NCT04311697 |
| COVID-19 | Sildenafil citrate tablets | inhibitor of cGMP-specific phosphodiesterase type 5, improve pulmonary oxygenation | Phase 3 | NCT04304313 | |
| Immunoregulation therapy | Moderate COVID-19 | thalidomide | anticancer drug for multiple myeloma | Phase 2 | NCT04273529 |
| Severe COVID-19 | thalidomide combined with Low-dose Hormones | anti-inflammatory, anti-fibrotic, anti-angiogenesis, and immune regulation | Phase 2 | NCT04273581 | |
| COVID-19 | Fingolimod | immunomodulating drug for multiple sclerosis, phingosine-1-phosphate receptor regulators, effective immunology modulator | Phase 2 | NCT04280588 | |
| severe COVID-19 with lymphocytopenia | PD-1 blocking antibody, Thymosin | PD-1 blocking antibody: immune checkpoint inhibitor, prevent T cell exhaustion; Thymosin: regulate cellular immunity | Phase 2 | NCT04268537 | |
| COVID-19 | Ozanimod | immune modulator | Phase 2 | NCT04405102 | |
| COVID-19 | recombinant human interferon-α2β | an antiviral or antineoplastic drug for a wide range of viral infections and cancers | Early Phase 1 | NCT04293887 | |
| Severe COVID-19 | Intravenous Immunoglobulin | passive immunity and anti-inflammatory, immunomodulatory effect | Phase 2|Phase 3 | NCT04261426 | |
| Moderate to Severe COVID-19 | COVID Convalescent Plasma | neutralizing antibodies directly bind to the spike protein to prevent viral entry | Early Phase 1 | NCT04412486 | |
| Severe COVID-19 | Immunoglobulin of cured patients | Not Applicable | NCT04264858 | ||
| COVID-19 | Anti-SARS-CoV-2 Inactivated Convalescent Plasma | Not Applicable | NCT04292340 | ||
| Immunoregulation therapy | Severe COVID-19 | Anti-SARS-CoV-2 convalescent plasma | neutralizing antibodies directly bind to the spike protein to prevent viral entry | Phase 2 | NCT04354831 |
| Severe COVID-19 | convalescent plasma | Phase 2 | NCT04415086 | ||
| critical Covid-19 | hyperimmune plasma | completed | NCT04321421 | ||
| COVID-19 | Eculizumab | modulate the activity of the distal complement preventing the formation of the membrane attack complex | Not Applicable | NCT04288713 | |
| Severe COVID-19 | CD24Fc | innate checkpoint against the inflammatory response | Phase 3 | NCT04317040 | |
| Severe COVID-19 | Methylprednisolone | Methylprednisolone: used for a wide range of conditions. suppress the immune system and decrease inflammation | Phase 4 | NCT04263402 | |
| COVID19 Critically Ill Patients With Severe Acute Respiratory Failure | Methylprednisolone | Phase 2|Phase 3 | NCT04244591 | ||
| COVID-19 | Sarilumab | an inhibitor of the receptor of interleukin 6 | Phase 2|Phase 3 | NCT04315298 | |
| Critical COVID-19 | Naproxen | anti-inflamatory | Phase 3 | NCT04325633 | |
| Cytokine Release Syndrome in COVID-19 | Tocilizumab | an inhibitor of the receptor of interleukin 6 | Not Applicable | NCT04306705 | |
| COVID-19 with viral Inflammation | Stem Cell Educator-Treated Mononuclear Cells Apheresis | reverse the autoimmune response | Phase 2 | NCT04299152 | |
| COVID-19 | Natural killer Cells | mobilize the immune system to actively kill the invading viruses and the infected cells. | Phase 1 | NCT04280224 | |
| COVID-19 | NKG2D-ACE2 CAR-NK cells | secreting super IL15 superagonist and GM-CSF neutralizing scFv | Phase 1 Phase 2 |
NCT04324996 | |
| COVID-19 | FT516 | anti-viral activity of NK cells | Phase 1 | NCT04363346 | |
| COVID-19 | SARS-CoV-2 specific T cells from convalescent donors | adoptive T-cell therapy | Not Applicable | NCT04351659 | |
| COVID-19 | PUL-042 Inhalation Solution | innate immune stimulant | Phase 2 | NCT04313023 | |
| Severe COVID-19 | PUL-042 Inhalation Solution | Phase 2 | NCT04312997 | ||
| vaccine | COVID-19 | Injection and infusion of LV-SMENP-DC vaccine and antigen-specific CTLs | LV-SMENP-DC vaccine is made by modifying DC with lentivirus vectors expressing Covid-19 minigene SMENP and immune modulatory genes. CTLs will be activated by LV-DC presenting Covid-19 specific antigens. | Phase 1|Phase 2 | NCT04276896 |
| COVID-19 | Recombinant Novel Coronavirus Vaccine | Adenovirus Type 5 Vector | Phase 1 |
NCT04313127 NCT04398147 |
|
| COVID-19 | Pathogen-specific aAPC | lentivirus modification including immune modulatory genes and the viral minigenes | Phase 1 | NCT04299724 | |
| COVID-19 | mRNA-1273 | a novel lipid nanoparticle -encapsulated mRNA-based vaccine that encodes for a full-length, prefusion stabilized spike protein of SARS-CoV-2 | Phase 1 |
NCT04283461 NCT04405076 |
|
| COVID-19 | SARS-CoV-2 inactivated vaccine | inactivated vaccine | Phase 1 Phase 2 |
NCT04383574 NCT04352608 NCT04412538 | |
| COVID-19 | SCB-2019 | a Recombinant SARS-CoV-2 Trimeric S Protein Subunit Vaccine | Phase 1 | NCT04405908 | |
| COVID-19 | Measles Vaccine | cross-protective antibodies | Phase 3 | NCT04357028 | |
| COVID-19 | BNT162a1, BNT162b1, BNT162b2, BNT162c2 | SARS-CoV-2 RNA vaccine | Phase 1 | NCT04368728 | |
| COVID-19 | V-SARS | vaccine made from heat-inactivated plasma from donors with COVID-19 | Phase 1 Phase 2 |
NCT04380532 | |
| COVID-19 | bacTRL-Spike | plasmids containing synthetic DNA encoding spike protein | Phase 1 | NCT04334980 | |
| vaccine | COVID-19 | BCG Vaccine | stimulate broad, innate components of the immune system | Phase 3 Phase 4 |
NCT04348370 NCT04384549 NCT04373291 |
| Mesenchymal Stem Cells (MSCs) | COVID-19 | Human UC-MSCs | regulate the immune response, reduce inflammation and affect tissue regeneration | Phase 1 | NCT04293692 |
| Severe COVID-19 | MSCs | Phase 1 |Phase 2 |
NCT04288102 | ||
| COVID-19 | MSCs | Phase 1 | NCT04252118 | ||
| Severe COVID-19 | Human UC-MSCs | Not Applicable | NCT04273646 | ||
| COVID-19 Patients Using Wharton's Jelly-Mesenchymal Stem Cells | Wharton's Jelly-MSCs | Phase 1 | NCT04313322 | ||
| COVID-19 | UC-MSCs | Phase 2 | NCT04269525 | ||
| Severe COVID-19 | Dental Pulp MSCs | Early Phase 1 | NCT04302519 | ||
| Severe COVID-19 | MSCs-derived exosomes | Phase 1 | NCT04276987 | ||
| TCM | COVID-19 | Huaier Granule | an adjuvant drug for primary hepatic carcinoma | Phase 2 |Phase 3 |
NCT04291053 |
| COVID-19 with Pulmonary Fibrosis | N-acetylcysteine + Fuzheng Huayu Tablet | inhibit MMP activity to protect subepithelial basement membrane which plays a key role in lung injury and interstitial fibrosis. | Phase 2 | NCT04279197 | |
| Mild/Common COVID-19 | Yinhu Qingwen Decoction | antiviral activity | Phase 2 |Phase 3 |
NCT04278963 | |
| COVID-19 | T89 capsule | Improve cardiovascular function and relieve angina | Not Applicable | NCT04285190 | |
| General/Severe COVID-19 | Xiyanping Injection | treatment for bronchitis, has anti-inflammatory and immune regulation effects | Not Applicable | NCT04295551 | |
| 2019-nCoV Pneumonia | Xiyanping injection | Not Applicable | NCT04275388 | ||
| mild and severe neo-coronary pneumonia | Tetrandrine | anti-fibrosis | Phase 4 | NCT04308317 | |
| VC | Severe COVID-19 | Vitamin C Infusion | essential nutrient | Phase 2 | NCT04264533 |
| NO | COVID-19 | Nitric Oxide Gas Inhalation | gaseous signaling molecule | Phase 2 | NCT04388683 |
| Severe Acute Respiratory Syndrome in COVID-19. | Nitric Oxide Gas Inhalation | Phase 2 | NCT04290871 | ||
| Mild/Moderate COVID19 | Nitric Oxide Gas Inhalation | Phase 2 | NCT04290858 | ||
| washed microbiota transplantation | COVID-19 | washed microbiota transplantation | immunomodulatory effect | Not Applicable | NCT04251767 |
Data were collected from https://clinicaltrials.gov by June 9, 2020.
7.1. Direct-acting antiviral
7.1.1. Remdesivir
Remdesivir, as a nucleotide analogue inhibiting RNA-Dependent RNA Polymerase, has shown broad-spectrum antiviral activities against RNA viruses including SARS-CoV, MERS-CoV and Ebola virus [40,74]. Xiao’s group firstly found that remdesivir and chloroquine are potently effective against SARS-CoV-2 infection with rather low EC50 value and high selective index in vitro [75]. Notably, intravenously administration of remdesivir after development of pneumonia remarkably improved clinical condition, reported in the first case of novel coronavirus infection in the united states [76], though this could not be definitely ascribed to the effect of the drug. Currently, clinical trials are ongoing evaluating the efficacy and safety of remdesivir in patients hospitalized with mild, moderate (NCT04252664) and severe (NCT04257656) COVID-19. Up to now, several results of clinical trials on the efficacy of remdesivir for COVID-19 have published. Beigel et al. have reported a multicenter trial enrolling 1063 patients that conducted in 60 sites and 13 subsites globally [77]. In the remdesivir treated group, the time to recovery was significantly shorter compared to the placebo (NCT04280705). Gilead Sciences company reported the improved clinical outcomes of the patients with severe COVID-19 treated with remdesivir in a compassionate-use program. No new safety signal was observed during the therapy [78]. In contrast, in a multicenter trial conducted at ten hospitals in Hubei, China, use of remdesivir in severe COVID-19 patients was not statistically significantly correlated to improve the time to clinical improvement, mortality, or time to eliminate virus [79]. The opposite clinical outcomes resulting from various studies may mainly ascribe to different clinical parameters setting, outcome measures, and eligibility criteria for enrollment. Larger sample sizes and more rigorous clinical trial designs are required to further confirm the potency of remdesivir against SARS-CoV-2 infection.
7.1.2. Arbidol
Arbidol is licensed in Russia and China mainly for treatment of influenza infection and other respiratory viral infections [80]. It is both a direct-acting antiviral and host-targeting agent with broad-spectrum activity interacting with membrane and viral or cellular proteins, exerting inhibition ability involved in viral entry, viral fusion, replication, assembly, budding, and stimulation of host immune responses [81]. In February 2020, Li Lanjuan, a famous Chinese epidemiologist, claimed that arbidol and darunavir in preliminary tests could inhibit the replication of new coronavirus in vitro. She proposed using arbidol and darunavir for treating pneumonia patients suffering from SARS-CoV-2 infection. Prominently, novel coronavirus infected patients in Wuhan and Shanghai receiving arbidol showed significant signs of improvement in pneumonia [53,82]. Phase IV clinical trials involving arbidol/arbidol hydrochloride alone or in combination with interferon atomization, oseltamivir or Lopinavir/Ritonavir for treatment of SARS-CoV-2 viral pneumonia are under investigation (NCT04255017, NCT04260594, NCT04254874).
7.1.3. Lopinavir/ritonavir
Lopinavir/ritonavir is a fixed dose combination medication approved by FDA acting as a potent protease inhibitor for HIV-1 infection [83]. Lopinavir/ritonavir are potential candidates for SARS-CoV and MERS-CoV infection [84,85]. Molecular dynamics simulations studies have showed that lopinavir/ritonavir could bind to the active site of SARS-CoV 3CLpro [86]. An open clinical trial conducted in Hong Kong during the SARS-CoV outbreak in 2003 revealed that patients treated with lopinavir/ritonavir had a lower viral load and were associated with better clinical outcomes and fewer adverse effect [84]. Lopinavir/ritonavir combined with recombinant interferon beta-1b is also being investigated for treatment of MERS-CoV infection (NCT02845843) [87]. Since the protein sequence of main protease of SARS-CoV and SARS-CoV-2 are closely related with 96 % identity, it is possible that lopinavir/ritonavir can be applied to novel coronavirus infection. However, a clinical trial conducted in hospitalized adult patients with severe COVID-19 in China showed no benefit with lopinavir/ritonavir treatment over standard care (ChiCTR2000029308) [88]. Whether combination of lopinavir/ritonavir with other antivirals would have improvement of clinical outcomes remains to be further determined.
7.2. Host target agent
7.2.1. Chloroquine
Chloroquine, a FDA-approved drug for anti-malarial and autoimmune disease [89], is also known as a potent antiviral of SARS-CoV infection in vitro for interrupting the glycosylation of cell surface receptor, ACE2 [90]. Chloroquine also showed inhibitory effect on the replication of MERS-CoV in vitro [91]. Recently, chloroquine were tested against SARS-CoV-2 with EC90 at low micromolar concentration and may restrict the viral entry and post entry processes in vitro [75]. Chloroquine is an immunomodulation agent that may enhance its antiviral activity as well. In light of the safety and potent efficacy, clinical study testing chloroquine against pneumonia caused by SARS-CoV-2 is being conducting (NCT04261517). Preliminary results from the multicenter clinical trials conducted in China have demonstrated that chloroquine phosphate effectively inhibited the progression of pneumonia, eliminated the virus, and shortened the duration of diseases without any severe adverse effects [92,93]. However, data of an open label, randomized controlled trial conducted in China (ChiCTR2000029868) enrolling patients with mild to moderate COVID-19 and two observational studies involving a large number of consecutive hospitalized patients with COVID-19 in New York City did not support the use of hydroxychloroquine because it was not associated with either a significantly higher or lower risk of intubation or death, or lower in-hospital mortality [[94], [95], [96]]. In the absence of evidence of therapeutic effects, we particularly need to understand whether the drug’s safety risks are worth taking.
7.2.2. Recombinant human angiotensin-converting enzyme 2 (rhACE2)
ACE2, an important regulatory component in the renin-angiotensin-aldosterone system (RAAS), not only mediates virus infection, but also directly participates in the progression of acute lung injury that highly correlated with the high mortality [97]. SARS-CoV-2 infection has been shown to significantly reduce the expression of ACE2 in lung, resulting in aggravated acute lung injury. Conversely, overexpression of ACE2 in the knockdown mice model of acute respiratory distress syndrome offered some protection, suggesting the potential therapeutic effect of rhACE2 in SARS-CoV-2 infection [98]. On the one hand, elevated expression of ACE2 may increase the risk of viral infection; on the other hand, higher expression of ACE2 may have a protective effect against acute lung injury. So far the exact role of ACE2 expression remains unknown, and the impacts on susceptibility of SARS-CoV-2 and prognosis of patients need more basic and clinical studies for further confirmation (NCT04287686).
7.2.3. Anti-CD147 antibody
Except for ACE2 as a receptor of host cells for SARS-CoV-2 attachment and entry, it is reported that CD147, which expresses in many human cell types including epithelial cells, endothelial cells and leukocytes, also facilities the invasion of SARS-CoV-2 via binding to spike protein [99]. Blocking the interaction between spike protein of SARS-CoV-2 and CD147 via anti-CD147 humanized antibody was proved to be able to inhibit the replication of SARS-CoV-2 in vitro, offering a novel target for antiviral therapy (NCT04275245) [100].
7.3. Immunoregulation therapy
7.3.1. Methylprednisolone
Methylprednisolone is a corticosteroids medication for inhibiting the immunity and reducing inflammation [101]. New coronavirus infected patients are complicated by acute lung injury and acute respiratory distress syndrome, which are partly caused by host overactive immune responses [102]. Corticosteroids can decrease inflammation of the lungs but also suppress the immune response and increase viral replication. Treatments of SARS and MERS-CoV infected patients with corticosteroids showed that inflammation persisted after clearance of virus. No sufficient evidence supported that SARS-CoV-2 infected patients would benefit from corticosteroids, and contrarily they may suffer from this therapy [103,104]. Two trials are recruiting critically ill patients with severe acute respiratory failure caused by novel coronavirus infection to test the efficacy and safety of methylprednisolone (NCT04263402, NCT04244591). However, corticosteroids should be used with caution for treating novel coronavirus. While considering the possible benefits, the risk of complications including psychosis, diabetes, avascular necrosis must be balanced against.
7.3.2. Immunoglobulin/ immunoglobulin of cured patients/Convalescent plasma
Immunoglobulin therapy is to use a mixture of normal human immunoglobulins for improving the immunity, which is especially effective for acute infections. This passive immunity through immunoglobulin injection can be acquired immediately but the duration is short [105]. Intravenous immunoglobulin suggested for treating severe and critically ill COVID-19 patients was proved to improve the clinical outcomes and prognosis [82]. Whereas SARS-CoV-2 is a novel virus and almost no antibodies are present in the healthy populations, intravenous immunoglobulin acting as a non-specific immune enhancer on mild or common patients may only have limited efficacy.
After virus infects the body, many different antibodies against viral proteins can be produced in the body, but most antibodies do not actually have an antiviral effect except for those that recognize proteins on the surface of the virus particles [106]. During the outbreak of SARS-CoV, infected patients treated with convalescent plasma carrying SARS-CoV-specific antibodies from cured patients have been suggested to yield clinical benefits with reduced viral load and lower mortality rate [107]. The underlying mechanism for the protective effect of convalescent plasma seems to be that the neutralizing antibodies produced in previously infected patients can directly bind to the spike protein on the virus particles to prevent them from entering the cells [108]. In addition to the neutralizing antibodies, other surface antibodies can bind to the virus and mediate the phagocytosis of the virus by macrophages, and further hydrolyze the viral nucleic acids and proteins. However, virus can also evolve a set of strategies to escape the degradation through macrophages and replicate to form new ones. Worse still, the viral replication in macrophages may promote the release of excess pro-inflammatory factors leading to cytokine storms, which is the major cause of acute respiratory distress syndrome and multiple organ failure [109].
At present, several clinical trials are ongoing testing the efficacy and safety of the anti-SARS-CoV-2 inactivated convalescent plasma (eg, NCT04292340) and the immunoglobulin from patients who recovered from SARS-CoV-2 pneumonia (NCT04264858) on the treatment of patients with acute severe SARS-CoV-2 pneumonia. Though the use of convalescent plasma or hyperimmune immunoglobulin may be a new strategy especially effective for the treatment of SARS-CoV-2 pneumonia, the potential risks of promotion of cytokine storms and transfusion-transmitted infection should be taken seriously.
7.3.3. PD-1 blocking antibody
Virus attacking the respiratory system and immune system causes the damage of immunological function with lymphocytopenia, decreased CD8 and CD4 T cell count that associated with severe SARS-CoV-2 pneumonia [14,51,54,103]. The host fights against the viral infection through virus-specific cytotoxic T lymphocytes. Reversion of T cell exhaustion is helpful to promote the antiviral immunity. PD-1 blocking antibody is one of the anticancer drugs that prevents the interaction between PD-L1 on the tumor cells and PD-1 on the T cells. Blocking the interaction of these cell surface proteins can modulate the immunological tolerance of tumors, thus preventing the cancer cells from evading the surveillance of immune system [110]. Diao et al reported that the expression levels of T cell exhaustion marker PD-1 from COVID-19 patients were much higher as compared to healthy controls [111]. Therefore, blocking PD-1 to prevent T cell exhaustion resulting in activation of antiviral immune responses may be a novel therapeutic strategy for COVID-19. However, before applying the PD-1 blocking antibody for treatment of lymphocytopenia induced by SARS-CoV-2 infection (NCT04268537), the correlation between PD-1 and T cell function should be measured to predict the response rate and the adverse effects such as cytokine-release syndromes and pneumonitis.
7.3.4. Mesenchymal Stem Cells (MSCs)/MSCs-derived exosomes
Patients infected with SARS-CoV-2 admitted to the intensive care unit had cytokine storms with increased secretion of IL2, IL7, IL10, GSCF, IP10, MCP1, MIP1A, and TNFα, associated with severe illness including acute respiratory distress syndrome and organ failure [102]. Mesenchymal stem cells (MSCs) can be an immunomodulatory agent inhibiting the secretion of cytokines, suppressing the overactive immune responses, and decreasing the inflammation. MSCs are safe and seldom induce rejection reactions to human body due to the inherently poor immunogenicity properties [112]. Regenerative potential of MSCs is helpful to repair the alveolar epithelial cells and vascular endothelial cells, leading to reduction of lung damage [113]. Owing to the immunoregulation, anti-inflammation, damaged tissue repair and regeneration properties, MSCs-based treatments have been applied to a variety of refractory diseases and are expected to have a critical role in novel coronavirus-induced severe pneumonia (NCT04293692, NCT04288102, NCT04252118, NCT04273646, NCT04269525).
MSCs-derived exosomes contain abundant biologically active substances such as signaling lipids, growth factors, cytokines, and RNA. Recent studies have revealed that exosomes are also important mediators for cell-cell communication via paracrine or endocrine manner [114]. MSCs-derived exosomes have similar biological functions as mesenchymal stem cells, for example, repairing and regeneration of tissues, suppression of inflammatory response and regulation of immunity [115]. MSCs-derived exosomes have been applied to the treatment of various lung diseases including pulmonary fibrosis, pulmonary arterial hypertension, lung cancer, bronchopulmonary dysplasia, and acute respiratory distress syndrome [116]. Studies have demonstrated that MSCs-derived exosomes could significantly reduce lung pathologic damage and increase the survival rate of mice with pulmonary fibrosis [117]. Compared to MSCs-based therapy, its derived exosomes as cell-free therapy is much safer and easier to pass through the physical barriers due to their nano size [118], suggesting a broad application prospect in a variety of diseases especially the pulmonary diseases (NCT04276987).
7.4. TCMs
TCMs have a long history of medical use and are frequently used for the prevention and treatment of infection diseases in China. Based on TCM therapy concept of “multiple compounds-multiple targets-multiple effects”, a TCM prescription often contains multiple herbs working together to battle a disease. Within these mixture components, some work directly on the viral targets involving the life cycle including attachment, entry, transcription of viral mRNA, replication of viral genome, translation of viral protein, and the assembly, maturation and release of progeny virus particles [119]. To infect the host efficiently, viruses have evolved a series of strategies to escape the host’s innate and adaptive immune responses against viral infection. On the other hand, to fight against the virus, the host may release excessive or uncontrolled proinflammatory cytokines that correlated to tissue damage or organ failure [102]. Except working as direct-acting antivirals, most of the molecules in TCM are immunomodulators that could block the viral immune evasion or enhance the host antiviral immune responses, while the others may contribute to reducing the toxicity or enhancing the bioavailability [119]. Despite not often being recognized as a real medicine, TCMs actually have exhibited excellent anti-SARS-CoV-2 efficacy during the outbreak of COVID-19 as evidenced by the high recovery rate and low mortality rate of confirmed cases taking TCM in China [82,120]. Xiyanping is a licensed TCM injection in China used in the treatment of bronchitis, tonsillitis, bacterial dysentery. It mainly contains active compounds extracted from Andrographis paniculata (Chuan Xin Lian) [121]. In the 7th edition of the diagnostic and treatment guidelines for the COVID-19 issued by Chinese health authorities, Xiyanping injection is recommended for treating severe and critically ill patients with bacterial co-infection attributed to its antiviral, anti-inflammatory, antibacterial, and immune regulation activities. In addition to the significant antiviral efficacy, what calls for special attention is that the indication, dosage, and solvents of TCM injection should be used rationally to reduce the occurrence of side effects [122].
7.5. Vaccine
Strategies to develop vaccine for COVID-19 are mainly focused on the spike protein of SARS-CoV-2, which is crucial for virus entry. Zhu et al. have reported a first-in-human clinical trial of a replication defective adenovirus type-5 (Ad-5) vectored COVID-19 vaccine expressing the full-length spike glycoprotein of SARS-CoV-2 (NCT04313127). This vaccine was showed to be tolerated and the adverse reactions were transient and self-limiting. Vaccination could induce humoral and T-cell responses rapidly but partly diminished in some participants by reason of pre-existing anti-Ad5 immunity [123]. Mckay et al. have developed a self-amplifying RNA encoding pre-fusion stabilized spike protein of SARS-CoV-2 encapsulated within a lipid nanoparticle as a vaccine. Immunizations of self-amplifying RNA lipid nanoparticle have been shown to induce large amount of SARS-CoV-2 specific lgG antibodies, robust cellular responses and secretion of pluripotent cytokines in mice. Vaccination was also found to be capable of potently neutralizing a pseudotyped HIV virus expressing the spike protein. Unlike messenger RNA based vaccine (NCT04283461), self-amplifying RNA is able to encode any antigen of interest and only requires a minimum dose. More importantly, not as plasmid DNA requiring electroporation, this lipid nanoparticle-formulated vaccine is more convenient to use because it can be injected directly with a syringe and needle [124]. Smith et al. have reported the preclinical development of a synthetic DNA-based COVID-19 vaccine encoding SARS-CoV-2 spike glycoprotein and an N-terminal IgE leader for enhancing the expression and immunogenicity. Immunization of mice and guinea pigs could promote durable production of functional antibodies that strongly neutralize the pseudovirus and live virus and block the ACE2 binding to spike protein of SARS-CoV-2. Specific antibodies of anti-SARS-CoV-2 were found to mainly distribute in the lungs after immunization. SARS-CoV-2 S protein-specific T cell responses were also stimulated in immunized mice [125].
Except for targeting S protein, SARS-CoV-2 inactivated vaccine (NCT04383574, NCT04352608, NCT04412538) and SARS-CoV-2 RNA vaccine (NCT04368728) are also being investigated in the clinical trials. What’s more, repurposing other vaccines, such as BCG vaccine (NCT04384549, NCT04348370) and MMR vaccine (NCT04357028), may be a potential alternative strategy for treating COVID-19. For the outbreak that likely to stage a come back in the future, clinical trials of SARS-CoV-2 vaccines have been launched in many countries around the world, but it still takes time until the safe and effective vaccine against COVID-19 is available.
7.6. Other treatments
7.6.1. Washed microbiota transplantation
ACE2 not only expresses in the lung tissue, but also distributes in the intestinal epithelium [126]. Apart from invading the host's lung tissues, SARS-CoV-2 also interferes with the absorption of nutrients in the human body by binding to ACE2 of the intestinal epithelium, causing gastrointestinal discomfort [14]. The intestinal flora can regulate various lung diseases through the intestinal-lung axis with immunomodulatory effect, including viral pneumonia, asthma, tuberculosis, and chronic obstructive pulmonary disease. Maintaining the normal communication of intestinal-pulmonary axis is helpful to alleviate lung disease [127,128]. Intestinal malnutrition often occurs in patients with COVID-19 and sometimes develops to secondary bacterial infections and antibiotic-related diarrhea, suggesting the crucial connection between intestinal tract and respiratory tract. Washed microbiota transplantation as a rescue therapy for critically ill patients with diarrhea may have important clinical benefits (NCT04251767).
7.6.2. Vitamin C
Vitamin C, a kind of antioxidant, is essential for tissue repair and immune system function [129]. Vitamin C can prevent oxidative damage induced by virus infection and improve the immunity of patients with pneumonia. It has been reported that enterovirus/rhinovirus infection can cause devastating acute lung injury. High dose of intravenous vitamin C could attenuate the acute respiratory distress syndrome and relieve lung damage produced by enterovirus/rhinovirus infection. Down-regulation of reactive oxygen, nitrogen species, and pro-inflammatory genes by vitamin C may contribute to these curative effect [130,131]. Nevertheless, the role of vitamin C as monotherapy in the recovered patients is still uncertain. Before vitamin C can be applied as a therapy, the side effects of high dosage, such as nausea, indigestion, abdominal cramps, and diarrhea owing to the unabsorbed vitamin C in the intestine should be considered. Since the leading causes of death of SARS-CoV-2 infected patients are associated with acute respiratory distress and multiple organ failure, further investigations and larger trials should be performed to uncover the underlying mechanism and evaluate the exact efficacy of vitamin C.
The strong infectivity of SARS-CoV-2 highlights the need of early intervention with effective treatment. Although abundant clinical trials are registered and underway since the outbreak of COVID-19, the exact pathogenesis of SARS-CoV-2 has not been elucidated clearly yet, along with no approved drug or vaccine for either SARS-CoV or MERS-CoV as a reference, raising a significant challenge for specific and effective anti-SARS-CoV-2 drug development.
8. Conclusions
(1) SARS-CoV-2 infections currently have spread to more than 200 countries across 6 continents and is posing a profound threat to the global public health and economy. Proper managements to decrease the prevalence rate of COVID-19 patients have become more urgent with the expanding geographic distribution. Unfortunately, control measures available to prevent SARS-CoV-2 infection are very limited owing to the strong infectivity of the virus and the pattern of person-to-person transmission.
(2) Currently there is no vaccine or specific antivirals available for COVID-19. Though mountains of novel findings on COVID-19 are accumulating, it is clear that there are still fundamental gaps in our knowledge. A better understanding of the aetiology and pathogenesis of COVID-19 will help us to develop effective strategies for the prevention and treatment of the disease. There is still a long way to go to curb and combat this novel coronavirus.
Declarations of Competing Interest
None.
References
- 1.Guan W.J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linton N.M., et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J. Clin. Med. 2020;9(2) doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauer S.A., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020 doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan J.F., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4) doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X., et al. Potential of large "first generation" human-to-human transmission of 2019-nCoV. J. Med. Virol. 2020;92(4):448–454. doi: 10.1002/jmv.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothe C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu P., et al. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiura H., et al. The extent of transmission of novel coronavirus in Wuhan, China, 2020. J. Clin. Med. 2020;9(2) doi: 10.3390/jcm9020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai Y., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furukawa N.W., Brooks J.T., Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.To K.K., et al. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W., et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao F., et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Y., et al. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 20.Liang W., et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020 doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 21.Chen W., et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg. Microbes Infect. 2020;9(1):469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu C.W., Liu X.F., Jia Z.F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia J., et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng W., et al. 2020. Rhesus Macaques Can Be Effectively Infected With SARS-CoV-2 Via Ocular Conjunctival Route. [Google Scholar]
- 25.Chen H., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet. 2020;395(10226):760–762. doi: 10.1016/S0140-6736(20)30365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu N., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan J.F., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen N., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceraolo C., Giorgi F.M. Genomic variance of the 2019-nCoV coronavirus. J. Med. Virol. 2020;92(5):522–528. doi: 10.1002/jmv.25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helmy Y.A., et al. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020;9(4) doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang X., et al. On the origin and continuing evolution of SARS-CoV-2. Sci. Rev. 2020 doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forster P., et al. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delanghe J.R., Speeckaert M.M., De Buyzere M.L. The host’s angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin. Chim. Acta. 2020;505:192–193. doi: 10.1016/j.cca.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delanghe J.R., Speeckaert M.M., De Buyzere M.L. ACE Ins/Del genetic polymorphism and epidemiological findings in COVID-19. Clin. Chem. Lab. Med. 2020;58(7):1129–1130. doi: 10.1515/cclm-2020-0605. [DOI] [PubMed] [Google Scholar]
- 36.Delanghe J.R., Speeckaert M.M., De Buyzere M.L. COVID-19 infections are also affected by human ACE1 D/I polymorphism. Clin. Chem. Lab. Med. 2020;58(7) doi: 10.1515/cclm-2020-0425. [DOI] [PubMed] [Google Scholar]
- 37.Delanghe J.R., De Buyzere M.L., Speeckaert M.M. C3 and ACE1 polymorphisms are more important confounders in the spread and outcome of COVID-19 in comparison with ABO polymorphism. Eur. J. Prev. Cardiol. 2020 doi: 10.1177/2047487320931305. p. 2047487320931305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur. J. Prev. Cardiol. 2020 doi: 10.1177/2047487320922370. p. 2047487320922370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C., et al. Viral architecture of SARS-CoV-2 with post-fusion spike revealed by Cryo-EM. bioRxiv. 2020 [Google Scholar]
- 40.Warren T.K., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 42.Angeletti S., et al. COVID-2019: the role of the nsp2 and nsp3 in its pathogenesis. J. Med. Virol. 2020 doi: 10.1002/jmv.25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann M., et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020 [Google Scholar]
- 44.Hoffmann M., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coutard B., et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. p. 104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu A., et al. Mutations, recombination and insertion in the evolution of 2019-nCoV. BioRxiv. 2020 [Google Scholar]
- 47.Gordon D.E., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang N., et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guan W., et al. Comorbidity and its impact on 1,590 patients with COVID-19 in China: a Nationwide Analysis. MedRxiv. 2020 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao L., et al. 2020. Neurological Manifestations of Hospitalized Patients With COVID-19 in Wuhan, China: a Retrospective Case Series Study. [Google Scholar]
- 51.Liu Y., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020 doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Z., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z., et al. Clinical features and treatment of 2019-nCov pneumonia patients in Wuhan: report of a couple cases. Virol. Sin. 2020 doi: 10.1007/s12250-020-00203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu D.K.W., et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shirato K., et al. Development of genetic diagnostic methods for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020 doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- 57.Corman V.M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li D., et al. False-negative results of real-time reverse-transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2: role of deep-learning-Based CT diagnosis and insights from two cases. Korean J. Radiol. 2020 doi: 10.3348/kjr.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung M., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020 doi: 10.1148/radiol.2020200230. p. 200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang Y., et al. CT manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200280. p. 200280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020 doi: 10.1148/radiol.2020200241. p. 200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei J., et al. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200236. p. 200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan Y., Guan H. Imaging changes in patients with 2019-nCov. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan Y., et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie X., et al. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. p. 200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Long Q.X., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020 doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 67.Zhao R., et al. Early detection of SARS-CoV-2 antibodies in COVID-19 patients as a serologic marker of infection. Clin. Infect. Dis. 2020 [Google Scholar]
- 68.Padoan A., et al. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0443. [DOI] [PubMed] [Google Scholar]
- 69.Perera R.A., et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill. 2020;25(16) doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nie J., et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020;9(1):680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou T., et al. Development and evaluation of a CRISPR-based diagnostic for 2019-novel coronavirus. MedRxiv. 2020 [Google Scholar]
- 72.Lamb L.E., et al. Rapid detection of novel coronavirus (COVID-19) by reverse transcription-loop-Mediated isothermal amplification. MedRxiv. 2020 doi: 10.1371/journal.pone.0234682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu L., et al. Rapid colorimetric detection of COVID-19 coronavirus using a reverse tran-scriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic plat-form: iLACO. MedRxiv. 2020 doi: 10.1093/clinchem/hvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheahan T.P., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holshue M.L., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beigel J.H., et al. Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 78.Grein J., et al. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leneva I.A., et al. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res. 2009;81(2):132–140. doi: 10.1016/j.antiviral.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 81.Blaising J., Polyak S.J., Pecheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z., et al. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci. Trends. 2020 doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 83.Yang W., et al. Evaluation of pharmacokinetic interactions between long-acting HIV-1 fusion inhibitor albuvirtide and lopinavir/ritonavir, in HIV-infected subjects, combined with clinical study and simulation results. Xenobiotica. 2017;47(2):133–143. doi: 10.3109/00498254.2016.1166532. [DOI] [PubMed] [Google Scholar]
- 84.Chu C.M., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan J.F., et al. Treatment with Lopinavir/Ritonavir or Interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nukoolkarn V., et al. Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS-CoV 3CL(pro) inhibitors. J. Theor. Biol. 2008;254(4):861–867. doi: 10.1016/j.jtbi.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arabi Y.M., et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-beta1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21(1):8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cao B., et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plowe C.V. Antimalarial drug resistance in Africa: strategies for monitoring and deterrence. Curr. Top. Microbiol. Immunol. 2005;295:55–79. doi: 10.1007/3-540-29088-5_3. [DOI] [PubMed] [Google Scholar]
- 90.Vincent M.J., et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang R., et al. Development of small-molecule MERS-CoV inhibitors. Viruses. 2018;10(12) doi: 10.3390/v10120721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 93.Cortegiani A., et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang W., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369 doi: 10.1136/bmj.m1849. p. m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenberg E.S., et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020 doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Geleris J., et al. Observational study of Hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu R., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Imai Y., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yurchenko V., Constant S., Bukrinsky M. Dealing with the family: CD147 interactions with cyclophilins. Immunology. 2006;117(3):301–309. doi: 10.1111/j.1365-2567.2005.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang K., et al. 2020. SARS-CoV-2 Invades Host Cells Via a Novel Route: CD147-spike Protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chan O.W., et al. Methylprednisolone pulse therapy as an adjuvant treatment of Streptococcus pneumoniae meningitis complicated by cerebral infarction-a case report and review of the literature. Childs Nerv. Syst. 2020;36(2):229–233. doi: 10.1007/s00381-019-04485-6. [DOI] [PubMed] [Google Scholar]
- 102.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kui L., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Orange J.S., et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J. Allergy Clin. Immunol. 2006;117(4 Suppl):S525–53. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 106.Sanna P.P., Burton D.R. Role of antibodies in controlling viral disease: lessons from experiments of nature and gene knockouts. J. Virol. 2000;74(21):9813–9817. doi: 10.1128/jvi.74.21.9813-9817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mair-Jenkins J., et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J. Infect. Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen L., et al. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020 doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Syn N.L., et al. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18(12):e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 111.Diao B., et al. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019. COVID-19). 2020 doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang M., Yuan Q., Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int. 2018;2018 doi: 10.1155/2018/3057624. p. 3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Han Y., et al. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8(8) doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Phinney D.G., Pittenger M.F. Concise review: MSC-Derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 115.Yin K., Wang S., Zhao R.C. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark. Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mendt M., Rezvani K., Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54(Suppl 2):789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 117.Zhu Y.G., et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32(1):116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xin H., et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013;33(11):1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li T., Peng T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antiviral Res. 2013;97(1):1–9. doi: 10.1016/j.antiviral.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. p. 104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiao X.W., et al. Potential anti-angiogenic sulfates of andrographolide. J. Asian Nat. Prod. Res. 2013;15(8):809–818. doi: 10.1080/10286020.2013.803473. [DOI] [PubMed] [Google Scholar]
- 122.Wang Z.F., Xiang Y.Y., Xie Y.M. [Research on early warning signals of adverse drug reactions to parenterally administered xiyanping based on spontaneous reporting system (SRS) data] Zhongguo Zhong Yao Za Zhi. 2013;38(18):3008–3012. [PubMed] [Google Scholar]
- 123.Zhu F.C., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McKay P.F., et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine induces equivalent preclinical antibody titers and viral neutralization to recovered COVID-19 patients. bioRxiv. 2020 [Google Scholar]
- 125.Smith T.R.F., et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020;11(1):2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hamming I., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.O’Dwyer D.N., Dickson R.P., Moore B.B. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J. Immunol. 2016;196(12):4839–4847. doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marsland B.J., Trompette A., Gollwitzer E.S. The gut-lung Axis in respiratory disease. Ann. Am. Thorac. Soc. 2015;12(Suppl 2):S150–6. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 129.Padayatty S.J., et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 130.Fowler Iii A.A., et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J. Crit. Care Med. 2017;6(1):85–90. doi: 10.5492/wjccm.v6.i1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Berger M.M., Oudemans-van Straaten H.M. Vitamin C supplementation in the critically ill patient. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18(2):193–201. doi: 10.1097/MCO.0000000000000148. [DOI] [PubMed] [Google Scholar]