Abstract
Objective
Add to available understanding of COVID-19 to help decrease further spread of SARS-CoV-2 by providing protocol providers can consider when giving patients recommendations to retest as well as length of time for self-isolation.
Methods
We retrospectively collected data from the electronic medical record of patients in the Mayo Clinic Florida's COVID Virtual Clinic. Hundred and eighteen patients with detectable results for the virus were followed. Data reviewed in this study included (1) length of time from detectable to undetectable results; (2) length of time from onset of symptoms to undetectable result; (3) length of time from resolution of fever to undetectable result.
Results
Fifty-three percent of studied patients eligible for discontinuation of self-isolation had detectable viral RNA, and therefore, underwent repeat testing. In these patients, the mean from the date of their first detectable result to attaining an undetectable result was 14.89 days. The mean time for onset of symptoms to undetectable testing was 21.5 days.
Conclusions
Hundred and eighteen patients with detectable results for SARS-CoV-2 were followed in the Mayo Clinic Florida COVID Virtual Clinic; 53% of patients still showed detectable viral RNA despite meeting CDC guidelines for discontinuation of self-isolation, prompting us to propose following a more cautious guideline that other providers could consider as a strategy to discontinue self-isolation, including increasing length of days since symptom onset.
Key Words: SARS-CoV-2, COVID 19, Coronavirus, PCR, Self-Isolation, Telemedicine
COVID-19, the novel coronavirus SARS-CoV-2 infection, was first reported in Wuhan China on December 31, 2019, and has since spread, infecting over 9.2 million people worldwide as of June 24, 2020.1 There has been a global effort to better understand the disease. Some studies have shown patterns of transmission with viral shedding and the concern of asymptomatic transmission.2 , 3 It is vital to identify and isolate infected patients as early as possible, and maintain isolation through the full duration of illness. To better understand this new virus, further studies are needed to characterize its epidemiology and clinical presentation in the health care setting with hopes of developing improved guidelines for management in defined populations.
The CDC currently gives 2 guidelines for release from medical-isolation for those who have symptoms with a proven infection - a “test-based strategy” and a “symptom-based strategy.” With regard to release from self-isolation by the test-based strategy, patients are recommended to undergo repeat testing for viral detection via nasopharyngeal swab after they have met the following criteria: (1) fever-free for 72 hours, without fever reducing medications and the individual's other respiratory symptoms have improved AND, (2) the individual has had 2 consecutive negative RT-PCR swab tests >24 hours apart. For those undergoing “symptom-based strategy” the recommendations are (1) the individual is afebrile for 72 hours, (2) symptoms have improved, (3) and at least 10 days have passed since symptoms first appeared (recently increased from 7 days).4
However, there are still gaps in knowledge on the incubation period of the virus (time from exposure to onset of illness), serial interval distribution (delay between illness onset dates in successive cases in chains of transmission), and duration of infection.5 , 6 Prior studies on differing patient populations were able to calculate incubation periods with mean values varying from 5.2 days to as much as 7.1 days, while other studies had a range of 3.6-6.4 days. These studies also showed serial intervals ranging from 4.0 to 7.5 days.6 , 7 We have observed patients that had detectable results after the recommended timeline for discontinuation of isolation. This may be a contributing factor to the continued spread of this virus, as it has been documented in recent studies that, per person, the transmission rate of undocumented infections was 55% of documented infections (46%-62%). However, due to their greater numbers, undocumented infections were the infection source for 79% of documented cases.8 Therefore, adequately knowing a person's infectious status as well as proper management of positive patients is critical. Our hypothesis was that further guidelines were needed, likely including prolonging the duration of symptom following, to discontinue self-isolation. With a goal of decreasing further spread of the virus, data from the Mayo Clinic COVID Virtual Clinic (CVC) was retrospectively reviewed and interpreted to help develop additional, more conservative guidelines for discontinuation of self-isolation that providers may consider having their patients follow for self-isolation discontinuation.
Methods
Data collection
Data was retrospectively collected from the electronic medical records of Mayo Clinic Florida CVC patients. Our IRB application was deemed exempt. Patients were tested through an institutional drive-through testing site, the emergency room, or prior to discharge from the hospital. Testing performed using nasopharyngeal swabs with RNA detection via RT-PCR technique. The tests were reported as “detectable” and “undetectable.”
Those patients with detectable RNA were then followed throughout the course of their illness by multiple visits using telemedicine technology. Patients provided history to determine their demographics and risk factors. Documentation was regularly updated on their symptomatology, disease progression, and test results. The decision to discontinue self-isolation was made using the test-based strategy (Fig. 1 -3 ).
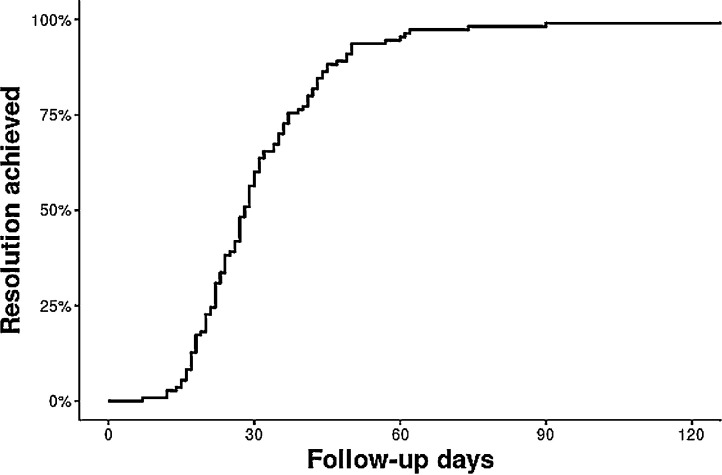
Fig 2.
. Kaplan-Meier curve of time to resolution.
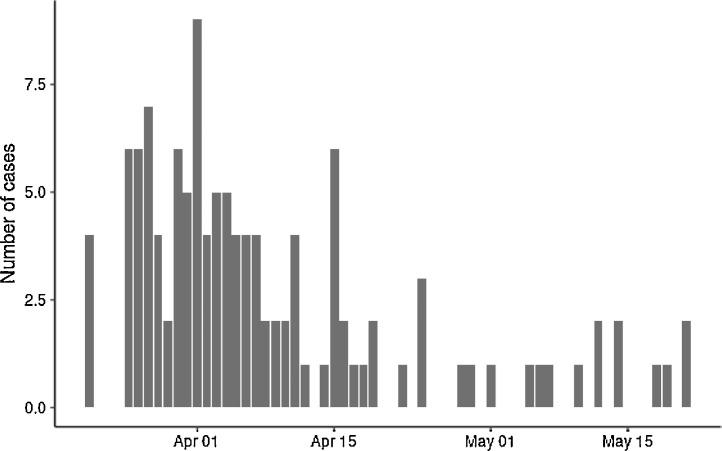
Fig 1.
.Number of cases by day.
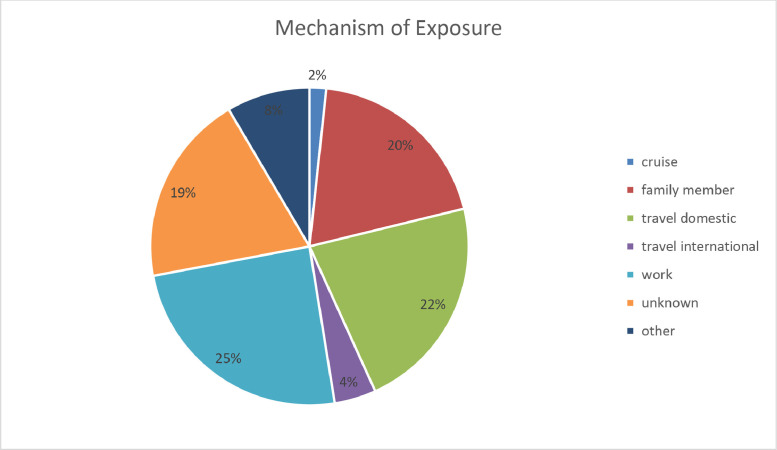
Fig 3.
. Mechanism of Exposure.
Data analysis
Clinical features and laboratory findings were compared between several patient groups. Data reviewed in this study included the following: (1) length of time from detectable to undetectable result, (2) length of time from onset of symptoms to undetectable results; (3) length of time from resolution of fever to confirmed undetectable result. Statistical analysis on duration between these points was conducted. Continuous variables were summarized as a mean (standard deviation) and median (range) and categorical variables were reported as frequency (percentage). Time to resolution was calculated as the days from onset of symptom to second undetectable test. Kaplan-Meier method was used to draw the time to resolution curve. R3.6.1 was used for analysis.
Results
As of May 15, 2020, there were 118 patients in the Mayo Clinic FL Positive COVID Registry. Of those, 8 were lost to follow-up. Analysis of the first 100 patients revealed that 87% met CDC criteria for the symptom-based strategy for release from isolation (fever-free greater than 72 hours, improved symptoms, and 10 days from first symptoms). Fifty-three percent (N = 31) of eligible patients continued to have detectable results and require repeat testing. Of eligible repeat testers, only 48.75% (N = 39) had undetectable results. The mean and median time for onset of symptoms in these patients to undetectable testing was 21.5 and 20.5 days, respectively. The mean and median time from the date of their first detectable result to undetectable result was 14.89 and 14 days, respectively. Lastly, of those 39 that were retested, 33.33% (N = 13) reported fever as a resolved symptom, and had a mean and median time to negative test of 14 and 17 days, respectively. Of all the CVC patients, risk factors identified included asthma (11%), end-stage renal disease on dialysis (2.5%), chronic lung disease (1.7%), diabetes (9.3%), hemoglobin disorders (0.8%), immunocompromised state (5.9%), liver disease (1.7%), resident of a nursing home or long-term care facility (0.8%), serious heart condition (5.1%), or morbid obesity (0.8%). Furthermore, at least 30 households had more than one detectable case. In regards to exposure, although this cannot be confirmed, presumed mechanisms were found to include (1) cruise (1.7%), family member (19.5%), domestic travel (22%), international travel (4.2%), work (24.6%), unknown (19.5%), or other (8.4%) (Tables 1 and 2 ).
Table 1.
. Patient demographics and risk factors
| Overall (N = 118) | |
|---|---|
| Age | |
| N | 118 |
| Mean (SD) | 51.2 (16.1) |
| Median (Range) | 52.0 (17.0, 86.0) |
| Gender | |
| Male | 58 (49.2%) |
| Female | 60 (50.8%) |
| Race | |
| Asian | 6 (5.1%) |
| American Indian or Alaska Native | 0 (0.0%) |
| Black or African American | 21 (17.8%) |
| White | 82 (69.5%) |
| Native Hawaiian or other Pacific Islander | 0 (0.0%) |
| Other or unknown | 9 (7.6%) |
| Ethnicity | |
| Hispanic/Latino | 6 (5.1%) |
| Not Hispanic/Latino | 112 (94.9%) |
| Referral | |
| N-Miss | 1 |
| ER | 22 (18.8%) |
| Hospital | 2 (1.7%) |
| Outside testing | 12 (10.3%) |
| Drive-thru | 81 (69.2%) |
| Risk factors | |
| Asthma | 13 (11.0%) |
| ESRD on dialysis | 3 (2.5%) |
| Chronic lung disease | 2 (1.7%) |
| Diabetes | 11 (9.3%) |
| Hemoglobin disorders | 1 (0.8%) |
| Immunocompromised | 7 (5.9%) |
| Liver disease | 2 (1.7%) |
| Nursing home or long-term care facilities | 1 (0.8%) |
| Serious heart condition | 6 (5.1%) |
| Morbid obesity | 1 (0.8%) |
Table 2.
. Patient outcomes
| Overall (N = 118) | |
|---|---|
| Hospitalized? | |
| Yes | 14 (11.9%) |
| No | 104 (88.1%) |
| Length of hospital stay | |
| N | 13 |
| Mean (SD) | 9.4 (10.9) |
| Median (Range) | 6.0 (2.0, 44.0) |
| ICU stay? | |
| Yes | 2 (1.7%) |
| No | 116 (98.3%) |
| Length of ICU stay | |
| N | 2 |
| Mean (SD) | 3.5 (0.7) |
| Median (Range) | 3.5 (3.0, 4.0) |
| Time to resolution (days) | |
| N | 110 |
| Mean (SD) | 31.6 (16.7) |
| Median (Range) | 28.0 (7.0, 135.0) |
Discussion
Based on this data, we propose following more cautious guidelines. Despite the current CDC guidelines for symptom-based strategy being 10 days since onset of symptoms, with 72 hours of no fever, we recommend discontinuing self-isolation no sooner than 21 days after onset of symptoms. We also recommend that patients not be retested until at least 14 days from the onset of symptoms. Mostly all patients we have encountered with COVID-19 have added to their symptomatology some level of increased anxiety as a result of concerning news reports, and an unknown duration or severity of illness. A patient with COVID-19 is more likely than not to feel anxious. The verified figures of over 478,000 deaths globally as of June 24, 2020 do not help alleviate concern, for providers too.1 , 9 There has been some progress made on clinical trials and understanding this virus better, but still much unknown remains.10 Something that can at least partially alleviate the anxiety is knowing when the infection will pass. This also facilitates those who have been out of work to return to work.
With the test-based strategy, there has been an unanswered question – when is the best time to implement? It has been difficult for any official statement to be made up to this point, not only because of resource availability, but also because the timing could be considered to be based on the essential need for someone to be back to work. If a patient is a nonessential worker, and has an aversion to testing more than necessary, this could affect the provider's recommendation on timing. As such, there are 2 points to consider for this problem. First, if testing is too soon, there is a higher likelihood of a positive result, and thus using resources unnecessarily. Added to this, we have had patients complain of the invasiveness of the testing, and even injuries by trauma to the nasopharynx from swabbing, which caused epistaxis and pain. So testing too soon will increase the potential for suffering from further injury by repeated swabbing. Second, if testing is too late, then time is being lost. This time could be important if an essential job is being vacated, or mental health is further strained from the effects of prolonged isolation. Thus, it is imperative for the provider to get the timing as accurate as possible.
Our results are consistent with the experience from other studies where viral detection could be identified at a median of 20 days after symptom onset although infectiousness may be unknown.11, 12, 13, 14 Recent studies from China where PCR and viral loads were assessed in patients and their analysis shows that viral shedding may begin before the onset of symptoms and decreasing subsequently.11, 12, 13 This pattern is characterized by being most infectious before the onset of symptoms similar to the viral shedding pattern typical for influenza.11 , 12
The data we have come across in our COVID-19 outpatient clinic shows that most patients are undetectable if tested outside of 14 days. By this information, we are able to help guide other providers.
Considerations
-
-
Test-based strategy: Persons who have COVID-19 with associated symptoms may discontinue isolation under the following conditions: resolution of fever without the use of fever-reducing medications, and improvement in respiratory symptoms, and negative results of an FDA emergency use authorized molecular assay for COVID-19 from at least 2 consecutive nasopharyngeal swab specimens collected ≥24 hours apart, no sooner than 14 days from onset of symptoms.
-
-
Symptom-based strategy: Persons with COVID-19 who have symptoms may discontinue isolation under the following conditions: at least 3 days (72 hours) have passed since recovery defined as resolution of fever without the use of fever-reducing medications, and improvement in respiratory symptoms; and, at least 21 days have passed since symptoms first appeared.
Limitations of this study
As a retrospective study, we accept the inferior level of evidence to a prospective study. Additionally, the sample size is small at 118. When taking history from the patient, there is a risk of recall bias, or misclassification bias based on their recount of the onset of symptoms. There is also the possibility of a selection bias due to the demographic of individuals enrolled in our clinic, which may engender a type of convenience sampling. The correspondence of PCR detection and infectivity is assumed, based on detecting presence of virus RNA. This limitation could be minimized with the use of viral cultures; however, given the increased risk of viral infection among laboratory staff, this testing method is deemed too unsafe to warrant those risks. Additionally, the exact false negative rate is unclear.15 Some patients were noted to have undetectable results followed by detectable results. The first well known study that conducted an investigation into isolating live virus by the timing of onset of symptoms showed no growth after 8 days. This was also conducted with a very small sample size of 9 patients.14 There is no evidence we are aware of that shows detectable viral RNA by PCR after 8 days correlating with an active infection. But also as noted, we do not have either any large body of evidence that shows at what time specifically positive viral PCR no longer signifies live viral replication. Some suggest using cycle threshold for future analysis on viral activity by PCR. This would be a worthwhile investigation in our view.16
Conclusions
In this study, we followed 118 patients who tested positive for SARS-CoV-2. They were followed in the Mayo Clinic Florida CVC.17 Data was collected on symptomology and follow-up testing results, and recommendations were made for self-isolation. Despite meeting CDC guidelines for a symptom-based method of release from isolation, only 48.75% of eligible patients had negative test results, with 53% still showing detectable viral RNA. With these results, we decided to take a more cautious plan for testing, and discontinuing patients from isolation. It is difficult to ascertain if the patients with prolonged positive tests are shedding unviable viral material, unviable virions or truly infectious viral particles. More research is needed. Future studies could include evaluating the rate of detectable retests to 2 consecutive undetectable results, the character of presenting symptoms to the timing of a negative test (do more severe symptoms at onset correlate to longer duration of viral shedding), and improved management of patients without symptoms. It would also be useful to know the most common symptoms and duration of illness in patients with and without comorbidities; and what effect does hospitalization have on the time to negative testing.
Acknowledgments
We would like to thank Dawn Francis, M.D., Zhuo Li, M.S., and Ilana Logvinov, D.N.P. for their assistance in completing this study.
Footnotes
Financial support: None to report.
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaijin Xu, Yanfei Chen, Jing Yuan, et al. Factors associated with prolonged viral RNA shedding in patients with Coronavirus Disease 2019 (COVID-19), [e-pub ahead of print]. Clin Infect. Dis. doi: 10.1093/cid/ciaa351. Accessed July 23, 2020. [DOI] [PMC free article] [PubMed]
- 3.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medical Isolation of Confirmed or Suspected COVID-19 Cases. Available at:https://www.cdc.gov/coronavirus/2019-ncov/community/correction-detention/guidance-correctional-detention.html. Accessed July 23, 2020
- 5.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tindale L. Transmission Interval Estimates Suggest Pre-Symptomatic Spread of COVID-19. 2020, Available at: www.medrxiv.org/content/10.1101/2020.03.03.20029983v1. Accessed June 1, 2020.
- 7.Li Qun, Guan Xuhua, Wu Peng, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. New Eng J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020,323: 2133–2134. [DOI] [PubMed]
- 10.Jogalekar M.P., Veerabathini A., Gangadaran P. Novel 2019 coronavirus: genome structure, clinical trials, and outstanding questions. Exp Biol Med. 2020;245:964–969. doi: 10.1177/1535370220920540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X., Lau E.H.Y., Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 12.Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.To K.K.-W., Tsang O.T-Y., Leung W-S., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wölfel R, Corman V, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:464–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 15.West CP, Montori VM, Sampathkumar P, et al. COVID-19 testing: the threat of false-negative results. Mayo Clin Proc. 2020;95:1127–1129. doi: 10.1016/j.mayocp.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MR Tom, MJ Mina, To interpret the SARS-CoV-2 test, consider the cycle threshold value, clinical infectious diseases, ciaa619. [DOI] [PMC free article] [PubMed]
- 17.Francis D, et al. The COVID-19 virtual clinic experience. Mayo Clin Dept Med Grand Rounds. 2020 https://mssvideoupload.mayo.edu/media/fla-medical-grand-rounds-may-27-2020-hq-720p/0_x3jvkenf Available at: Accessed July 23, 2020. [Google Scholar]