Abstract
Background
Convalescent plasma (CP) has been used successfully to treat many types of infectious disease, and has shown initial effects in the treatment of the emerging 2019 coronavirus disease (COVID-19). However, its curative effects and feasibility have yet to be confirmed by formal evaluation and well-designed clinical trials. To explore the effectiveness of treatment and predict the potential effects of CP with COVID-19, studies of different types of infectious disease treated with CP were included in this systematic review and meta-analysis.
Methods
Related studies were obtained from databases and screened according to the inclusion criteria. The data quality was assessed, and the data were extracted and pooled for analysis.
Results
40 studies on CP treatment for infectious diseases were included. Our study found that CP treatment could reduce the risk of mortality, with a low incidence of adverse events, promote the production of antibodies, lead to a decline in viral load, and shorten the disease course. A meta-analysis of 15 controlled studies showed that there was a significantly lower mortality rate in the group treated with CP (pooled OR = 0.32; 95% CI = 0.19–0.52; p < 0.001, I2 = 54%) compared with the control groups. Studies were mostly of low or very low quality, with a moderate or high risk of bias. The sources of clinical and methodological heterogeneity were identified. The exclusion of heterogeneity indicated that the results were stable.
Conclusions
CP therapy has some curative effect and is well tolerated in treating infectious diseases. It is a potentially effective treatment for COVID-19.
Keywords: Convalescent plasma (CP), Coronavirus disease 2019 (COVID-19), Infectious disease, Meta-analysis
Background
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease caused by novel coronavirus (SARS-CoV-2). It has an insidious onset and high infectivity, which can lead to death in severe cases (Malik et al., 2020). The epidemic — causing more than 16 million infections and 640 thousand deaths so far — has spread quickly worldwide since December 12, 2019, and the number of infections continues to increase throughout the world. To date, there are no approved specific antiviral agents for COVID-19. Convalescent plasma (CP) therapy has shown some effectiveness, with great potential for use in treating COVID-19. The China National Biotech Group reported on February 13, 2019 that it had detected high titers of virus-neutralizing antibodies as a result of CP. More than 10 patients with severe disease had significantly improved clinical outcomes 12–24 h after CP transfusion, which is of great relevance in the fight against COVID-19.
CP therapy is a form of passive immunization in which antibody-rich blood is collected from recovered patients and then processed to transfuse into other patients. Neutralizing antibodies are the key factors: these block the entry of the virus into a cell by binding to the virus, and regulate the immune system to mediate the phagocytosis of immune cells and remove the virus. In this way CP therapy has been effective in treating diphtheria and tetanus since the late 19th century, but the earliest complete record dates back to the outbreak of the Spanish influenza pandemic in 1918. Later, CP was used to treat Ebola, SARS, MERS, pandemic influenza, and other unexpected major infectious diseases; additionally, some progress has been made in related research (Leider et al., 2010, Stockman et al., 2006, Arabi et al., 2015). Two systematic reviews on respiratory infection revealed a significant reduction in the pool odds of mortality following CP therapy (Luke et al., 2006, Jenkins et al., 2016). These experiences raise the hypothesis that use of CP transfusion could be beneficial in patients infected with SARS-CoV-2. The Food and Drug Administration (FDA) has approved use of CP to treat severe COVID-19 patients (Tanne, 2020). However, its curative effects and feasibility have yet to be confirmed in a large clinical trial, and further studies are required to develop specific treatment criteria. To predict the potential effect of CP on COVID-19, we conducted a systematic review and meta-analysis of different types of infectious disease treated with CP, and further investigated the key points of CP treatment.
Methods
Literature collection
According to the literature retrieval strategies recommended by the Cochrane Collaboration, databases such as PubMed, Web of Science, Embase, and the Cochrane Library were comprehensively searched for journal papers published from the time the databases were created to March 30, 2020, using the keywords “convalescent plasma”, “SARS”, “MERS”, “Ebola”, “H1N1”, “H5N1”, “H7N9”, and “influenza”. Additionally, the references for selected studies were searched to identify other eligible studies.
Study selection
The studies fulfilled the following inclusion criteria: (i) The population of interest comprized human subjects of any age or sex who were diagnosed with SARS, MERS, Ebola, influenza, and other epidemic diseases with a laboratory-confirmed or suspected viral etiology. (ii) Study designs included randomized controlled trials (RCTs), non-randomized single-arm intervention studies, prospective and retrospective cohort studies, case reports and case series, and studies with no control group. (iii) The intervention measure was convalescent blood product containing CP. (iv) Reporting of at least one outcome of interest (mortality, symptom duration, hospital length of stay, antibody levels, viral load, adverse events, and other specific outcomes of CP therapy). Excluded studies included: (i) reviews and guiding documents, including clinical guidelines and expert consensus; (ii) animal or in vitro cell studies; (iii) studies for which the full text was not available; and (iv) studies with insufficient data on clinical information. Two investigators independently screened the titles and abstracts of the retrieved citations and then assessed the full-text manuscripts that were considered potentially eligible.
Data extraction and quality assessment
The following information was extracted from the collected literature: article title, first author's name, year of publication, study methods, number of patients, types of infectious disease, details of treatment, and clinical outcomes. The Cochrane bias risk assessment tool (version 5.1) was used to assess the quality of randomized or prospective controlled studies (The Cochrane Collaboration, 2011). The Newcastle-Ottawa scale (NOS) was used for other clinical observational studies (Tugwell and Wells, 2020). The risk of bias in the included studies was independently assessed by two investigators. Differences were solved by discussion or through consultation with the senior investigator.
Data analysis
Meta-analysis was conducted using Review Manager 5.3 software. The Mantel-Haenszel method was used to determine the odds ratio (OR) and 95% confidence interval (95% CI). We considered p ≤ 0.05 to be statistically significant. The assessment of inter-study statistical heterogeneity was based on the I 2 statistic. A high value for I 2 (>50%) indicated heterogeneity, in which case the random effects model was used, and subgroup analysis was performed according to the factors that may have been the source of heterogeneity. In contrast, for I 2 ≤ 50%, the fixed-effect model was recommended. Sensitivity and sources of the heterogeneity were evaluated by (1) changing the analysis model and (2) screening the included studies to assess the impact of each study on the outcomes.
Results
Study inclusion and characteristics
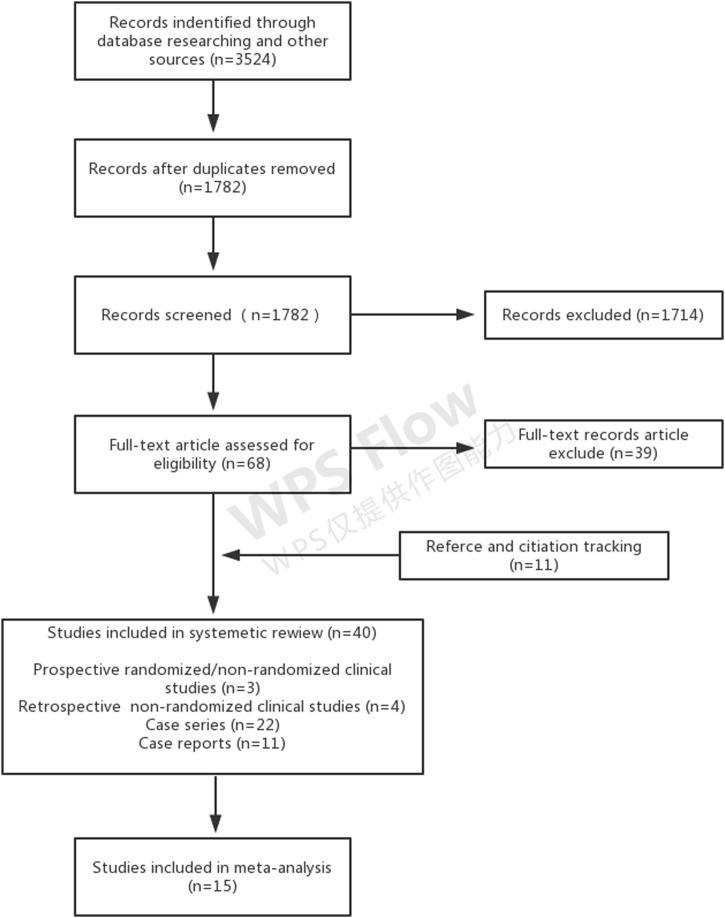
According to the search criteria, a total of 3524 studies were initially selected, from which 40 studies were included. The screening process is shown in Figure 1 . Seven studies reported outcomes for 174 patients infected with severe acute respiratory syndrome coronavirus (SARS-CoV) (Soo et al., 2004, Cheng et al., 2005, Kong, 2003, Wong et al., 2003, Yeh et al., 2005, Wong and Yuen, 2008, Zhou et al., 2003), four studies reported outcomes for 104 patients infected with 2009 pandemic influenza A (H1N1) (influenza A [H1N1] pdm09) (Hung et al., 2011, Sang et al., 2011, Wiesneth et al., 2010, Chan et al., 2010), four studies reported outcomes for 29 patients with avian influenza A (H5N1) (Kong, 2006, Yu et al., 2008, Zhang et al., 2009, Zhou et al., 2007), and 15 studies reported outcomes for 1803 patients with Spanish influenza A (H1N1) (Gould, 1919, O’Malley and Hartman, 1919, Ehrenberg and Barkman, 1919, Holst, 1919, Huff-Hewitt, 1919, Kahn, 1919, Miller and McConnell, 1919, Sanborn, 1920, McGuire and Redden, 1918, McGuire and Redden, 1919, Stoll, 1919, Ross and Hund, 1918, Ross and Hund, 1919, Bang, 1920, Redden, 1919). Clinical outcomes for one patient with avian influenza A (H7N9) were reported (Wu et al., 2015). One study including 87 patients with diverse severe influenza was found (Beigel et al., 2017). For infection with Ebola virus, six studies reporting outcomes for 583 patients were included (Griensven et al., 2016, Sahr et al., 2017, Mupapa et al., 1999, Kraft et al., 2015, Rillo et al., 2015, Florescu et al., 2015). Finally, four studies of 31 patients infected with SARS-CoV-2 were included (Kai et al., 2020, Zhang et al., 2020, Shen et al., 2020). There were two non-randomized prospective studies, one randomized prospective study, 12 non-randomized intervention and cohort studies with control groups, and 25 case series and case reports with no control groups. Supplementary Table 1 shows the characteristics of the included studies. All included studies reported the use of convalescent plasma except for 14 studies, of which 12 used convalescent serum to treat Spanish influenza A (H1N1) infection, one of which reported the use of convalescent blood to treat Ebola infection, and one reported the use of immune plasma to treat severe influenza.
Figure 1.
Flow diagram of the systematic review.
Risk of bias within studies
The risks of bias for the three prospective controlled studies were considered to be moderate according to the Cochrane Collaboration tool, and all of them were at a high risk of bias in terms of allocation concealment and blinding (Supplementary Table 2). Supplementary Table 3 summarizes the results of the 37 observational studies for which the NOS was used for quality assessment. Most of the studies had a moderate-to-high risk of bias, among which the expected absence of random and blinded intervention was the common caveat, and 25 studies were at extremely high risk of bias due to the lack of a control group.
Mortality outcomes
A retrospective controlled study on SARS-CoV showed no deaths in 19 patients who received CP therapy, and there was a statistically significant difference in the case fatality ratio (CFR) compared with the control group (0% vs. 23.8%; 95% CI 6–42; p = 0.049) (Soo et al., 2004). Cheng et al. reported a CFR of 12.5% in 80 patients who received CP therapy in Hong Kong, but the SARS-related CFR in Hong Kong was 17% over the same period (Cheng et al., 2005). No deaths in those treated with CP were reported in all four studies that enrolled fewer than five patients infected with SARS (Kong, 2003, Wong et al., 2003, Yeh et al., 2005, Wong and Yuen, 2008). In the retrospective controlled study conducted by Hung et al. on patients with influenza A (H1N1) pdm09 who underwent CP therapy using an antibody titer higher than 1:160, the multivariate analysis showed that the intervention group had a significantly lower CFR than the control group (20% vs. 54.8%; OR = 0.20; 95% CI 6–69; p = 0.011) (Hung et al., 2011). In addition, in the three small case series or case reports on CP therapy for patients with influenza A (H1N1) pdm09, only one death was reported by Sang et al. (Sang et al., 2011, Wiesneth et al., 2010, Chan et al., 2010). However, there was no significant difference (95% CI 52–89; p = 0.11) between the two groups in a case series of patients with avian influenza A (H5N1); two out of 26 patients received CP therapy, and the CFRs for the intervention group and control group were 0% and 70%, respectively (Yu et al., 2008). The absolute reduction of CFR in the CP group was observed in three non-randomized controlled studies on Spanish influenza A (H1N1) (6.7% vs. 28.3%, p = 0.008, 95% CI 11–32; 6.5% vs. 25%, p < 0.001, 95% CI 8–30; 4.0% vs. 30%, p < 0.001, 95% CI 21–31) (Gould, 1919, O’Malley and Hartman, 1919, McGuire and Redden, 1918, McGuire and Redden, 1919). In a randomized, prospective, phase II clinical study on CP therapy for severe influenza conducted by John et al., CRF was 2% (1/49) in the treatment group, which was lower than the 10% (5/49) found in the control group, but the difference was not statistically significant (p = 0.093) (Beigel et al., 2017). A study of CP therapy for patients with Ebola virus showed that the risk of death was 31% in the CP group and 38% in the control group from day 3 to day 16 after diagnosis (RD −7%; 95% CI 18 to −4), and the difference was reduced after adjustment for age and cycle-threshold value (RD −3%; 95% CI −13 to 8)(Griensven et al., 2016). Another controlled study on Ebola virus showed that the CFR was 28% (12/43) in the CP group and 44% (11/25) in the control group. However, there was no significant difference between the intervention and control groups in these two studies (Sahr et al., 2017). Mupapa et al. reported one death (CFR 12.5%) in eight patients with Ebola hemorrhagic fever after treatment with convalescent whole blood, while the overall CFR for this epidemic was 80% (Mupapa et al., 1999). No deaths were reported in patients infected with SARS-CoV-2 using CP therapy (Table 1, Table 2) .
Table 1.
Outcomes of studies with control group (n = 15).
| Author (year) | Viral etiology | CFR of intervention group (n) | CFR of control group (n) | Viral load | Antibody level | Length of Hospital Stay | Adverse event | Others |
|---|---|---|---|---|---|---|---|---|
| Soo et al. (2004) | SARS-CoV | 0% (0/19) | 23 8% (5/21) | Not known | Not known | 74% of patients were discharged by day 22, compared with 19% in the steroid group (p < 0.001) | No adverse events were observed with CP | Patients receiving CP after day 16 had a poor clinical response |
| Zhou et al. (2003) | SARS-CoV | 0% (0/1) | 7% (2/28) | Not known | Not known | Not known | No adverse events were observed with CP | The patient recovered within 21 days having a shorter disease course |
| Hung et al. (2011) | Influenza A(H1N1)pdm09 | 20% (4/20) | 54 79% (40/73) | Viral loads measured on day 3, 5, and 7 after ICU admission were significantly lower in the treatment than in the control group (p = 0.001, p = 0.02, and p = 0.04, respectively) | Not known | Not known | No adverse events were observed with CP | The levels of IL-6, IL-10, and TNF-α were lower in the intervention group than the control group |
| Chan et al. (2010) | Influenza A(H1N1)pdm09 | 0 (0/3) | 33 33% (1/3) | Not known | Not known | All samples were discharged by day 31 (25–55) | No adverse effects were reported | NA |
| Yu et al. (2008) | Avian influenza A(H5N1) | 0% (0/2) | 70% (17/24) | Not known | Not known | Nonfatal cases were discharged at a median of 41 days (31.5–64.0) after illness onset | No adverse effects were reported | NA |
| Kahn et al. (1919) | Spanish influenza A(H1N1) | 48% (12/25) | 66 66% (12/18) | Not known | Not known | Not known | No adverse effects were reported | NA |
| Gould et al. (1918) | Spanish influenza A(H1N1) | 6 66% (2/30) | 28 27% (82/290) | Not known | Not known | Not known | Infrequently experienced a chill and temporary increase in temperature. 1 case of jaundice and phlebitis was related to transfusion | Early transfusion resulted in distinct improvement in clinical signs and symptoms, fever ended 1.83days after transfusion (shorter compared with controls) |
| O’Malley et al. (1919) | Spanish influenza A(H1N1) | 6% (3/46) | 25% (28/111) | Not known | Not known | Not known | 75% patients had a slight or frank chill with a temporary increase in temperature, transfusion may aggravated serious symptoms in terminally patients | NA |
| Stoll et al. (1919) | Spanish influenza A(H1N1) | 44.6% (25/56) | 53% (201/379) | Not known | Not known | Not known | 16%patients had a chill, shake and temporary increase temperature. Transfusion reaction possibly hasten death in 4 seriously patients | Early transfusions resulted in distinct improvement in clinical signs and symptoms |
| Ross et al. (1919) | Spanish influenza A(H1N1) | 21.4 (6/28) | 42.8 (9/21) | Not known | Not known | Not known | Chill, temporary increase temperature | Early transfusions resulted in distinct improvement in clinical signs and symptoms |
| McGulre et al. (1919) | Spanish influenza A(H1N1) | 4% (6/151) | 30% (120/400) | Not known | Not known | Not known | 10% patients had a mild chill reaction | Early transfusions resulted in distinct improvement in clinical signs and symptoms, fever ended in 1or2 days in treated survivors |
| Sahr et al. (2016) | Ebola | 27.9% (12/43) | 44% (11/25) | There was a significant difference between admission viral load and after the first 24 h of treatment with intervention group (p < 0.01) | Not known | Not known | No adverse events were observed with CP | Patients treated with convalescent whole blood took an average of 10.6 ± 3.4 days to recover while the control patients took an average of 12.23 ± 4.8 days to recover |
| Griensven et al. (2016) | Ebola | 31% (26/84) | 38% (158/418) | One day after the transfusion of CP, the median Ct value increased by 3.5 cycles | Not known | Not known | 8% patients had an adverse reaction during or early after the transfusion including increase in temperature itching or skin rash nausea | The mortality difference was reduced after adjustment for age and cycle-threshold value (adjusted risk difference, −3 percentage points; 95% CI, −13 to 8) |
| Beigel et al. (2017) | Severe Influenza | 2% (1/49) | 10% (5/49) | There was no significant difference in time when no virus is detected | Not known | There were fewer days in the hospital in intervention group (median 6 vs. 11, p = 0 13) | 9 (20%) had SAEs The most common SAEs were acute respiratory distress syndrome and stroke | Hospital readmissions (2 vs. 7, p = 0 096), fewer participants with ICU admissions (57% vs. 69%, p = 0 097), and fewer days on mechanical ventilation (median 0 vs. 3, p = 0 14). 67% participants randomized to receive plasma had resolution of tachypnea and hypoxia by day 28, compared to 53%of control participants (p = 0 069) |
| Duan et al. (2020) | SARS-CoV-2 | 0 (0/10) | 30% (3/10) | Virus RNA was positive in 7 patients before transfusion. Virus RNA was decreased to an undetectable level in 3 patients on day 2, 3 patients on day 3 and 1 patients on day 6 after intervention | After CP transfusion, the level of neutralizing antibody increased rapidly up to 1:640 in five cases, while that of the other four cases maintained at a high level (1:640) | 3 cases discharged, while 7 cases in much improved status and ready for discharge in CP group. No patient in control group were eligible to be discharged | No adverse events were observed with CP | The symptoms were significantly improved within 3 days. Several parameters tended to improve as compared to pre-transfusion, including increased lymphocyte counts (0.65 × 109 L−1 vs. 0.76 × 109 L−1) and decreased C-reactive protein (55.98 mg/L vs. 18.13 mg/L) |
NA: not applicable.
Table 2.
Outcomes of studies without control group (n = 25).
| Author (year) | Viral etiology | CFR treated group (n) | Viral load | Antibody levels | Length of hospital stay | Adverse event | Others |
|---|---|---|---|---|---|---|---|
| Cheng et al. (2005) | SARS-CoV | 12 5% (10/80) | Not known | There was no correlation between clinical outcome and either the volume of plasma infused or the coronavirus antibody titers of the donors. | A higher day-22 discharge rate was observed among patients who were given CP before day 14 of illness (58.3% vs. 15.6%; p < 0.001) | No adverse events were observed with CP | Patients given CP before day 14 had a better outcome than those given plasma after day 14. The mortality rates in the two groups were 6.3% and 21.9%, respectively (p = 0.08) |
| Wong et al. (2003) | SARS-CoV | 0% (0/1) | Not known | Not known | Not known | No adverse effects were reported | NA |
| Yeh et al. (2005) | SARS-CoV | 0% (0/3) | Viral load dropped from 495 × 103, 76 × 103 or 650 × 103 copies/ml to zero or 1 copy/ml one day after transfusion. | Anti-SARS-CoV IgM and IgG increased in a time-dependent manner following transfusion. | Not known | No adverse events were observed with CP | After 1 day of CP transfusion, body temperature decreased from >38 to <37 °C. Radiological improvement was also observed after the CP transfusion |
| Kong et al. (2003) | SARS-CoV | 0% (0/1) | Not known | Not known | 60 days | No adverse effects were reported | NA |
| Wong et al. (2003) | SARS-CoV | 0% (0/1) | Not known | Not known | Not known | No adverse effects were reported | NA |
| Sang et al. (2011) | Influenza A(H1N1)pdm09 | 25% (1/4) | Not known | Not known | Not known | No adverse effects were reported | NA |
| Weisneth et al. (2010) | Influenza A(H1N1)pdm09 | 0% (0/1) | Viral load became rapidly undetectable in respiratory tract specimens | Not known | Not known | No adverse effects were reported | NA |
| Kong et al. (2006) | Avian influenza A(H5N1) | 0% (1) | Between the 7th and the 16th days of treatment with CP, the virus became undetectable in his the serum | Between the 7th and the 16th days of treatment with CP specific antibodies to H5N1 appeared | Not known | No adverse effects were reported | NA |
| Zhang et al. (2009) | Avian influenza A(H5N1) | 0% (0/1) | Not known | Not known | The patient was discharged from the hospital 99 days after onset of illness (at the 94th hospital day). | No adverse effects were reported | NA |
| Zhou et al. (2007) | Avian influenza A(H5N1) | 0% (1) | the patient's viral load was reduced by a factor of approximately 12 (from 1.68 × 105 to 1.42 × 104 copies/ml) during the first 8 h (from 2 a.m. to 10 a.m. on June 15) and was undetectable within 32 h | The neutralizing-antibody titer was negative before treatment, then it rose steadily and was between 1:40 and 1:80 in 5 days. | The patient was discharged from the hospital 53 days | No adverse effects were reported | NA |
| Bang et al. (1920) | Spanish influenza A(H1N1) | 20% (2/10) | Not known | Not known | Not known | No adverse effects were reported | NA |
| Ehrenberg et al. (1919) | Spanish influenza A(H1N1) | 20% (2/10) | Not known | Not known | Not known | No adverse effects were reported | NA |
| Holst et al. (1919) | Spanish influenza A(H1N1) | 35% (7/20) | Not known | Not known | Not known | No adverse effects were reported | NA |
| Huff-Hewitt et al. (1919) | Spanish influenza A(H1N1) | 0% (0/4) | Not known | Not known | Not known | No adverse effects were reported | NA |
| Miller et al. (1919) | Spanish influenza A(H1N1) | 0% (0/2) | Not known | Not known | Not known | No adverse effects were reported | 2 children treated with CP had rapid improvement in signs and symptom, one woman had gradual improvement |
| Redden et al. (1919) | Spanish influenza A(H1N1) | 16% (16/100) | Not known | Not known | Not known | No adverse effects were reported | The majority was treated early, 13deaths were among late-treated patients |
| Sanborn et al. (1920) | Spanish influenza A(H1N1) | 33% (33/101) | Not known | Not known | Not known | Chill, increased temperature | NA |
| Kraft et al. 2015 | Ebola | 0% (0/2) | Ct value was presented a linear increase after plasma infusion. Plasma tested negative for EBOV RNA on illness days 22, 24, and 25 | Not known | On day 28, 44 respectively | Transfusion of the initial 500 mL was associated with worsening shortness of breath and increasing oxygen requirements in 1 patient | 1 patient's respiratory status slowly improved and he was extubated on day 21 of illness |
| Mupapa et al. (2016) | Ebola | 12.5% (1/8) | In 5 (83%) of 6 patients tested, EBO antigens had disappeared before day 4 after transfusion | In 4 (57%) of 7 transfusion recipients tested, EBO IgG or IgM antibodies were present before transfusion (only 2 of them had both IgG and IgM antibodies). After transfusion, IgG and IgM antibodies were detected in 7 (87.5%) of the 8 blood recipients. IgM antibodies were never detected in the 1 patient who died | The patient was discharged from the hospital 21–52 days | No adverse effects were reported | NA |
| Rillo et al. (2015) | Ebola | 0% (0/1) | Not known | Not known | The patient was discharged on day 34 of illness | On day 10 of illness, the patient had ARDS possibly caused by transfusion-related acute lung injury, which was managed without mechanical ventilation | NA |
| Florescu et al. (2015) | Ebola | 0% (0/1) | Ct value became negative on 17day illness | IgM antibodies increased almost linearly after CP therapy and stabilized after peaking on day 10, while the level of IgG was lower and had not significant increased | On illness day 20 | No adverse effects were reported | NA |
| Wu et al. (2015) | Influenza A (H7N9) | 0% (0/1) | H7N9 virus was undetectable after 4 days of CP treatment | At discharge, the patient had a neutralizing antibody titer greater than 1:80 | The patient was discharged from the hospital on day16 | No adverse effects were reported | Combination of CP and antiviral drugs may be effective for the treatment of avian-origin H7N9 infection |
| Zhang et al. (2020) | SARS-CoV-2 | 0% (0/4) | In one patient, the viral load decreased from 55 × 105 copies/ml to 180 copies/ml 5 days after completion of CP infusion. RT-PCR was negative on day 10 after completion of CP infusion | Antibody testing indicated positive IgG during the infusion interval in one patient. IgM changed from positive to weakly positive to negative, while IgG was persistently positive | There patients were discharged from the hospital on day 42, 18, 27 respectively | No adverse events were observed with CP | NA |
| Shen et al. (2020) | SARS-CoV-2 | 0% (0/5) | Ct value increased within 1 day after transfusion. The Ct value of patient 5 became negative on posttransfusion day 1, patient 3 and patient 4 became negative on day 3, and patient 1 and patient 2 became negative on day 12 after the transfusion | The titers of IgG and IgM in the sera increased in a time-dependent manner at 3 days after transfusion and maintained a high level at 7 days after transfusion. The neutralizing antibody titers increased following the transfusion (range, 40–60 before and 80–320 on day 7) | Three have been discharged from the hospital (length of stay: 53, 51, and 55 days), and 2 are in stable condition at 37 days after transfusion | No adverse effects were reported | Temperature normalized within 3 days in 4 patients, SOFA score decreased, and PAO2/FIO2 increased within 12 days (172–276 before and 284–366 after). ARDS resolved in 4 patients at 12 days after transfusion, and 3 patients were weaned from mechanical ventilation within 2 weeks of treatment |
| Ahn et al. (2020) | SARS-CoV-2 | 0% (0/2) | In 1 patient, Ct value changed from 24.98 before CP infusion to 33.96 on day 9 after infusion, and the viral was negative after on day 15 after CP infusion. Ct value of another patient changed from 20.51 before CP infusion to 36.33 on day 3 after plasma infusion | Not known | 1 patient was discharged from the hospital on day24 | No adverse events were observed with CP | In 1 patient, the fever subsided, and oxygen demand decreased after 1 day of CP transfusion. CRP and IL-6decreased to normal range on 7day after CP infusion. In another patient, leukocytosis and lymphopenia were immediately recovered after CP infusion. The level of CRP and IL-6 also recovered to the normal range |
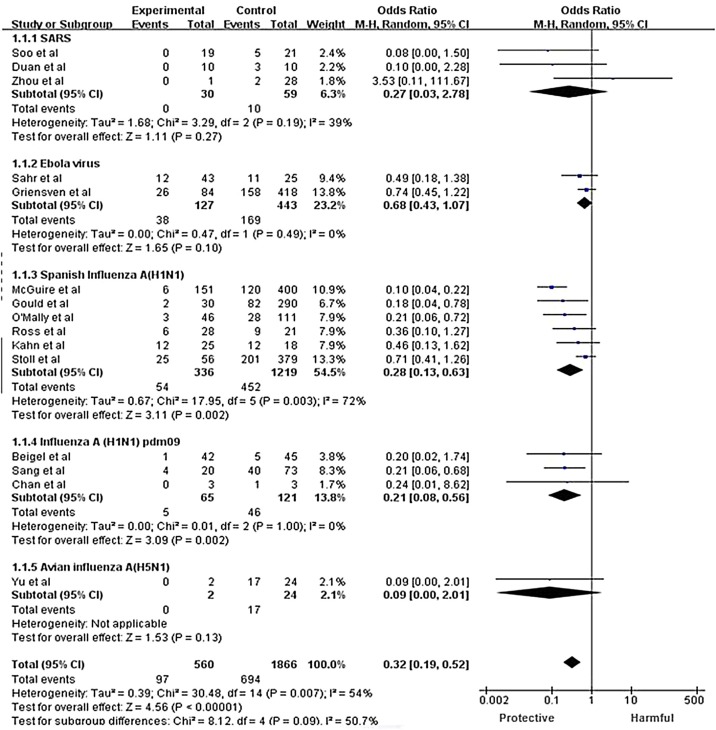
Fifteen controlled studies were included for meta-analysis: two studies of SARS-CoV infection (Soo et al., 2004, Zhou et al., 2003), two of influenza A (H1N1) pdm09 infection (Sang et al., 2011, Chan et al., 2010), one of avian influenza A (H5N1) infection (Yu et al., 2008), six of Spanish influenza A (H1N1) infection (Gould, 1919, O’Malley and Hartman, 1919, Kahn, 1919, McGuire and Redden, 1918, McGuire and Redden, 1919, Stoll, 1919, Ross and Hund, 1918, Ross and Hund, 1919), one of severe influenza (Beigel et al., 2017), two of Ebola infection (Griensven et al., 2016, Sahr et al., 2017), and one of SARS-CoV-2 infection (Kai et al., 2020). There was a significantly lower CFR in the group treated with CP (pooled OR = 0.32; 95% CI 0.19–0.52; p < 0.001; I 2 = 54%; Figure 2 ).
Figure 2.
Forest plot of pooled odds ratios (ORs) for mortality following treatment with convalescent plasma or convalescent serum. The labels ‘Protective’ and ‘Harmful’ on x-axis represent the convalescent plasma or convalescent serum group and the control group, respectively.
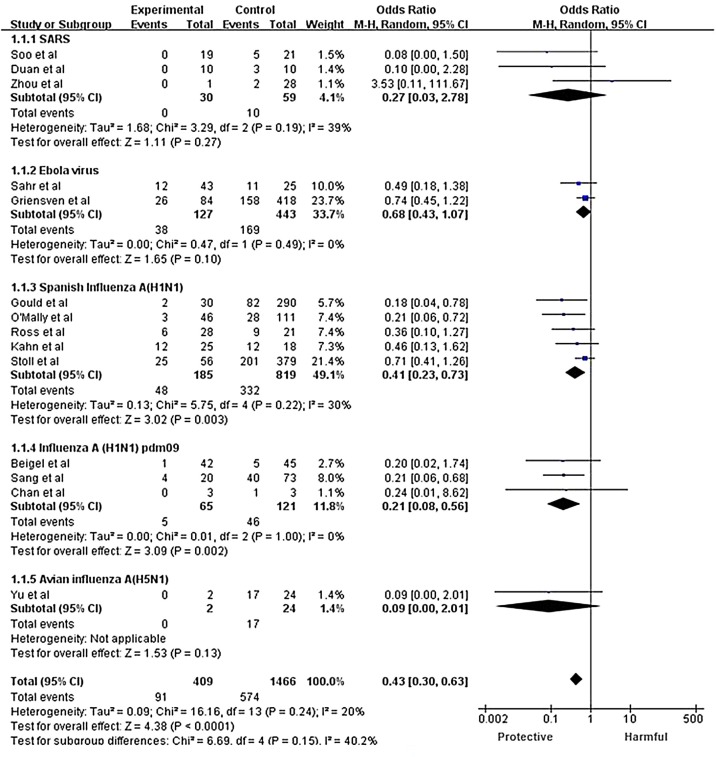
Due to the high heterogeneity, the random effects model was used for subgroup analysis according to the type of infectious disease (one randomized controlled study with severe influenza patients was included in the influenza A (H1N1) pdm09 subgroup, as the subjects in the study were H1N1, H3N2, and influenza B patients). Significant heterogeneity was found among subgroups, indicating that the different types of infectious disease may be a source of heterogeneity. In the subgroup analysis, heterogeneity existed in the Spanish influenza A (H1N1) group, in which we excluded the studies one by one. The sensitivity analyses that excluded the study conducted by McGuire et al. (McGuire and Redden, 1918) demonstrated homogeneity among the remaining studies (p = 0.24; I 2 = 20%) and little variation or change in pooled results (OR = 0.43; 95% CI 0.30–0.63; p < 0.001; Figure 3), which supports the conclusion that CP therapy could reduce the risk of mortality compared with the control group.
Figure 3.
Forest plot of pooled odds ratios (ORs) for mortality following treatment with convalescent plasma or convalescent serum, excluding a study with high heterogeneity.
Viral load
Viral loads are highly correlated with disease severity and progression (Ng et al., 2018). The indicators of viral load were tested before and after CP therapy in several studies. Yeh et al. found that the viral loads decreased from 495 × 103, 76 × 103, and 650 × 103 copies/ml to 0 or 1 copy/ml in three patients with SARS-CoV on day 1 after CP transfusion (Yeh et al., 2005). Hung et al. reported that the viral loads of patients with influenza A (H1N1) pdm09 were significantly lower in the CP therapy group than in the control group on days 3, 5, and 7 after admission to the ICU (p = 0.001, p = 0.02, and p = 0.04, respectively) (Hung et al., 2011). The virus was not detected in the serum of one patient with avian influenza A (H5N1) on days 7 and 16 after CP therapy (Kong, 2006). The viral load was reduced by approximately 92% (from 1.68 × 105 to 1.42 × 104 copies/ml) in another H5N1-infected patient within 8 hours of CP therapy, and no virus was detected within 32 h (Zhou et al., 2007). Wu et al. reported that no avian influenza A (H7N9) virus was detected in an infected patient on day 4 after CP therapy. However, in randomized controlled trials of CP therapy for multiple severe influenzas, there was no significant difference between the intervention group and the control group regarding the time when no virus was detected (Wu et al., 2015). In a controlled study of Ebola, the PCR cycle threshold increased by 3.5 cycles on day 1 after CP transfusion (the Ct value is inversely proportional to the viral load) (Griensven et al., 2016). Another study of convalescent whole blood treatment for Ebola virus showed that there was a significant difference between the virus quantification at admission and that within the first 24 h (p < 0.01). In the intervention group, the mean Ct value was 23.37 ± 5.0 at admission compared with 29.99 ± 5.9 at 24 h after blood transfusion. In the control group, the mean Ct value was 31.97 ± 8.4 at admission vs. 31.25 ± 7.5 at 24 h after admission (Sahr et al., 2017). Kraft et al. reported that the plasma of two Ebola-infected patients who received CP therapy was negative for EBOV RNA on days 22, 24, and 25 of the disease (Rillo et al., 2015). In the study conducted by Diana et al. on combined CP and brincidofovir therapy for Ebola-infected patients, the patients had a moderately high EBOV load at admission but were negative for EBOV RNA on day 17, according to three consecutive tests (Florescu et al., 2015). A case series on CP therapy for Ebola hemorrhagic fever reported that EBOV antigens disappeared in five of the six tested patients by day 4 after blood transfusion (Mupapa et al., 1999). A recent pilot study on COVID-19 showed that SARS-CoV-2 RNA was decreased to an undetectable level in seven patients who had previous viremia (Kai et al., 2020). Zhang et al. reported that the viral load of one patient infected with SARS-CoV-2 decreased from 55 × 105 copies/ml to 180 copies/ml 5 days after the completion of CP transfusion, and RT-PCR was negative on day 10 after the completion of CP transfusion (Zhang et al., 2020). A case series of five COVID patients by Shen et al. reported that the Ct value increased within 1 day after transfusion and became negative on post-transfusion days 1–3 in three patients, and two became negative on day 12 after transfusion. They also found that SARS-CoV-2 was still detectable in all five patents even though antiviral treatment had been given for at least 10 days; however, viral load decreased and became undetectable soon after CP treatment, highlighting the possibility that CP had contributed to the clearance of the virus (Shen et al., 2020). Case reports from Korea recorded the Ct value of two patients with SARS-CoV-2 before and after CP therapy. In one patient, the Ct value changed from 24.98 to 33.96 on day 9 after CP infusion, and viral testing was negative after day 15. Similarly, the Ct value in another patient changed from 20.51 before CP infusion to 36.33 on day 3 after plasma infusion (Ahn et al., 2020). Based on the above results, it can be concluded that CP therapy can reduce the viral load of infectious diseases to some extent (Table 1, Table 2).
Antibody levels
Some of the included studies described the level of antibodies after CP therapy but provided no data on comparisons between the intervention group and the control group. Yeh et al. reported that SARS-CoV IgG and IGM antibodies in patients increased in a time-dependent manner and reached a peak on days 3–5 after CP therapy (Yeh et al., 2005). Testing of antibody levels in a patient with avian influenza A (H5N1) from Hong Kong, who received CP therapy, showed that specific antibodies to H5N1 appeared between the 7th and 16th days of following treatment with CP (Kong, 2006). Zhou et al. also reported that specific antibodies rose from negative to a titer of 1:40–1:80 within 5 days following CP therapy in one H5N1-infected patient (Zhou et al., 2007). One patient with avian influenza A (H7N9) was found to have a neutralizing antibody titer of more than 1:80 at discharge on day 16 after CP therapy (Wu et al., 2015). A case report of an Ebola-infected patient found that IgM antibodies increased almost linearly after CP therapy and stabilized after peaking on day 10 (Florescu et al., 2015). When antibody tests were performed on seven out of eight Ebola-infected patients before CP therapy, IgG or IgM antibodies were detected in four patients (57%), and only two of these had both IgG and IgM antibodies. After transfusion, IgG and IgM antibodies were detected in seven (87.5%) of the eight blood recipients. IgM antibodies were undetectable in the one patient who died (Mupapa et al., 1999). For SARS-CoV-2, Duan et al. determined neutralizing antibody titers in nine patients. After CP transfusion, levels of neutralizing antibodies increased rapidly up to 1:640 in five cases, while those in the other four cases were maintained at a similarly high level (1:640) (Kai et al., 2020). Another study on COVID-19 demonstrated that titers of IgG and IgM in the sera increased in a time-dependent manner at 3 days after transfusion and maintained a high level at 7 days after transfusion. The neutralizing antibody titers increased following the transfusion (range 40–60 before and 80–320 on day 7) (Shen et al., 2020). Generally, CP therapy is likely to increase the levels of specific antibodies.
Time point of treatment
In a study of CP therapy for SARS-CoV-infected patients, patients receiving CP during the initial 14 days from diagnosis had a better outcome than those receiving CP after day 14 from diagnosis (day-22 discharge rate: 58.3% vs. 15.6%; p < 0.001). The CFR in the two groups was 6.3% and 21.9%, respectively (p = 0.08) (Cheng et al., 2005). Another controlled study on SARS-CoV also showed poor clinical responses in patients who received CP therapy after day 16 (Soo et al., 2004). Four studies on Spanish influenza A (H1N1) showed that early treatment with CP could significantly improve the prognosis, with two of these studies providing data showing that patients who received the therapy before day 4 had a lower risk of mortality than those who received the therapy after day 4 (32% vs. 60%; 95% CI 2%–53%; p = 0.85 and 14% vs. 40%; 95% CI −2% to 72%; p = 0.86) (Sanborn, 1920, McGuire and Redden, 1918, McGuire and Redden, 1919, Stoll, 1919, Ross and Hund, 1918, Ross and Hund, 1919). In a study of 16 patients with Spanish influenza A (H1N1) who died after CP therapy, the transfusion was provided at quite a late stage in 13 of the patients (Redden, 1919). Based on the above results, it can be concluded that the early use of CP may improve the outcomes of severe infectious diseases (Table 1, Table 2).
Length of hospital stay
A study involving patients infected with SARS-CoV showed that 74% of those receiving CP were discharged by day 22, compared with 19% in the control group (p = 0.001) (Soo et al., 2004). Zhou et al. reported one case of a SARS-CoV-infected patient who recovered within 21 days after having a shorter disease course (Zhou et al., 2003). In a study on Ebola virus, the average recovery time was 10.6 ± 3.4 days for patients treated with convalescent whole blood, compared with 12.23 ± 4.8 days for the control group (Sahr et al., 2017). Chan et al. reported that the average length of hospital stay after CP transfusion was shorter than that in the control group in three patients infected with influenza A (H1N1) pdm09 (36.6 days vs. 60 days; p = 0.23)(Chan et al., 2010). In the included studies of patients with severe influenza, there were fewer days spent in hospital after randomization (median 6 days vs. 11 days; p = 0.13)(Beigel et al., 2017). To some degree, CP therapy for infectious diseases can reduce the length of hospital stay, shorten the course of disease, and contribute to the recovery of patients (Table 1, Table 2).
Adverse events
No serious adverse events (SAE) related to CP therapy were reported in most of the included studies. According to some relevant studies on Spanish influenza A (H1N1), the most common CP-related adverse events were chills and a temporary increase in temperature, which occured mainly 30–120 min after blood transfusion. Gould et al. found that the occurrence of jaundice and phlebitis might be associated with blood transfusion (Gould, 1919, O’Malley and Hartman, 1919, Ehrenberg and Barkman, 1919, Miller and McConnell, 1919, Sanborn, 1920, McGuire and Redden, 1918, McGuire and Redden, 1919, Stoll, 1919, Ross and Hund, 1918, Ross and Hund, 1919). Two studies on Spanish influenza showed that transfusion might aggravate serious symptoms or hasten death in terminally ill patients (O’Malley and Hartman, 1919, Stoll, 1919). Kraft et al. reported that CP transfusion was associated with worsening shortness of breath and increasing oxygen requirements in one patient with Ebola virus (Kraft et al., 2015). A case report described one Ebola-infected patient who had ARDS, possibly caused by transfusion-related acute lung injury, which was managed without mechanical ventilation (Rillo et al., 2015). A study of severe influenza cases reported that the incidence of SAE was 20% in 42 patients after CP therapy, including ARDS and stroke (Beigel et al., 2017). In general, CP infusion is well tolerated, and it is rare to observe serious CP-related adverse events. Attention should be paid to terminally ill patients in terms of possible exacerbation of their disease symptoms (Table 1, Table 2).
Discussion
According to the results of the pooled analysis of different types of infectious disease, CP therapy is effective in reducing mortality rate and can have a significant effect on adjusting the immune system and decreasing the viral load. Analysis of the length of hospital stay indicates that CP therapy can shorten the course of disease and contribute to patient recovery. A low incidence of serious adverse events, which are mostly controllable, has been demonstrated during and after CP infusion. SARS-CoV immunoglobulin was prepared successfully using CP in 2004, and has been approved by the Chinese Food and Drug Administration as an emergency rescue drug for the treatment of SARS-CoV. The World Health Organization (WHO) has identified CP as a treatment for Middle East respiratory syndrome coronavirus (MERS-CoV), and the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) has recommended CP infusion as a potential treatment for reducing the clinical symptoms of MERS-CoV infection (Xiaoming and Jifeng, 2020). In response to the SARS-CoV-2 outbreak, the administration of CP to severe patients was included in Chinese guidelines for the treatment of COVID-19 (General Office of the National Health Commission, 2020). The FDA also provided emergency access to CP for patients with serious or immediately life-threatening COVID-19 infections (Tanne, 2020).
The practice of treating severe infectious diseases with blood products collected from recovered patients reveals the importance of antibodies. The curative effect of CP therapy is attributed to the protective antibodies that block the virus persistently and efficiently. It has been reported that the immune response is associated with the neutralizing activity of antibodies. In one study, after infusion with a 1:80 CP titer in a plaque-reduction neutralization test (PRNT), MERS-infected patients showed a significant immune response, while plasma with a titer of 1:40 did not produce a similar response (Ko et al., 2018). Patients who showed no significant improvement in survival after CP infusion possibly had a lower titer of neutralizing antibodies. Therefore, to make CP more effective, optimum neutralizing antibody titers of CP need to be further explored, and the level of neutralizing antibodies in donor plasma should be determined before transfusion.
Whether the antibodies in CP are definitely beneficial for treatment remains unclear. Although the antibodies probably do have an impact on disease severity, there are potential risks resulting from the complexity of blood products, such as allergic reactions and pathogen transmission (Andrew and Vanessa, 2020). Evidence from animal models of SARS-CoV infection suggests that the role of antibodies was related to the development of more severe acute lung injury (Liu et al., 2019). Studies on the pathogenesis of SARS-CoV-2 have shown that as the virus attacks the human body, it can trigger a specific immune response; subsequently, a variety of cytokines are produced abundantly. While killing pathogens, cytokines also damage normal tissues and organs in an effect called a cytokine storm. Clinical data on SARS-CoV-2 infections in China have shown that cytokine storms are observed in patients with severe disease (Chen et al., 2020). There are neutralizing antibodies in CP that prevent the virus from attacking the human body, while non-neutralizing antibodies mainly mediate the virus's entry into macrophages. However, when the virus multiplies rapidly in the macrophages, the latter can release excessive pro-inflammatory factors that aggravate the cytokine storm (Vial and Descotes, 1995, Channappanavar et al., 2016). This may explain how CP therapy can worsen symptoms and hasten the death of terminally ill patients with Spanish influenza A (H1N1), and is potential factor behind CP-related ARDS in Ebola-infected patients. Therefore, we need to be alert to cytokine storms when applying CP therapy. Avoiding cytokine storms and the reasonable application of CP are vital for this treatment.
Collection of and treatment with CP should be performed at the right time to ensure effective antibody titers and a boost to the patient's immune response in the most timely manner. Various studies have shown that early treatment with CP resulted in better clinical outcomes than later intervention. There is a 10-day incubation period before the antigen stimulates the primary immune response. Later, low-affinity IgM and then low-affinity IgG antibodies are produced and peak on day 21. High-affinity IgG antibodies can be produced quickly (in 3–5 days) only as a secondary response (Xiaoming and Jifeng, 2020). Therefore, CP should be given early in the course of the disease, when IgG antibodies have not yet been produced in the body. At this time, the passive infusion of high-level and high-affinity IgG can improve the humoral immune response, reduce the repeated stimulation of killer T cells in the immune system, avoid cytokine storms, and prevent the disease from worsening or progressing to a critical stage.
In China, the collection of blood products is highly regulated. In addition to conventional pathogens and biological indicators, more than 30 types of pathogen from the respiratory, digestive, and urogenital systems are screened in the plasma of donors who have recovered from COVID-19. Furthermore, plasma needs to undergo viral inactivation to ensure the safety of CP (Xiaoming and Jifeng, 2020). CP collection is an established method in which only plasma is collected, and blood cells are transfused back into the donor. Plasma donation has little effect on recovered patients, and plasma transfusion is a routine and safe medical procedure.
COVID-19 presents an ongoing global health emergency. Without the availability of specific agents and vaccines, it is essential to explore effective treatments for infection. Based on the successful outcomes of CP therapy for treating acute viral infectious diseases, CP should be reasonably used as early as possible to treat COVID-19 patients in a serious condition.
Limitations
Our study had several limitations. The lack of high-quality studies weakened our analysis, with the majority of included studies being at a moderate to high risk of bias, and some lacking a control group. The absence of blind interventions in controlled studies exacerbates this situation. Given the limitations of database searches and manual retrieval, we cannot be certain that all published reports on CP therapy were included, especially those on Spanish influenza from 1918 to 1920. Since the recording methods of the various studies were not unified, some clinical outcomes could not be analyzed quantitatively. Treatments for infectious diseases are diverse and individualized, and we did not exclude factors that might influence clinical outcomes, which weakened our evaluation of CP therapy.
Conclusions
According to our analysis and predictions, CP has some curative effect and is a safe method for treating infectious diseases early after symptom onset. CP is a potentially effective treatment offers a promising rescue option for severe COVID-19 cases. Well-designed clinical trials and further investigations of CP therapy are warranted in the future.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding sources
Yinghui Xu received support for this study from the Youth Foundation of The First Hospital of Jilin University (Grant ID: JDYY82017020) and Xisike Clinical Oncology Research Foundation (CSCO-Haosen) (Grant ID: Y-HS2017-062).
Ethical approval
This study did not require ethical approval because the meta-analysis was based on published research, and the original data were anonymous.
Contributions
Study design: Mengyao Sun, Yinghui Xu, Kewei Ma, Xu Wang
Data collection: Mengyao Sun, Yinghui Xu, Hua He, Li Zhang, Xu Wang, Qing Qiu
Data analysis: Mengyao Sun, Hua He, Chao Sun, Ye Guo, Shi Qiu
Writing: Mengyao Sun, Yinghui Xu, Kewei Ma, Li Zhang
Declaration of Competing Interest
The authors report no declarations of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2020.06.107.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;13;35(14):e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew B.F., Vanessa R. Current studies of convalescent plasma therapy for COVID-19 may underestimate risk of antibody-dependent enhancement. J Clin Virol. 2020;127(1043):88. doi: 10.1016/j.jcv.2020.104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y., Balkhy H., Hajeer A.H., Bouchama A., Hayden F.G., Omari A.A., et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4(1):709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang O. Convalescent serum in the treatment of influenza pneumonia. Nor Mag Laegevidenskaben. 1920;81:255–261. [Google Scholar]
- Beigel J.H., Tebas P., Elie-Turenne M., Bajwa E., Bell T.E., Cairns C.B., et al. A randomised study of immune plasma for the treatment of severe influenza. Lancet Respir Med. 2017;5(6):500–511. doi: 10.1016/S2213-2600(17)30174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.K., Lee K., Lam P.K., Law K., Joynt G.M., Yan W. Hong Kong’s experience on the use of extracorporeal membrane oxygenation for the treatment of influenza A (H1N1) Hong Kong Med J. 2010;16:447–454. [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbes. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhang X.R., Ju Z.Y., He W.F. Advances in the research of the cytokine storm mechanism induced by coronavirus disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Wong R., Soo Y.O., Wong W.S., Lee C.K., Ng M.H., et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg L., Barkman A. Convalescent serum in the prevention and treatment of influenza. Hygiea. 1919;81:113–123. [Google Scholar]
- Florescu D.F., Kalil A.C., Hewllet A.L., Schuh A.J., Stroher U., Uyeki T.M., et al. Administration of brincidofovir and convalescent plasma in a patient with ebola virus disease. Clin Infect Dis. 2015;15;61(6):969–973. doi: 10.1093/cid/civ395. [DOI] [PubMed] [Google Scholar]
- General Office of the National Health Commission; 2020. Diagnosis and Treatment Plan for Novel Coronavirus Pneumonia (Fifth Edition for Trial Implementation) [Google Scholar]
- Gould E.W. Human serum in the treatment of influenza bronchopneumonia. N Y Med J. 1919;109:666–667. [Google Scholar]
- Griensven J., Edwards T., Lamballerie X., Semple M.G., Gallian P., Baize S., et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J. Convalescent serum in the treatment of influenza. Nor Mag Laegevidenskaben. 1919;80:31–561. [Google Scholar]
- Huff-Hewitt W. Human serum in influenza. Br Med J. 1919;1:575. [Google Scholar]
- Hung I.F., To K.K., Lee C.K., Lee K.L., Chan K., Yan W.W., et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J.M., Campos M.S., Baillie J.K., Cleary P., Khaw F.M., Lim W.S., et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2016;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M.H. Serum treatment of postinfluenzal bronchopneumonia. J Am Med Assoc. 1919;72:102–103. [Google Scholar]
- Kai D, Bende L, Cesheng L, et al. TheThe feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv. preprint doi: 10.1101/2020.03.16.20036145. [DOI]
- Ko J.H., Seok H., Cho S.Y., Ha Y.E., Baek J.Y., Kim S.H., et al. Challenges of convalescent plasma infusion therapy inMiddle East respiratory coronavirus infection: a single-centre experience. Antivir Therapy. 2018;23(7):617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- Kong L.K. Letter to the editor. Transfus Apher Sci. 2003;29:101. doi: 10.1016/S1473-0502(03)00109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L.K. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med J. 2006:12. [PubMed] [Google Scholar]
- Kraft C.S., Hewlett A.L., Koepsell S. The use of TKM-100802 and convalescent plasma in 2 patients with ebola virus disease in the United States. Clin Infect Dis. 2015;15;61(4):496–502. doi: 10.1093/cid/civ334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leider J.P., Brunker P.A., Ness P.M. Convalescent transfusion for pandemic influenza: preparing blood banks for a new plasma product. Transfusion. 2010;50(6):1384–1398. doi: 10.1111/j.1537-2995.2010.02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H., et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4):e123158. doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish inflfluenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- Malik Y.S., Sircar S., Bhat S., Sharun K., Dhama K., Dadar M., et al. Emerging novel coronavirus (2019-nCoV): current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 2020;8:1–12. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire L.W., Redden W.R. Treatment of influenza pneumonia by the use of convalescent human serum: preliminary report. J Am Med Assoc. 1918;71:1311–1312. doi: 10.2105/ajph.8.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire L.W., Redden W.R. Treatment of influenzal pneumonia by the use of convalescent human serum. JAMA. 1919;72:709–713. doi: 10.2105/ajph.8.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O., McConnell W. Report of influenza treated with serum from recovered cases. Ky Med J. 1919;17:218–219. [Google Scholar]
- Mupapa K., Massamba M., Kibadi K., Kuvula K., Bwaka A., Kipasa M., et al. Treatment of ebola hemorrhagic fever with blood transfusions from convalescent patients. J Infect Dis. 1999;179(Suppl. 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- Ng K.T., Oong X.Y., Lim S.H., Chook J.B., Takebe Y., Chan Y.F., et al. Viral load and sequence analysis reveal the symptom severity, diversity, and transmission clusters of rhinovirus infections. Clin Infect Dis. 2018;67(2):261–268. doi: 10.1093/cid/ciy063. [DOI] [PubMed] [Google Scholar]
- O’Malley J., Hartman F. Treatment of influenzal pneumonia with plasma of convalescent patients. J Am Med Assoc. 1919;72:34–37. [Google Scholar]
- Redden W.R. Treatment of influenza-pneumonia by use of convalescent human serum. Boston Med Surg J. 1919;161:688–691. [Google Scholar]
- Rillo M.M., Arsuaga M., Olivencia G.R., Calle F., Borobia A.M., Seco P.S., et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir Med. 2015;3(7):554–562. doi: 10.1016/S2213-2600(15)00180-0. [DOI] [PubMed] [Google Scholar]
- Ross C.W., Hund E.J. Transfusion on the desperate pneumonias complicating influenza—preliminary report on the successful use of total immune citrated blood. JAMA. 1918;71:1992–1993. [Google Scholar]
- Ross C.W., Hund E.J. Treatment of pneumonic disturbance complicating influenza. JAMA. 1919;72:640–645. [Google Scholar]
- Sahr F., Ansumana R., Massaquoi T.A., Idriss B.R., Sesay F.R., Lamin J.M., et al. Evaluation of convalescent whole blood for treating Ebola Virus Disease in Freetown, Sierra Leone. J Infect. 2017;74(3):302–309. doi: 10.1016/j.jinf.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn G. The use of the serum of convalescents in the treatment of influenza pneumonia: a summary of the results in a series of one hundred and one cases. Boston Med Surg J. 1920;183:171–177. [Google Scholar]
- Sang L.-Y., Zhuang P., Fang F. Retrospective study on collecting convalescent donor plasma for the treatment of patients with pandemic influenza A (H1N1) virus infection. Chin J Nosocomiol. 2011;21:4684–4686. [Google Scholar]
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed]
- Soo Y.O., Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K., et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll H.F. Value of convalescent blood and serum in treatment of influenza pneumonia. JAMA. 1919;73:478–483. [Google Scholar]
- Tanne J.H. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;26 doi: 10.1136/bmj.m1256. 368:m1256. [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration; Oxford, UK: 2011. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Updated March 2011. http://www.cochrane-handbook.org [accessed 12.06.13] [Google Scholar]
- Tugwell P., Wells G. 2020. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 20.06.13] [Google Scholar]
- Vial T., Descotes J. Immune-mediated side-effects of cytokines in humans. Toxicology. 1995;20;105(1):31–57. doi: 10.1016/0300-483x(95)03124-x. [DOI] [PubMed] [Google Scholar]
- Wiesneth M., Harter G., Schulz A., Rauch S., Mertens T., Schrezenmeier H. Convalescent plasma for prophylaxis and treatment of severe pandemic influenza A (H1N1) 2009 infection: case reports. Vox Sang. 2010;99:238. [Google Scholar]
- Wong S.S., Yuen K.Y. The management of coronavirus infections, with particular reference to SARS. J Antimicrob Chemother. 2008;62:437–441. doi: 10.1093/jac/dkn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V.W.S., Dai D., Wu A.K.L., Sung J.J.Y. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9(3):199–201. [PubMed] [Google Scholar]
- Wu X.X., Gao H.N., Wu H.B., Peng X.M., Ou H.L., Li L.J. Successful treatment of avian-origin influenza A (H7N9) infection using convalescent plasma. Int J Infect Dis. 2015;41:3–5. doi: 10.1016/j.ijid.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Xiaoming Y., Jifeng H. The current state of the use of blood plasma for acute infectious diseases during recovery and the prospects of its use for the treatment of novel coronavirus pneumonia [J/OL] Chin J Biol. 2020:1–5. doi: 10.13200/j.cnki.cjb.002999/. [DOI] [Google Scholar]
- Yeh K.M., Chiueh T.S., Siu L.K., Lin J.C., Chan P.K., Peng M.Y., et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Gao Z., Feng Z., Shu Y., Xiang N., Zhou L., et al. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS ONE. 2008;3:e2985. doi: 10.1371/journal.pone.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.P., Zeng Y.M., Lin Z.S., Chen W., Liang J.S., Zhang H., et al. Clinical characteristics and therapeutic experience of a case of severe highly pathogenic A/H5N1 avian influenza with bronchopleural fistula. Zhonghua Jie He Hu Xi Za Zhi. 2009;32:356–359. [PubMed] [Google Scholar]
- Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L., et al. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. CHEST. 2020 doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.Z., Zhao M., Wang F.S., Jiang T.J., Li Y.G., Nie W.M., et al. Epidemiologic features, clinical diagnosis and treatment of the first cluster of patients with severe acute respiratory syndrome in the Beijing area. Zhonghua Yi Xue Za Zhi. 2003;83:1018–1022. [PubMed] [Google Scholar]
- Zhou B., Zhong N., Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.