Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2, has inevitable consequences for medical care of patients without COVID-19. To assess the impact of this pandemic on oncological care, a nationwide survey was conducted among patients with cancer in the Netherlands.
Methods
The patients' perspective on oncological care was investigated using an online survey between March 29th 2020 and April 18th 2020. The survey consisted of 20 questions on four topics: patients’ characteristics, contact with the hospital, consequences of the COVID-19 pandemic and concerns about COVID-19.
Results
Five thousand three hundred two patients with cancer completed this nationwide survey. Overall, 30% of patients reported consequences for their oncological treatment or follow-up. In the majority of cases, this resulted in conversion from hospital visit to consultation by phone or video. The most frequently adjusted treatments were chemotherapy (30%) and immunotherapy (32%). Among patients with delay and discontinuation of treatment, 55% and 63% of patients, respectively, were (very) concerned about these consequences of the COVID-19 pandemic. Consequences were independent of regional differences in COVID-19 incidence. However, patients in regions with high COVID-19 incidence were significantly more concerned.
Conclusion
This is the first study investigating perspectives of patients with cancer during the COVID-19 pandemic. The study demonstrates the significant impact of the COVID-19 crisis on oncological care, indicating the need for psycho-oncological support during this pandemic.
Keywords: Coronavirus, COVID-19, Pandemic, Cancer, Patients' perspective
Highlights
-
•
Impact of COVID-19 on oncological care from cancer patients’ perspective.
-
•
5302 patients with cancer completed a nationwide online survey.
-
•
30% of the patients reported consequences for oncological treatment or follow-up.
-
•
The consequences were independent of regional differences in COVID-19 incidence.
-
•
This study indicates the need for psycho-oncological support during this pandemic.
1. Introduction
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2], is exhausting healthcare system capacities and has major consequences on non–COVID-19 medical care [3]. Besides limiting capacity of medical care, in general, the COVID-19 pandemic has specific impact on oncological care [4].
First, it has been reported that patients with an (active) malignancy may have an increased risk of COVID-19 [[5], [6], [7]]. Second, malignancy may be an independent risk factor of a more severe course of COVID-19 [5,[7], [8], [9]]. Third, systemic anti-cancer treatment, such as chemotherapy, may increase the risk of a severe infection [10]. Fourth, hospital visits that are required for many cancer treatments may put patients with cancer at risk to be infected with SARS-CoV-2 [5,8].
Considering these factors, physicians and patients are confronted with unprecedented uncertainties about the safety of cancer treatment during this pandemic, balancing between the risk of exposure to SARS-CoV-2 and the risks of postponing life-saving or life-prolonging cancer treatments. Therefore, several (inter)national societies and committees have developed guidelines for oncology physicians [[11], [12], [13], [14]]. Until now, these guidelines are only based on expert opinions. Similarly, scientific evidence has not yet been generated on the impact of COVID-19 on oncological care and patients’ perspectives.
In this study, the impact of the COVID-19 pandemic on patients with cancer and the consequences for their treatment was investigated in the Netherlands. To assess patients’ perspectives on the consequences of the COVID-19 pandemic on cancer treatment and follow-up, a nationwide survey was conducted among patients with cancer during the national lockdown [15,16].
2. Methods
2.1. Survey
A survey for patients with cancer was developed to evaluate the impact of the COVID-19 pandemic on hospital appointments and cancer treatment. The survey contained 20 questions on four topics: patients’ characteristics, contact with the hospital, consequences of the COVID-19 pandemic (consultations, treatment and follow-up) and concerns about COVID-19 (appendix 1).
This online survey was developed by the Dutch Federation of Cancer Patients Organisations (Nederlandse Federatie van Kankerpatiëntenorganisaties [NFK]1 [17]) in close collaboration with oncology physicians and representatives of patient advocacy groups and the Dutch Multidisciplinary Oncology Foundation (Stichting Oncologische Samenwerking (SONCOS)2 [18]). NFK, Dutch hospitals, Dutch Cancer Society and cancer-specific patient advocacy groups distributed the survey to patients by direct mailing, announcements on websites and social media. The survey was open between March 29th 2020 and April 18th 2020.
2.2. Privacy
No personal data were collected, and questionnaires could not be traced back to the patients.
2.3. Regional classification of COVID-19 in the Netherlands
To evaluate the impact of COVID-19 incidence on hospital visits and treatment, regions in the Netherlands were categorised according to the number of patients with COVID-19. In the Netherlands, the COVID-19 pandemic is monitored by the National Institute for Public Health and the Environment [19]. Patients are registered COVID-19 positive when they have a positive test for SARS-CoV-2 using reverse transcription polymerase chain reaction.
The country of the Netherlands has 17.4 million inhabitants [20] and is divided into twelve regions, called provinces, according to geographic location. For the analyses in the present study, provinces were classified according to the number of COVID-19–positive patients. A province was defined as code red when there were ≥100 COVID-19–positive patients per 100,000 inhabitants in that particular province on March 29th 2020 [19]. To determine the regional development of COVID-19 in the three-week period of the survey, the number of COVID-19–positive patients per 100,000 inhabitants per province on April 18th 2020 was calculated.
2.4. Data analysis
Baseline characteristics and survey responses were analysed using descriptive statistics. Cancer diagnosis not represented by a patient advocacy group (mainly rare cancers) or reported as unknown was grouped as ‘other’. The following subgroups were analysed: age (age <65 vs. age ≥65 years), treatment setting (awaiting treatment, under treatment, follow-up), disease setting (cured, curable, incurable) and region of the patients' hospital (code red vs. other). Regarding questions on concerns, the four selectable answers were categorised into ‘not/slightly concerned’ (i.e. not or slightly concerned) and ‘(very) concerned’ (i.e. concerned and very concerned). The Pearson's chi-square test was used to test for differences between specific groups. All statistical tests were performed two sided. P-values of <0.05 were considered statistically significant. All data were analysed using IBM SPSS statistics, version 25.
3. Results
3.1. COVID-19 in the Netherlands
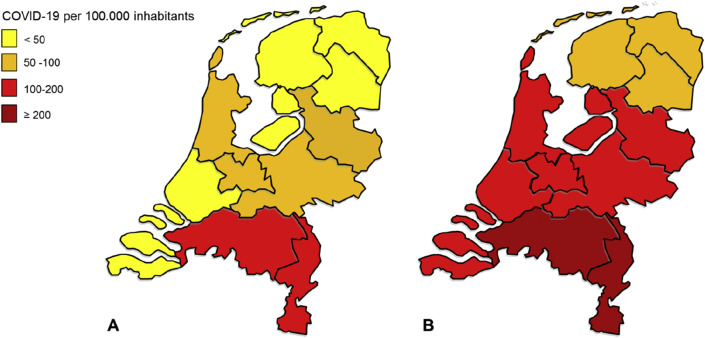
At the time the survey was launched on March 29th 2020, the southern part of the Netherlands suffered severely from the COVID-19 pandemic. On March 29th 2020, code red was applicable to two provinces (i.e. Noord-Brabant and Limburg) (118 and 107 patients with COVID-19 per 100,000 inhabitants, respectively; Fig. 1 a). At the time the survey was closed on April 18th 2020, Noord-Brabant and Limburg still had the highest number of patients with COVID-19 per 100,000 inhabitants (262 and 293 patients with COVID-19, respectively; Fig. 1b). In the three-week period of the survey, the incidence of patients with COVID-19 also increased in other regions. For example, in Friesland, a northern province with the lowest incidence, the number of patients with COVID-19 increased from 18 to 70 per 100,000 inhabitants.
Fig. 1.
Number of patients with a positive test for SARS-CoV-2 in the Netherlands during the three-week period of the survey on (a) March 29th 2020 and (b) April 18th 2020. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
3.2. Patients’ characteristics
The survey was completed by 5302 patients with cancer (Table 1 ). Most (62%) respondents completed the survey in the first week, that is, March 29th to April 3rd. In total, 3413 (64%) patients were women, and most patients (65%) were <65 years. Haematological malignancies (31%), breast cancer (22%) and lung cancer (12%) were the most frequent cancer diagnoses. Only 21 (0.4%) patients reported to have been tested positive for SARS-CoV-2.
Table 1.
Patients' characteristics.
| Number | % | |
|---|---|---|
| Gender | ||
| Women | 3413 | 64 |
| Men | 1887 | 36 |
| Age | ||
| <65 years | 3422 | 65 |
| ≥65 years | 1880 | 35 |
| Region in the Netherlands | ||
| Code red according COVID-19 | 834 | 16 |
| Other | 4468 | 84 |
| Disease setting | ||
| Cured | 2004 | 38 |
| Curable disease | 793 | 15 |
| Incurable disease | 1907 | 36 |
| Unknown | 598 | 11 |
| Treatment setting | ||
| Awaiting treatment | 250 | 5 |
| Under treatment | 2391 | 45 |
| Follow-up | 2661 | 50 |
| Cancer diagnosis | ||
| Bladder and renal cell cancer | 214 | 4 |
| Brain tumor | 45 | 1 |
| Breast cancer | 1187 | 22 |
| Colorectal cancer | 296 | 6 |
| Gastric and oesophageal cancer | 92 | 2 |
| Gynaecological cancer | 177 | 3 |
| Head and neck cancer | 77 | 1 |
| Haematological malignancy | 1660 | 31 |
| Lung cancer | 622 | 12 |
| Melanoma and skin cancer | 209 | 4 |
| Mesothelioma | 34 | 1 |
| Pancreatic cancer | 55 | 1 |
| Prostate cancer | 160 | 3 |
| Sarcoma | 56 | 1 |
| Testicular cancer | 37 | 1 |
| Other | 381 | 7 |
COVID-19 = coronavirus disease 2019.
According to disease setting, 36% of patients had incurable disease, whereas 15% and 38% of patients had curable or cured disease, respectively. For 11% of patients, the (expected) outcome of their disease was ‘unknown’. As the intention of treatment (curative vs. palliative) could affect the nature of adjustments in treatment, the ‘unknown’ group was excluded from further analyses. Elderly patients more frequently reported to have incurable disease than younger patients (51% vs. 35%, p < 0.05). According to treatment setting, 2661 (50%) patients had completed oncological treatment and were in follow-up, whereas 250 (5%) and 2391 (45%) patients were awaiting or currently under treatment, respectively.
3.3. Contact with the hospital
Half of the patients (n = 2664, 50%) had been in contact with their hospital about the consequences of the COVID-19 pandemic for their individual situation in relation to their cancer treatment or follow-up. Among patients who were in follow-up, 36% of patients had contact with the hospital, whereas 52% and 69% of patients who were awaiting and under treatment had contact with the hospital, respectively (p < 0.05). Patients who completed the survey between April 4th and April 17th had more frequently contact with the hospital than patients who completed the survey in the first week (57% vs. 48%, p < 0.05). Overall, 75% of all patients reported that the COVID-19 pandemic did not influence their behaviour to contact the hospital; however, 19% of patients reported that they were less inclined to contact the hospital.
3.4. Consequences for treatment and hospital visits
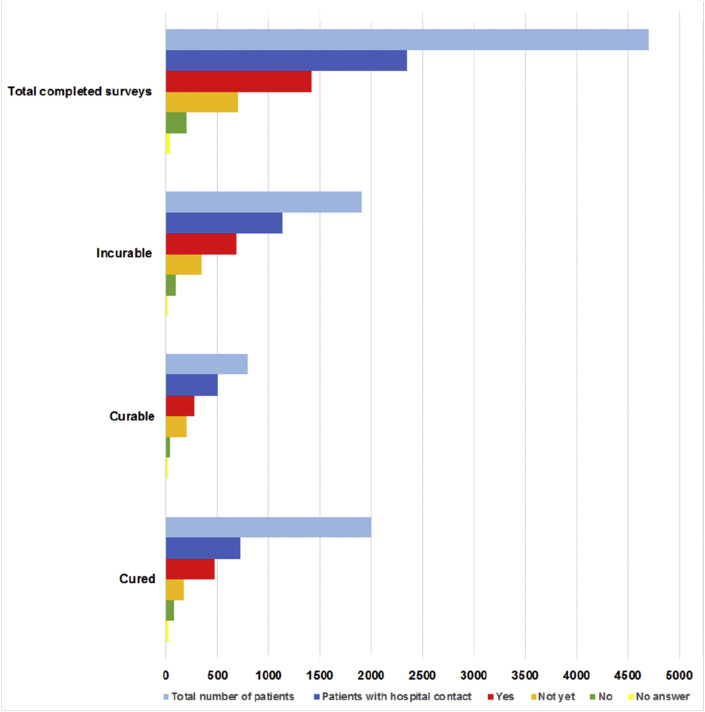
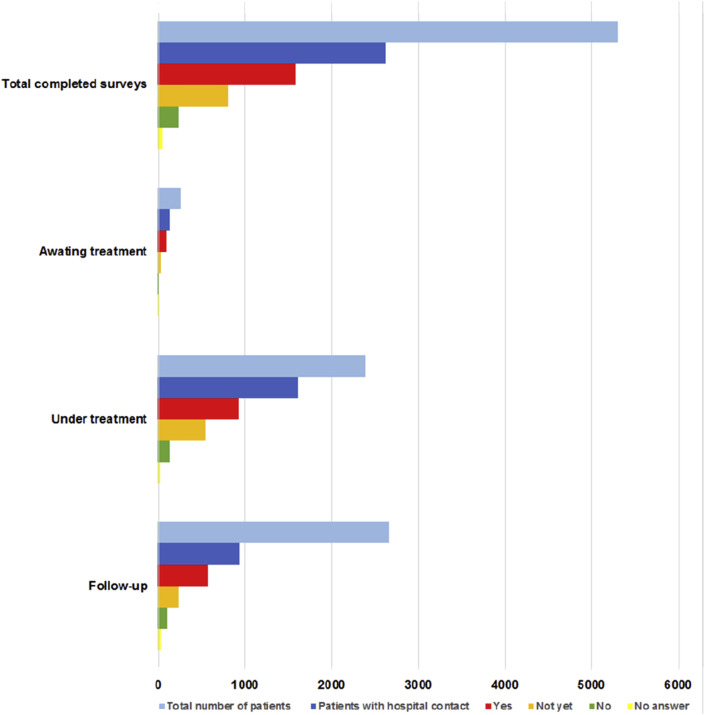
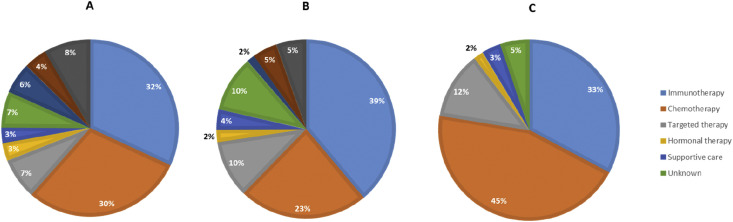
The COVID-19 pandemic led to consequences for treatments and hospital visits, mostly initiated by the hospital (≥79%). The majority of patients (≥72%) reported that they had been informed adequately by the hospital about the reason for the consequences for treatment and hospital visits. Among the 2664 patients who had had contact with the hospital, 1583 patients (i.e. 30% of all respondents) experienced consequences for their treatment or follow-up. Patients with incurable disease or under treatment most frequently experienced consequences (Fig. 2, Fig. 3 ). Overall, the most frequently reported consequence was the conversion from hospital visit to consultation by phone or video, which was reported in 817 of 1583 (52%) patients who experienced consequences. Treatment was adjusted in 7 of 250 patients (3%) and in 162 of 2391 patients (7%) who were awaiting and under treatment, respectively. Treatment was postponed in 39 of 250 patients (16%) and in 279 of 2391 patients (12%) who were awaiting and under treatment, respectively. Treatment changes included adjustment, delay and discontinuation of treatment. Overall, treatment was changed in 49 of 250 patients (20%) and in 480 of 2391 patients (20%) who were awaiting and under treatment, respectively. In patients with adjusted treatment, chemotherapy (30%) and immunotherapy (32%) were most frequently adjusted (Fig. 4 a). Delay and discontinuation of treatment also mainly included chemotherapy and immunotherapy (Fig. 4b and c).
Fig. 2.
Experienced consequences for treatment and follow-up visits of 4704 patients according to disease setting. Patients with ‘unknown’ disease setting were excluded. Patients answered whether they experienced consequences as ‘yes’, ‘not yet’ or ‘no’.
Fig. 3.
Experienced consequences for treatment and follow-up visits of 5302 patients according to treatment setting. Patients answered whether they experienced consequences as ‘yes’, ‘not yet’, or ‘no’.
Fig. 4.
Adjusted treatment (a; n = 213), postponed treatment (b; n = 406) and cancelled treatment (c; n = 58) according to treatment modality.
3.5. Concerns
Many patients were concerned about the consequences of the COVID-19 pandemic for their cancer treatment or follow-up. Among patients with delay and discontinuation of treatment, 55% and 62% of patients were concerned, respectively. Among patients who did not experience consequences yet, 24% of patients were (very) concerned about potential consequences for their treatment or follow-up. In the setting of cured disease or follow-up, 87% and 83% of patients were not/slightly concerned, respectively.
In total, 47% of respondents were (very) concerned to be infected with SARS-CoV-2. Patients who were under treatment were more often (very) concerned to be infected than patients in follow-up (54% vs. 40%, p < 0.05). Patients with incurable disease were also more often (very) concerned than patients with curable or cured disease (55% vs. 50% and 37%, respectively, p < 0.05).
3.6. Patients’ perspectives in most affected COVID-19 regions
According to regional classification, surveys were completed by 834 (16%) patients in code red regions (23 completed surveys per 100,000 inhabitants) and 4468 (84%) patients in other regions (33 completed surveys per 100,000 inhabitants). In regions with code red, appointments were more frequently cancelled and treatments were more frequently adjusted, but these differences were not statistically significant as compared with other regions. In three specific situations, however, differences were found between code red and other regions. First, cancellation of appointments was more frequently initiated by hospitals in code red regions (99% vs. 92%, p < 0.05). In code red regions, patients more frequently reported symptoms probably related to COVID-19 (13.3% vs. 10%, p < 0.05). Finally, patients in code red regions were more concerned to be infected with SARS-CoV-2 than patients in the other regions (51% vs. 46%, p < 0.05).
4. Discussion
According to many expert opinions, oncology physicians are very concerned about the impact of the COVID-19 pandemic on oncological care, in particular, because more COVID-19 outbreaks are expected. Nevertheless, a comprehensive study on patients’ perspectives has been lacking. To the best of our knowledge, this is the first study investigating perspectives of patients with cancer during the COVID-19 pandemic. The survey was conducted among 5302 patients with cancer in the Netherlands. As reported by these patients, the COVID-19 pandemic has significant impact on their oncological care. Overall, 30% of patients experienced consequences regarding their oncological treatment or follow-up, mostly initiated by the hospital. The most frequently adjusted therapies were chemotherapy and immunotherapy.
Although 50% of patients had had contact with their hospital about their individual situation, 19% of patients was more reluctant to contact the hospital during the COVID-19 pandemic. This hesitancy to consult the hospital for non–COVID-19 diseases is an issue of international concern. For example, a large reduction in hospital admissions for acute coronary syndrome was noticed since the COVID-19 outbreak in Italy [21]. In addition, the incidence of new cancer diagnoses has decreased significantly [22], which is also the result of temporarily discontinuation of screening programmes for cancer [23]. In the Netherlands, a 30% decrease in the incidence of new cancer diagnosis was observed in March and April 2020 [24].
Most patients with curable disease continued their treatment unchanged, whereas treatment was more frequently postponed in patients with incurable disease. As compared with patients with curable or cured disease, patients with incurable disease were more concerned about the COVID-19 pandemic and the risk of infection with SARS-CoV-2. These concerns may be explained by fear not to be admitted to the intensive care unit in case of severe COVID-19. This fear is conceivable, as strict triage criteria were expected due to capacity issues and restrictive national guidelines for the treatment of patients with incurable malignancies.
Although patients treated in code red regions were more concerned about the consequences of their oncological treatment or follow-up, the absolute differences in regional adjustments were negligible. It is conceivable that these nationwide adjustments are the result of national advices on oncological treatment during the COVID-19 pandemic. On March 22nd, the Dutch Multidisciplinary Oncology Foundation (SONCOS) and other organisations have issued guidelines with practical adjustments to preserve the continuity of cancer care as much as possible. To reduce the risk for in-hospital transmissions, treatment-related hospitalisations were limited, thereby anticipating on the expected capacity issues. In addition, the lack of scientific evidence and concerns about safety of cancer treatments may have contributed to nationwide adjustments of treatment.
Although the Netherlands is a relatively small European country, the present study serves as a representative model to evaluate the impact of the COVID-19 pandemic on oncological care. As all patients have equal access to medical care in the Netherlands, reimbursement issues have most likely not contributed to the results of the present study. Therefore, the impact of the COVID-19 pandemic on oncological care is expected to be even higher in countries with unequal access to oncological care [25]. As comparable with other countries, there were significant differences in COVID-19 incidence between regions in the Netherlands (Fig. 1a and b). Therefore, the present study, which has nationwide coverage, serves as a representative model for patients’ perspectives in a country during the COVID-19 pandemic. Nevertheless, cultural differences and different national recommendations on cancer treatment may significantly influence the behaviour of patients with cancer and oncology physicians during the COVID-19 pandemic.
As no personal data were collected and questionnaires could not be traced back to patients, clinical data could not be monitored. In particular, it can be difficult for patients to distinguish incurable (i.e. palliative) from curable disease setting. Besides disease setting, treatment restrictions, such as restrictions on resuscitation and mechanical ventilation, could have influenced treatment adjustments and patients’ perspectives. As the survey did not contain questions about such treatment restrictions, this is a potential limitation of the survey. Another limitation is a potential selection bias, which may be induced by the design of this study. In the Netherlands, 347,121 people are suffering from cancer [26], whereas only a fraction, 5302 (0.9%) has participated in the current survey. Nevertheless, the survey had nationwide coverage, and patients from all regions in the Netherlands participated in the survey.
In general, most patients were (very) concerned about the impact of the COVID-19 pandemic on their oncological treatment or follow-up. In particular, patients treated in code red regions were more concerned than patients in other regions, independent of the treatment adjustments. These findings indicate that all patients could benefit from more psycho-oncological support and information, for example, by use of webinars. Additional support for patients facing the daily consequences of the COVID-19 pandemic would be beneficial.
This COVID-19 pandemic has inevitable consequences for healthcare systems and, as underscored by the present study, adjustments in non–COVID-19 medical care cannot be avoided. In the near future, it will be a challenge to reorganise oncological care while still facing the COVID-19 pandemic. In the meantime, we will be awaiting the impact of all adjustments of oncological care on survival and quality of life of patients with cancer.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
K.de.J., D.W.D., A.C.D. and A.A.M.v.d.V. contributed to literature search, data collection, data analysis, data interpretation and writing of the manuscript. V.E., H.J.B., M.V., H.W.M.v.L., I.H.D., A.C.D. and A.A.M.v.d.V. contributed to the study design. V.E. and I.H.D. made contributions to collection of the data and the data analysis. H.J.B., M.V. and H.W.M.v.L. participated in drafting the article and revising it critically for important intellectual content. All authors reviewed the manuscript and gave final approval of the submitted version.
Conflict of interest statement
D.W.D. reports receiving personal fees and speaker fee from MSD, Roche, AstaZeneca, BMS, Novartis and Pfizer, outside the submitted work. A.C.D. reports receiving personal fees from Roche, PharmaMar, Boehringer Ingelheim, Novartis, Takeda, Pfizer and Eli Lilly; grants from BMS and Amgen and non-financial support from Abbvie,grants fees outside the submitted work;
A.A.M.v.d.V. reports receiving other grants from BMS, MSD, Merck, Pfizer, Ipsen, Eisai, Pierre Fabre, Roche, Novartis and Sanofi, outside the submitted work. All other authors report no conflict of interest.
Footnotes
NFK federates 19 national patient advocacy groups for cancer and advises the government, health authorities and health care professionals in the Netherlands.
SONCOS, an initiative by the Dutch societies of medical oncology, radiation oncology and surgical oncology, represents 29 scientific societies/organisations involved in cancer care and acts as a national platform to improve the quality of cancer care.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2020.06.019.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. Coronavirus disease (COVID-19) outbreak. [Google Scholar]
- 3.World Health Organization . 2020. Coronavirus disease (COVID-2019) situation reports. [Google Scholar]
- 4.Indini A., Aschele C., Cavanna L., Clerico M., Daniele B., Fiorentini G. Reorganisation of medical oncology departments during the novel coronavirus disease-19 pandemic: a nationwide Italian survey. Eur J Cancer. 2020;132:17–23. doi: 10.1016/j.ejca.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020 Jul;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020 Jun;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whisenant J.G., Trama A., Torri V., De Toma A., Viscardi G., Cortellini A. TERAVOLT: thoracic cancers international COVID-19 collaboration. Cancer Cell. 2020 Jun 8;37(6):742–745. doi: 10.1016/j.ccell.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolston K.V. Infections in cancer patients with solid tumors: a review. Infect Dis Ther. 2017;6:69–83. doi: 10.1007/s40121-017-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banna G., Curioni-Fontecedro A., Friedlaender A., Addeo A. How we treat patients with lung cancer during the SARS-CoV-2 pandemic: primum non nocere. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guckenberger M., Belka C., Bezjak A., Bradley J., Daly M.E., DeRuysscher D. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: an ESTRO-ASTRO consensus statement. Radiother Oncol. 2020 May;146:223–229. doi: 10.1016/j.radonc.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dingemans A.C., Soo R.A., Jazieh A.R., Rice S.J., Kim Y.T., Teo L.L. Treatment guidance for lung cancer patients during the COVID-19 pandemic. J Thorac Oncol. 2020 Jul;15(7):1119–1136. doi: 10.1016/j.jtho.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng L., Zagorac S., Stebbing J. Managing patients with cancer in the COVID-19 era. Eur J Cancer. 2020;132:5–7. doi: 10.1016/j.ejca.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruins B., van Engelshoven I. Ministerie van Volksgezondheid Welzijn en Sport. 2020. COVID-19 nieuwe aanvullende maatregelen. [Google Scholar]
- 16.Kraaijenbrink J. Forbes; 2020. The Dutch answer to COVID-19: the ‘1.5 meter economy’.https://www.forbes.com/sites/jeroenkraaijenbrink/2020/04/14/the-dutch-answer-to-covid-19-the-15-meter-economy/#2a494a044627 [Google Scholar]
- 17.Nederlandse Federatie van Kankerpatiëntenorganisaties. 'Gevolgen van de coronacrisis voor kankerpatiënten, wat is jouw ervaring?'Website: https://nfk.nl/resultaten/gevolgen-van-de-coronacrisis-voor-kankerpati%C3%ABnten-wat-is-jouw-ervaring.
- 18.Stichting Oncologische Samenwerking. COVID-19. Website: https://www.soncos.org/covid-19/.
- 19.Rijksinstituut voor Volksgezondheid en Milieu. Actuele informatie over het nieuwe coronavirus (COVID-19). Website: https://www.rivm.nl/coronavirus-covid-19/actueel.
- 20.Het Centraal Bureau voor de Statistiek. StatLine databank CBS. Website: https://opendata.cbs.nl/statline/#/CBS/nl/.
- 21.De Filippo O., D’Ascenzo F., Angelini F., Bocchino P.P., Conrotto F., Saglietto A. Reduced rate of hospital admissions for ACS during covid-19 outbreak in northern Italy. N Engl J Med. 2020 Jul 2;383(1):88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The IQVIA Institue. COVID-19 – Shifts in Healthcare Demand, Delivery, and Care During the COVID 19 Era. Website: https://www.iqvia.com/insights/the-iqvia-institute/covid-19/shifts-in-healthcare-demand-delivery-and-care-during-the-covid-19-era.
- 23.Cancer Research UK . 2020. Cancer screening and coronavirus (COVID-19) [Google Scholar]
- 24.Dinmohamed A.G., Visser O., Verhoeven R.H.A., Louwman M.W.J., van Nederveen F.H., Willems S.M. Fewer cancer diagnoses during the COVID-19 epidemic in The Netherlands. Lancet Oncol. 2020 Jun;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The American Society for Clinical Oncology (ASCO) post (April 2020). ‘Survey Shows COVID-19 Pandemic Is Affecting Patients’ Access to Cancer Care.Website: https://www.ascopost.com/news/april-2020/survey-shows-covid-19-pandemic-is-affecting-patients-access-to-cancer-care/.
- 26.Nederlandse Kankerregistratie (NKR) Prevalentie, Alle kankersoorten, 5-jaarsprevalentie. https://www.iknl.nl/nkr2017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.