Abstract
The novel coronavirus disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), first appeared in December 2019, in Wuhan, China and evolved into a pandemic. As Angiotensin-Converting Enzyme 2 (ACE2) is one of the potential target receptors for SARS-CoV-2 in human body, which is expressed in different tissues, multiple organs might become affected. In the initial phase of the current pandemic, a handful of post-mortem case-series revealed COVID-19-related pathological changes in various organs. Although pathological examination is not a feasible method of diagnosis, it can elucidate pathological changes, pathogenesis of the disease, and the cause of death in COVID-19 cases. Herein, we thoroughly reviewed multiple organs including lung, gastrointestinal tract, liver, kidney, skin, heart, blood, spleen, lymph nodes, brain, blood vessels, and placenta in terms of COVID-19-related pathological alterations. Also, these findings were compared with SARS and MERS infection, wherever applicable. We found a diverse range of pathological changes, some of which resemble those found in SARS and MERS.
Keywords: COVID-19, SARS-CoV-2, Pathology, Histopathology
1. Introduction
The novel coronavirus disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), first appeared in December 2019, in Wuhan, China and evolved into a pandemic [6]. So far, almost 10 million infected cases and 500,000 consequent deaths have been reported globally.
SARS-CoV-2 is the seventh member of coronavirus family leading to a wide range of clinical symptoms including respiratory, hepatic, enteric, and neurologic manifestations. Earlier in the last two decades, two other members of this family, severe acute respiratory syndrome coronavirus (SARS‐CoV) and the Middle East respiratory syndrome coronavirus (MERS‐CoV), leaded to global outbreaks with high mortality and morbidity [16,20]. Sharing similar clinical manifestations, these coronaviruses may resemble each other in terms of pathogenesis and pathological features [21].
Besides, Angiotensin-Converting Enzyme 2 (ACE2) is one of the potential target receptors for SARS-CoV-2 in human body, which is expressed in different tissues including lung, gastrointestinal tract, vessels, brain, liver, kidney, spleen, and skin [9,13]. This may lead to possible short-term and long-term involvement of multiple human organs.
Based on the autopsy results SARS-CoV-2 seems to affect several organs, such as lung, heart, blood vessels, etc [24]. Previous post-mortem case-series revealed a diverse range of pathological changes after being infected with SARS-CoV-2. Currently, diagnosis of COVID-19 is mainly based on CT-imaging, laboratory tests, and history taking. Although pathological examination is not a feasible method of diagnosis, it can elucidate pathological changes, pathogenesis of the disease, and the cause of death in COVID-19 cases [22]. Herein, we discuss histopathologic changes in different tissues after being infected by SARS-CoV-2 to broaden the knowledge of SARS-CoV-2 pathological changes.
2. Lung
Since SARS-CoV-2 is considered as a respiratory virus, lung is one of the well-studied organs in autopsies and biopsies of infected cases. Acute respiratory distress syndrome (ARDS) and pneumonia are usually the clinical diagnosis of infected cases [15,38]. In autopsy samples, macroscopic findings revealed an increase in lung weight, copious amounts of gray-white viscous fluid, vascular engorgement, consolidation, edema, pleurisy, mildly erythematous trachea, presence of white mucous in lungs, and pink froth in airways with dark-colored hemorrhage [10,14,38]. These findings may vary regarding the phenotype of the virus, explained as L and H phenotypes. L type is described with the following characteristics: low elastance, a low ventilation-to-perfusion ratio, a low lung weight and a low lung recruitability. However, H type is described with high elastance, a high right-to-left shunt, a high lung weight and a high lung recruitability. Also, H type tends to accompany more adverse outcomes compared to L type [5]. Microscopic autopsy findings have also been reported with a great variability. Diffuse alveolar damage (DAD), infiltration of lymphocytes in interstitial regions, giant pneumocytes adjacent to multinucleated giant cells, hyperplasia of pneumocytes, intra-alveolar fibrin deposition, exudate formation, lymphocytic inflammation, loose connective tissue within the alveolar ducts and bronchioles, intra-alveolar fibrin surrounded by fibroblasts, viral particles within pneumocytes, and hyaline membrane formation were the most commonly observed histopathological changes described by previous studies [3,5,14,15,38]. In addition, cytoplasmic vacuolization in pulmonary arteries, megakaryocyte with multinuclear appearance within the branching small vessels (in exudative phase), pulmonary microangiopathy, fibrin clotting within small capillaries around alveoli, capillary congestion (in exudative phase), small vessel thrombosis with alveolar hemorrhage, and thickening of alveolar capillaries in lungs were found in different post-mortem studies [3,5,10,18]. Similar findings have been reported in SARS, and Middle East Respiratory Syndrome (MERS). predominant visceral macroscopic changes in fatal SARS‐CoV cases have been reported as edematous lungs with increased gross weights and multiple areas of congestion, enlargement of lymph nodes in the pulmonary hila and the abdominal cavity [7,26]. Also, bronchial epithelial denudation, loss of cilia, and squamous metaplasia were present among microscopic findings [26]. Similar findings were observed in MERS-CoV, including exudative diffuse alveolar damage with hyaline membranes, pulmonary edema, type II pneumocyte hyperplasia, interstitial pneumonia and multinucleate syncytial cells [1,25]. Both SARS‐CoV and MERS-CoV particles were present in pneumocytes in electron microscopic examination [30]; same results were observed for SARS-CoV-2 [40]. In addition, DAD is among the most common pathological findings reported in Human Influenza A (H1N1), which tends to be commonly present among SARS-CoV-2 cases [23].
3. Gastrointestinal (GI) tract
COVID-19 infection may also affect Gastrointestinal (GI) tract since ACE2 is abundantly present in the enterocytes [13]. SARS-CoV-2 has been found in stool specimen and diarrhea was reported in several infected cases [36,37]. Endoscopy was performed on patients and hematoxylin and eosin (H&E) staining revealed no significant damage in mucous epithelium of GI tract; however, infiltration of lymphocytes in the esophageal squamous epithelium, and lamina propria of the stomach, duodenum, and rectum was seen. In addition, interstitial edema was observed. Besides, viral nucleocapsid protein was seen in glandular epithelial cell of stomach, duodenum, and rectum, but not in the esophageal epithelium [36]. As ACE2 is highly distributed in glandular epithelial cell [36], it is worth mentioning that there are evidences suggesting that SARS-CoV-2 may cause acute and chronic sialadenitis in infected cases [35].
4. Liver
Moreover, high level of hepatic enzymes, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) in infected cases suggests involvement of liver tissue. Macroscopic findings of autopsy samples showed dark red liver with hepatomegaly. Histopathologic examinations showed hepatocyte degeneration with lobular focal necrosis, congestion of hepatic sinuses with microthrombus, fibrosis of portal tract, proliferation of portal vein branches, and mononuclear leukocyte and neutrophil infiltration within the portal area [19,31]. Sonzogni et al. found alteration of vascular structure, both acute (thrombosis, luminal ectasia) and chronic (fibrous thickening of vascular wall or phlebosclerosis, and abnormal asset of portal intrahepatic system). Atypical arrangement of intrahepatic blood vessels was also observed with CD34 staining, decorating a peri-portal network of sinusoidal vessels, which may show increased arterial pressure [31]. Also, biopsy findings of patients with Covid-19 revealed moderate microvascular steatosis and mild lobular and portal activity. It is also worth mentioning that SARS and MERS showed similar liver pathological characteristics to SARS-CoV-2 [39].
5. Kidney
COVID-19 may also involve the kidney tissue of infected cases. Histopathologic findings of biopsy samples showed proximal acute tubule injury (ATI) in renal tissues. Luminal brush border sloughing, vacuole degeneration, tubular necrosis, infiltration of lymphocytes (mainly CD8 + T cells) and CD68+ macrophages in the tubulointerstitial and sub-capsular area, interstitial fibrosis in cortical parenchyma, strong complement membrane attack complex (MAC) deposition in tubules, mild focal tubular atrophy, hypertrophy and hyperplasia of glomerular epithelial cells, hemosiderin granules in tubular epithelium, and podocyte vacuolation were reported. Moreover, infrastructural changes were also present; cellular swelling in infected renal tissues with the expansion of mitochondria and lysosome, as well as dilation of smooth endoplasmic reticulum (SER) and rough endoplasmic reticulum (RER) were seen among samples [6,28,32]. In addition, vascular analysis visualized infiltration of inflammatory cells in the arcuate artery, and dilated and swollen capillary vessels in glomeruli. Regarding vascular changes, segmental fibrin thrombus in glomerular capillary loops, endothelial hyperplasia, and foamy-like appearance of endothelial cells were seen in microscopic analysis [6,32]. It is also worth mentioning that collapsing glomerulopathy (CG) could be the result of COVID-19 infection in several patients [28]. Previous studies on MERS-CoV reported acute tubulointerstitial nephritis and acute tubular sclerosis with proteinaceous cast formation [4].
6. Skin
COVID-19 also causes Skin tissue alterations. Skin macroscopic manifestations can be divided into viral exanthems (morbilliform rash, petechial rash co-existing with thrombocytopenia, erythematous-to-purpuric coalescing macules, widespread urticaria, and varicella-like vesicles) and vasculopathy-related skin manifestations (peripheral cyanosis with bullae and dry gangrene, transient unilateral livedo reticularis, and red papules on fingers resembling chilblains) [33]. A recent study classified skin lesions as acral areas of erythema with vesicles or pustules (i.e. Pseudo-chilblain, appearing late in the evolution of the disease), vesicular eruptions (early manifestation), urticarial lesions, maculopapular eruptions (most common lesions), and livedo or necrosis [11]. Regarding histopathological findings of biopsy samples, superficial and deep perivascular dermatitis, blood vessels surrounded by lymphocytes, focal acantholytic suprabasal clefts, dyskeratotic and ballooning herpes-like keratinocytes, necrosis of keratinocytes, mucin deposition in the dermis and hypodermis, and nests of Langerhans cells within the epidermis were reported among infected cases. Moreover, thrombus formation and extravasation of erythrocyte in mid-dermis blood vessels was found. Also, swollen thrombosed blood vessels in the dermis with the presence of eosinophils, neutrophils and nuclear debris was visualized [8,12].
7. Heart
COVID-19 also tends to change the macroscopic and microscopic features of the heart. Cardiomegaly and right ventricular dilation were prominent in some patients. Autopsies showed firm myocardium with red-brown appearance without injury. Microscopic findings revealed dispersed area of myocyte necrosis near to lymphocytes in few cases (suggesting microvascular blood supply failure in heart). Also, typical viral myocarditis pattern (brisk lymphocytic inflammatory infiltrate) was not present [10]. Infiltration of mononuclear leukocytes in interstitial areas was also reported by one study [37].
8. Blood
Blood specimen is used to detect SARS-CoV-2 in suspected cases. Although leukopenia and lymphocytopenia occurred in infected cases, blood flow cytometry detected high levels of T-helper 17 and CD8 + T-cells [19]. Interestingly, other flow cytometric analyses revealed that monocytes count did not change in patients; however, they were larger in comparison to normal monocytes (the presence of high forward scatter [FSC-high] monocytes, which are consistent with an inflammatory phenotype) [41]. Besides, autopsy findings of bone marrow showed reactive left-shifted myelopoiesis and hyperplasia of CD8 + T cells [24].
9. Spleen and lymph nodes
Studies showed that viral nucleocapsid protein (NP) could be seen in splenic tissue. Viral NP+ Cells were distributed in both red (primarily) and white pulp. Also, NP antigen was found in macrophages within the lymph nodes [9]. Histopathologic examinations of autopsy samples showed reduction of cell composition, atrophy of white pulp, neutrophil and plasma cell infiltration, reduction or absence of lymph follicles, increase in red pulp to white pulp proportion, reduction of T and B cells due to necrosis and apoptosis, and atrophy of corpuscles in the spleen of infected cases. Besides, in microscopic examination of vessels, splenic infarction due to arterial thrombosis, CD20 + B cells surrounding splenic artery, and proliferation of fibrotic tissues in sinuses were seen [9,38]. In addition, congestion and hemorrhagic appearance were visualized in the spleen [9]. However, previous studies showed a diminished spleen size and reduced spleen weights among SARS-CoV cases [7].
10. Brain
Viral particles of SARS-CoV-2 were also found in the frontal lobe of the brain and brain capillary endothelial cells [27]. Moreover, Histopathologic examination of autopsy samples of the brain showed no infiltration of inflammatory cells or neural cell degeneration. However, mild hypoxic manifestations were present in several autopsies [24]. Also, microglia and type I astrocytes, infected by murine coronavirus (MHV-A59), can produce pro-inflammatory cytokines [17]. Autopsy findings of a patient with post-operational complications, positive for Covid-19, showed hemorrhagic white matter lesions with axonal injuries and white blood cells such as macrophages. Perivascular acute disseminated encephalomyelitis (ADEM)-like appearance as well as neocortical microscopic infarcts were also seen in autopsy findings [29].
11. Blood vessels and placenta
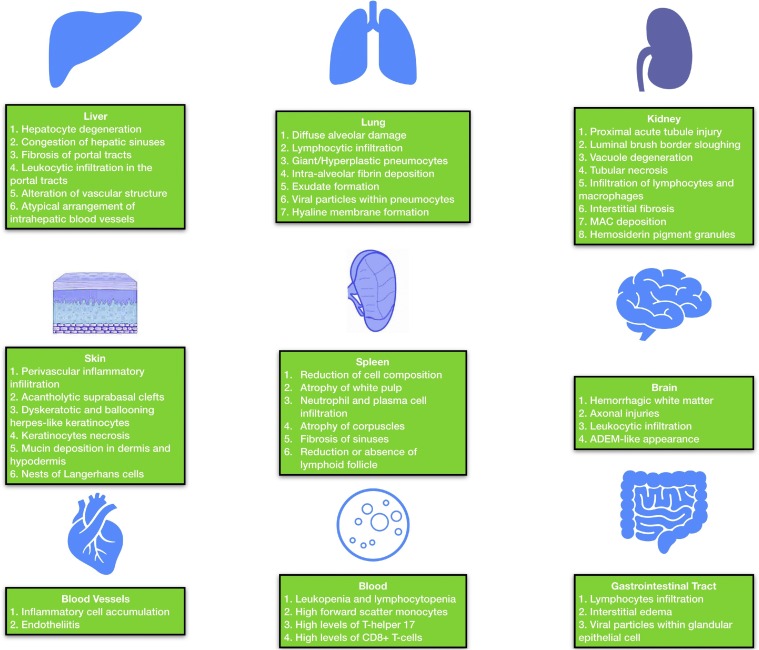
Pathological findings of SARS-CoV-2 have been discussed in each section, but there are also other tissue alternations due to SARS-CoV-2 in blood vessels. Autopsy findings revealed accumulation of inflammatory cells and endothelial and inflammatory cell death, which are the features of endotheliitis [34]. SARS-Cov-2 also alter placenta in pregnant women. Pathologic findings of biopsy samples revealed low grade fetal vascular malperfusion, intramural fibrin deposition, intramural nonocclusive thrombi, and meconium macrophages. In human SARS, fetal thrombotic vasculopathy has also been reported [2]. A summary of microscopic COVID-19-related findings is illustrated in Fig. 1 .
Fig. 1.
A summary of most common microscopic findings of COVID-19 in various organs.
MAC: Membrane Attack Complex; ADEM: Acute Disseminated Encephalomyelitis.
12. Conclusion
COVID-19 infection affects not only the lung but also other organs, such as GI tract, liver, kidney, skin, heart, blood, and spleen. We found a diverse range of pathological changes, some of which resemble those found in SARS and MERS. In addition to pulmonary adverse reactions, short and long-term effects of the SARS-CoV-2 on other organs should be considered.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
None to declare.
References
- 1.Alsaad K.O., Hajeer A.H., Al Balwi M., Al Moaiqel M., Al Oudah N., Al Ajlan A., AlJohani S., Alsolamy S., Gmati G.E., Balkhy H. Histopathology of Middle East respiratory syndrome coronovirus (MERS‐CoV) infection–clinicopathological and ultrastructural study. Histopathology. 2018;72:516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baergen R.N., Heller D.S. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatric Dev. Pathol. 2020;23:177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carsana L., Sonzogni A., Nasr A., Rossi R., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M. medRxiv; 2020. Pulmonary Post-Mortem Findings in a Large Series of COVID-19 Cases from Northern Italy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cha R.-h., Yang S.H., Moon K.C., Joh J.-S., Lee J.Y., Shin H.-S., Kim D.K., Kim Y.S. A case report of a Middle East respiratory syndrome survivor with kidney biopsy results. J. Korean Med. Sci. 2016;31:635–640. doi: 10.3346/jkms.2016.31.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copin M.-C., Parmentier E., Duburcq T., Poissy J., Mathieu D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-06057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diao B., Feng Z., Wang C., Wang H., Liu L., Wang C., Wang R., Liu Y., Liu Y., Wang G. medRxiv; 2020. Human Kidney Is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Li X., Kang W., Weng D. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Hachem M., Diociaiuti A., Concato C., Carsetti R., Carnevale C., Ciofi Degli Atti M., Giovannelli L., Latella E., Porzio O., Rossi S. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Z., Diao B., Wang R., Wang G., Wang C., Tan Y., Liu L., Wang C., Liu Y., Liu Y. medRxiv; 2020. The Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Directly Decimates Human Spleens and Lymph Nodes. [Google Scholar]
- 10.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. MedRxiv; 2020. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galván Casas C., Català A., Carretero Hernández G., Rodríguez‐Jiménez P., Fernández Nieto D., Rodríguez‐Villa Lario A., Navarro Fernández I., Ruiz‐Villaverde R., Falkenhain D., Llamas Velasco M. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020 doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianotti R., Veraldi S., Recalcati S., Cusini M., Ghislanzoni M., Boggio F., Fox L. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm. Venereol. 2020 doi: 10.2340/00015555-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanley B., Lucas S.B., Youd E., Swift B., Osborn M. Autopsy in suspected COVID-19 cases. J. Clin. Pathol. 2020;73:239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 15.Joob B., Wiwanitkit V. Pulmonary pathology of early phase 2019 novel coronavirus pneumonia. J. Thoracic Oncol. 2020;15:e67. doi: 10.1016/j.jtho.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kupferschmidt K. American Association for the Advancement of Science; 2013. Researchers Scramble to Understand Camel Connection to MERS. [DOI] [PubMed] [Google Scholar]
- 17.Lavi E., Cong L. Type I astrocytes and microglia induce a cytokine response in an encephalitic murine coronavirus infection. Exp. Mol. Pathol. 2020 doi: 10.1016/j.yexmp.2020.104474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G., Fox S.E., Summa B., Hu B., Wenk C., Akmatbekov A., Harbert J.L., Vander Heide R.S., Brown J.Q. bioRxiv; 2020. Multiscale Three-Dimensional Pathology Findings of COVID-19 Diseased Lung Using High-Resolution Cleared Tissue Microscopy. [Google Scholar]
- 19.Li J., Fan J.-G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J. Clin. Transl. Hepatol. 2020;8:13. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 21.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. J. Med. Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo W., Yu H., Gou J., Li X., Sun Y., Li J., Liu L. 2020. Clinical Pathology of Critical Patient With Novel Coronavirus Pneumonia (COVID-19) Preprints 2020. [Google Scholar]
- 23.Mauad T., Hajjar L.A., Callegari G.D., da Silva L.F., Schout D., Galas F.R., Alves V.A., Malheiros D.M., Auler J.O., Jr, Ferreira A.F. Lung pathology in fatal novel human influenza A (H1N1) infection. Am. J. Respir. Crit. Care Med. 2010;181:72–79. doi: 10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]
- 24.Menter T., Haslbauer J., Nienhold R., Savic S., Hopfer H., Deigendesch N., Frank S., Turek D., Willi N., Pargger H. Post‐mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng D.L., Al Hosani F., Keating M.K., Gerber S.I., Jones T.L., Metcalfe M.G., Tong S., Tao Y., Alami N.N., Haynes L.M. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am. J. Pathol. 2016;186:652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paniz‐Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) J. Med. Virol. 2020 doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peleg Y., Kudose S., D’Agati V., Siddall E., Ahmad S., Kisselev S., Gharavi A., Canetta P. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int. Rep. 2020 doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shieh W.-J., Hsiao C.-H., Paddock C.D., Guarner J., Goldsmith C.S., Tatti K., Packard M., Mueller L., Wu M.-Z., Rollin P. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum. Pathol. 2005;36:303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonzogni A., Previtali G., Seghezzi M., Alessio M.G., Gianatti A., Licini L., Zerbi P., Carsana L., Rossi R., Lauri E. 2020. Liver and COVID 19 Infection: A Very Preliminary Lesson Learnt from Histological Post-Mortem Findings in 48 Patients. [Google Scholar]
- 32.Su H., Yang M., Wan C., Yi L.-X., Tang F., Zhu H.-Y., Yi F., Yang H.-C., Fogo A.B., Nie X. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suchonwanit P., Leerunyakul K., Kositkuljorn C. Cutaneous manifestations in COVID-19: lessons learned from current evidence. J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C., Wu H., Ding X., Ji H., Jiao P., Song H., Li S., Du H. Does infection of 2019 novel coronavirus cause acute and/or chronic sialadenitis? Med. Hypotheses. 2020 doi: 10.1016/j.mehy.2020.109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. e1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X., Barth R.F., Buja L.M. A call to action: the need for autopsies to determine the full extent of organ involvement associated with COVID-19 infections. Chest. 2020 doi: 10.1016/j.chest.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X., Chang X., Pan H., Su H., Huang B., Yang M., Luo D., Weng M., Ma L., Nie X. Pathological changes of the spleen in ten patients with new coronavirus infection by minimally invasive autopsies. Zhonghua Bing li xue za zhi. 2020;49 doi: 10.3760/cma.j.cn112151-20200401-00278. E014–E014. [DOI] [PubMed] [Google Scholar]
- 39.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao X.-H., He Z.-C., Li T.-Y., Zhang H.-R., Wang Y., Mou H., Guo Q., Yu S.-C., Ding Y., Liu X. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020;30:541–543. doi: 10.1038/s41422-020-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y., Dai T., Zhang T., Lai Y., Wang J. medRxiv; 2020. COVID-19 Infection Induces Readily Detectable Morphological and Inflammation-Related Phenotypic Changes in Peripheral Blood Monocytes, the Severity of Which Correlate With Patient Outcome. [Google Scholar]