Abstract
Introduction
Current evidence on the association between COVID-19 and dementia is sparse. This study aims to investigate the associations between COVID-19 caseload and the burden of dementia.
Methods
We gathered data regarding burden of dementia (disability-adjusted life years [DALYs] per 100,000), life expectancy, and healthy life expectancy (HALE) from the Global Burden of Disease (GBD) 2017 study. We obtained COVID-19 data from Our World in Data database. We analyzed the association of COVID-19 cases and deaths with the burden of dementia using Spearman's rank correlation coefficient.
Results
Globally, we found significant positive (p < .001) correlations between life expectancy (r = 0.60), HALE (r = 0.58), and dementia DALYs (r = 0.46) with COVID-19 caseloads. Likewise, we found similar correlations between life expectancy (r = 0.60), HALE (r = 0.58) and dementia DALYs (r = 0.54) with COVID-19 mortality.
Conclusion
Health policymakers should clarify a targeted model of disease surveillance in order to reduce the dual burden of dementia and COVID-19.
Keywords: Dementia, COVID-19, Global burden of disease, Mortality
Abbreviations: SARS-CoV, Severe acute respiratory syndrome (SARS) coronavirus; GBD, Global Burden of Diseases; HALE, Healthy life expectancy; DALYs, Disability-adjusted life years; UIs, Uncertainty intervals; CI, Confidence Intervals
Highlights
-
•
We assessed the burden of dementia on COVID-19 caseload and mortality worldwide.
-
•
Global burden of dementia correlates with COVID-19 caseloads.
-
•
Global burden of dementia correlates with COVID-19 mortality.
-
•
Healthy life expectancy correlates with COVID-19 cases and mortality.
-
•
We recommend a targeted approach to reduce the burden of COVID-19 in dementia.
1. Introduction
With the emergence of COVID-19 pandemic, it is crucial to identify those at increased risk of infection and death. The worldwide prevalence of dementia has increased dramatically over the past three decades, mainly due to an ageing population [1]. Patients with dementia are among the most vulnerable populations due to ageing, frailty [2], chronic immune dysregulation [3] and comorbidity of other chronic diseases, particularly vascular disorders [4]. Individuals with dementia are faced with a “double burden”, as the pandemic exacerbates both their vulnerability due to increased morbidity and mortality and breakdown of their social supports and access to their healthcare system due to lockdown and social distancing measures [5].
Epidemiological evidence has shown a significant association between communicable diseases and dementia [[6], [7], [8], [9]]. Those with dementia had poor clinical outcomes and increased hospitalization rate after respiratory syncytial virus disease [6], severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) [7], and influenza A infections [[8], [9], [10]]. Recent evidence showed that there is a high prevalence of dementia among COVID-19 patients [11,12]. In addition, case fatality of COVID-19 is significantly higher in the elderly with dementia [13,14]. However, the nature of this association has not yet been clearly evaluated on a global perspective and it may take several months to gather enough epidemiological and clinical data to address this issue.
In a recent study, using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017 database [15], we observed a significant yet weak correlation between all non-communicable diseases, including ischemic stroke and ischemic heart diseases with COVID-19 confirmed cases and deaths. Furthermore, life expectancy and healthy life expectancy (HALE) had independent positive associations with COVID-19 caseload and mortality.
The purpose of this study is to assess the association between the national and global burden of dementia and COVID-19 confirmed cases and death. We will also provide a brief review of the literature regarding the association between COVID-19 and dementia and summarize available recommendations to reduce the dual burden of dementia and COVID-19.
2. Materials and methods
We gathered data regarding regional and national mortality of Alzheimer's disease and related dementias (later regarded as dementia) from the GBD 2017 study database [16]. We used disability-adjusted life years (DALYs) per 100,000 (with 95% uncertainty intervals [UIs]) to measure the burden of dementia. Using the GBD 2017 database, we also collected data regarding national and regional HALE and life expectancy with their 95% UIs. Data regarding the COVID-19 confirmed cases, and deaths per million population, and the percentage of population aged ≥65 and aged ≥70 years was retrieved using Our World in Data. We categorized all countries into a) seven GBD super regions, including 1. South Asia, 2. Southeast Asia, East Asia & Oceania, 3. Central Europe, Eastern Europe & Central Asia, 4. North Africa & Middle East, 5. Sub-Saharan Africa, 6. Latin America & Caribbean, and 7. high-income; b) world Bank income levels, including (1. Low-income countries, 2. lower middle-income countries, 3. upper middle-income countries, and 4. high-income countries [17].
We calculated COVID-19 confirmed cases and deaths per million population with their 95% confidence intervals (CIs) by each region. We used Spearman's rank correlation coefficient with Bonferroni correction to analyse the association of COVID-19 caseload and deaths with the burden and mortality of dementia. We also assessed correlations between national and global life expectancy and HALE with COVID-19 cases and death using Spearman's rank correlation coefficient. We used STATA software (version 13) for all statistical analysis. Data reported in this paper are publicly available with no personal identifiers.
3. Results
Table 1 summarizes regional and national data regarding COVID-19 confirmed cases, deaths, and the burden of dementia. According to the GBD 2017 data, 4% and 1% of mortality and burden of all disease, respectively, were attributed to dementia. The highest rate of dementia DALYs per 100,000 was observed in the high-income region (955.65), followed by Central Europe, Eastern Europe, and Central Asia (670.18), and Southeast Asia, East, East Asia, and Oceania (438.06) (Table 1.A). The greatest rate of dementia deaths were also observed in the high-income GBD region (93.74), followed by Central Europe, Eastern Europe, and Central Asia (56.12), and Latin America and the Caribbean (30.27) (Table 1.A). Likewise, using the World Bank classification, we found higher dementia DALYs and deaths in high-income countries compared to low- and middle-income countries (Table 1.B). In 2017, HALE were significantly higher in high-income countries as compared to low-income countries (69.4 [66.15 to 72.18] vs. 55.66 [53.17 to 57.97]). We found similar findings regarding life expectancy. Likewise, the highest percentage of the population aged ≥65 and aged ≥70 years was seen in the high-income region and high-income countries.
Table 1.
Global burden and mortality of Alzheimer's disease and other dementias, population age structure, and COVID-19: Results classified according to the A) Global burden of disease super region classification and B) World Bank classification.
| A. Global Burden of Disease Study super regions | |||||||
|---|---|---|---|---|---|---|---|
| Super regions | COVID-19 |
Alzheimer's disease and other dementias |
Population age structure |
||||
| Cases per million (95% CI) | Deaths per million (95% CI) | DALY per 100,000 | Death per 100,000 | Age, median (IQR) | Population aged ≥ 65 years (%) | Population age d ≥ 70 years (%) | |
| Central Europe, Eastern Europe, and Central Asia region | 1747.25 (1743.21–1751.30) | 30.94 (30.40–31.48) | 670.18 (625.90–716.85) | 56.12 (55.11–56.93) | 40.75 (36.65–43.15) | 13.49 | 8.81 |
| High-income region | 3453.27 (3449.78–3456.77) | 269.23 (268.25–270.2) | 955.65 (894.67–1020.10) | 93.74 (91.84–95.25) | 41.60 (37.9–43.3) | 18.31 | 12.24 |
| Latin America and Caribbean region | 2128.03 (2124.28–2131.78) | 113.82 (112.95–114.69) | 367.79 (344.97–391.97) | 30.27 (29.74–30.85) | 29.35 (26.9–32.85) | 7.61 | 4.62 |
| North Africa and Middle East region | 1320.08 (1317.01–1323.14) | 32.55 (32.07–33.03) | 241.49 (225.20–258.43) | 17.42 (16.85–17.94) | 30.7 (23.2–32.4) | 5.38 | 3.21 |
| South Asia region | 265.78 (265.03–266.53) | 6.20 (6.08–6.31) | 176.06 (161.4–193.27) | 12.57 (11.72–13.87) | 26.25 (23.5–28.2) | 5.65 | 3.27 |
| Southeast Asia, East Asia, and Oceania region | 72.65 (72.29–73.01) | 3.60 (3.52–3.68) | 438.06 (406.77–472.66) | 31.22 (30.29–32.09) | 29.3 (25.2–34.1) | 9.21 | 5.19 |
| Sub-Saharan Africa | 133.004 (132.33–133.67) | 2.96 (2.86–3.06) | 124.36 (110.22–140.48) | 9.22 (8.06–10.47) | 19.25 (17.9–21.75) | 3.11 | 1.78 |
| B. World Bank classifications | |||||||
|---|---|---|---|---|---|---|---|
| Income levels | COVID-19 |
Alzheimer's disease and other dementias |
Population age structure |
||||
| Cases per million (95% CI) | Deaths per million (95% CI) | DALY per 100,000 | Death per 100,000 | Age, median (IQR) | Population age older than 65 (%) | Population age older than 70 (%) | |
| High-income countries | 3389.12 (3385.82–3392.41) | 246.31 (245.42–247.20) | 917.88 (858.15–979.11) | 89.33 (87.57–90.75) | 41.1 (36.2–43.2) | 17.73 | 11.79 |
| Upper middle-income countries | 883.05 (881.92–884.17) | 35.52 (35.29–35.75) | 463.36 (430.6–498.32) | 34.70 (33.99–35.36) | 31.25 (28.8–38.35) | 9.77 | 5.68 |
| Lower middle-income countries | 226.57 (266.05–277.09) | 6.14 (6.05–6.22) | 204.29 (191.38–218.26) | 14.94 (15.60–14.33) | 25.05 (20.35–28.4) | 5.38 | 3.16 |
| Low-income countries | 90.44 (89.73–91.15) | 1.72 (1.63–1.82) | 132.27 (114.88–146.66) | 9.18 (7.78–10.21) | 18.75 (17.5–19.4) | 3.10 | 1.80 |
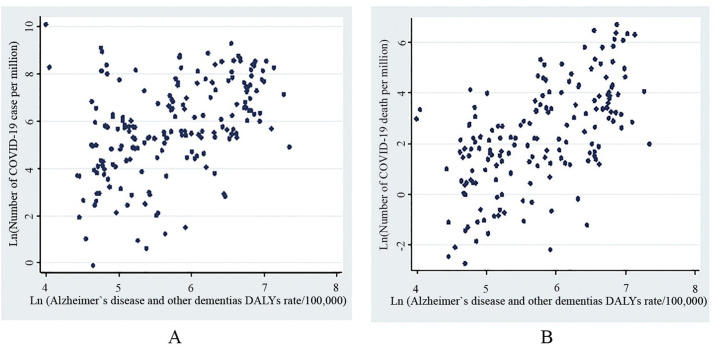
As of June 10, 2020, the highest number of COVID-19 confirmed cases (3453.27) and deaths (269.23) were reported in high-income regions (Table 1.A). Globally, we found significant moderate positive correlations between life expectancy (r = 0.60, p < .001), HALE (r = 0.58, P < .001), and dementia DALYs (r = 0.46, p < .001) with COVID-19 caseloads (Fig. 1 .A). Likewise, there were significant correlations between COVID-19 mortality with life expectancy (r = 0.60; p < .001) HALE (r = 0.58; P < .001), and dementia DALYs (r = 0.54; P < .001) (Fig. 1.B).
Fig. 1.
A. Correlation between Alzheimer's disease and other dementias DALYs rate and: A. Total number of COVID-19 cases per million. B. Total number of COVID-19 deaths per million.
4. Discussion
The results of this study have major public health implications. Using available GBD 2017 data regarding dementia and COVID-19 in 185 countries, we found a significant correlation between the burden of dementia and COVID-19 cases and deaths. There were also positive correlations between life expectancy and HALE with COVID-19 cases and deaths. To the best of our knowledge, this is the first study reporting a correlation between COVID-19 and dementia on a global scale.
The world is facing an ageing population [18]. Among age-related health conditions, dementia is one of the most burdensome in the world with significant health and social well-being implications [19]. In 2016, about 43.8 million people were reported to be living with dementia [1] with huge social and personal costs [20]. Older adults with underlying health issues are known to be at increased risk of severe COVID-19 and mortality [3]. Therefore, access to accurate data regarding dementia and ageing population is a research priority worldwide, particularly in the era of COVID-19 pandemic.
We found that life expectancy and HALE had significant correlations with COVID-19 case numbers and deaths. As reported earlier [21,22], HALE and life expectancy were higher in high-income countries than those of low- and middle-income countries. In addition, the highest percentage of citizens aged ≥65 and ≥ 70 years were seen in high-income countries followed by upper middle-income countries. These findings may partially explain a higher rate and rapid spread of COVID-19 pandemic in many high-income countries [23,24]. Older adults have slower and less efficient immune responses, making them more vulnerable to COVID-19 consequences [3]. Additionally, a substantial number of COVID-19 cases and deaths occured in the elderly residing in long-term care facilities and assisted living [13,25,26] often available in high-income countries. Data from the National Survey of Residential Care Facilities in the United States showed that seven out of ten individuals in assisted living had some cognitive impairment, ranging from mild (29%) to severe cognitive impairment (19%) [27]. A combination of ageing, frailty and comorbidities make these individuals more susceptible to COVID-19 severe consequences.
Our results also suggest a significant correlation between dementia DALYs and COVID-19 cases. The rate of dementia in hospitalized cases COVID-19 varied from 6.8% [11] to 13.1% [12]. A higher rate of COVID-19 among patients with dementia might be partially explained by problems with appropriate safeguard, self-quarantine procedures, less adherence to social distancing, and living in long-term care facilities [28].
Dementia is a strong predictor of COVID-19 mortality [29]. In our study, we found a significant correlation between dementia DALYs and COVID-19 mortality. In the UK Biobank study, a large community-based cohort (n = 274,356 participants aged 65+, including 448 hospitalized COVID-19 patients) pre-existing dementia was associated with COVID-19 severity (odds ratio = 3.07, 95% CI: 1.71 to 5.50) [29] and increased mortality rate. Likewise, among 627 Italian patients with COVID-19, the mortality rate increased significantly (p < .001) in those with dementia (62.2%) as compared to those without (26.2%). Among 20,133 cases with COVID-19 admitted in 208 hospitals in the UK, chronic non-communicable diseases including dementia increased hospital mortality [30]. Case fatality rate in long-term care facilities is high, ranging from 20% [14] to 33.7% [13]. Increased risk of adverse outcomes and patient safety in hospitalized cases with dementia has been a challenging condition even prior ot COVID-19 pandemic [31]. A high mortality rate of COVID-19 among demented patients could be partially explained by ageing and chronic comorbidities, particularly cardio or cerebr-vascular diseases [32,33]. In addition, using the UK Biobank databasebase and after adjusting for pre-existing dementia, cardiovascular diseases, and type-2 diabetes, Kuo et al. showed that the ApoE e4e4 allele is associated with severe COVID-19 infection [34].
There is no silver bullet solution to reduce the dual burden of dementia and COVID-19. However, it is still possible to reduce the burden of COVID-19 among patients with dementia and their caregivers [5]. Prevention of COVID-9 in the elderly, particularly those with dementia, or residents of long-term care facilities requires a coordinated approach with a close collaboration between family members and health professionals. Strict environmental clearance, hand hygiene, safe distance between residents to reduce droplet contamination can prevent COVID-19 spread [35].
Those with cognitive impairment may need additional support in order to adequately practice infection control procedures during the COVID-19 era. These procedures are also crucial for caregivers of patients with dementia who may be at risk of COVID-19 [13]. Active surveillance/testing of patients, caregivers, and staff in long-term care facilities, are highly recommended to reduce the chance of COVID-19. Older adults with COVID-19 may be afebrile with atypical symptoms [36]. In addition, patients with dementia who develop COVID-19 may present with non-respiratory symptoms such as delirium (particularly the hypoactive form) or isolated functional decline in the absence of any obvious physical symptoms [36]. These atypical presentations of COVID-19 may reduce early recognition of disease, increase COVID-19 spread and mortality among patients and caregivers. Moreover, fear of presenting to hospitals and medical facilities for patients and their caregiver may increase the rate of spread and mortality of COVID-19 among the elderly. Hospital administration and public health officials should encourage patients/caregivers to seek medical attention, reassure them that hospitals are safe and they will ensure patient safety while in hospital.
Compared to the pre-COVID-19 era, residents of long-term care facilities and patients with dementia should have a higher frequency of vital signs monitoring and observations for any changes in clinical status [36]. Long-term care facilities and caregivers should implement frequent check ups using simple questionnaires or checklists regarding activity level, potential exposure, change in appetite, generalized weakness, change in temperature or pulse rate to identify suspected cases with COVID-19. These screening approaches may be linked to national and local public health screening tools (if available) to provide an active surveillance model for the elderly. Furthermore, staff that work in residential care facilities are often poorly trained, unregulated and overworked [37], which creates further risk for COVID-19 transmission. Thus, there is a need to dedicate funds and regulate training for staff at residential and long term care facilities.
While social distancing and safeguard procedures are still the most effective approaches to prevent COVID-19 infection, these approaches should not endanger the safety of patients with dementia [38]. Older individuals with cognitive impairment are among the most vulnerable persons and often need help in their activities of daily living, medication adherence, social and leisure activities [39]. Due to the COVID-19 pandemic, family, friends, or professional caregivers may self isolate themselves due to fear of infection, and may refrain from visiting due to changes in their lifestyle due to COVID-19. The lack of adequate and previously established formal and informal support in these patients may further endanger their lives due to risk of falls [40], aspiration pneumonia [41] and even suicide [42]. In addition, lack of in-person support, face-to-face visits with friends and family members, group activities, and exercise with peers may increase the sense of loneliness [43], isolation and depression particularly in the elderly [44]. These conditions may increase the chance of delirium [45] and aggressive behaviors leading to self injuries [46].
Whenever possible, individuals with dementia should be encouraged to participate in their daily routine, remain physically active, maintain proper sleep hygiene, and engage in leisure activities [47]. Telehealth home support during COVID-19 may assist those with milder cognitive impairment due to its recreational component and intellectual activity [47]. For individuals with milder forms of cognitive impairment, activities such as relaxation, meditation, group exercise, and social interactions using cross-platform mobile applications and video-call technology may prevent a sense of loneliness and maintain mental and physical health [48]. However, barriers to technology use including fear and lack of familiarity, in addition to hearing/vision loss may reduce the effectiveness of these approaches.
This study has some limitations. The data for COVID-19 is dynamic and some of the most readily accessible data is publicly available aggregate population level data. Given the ecological design of the study, the results need to be interpreted as correlational and not causal, due to the potential for ecological fallacy [49]. There is an observed difference between the number of COVID-19 cases and deaths reported in low- and middle-income countries vs. high-income countries. This under-reporting may be due to decreased capacity for testing and active surveillance in the low and middle-income countries. Another limitation is the fact that we did not adjust for potential confounders that may be contributing to the correlation. There is a concern that confounding may further distort or create misleading results when comparing COVID-19 rates in rich versus poorer countries [50]. Adjusting for multiple potential confounders may create instability in adjusted models. The major strength of our study is based on combining multiple available databases regarding COVID-19 pandemic and GBD 2017 study. This information provides the opportunity to assess the correlations between dementia and COVID-19 at a global scale to inform policy makers, researchers, and clinicians about global differences in dementia trends.
5. Conclusions
In the current study, we found significant correlations between global and national burden of dementia and COVID-19 cases and death. In this critical stage of the pandemic when countries are planning to end their national lockdown and begin to open their borders, health policy makers must have comprehensive plans to identify those at risk (including older people) and reduce the chance of infection while maintaining appropriate attention to medical and psychosocial wellbeing. If a vaccination becomes available, such patients could also be prioritized given their risk status.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare no conflicts of interest to disclose.
References
- 1.GBD 2016 Dementia Collaborators Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers N.T., Steptoe A., Cadar D. Frailty is an independent predictor of incident dementia: evidence from the English longitudinal study of ageing. Sci. Rep. 2017;7:15746. doi: 10.1038/s41598-017-16104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azarpazhooh M.R., Avan A., Cipriano L.E., Munoz D.G., Sposato L.A., Hachinski V. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Dement. 2018;14:148–156. doi: 10.1016/j.jalz.2017.07.755. [DOI] [PubMed] [Google Scholar]
- 5.Brown E.E., Kumar S., Rajji T.K., Pollock B.G., Mulsant B.H. Anticipating and mitigating the impact of the COVID-19 pandemic on Alzheimer’s disease and related dementias. Am. J. Geriatr. Psychiatry. 2020;S1064-7481(20) doi: 10.1016/j.jagp.2020.04.010. (30294-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falsey A.R., Walsh E.E. Respiratory syncytial virus infection in elderly adults. Drugs Aging. 2005;22:577–587. doi: 10.2165/00002512-200522070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiris J.S.M., Yuen K.Y., Osterhaus A.D.M.E., Stöhr K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 8.Wise J. Dementia and flu are blamed for increase in deaths in 2015 in England and Wales. BMJ. 2016;353:i2022. doi: 10.1136/bmj.i2022. [DOI] [PubMed] [Google Scholar]
- 9.Bouza C., Martínez-Alés G., López-Cuadrado T. The impact of dementia on hospital outcomes for elderly patients with sepsis: a population-based study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manabe T., Fujikura Y., Mizukami K., Akatsu H., Kudo K. Pneumonia-associated death in patients with dementia: a systematic review and meta-analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 12.Bianchetti A., Rozzini R., Guerini F., Boffelli S., Ranieri P., Minelli G. Clinical presentation of COVID19 in dementia patients. J. Nutr. Health Aging. 2020:1–3. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N. Engl. J. Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu A.T., Lane N.E. Article in LTCcovidOrg, International Long-Term Care Policy Network, CPEC-LSE; 2020. Report: Impact of COVID-19 on Residents of Canada’s Long-Term Care Homes — Ongoing Challenges and Policy Response.https://ltccovid.org/2020/04/15/impact-of-covid-19-on-residents-of-canadas-long-term-care-homes-ongoing-challenges-and-policy-response/ (accessed May 24, 2020) [Google Scholar]
- 15.Azarpazhooh M.R., Morovatdar N., Avan A., Phan T.G., Divani A.A., Yassi N. COVID-19 pandemic and burden of non-communicable diseases: an etiological study on data of 185 countries. J. Stroke Cerebrovasc. Dis. 2020 Sep;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020.105089. Published online 2020 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The World Bank World Bank Country and Lending Groups – World Bank Data Help Desk. 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed May 25, 2020)
- 18.United Nations, Department of Economic and Social Affairs, Population Division World Population Ageing 2019 (ST/ESA/SER.A/444) 2020. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2019_worldpopulationageing_report.pdf (accessed May 25, 2020)
- 19.Cognitive Aging Dementia, and the Future of an Aging Population - Future Directions for the Demography of Aging - NCBI Bookshelf. 2020. https://www.ncbi.nlm.nih.gov/books/NBK513075/ (accessed May 28, 2020)
- 20.Sköldunger A., Fastbom J., Wimo A., Fratiglioni L., Johnell K. The impact of dementia on drug costs in older people: results from the SNAC study. BMC Neurol. 2016;16:28. doi: 10.1186/s12883-016-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islam M.S., Mondal M.N.I., Tareque M.I., Rahman M.A., Hoque M.N., Ahmed M.M. Correlates of healthy life expectancy in low- and lower-middle-income countries. BMC Public Health. 2018;18:476. doi: 10.1186/s12889-018-5377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . (WHO/DAD/2019.1); Geneva: 2019. World Health Statistics Overview 2019: Monitoring Health for the SDGs, Sustainable Development Goals.https://apps.who.int/iris/bitstream/handle/10665/311696/WHO-DAD-2019.1-eng.pdf?ua=1 Licence: CC BY-NC-SA 3.0 IGO. (accessed May 28, 2020) [Google Scholar]
- 23.Clark A., Jit M., Warren-Gash C., Guthrie B., Wang H.H., Mercer S.W. How many are at increased risk of severe COVID-19 disease? Rapid global, regional and national estimates for 2020. MedRxiv. 2020 doi: 10.1101/2020.04.18.20064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 25.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimball A., Hatfield K.M., Arons M., James A., Taylor J., Spicer K. Asymptomatic and Presymptomatic SARS-CoV-2 infections in residents of a Long-term care skilled nursing facility - King County, Washington, march 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmerman S., Sloane P.D., Reed D. Dementia prevalence and care in assisted living. Health Aff. (Millwood) 2014;33:658–666. doi: 10.1377/hlthaff.2013.1255. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerman S., Sloane P.D., Katz P.R., Kunze M., O’Neil K., Resnick B. The need to include assisted living in responding to the COVID-19 pandemic. J. Am. Med. Dir. Assoc. 2020;21:572–575. doi: 10.1016/j.jamda.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkins J.L., Masoli J.A., Delgado J., Pilling L.C., Kuo C.-L.C., Kuchel G. Preexisting comorbidities predicting severe covid-19 in older adults in the Uk biobank community cohort. MedRxiv. 2020 doi: 10.1101/2020.05.06.20092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO clinical characterisation protocol. MedRxiv. 2020 doi: 10.1101/2020.04.23.20076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George J., Long S., Vincent C. How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. J. R. Soc. Med. 2013;106:355–361. doi: 10.1177/0141076813476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abootalebi S., Aertker B.M., Andalibi M.S., Asdaghi N., Aykac O., Azarpazhooh M.R. Call to action: SARS-CoV-2 and cerebrovascular DisordErs (CASCADE) J. Stroke Cerebrovasc. Dis. 2020;104938 doi: 10.1016/j.jstrokecerebrovasdis.2020.104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Divani A.A., Andalib S., Di Napoli M., Lattanzi S., Hussain M.S., Biller J. Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J. Stroke Cerebrovasc. Dis. 2020;104941 doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo C.-L., Pilling L.C., Atkins J.L., Masoli J.A., Delgado J., Kuchel G.A. Apoe e4 genotype predicts severe covid-19 in the Uk biobank community cohort. MedRxiv. 2020 doi: 10.1101/2020.05.07.20094409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang H.-T., Chen T.-C., Liu T.-Y., Chiu C.-F., Hsieh W.-C., Yang C.-J. How to prevent outbreak of a hospital-affiliated dementia day-care facility in the pandemic COVID-19 infection in Taiwan. J. Microbiol. Immunol. Infect. 2020;53(3):394–395. doi: 10.1016/j.jmii.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Adamo H., Yoshikawa T., Ouslander J.G. Coronavirus disease 2019 in geriatrics and long-term care: the ABCDs of COVID-19. J. Am. Geriatr. Soc. 2020;68:912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
- 37.Shah H.B. 2017. Understaffed and Overworked: Poor Working Conditions and Quality of Care in Residential Care Facilities for theElderly; p. 788.https://digitalcommons.law.ggu.edu/cgi/viewcontent.cgi?article=1789&context=pubs Publications. (accessed June 16, 2020) [Google Scholar]
- 38.Steinman M.A., Perry L., Perissinotto C.M. Meeting the care needs of older adults isolated at home during the COVID-19 pandemic. JAMA Intern. Med. 2020 Apr 16 doi: 10.1001/jamainternmed.2020.1661. [DOI] [PubMed] [Google Scholar]
- 39.Marshall G.A., Amariglio R.E., Sperling R.A., Rentz D.M. Activities of daily living: where do they fit in the diagnosis of Alzheimer’s disease? Neurodegen. Dis. Manag. 2012;2:483–491. doi: 10.2217/nmt.12.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allan L.M., Ballard C.G., Rowan E.N., Kenny R.A. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arcand M. End-of-life issues in advanced dementia: part 1: goals of care, decision-making process, and family education. Can. Fam. Physician. 2015;61:330–334. [PMC free article] [PubMed] [Google Scholar]
- 42.Conejero I., Navucet S., Keller J., Olié E., Courtet P., Gabelle A. A complex relationship between suicide, dementia, and amyloid: a narrative review. Front. Neurosci. 2018;12:371. doi: 10.3389/fnins.2018.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Health Quality Ontario Social isolation in community-dwelling seniors: an evidence-based analysis. Ont. Health Technol. Assess Ser. 2008;8:1–49. [PMC free article] [PubMed] [Google Scholar]
- 44.Ye L., Zhang X. Social Network Types and Health among Older Adults in Rural China: the mediating role of social support. Int. J. Environ. Res. Public Health. 2019;31(16):410. doi: 10.3390/ijerph16030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaHue S.C., James T.C., Newman J.C., Esmaili A.M., Ormseth C.H., Ely E.W. Collaborative delirium prevention in the age of COVID-19. J. Am. Geriatr. Soc. 2020;68:947–949. doi: 10.1111/jgs.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kales H.C., Lyketsos C.G., Miller E.M., Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer’s disease: an international Delphi consensus. Int. Psychogeriatr. 2019;31:83–90. doi: 10.1017/S1041610218000534. [DOI] [PubMed] [Google Scholar]
- 47.Goodman-Casanova J.M., Durá-Pérez E., Guzmán-Parra J., Cuesta-Vargas A., Mayoral-Cleries F. Telehealth home support during COVID-19 confinement: survey study among community-dwelling older adults with mild cognitive impairment or mild dementia. J. Med. Internet Res. 2020;22(22) doi: 10.2196/19434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padala S.P., Jendro A.M., Orr L.C. Facetime to reduce behavioral problems in a nursing home resident with Alzheimer’s dementia during COVID-19. Psychiatry Res. 2020;288:113028. doi: 10.1016/j.psychres.2020.113028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakefield J. Ecologic studies revisited. Annu. Rev. Public Health. 2008;29:75–90. doi: 10.1146/annurev.publhealth.29.020907.090821. [DOI] [PubMed] [Google Scholar]
- 50.Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Annu. Rev. Public Health. 1995;16:61–81. doi: 10.1146/annurev.pu.16.050195.000425. [DOI] [PubMed] [Google Scholar]