Abstract
The United States Environmental Protection Agency Contaminant Candidate List 3 lists strontium as a contaminant for potential regulatory consideration in drinking water. Very limited data is available on strontium removal from drinking water and as a result, there is an immediate need for treatment information. The objective of this work is to evaluate the effectiveness of coagulation/filtration and lime-soda ash softening treatment methods to remove strontium from surface and ground waters. Coagulation/filtration jar test results on natural waters showed that conventional treatment with aluminum and iron coagulants were able to achieve only 12% and 5.9% strontium removal, while lime softening removed as high as 78% from natural strontium-containing ground water. Controlled batch experiments on synthetic water showed that strontium removal during the lime-soda ash softening was affected by pH, calcium concentration and dissolved inorganic carbon concentration. In all softening jar tests, the final strontium concentration was directly related to the initial strontium concentration and the removal of strontium was directly associated with calcium removal. Precipitated solids showed well-formed crystals or agglomerates of mixed solids, two polymorphs of calcium carbonate (vaterite and calcite), and strontianite, depending on initial water quality conditions. X-ray diffraction analysis suggested that strontium was likely incorporated in the calcium carbonate crystal lattice and was likely responsible for removal during lime softening.
Keywords: Strontium, Drinking water, Coagulation, Lime softening, Jar test
1. Introduction
Strontium is not currently regulated in drinking water. In 2009, the Environmental Protection Agency (EPA) listed strontium on the Contaminant Candidate List 3 (CCL 3) (USEPA, 2008) for additional data collection and potential future regulatory consideration. The CCL is a list of unregulated contaminants that are currently not subject to any proposed or promulgated national primary drinking water regulations, but are known or anticipated to occur in public water systems. Supported by a 1992 EPA health assessment from the Integrated Risk Information System (IRIS), a health reference level (HRL) of 4000 μg/L for strontium in drinking water was established due to the susceptibility of infants, children and adolescents to abnormal skeletal developments associated with its consumption (USEPA, 1990). Exacerbated by inadequate calcium levels, strontium substitutes for calcium during bone calcification or displaces calcium from the existing calcified matrix (El Solh and Rousselet, 1981).
On October 20, 2014, the EPA published the Preliminary Regulatory Determinations for Contaminants on the Third Drinking Water Contaminant Candidate List Federal Register notice and announced a preliminary regulatory determination to regulate strontium and to not regulate four additional contaminants (USEPA, 2014). In the notice, the HRL for strontium was lowered to 1500 μg/L, supported by an updated assessment from the EPA’s Office of Water. The two points of departure from the 1992 IRIS assessment were (1) the oral reference dose (RfD) was lowered from 0.6 to 0.3 mg/kg/day based on the critical study by Marie et al. (1985) for decreased bone calcification rate in male weanling rats, and (2) age-specific exposure factors included to account for the sensitive population and life-stage of birth through 18 years to reflect the most active period of bone growth and development. The lower HRL means that the acceptable daily oral exposure of strontium (meaning there would be no significant risk of toxic effects) during a human lifespan was lower than previously believed (USEPA, 2014). In December 2015, the EPA announced the postponement of strontium regulation in order to further research the feasibility of strontium removal and the health risks leaving it unregulated poses (USEPA, 2015).
Monitoring data is being collected for strontium from 2013 through 2015 under the Unregulated Contaminant Monitoring Rule 3 (UCMR 3). Based on the data available as of June 2015, the UCMR 3 summary report results indicate that 2.7% of 48,982 samples had strontium values over the HRL (1500 μg/L), while public water systems (PWSs) reported having 5.6% of 4413 systems over the HRL (USEPA, 2015). All but one PWS reported strontium levels above the UCMR 3 minimum reporting level of 0.3 μg/L.
Strontium (Sr) is an alkaline earth metal and the 15th most abundant element in Earth’s crust. Strontium is relatively widely distributed in groundwater across the United States (Fig. 1), as well as in some surface waters near coastal regions. Given the potential for regulatory action, an obvious need exists to examine the effectiveness of drinking water treatment processes to remove strontium from drinking water. Such information would be useful in the regulatory determination process (and potential regulation development), and valuable to public water systems that have elevated strontium levels in their source water.
Figure 1.
Strontium occurrence in surface and ground waters in the United States (USGS NWIS and EPA STORET databases, 2003–2013, www.waterqualitydata.us).
The majority of strontium treatment studies in water has been associated with the synthetic radioactive isotope 90Sr, which has a half-life of nearly 29 years and the same physical properties as stable strontium (Lide, 2005). Radioactive strontium is a product of nuclear fallout, and 90Sr been regulated since 1977 due to associated health exposure concerns (USEPA, 2000). Research on the removal of radioactive strontium from drinking water had been explored by a variety of methods including sorption (Ghaemi et al., 2011, Sivaiah et al., 2005, Tel et al., 2010, Cheng et al., 2012, Gürboğa and Tel, 2005, Li et al., 2010, Merceille et al., 2012), nanofiltration (Hwang et al., 2002), and biological treatment (Paterson-Beedle et al., 2006, Thorpe et al., 2012, Achal et al., 2012).
An extensive review of radioactive strontium removal can be found in EPA’s treatability database (USEPA 2011).
Past research on natural strontium treatment has primarily focused on industrial wastewater applications. Those studies have considered adsorption (Chegrouche et al., 2009), ion exchange (Nikolaev et al., 1992, Sayed, 1996, Zhang et al., 1999) and biological treatment (Chen and Wang, 2008) processes. Limited data exists on the removal of natural strontium from drinking water. Strontium has similar physical and chemical properties as calcium and barium, therefore, water treatment strategies are expected to be similar. The EPA Treatability Database contains data showing that bench-scale adsorption (hydrated manganese oxide) and reverse osmosis methods were effective (USEPA, 2011). In 1954, one drinking water treatment study compared removals from raw source waters to tap water using a variety of treatment methods, including alum, ferrous sulfate, lime, phosphate, ion exchange, activated carbon, chlorine, ammonia, fluoride, soda ash, sodium silicate, copper sulfate and caustic soda (Alexander et al., 1954). Waters from 50 metropolitan cities with initial strontium concentrations between 0.0058 and 1.9 mg/L were tested. Lime softening was found to be the most effective treatment process, removing strontium by 75% (Alexander et al., 1954). Parks and Edwards (2006) examined the impact of sodium carbonate addition to pH 10.3 to remove strontium from drinking water sources. The study provided some useful information regarding strontium removal during softening. The median observed removal of strontium (373 waters in survey) was 99%. O’Donnell and Lytle (2014) recently presented monitoring data over a six month period from a full-scale lime softening plant in Ohio treating ground water that contained 3.5 mg/L strontium (and 122 mg/L calcium and 46 mg/L magnesium). This plant consistently reduced strontium levels down to 0.94 mg/L or achieved 72% strontium removal. During the same time period, the plant achieved 82% and 73% reduction of calcium and magnesium, respectively.
Given the potential for regulatory action and lack of information on the removal of strontium from drinking water, an obvious need exists to perform treatment studies. Therefore, the objective of this research was to perform bench-scale experiments to evaluate the effectiveness of conventional coagulation/filtration treatment (aluminum and iron coagulants) and lime softening on strontium removal from surface and groundwater sources. Solids analysis techniques including scanning electron microscopy (SEM) and x-ray diffraction (XRD) were used to characterize solid precipitates and provide insights into strontium removal mechanisms.
2. Material and methods
2.1. Conventional coagulation/filtration and lime softening jar tests
Bench-scale studies were performed using a series of jar tests to simulate full-scale conventional water treatment (i.e., rapid mix, flocculation, sedimentation and filtration) and lime-soda ash softening. The impact of coagulant dose and pH on strontium removal was examined during coagulation/filtration treatment jar tests. The impact of pH, calcium, dissolved inorganic carbon (DIC) and lime dose on strontium removal was considered during softening jar tests. A PB-700 series six-paddle standard jar-tester (Phipps & Bird, Richmond, VA) was used to perform all jar-testing experiments. Each stainless steel paddle (1.0 in. × 3.0 in.) was adjusted to the maximum depth of 9 inches inside a 2.5 L B-KER acrylic square-testing jar.
Target experimental water quality conditions for all jar-testing experiments are summarized in Table 1. The water was prepared by adding appropriate amounts of analytical grade aluminum sulfate (alum), ferric chloride, calcium hydroxide (slaked lime), sodium carbonate (soda ash) and strontium reference standard solution (Fisher Scientific, Fair Lawn, NJ) to 2.0 L of source water at room temperature (∼23.0 °C). The chemical characteristics of the source waters (natural ground and surface waters) are summarized in Table 2. After collection of the test waters in the field, the samples were stored in separate 40 L plastic storage containers. Prior to use, the plastic storage containers were manually shaken for several minutes in all directions to loosen and re-suspend particles and sediment.
Table 1.
Experimental jar testing conditions.
| Source water | Chemical | Chemical doses (mg/L) | pH | Turbidity (NTU) | Strontium (mg/L) | Varied target goals of variable parameters |
|---|---|---|---|---|---|---|
| Ohio river (surface) | Alum or FeCl3 | Variable | 7.70 | Ambient | 5.0 | 0, 10, 20, 30, 40, 50 |
| Optimum | Variable | Ambient | 5.0 | 6.5, 7.0, 7.5, 8.0, 8.5, 9.0 | ||
| Optimum | Optimum | Variable | 5.0 | 25, 50, 75, 100, 125, 150 | ||
| Optimum | Optimum | Ambient | Variable | 1.0, 2.5, 4.0, 6.0, 8.0, 10.0 | ||
| Natural ground waters | Lime & soda ash | Variable | Ambient | Ambient | Ambient | 0, 50, 100, 150, 200, 250, 300, 250, 400, 450 |
| Bolton plant (ground) | Lime & soda ash | Variable | Ambient | Ambient | 5.0 | 0, 50, 100, 150, 200, 250, 300, 350, 400, 450 |
| Optimum | Ambient | Ambient | Variable | 1.0, 2.5, 4.0, 6.0, 8.0, 10.0 |
Table 2.
Average water quality of test waters used in jar testing.
| Strontium (mg/L) | Calcium (mg/L) | Magnesium (mg/L) | Iron (mg/L) | Sodium (mg/L) | Manganese (mg/L) | Total alkalinity (mg/L as CaCO3) | pH | Sulfate (mg/L) | Silicate (mg SiO2/L) | Turbidity (NTU) | Total hardness (mg CaCO3/L) | Dissolved inorganic carbon (mg C/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ohio river | 0.21 | 26.4 | 9.88 | 1.0 | 19.6 | 0.13 | 60.7 | 7.71 | 56.7 | 0.616 | 24.7 | 107 | 15 |
| Local lake | 0.25 | 66.4 | 14.3 | 2.7 | 32.5 | 0.44 | – | 7.53 | 29.2 | 14.6 | 75 | 225 | |
| Private well | 4.2 | 127 | 32.6 | 0.96 | 213 | 0.06 | 296 | 7.22 | 157 | 10.7 | 15.1 | 451 | 80 |
| Bolton plant | 0.42 | 61.9 | 23.6 | 0.028 | 31.7 | 0.21 | 218 | 6.72 | 57.5 | 8.48 | 0.75 | 252 | 74 |
| Site 1 | 21.8 | 102 | 43.7 | 1.33 | 26.2 | 0.06 | 318 | 7.29 | 190 | 16.7 | 12.5 | 435 | 85 |
| Site 2 | 10.9 | 112 | 32.5 | 0.67 | 7.58 | 0.14 | 340 | 7.3 | 148 | 14.5 | 9.98 | 414 | 90 |
The primary source water used for the coagulation/filtration treatment experiments was Ohio River water collected from the Greater Cincinnati (Ohio) Water Works Richard Miller Treatment Plant (Table 2). A second surface water supply was collected from a local lake in a city park (Cincinnati, Ohio). The park water was primarily used as a source of particles to adjust initial turbidity of jar tests performed using Ohio River water. The lake water was left undisturbed for 24 h to allow particles to settle after which the top two-thirds of the water was removed. The settled particles were used to adjust the initial turbidity of Ohio River water during experiments designed to evaluate the impact of initial turbidity on strontium removal. Lime-soda ash softening experiments were performed on untreated ground waters that contained elevated strontium levels (Table 2) collected from a local private homeowner’s well (Cleves, Ohio), and drinking water sources from two cities in Ohio identified as site 1 and site 2. Also, untreated Great Miami Aquifer water collected from the Greater Cincinnati Water Works Bolton Treatment Plant (Fairfield, Ohio) was spiked with strontium for use in additional jar tests. All groundwater sources were considered very hard (USGS, 2015) (Table 2) with a total hardness over 180 mg CaCO3/L.
All jar tests were conducted with 2.0 L of test source water in each of the six jars. The jar test paddles were run at a rate of 80 revolutions per minute (rpm) (94 sec−1 velocity gradient) to simulate rapid mixing. For conventional treatment, stock strontium solution and local lake water particles were added to achieve the desired initial strontium and turbidity values prior to coagulant addition. The pH was pre-adjusted manually with sodium hydroxide, NaOH, prior to coagulant addition to account for the impact of coagulant on final pH (i.e., to maintain final pH relatively consistent across conditions). After water quality was adjusted, alum or ferric chloride was added between 0 and 50 mg/L and allowed to rapid mix for 5 min to ensure sufficient mixing. During lime-soda ash softening jar tests, strontium was only added to Bolton plant ground water to give a strontium concentration between 1 mg/L and 10 mg/L. Calcium hydroxide or slaked lime, Ca(OH)2 (Acros Organics, Morris Plains, NJ) (98% purity) was added to the test waters at initial concentrations between 0 and 450 mg/L during rapid mixing. In order to remove noncarbonate hardness, 45 mg/L sodium carbonate or soda ash, Na2CO3 (Fisher Scientific Company, Fair Lawn, NJ) (99.995% purity) was added to the three ground waters that contained strontium and 50 mg/L was added to Bolton plant water.
Water samples for initial water quality analysis were collected immediately after the 5 min rapid-mixing phase. A 60 mL water sample was collected from each jar in a HDPE bottle for inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis (i.e., Al, Fe, Ca, Mn, Mg and Sr). An additional 250 mL sample was collected in a HDPE bottle from the lime-soda ash softening experiments for total alkalinity and chloride analysis. Color, turbidity, temperature, pH and dissolved oxygen (DO) were analyzed immediately after rapid mixing. Color was measured with a Hach (Loveland, CO) DR 2700 Portable Spectrophotometer. Turbidity was measured with a Hach 2011N Laboratory Turbidimeter. Temperature, pH and DO were recorded with a Hach HQ40d portable pH, conductivity, optical dissolved oxygen, oxidation reduction potential and ion-selective electrode multi-parameter meter. Measurements were made with a Hach IntelliCAL™ PHC281 pH ultra-refillable pH electrode and a Hach IntelliCAL™ LDO101 standard luminescent/optical dissolved oxygen electrode.
After rapid-mixing, the paddle speed was reduced to 20 rpm for 30 min to simulate flocculation. Immediately after the 30 min, color, turbidity, temperature, pH and DO were measured. The paddle mixer was then turned off and the water was allowed to settle undisturbed for an additional 30 min to simulate sedimentation. After the 30 min were up, supernatant water was extracted from the jar at a depth of 8.5 inches from the top of the water surface. A 60 mL sample was collected for ICP-AES analysis, and color, turbidity, temperature, pH and DO were analyzed upon collection. A subsample of the supernatant water was passed through a 0.2 μm Nuclepore® 47 mm polycarbonate track-etched membrane filter (Whatman Inc., Clifton, NJ) using an in-house vacuum line to simulate filtration. A 60 mL filtered water sample was collected and submitted for ICP-AES analysis.
2.2. Controlled lime softening batch studies
A series of controlled batch tests were performed to evaluate strontium removal during lime-soda ash softening and identify removal mechanisms. Tests were conducted on 2.0 L of test water in a 3.0 L glass beaker. Appropriate amounts of analytical-grade sodium bicarbonate, calcium chloride dihydrate and a strontium reference standard (1000 mg/L) solution (Fisher Scientific, Fair Lawn, NJ) were added to 2.0 L of doubled-deionized (DDI) water at room temperature (∼23 °C) to achieve desired initial target conditions. DDI water was prepared by passing building-demineralized water through a Milli-Q Academic with a resistivity ≥18.2 MΩ cm that uses a Quantium EX cartridge filter and Q-Gard purification packs (EMD Millipore Crop., Billerica, MA).
The glass beaker was placed on a stir plate and rapid mixed at 80 rpm for 5 min. A pH electrode and an injection line for both acid and base addition were secured to the top of the beaker. A dual TITRONIC (Schott Instruments GmbH, Mainz, Germany) motor-driven burette system with a Sensor-Interface SensoLab® pH controller (Jensen Systems, Inc., Sylmar, CA) and Multi-T 2.2™ software program (Jensen Systems) was used to adjust the initial pH and continuously compensate for pH changes due to chemical additions and reactions. Adjustments were made with the addition of 0.6 N hydrochloric acid or 0.6 N sodium hydroxide by aliquot. The titration system was programmed to incrementally increase the pH by 1.00 ± 0.05 between pH 5.0 and 12.0.
Once the pH stabilized at 5.00 ± 0.05 for 2 min, the strontium standard solution was added to achieve an initial strontium concentration of 1.0, 5.0 or 10 mg/L. An appropriate amount of sodium bicarbonate was added at an initial DIC of either 10 or 85 mg C/L. Calcium chloride dihydrate was added to calcium concentrations of either 0, 50, 100 or 150 mg/L. After the water was rapid mixed for two additional minutes, 60 mL unfiltered and filtered samples were collected for ICP-AES analysis. The filtered sample ran the water through a 0.2 μm polypropylene syringe 25 mm disk filter (Whatman Inc., Clifton, NJ) to distinguish between soluble and particulate calcium and strontium. An additional 250 mL sample was collected when calcium was present and the pH reached 11 for solids analysis. The water was filtered through a 0.2 μm Nuclepore® 47 mm polycarbonate membrane filter (Whatman Inc., Clifton, NJ) using an in-house vacuum line to collect any precipitate solids. The precipitate on the membrane filter was air-dried. The solid was photographed and analyzed by scanning electron microscopy (SEM) and x-ray diffraction (XRD) to determine the elemental composition, particle size and morphology, and mineral identification.
2.3. Water analysis
Water samples submitted for ICP-AES analyses were preserved with analytical grade ultrapure nitric acid (0.15% v/v) and were analyzed on a Thermo Elemental (Franklin, MA) model 6500 Duo ICP-AES, per USEPA Method 200.7 (USEPA, 1983). Water samples designated for chloride and total alkalinity analyses were kept at less than 4 °C. Chloride analyses were performed on a Metrohm (Riverview, FL) model 751 Titrino autotitrator using a potentiometric titration with silver nitrate, per Standard Method 4500-Cl D (APHA, 1992). Total alkalinity analyses were performed on a Metrohm (Riverview, FL) model 751 Titrino autotitrator using an acid titration to the carbonate equivalence point, according to Standard Method 2320 B (APHA, 1992).
2.4. Solids analysis
For the SEM analysis, 12 mm carbon tabs were affixed to 10 mm aluminum stubs. Precipitate from the experiments was pressed into the carbon tabs. Samples were imaged with a JEOL 6490LV Scanning Electron Microscope (JEOL, Tokyo, Japan). Typical accelerating voltage was 3–5 kV. Micrographs were collected in Secondary Electron Imaging mode (SEI). Elemental analysis was conducted by Energy Dispersive X-ray Analysis (EDXA) with an Oxford Instruments system consisting of a 50 mm2 silicon drift detector running INCA analytical software (Oxford Analytical, Oxfordshire, UK). Filters were cut to fit and affixed to sample holders for x-ray diffraction. A PANalytical X’Pert Pro Diffractometer (Almelo, The Netherlands) using Cu Kα radiation was used to conduct the powdered x-ray diffraction experiments. The x-ray tube was operated at 35 kV and 40 mA for the analyses. Scans were performed over a 2-theta range between 5° and 90° with a step of 0.02 °C and a 1-s count time at each step. Pattern analysis was performed generally following American Society for Testing and Materials procedures using the computer software Jade (Versions 5 to 7, Materials Data, Inc.) with reference to the 1995–2002 ICDD PDF-2 data files (International Center for Diffraction Data, Newtown Square, PA). An attempt was made to offset the thickness of the filters to the mounted sample holder to minimize sample displacement.
3. Results
3.1. Conventional coagulation/filtration water treatment
3.1.1. Effect of coagulant dose on strontium removal
Conventional coagulation and filtration jar tests were tested to verify this method would be ineffective due to chemical similarities between strontium and calcium. Jar tests were conducted on Ohio River water spiked with 5 mg/L of strontium to evaluate the impact of coagulant type (alum and ferric chloride) and dose (up to 50 mg/L). Two control jar tests (no coagulant added) were also conducted to determine whether strontium removal occurred naturally under the test conditions. Strontium removal in the control studies were very low at 1.2% and 2.0%, demonstrating that strontium did not precipitate out of the test water without the addition of coagulants.
The average initial and settled pH values of all alum coagulation jar tests were 7.71 ± 0.044 and 7.93 ± 0.021, respectively. The average initial turbidity and strontium concentrations were 4.0 NTU ±2.5 and 4.93 mg/L ± 0.13, respectively. The settled water turbidity was consistent across all coagulant doses with an average of 2.52 NTU ±0.38. The highest strontium reduction achieved during alum coagulation/filtration (Supplemental Fig. A) was only 1.6% and 3.1% for settled and filtered water samples, respectively, which was nearly the same as the control tests.
The average initial and settled pH values of all ferric chloride coagulation jar tests were 7.74 ± 0.048 and 7.67 ± 0.044, respectively. The average initial turbidity and strontium concentrations were 41.1 NTU ±1.9 and 4.72 mg/L ± 0.13, respectively. The settled turbidity decreased from 8 NTU to 1.1 NTU with an increased coagulant dose (not shown). Although turbidity reduction increased with dose, strontium reduction during ferric chloride coagulation was very low (Supplemental Fig. A). The highest strontium removals achieved of the ferric chloride jar tests were negligible at only 2.6% and 3.4% for settled and filtered samples, respectively.
Conventional treatment jar tests using both coagulants were repeated as a quality control measure and showed similar results as described above (not shown). Additional experiments were needed to ensure no other factors during coagulation affected strontium removals.
3.1.2. Effect of pH on strontium removal
Jar tests were performed to evaluate the impact of initial pH (6–9) on strontium removal from Ohio River water by conventional water treatment with alum and ferric chloride as the coagulants. Tests were performed at a single “optimal” coagulant dose of 30 mg/L based on turbidity reductions identified in jar test described in the previous section.
For alum coagulant tests, the average initial turbidity and strontium concentrations were 73.5 NTU ±16 and 4.94 mg/L ± 0.16, respectively. The settled turbidity was consistent across all evaluated pH values and were below 1.0 NTU. Strontium reductions for the alum coagulant (Supplemental Fig. A) were less than 3.1% and 4.9% for settled and filtered waters samples, respectively. An increase in the settled pH appeared to slightly increase the amount of strontium removed, but removals remained very low and inconsequential.
For ferric chloride coagulation jar tests, the average initial turbidity and strontium concentrations were 15.4 NTU ±5.0 and 4.78 mg/L ± 0.23, respectively. The initial turbidity was lower than alum tests due to the differences in the mixing of the source water. The settled turbidity was consistently below 1 NTU across all pH values evaluated. Strontium reduction during ferric chloride coagulation were less than 3.1% and 3.8% for settled and filtered water samples, respectively (Supplemental Fig. A). The settled pH value did not appear to influence strontium removal.
Duplicate experiments with both coagulants were run as a quality control measure and showed similar results as indicated above. In conclusion, despite good turbidity reduction at all pH values tested, removal of strontium by conventional water treatment was negligible and independent of pH.
3.1.3. Effect of initial strontium concentration on strontium removal
Jar tests were performed to evaluate the impact of initial strontium concentration (1.2, 4, and 7.8 mg/L) on strontium removal from Ohio River water by conventional water treatment using alum and ferric chloride. Tests were performed at a pH of 7.70 and a coagulant dose of 30 mg/L. The average initial and settled pH values of all alum coagulant jar tests were 7.70 ± 0.012 and 7.70 ± 0.042, respectively. The average initial turbidity concentration was 4.28 NTU ±0.63. The settled turbidity was consistent across all coagulant doses with an average of 3.03 NTU ±0.31. Strontium reduction for the alum coagulant (Supplemental Fig. A) was 0% for settled samples and less than 1.6% for filtered samples. Strontium reduction was not impacted by initial strontium concentration.
The average initial and settled pH values of all ferric chloride coagulant jar tests were 7.72 ± 0.01 and 7.69 ± 0.065, respectively. The average initial turbidity concentration was 10.1 NTU ±4.3. The settled turbidity was consistent across all coagulant doses with an average of 9.82 NTU ±3.5. Strontium reductions for the ferric chloride coagulant (Supplemental Fig. A) were less than 3.5% and 6.2% for settled samples and filtered waters, respectively. Strontium reduction did not appear to be impacted by initial strontium concentration.
The results of duplicate experiments with both coagulants were similar indicating that the initial strontium concentration had no impact on strontium removal from water by either alum or iron coagulation/filtrations.
3.1.3. Effect of initial strontium concentration on strontium removal
Jar tests were performed to evaluate the impact of initial strontium concentration (1.2, 4, and 7.8 mg/L) on strontium removal from Ohio River water by conventional water treatment using alum and ferric chloride. Tests were performed at a pH of 7.70 and a coagulant dose of 30 mg/L. The average initial and settled pH values of all alum coagulant jar tests were 7.70 ± 0.012 and 7.70 ± 0.042, respectively. The average initial turbidity concentration was 4.28 NTU ±0.63. The settled turbidity was consistent across all coagulant doses with an average of 3.03 NTU ±0.31. Strontium reduction for the alum coagulant (Supplemental Fig. A) was 0% for settled samples and less than 1.6% for filtered samples. Strontium reduction was not impacted by initial strontium concentration.
The average initial and settled pH values of all ferric chloride coagulant jar tests were 7.72 ± 0.01 and 7.69 ± 0.065, respectively. The average initial turbidity concentration was 10.1 NTU ±4.3. The settled turbidity was consistent across all coagulant doses with an average of 9.82 NTU ±3.5. Strontium reductions for the ferric chloride coagulant (Supplemental Fig. A) were less than 3.5% and 6.2% for settled samples and filtered waters, respectively. Strontium reduction did not appear to be impacted by initial strontium concentration.
The results of duplicate experiments with both coagulants were similar indicating that the initial strontium concentration had no impact on strontium removal from water by either alum or iron coagulation/filtrations.
3.1.4. Effect of initial turbidity on strontium removal
Jar tests were performed to evaluate the impact of initial turbidity level (14.7–322 NTU) on strontium removal from Ohio River water by conventional water treatment using alum and ferric chloride coagulants. The initial strontium concentration was 5.0 mg/L, initial pH was 7.70 ± 0.05 and the coagulant dose was 30 mg/L.
For alum coagulant tests, the average initial and settled pH values were 7.69 ± 0.023 and 7.70 ± 0.045, respectively. The average initial strontium concentration was 4.9 mg/L ± 0.43. Strontium reductions for the alum coagulant were between 0% and 18% for filtered samples. Although there was some scatter in the data, strontium reduction appeared to improve slightly with increasing initial turbidity up to 50 NTU. For ferric chloride tests, the average initial and settled pH values were 7.68 ± 0.030 and 7.61 ± 0.036, respectively. The average initial strontium concentration was 4.95 mg/L ± 0.34. Strontium reductions for the ferric chloride coagulant were between 0% and 5.9% for filtered samples. The maximum strontium removals occurred after filtration when the initial turbidities were 246 NTU (18%) and 89.6 NTU (5.9%) for alum and iron coagulants, respectively (Fig. 2). Although initial turbidity had a very slight impact on strontium removal, removals were very low and coagulation/filtration was not found to be a viable strontium removal strategy.
Figure 2.
Effect of initial turbidity on strontium removal in coagulation jar tests.
In summary, very little strontium removal could be achieved by conventional drinking water treatment using alum and ferric chloride up to doses of 50 mg/L under the conditions evaluated in this work.
3.2. Lime-soda ash softening in natural waters
3.2.1. Effect of lime dose and pH on strontium removal from ground waters
Jar tests were conducted on three Ohio ground waters that contained naturally elevated levels of strontium (4.2, 21.8, and 10.9 mg/L) (Table 2). The tests evaluated the effect of the lime dose (0–450 mg/L) on strontium removal. A set of “control” jar tests (no lime was added) were also conducted on each test water and the results showed that strontium was not removed during the softening protocol in the absence of lime and soda ash.
The initial pH of the private well water averaged 7.2 and the initial strontium, calcium and magnesium concentrations averaged 4.1 mg/L, 126 and 32 mg/L, respectively. Following lime addition, total calcium levels increased to as high as 337 mg/L for the highest lime dose (450 mg/L). The pH during all softening jar tests increased with increasing lime dose. In the case of the private well water, the pH increased to 11 (Fig. 3a) at the highest lime dose of 450 mg/L.
Figure 3.
Lime-soda ash softening jar test results for private well water: (a) relationship between lime dose and pH, (b) final strontium concentration, (c) % strontium reduction, and (d) % calcium and magnesium reductions. Private well water had an initial pH of 7.22 and contained 4.2 mg/L strontium, 127 mg/L calcium, and 33 mg/L magnesium.
Strontium concentrations in the private well water decreased rapidly and in a linear manner (R2 = 0.87) between pH 8.8 and 10 from 3.8 mg/L to 1.1 mg/L (Fig. 3b). Very little additional strontium reduction was observed as the pH was increased to 10.7 where the final strontium concentration was 0.9 mg/L resulting in a 78.2% removal (Fig. 3c). Calcium reduction nearly paralleled that of strontium in both trend and values (Fig. 3d). Calcium reduction increased rapidly from 12.7% to a maximum of 86.4% between pH 8.6 and 10.7. Very little magnesium removal was observed up to pH 9.8 (Fig. 3d). Magnesium reduction increased slightly to a maximum of 15.5% as the pH increased above pH 9.8. Magnesium reduction was not realized until the pH was sufficiently high to precipitate Mg(OH)2.
Triplicate jar tests were conducted with site 1 ground water following the same test procedure. The initial strontium concentration in the water averaged 21.8 mg/L. The initial pH of the source water averaged 7.29, and magnesium and calcium concentrations averaged 43.7 mg/L and 102 mg/L, respectively. The pH increased with increasing lime dose (Fig. 4a) although there was some variability in trends of replicate jar tests. Discrepancies were most likely associated with the differences in pH of the waters at the start of the jar tests (Fig. 4a, 0 mg/L lime) which could have occurred as a result of changes in DIC as result of interaction with the air during the water storage period.
Figure 4.
Lime-soda ash softening jar test results for site 1 water: (a) relationship between lime dose and pH, (b) final strontium concentration, (c) % strontium reduction, and (d) % calcium and magnesium reductions. Site 1 water had an initial pH of 7.29 and contained 21.8 mg/L strontium, 102 mg/L calcium, and 43.7 mg/L magnesium.
Strontium reduction from the site 1 water increased with increasing pH from the initial pH values to pH 10 in an exponential pattern (R2 = 0.72) (Fig. 4b). There were differences in final strontium concentrations between the three tests up to pH 9 as the result of the different starting pH values. Final strontium concentrations during replicate tests merged together near pH 9.5. The strontium concentration reached a minimum level of approximately 4.8 mg/L between pH 10 and 10.8. At pH 11.9, strontium re-solubilized to 13.7 mg/L. As a percentage, strontium removal increased with increasing pH to level-off at a maximum of 77.7% at pH 10.7 (Fig. 4c). Strontium removal dropped to 37.0% at pH 11.9. Calcium reduction (%) nearly paralleled that of strontium in both trend and values (Fig. 4d). Calcium reduction increased rapidly to nearly 82.4% with increasing pH to pH 9.7 where it remained to pH 10.7. Above pH 10.7, reduction began to decrease to as low as 59.2% as calcium re-solubilized, and at pH 11.9 calcium levels actually increased above the initial concentration reflecting lime contribution. The increase in solubility of calcium carbonate was likely a result of changing dissolved carbonate levels as calcium carbonate precipitated and calcium levels increased as lime was added to achieve upper pH values. Very little magnesium removal was observed up to pH 9.8 (Fig. 4d). Magnesium reduction increased slightly with increasing pH to approximately 25.3% between the initial pH and pH 10.7 (Fig. 4d). Above pH 10.7, magnesium reduction rapidly increased to nearly 98.9% at pH 11.9.
Duplicate jar tests were performed with the site 2 ground water. The water had an initial pH of 7.30 and the initial strontium concentration averaged 10.9 mg/L. Initial magnesium and calcium concentrations were 32.5 mg/L and 111.9 mg/L, respectively. Test water pH increased with increasing lime dose (Fig. 5a). The pH trends in the duplicate jar tests were in reasonable agreement at pH values less than 9, but became less reproducible at higher pH values for unknown reasons.
Figure 5.
Lime-soda ash softening jar test results for site 2 water: (a) relationship between lime dose and pH, (b) final strontium concentration, (c) % strontium reduction, and (d) % calcium and magnesium reductions. Site 2 water had an initial pH of 7.30 and contained 10.9 mg/L strontium, 112 mg/L calcium, and 32.5 mg/L magnesium.
Strontium levels decreased in an exponential decay trend (R2 = 0.80) with increasing pH to a minimum of 2.3 mg/L between pH 9.8 and 10.7 (Fig. 5b). Above pH 10.7, strontium levels increased to 7 mg/L at pH 11.9. Expressed as a percentage, strontium removal values increased with increasing pH to level-off at a maximum of 78.5% at pH 9.79 (Fig. 5c). Similar to jar test results of other source waters, calcium removal percentages and trends were nearly identical to the strontium removal (Fig. 5d). Maximum calcium removals of approximately 83.2% were achieved at pH 9.79. Magnesium reduction increased slightly with increasing pH to approximately 20.8% between the initial pH and pH 10.7 (Fig. 4d). Above pH 10.7, magnesium reduction rapidly increased to nearly 99.2% at pH 11.9 in Run 1. It is worth noting that magnesium reduction varied with test. The degree of magnesium removal by Mg(OH)2 precipitation during softening is dependent on pH and favored at higher pH values. Maximum pH values varied with jar test which explains the differing degrees of magnesium removal. It is apparent, however, that strontium removal was not associated with magnesium removal or some other mechanism at elevated pH values where magnesium removal was observed.
An interesting and consistent observation in all three natural test waters was that strontium and calcium removals (percentages) during softening jar tests were nearly identical. The finding suggests that strontium removal was directly related to calcium removal. The relationship was emphasized in a comparison of percent strontium reduction and the percentage of calcium removal for all jar test data (Fig. 6). The relationship was linear with a correlation coefficient, R2, value of 0.960, and the regression line fell very close to the 1:1 line where percent strontium removal is identical to percent calcium removal (Fig. 6). Another important observation was that the minimum strontium concentration achieved in each water appeared to be related to the initial strontium concentration while the maximum strontium removal expressed as percentages were approximately the same at different pH values even though the initial concentrations varied widely. Specifically, initial and final strontium levels were 4.1, 10.9 and 21.8 mg/L and 0.9, 2.3 and 4.8 mg/L, respectively. Although the initial calcium concentrations and pH values (or lime dose) where minimum strontium levels were achieved during softening of all waters were similar, the final strontium concentration varied by over 500%. In contrast, corresponding final calcium levels were between 17 and 19 mg//L which were in reasonable proximity to the solubility of calcium carbonate.
Figure 6.
Strontium removal plotted against calcium removal (%) for all lime-soda ash jar tests performed with ground waters that contained natural strontium.
3.2.2. Effect of lime dose and pH on strontium removal from strontium-spiked water
Jar tests were performed to evaluate the effect of the lime dose (0 mg/L to 450 mg/L) on strontium removal from Bolton plant water that was spiked with 4.9 mg/L strontium. Two “control” jar tests conducted with an initial pH of 6.69–6.75 demonstrated that strontium was not removed from the water during the mechanical softening steps in the absence of soda ash and lime (not shown).
Lime-soda ash softening jar tests were performed with varying lime doses using raw Bolton groundwater. The average initial pH of the Bolton plant water was 6.75 ± 0.05. The average initial strontium spike was 4.92 mg/L ± 0.11, and the average calcium and magnesium concentrations were 62 mg/L and 24 mg/L, respectively (Table 2). The pH during the jar tests increased with increasing lime dose (Fig. 7a) up to pH 12 at the highest dose of 450 mg/L.
Figure 7.
Lime-soda ash softening jar test results for strontium-spiked Bolton ground water: (a) relationship between lime dose and pH, (b) final strontium concentration, (c) % strontium reduction, and (d) % calcium and magnesium reductions. Bolton had an initial pH of 6.72 and contained 0.42 mg/L strontium, 62 mg/L calcium, and 24 mg/L magnesium. Water was spiked with 4.9 mg/L strontium.
Strontium reduction from spiked Bolton water decreased with increasing pH from the initial pH values to pH 10.6 in a near exponential decay pattern (Fig. 7b). The strontium concentration reached a minimum level of approximately 1.6 mg/L at pH around 11. As pH increased above 11, strontium was released back to the water to as high as 3.5 mg/L at pH 12. Expressed as a percentage, strontium removal increased in an exponential trend with increasing pH to level-off at a maximum of 70% at pH 10.6 (Fig. 4c) then strontium removal dropped to 35% by pH 12. Calcium reduction was similar to that of strontium in both trend and values (Fig. 7d). Calcium reduction increased rapidly to nearly 60% with increasing pH to pH 9.7. Above pH 9.7, soluble calcium reduction began to decrease to as low as 20% as it re-solubilized, and at above pH 11.2 soluble calcium actually increased. Very little magnesium was observed up to pH 9 (Fig. 7d). Magnesium reduction increased slightly with increasing pH to approximately 22% between the initial pH and pH 10.7 (Fig. 4d). Above pH 10.7, magnesium reduction rapidly increased to nearly 98.9% at pH 11.9.
3.2.3. Effect of initial strontium concentration on strontium removal
Jar tests were conducted to evaluate the impact of initial strontium concentration (1–10 mg/L) on strontium removal from Bolton plant water (Table 2). The tests were conducted using lime and soda ash doses of 150 mg/L and 50 mg/L, respectively, previously identified for optimal calcium and strontium removal. Lime softening achieved 39%–53% strontium removal (Fig. 8a). Calcium and magnesium reductions ranged between 59% to 77% and 37%–82%, respectively (Fig. 8.a). The average settled pH value was 11.4 ± 0.01 which was higher than the anticipated pH and pH where optimal strontium removal was observed. Although there was some variability in strontium removal, there was not a relationship between initial strontium concentration and percent removal (Fig. 8a). Initial strontium concentration, however, did impact final strontium concentration (Fig. 8b). A linear relationship existed between initial and final strontium levels with an R-squared of 0.988 (Fig. 8b). A similar relationship was noted in jar test results on natural waters.
Figure 8.
The effect of initial strontium concentration on the removal of strontium, calcium and magnesium from lime-soda ash softening jar tests.
3.3. Controlled batch studies
A series of batch studies were performed in which the initial water conditions were varied in order to better understand the relationship between important water quality parameters and strontium removal during lime softening, and removal mechanism(s).
3.3.1. Effect of initial strontium concentration and pH on strontium removal in absence of calcium
Controlled batch tests were conducted using laboratory prepared water to determine whether strontium (three different initial concentrations) could be removed through precipitation from water with 85 mg C/L DIC between pH 5 to 11 in the absence of calcium (pH adjusted with sodium hydroxide). A DIC of 85 mg C/L was selected because it was similar to the DIC of natural waters used in this work. Effective strontium removal from natural source waters during lime softening was previously demonstrated in ground water with a very similar DIC (ground water site #1) under these conditions. Average strontium reduction efficiencies were only 4.5%, 7.2% and 5.3% in waters for waters with initial strontium concentrations of 0.81, 4.8 and 9.5 mg/L, respectively. There was not an apparent impact of pH on strontium removal (Supplemental Fig. B). These results show that very little strontium removal occurred in the absence of calcium.
3.3.2. Effect of initial strontium and calcium concentrations, and pH on strontium removal
Lime softening batch tests were conducted to identify the impact of calcium concentration on strontium removal in water containing 85 mg C/L DIC and an initial calcium concentration of approximately 100 mg/L over a pH range of 7–12. Tests were also conducted with initial strontium concentrations of 0.99, 5.1 and 9.6 mg/L. No strontium and calcium removal was observed between pH 7 to 9 (Fig. 9a and b). Between pH 9 and 10, both strontium and calcium reductions dramatically increased and then leveled-off between pH 10 and 12. At pH 12, the maximum strontium reduction for the initial strontium concentrations of 0.99, 5.1 and 9.6 mg/L were 64.6%, 62.9% and 55.2%, respectively, and the maximum calcium reductions were 98.0%, 96.3% and 97.7%, respectively. The results clearly demonstrate the necessity of calcium precipitation to strontium removal and the importance of pH particularly as it impacts calcium carbonate solubility. Interestingly, the initial strontium concentration impacted the final strontium concentration. An increase in initial strontium concentration resulted in a greater treated water concentration. However, initial strontium concentrations did not have an impact on the percent removals of strontium which were relatively consistent. Both the final concentration of calcium and percent reductions of calcium were also relatively consistent.
Figure 9.
Effect of pH on a) strontium removal and b) calcium removal during controlled batch tests.
Lime softening batch tests were conducted to determine the impact of initial calcium concentration on strontium removal. When the initial calcium concentration was reduced from an average of 95.3 mg/L to 50.1 mg/L in high strontium-containing water (10.3 mg/L and 9.59 mg/L), the corresponding minimum strontium levels were 4.3 mg/L and 3.3 mg/L, or strontium reductions of 55.0% and 68.2%, respectively (Fig. 10a) while the calcium was similarly very good at 97.7%–95.3%, respectively (Fig. 10b). When the initial calcium concentration was increased from 101 mg/L to 157 mg/L in moderate strontium-containing water (4.95 mg/L and 3.85 mg/L), the corresponding minimum strontium levels were 1.9 mg/L to 1.8 mg/L, or from 61.9% to 52.7%, respectively (Fig. 10a) while the calcium reduction increased very slightly from 96.2% to 98.5% (Fig. 10b). The results suggest that calcium plays a very important and necessary part in strontium’s removal mechanism.
Figure 10.
Effect of pH and (a) initial calcium concentration on strontium removal, (b) initial calcium concentration on calcium removal, (c) initial dissolved inorganic concentration on strontium removal, and (d) initial dissolved inorganic concentration on calcium removal during controlled batch tests.
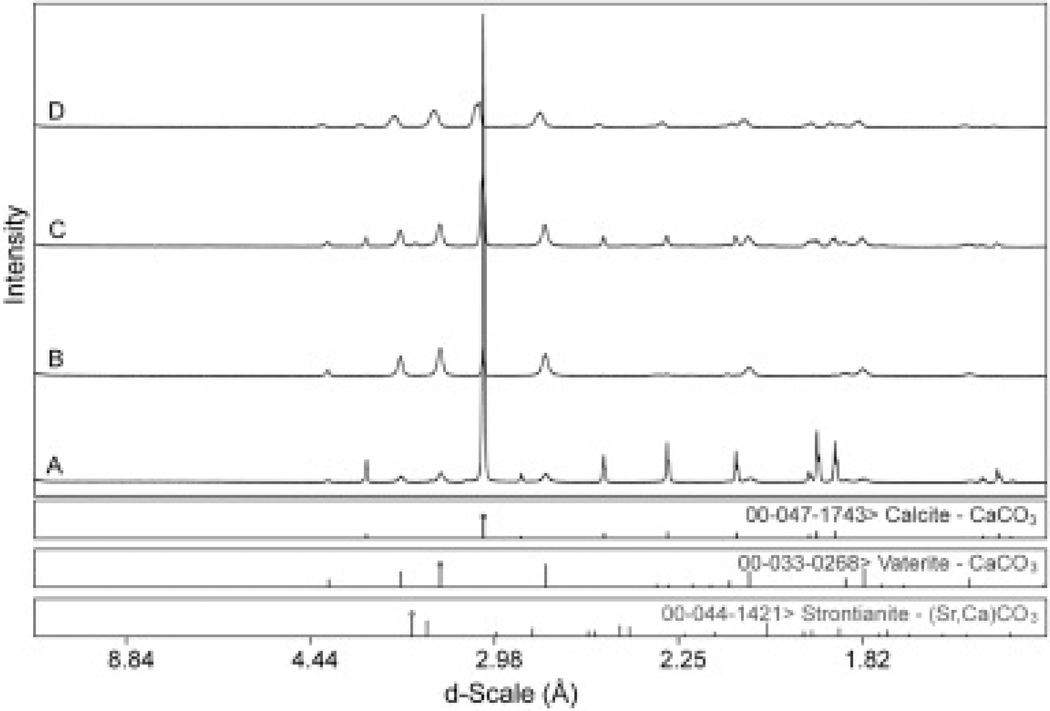
X-ray diffraction was used to identify the crystalline solids present in the filter precipitate. Two dominate distinct crystal phases were identified: calcite and vaterite, both polymorphs of calcium carbonate (CaCO3). The mineral with the rhombohedrum crystal habit was identified as calcite and was dominant when strontium was not present Fig. 11, Fig. 12a). The spherical particles were identified as vaterite, which was the dominant form where strontium and calcium concentrations were 5.08 mg/L and 97.2 mg/L, respectively (Fig. 11, Fig. 12b). Calcite is the most thermodynamically stable polymorph while vaterite is the least stable and less common in nature. It is possible that the presence of strontium in solution may have influenced the mineralogy change from calcite to vaterite in these experiments. Strontianite (SrCO3) was also found but only in the sample with the lowest initial calcium concentration (data not shown). Particle and crystal shape could impact particle interactions, settling properties and sludge volume properties (Komar and Reimers, 1978, Namer and Ganczarczyk, 1993, Dietrich, 1982, Plumpton, 1988).
Figure 11.
SEM images from controlled batch tests of solids formed at pH 11 with initial strontium and calcium concentrations of (a) 0 and 100 mg/L, (b) 5.08 and 97.2 mg/L, (c) 10.3 and 50.1 mg/L, and (d) 3.85 and 157 mg/L, respectively.
Figure 12.
XRD patterns of solids formed during controlled batch studies at pH 11 with initial strontium and calcium concentrations of (a) est. 0 and 100 mg/L, (b)5.08 and 97.2 mg/L, (c) 10.3 and 50.1 mg/L, and (d) 3.85 and 157 mg/L, respectively.
Precipitate from waters with 10.3 mg Sr/L and 50.1 mg Ca/L, and 3.85 mg Sr/l and Ca 157 mg Ca/L consisted of both calcite and vaterite. XRD patterns for calcite showed a trend of d-space shifting to larger d-spacing, which could be an indication of solid-solution with the larger atomic radius (AR) of strontium (200 pm) substituted for calcium (AR = 180 pm) in the crystal lattice (Fig. 12).
Others have reported that vaterite’s crystal shape and size can be affected when there are alkaline-earth cations, including strontium, in solution. The changes in morphology are most likely caused by the substitution of Ca2+ with Sr2+ (Noethig-Laslo and Brecevic, 1998). When calcium carbonate was precipitated under supersaturated conditions, vaterite readily formed initially. However, vaterite transformed into calcite over time, which could be due to the vaterite crystals dissolving and the calcite crystals recrystallizing. When the super-saturation levels were higher, the formation of vaterite was more prevalent (Spanos and Koutsoukos, 1998).
4. Conclusions
As anticipated, conventional coagulation/filtration drinking water treatment was not effective in removing strontium from surface waters. This result was generally independent of the initial strontium concentration, pH, and turbidity. At best, conventional treatment using alum and ferric chloride can only achieve strontium removals up to 12% and 5.9%, respectively.
Lime-soda ash softening was an effective approach to reduce strontium from three strontium-containing natural waters, a strontium-spiked ground water and a laboratory-prepared water. Soda ash was necessary to achieve sufficient carbonate hardness so calcium and strontium carbonate could precipitate. Strontium removal as great as 78% was achieved.
Strontium removal during lime softening jar tests with natural strontium containing ground waters was pH dependent. Percent strontium removal increased to a maximum between pH 10 and 11. Strontium removal trended with calcium removal both in optimal removal and percent removal, where magnesium removal was independent. Optimum strontium removal corresponded to optimum calcium removal or minimum calcium solubility.
Strontium removal (%) did not appear to be related to initial strontium concentration in tests with natural waters and strontium-spiked water. Final strontium concentration, however, was dependent on initial strontium concentration (greater initial concentrations result in greater final concentrations). Some waters with very high strontium levels may have difficulty reducing strontium to desired levels (e.g., HRL).
Controlled batch experiments further illustrated the importance of calcium concentration on strontium removal. Strontium was not removed from water in the absence of calcium or sufficient DIC to precipitate calcium. In batch experiments, strontium and calcium removals increased with increasing pH above pH 11–65% and 98%, respectively, at 85 mg C/L of initial dissolved inorganic carbon. At a low initial dissolved inorganic carbon of 10 mg C/L, both strontium and calcium removal efficiencies greatly reduced to 8.1% and 29%, respectively. The precipitation of strontium depends on the precipitation of calcium.
Scanning electron microscope micrographs and x-ray diffraction patterns of solids collected during controlled batch studies revealed that at pH 11 with 5.08 mg Sr/L and 97.2 mg Ca/L spherical vaterite, the least stable polymorph of calcium carbonate, was prevalent in the samples. Only small amounts of rhombohedron calcite, the most stable polymorph, were found in the samples. A relatively small amount of strontianite was identified in one case where calcium concentration was lowest.
X-ray diffraction patterns revealed that the calcite and vaterite d-spacing were shifted, indicating possible SrCa solid solution inside the crystal lattice.
Supplementary Material
Highlights.
Conventional coagulation/filtration was not effective in removing strontium from surface water.
Lime-soda ash softening was an effective approach to reduce strontium from natural ground waters.
Strontium removal during lime softening was pH, DIC and calcium dependent.
Strontium removal (%) during softening was not related to initial strontium concentration.
Final strontium concentration during softening was dependent on initial strontium concentration.
Acknowledgments
The authors wish to thank Maily Pham, Christopher Parrett, Daniel Williams, Christy Muhlen and Keith Kelty EPA Office of Research and Development (ORD) and Water Supply and Water Resources Division (WSWRD) for conducting the water and solids analyses, and assistance in the laboratory. We would also like to thank Thomas Sorg of the WSWRD, and Zeno Bain and Lili Wang of EPA’s Office of Water for providing technical reviews of the manuscript. Lastly, we would also like to thank Mitch Wilcox of Pegasus Technical Services, Inc. for providing editorial reviews of the manuscript.
Notice
The U.S. Environmental Protection Agency, through its Office of Research and Development, funded and managed, or partially funded and collaborated in, the research described herein. It has been subjected to the Agency’s administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the author(s) and do not necessarily reflect the views of the Agency, therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Achal V, Pan X, Zhang D Bioremediation of strontium (Sr) contaminated aquifer quartz sand based on carbonate precipitation induced by Sr resistant Halomonas sp Chemosphere, 89 (6) (2012), pp. 764–768 [DOI] [PubMed] [Google Scholar]
- Alexander GV, Nusbaum RE, MacDonald NS Strontium and calcium in municipal water supplies J. Am. Water Works Assoc, 46 (7) (1954), pp. 643–654 [Google Scholar]
- APHA Standard Methods for the Examination of Water and Wastewater (eighteenth ed.), American Public Health Association, Washington, D.C: (1992) [Google Scholar]
- Chegrouche S, Mellah A, Barkat M Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies Desalination, 235 (1–3) (2009), pp. 306–318 [Google Scholar]
- Chen C, Wang J Removal of Pb2+, Ag+, Cs+ and Sr2+ from aqueous solution by brewery’s waste biomass J. Hazard. Mater, 151 (1) (2008), pp. 65–70 [DOI] [PubMed] [Google Scholar]
- Cheng Z, et al. Preparation of magnetic Fe3O4 particles modified sawdust as the adsorbent to remove strontium ions Chem. Eng. J, 209 (0) (2012), pp. 451–457 [Google Scholar]
- Dietrich WE Settling velocity of natural particles Water Resour. Res, 18 (6) (1982), pp. 1615–1626 [Google Scholar]
- El Solh N, Rousselet F Skoryna SC (Ed.), Handbook of Stable Strontium, Springer; (1981), pp. 515–544 [Google Scholar]
- Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder J. Hazard. Mater, 190 (1–3) (2011), pp. 916–921 [DOI] [PubMed] [Google Scholar]
- Gürboğa G, Tel H Preparation of TiO2–SiO2 mixed gel spheres for strontium adsorption J. Hazard. Mater, 120 (1–3) (2005), pp. 135–142 [DOI] [PubMed] [Google Scholar]
- Hwang E-D, et al. Effect of precipitation and complexation on nanofiltration of strontium-containing nuclear wastewater Desalination, 147 (1–3) (2002), pp. 289–294 [Google Scholar]
- Komar PD, Reimers CE Grain shape effects on settling rates J. Geol, 86 (2) (1978), pp. 193–209 [Google Scholar]
- Li Q, et al. Strontium and calcium ion adsorption by molecularly imprinted hybrid gel Chem. Eng. J, 157 (2–3) (2010), pp. 401–407 [Google Scholar]
- Lide DR CRC Handbook of Chemistry and Physics (85 ed), CRC Press, Boca Raton, FL: (2005), p. 2656 [Google Scholar]
- Marie PJ, Garba MT, Hott M, Miravet L Effect of low doses of stable Sr on bone metabolism in rats. Min. Electrolyte Metab, 11 (1985), pp. 5–13 [PubMed] [Google Scholar]
- Merceille A, et al. The sorption behaviour of synthetic sodium nonatitanate and zeolite A for removing radioactive strontium from aqueous wastes Sep. Purif. Technol, 96 (0) (2012), pp. 81–88 [Google Scholar]
- Namer J, Ganczarczyk JJ Settling properties of digested sludge particle aggregates. Water Res., 27 (8) (1993), pp. 1285–1294 [Google Scholar]
- Nikolaev NP, Ivanov VA, Gorshkov VI, Nikashina VA, Ferapontov NB Counter-current ion-exchange separation of strontium from brines with acrylic-type cation-exchange resins React. Polym, 18 (1) (1992), pp. 25–33 [Google Scholar]
- Noethig-Laslo V, Brecevic L Mode and sites of incorporation of divalent cations in vaterite J. Chem. Soc. Faraday Trans, 94 (14) (1998), pp. 2005–2009 [Google Scholar]
- O’Donnell AJ, Lytle DA Full-scale and bench-scale studies on the removal of strontium from water Proceedings American Water Works Annual Conference Boston, MA June 11, 2014 (2014) [Google Scholar]
- Parks JL, Edwards M Precipitative removal of As, Ba, B, Cr, Sr, and V using sodium carbonate. J. Environ. Eng, 132 (5) (2006), pp. 489–496 [Google Scholar]
- Paterson-Beedle M, et al. Utilisation of a hydrogen uranyl phosphate-based ion exchanger supported on a biofilm for the removal of cobalt, strontium and caesium from aqueous solutions Hydrometallurgy, 83 (1–4) (2006), pp. 141–145 [Google Scholar]
- Plumpton AJ (Ed.), Production and Processing of Fine Particles, Pergamon Press, New York: (1988), pp. 404–407 [Google Scholar]
- Sayed SA Removal of Sr2+, Ca2+, and Mg2+ from aqueous and aqueous-alcoholic saline media. Zeolites, 17 (4) (1996), pp. 361–364 [Google Scholar]
- Sivaiah MV, et al. Ion exchange properties of strontium on in situ precipitated polyantimonic acid in amberlite XAD-7 Sep. Purif. Technol, 44 (1) (2005), pp. 1–9 [Google Scholar]
- Spanos N, Koutsoukos PG The transformation of vaterite to calcite: effect of the conditions of the solutions in contact with the mineral phase J. Cryst. Growth, 191 (4) (1998), pp. 783–790 [Google Scholar]
- Tel H, et al. Preparation of ZrO2 and ZrO2–TiO2 microspheres by the sol–gel method and an experimental design approach to their strontium adsorption behaviours Chem. Eng. J, 161 (1–2) (2010), pp. 151–160 [Google Scholar]
- Thorpe CL, et al. Strontium sorption and precipitation behaviour during bioreduction in nitrate impacted sediments Chem. Geol, 306–307 (0) (2012), pp. 114–122 [Google Scholar]
- USEPA Methods for Chemical Analysis of Water and Wastes. EPA-600/14–79-020 (1983) [Google Scholar]
- USEPA Drinking Water Criteria Document for Stable Strontium Prepared by the Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office, Cincinnati, OH for the Office of Drinking Water, Washington, DC: (1990) [Google Scholar]
- 2000. USEPA National Primary Drinking Water Regulations; Radionuclides; Final Rule. Office of the Federal Register, National Archives and Records Administration; 76708–76753 [Google Scholar]
- USEPA Drinking water contaminant candidate list 3—Draft Fed. Regist, 73 (35) (2008), pp. 9628–9654 [Google Scholar]
- USEPA Drinking Water Treatability Database: Strontium (2011) http://iaspub.epa.gov/tdb/pages/contaminant/contaminantOverview.do
- USEPA Announcement of preliminary regulatory determinations for contaminants on the third drinking water contaminant candidate list; proposed Rule Fed. Regist, 79 (202) (2014), pp. 62715–62750 [Google Scholar]
- USEPA The Third Unregulated Contaminant Monitoring Rule (UCMR 3): Data Summary EPA 815-S-15–002 (2015) [Google Scholar]
- USGS U.S. Geological Survey Office of Water Quality. “USGS Water-quality Information: Water Hardness and Alkalinity” (2015) [Google Scholar]
- Zhang L, Zhou J, Zhou D, Tang Y Adsorption of cadmium and strontium on cellulose/alginic acid ion-exchange membrane J. Membr. Sci, 162 (1–2) (1999), pp. 103–109 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.