Abstract
Anxiety disorders are often preceded by interpersonal stress; however, most individuals who experience stress do not develop anxiety, making it difficult to predict who is most susceptible to stress. One proposed trans-diagnostic neural risk marker for anxiety is the error-related negativity (ERN), a negative defection in the event-related potential waveform occurring within 100 ms of error commission. The present study sought to investigate whether interpersonal stress experienced over the course of a year interacts with ERN magnitude to prospectively predict anxiety symptoms. A sample of 57 emerging adults performed an arrow flanker task to elicit the ERN at the start of the academic school year (time one). Toward the end of the academic year (time two), participants reported on past-year interpersonal stress and anxiety symptoms. Stress interacted with ERN magnitude to predict anxiety symptoms, whereby, for individuals with an enhanced ERN at time one, greater interpersonal stress over the course of a year was significantly associated with increased anxiety symptoms at time two, even controlling for anxiety symptoms at time one. These findings suggest that enhanced performance monitoring may render individuals more susceptible to the adverse effects of interpersonal stress, thereby increasing risk for heightened anxiety.
Keywords: Error-related negativity, Interpersonal stress, Anxiety
1. Introduction
Anxiety disorders are among the most common and persistent forms of mental illness worldwide (Baxter et al., 2013; Kessler et al., 2005; Polanczyk et al., 2015). In addition to being associated with emotional distress and severe impairments in interpersonal functioning and job performance (Antony and Stein, 2008), anxiety disorders place significant economic strain on the health care system (Kessler and Greenberg, 2002). Anxiety is often preceded by episodes of life stress (Faravelli, 1985; Faravelli and Pallanti, 1989; Finlay-Jones and Brown, 1981; Green et al., 2010; Hankin et al., 2004; Young and Dietrich, 2015), and interpersonal stressors such as entrapment, humiliation, and peer victimization are particularly salient in predicting symptoms (Farmer and Kashdan, 2015; Hamilton et al., 2016; Kendler et al., 2003; Siegel et al., 2009; Uliaszek et al., 2010). Prior research demonstrates relationships between interpersonal stress and panic disorder (Klauke et al., 2010), social anxiety disorder (Brook and Schmidt, 2008; Siegel et al., 2009), agoraphobia (Kleiner and Marshall, 1987; Last et al., 1984), and obsessive-compulsive disorder (Cromer et al., 2007; Real et al., 2011), suggesting that the link is not symptom-or disorder-specific.
However, not everyone who experiences life stress goes on to develop psychopathology (Ingram and Luxton, 2005; Harkness et al., 2015; Harkness and Monroe, 2016) — in fact, most will not — making it difficult to predict who is at risk of increased anxiety following stress exposure. Diathesis-stress models of psychopathology suggest that certain vulnerability factors, such as a genetic liability, and significant stress exposure (e.g., a divorce), interact to place individuals at risk of developing psychopathology (Ingram and Luxton, 2005; Monroe and Simons, 1991). Assessing both proposed diatheses and stressors may thus be important for understanding the etiology of anxiety.
Recently, there has been increased interest in elucidating neural systems involved in the development and maintenance of anxiety (Pine, 2007). One proposed neural marker of risk for anxiety is the error-related negativity (ERN; Olvet and Hajcak, 2008; Riesel et al., 2011, 2015), an event-related potential (ERP) component that is larger for erroneous than correct responses between 0 and 100 ms following the response (Falkenstein et al., 1991; Gehring et al., 1993). The ERN is a negative defection in the ERP waveform that is maximal at frontocentral electrode sites and is thought to reflect activity of the anterior cingulate cortex (ACC; Brázdil et al., 2005; Dehaene et al., 1994; Stemmer et al., 2004). It is hypothesized to represent an alarm signal generated by a neural network engaged in performance monitoring, signifying that an error has been made and increased cognitive control is needed to adjust behaviour (Carter and van Veen, 2007; Dehaene, 2018; Holroyd and Coles, 2002; Lo, 2018).
An enhanced ERN has been observed in trait anxious adults and children (Hajcak et al., 2003; Meyer et al., 2012, 2016; Moser et al., 2013; Olvet and Hajcak, 2008), as well as individuals with obsessive-compulsive disorder (OCD; Carrasco et al., 2013; Endrass et al., 2010, 2014; Gehring et al., 2000; Hajcak and Simons, 2002; Hajcak et al., 2008; Riesel, 2019; Riesel et al., 2011), generalized anxiety disorder (GAD; Weinberg et al., 2012, 2015b; Xiao et al., 2011), and social anxiety disorder (SAD; Endrass et al., 2014; Kujawa et al., 2016). A larger ERN is also associated with heightened negative affect (Hajcak et al., 2004; Luu et al., 2000; Wiswede et al., 2009), a transdiagnostic characteristic of anxiety disorders (Clark and Watson, 1991).
This enhanced ERN is not only evident in already-affected anxious individuals, but may also be a viable risk marker for anxiety (Olvet and Hajcak, 2008; Riesel et al., 2011, 2015). For instance, the ERN is heritable, with genes accounting for approximately 50% of the variation in its magnitude (Anokhin et al., 2008), and an enhanced ERN in childhood and adolescence can prospectively predict subsequent increases in anxiety (Lahat et al., 2014; McDermott et al., 2009; Meyer, 2017; Meyer et al., 2015, 2018). However, an enhanced ERN is also observed in unaffected first-degree relatives of individuals with OCD (Carrasco et al., 2013; Riesel et al., 2011), as well as in individuals in remission from clinically-significant anxiety (Hajcak et al., 2008; Kujawa et al., 2016; Riesel et al., 2015), suggesting that not everyone with an enhanced ERN is anxious. Therefore, a larger ERN appears not to be a symptom, state marker, or “scar” of psychopathology, but rather a latent vulnerability for anxiety and anxiety-related disorders (Olvet and Hajcak, 2008; Riesel et al., 2011, 2015) that can interact with other factors, including stressful events, to set the stage for heightened symptoms (Meyer et al., 2017a). However, it is currently unclear what social-environmental circumstances trigger heightened anxiety in emerging adults with this vulnerability marker.
Taken together, previous research indicates that interpersonal stress often precedes anxiety, and that the ERN may be a viable neural risk marker for anxiety; however, the extent to which interpersonal stress and ERN magnitude might interact to predict anxiety is not yet clear. Further, very little is known about whether ERN magnitude can track symptom changes in adult populations. To address these issues, we examined the extent to which an enhanced ERN in combination with greater interpersonal stress exposure predicts subsequent symptoms of anxiety in a sample of first-year undergraduate students. Neural systems implicated in performance monitoring mature substantially in late adolescence and young adulthood (e.g., Hogan et al., 2005; Kelly et al., 2009; Ladouceur et al., 2007; Segalowitz and Dywan, 2009; Steinberg, 2005), which is a time of increased stress sensitivity (Walker et al., 2004), and heightened risk for psychopathology (Birmaher et al., 1996; Braet et al., 2013; Kessler et al., 2001; Wagner and Compas, 1990). Importantly, the entry to university is a time of heightened interpersonal stress (Bouteyre et al., 2007; Fisher and Hood, 1988; Schlossberg, 1989; Wilcox et al., 2005), and first-year students endorse more symptoms of psychopathology than students in later years (Adlaf et al., 2001). All of these factors make our sample an important population in which to investigate how interpersonal stress interacts with error monitoring to predict anxiety.
To that end, we measured ERN magnitude at the beginning of the academic year (i.e., in the first month and a half at university), interpersonal and non-interpersonal stressors experienced across the first year at university, and anxiety symptoms toward the end of the academic year. Because of prior research indicating that the ERN is a transdiagnostic marker of anxiety (Meyer, 2017; Riesel et al., 2017, 2019; Weinberg et al., 2016), we investigated a variety of anxiety symptoms as a composite score. We hypothesized that, for undergraduate students with a large ERN at baseline, greater interpersonal stress exposure over the year would predict more subsequent symptoms of anxiety at the end of the year while controlling for baseline anxiety levels. In order to determine if these effects were specific to social stressors, we also conducted exploratory analyses to investigate whether this effect is evident for non-interpersonal stressors.
2. Method
Two hundred and fifty-six first-year undergraduate students from McGill University were recruited at the start of the academic year (Time1) over three consecutive years. The first (N = 92), second (N = 73), and third (N = 91) wave of participants were recruited in 2016, 2017, and 2018, respectively. Participants were recruited from the University’s psychology human participant pool, verbal advertisements in classrooms, and flyers posted around the campus. Participants either received course credit or monetary compensation for their time. For those requesting monetary compensation, $23 was given to wave one and two participants, and $28 was given to wave three participants. Permission to recontact was obtained from 211 participants at Time 1. Toward the end of the first academic year, approximately six months after the initial lab visit (Time 2), those 211 participants were re-contacted several times via email with an invitation to complete online questionnaires. Participants were compensated $10 for participating in the Time 2 questionnaires, and entered into a draw to win a $100 gift card. Informed consent was obtained prior to participation and the research protocol was approved by the Research Ethics Board at McGill University.
At Time 1, two participants were excluded due to excessive noise in the electroencephalogram (EEG) data, 28 participants were excluded due to committing too few errors (i.e., fewer than 6; Olvet and Hajcak, 2009), 11 were excluded because they were taking psychotropic medication (i.e., anti-depressant/anxiety medication; De Bruijn et al., 2004; Zirnheld et al., 2004), and one participant was excluded because their Time 1 anxiety was more than three standard deviations above the sample mean. Of the remaining 214 participants, 59 participants (28% of the original sample) completed the online questionnaires at the end of the year. Two of these 59 participants were excluded because their scores on either the Time 2 anxiety or stress exposure measures were more than three standard deviations above the sample mean. Therefore, the final number of participants with usable data at Time 2 was 57.1 Because of the size of this final sample, we conducted a sensitivity analysis using G*Power (Faul et al., 2007), to establish the smallest effect size we had at least 80% power to detect. With a total sample size of 57, eight predictors in a multiple regression, and α error probability set to 0.05, the smallest effect size we could detect was f2 = 0.11. Meta-analyses investigating the association between ERN magnitude and anxiety report effect sizes ranging from f2 = 0.18 (Moser et al., 2016) to f2 = 0.26 (Moser et al., 2013), suggesting our sample would support this investigation.
The mean age of this final sample was 18.12 years old (SD = 0.47) at the start of the year, and 84% of participants were female. Forty-four percent of participants were Caucasian, 28% were Chinese, 7% were South East Asian, 5% were South Asian, 2% were Caribbean, 2% were Arab/West Asian, 2% were Hispanic, 2% were Korean, and 8% indicated they were another ethnicity. The median annual family income of the sample was between $100,000 and $149,999 (range: $50,000 to $250,000 or greater). For comparison, the median family income in Canada in 2017 was $92,990 for families consisting of a couple (and children, if applicable) living at the same address, and $46,140 for single-parent families (Statistics Canada, n.d.). However, we did not adjust participants’ reported income by number of people in their immediate family, and did not collect information about number of wage-earners in their family.
2.1. Measures
2.1.1. Questionnaires
At Time 1 and 2, participants completed the Inventory of Depression and Anxiety Symptoms (IDAS-II; Watson et al., 2012). The IDAS-II is a 99-item self-report measure of 18 empirically derived internalizing dimensions of depression and anxiety. Items assess symptoms over the past two weeks and participants make their responses using a 5-point Likert-type scale ranging from 1 (not at all) to 5 (extremely). The IDAS-II has demonstrated good internal consistency, test-retest reliability, and convergent and discriminant validity with diagnoses and self-report measures in similar populations (Watson et al., 2012). The present study focused on a composite measure of anxiety symptoms by summing across the eight anxiety subscales of the IDAS-II. This composite score represents the total sum of panic (8 items; range: 8–40), social anxiety (6 items; range: 5–30), claustrophobia (5 items; range: 5–25), traumatic intrusions (4 items; range: 4–20), traumatic avoidance (4 items; range: 4–20), checking (3 items; range: 3–15), ordering (5 items; range: 5–25), and cleaning (7 items; range: 7–35) subscales; therefore, 42 items were included in our composite anxiety score (range: 42–210; Time 1 α = 0.94; Time 2 α = 0.90). We used this composite anxiety score because interpersonal stress is associated with multiple forms of anxiety and symptom profles (e.g., panic disorder [Klauke et al., 2010], social anxiety disorder [Brook and Schmidt, 2008; Siegel et al., 2009], agoraphobia [Kleiner and Marshall, 1987; Last et al., 1984], and obsessive-compulsive disorder [Cromer et al., 2007; Real et al., 2011]). Additionally, an enhanced ERN has been found in individuals with a broad range of anxiety symptoms and disorders (Carrasco et al., 2013; Endrass et al., 2010; Hajcak and Simons, 2002; Weinberg et al., 2012, 2015b; Endrass et al., 2014; Kujawa et al., 2016). This anxiety composite score allowed us to examine potential moderating effects of the ERN on a broader measure of anxiety symptoms following exposure to past-year stress.
At Time 2, participants also completed the past-year version of the Stress and Adversity Inventory for Adults (Adult STRAIN; Slavich and Shields, 2018). The STRAIN is an online interview that assesses the severity and frequency of individuals’ exposure to different stressors over the entire lifetime, and the past-year version of the STRAIN used here includes the same stressor questions but focuses specifically on the past 12 months. Participants respond to questions probing 55 different types of acute life events and chronic difficulties; for each stressor that is endorsed, follow-up questions are asked about its timing, severity, duration, and frequency. Summary scores can be computed that reflect the count and severity of total, acute, and chronic stress experienced across 12 major life domains (i.e., housing, education, work, treatment/health, marital/partner, reproduction, financial, legal/crime, other relationships, death, life-threatening situations, possessions) and 5 social-psychological characteristics (i.e., interpersonal loss, physical danger, humiliation, entrapment, role change/disruption). The STRAIN has demonstrated excellent test-retest reliability and concurrent and discriminant validity in community and clinical samples (Slavich and Shields, 2018; Slavich et al., 2019), as well as excellent predictive validity in relation to a variety of cognitive, biological, and clinical out-comes including anxiety levels (e.g., Mayer et al., 2019; Stewart et al., 2019; Sturmbauer et al., 2019).
The present study focused on the total count of stressors experienced over the past year within interpersonal and non-interpersonal life domains, separately. To address our specific research question, and in line with prior work involving the STRAIN (Pegg et al., 2019), we created distinct subscales for interpersonal and non-interpersonal stress. Interpersonal stressors included all acute life events and chronic difficulties occurring in the marital/partner life domain (e.g., divorce or serious break-up, ongoing arguments with a spouse or partner) and other relationships domain (e.g., major interpersonal fights with roommate(s) or suitemates). In turn, non-interpersonal stressors included all acute life events and chronic difficulties occurring in the life domains of housing, education, work, treatment/health, reproduction, financial, legal/crime, life-threatening situations, death, and possessions. Higher scores on these two variables indicate greater past-year life stress exposure.
2.2. Task and materials
Participants completed an arrow version of the flanker task (Eriksen and Eriksen, 1974) on an Intel Core i7 computer using Presentation software (Neurobehavioural Systems, Inc.; Albany, CA). All stimuli were displayed on a 19-in. (48.3 cm) computer monitor. On each trial, five horizontally aligned arrowheads were presented in the center of the screen, and targets were always the center arrow. Half of these trials were congruent (“ < < < < < ” or “ > > > > > ”) and half were incongruent (“ > > < > > ” or “ < < > < < ”); the order of congruent and incongruent trials was random. Participants were instructed to use the computer mouse to quickly indicate the direction of the target arrow using the right or left mouse button (i.e., they pressed the right mouse button if the arrow pointed to the right). All stimuli were presented for 200 ms, followed by a black screen that either terminated following response selection or after 1800 ms had elapsed. An intertrial interval ranging at random between 1000 and 2000 ms was then presented. Participants were presented with a black screen with a white fixation cross in the center during response and intertrial periods. Participant response type (correct or incorrect) and reaction time (in ms) on every trial was recorded for later analysis.
2.3. Procedure
Participants visited the lab to complete the EEG assessment within the first month and a half of the academic year. Participants completed multiple computer tasks during the experiment, with the order of the tasks counterbalanced across participants. Other tasks included a social feedback task (as described in Ethridge and Weinberg, 2018), a monetary reward task (also described in Ethridge and Weinberg, 2018), and an emotional picture viewing task (as described in Sandre et al., 2019). Participants completed a 6-trial practice block and were told to be both as fast and as accurate as possible. The actual task consisted of five blocks of 30 trials (150 trials total), and each block was initiated by the participant. At the end of every block, participants received feedback based on their performance on the screen; if accuracy was 75% or lower, the message “Please try to be more accurate” was displayed to increase attention to the task; when more than 80% of responses were correct, the message “Please try to respond faster” was shown to increase the likelihood of the participant committing more errors; otherwise, the message “You are doing a great job” was presented.
Approximately six months after the first lab visit (Mdays = 176.05, SD = 13.65, range = 149–208), during the final weeks of the academic year, all participants were re-contacted and invited to complete an online version of the IDAS-II and STRAIN.
2.4. Electroencephalogram recording and data processing
Continuous EEG was recorded with a 32-electrode cap and a BrainVision actiCHamp system. The cap used the standard 10/20 layout and the ground electrode was placed at Fpz. The electrooculogram (EOG) generated from blinks and eye movements was recorded using facial electrodes placed around 1 cm to the left and right of both eyes (HEO) and 1 cm below and above one eye (VEO). Data were recorded with a sampling rate of 1000 Hz. Across all participants, the average electrode impedance was below 10 kΩ.
EEG data were analyzed offline using BrainVision Analyzer software (Brain Products, Munich, Germany). Continuous (unsegmented) data were band-pass filtered with fourth order low and high cut-offs of 0.01 and 30 Hz, respectively, using a Butterworth zero phase filter with a 24 dB/octave roll-off. Following this, for each trial, the EEG was segmented into 1500 ms windows starting 500 ms before each response onset and continuing for 1000 ms post-response. Then data were referenced offline to the average of left (TP9) and right (TP10) mastoids. Ocular and eye-blink corrections were conducted using HEO and VEO using the method developed by Miller et al. (1988). A semi-automatic artifact rejection procedure was conducted in which data from individual channels were automatically rejected if there was a voltage step greater than 50 μV/ms, a difference greater than 175 μV within 400 ms, or activity of less than 0.5 μV in 100 ms intervals. Visual inspection of the data by trained research assistants was then conducted to detect and reject any remaining artifacts.
Error and correct trials were then averaged separately. The mean voltage in the 200 ms window from −500 to −300 ms before response onset served as a baseline and was subtracted from each data point (Gorka et al., 2017; Meyer et al., 2014; Weinberg and Hajcak, 2011). Based on visual inspection of the grand averaged data, the ERN was quantified on error trials as the average activity from 0 to 100 ms at electrode site Cz, where error-related brain activity has been shown to be maximal and have high internal consistency reliability (Riesel et al., 2013; Sandre et al., revise & resubmit). In addition, the correct-response negativity (CRN) was evaluated in the same time window and electrode site on correct trials. The CRN is a negative defection in the ERP that typically follows both error and correct responses (Burle et al., 2008) and appears to reflect generic response monitoring (Simons, 2010). Therefore, to isolate error-specific neural activity, we used a regression-based procedure to compute unstandardized residuals of the ERN (Meyer et al., 2017b). To calculate the ERNresid, participants’ CRN was entered as the predictor, and the ERN was the dependent variable; the ERNresid scores are the saved unstandardized residuals from this regression.
Internal consistency (split-half reliability) of the ERP components of interest were calculated by examining correlations between averages based on odd-and even-numbered trials for each response type (i.e., error and correct), corrected using the Spearman-Brown prophecy formula (Nunnally et al., 1967). The ERN (r = 0.84), CRN (r = 0.98), and ERNresid (r = 0.75) demonstrated good internal consistency in the present sample.
Behavioural measures on the flanker task included the number of error trials for each participant, as well as accuracy expressed as a percentage of correct trials out of the total number of trials. Accuracy following error and correct responses was also calculated (post-error accuracy and post-correct accuracy). Average reaction times (RTs) on error and correct trials were calculated separately. Post-error slowing was calculated as the average of [RT (E+1) – RT (E−1)] for all errors, where (E+1) is the trial after the error and (E–1) is the trial before the error (Dutilh et al., 2012). Trials were removed from analyses if RTs were faster than 200 ms or slower than 1000 ms.
2.5. Data analysis
All statistical analyses were conducted using SPSS General Linear Model Software (Version 23). Paired-sample t-tests were used to compare within-subject conditional ERN and CRN magnitude, reaction times (RTs) on error and correct trials, as well as RTs and accuracy following each response type. Pearson coefficients were used to examine zero-order correlations between ERPs (at Time 1), anxiety symptoms (at Time 1 and Time 2), and total past-year interpersonal and non-interpersonal stress (at Time 2).
To examine whether the magnitude of the ERNresid at Time 1 moderated the association between past-year stress exposure and anxiety symptoms at Time 2, we conducted a simultaneous multiple regression with Time 2 anxiety symptoms as the dependent variable. ERNresid magnitude, past-year interpersonal stress, the interaction between ERNresid magnitude and past-year interpersonal stress, past-year non-interpersonal stress, and the interaction between ERNresid magnitude and past-year non-interpersonal stress were entered as predictors. Anxiety symptoms at baseline (Time 1), as well as time between baseline and follow-up assessments (in days) were included as covariates. We also entered gender (0 = male; 1 = female) as a covariate given evidence of gender differences in the ERN and its association with individual differences (Fischer et al., 2016; Larson et al., 2011; Moser et al., 2016; Sandre et al., revise & resubmit).
3. Results
3.1. Life stress exposure
Over the past year, participants experienced an average of 4.25 total stressors (SD = 3.26; range = 0–14), with an average total stressor severity score of 11.65 (SD = 10.39; range = 0–42). On average, participants experienced 1.23 interpersonal stressors (SD = 1.18; range = 0–5) and 3.02 non-interpersonal stressors (SD = 2.77; range = 0–12) over the past year.
3.2. Flanker task performance
Participants made an average of 14.47 errors (SD = 6.15; range = 6–34) and 134.98 correct responses (SD = 6.65, range = 109–144). Mean post-error slowing was 45.30 ms (SD = 44.06). Participants were faster on error (M = 302.07, SD = 30.15) as compared to correct trials (M = 376.89, SD = 37.07; t (56) = 16.34, p < .001), and were slower to respond following error trials (M = 389.79, SD = 48.43) compared to trials following correct trials (M = 366.67, SD = 36.71; t(56) = 5.13, p < .001). Additionally, participants were more accurate following error trials (M = 0.93, SD = 0.08) than following correct trials (M = 0.90, SD = 0.04; t(56) = 3.27, p = .002).
Fig. 1A depicts response-locked ERP activity at Cz and Fig. 1B shows the scalp distribution of the error minus correct difference from 0 to 100 ms for the full sample. As depicted, the ERN was observed as a larger negativity in the waveform compared to the CRN (t(56) = 11.24, p < .001). Table 1 reports the means, standard deviations, and ranges for all Time 1 and Time 2 measures, as well as bivariate associations among these variables.
Fig. 1.
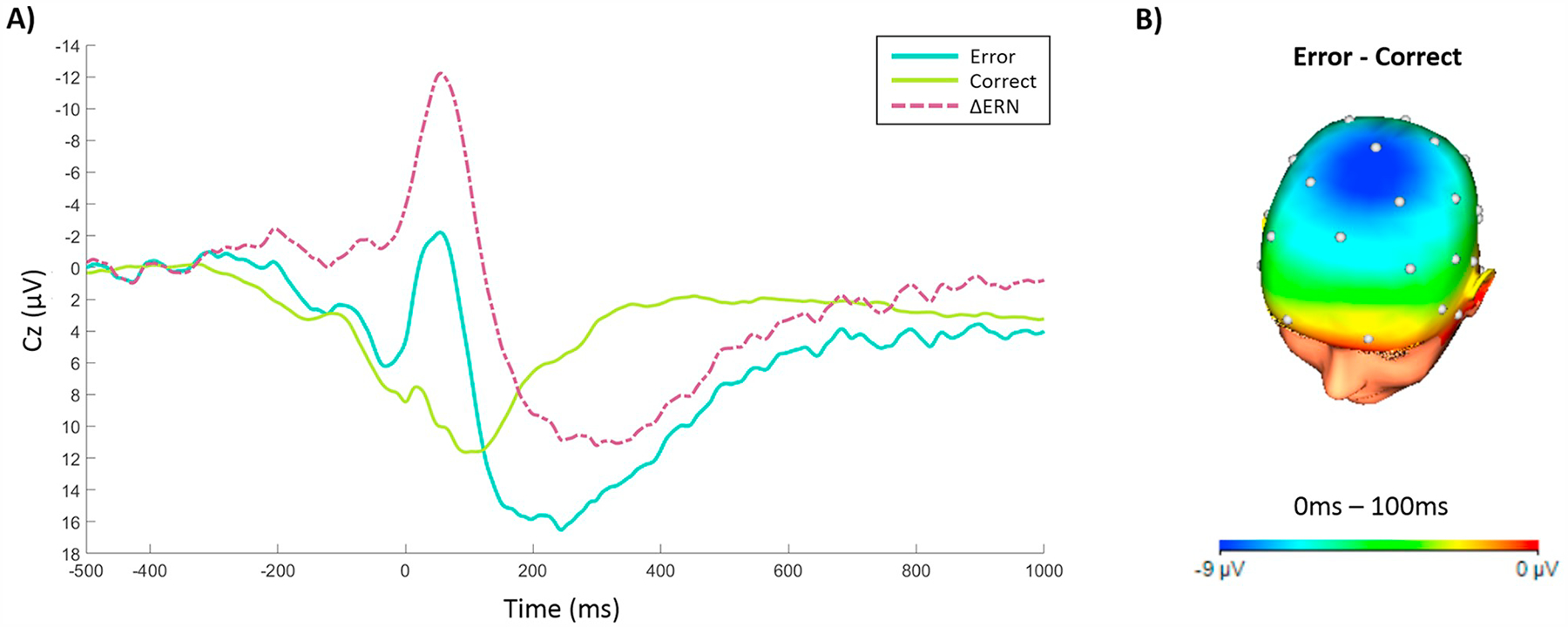
A) Response-locked ERP average waveforms following error and correct responses, as well as the error minus correct difference wave (i.e., ΔERN), at electrode Cz. B) Topographic map depicting the average difference (μV) between error and correct responses from 0 ms to 100 ms post-response onset.
Table 1.
Descriptive statistics and bivariate correlations for neural activity at Time 1, anxiety symptoms at Times 1 and 2, total count of past-year interpersonal and non-interpersonal stressors, and time between assessments.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean | SD | Range | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. ERN (T1) | 0.42 | 7.10 | −17.47–17.61 | |||||||
| 2. CRN (T1) | 0.60** | 9.58 | 6.58 | −7.25–29.86 | ||||||
| 3. ERNresid (T1) | 0.80** | 0.00 | 0.00 | 5.69 | −16.15–16.22 | |||||
| 4. Anxiety symptoms (T1) | −0.16 | −0.18 | −0.06 | 74.84 | 22.20 | 47.00–139.00 | ||||
| 5. Anxiety symptoms (T2) | −0.08 | −0.10 | −0.02 | .0.71 | 65.86 | 16.72 | 44.00–119.00 | |||
| 6. Total past-year interpersonal stressors (T2) | −0.15 | −0.10 | −0.12 | 0.24 | 0.32* | 1.23 | 1.18 | 0.00–5.00 | ||
| 7. Total past-year non-interpersonal stressors (T2) | −0.03 | −0.12 | 0.05 | 0.18 | 0.29* | 0.24 | 3.02 | 2.77 | 0.00–12.00 | |
| 8. Time between symptom assessment (days) | −0.06 | 0.00 | −0.07 | −0.04 | −0.01 | 0.07 | 0.15 | 176.05 | 13.65 | 149.00–208.00 |
Note. T1 = Time one; T2 = Time two; ERN = error-related negativity; ERNresid = error-related negativity residual; CRN = correct-response negativity; SD = standard deviation.
p < .01.
p < .05.
3.3. Moderation analyses
Moderated multiple regression analysis was used to examine whether the magnitude of the ERNresid at the start of the year moderated the effects of total past-year interpersonal and non-interpersonal stress exposure in predicting anxiety symptoms at follow-up, adjusting for baseline anxiety symptoms, gender, and time between symptom assessments (in days). As indicated in Table 2, the ERNresid X total past-year interpersonal stress interaction term significantly predicted anxiety symptoms at follow-up, controlling for the interaction between ERNresid and total past-year non-interpersonal stress exposure. In contrast, the ERNresid X total past-year non-interpersonal stress interaction did not significantly predict anxiety symptoms at Time 2.
Table 2.
Results of a simultaneous multiple regression investigating whether the residual error-related negativity at Time 1 interacts with total past-year interpersonal and non-interpersonal stress exposure to predict anxiety symptoms at Time 2.
| β | t | p | 95% CI | |
|---|---|---|---|---|
| Gender | 0.01 | 0.11 | 0.92 | −8.16, 9.07 |
| Time between symptom assessment (days) | −0.02 | −0.26 | 0.80 | −0.26, 0.20 |
| Anxiety symptoms (T1) | 0.65 | 6.89 | 0.00 | 0.34, 0.63 |
| ERNresid (T1) | 0.35 | 2.01 | 0.05 | −0.001, 2.07 |
| Total past-year interpersonal stress (T2) | −0.04 | −0.36 | 0.72 | −3.81, 2.66 |
| ERNresid (T1) × Total past-year interpersonal stress (T2) | −0.54 | −2.97 | 0.01 | −1.76, −0.34 |
| Total past-year non-interpersonal stress (T2) | 0.20 | 1.98 | 0.05 | −0.02, 2.38 |
| ERNresid (T1) × Total past-year non-interpersonal stress (T2) | 0.10 | 0.59 | 0.56 | −0.11, 0.21 |
| R = 0.79 | R2 = 0.62 | |||
Note. β is a standardized regression coefficient. T1 = Time one; T2 = Time two; ERNresid = error-related negativity residual; CI = confidence interval. The dependent variable is anxiety symptoms at Time 2.
Simple slopes were calculated at small (1 SD above the mean, as the ERN is a negative-going component; M + 1 SD = 5.69), intermediate (mean; M = 0), and large (1 SD below the mean; M − 1 SD = −5.69) residual ERN values; results are reported in Table 3. As hypothesized, the conditional effect of past-year interpersonal stress exposure on Time 2 anxiety scores was significant at large (i.e., more negative) residual ERN magnitude, b = 5.40 (SE = 1.81), p = .004, whereby greater interpersonal stress exposure was associated with more anxiety. In contrast, at smaller (i.e., less negative) residual ERN magnitude, greater stress exposure was significantly associated with fewer symptoms of anxiety, b = −6.57 (SE = 3.16), p = .04. Fig. 2 displays simple slopes, adjusting for effects of gender, time between assessments, baseline anxiety, past-year non-interpersonal stress, and the interaction between past-year non-interpersonal stress and residual ERN magnitude.2
Table 3.
Results of simple slopes analyses showing slope of past-year interpersonal stress at three values of residual ERN magnitude, controlling for effects of gender, time between assessments, non-interpersonal stress experienced over the past year, and the interaction between residual ERN magnitude and past-year non-interpersonal stressors.
| ERN magnitude (μV) | Effect | Standard Error | t | p | 95% CI |
|---|---|---|---|---|---|
| −5.69 (large) | 5.40 | 1.81 | 2.99 | 0.004 | 1.77, 9.04 |
| 0 | −0.58 | 1.61 | −0.36 | 0.72 | −3.82, 2.66 |
| 5.69 (small) | −6.57 | 3.16 | −2.08 | 0.04 | −12.93, −0.21 |
Note. ERN magnitudes −5.69 and 5.69 represent values 1 standard deviation below and above the sample mean, respectively.
Fig. 2.
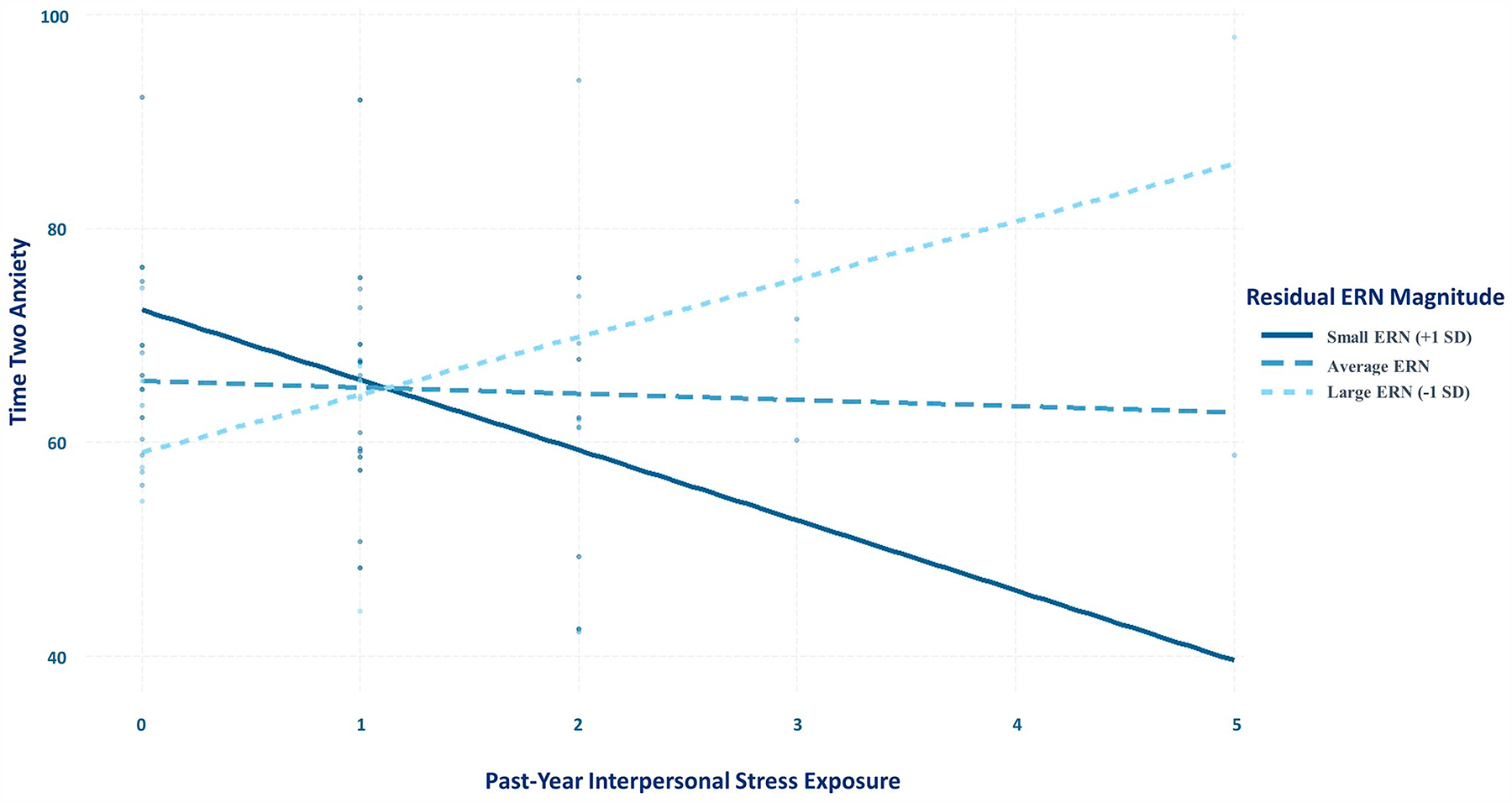
Simple slopes depicting conditional effect of past-year interpersonal stress on Time 2 anxiety at large (−5.69), medium (0), and small (5.69) ERN magnitude values, controlling for Time 1 anxiety, gender, days between assessments, past-year non-interpersonal stress, and the interaction between residual ERN magnitude and past-year non-interpersonal stress.
4. Discussion
In a group of first-year university students, we examined whether ERN magnitude at the start of the academic year interacted with interpersonal stress experienced over the year to predict symptoms of anxiety toward the end of the academic year. As hypothesized, we found evidence for an interaction, whereby, for those individuals with a larger ERN (i.e., more negative values), greater interpersonal stress exposure was significantly associated with more symptoms of anxiety toward the end of the year, even when controlling for the interaction between ERN magnitude and non-interpersonal stress, baseline anxiety symptoms, and relevant demographic factors.
These findings are consistent with research indicating that interpersonal stress is a strong predictor of heightened anxiety (Farmer and Kashdan, 2015; Hamilton et al., 2016; Kendler et al., 2003; Siegel et al., 2009; Uliaszek et al., 2010), but that experiencing interpersonal stress does not always precipitate increases in anxiety (Broeren et al., 2014; Brozina and Abela, 2006). Our results are also consistent with data suggesting that an enhanced ERN is a vulnerability marker for anxiety (Olvet and Hajcak, 2008; Riesel et al., 2011; Riesel et al., 2015), but is not itself a diagnostic marker of anxiety, as it is also seen in first-degree relatives of those with OCD who do not have the disorder (Carrasco et al., 2013; Riesel et al., 2011), is unrelated to OCD symptom severity (Riesel et al., 2014), and is observed among remitted individuals (Hajcak et al., 2008; Kujawa et al., 2016; Riesel et al., 2015). Instead, our results suggest that the interaction between ERN magnitude and interpersonal stress exposure might be particularly potent in predicting later anxiety symptoms – that is, the ERN may represent a latent vulnerability for anxiety that is triggered by stressful experiences (Meyer et al., 2017a).
Although interpersonal and non-interpersonal stress were both significantly associated with increased Time 2 anxiety levels, exploratory analyses revealed that non-interpersonal stress did not significantly interact with ERN magnitude to predict anxiety, suggesting that the characteristics of interpersonal stressors specifically may be particularly important to consider. Humans are motivated to perform well in social settings (Barker et al., 2018; Blascovich et al., 1999; Blascovich and Tomaka, 1996), as errors in interpersonal contexts may threaten safety or social standing (Hajcak, 2012; Lim et al., 2015). Consistent with this finding, research suggests that errors are more significant in social situations than non-interpersonal contexts: The ERN is enhanced when participants are told that their behaviour in error-eliciting tasks is being observed or evaluated (Barker et al., 2015; Buzzell et al., 2017; Hajcak et al., 2005; Kim et al., 2005; Meyer et al., 2019; Schillinger et al., 2016; Van Meel and Van Heijningen, 2010). Performance monitoring may thus be particularly important in stressful social situations relative to situations that are non-interpersonal in nature. And in fact, in our sample, participants experienced fewer interpersonal than non-interpersonal stressors, suggesting that it is the interpersonal qualities of the stressors, as opposed to the number of stressors experienced, that interacts with an enhanced ERN to predict heightened anxiety.
It is possible that individuals who exhibit an enhanced ERN are more emotionally reactive to interpersonal stressors that have a social-evaluative component, which may help to explain why an enhanced ERN interacts with interpersonal (but not non-interpersonal) stress exposure to predict anxiety levels. Indeed, some evidence suggests that individuals with social anxiety – which is associated with an enhanced ERN (Endrass et al., 2014; Kujawa et al., 2016) – are more sensitive and emotionally reactive to daily social stressors than their non-anxious counterparts (Farmer and Kashdan, 2015). Combined with our findings, these data suggest that increased performance monitoring may enhance negative affective responses to social stressors, leading to increased anxiety over time. However, future research is needed to directly test this possibility.
Limitations of the present study suggest avenues for future research. First, although our attrition rate was comparable to those from other similar prospective studies (e.g., LeMoult et al., 2015; McLaughlin et al., 2014; Meyer et al., 2017a; Sandre et al., 2019), we lost a substantial portion of our participants between the in-lab assessment at Time 1 and the follow-up assessment at Time 2. We can only speculate on reasons for this attrition, but possible explanations include university drop-out, a lack of time to complete the Time 2 assessments, or insufficient compensation. Although participants who were lost to follow-up did not differ significantly from those who completed the follow-up session on demographic variables, baseline anxiety symptoms, or ERP values, it is nevertheless possible that our results were impacted by our low retention. It will be important to replicate the present results in a larger sample, and to prevent attrition through methods like increased participant compensation, to address these issues.
Second, participants in our sample were mostly female, and women have been found to experience more interpersonal stressors on the STRAIN (Slavich and Shields, 2018), to respond differently to social stress (Rudolph, 2002; Stroud et al., 2002; Troisi, 2001), and to experience more anxiety than men (Kessler et al., 2005; McLean et al., 2011). Our results may thus reflect the effects of the interaction between performance monitoring and social stress on anxiety mostly for women. Third, our sample was 44% Caucasian, with a median family income that is above the national median (Statistics Canada, n.d.), which may limit the generalizability of our findings. Future studies will need to examine the extent to which these effects extend to more di-verse samples.
Fourth, although there is evidence that responses on the STRAIN are largely independent of participants’ mood state and personality characteristics (Slavich and Shields, 2018), these factors could have nonetheless played a role here. Future studies could seek to replicate these effects using interview-based measures of interpersonal life stress (Hammen, 1991; Hammen et al., 1989). Relatedly, the interpersonal life stress variable we used included a range of stressors that possess different social-psychological characteristics (e.g., social evaluation, isolation, rejection). As a result, it is not clear if the present results are more strongly driven by some interpersonal stressors, or stressor qualities, than others (Slavich, 2019). In addition, since participants were not interviewed about the characteristics of each stressor that they experienced, it is possible that our non-interpersonal stress exposure variable could have included some interpersonal elements (e.g., a major financial problem that, at some point, triggered an interpersonal argument).
Lastly, because we used a composite measure of anxiety symptoms, our results cannot speak to the ability of the ERN and interpersonal stress to interact to predict specific symptoms of anxiety. This composite included symptoms associated with disorders that have been consistently linked to an enhanced ERN (e.g., OCD and SAD symptoms; Carrasco et al., 2013; Endrass et al., 2010; Hajcak and Simons, 2002; Weinberg et al., 2012, 2015b; Endrass et al., 2014; Kujawa et al., 2016), but also symptoms less consistently associated with a heightened ERN (e.g., trauma-related symptoms; Gorka et al., 2016; Khan et al., 2018; Lackner et al., 2018; Meyer et al., 2013; Rabinak et al., 2013; Swick et al., 2015). It is possible that certain categories of anxiety symptoms are better predicted by an interaction between ERN magnitude and interpersonal stress. Future studies looking across anxiety diagnoses in a clinical sample will be important for more fully understanding the specificity of the ERN as a predictor of later anxious dysfunction. However, prior research suggests that the ERN is a transdiagnostic risk marker for anxiety (Meyer, 2016; Riesel et al., 2017; Weinberg et al., 2015a), rather than a marker of specific forms of dysfunction, suggesting that a composite anxiety symptom score is appropriate to investigate our research questions.
In sum, the present results indicate that ERN magnitude at the start of the academic year interacts with past-year interpersonal (but not non-interpersonal) stress exposure to predict anxiety symptoms six months later, controlling for baseline anxiety symptoms. Specifically, experiencing more interpersonal stress was significantly related to subsequently heightened symptoms of anxiety, but only for individuals with an enhanced ERN. These findings are consistent with diathesis-stress models, whereby enhanced error monitoring renders individuals more susceptible to the negative effects of interpersonal stress, enhancing risk for heightened anxiety (Olvet and Hajcak, 2008; Riesel et al., 2011, 2015). This framework can be used by future studies to examine mechanisms through which stress may interact with the ERN to predict anxiety, with the aim of identifying individuals at risk of developing anxiety disorders.
Funding
This work was supported by the Canada Research Chairs Program awarded to Dr. Anna Weinberg, and a Canada Graduate Scholarships - Master’s Program scholarship awarded to Iulia Banica. Dr. George Slavich was supported by a Society in Science—Branco Weiss Fellowship, NARSAD Young Investigator Grant #23958 from the Brain & Behavior Research Foundation, and National Institutes of Health grant K08 MH103443.
Footnotes
Declaration of competing interest
None.
The participants who did not complete the questionnaires at Time 2 (N = 155) did not differ in age (t208 = 0.14, p = .89), ethnicity (χ2(10) = 18.59, p = .05), annual family income (χ2(13) = 13.20, p = .43), or baseline symptoms of anxiety (t209 = 1.45, p = .15) compared to those who completed the questionnaires at Time 2 (N = 57; exclusive of the two participants excluded at Time 2). Gender was a significant predictor of attrition (χ2(1) = 4.28, p = .04), with 108 females and 46 males lost to follow-up versus 48 females and 9 males who completed the follow-up. The magnitude of the ERN and CRN did not differ between participants who did and did not complete the follow-up questionnaires at Time 2 (ERN, t210 = 1.59, p = .11; CRN, t210 = 0.23, p = .82).
The results of the regression were similar, and the effect size for the interaction term was in the same direction and of a similar magnitude, when including the participant excluded for reporting Time 2 anxiety scores more than three SD above the sample mean, though the interaction term was no longer a statistically significant predictor (p = .10).
References
- Adlaf EM, Gliksman L, Demers A, Newton-Taylor B, 2001. The prevalence of elevated psychological distress among Canadian undergraduates: findings from the 1998 Canadian Campus Survey. J. Am. Coll. Heal 50 (2), 67–72. 10.1080/07448480109596009. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykni S, Heath AC, 2008. Heritability of frontal brain function related to action monitoring. Psychophysiology 45, 524–534. 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Antony MM, Stein MB (Eds.), 2008. Oxford Handbook of Anxiety and Related Disorders. Oxford University Press, New York. [Google Scholar]
- Barker TV, Troller-Renfree S, Pine DS, Fox NA, 2015. Individual differences in social anxiety affect the salience of errors in social contexts. Cognitive, Affective, & Behavioral Neuroscience 15 (4), 723–735. 10.3758/s13415-015-0360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TV, Troller-Renfree SV, Bowman LC, Pine DS, Fox NA, 2018. Social influences of error monitoring in adolescent girls. Psychophysiology, e13089 10.1111/psyp.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AJ, Scott KM, Vos T, Whiteford HA, 2013. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol. Med 43 (5), 897–910. 10.1017/S003329171200147X. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, … Nelson B, 1996. Childhood and adolescent depression: a review of the past 10 years. Part I. Journal of the American Academy of Child & Adolescent Psychiatry 35 (11), 1427–1439. 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Tomaka J, 1996. The biopsychosocial model of arousal regulation In: Advances in Experimental Social Psychology. 28 Academic Press, New York, pp. 1–51. 10.1016/S0065-2601(08)60235-X. [DOI] [Google Scholar]
- Blascovich J, Mendes WB, Hunter SB, Salomon K, 1999. Social “facilitation” as challenge and threat. J. Pers. Soc. Psychol 77 (1), 68–77. 10.1037/0022-3514.77.1.68. [DOI] [PubMed] [Google Scholar]
- Bouteyre E, Maurel M, Bernaud JL, 2007. Daily hassles and depressive symptoms among first year psychology students in France: the role of coping and social support. Stress. Health 23 (2), 93–99. 10.1002/smi.1125. [DOI] [Google Scholar]
- Braet C, Vlierberghe LV, Vandevivere E, Theuwis L, Bosmans G, 2013. Depression in early, middle and late adolescence: differential evidence for the cognitive diathesis–stress model. Clinical Psychology & Psychotherapy 20 (5), 369–383. 10.1002/cpp.1789. [DOI] [PubMed] [Google Scholar]
- Brázdil M, Roman R, Daniel P, Rektor I, 2005. Intracerebral error-related negativity in a simple Go/NoGo task. J. Psychophysiol 19 (4), 244–255. 10.1027/0269-8803.19.4.244. [DOI] [Google Scholar]
- Broeren S, Newall C, Dodd HF, Locker R, Hudson JL, 2014. Longitudinal investigation of the role of temperament and stressful life events in childhood anxiety. Dev. Psychopathol 26 (2), 437–449. 10.1017/S0954579413000989. [DOI] [PubMed] [Google Scholar]
- Brook CA, Schmidt LA, 2008. Social anxiety disorder: a review of environmental risk factors. Neuropsychiatr. Dis. Treat 4 (1A), 123–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozina K, Abela JR, 2006. Behavioural inhibition, anxious symptoms, and depressive symptoms: a short-term prospective examination of a diathesis-stress model. Behav. Res. Ther 44 (9), 1337–1346. 10.1016/j.brat.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Burle B, Roger C, Allain S, Vidal F, Hasbroucq T, 2008. Error negativity does not reflect conflict: a reappraisal of conflict monitoring and anterior cingulate cortex activity. J. Cogn. Neurosci 20 (9), 1637–1655. 10.1162/jocn.2008.20110. [DOI] [PubMed] [Google Scholar]
- Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, … Fox NA, 2017. A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety. Journal of the American Academy of Child & Adolescent Psychiatry 56 (12), 1097–1105. 10.1016/j.jaac.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL, 2013. Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depression and Anxiety 30 (1), 39–46. 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V, 2007. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, & Behavioral Neuroscience 7 (4), 367–379. 10.3758/CABN.7.4.367. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, 1991. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J. Abnorm. Psychol 100 (3), 316–336. 10.1037/0021-843X.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cromer KR, Schmidt NB, Murphy DL, 2007. An investigation of traumatic life events and obsessive-compulsive disorder. Behav. Res. Ther 45 (7), 1683–1691. 10.1016/j.brat.2006.08.018. [DOI] [PubMed] [Google Scholar]
- De Bruijn ER, Hulstijn W, Verkes RJ, Ruigt GS, Sabbe BG, 2004. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology 177 (1–2), 151–160. 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- Dehaene S, 2018. The error-related negativity, self-monitoring, and consciousness. Perspect. Psychol. Sci 13 (2), 161–165. 10.1177/1745691618754502. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM, 1994. Localization of a neural system for error detection and compensation. Psychol. Sci 5 (5), 303–305. 10.1111/j.1467-9280.1994.tb00630.x. [DOI] [Google Scholar]
- Dutilh G, van Ravenzwaaij D, Nieuwenhuis S, van der Maas HLJ, Forstmann BU, Wagenmakers E-J, 2012. How to measure post-error slowing: a confound and a simple solution. J. Math. Psychol 56 (3), 208–216. 10.1016/j.jmp.2012.04.001. [DOI] [Google Scholar]
- Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, Kathmann N, 2010. Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biol. Psychol 84 (2), 257–263. 10.1016/j.biopsycho.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Endrass T, Riesel A, Kathmann N, Buhlmann U, 2014. Performance monitoring in obsessive–compulsive disorder and social anxiety disorder. J. Abnorm. Psychol 123 (4), 705–714. 10.1037/abn0000012. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW, 1974. Effects of noise letters upon the identification of a target letter in a non-search task. Percept. Psychophys 16 (1), 143–149. 10.3758/BF03203267. [DOI] [Google Scholar]
- Ethridge P, Weinberg A, 2018. Psychometric properties of neural responses to monetary and social rewards across development. Int. J. Psychophysiol 132, 311–322. 10.1016/j.ijpsycho.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L, 1991. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr. Clin. Neurophysiol 78 (6), 447–455. 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Faravelli C, 1985. Life events preceding the onset of panic disorder. J. Affect. Disord 9(1), 103–105. 10.1016/0165-0327(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Faravelli C, Pallanti S, 1989. Recent life events and panic disorder. Am. J. Psychiatry 146 (5), 622–626. 10.1176/ajp.146.5.622. [DOI] [PubMed] [Google Scholar]
- Farmer AS, Kashdan TB, 2015. Stress sensitivity and stress generation in social anxiety disorder: a temporal process approach. J. Abnorm. Psychol 124 (1), 102–114. 10.1037/abn0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A, 2007. G*Power 3: a fexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Finlay-Jones R, Brown GW, 1981. Types of stressful life event and the onset of anxiety and depressive disorders. Psychol. Med 11 (4), 803–815. 10.1017/S0033291700041301. [DOI] [PubMed] [Google Scholar]
- Fischer AG, Danielmeier C, Villringer A, Klein TA, Ullsperger M, 2016. Gender influences on brain responses to errors and post-error adjustments. Sci. Rep 6, 24435 10.1038/srep24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S, Hood B, 1988. Vulnerability factors in the transition to university: self-re-ported mobility history and sex differences as factors in psychological disturbance. Br. J. Psychol 79 (3), 309–320. 10.1111/j.2044-8295.1988.tb02290.x. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E, 1993. A neural system for error detection and compensation. Psychol. Sci 4 (6), 385–390. 10.1111/j.1467-9280.1993.tb00586.x. [DOI] [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG, 2000. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol. Sci 11 (1), 1–6. 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gorka SM, MacNamara A, Aase DM, Proescher E, Greenstein JE, Walters R, … DiGangi JA, 2016. Impact of alcohol use disorder comorbidity on defensive re-activity to errors in veterans with posttraumatic stress disorder. Psychology of Addictive Behaviors 30 (7), 733–742. 10.1037/adb0000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Burkhouse KL, Afshar K, Phan KL, 2017. Error-related brain activity and internalizing disorder symptom dimensions in depression and anxiety. Depression and Anxiety 34 (11), 985–995. 10.1002/da.22648. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC, 2010. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry 67 (2), 113–123. 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, 2012. What we’ve learned from mistakes: insights from error-related brain activity. Curr. Dir. Psychol. Sci 21 (2), 101–106. 10.1177/0963721412436809. [DOI] [Google Scholar]
- Hajcak G, Simons RF, 2002. Error-related brain activity in obsessive–compulsive undergraduates. Psychiatry Res. 110 (1), 63–72. 10.1016/S0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF, 2003. Anxiety and error-related brain activity. Biol. Psychol 64 (1–2), 77–90. 10.1016/S0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF, 2004. Error-related psychophysiology and negative affect. Brain Cogn. 56 (2), 189–197. 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF, 2005. On the ERN and the significance of errors. Psychophysiology 42 (2), 151–160. 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF, 2008. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. Am. J. Psychiatry 165 (1), 116–123. 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hamilton JL, Potter CM, Olino TM, Abramson LY, Heimberg RG, Alloy LB, 2016. The temporal sequence of social anxiety and depressive symptoms following interpersonal stressors during adolescence. J. Abnorm. Child Psychol 44 (3), 495–509. 10.1007/s10802-015-0049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, 1991. Generation of stress in the course of unipolar depression. J. Abnorm. Psychol 100 (4), 555–561. 10.1037/0021-843X.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen C, Ellicott A, Gitlin M, Jamison KR, 1989. Sociotropy/autonomy and vulnerability to specific life events in patients with unipolar depression and bipolar disorders. J. Abnorm. Psychol 98 (2), 154–160. 10.1037/0021-843X.98.2.154. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Miller N, Haefel GJ, 2004. Cognitive vulnerability-stress theories of depression: examining affective specificity in the prediction of depression versus anxiety in three prospective studies. Cogn. Ther. Res 28 (3), 309–345. 10.1023/B:COTR.0000031805.60529.0d. [DOI] [Google Scholar]
- Harkness KL, Monroe SM, 2016. The assessment and measurement of adult life stress: basic premises, operational principles, and design requirements. J. Abnorm. Psychol 125 (5), 727–745. 10.1037/abn0000178. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Hayden EP, Lopez-Duran NL, 2015. Stress sensitivity and stress sensitization in psychopathology: an introduction to the special section. J. Abnorm. Psychol 124 (1), 1–3. 10.1037/abn0000041. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Vargha-Khadem F, Kirkham FJ, Baldeweg T, 2005. Maturation of action monitoring from adolescence to adulthood: an ERP study. Dev. Sci 8 (6), 525–534. 10.1111/j.1467-7687.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG, 2002. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev 109 (4), 679–709. 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Luxton DD, 2005. Vulnerability-stress models In: Hankin BL, Abela JRZ (Eds.), Development of Psychopathology: A Vulnerability-stress Perspective. Sage Publications, California. [Google Scholar]
- Kelly AC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, … Milham MP, 2009. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex 19 (3), 640–657. 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA, 2003. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch. Gen. Psychiatry 60 (8), 789–796. 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Greenberg PE, 2002. The economic burden of anxiety and stress disorders. In: Neuropsychopharmacology: The Fifth Generation of Progress. 67 pp. 982–992. [Google Scholar]
- Kessler RC, Avenevoli S, Merikangas KR, 2001. Mood disorders in children and adolescents: an epidemiologic perspective. Biol. Psychiatry 49 (12), 1002–1014. 10.1016/S0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62 (6), 593–602. 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Khan NI, Burkhouse KL, Lieberman L, Gorka SM, DiGangi JA, Schroth C, … Proescher E, 2018. Individual differences in combat experiences and error-related brain activity in OEF/OIF/OND veterans. International Journal of Psychophysiology 129, 52–57. 10.1016/j.ijpsycho.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Uno H, Fujita T, 2005. Error-related negativity in children: effect of an observer. Dev. Neuropsychol 28 (3), 871–883. 10.1207/s15326942dn2803_7. [DOI] [PubMed] [Google Scholar]
- Klauke B, Deckert J, Reif A, Pauli P, Domschke K, 2010. Life events in panic disorder—an update on “candidate stressors”. Depression and Anxiety 27 (8), 716–730. 10.1002/da.20667. [DOI] [PubMed] [Google Scholar]
- Kleiner L, Marshall WL, 1987. The role of interpersonal problems in the development of agoraphobia with panic attacks. Journal of Anxiety Disorders 1 (4), 313–323. 10.1016/0887-6185(87)90011-9. [DOI] [Google Scholar]
- Kujawa A, Weinberg A, Bunford N, Fitzgerald KD, Hanna GL, Monk CS, Kennedy AE, Klumpp H, Hajcak G, Phan KL, 2016. Error-related brain activity in youth and young adults before and after treatment for generalized or social anxiety disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 71, 162–168. 10.1016/j.pnpbp.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner CL, Santesso DL, Dywan J, O’Leary DD, Wade TJ, Segalowitz SJ, 2018. Adverse childhood experiences are associated with self-regulation and the magnitude of the error-related negativity difference. Biol. Psychol 132, 244–251. 10.1016/j.biopsycho.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS, 2007. Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev. Sci 10 (6), 874–891. 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA, 2014. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. J. Am. Acad. Child Adolesc. Psychiatry 53 (4), 447–455. 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, South M, Clayson PE, 2011. Sex differences in error-related performance monitoring. Neuroreport 22 (1), 44–48. 10.1097/WNR.0b013e3283427403. [DOI] [PubMed] [Google Scholar]
- Last CG, Barlow DH, O’Brien GT, 1984. Precipitants of agoraphobia: role of stressful life events. Psychol. Rep 54 (2), 567–570. 10.2466/pr0.1984.54.2.567. [DOI] [PubMed] [Google Scholar]
- LeMoult J, Carver CS, Johnson SL, Joormann J, 2015. Predicting change in symptoms of depression during the transition to university: the roles of BDNF and working memory capacity. Cognitive, Affective, & Behavioral Neuroscience 15 (1), 95–103. 10.3758/s13415-014-0305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Hart H, Mehta MA, Simmons A, Mirza K, Rubia K, 2015. Neural correlates of error processing in young people with a history of severe childhood abuse: an fMRI study. Am. J. Psychiatr 172 (9), 892–900. 10.1176/appi.ajp.2015.14081042. [DOI] [PubMed] [Google Scholar]
- Lo SL, 2018. A meta-analytic review of the event-related potentials (ERN and N2) in childhood and adolescence: providing a developmental perspective on the conflict monitoring theory. Dev. Rev 48, 82–112. 10.1016/j.dr.2018.03.005. [DOI] [Google Scholar]
- Luu P, Collins P, Tucker DM, 2000. Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J. Exp. Psychol. Gen 129 (1), 43–60. 10.1037/0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Mayer SE, Prather AA, Puterman E, Lin J, Arenander J, Coccia M, Shields GS, Slavich GM, Epel ES, 2019. Cumulative lifetime stress exposure and leukocyte telomere length attrition: the unique role of stressor duration and exposure timing. Psychoneuroendocrinology 104, 210–218. 10.1016/j.psyneuen.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA, 2009. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol. Psychiatry 65 (5), 445–448. 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA, 2014. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression and Anxiety 31 (10), 834–842. 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG, 2011. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res 45 (8), 1027–1035. 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, 2016. Developing psychiatric biomarkers: a review focusing on the error-re-ated negativity as a biomarker for anxiety. Current Treatment Options in Psychiatry 3 (4), 356–364. 10.1007/s40501-016-0094-5. [DOI] [Google Scholar]
- Meyer A, 2017. A biomarker of anxiety in children and adolescents: a review focusing on the error-related negativity (ERN) and anxiety across development. Developmental Cognitive Neuroscience 27, 58–68. 10.1016/j.dcn.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G, 2012. The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience 2 (1), 152–161. 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Buferd S, … Klein DN, 2013. Increased error-related brain activity in six-year-old children with clinical anxiety. Journal of Abnormal Child Psychology 41 (8), 1257–1266. 10.1007/s10802-013-9762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress JN, Proudft GH, 2014. Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology 51 (7), 602–610. 10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, Klein DN, 2015. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. J. Abnorm. Psychol. 124 (2), 266 10.1037/abn0000044266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Glenn CR, Kujawa AJ, Klein DN, 2016. Error-related brain activity is related to aversive potentiation of the startle response in children, but only the ERN is associated with anxiety disorders. Emotion 17 (3), 487–496. 10.1037/emo0000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Danielson CK, Danzig AP, Bhatia V, Black SR, Bromet E, … Klein DN, 2017a. Neural biomarker and early temperament predict increased internalizing symptoms after a natural disaster. Journal of the American Academy of Child & Adolescent Psychiatry 56 (5), 410–416. 10.1016/j.jaac.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, Hajcak G, 2017b. Considering ERP difference scores as individual difference measures: issues with subtraction and alternative approaches. Psychophysiology 54 (1), 114–122. 10.1111/psyp.12664. [DOI] [PubMed] [Google Scholar]
- Meyer A, Nelson B, Perlman G, Klein DN, Kotov R, 2018. A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. J. Child Psychol. Psychiatry 59 (11), 1162–1170. 10.1111/jcpp.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Carlton C, Chong LJ, Wissemann K, 2019. The presence of a controlling parent is related to an increase in the error-related negativity in 5–7 year-old children. J. Abnorm. Child Psychol 47 (6), 935–945. 10.1007/s10802-018-0503-x. [DOI] [PubMed] [Google Scholar]
- Miller GA, Gratton G, Yee CM, 1988. Generalized implementation of an eye movement correction procedure. Psychophysiology 25 (2), 241–243. 10.1111/j.1469-8986.1988.tb00999.x. [DOI] [Google Scholar]
- Monroe SM, Simons AD, 1991. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol. Bull 110 (3), 406–425. 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N, 2013. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front. Hum. Neurosci 7 (466), 1–19. 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Kneip C, Schroder HS, Larson MJ, 2016. Sex moderates the association between symptoms of anxiety, but not obsessive compulsive disorder, and error-monitoring brain activity: a meta-analytic review. Psychophysiology 53 (1), 21–29. 10.1111/psyp.12509. [DOI] [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH, Berge JMT, 1967. Psychometric Theory, 3rd ed. McGraw-Hill, New York, NY. [Google Scholar]
- Olvet DM, Hajcak G, 2008. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin. Psychol. Rev 28 (8), 1343–1354. 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G, 2009. The stability of error-related brain activity with increasing trials. Psychophysiology 46 (5), 957–961. 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Pegg S, Ethridge P, Shields GS, Slavich GM, Weinberg A, Kujawa A, 2019. Blunted social reward responsiveness moderates the effect of lifetime social stress exposure on depressive symptoms. Front. Behav. Neurosci 13, 178 10.3389/fnbeh.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, 2007. Research review: a neuroscience framework for pediatric anxiety disorders. J. Child Psychol. Psychiatry 48 (7), 631–648. 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA, 2015. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 56 (3), 345–365. 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Holman A, Angstadt M, Kennedy AE, Hajcak G, Phan KL, 2013. Neural response to errors in combat-exposed returning veterans with and without post-traumatic stress disorder: a preliminary event-related potential study. Psychiatry Res. Neuroimaging 213 (1), 71–78. 10.1016/j.pscychresns.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Real E, Labad J, Alonso P, Segalàs C, Jiménez-Murcia S, Bueno B, Menchón JM, 2011. Stressful life events at onset of obsessive–compulsive disorder are associated with a distinct clinical pattern. Depression and Anxiety 28 (5), 367–376. 10.1002/da.20792. [DOI] [PubMed] [Google Scholar]
- Riesel A, 2019. The erring brain: error-related negativity as an endophenotype for OCD—a review and meta-analysis. Psychophysiology 56 (4), e13348 10.1111/psyp.13348. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N, 2011. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. Am. J. Psychiatr 168 (3), 317–324. 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Meyer A, Hajcak G, 2013. The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biol. Psychol 93 (3), 377–385. 10.1016/j.biopsycho.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Riesel A, Kathmann N, Endrass T, 2014. Overactive performance monitoring in obsessive–compulsive disorder is independent of symptom expression. Eur. Arch. Psychiatry Clin. Neurosci 264 (8), 707–717. 10.1007/s00406-014-0499-3. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Auerbach LA, Kathmann N, 2015. Overactive performance monitoring as an endophenotype for obsessive-compulsive disorder: evidence from a treatment study. Am. J. Psychiatr 172 (7), 665–673. 10.1176/appi.ajp.2014.14070886. [DOI] [PubMed] [Google Scholar]
- Riesel A, Goldhahn S, Kathmann N, 2017. Hyperactive performance monitoring as a transdiagnostic marker: results from health anxiety in comparison to obsessive–compulsive disorder. Neuropsychologia 96, 1–8. 10.1016/j.neuropsychologia.2016.12.029. [DOI] [PubMed] [Google Scholar]
- Riesel A, Klawohn J, Grützmann R, Kaufmann C, Heinzel S, Bey K, … Kathmann N, 2019. Error-related brain activity as a transdiagnostic endophenotype for obsessive-compulsive disorder, anxiety and substance use disorder. Psychological Medicine 49 (7), 1207–1217. 10.1017/S0033291719000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, 2002. Gender differences in emotional responses to interpersonal stress during adolescence. J. Adolesc. Health 30 (4, Suppl), 3–13. 10.1016/S1054-139X(01)00383-4. [DOI] [PubMed] [Google Scholar]
- Sandre A, Bagot RC, Weinberg A, 2019. Blunted neural response to appetitive images prospectively predicts symptoms of depression, and not anxiety, during the transition to university. Biol. Psychol 145, 31–41. 10.1016/j.biopsycho.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Sandre A, Banica I, Riesel A, Flake J, Klawohn J, & Weinberg A (revise & resubmit). Comparing the effects of different methodological decisions on the error-related negativity and its association with behavior and gender. Int. J. Psychophysiol [DOI] [PubMed] [Google Scholar]
- Schillinger FL, De Smedt B, Grabner RH, 2016. When errors count: an EEG study on numerical error monitoring under performance pressure. ZDM Mathematic Education 48 (3), 351–363. 10.1007/s11858-015-0746-8. [DOI] [Google Scholar]
- Schlossberg NK, 1989. Marginality and mattering: key issues in building community. New Dir. Stud. Serv (48), 5–15. 10.1002/ss.37119894803. [DOI] [Google Scholar]
- Segalowitz SJ, Dywan J, 2009. Individual differences and developmental change in the ERN response: implications for models of ACC function. Psychological Research PRPF 73 (6), 857–870. 10.1007/s00426-008-0193-z. [DOI] [PubMed] [Google Scholar]
- Siegel RS, La Greca AM, Harrison HM, 2009. Peer victimization and social anxiety in adolescents: prospective and reciprocal relationships. Journal of Youth and Adolescence 38 (8), 1096–1109. 10.1007/s10964-009-9392-1. [DOI] [PubMed] [Google Scholar]
- Simons RF, 2010. The way of our errors: theme and variations. Psychophysiology 47 (1), 1–14. 10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Slavich GM, 2019. Stressnology: the primitive (and problematic) study of life stress exposure and pressing need for better measurement. Brain Behav. Immun 75, 3–5. 10.1016/j.bbi.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Shields GS, 2018. Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): an overview and initial validation. Psychosom. Med 80 (1), 17 10.1097/PSY.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Stewart JG, Esposito EC, Shields GS, Auerbach RP, 2019. The Stress and Adversity Inventory for Adolescents (Adolescent STRAIN): associations with mental and physical health, risky behaviors, and psychiatric diagnoses in youth seeking treatment. J. Child Psychol. Psychiatry 60 (9), 998–1009. 10.1111/jcpp.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada. (n.d.). Table 11-10-0012-01: distribution of total income by census family type and age of older partner, parent or individual. Retrieved from https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1110001201. doi: 10.25318/1110001201-eng. [DOI]
- Steinberg L, 2005. Cognitive and affective development in adolescence. Trends Cogn. Sci 9 (2), 69–74. 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Stemmer B, Segalowitz SJ, Witzke W, Schönle PW, 2004. Error detection in patients with lesions to the medial prefrontal cortex: an ERP study. Neuropsychologia 42 (1), 118–130. 10.1016/S0028-3932(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Stewart JG, Shields GS, Esposito EC, Cosby EA, Allen NB, Slavich GM, Auerbach RP, 2019. Life stress and suicide in adolescents. J. Abnorm. Child Psychol 47, 1707–1722. 10.1007/s10802-019-00534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES, 2002. Sex differences in stress responses: social rejection versus achievement stress. Biol. Psychiatry 52 (4), 318–327. 10.1016/S0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Sturmbauer SC, Shields GS, Hetzel EL, Rohleder N, Slavich GM, 2019. The Stress and Adversity Inventory for Adults (Adult STRAIN) in German: an overview and initial validation. PLoS One 14 (5), e0216419 10.1371/journal.pone.0216419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Honzel N, Turken U, 2015. Intact error monitoring in combat veterans with post-traumatic stress disorder. Psychiatry Res. Neuroimaging 234 (2), 227–238. 10.1016/j.pscychresns.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A, 2001. Gender differences in vulnerability to social stress: a Darwinian perspective. Physiol. Behav 73 (3), 443–449. 10.1016/S0031-9384(01)00459-0. [DOI] [PubMed] [Google Scholar]
- Uliaszek AA, Zinbarg RE, Mineka S, Craske MG, Sutton JM, Grifth JW, Hammen C, 2010. The role of neuroticism and extraversion in the stress–anxiety and stress–depression relationships. Anxiety, Stress, & Coping 23 (4), 363–381. 10.1080/10615800903377264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meel CS, Van Heijningen CA, 2010. The effect of interpersonal competition on monitoring internal and external error feedback. Psychophysiology 47 (2), 213–222. 10.1111/j.1469-8986.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- Wagner BM, Compas BE, 1990. Gender, instrumentality, and expressivity: moderators of the relation between stress and psychological symptoms during adolescence. Am. J. Community Psychol 18 (3), 383–406. [DOI] [PubMed] [Google Scholar]
- Walker EF, Sabuwalla Z, Huot R, 2004. Pubertal neuromaturation, stress sensitivity, and psychopathology. Dev. Psychopathol 16 (4), 807–824. 10.1017/S0954579404040027. [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Kofel E, Chmielewski M, Kotov R, … Ruggero CJ, 2012. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment 19 (4), 399–420. 10.1177/1073191112449857. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G, 2011. Longer term test–retest reliability of error-related brain activity. Psychophysiology 48 (10), 1420–1425. 10.1111/j.1469-8986.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G, 2012. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. J. Abnorm. Psychol 121 (4), 885–896. 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R, Riese A, 2015a. Error-related brain activity in the age of RDoC: a review of the literature. Int. J. Psychophysiol 98 (2), 276–299. 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Kotov R, Hajcak Proudft G, 2015b. Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. J. Abnorm. Psychol 124 (1), 172–185. 10.1037/abn0000019. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, Hajcak G, 2016. Error-related negativity (ERN) and sustained threat: conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology 53 (3), 372–385. 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox P, Winn S, Fyvie-Gauld M, 2005. ‘It was nothing to do with the university, it was just the people’: the role of social support in the first-year experience of higher education. Stud. High. Educ 30 (6), 707–722. 10.1080/03075070500340036. [DOI] [Google Scholar]
- Wiswede D, Münte TF, Goschke T, Rüsseler J, 2009. Modulation of the error-related negativity by induction of short-term negative affect. Neuropsychologia 47 (1), 83–90. 10.1016/j.neuropsychologia.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y,. Fromson JA, 2011. Error-related negativity abnormalities in generalized anxiety disorder and obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry 35 (1), 265–272. 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Young CC, Dietrich MS, 2015. Stressful life events, worry, and rumination predict depressive and anxiety symptoms in young adolescents. Journal of Child and Adolescent Psychiatric Nursing 28 (1), 35–42. 10.1111/jcap.12102. [DOI] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll CA, Kiefaber PD, O’donnell BF, Shekhar A, Hetrick WP, 2004. Haloperidol impairs learning and error-related negativity in humans. J. Cogn. Neurosci 16 (6), 1098–1112. 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]


