Ingestion of Ca2+ alleviates hyperkalemic periodic paralysis (HyperKPP) symptoms by an unknown mechanism. This study shows that lowering extracellular Ca2+ affects muscle contractility when the resting membrane potential is depolarized as in HyperKPP muscles and reduces the efficacy of salbutamol, a β2-adrenergic receptor agonist used to treat HyperKPP.
Abstract
Hyperkalemic periodic paralysis (HyperKPP) manifests as stiffness or subclinical myotonic discharges before or during periods of episodic muscle weakness or paralysis. Ingestion of Ca2+ alleviates HyperKPP symptoms, but the mechanism is unknown because lowering extracellular [Ca2+] ([Ca2+]e) has no effect on force development in normal muscles under normal conditions. Lowering [Ca2+]e, however, is known to increase the inactivation of voltage-gated cation channels, especially when the membrane is depolarized. Two hypotheses were tested: (1) lowering [Ca2+]e depresses force in normal muscles under conditions that depolarize the cell membrane; and (2) HyperKPP muscles have a greater sensitivity to low Ca2+-induced force depression because many fibers are depolarized, even at a normal [K+]e. In wild type muscles, lowering [Ca2+]e from 2.4 to 0.3 mM had little effect on tetanic force and membrane excitability at a normal K+ concentration of 4.7 mM, whereas it significantly enhanced K+-induced depression of force and membrane excitability. In HyperKPP muscles, lowering [Ca2+]e enhanced the K+-induced loss of force and membrane excitability not only at elevated [K+]e but also at 4.7 mM K+. Lowering [Ca2+]e increased the incidence of generating fast and transient contractures and gave rise to a slower increase in unstimulated force, especially in HyperKPP muscles. Lowering [Ca2+]e reduced the efficacy of salbutamol, a β2 adrenergic receptor agonist and a treatment for HyperKPP, to increase force at elevated [K+]e. Replacing Ca2+ by an equivalent concentration of Mg2+ neither fully nor consistently reverses the effects of lowering [Ca2+]e. These results suggest that the greater Ca2+ sensitivity of HyperKPP muscles primarily relates to (1) a greater effect of Ca2+ in depolarized fibers and (2) an increased proportion of depolarized HyperKPP muscle fibers compared with control muscle fibers, even at normal [K+]e.
Introduction
Hyperkalemic periodic paralysis (HyperKPP) is an autosomal dominant disease with nearly complete penetrance. The disease manifests as stiffness or subclinical myotonic discharges before or during periods of episodic muscle weakness or paralysis. Weakness and paralysis are prominent in the limbs, which can incapacitate patients for hours at a time, with the frequency of attacks ranging from 3 to 28 per month (Gamstorp et al., 1957; Miller et al., 2004; Charles et al., 2013). Paralysis is associated with an increased plasma [K+] in some, but not all, cases (Gamstorp et al., 1957; Poskanzer and Kerr, 1961). A key hallmark of the disease is the precipitation of paralytic attacks after potassium ingestion (Gamstorp et al., 1957; Poskanzer and Kerr, 1961; Wang and Clausen, 1976).
HyperKPP is caused by missense mutations in the SCN4A gene that encodes the α-subunit of the skeletal muscle Nav1.4 Na+ channel, which is responsible for generating and propagating action potentials (Cannon, 2006). The mutations cause three major functional defects in the Nav1.4 channel. First, the steady-state activation curve shifts toward more negative membrane potentials (Rojas et al., 1999), which lowers action potential threshold. Second, the steady-state slow inactivation shifts toward less negative membrane potentials, decreasing the extent of NaV1.4 channel inactivation when the membrane is depolarized (Hayward et al., 1999). Under voltage clamp, fast inactivation upon depolarization is impaired in mutant NaV1.4 channels when extracellular K+ ([K+]e) is increased to 10 mM, an effect not observed in normal NaV1.4 channels (Cannon et al., 1991). As a consequence of the first two defects, Nav1.4 channels open at greater frequency at rest and contribute to a large Na+ influx that depolarizes the cell membrane; this may largely explain why the mean resting membrane potential (resting EM) is significantly less negative in HyperKPP compared with wild type muscle fibers (Clausen et al., 2011; Lucas et al., 2014; Ammar et al., 2015). Less slow inactivation, greater depolarization, and the more negative firing threshold combine to facilitate excessive triggering of action potentials (i.e., myotonic discharges). As several action potentials are generated, the [K+]e increases, causing further depolarization that eventually renders the membrane less excitable. Given that HyperKPP muscles have a greater sensitivity to the K+-induced membrane depolarization and force depression compared with normal muscles (Hayward et al., 2008; Clausen et al., 2011; Lucas et al., 2014; Ammar et al., 2015), the ensuing depolarization may eventually trigger muscle paralysis. The reason as to why paralytic attacks last a few minutes to hours, on the other hand, is not fully understood.
Serum levels of Mg2+ and Ca2+ influence symptoms in patients affected by channelopathies. Myotonia caused by defective or blocked ClC-1 Cl− channels worsens when extracellular Ca2+ ([Ca2+]e) or Mg2+ ([Mg2+]e) concentrations are lowered (Skov et al., 2013, 2015). Hypocalcemia has been reported during a paralytic attack in an individual with HyperKPP (Dyken and Timmons, 1963). In another report, a 3-mo period of hypomagnesemia and hypocalcemia associated with severe HyperKPP symptoms was successfully treated with Mg2+ supplementation that normalized blood Ca2+ and Mg2+ levels and alleviated the symptoms (Mankodi et al., 2015). Ingestion or intravenous infusion of Ca2+ can also alleviate paralytic symptoms in HyperKPP patients (Herman and McDowell, 1963; Pearson, 1964).
Reducing [Ca2+]e or [Mg2+]e has two major effects on voltage-gated ion channels, including the NaV1.4 channel. The first effect involves a hyperpolarizing shift of the steady-state activation curve that shifts the action potential threshold closer to more negative resting EM (Frankenhaeuser and Hodgkin, 1957; Frankenhaeuser and Meves, 1958; Costantin, 1968; Campbell and Hille, 1976; Kostyuk et al., 1982; Hahin and Campbell, 1983). This is consistent with the observation that low [Ca2+]e and/or [Mg2+]e enhances myotonic discharges in individuals suffering of myotonia (Skov et al., 2013, 2015). The second effect is a hyperpolarizing shift of the steady-state inactivation curve that increases the extent of inactivation of voltage-gated channels (Frankenhaeuser and Hodgkin, 1957; Hahin and Campbell, 1983). Increased NaV1.4 channel inactivation will exacerbate muscle weakness in a large number of depolarized fibers.
It has also been proposed that the CaV1.1 channel, the t-tubular voltage sensor for Ca2+ release, has a Ca2+ extracellular binding site; the channel inactivates much faster in the absence of Ca2+ at the site, especially when the membrane is already depolarized resulting in a decrease in Ca2+ release (i.e., an inactivation of excitation-contraction coupling; Brum et al., 1988a; Dulhunty and Gage, 1988; Pizarro et al., 1989). Conceivably, this might be another mechanism by which lower [Ca2+]e depresses force during prolonged cell membrane depolarization caused by an increase in [K+]e.
Partial or complete removal of Ca2+ from solution has little effect on twitch and tetanic force in normal muscles (Dulhunty and Gage, 1988; Cairns et al., 1998; Zhao et al., 2005; Cairns and Lindinger, 2008). However, the studies on force were conducted using normal muscles under controlled conditions (i.e., normal resting EM), whereas the Ca2+ effects on ion channels described above are expected to be more pronounced on force if the cell membrane is depolarized. Indeed, lowering [Ca2+]e from 1.3 to 0.5 mM increases the force loss at 8 mM in mouse soleus tested at 25°C (Cairns et al., 2015). Considering that the mean resting EM is much less negative in HyperKPP than in wild type muscle (Lehmann-Horn et al., 1983; Ricker et al., 1989; Ammar et al., 2015), HyperKPP muscles may be expected to be more sensitive to reduced [Ca2+]e.
The first objective of this study was to test two hypotheses: (1) lowering [Ca2+]e depresses force in normal muscles under conditions that cause a membrane depolarization; and (2) HyperKPP muscles have a greater sensitivity to the Ca2+-induced force depression because their fibers are more depolarized, even at a normal [K+]e. To test the hypotheses, we exposed wild type and HyperKPP mouse soleus and extensor digitorum longus (EDL) muscles to various [K+]e known to depress force and determine whether lowering [Ca2+]e shifts the tetanic force–[K+]e relationship toward lower [K+]e.
At the present time, treatments for patients suffering of HyperKPP are not fully effective and sometimes lose efficacy over time (Clausen et al., 1980; Lehmann-Horn et al., 1983). For example, inhalation of salbutamol, a β2-adrenergic receptor agonist, constituted a satisfactory treatment for 6 out of 10 HyperKPP patients, the treatment being inadequate in four other patients (Clausen et al., 1980). It is possible that one reason for the lack of full efficacy is related to the patient calcemic state, for which hypocalcemia lowers treatment efficacy. Considering this, the second objective of this study was to test a third hypothesis: lowering [Ca2+]e reduces the efficacy of any treatment for HyperKPP. To test this hypothesis, we measured the effect of lowering [Ca2+]e on the efficacy of salbutamol to improve force generation in HyperKPP mouse muscle.
Materials and methods
Animals and approval for animal studies
HyperKPP mice (strain FVB.129S4(B6)-Scn4atm1.1Ljh/J) were generated by knocking in the equivalent of human missense mutation M1592V into the mouse genome (i.e., at position 1585), as previously described by Hayward et al. (2008). The FVB strain was used as wild type mouse. All mice were 1–2 mo old and weighed 20–25 g. The homozygous mutants generally do not survive beyond postnatal day 5, so knock-in mice were maintained as heterozygotes by crossbreeding with FVB mice. Mice were fed ad libitum and housed according to the guidelines of the Canadian Council for Animal Care. The Animal Care Committee of the University of Ottawa approved all experimental procedures used in this study. Prior to muscle excision, mice were anaesthetized with a single intraperitoneal injection of 65 mg ketamine/13 mg xylazine/2 mg acepromazine per kilogram body weight and sacrificed by cervical dislocation. EDL and soleus were then dissected out.
Genotyping
A 2-mm tail piece was incubated overnight with 500 µl tail digestion buffer (0.2 mM Na2EDTA and 25 mM NaOH, pH 12.3) and 50 µl Proteinase K (1 mg/ml) at 56°C. DNA extraction involved the addition of 650 µl of 1:1 phenol/chloroform:isoamyl alcohol (CIA) and centrifuged at 12,000 g for 10 min. Twice, 650 µl CIA was added to the pellet and centrifuged before suspending the resulting pellet in 750 µl isopropyl alcohol. After 10 min, the solution was centrifuged 15 min at 15,000 g. The alcohol was removed and the pellet was suspended in 750 µl 70% ethanol and centrifuged. After removing the alcohol, the pellet was left to dry for 30 min before addition of 200 µl TE buffer (10 mM Tris and 1 mM EDTA, pH 8.0) and incubated at 65°C for 2 h. PCR was then completed using the previously extracted DNA and the following primers: NC1F (forward), 5′-TGTCTAACTTCGCCTACGTCAA-3′; NC2R (reverse), 5′-GAGTCACCCAGTACCTCTTTGG-3′. PCR products were digested 6 h using the restriction digest enzyme NspI. The mutation that is knocked into HyperKPP mice causes the removal of one NspI cut site that is easily detected by agarose gel electrophoresis; two bands were visualized for wild type mice, which carry the cut site on both alleles, and three bands were seen for heterozygous HyperKPP mice harboring one normal allele and one mutant allele.
Physiological solutions
Control solution contained (in mM) 118.5 NaCl, 4.7 KCl, 2.4 CaCl2, 3.1 MgCl2, 25 NaHCO3, 2 NaH2PO4, and 5.5 D-glucose. Solutions containing different K+ concentrations were prepared by adding the appropriate amount of KCl. In one series of experiments, we tested the effect of a hypocalcemic condition; solutions containing lower [Ca2+]e (i.e., 1.3 and 0.3 mM) were prepared by adding less CaCl2. In a second series of experiments at 0.3 mM Ca2+, MgCl2 was increased to 5.2 mM to maintain the divalent cation content constant. Solutions containing 5 µM salbutamol were prepared by adding the proper amount of salbutamol. Solutions were continuously bubbled with 95% O2/5% CO2 to maintain a pH of 7.4. Experimental temperature was 37°C. Total flow of solutions in the muscle chamber was 15 ml/min being split just above and below the muscle in order to prevent any buildup of reactive oxygen species, which is quite large at 37°C and deleterious in terms of force generation (Edwards et al., 2007).
Stimulation
Electrical stimulations were applied across two platinum wires (4 mm apart) located on opposite sides of the fibers. They were connected to a Grass S88X stimulator and a Grass stimulation isolation unit (Grass Technologies/Astro-Med). Tetanic contractions were elicited with 200 ms trains of 0.3 ms and 10-V (supramaximal voltage) pulses at 140 and 200 Hz for soleus and EDL, respectively.
Force measurement
Muscles were positioned horizontally in a Plexiglas chamber. One end of the muscle was fixed to a stationary hook, while the other end was attached to a force transducer (model 400A; Aurora Scientific Canada). The transducer was connected to a KCP13104 data acquisition system (Keithley), and data were recorded at 5 kHz.
Muscle length was adjusted to give maximal tetanic force while muscles were stimulated every 100 s. After a 30 min equilibrium period, the time interval between contractions was increased to 5 min while determining how changes in [K+]e, [Ca2+]e, [Mg2+]e, and salbutamol affected tetanic contractions. Two protocols were used.
Protocol 1: Effects of Ca2+ on the tetanic force–[K+]e relationship
Following the equilibrium period in the presence of 4.7 mM K+ and 2.4 mM Ca2+, [Ca2+]e was either maintained at 2.4 mM Ca2+ or decreased to 1.3 or 0.3 mM. After 45 min, [K+]e was increased to various levels (between 6 and 14 mM), while 5 µM salbutamol was added 45 min later. To determine the reversibility of the K+ and Ca2+ effects, muscles were exposed to the original conditions of 4.7 mM K+ and 2.4 mM Ca2+.
Protocol 2: Effects of K+ on the tetanic force–[Ca2+]e relationship
Following the equilibrium period, [K+]e was either maintained at 4.7 mM or increased to 8 or 12 mM for 45 min, and [Ca2+]e was decreased to 1.3 mM and later to 0.3 mM.
Tetanic force, defined as the force developed during a tetanic contraction, was calculated as the difference between the maximum force during a contraction and the force measured 5 ms before the contraction was elicited. Unstimulated force, or the force generated in the absence of any electrical stimulation, was defined as the force measured 5 ms before a stimulation and calculated as the difference in unstimulated force before a contraction and the unstimulated force at the start of an experiment. Fade, defined as the decrease in force during the plateau phase after force had reached a peak, was calculated as the difference in force at 200 ms (the time of the last stimulation) and peak force, and expressed as a percentage of peak force within the same contraction.
Resting EM and action potential measurements
Resting EM and action potential were measured using glass microelectrodes. Microelectrodes tip resistances were 7–10 MΩ and that of the reference electrode was ∼1 MΩ. All electrodes were filled with 3 M KCl. A recording was rejected when the change in potential upon penetration was not a sharp drop or when the microelectrode potential did not return to zero upon withdrawal from the fiber. Stimulating electrodes for force measurements were disconnected. Instead, single action potentials were elicited using fine platinum wires 2 mm apart placed as close as possible to the surface fibers and moved laterally to stimulate fibers in the region where the microelectrode was positioned. This approach was used to reduce the extent of contracting fibers and prevent the breaking or dislodging of microelectrodes. To further reduce the number of contracting fibers, action potentials were triggered using a single 2–10-V, 0.3-ms square stimulating pulse; a low voltage was usually enough to trigger an action potential at 4.7 mM K+, while higher voltages were necessary at high [K+]e and/or low [Ca2+]e. When an action potential was not triggered, voltage was always increased to 10 V to properly verify that the fiber was unexcitable. Data were digitized using a KCP13104 data acquisition system (Keithley) at 400 kHz.
Protocol 3: Effects of Ca2+ and K+ on membrane potential
All muscles were first exposed to 4.7 mM K+ and 2.4 mM Ca2+ (equilibrium period of 30 min). [Ca2+]e was either maintained at 2.4 mM or reduced to 0.3 mM for 30 min. [K+]e was increased to 10 mM K+ and later to 5 µM salbutamol. Data for salbutamol were divided into early measurements (first 10 min) and late measurements (15–30 min) because the increase in force reached a peak within 10 min and decreased thereafter (Fig. 1). The reversibility of the K+ and Ca2+ effects was tested by returning to the initial conditions (i.e., 4.7 mM K+ and 2.4 mM Ca2+).
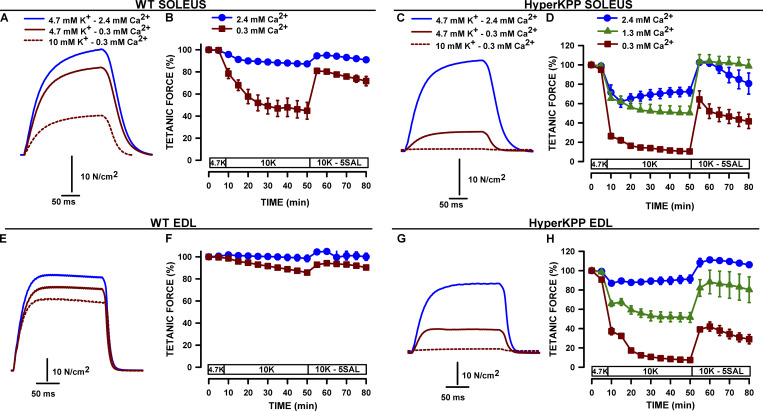
Figure 1.
Lowering [Ca2+]e enhanced the K+-induced force depression in wild type and HyperKPP soleus and EDL, the effects being greater in HyperKPP. After 45 min at 4.7 mM K+ and 2.4, 1.3, or 0.3 mM Ca2+, [K+]e was increased to 10 mM. All muscles were later exposed to 5 µM salbutamol (SAL; protocol 1 in Materials and methods). (A, C, E, and G) Examples of tetanic force traces. (B, D, F, and H) Changes in mean tetanic force over time. To clearly show the Ca2+ effects on the K+-induced force depression, mean tetanic force is expressed as a percent of the force measured at the [Ca2+]e just before the increase in [K+]e. Vertical bars represent the SE for five or six muscles.
The number of excitable fibers, resting EM, action potential overshoot, and maximum rate of depolarization (dV/dt) were determined in 10–20 fibers in each muscle and for each experimental condition. Overshoot was taken from the action potential peak, while dV/dt was first calculated by linear regression analysis of every 10 data points and taken from the peak value. Values from the same muscle were averaged, and the final mean was calculated from the muscle average values. For these parameters, the number of samples is reported as “number of tested fibers/number of muscles used.”
Statistics
Data are expressed as mean ± SE. The Fisher exact test was used to determine whether shifts in the frequency distribution of resting EM by changes in [K+]e, [Ca2+]e, and salbutamol were significant. For all other measurements, split-plot ANOVA designs were used to determine statistical differences, because as a consequence of the experimental design, we had two error terms (Steel and Torrie, 1980). Comparisons between wild type and HyperKPP involved muscles from different mice, and thus, the data were independent from one another. In this case, the SE for statistical differences was the population SE (whole plot). Comparisons between [K+]e, [Ca2+]e, [Mg2+]e, and salbutamol involved data from the same muscles, and thus, data were not independent from one another. In this case, the SE for statistical differences was the SE between muscles (split plot). Calculations were made using the GLM (general linear model) procedures of Statistical Analysis Software version 9.4 (SAS Institute). When a main effect or an interaction was significant, the least square difference (LSD) was used to locate the significant differences. The word “significant” refers only to a statistical difference (P < 0.05).
Results
Effect of lowering [Ca2+]e on tetanic force at normal [K+]e
All muscles were initially exposed to 4.7 mM K+ and 2.4 mM Ca2+. Under those conditions, the mean ± SE tetanic force values of wild type and HyperKPP EDL muscles were 37.8 ± 1.4 and 36.8 ± 1.6 N/cm2, respectively; for wild type and HyperKPP soleus, mean ± SE values were 34.8 ± 1.0 and 31.7 ± 1.0 N/cm2, respectively. After a 30-min equilibrium, [Ca2+]e was either maintained at 2.4 mM or decreased to 1.3 or 0.3 mM for 45 min (the effects of lowering [Ca2+]e while [K+]e is 4.7 mM is described in the next section).
Increasing [K+]e from 4.7 to 10 mM depressed mean tetanic force of wild type soleus by 55% at 0.3 mM Ca2+, a decrease much greater than the 13% decrease at 2.4 mM Ca2+ (Fig. 1, A and B). For HyperKPP soleus, the decreases were even greater, being 27%, 50%, and 90% at 2.4, 1.3, and 0.3 mM Ca2+, respectively (Fig. 1, C and D). A similar situation was observed for EDL. Increasing [K+]e to 10 mM reduced mean tetanic force of wild type EDL by 2% at 2.4 mM Ca2+ and 14% at 0.3 mM Ca2+ (Fig. 1, E and F), while the decreases for HyperKPP EDL were 9%, 48%, and 93% at 2.4, 1.3, and 0.3 mM Ca2+, respectively (Fig. 1, G and H). After 45 min at elevated [K+]e, muscles were exposed to 5 µM salbutamol. As previously reported (Clausen et al., 2011), the salbutamol addition allowed for a full force recovery in wild type soleus and EDL at 2.4 mM Ca2+. However, at 0.3 mM Ca2+, recovery was only partial. For HyperKPP muscle, force recovery was almost complete at 1.3 and 2.4 mM Ca2+, but not at 0.3 mM.
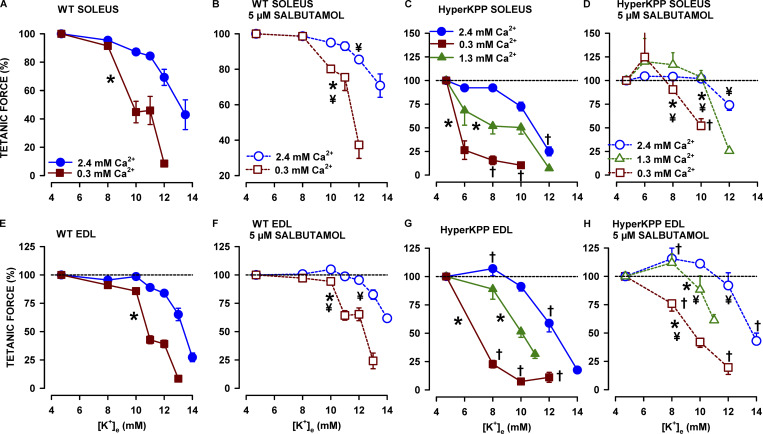
The major effect of lowering [Ca2+]e was a significant shift of the tetanic force–[K+]e relationship toward lower [K+]e, the effect being greater in HyperKPP than in wild type soleus and EDL (Fig. 2). Notably, the K+-induced force depressions in HyperKPP muscles at 1.3 mM Ca2+ were often as large as those in wild type muscles but at 0.3 mM Ca2+. For example, at 10 mM K+, mean tetanic force of HyperKPP soleus had decreased by 50% at 1.3 mM Ca2+ (Fig. 2 C) compared with 55% for wild type soleus, but at 0.3 mM Ca2+ (Fig. 2 A). Similarly at 10 mM, the force decrease for HyperKPP EDL at 1.3 mM Ca2+ was similar to that for wild type EDL at 0.3 mM (Fig. 2, E and G). Salbutamol allowed for force recovery at all [K+]e (Fig. 2, B, D, F, and H). On the one hand, salbutamol reduced the difference in the tetanic force–[K+]e relationships between [Ca2+]e. On the other hand, the lower [Ca2+]e, the less effective salbutamol was. As a final note, the K+-induced force depression at a given [Ca2+]e was always greater for HyperKPP than in wild type EDL, as previously reported (Hayward et al., 2008; Clausen et al., 2011; Ammar et al., 2015).
Figure 2.
Lowering [Ca2+]e significantly shifted the tetanic force-[K+]e relationship toward lower [K+]e, the effect being greater for HyperKPP than wild type soleus and EDL. Changes in [K+]e and [Ca2+]e were as described for protocol 1. To clearly show the Ca2+ effects on the K+-induced force depression, mean tetanic force is expressed as a percentage of the force measured after a 45-min exposure period to 2.4, 1.3, or 0.3 mM Ca2+; that is, just before the increase in [K+], as shown in Fig. 1. The tetanic force–[K+]e relationship (no salbutamol; A, C, E, and G) was obtained using the force measured 45 min after the increase in [K+]e (time 50 min; Fig. 1). The tetanic force–[K+]e relationship in the presence of 5 µM salbutamol (B, D, F, and H) was obtained using the force measured 10 min after salbutamol addition. For clarity, the relationships in the absence and presence of salbutamol were separated. The dashed horizontal line indicates 100%. Vertical bars represent the SE of five to nine muscles. *, Force–[K+]e relationship was significantly shifted to lower [K+]e compared with 2.4 mM Ca2+. †, Mean tetanic force of HyperKPP muscles was significantly different from that of wild type. ¥, Force–[K+]e relationship was significantly shifted to higher [K+]e by salbutamol. ANOVA and LSD, P < 0.05.
Effect of increasing [K+]e on the [Ca2+]e–tetanic force relationship
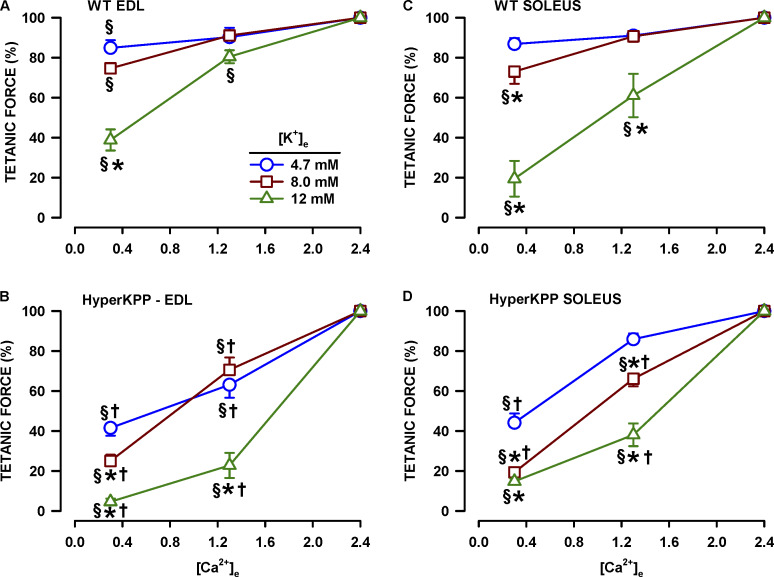
To further appreciate the effects of a reduction in [Ca2+]e, we performed other experiments in which [K+]e was kept at 4.7 mM or increased to either 8 or 12 mM while [Ca2+]e was 2.4 mM, and then [Ca2+]e was lowered from 2.4 to 1.3 to 0.3 mM. For each [K+]e, mean tetanic force at 1.3 and 0.3 mM [Ca2+]e was expressed as a percentage of the force at 2.4 mM [Ca2+]e. At 4.7 mM K+, mean tetanic force of wild type EDL and soleus decreased to only 90–91% when [Ca2+]e was lowered to 1.3 mM and to 85–87% at 0.3 mM (Fig. 3, A and C). The depressing effect of lowering [Ca2+]e was accentuated when [K+]e was increased. This was especially evident at 12 mM K+; at 0.3 mM Ca2+, mean tetanic force had decreased to 39% for EDL and 19% for soleus.
Figure 3.
Increasing [K+]e significantly enhanced the tetanic force depression at low [Ca2+], the effect being greater for HyperKPP than wild type soleus and EDL. Changes in [K+]e and [Ca2+]e were as described for protocol 2. To clearly show how K+ affects the Ca2+–tetanic force relationship, mean tetanic force is expressed as a percent of the force measured after a 45-min exposure to 4.7, 8, or 12 mM K+; that is, just before the decrease in [Ca2+]e. Vertical bars represent the SE of three to five muscles. §, Mean tetanic force significantly different from 100%. *, Mean tetanic force significantly different from that at 4.7 mM K+. †, Mean tetanic force of HyperKPP muscles significantly different that of wild type (same [K+]e and [Ca2+]e). ANOVA and LSD, P < 0.05.
The [Ca2+]e effects were significantly greater in HyperKPP EDL and soleus muscles (Fig. 3, B and D). For example, at 4.7 mM K+, lowering [Ca2+]e from 2.4 to 0.3 mM Ca2+ reduced mean tetanic force of HyperKPP soleus to 44% compared with 86% for the wild type counterpart. A similar situation was observed for 8 and 12 mM K+. Similar to wild type muscles, an increased [K+]e accentuated the depressing effect of lowering [Ca2+]e, but the decreases were significantly greater in HyperKPP muscles.
Effects of lowering [Ca2+]e on the plateau phase
As shown in Fig. 1 E, EDL force in wild type EDL increased rapidly during a tetanus, reaching on average a peak at 103 ± 2 ms. Thereafter, a small decrease in force occurred until the stimulation was stopped. Fade represents the force drop after peak force had been reached and was calculated as the difference in force between peak force and the force at 200 ms (end of the stimulation period). At 4.7 mM K+, fade in wild type EDL was 3% at both 0.3 and 2.4 mM Ca2+ (Fig. 4 A). Fade increased as [K+]e was raised, becoming significantly different from that at 4.7 mM K+ when [K+]e reached 8 and 12 mM K+ while [Ca2+]e was 0.3 and 2.4 mM, respectively. In general, fade appeared smaller in HyperKPP (Fig. 4 B) than in wild type EDL. However, peak force often occurred late during a tetanus in HyperKPP EDL (Fig. 1 G). On average, time to peak force was 172 ± 5 ms and often became longer at elevated [K+]e (data not shown), and therefore the time difference between time to peak and 200 ms was much shorter, giving rise to an apparent smaller fade. Contrary to tetanic force, the addition of 5 µM salbutamol had little alleviating effect on fade with very few significant effects. In regard to wild type and HyperKPP soleus, peak force occurred very late during a tetanus (Fig. 1, A and C), and thus little or no fade was observed for those muscles (data not shown).
Figure 4.
Lowering [Ca2+]e reduced the capacity of wild type and HyperKPP EDL to maintain a plateau phase at elevated [K+]e. Fade represents the force drop after peak force had been reached, was calculated as the difference in force between peak force and the force at 200 ms (end of the stimulation period), and was expressed as a percentage of the peak force. Changes in [K+]e and [Ca2+]e were according to protocol 1. The control fade–[K+]e relationship (i.e., no salbutamol [SAL]) was obtained using the force measured 45 min after the increase in [K+]e (time 50 min; Fig. 1). The fade–[K+]e relationship in the presence of 5 µM salbutamol was obtained using the force measured 10 min after the salbutamol addition. Vertical bars represent the SE of five to nine muscles. §, [K+]e at which mean fade became significantly greater than at 4.7 mM K+. *, Mean fade was significantly different from that at 2.4 mM Ca2+. ¥, Significant effect of salbutamol. ANOVA and LSD, P < 0.05.
Effect of lowering [Ca2+]e on unstimulated force and contractures
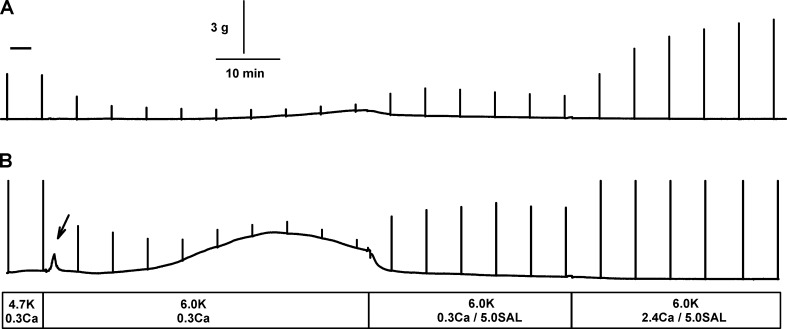
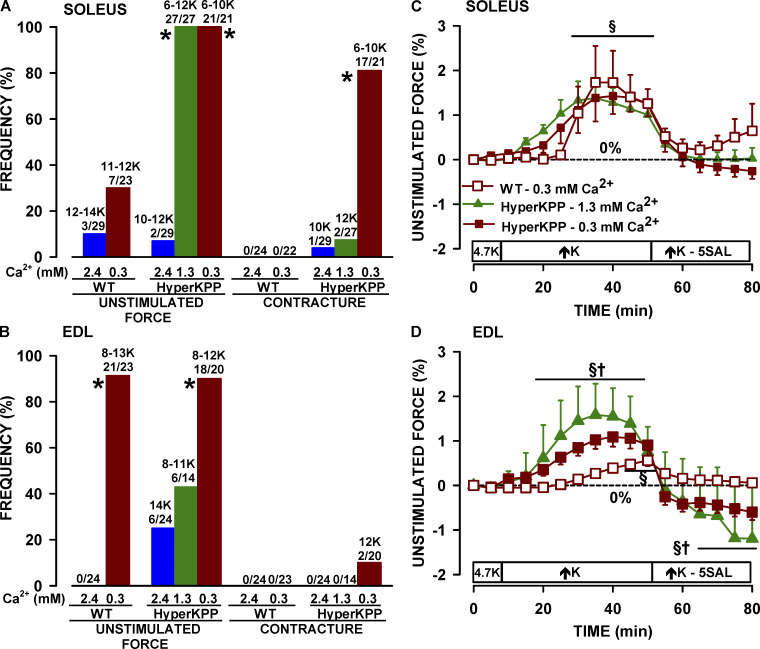
Another aspect of lowering [Ca2+]e was increases in force between elicited contractions (i.e., in the absence of any electrical stimulation). Two types of force increase were observed. The first type was a slow and continuous increase in baseline, named here as unstimulated force, that was either very small (Fig. 5 A) or quite large (Fig. 5 B). The second type was a fast and transient increase in baseline, here named contracture (Fig. 5 B). Addition of 5 µM salbutamol was always followed by a rapid decrease in unstimulated force. At 2.4 mM Ca2+, the occurrence of unstimulated force was small when [K+]e was increased in wild type and HyperKPP soleus and EDL (Fig. 6, A and B). A much greater number of muscles generated unstimulated force when [K+]e was increased from 4.7 to 6–14 mM K+ when [Ca2+]e was 1.3 mM; that is, all (27 of 27) HyperKPP solei and 6 out of 14 EDL (43%). At 0.3 mM Ca2+, an increase in [K+]e resulted in 7 of 23 (30%) wild type solei, 21 out of 23 (91%) wild type EDL, all HyperKPP solei, and 18 out of 20 (90%) HyperKPP EDL generating unstimulated force. The occurrence of contractures was much smaller than for unstimulated force and was prominent only in HyperKPP soleus at 0.3 mM Ca2+.
Figure 5.
Examples of force increase between elicited contractions in HyperKPP soleus when [K+]e was increased from 4.7 to 6 mM while [Ca2+]e was 0.3 mM. (A) Example of a slow increase in unstimulated force being in this case small and late after the increase in [K+]e. (B) Example of a fast and transient contracture (see arrow) followed by a large and slow increase in unstimulated force. Notice the decrease in unstimulated force following the addition of 5 µM salbutamol. The chart recorder trace was generated by averaging three data points every second for the baseline and drawing a vertical line from the minimum to the maximum force when a contraction was elicited.
Figure 6.
Lowering [Ca2+]e increased the occurrence frequency of unstimulated force and contractures. Occurrence frequency of unstimulated force and contractures in soleus (A) and EDL (B). Numbers at the top of the bars represent the range of [K+]e (in mM) that we observed an unstimulated force/contracture and the number of muscles that generated unstimulated force/contractures over the total number of muscles tested. Mean unstimulated force of soleus (C) and EDL (D). Unstimulated force was calculated from muscles that generated it and is expressed as a percentage of the tetanic force measured at 4.7 mM K+ and 2.4 mM Ca2+. Data from different [K+]e were pooled, because the magnitude of the unstimulated forces were not significant. *, Frequency of unstimulated force or contractures (A and B) was significantly different from that at 2.4 mM Ca2+. §, Mean unstimulated force was significantly different from that at time 0 min (C and D). †, Mean unstimulated force of HyperKPP muscles was significantly different that of wild type (C and D). ANOVA and LSD, P < 0.05.
Effects of lowering [Ca2+]e on membrane excitability
Fiber excitability
An excitable fiber was defined as a fiber generating an action potential upon stimulation (Fig. 7 A, i and ii), even when the action potential peak is just −30 mV (Fig. 7 A iii). An unexcitable fiber was defined as a fiber for which the membrane potential did not change at all following a stimulation (Fig. 7 A iv). As previously reported (Ammar et al., 2015), mean excitability was significantly less in HyperKPP than in wild type fibers regardless of [K+]e and [Ca2+]e (Fig. 7). At 4.7 mM K+ and 2.4 mM Ca2+, all penetrated wild type soleus fibers generated an action potential, whereas mean excitability of HyperKPP fibers was 85%, a mean significantly less than for wild type (Fig. 7 B i). Lowering [Ca2+]e to 0.3 mM caused a small but significant decrease in mean excitability to 89% in wild type but a substantially larger decrease to 29% for HyperKPP. An increase in [K+]e to 10 mM significantly reduced membrane excitability to a greater extent at 0.3 than at 2.4 mM Ca2+, completely abolishing excitability in HyperKPP fibers at 0.3 mM Ca2+ (Fig. 7 B, ii and iii). Considering that the increase in force following the addition of 5 µM salbutamol reached a peak within 10 min and is followed by a slow decline (Fig. 1), the salbutamol effect on membrane excitability was measured first within 10 min and then between 15 to 30 min. Adding 5 µM salbutamol resulted in small but significant transient increase excitability of wild type (2.4 mM Ca2+ only) and HyperKPP (0.3 and 2.4 mM Ca2+) fibers. At the end of all experiments, [K+]e and [Ca2+]e were respectively returned to 4.7 and 2.4 mM Ca2+. For fibers constantly exposed to 2.4 mM Ca2+, mean excitability completely returned to the initial mean measured at the start of the experiment (Fig. 7 B iv). However, for fibers exposed to 0.3 mM Ca2+, mean excitability increased but remained significantly less than the mean values measured at the start of the experiments. Overall, lowering [Ca2+]e increases the extent of fiber inexcitability to a greater extent in HyperKPP than in wild type soleus.
Figure 7.
Lowering [Ca2+]e significantly enhanced the K+-induced depression of membrane excitability to a greater extent in HyperKPP than in wild type soleus. (A) Examples of action potential traces generated by excitable fibers upon stimulation. Resting EM: −80 mV (i), −61 mV (ii), −54 mV (iii), −52 mV (iv); the last trace being an example of an unexcitable fiber that failed to generate an action potential. The little square change in EM preceding action potential was the stimulation artifact. (B) Mean fiber excitability expressed as a percentage of the number of fibers tested. Changes in [K+]e and [Ca2+]e were as described in protocol 3. Data for salbutamol (SAL) were divided into early measurements (first 10 min, E) and late measurements (15–30 min, L). The reversibility of the K+ and Ca2+ effects was tested by returning to the initial conditions (i.e., 4.7 mM K+ and 2.4 mM Ca2+). Vertical bars represent the SE of 71–144 fibers/5–6 muscles for high [K+]e, 277 fibers/26 muscles for wild type and 273 fibers/18 muscles for HyperKPP at 4.7 mM K+ and 2.4 mM. §, At similar [Ca2+]e, mean excitability significantly different from that at 4.7 mM K+ measured at the beginning of an experiment (i.e., in B ii). *, At similar [K+]e, mean excitability at 0.3 mM Ca2+ significantly different from that at 2.4 mM. †, At similar [K+]e and [Ca2+]e, mean excitability of HyperKPP fibers significantly different from that of wild type. ¥, Significant effect of salbutamol. ANOVA and LSD, P < 0.05.
Resting EM
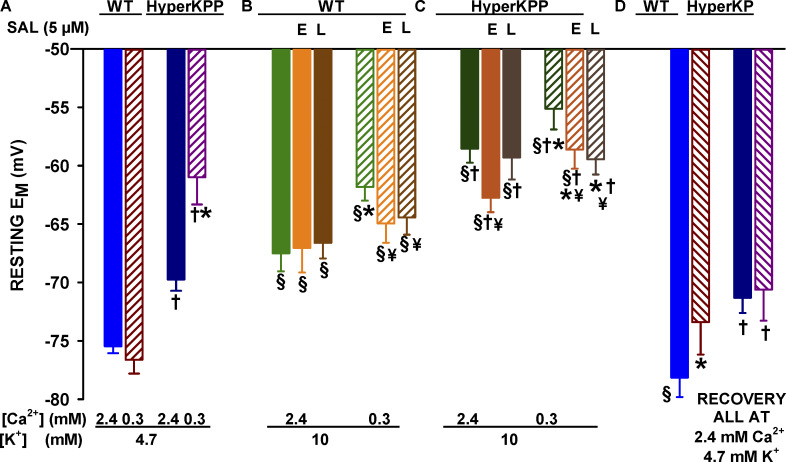
Mean resting EM was significantly less negative in HyperKPP than in wild type fibers regardless of [K+]e and [Ca2+]e (Fig. 8), in agreement with previous reports (Lehmann-Horn et al., 1983; Ricker et al., 1989; Ammar et al., 2015). At 4.7 mM K+, mean resting EM of wild type fibers was not affected by a decrease in [Ca2+]e from 2.4 to 0.3 mM, as the mean values were −76 and −77 mV, respectively (Fig. 8 A). In HyperKPP fibers, on the other hand, mean resting EM became significantly less negative at 0.3 mM Ca2+ as mean values changed from −70 to −61 mV. Increasing [K+]e to 10 mM caused significant depolarizations, which were greater at 0.3 than at 2.4 mM Ca2+ for both wild type and HyperKPP fibers (Fig. 8, B and C). Adding salbutamol resulted in a significant transient repolarization for HyperKPP, but not wild type, fibers at 2.4 mM Ca2+, whereas at 0.3 mM Ca2+ salbutamol caused a permanent repolarization for both wild type and HyperKPP fibers. Returning [K+]e to 4.7 mM was followed by a full recovery of mean resting EM in fibers only exposed to 2.4 mM Ca2+, whereas an incomplete recovery was observed when fibers had been exposed to 0.3 mM Ca2+ (Fig. 8 D).
Figure 8.
Lowering [Ca2+]e significantly enhanced the K+-induced membrane depolarization in both wild type and HyperKPP soleus. Mean resting EM values were calculated from all tested fibers (mean resting EM of unexcitable and excitable fibers are respectively given in Tables 1 and 2). All [K+]e and [Ca2+]e conditions are as described in Fig. 7. Data for salbutamol are divided into early measurements (first 10 min, E) and late measurements (15–30 min, L). The reversibility of the K+ and Ca2+ effects was tested by returning to the initial conditions (i.e., 4.7 mM K+ and 2.4 mM Ca2+). Vertical bars represent the SE of 71–144 fibers/5–6 muscles for high [K+]e, 277 fibers/26 muscles for wild type and 273 fibers/18 muscles for HyperKPP at 4.7 mM K+ and 2.4 mM. §, At similar [Ca2+]e, mean resting EM significantly different from that at 4.7 mM K+ measured at the beginning of an experiment (i.e., in A). *, At similar [K+]e, mean resting EM at 0.3 mM Ca2+ significantly different from that at 2.4 mM. †, At the same [K+]e and [Ca2+]e, mean resting EM of HyperKPP fibers significantly different that of wild type fibers. ¥, Significant effect of salbutamol. ANOVA and LSD, P < 0.05.
Resting EM was also divided in bins of 5 mV to determine the frequency distribution. At 4.7 mM K+, Ca2+ did not affect the distribution of resting EM in wild type soleus fibers (Fig. 9 A), whereas the frequency distribution for HyperKPP fibers was significantly shifted to less negative EM at 0.3 mM Ca2+ (Fig. 9 B). Moreover, the numbers of wild type fibers with resting EM less negative than −62 mV (mean resting EM of wild type unexcitable fibers; Table 1) were 7–8%, while the numbers of HyperKPP fibers with resting EM less negative than −57 mV (mean resting EM of HyperKPP unexcitable fibers; Table 1) at 2.4 and 0.3 mM Ca2+ were 21% and 59%, respectively. At 10 mM K+, lowering [Ca2+]e to 0.3 mM significantly shifted the frequency distribution toward less negative EM for both wild type and HyperKPP fibers. The number of wild type fibers with resting EM less negative than −63 mV was 66% at 0.3 mM Ca2+, a value significantly greater than the value of 35% at 2.4 mM Ca2+ (Fig. 9 C). The situation was worst for HyperKPP fibers as the number of fibers with resting EM less negative than −57 mV was 86% at both [Ca2+]e (Fig. 9 D). Salbutamol did not affect the frequency distribution of wild type resting EM at 10 mM K+ and 2.4 mM Ca2+ (Fig. 10 A), which is in agreement with the lack of effect on the mean resting EM. At 10 mM K+, salbutamol increased the number of wild type fibers with resting EM between −65 and −70 mV from 11% to 26% (Fig. 10 B). For HyperKPP fibers, a frequency shift toward more negative EM was significant in the presence of salbutamol at 10 mM K+ and at both 2.4 and 0.3 mM Ca2+ (Fig. 10, C and D).
Figure 9.
Lowering [Ca2+]e significantly increased the number of wild type soleus fibers exposed to 10 mM K+ and HyperKPP fibers exposed to both 4.7 and 10 mM K+ with resting EM less negative than the mean resting EM of unexcitable fibers. Resting EM values were divided in bin of 5 mV, and the number of fibers in each bin is expressed as a percentage of the total number of fibers tested. Mean resting EM of unexcitable wild type and HyperKPP fibers was −62 and −57 mV, respectively (Table 1), and is represented by a vertical dark yellow line. *, Frequency distribution at 0.3 mM Ca2+ significantly shifted compared with 2.4 mM Ca2+. Fisher test, P < 0.05.
Table 1. At 10 mM K+, mean resting EM of unexcitable fibers was less negative for HyperKPP soleus fibers than wild type fibers.
| [Ca2+]e (mM) | [Mg2+]e (mM) | Wild type soleus | HyperKPP soleus |
|---|---|---|---|
| 2.4 | 3.1 | −63.6 ± 1.3 (38/11) | −56.1 ± 1.2 (37/6)a |
| 0.3 | 3.1 | −61.7 ± 2.2 (62/8) | −55.1 ± 1.8 (75/6)a |
| 0.3 | 5.2 | −59.4 ± 1.3 (54/6) | −56.4 ± 1.1 (98/6)a |
Unexcitable fibers were fibers that did not generate an action potential upon stimulation. Mean resting EM values are expressed as mean ± SE (number of fibers/number of muscle). There was no significant effect of 5 µM salbutamol on mean resting EM of unexcitable fibers at 10 mM K+ (data not shown). ANOVA, P > 0.05.
Mean resting EM of unexcitable HyperKPP soleus fibers significantly different from that of wild type. ANOVA and LSD, P < 0.05.
Figure 10.
Salbutamol (SAL; 5 µM) at 10 mM K+ significantly shifted the frequency distribution of resting EM toward more negative potential only in HyperKPP solei. Resting EM values were divided in bin of 5 mV, and the number of fibers in each bin is expressed as a percent of the total number of fibers tested. Mean resting EM of unexcitable wild type and HyperKPP fibers was −62 and −57 mV, respectively (Table 1), and is represented by a vertical purple line. ¥, Salbutamol significantly shifted the frequency distribution of resting EM. Fisher test, P < 0.05.
Overshoot
At 4.7 mM K+, reducing [Ca2+]e to 0.3 mM significantly reduced mean action potential overshoot in both wild type and HyperKPP fibers (Fig. 11 A). A similar situation was observed at 10 mM K+, except that no overshoot was observed, as the action potential peak remained negative (Fig. 11, B and C). Interestingly, the lower action potential peaks at 0.3 mM Ca2+ were not associated with less negative mean resting EM among excitable fibers (Table 2). Salbutamol improved action potential peak of wild type fibers, but only at 0.3 mM Ca2+ (Fig. 11 B). Contrary to wild type, salbutamol improved action potential at 2.4 mM Ca2+ in HyperKPP (Fig. 11 C). None of the tested HyperKPP fibers generated action potential at 0.3 mM Ca2+ and 10 mM K+, but a few did with very small amplitude following the addition of salbutamol.
Figure 11.
Lowering [Ca2+]e significantly enhanced the K+-induced overshoot depression in both wild type and HyperKPP soleus. All [K+]e and [Ca2+]e conditions are as described in Fig. 7. Data in the presence of salbutamol were divided into early measurements (first 10 min, E) and late measurements (15–30 min, L). The reversibility of the K+ and Ca2+ effects was tested by returning to the initial conditions (i.e., 4.7 mM K+ and 2.4 mM Ca2+). Vertical bars represent the SE of 71–144 fibers/5–6 muscles for high [K+]e, 277 fibers/26 muscles for wild type and 273 fibers/18 muscles for HyperKPP at 4.7 mM K+ and 2.4 mM. §, At similar [Ca2+]e, mean overshoot significantly different from that at 4.7 mM K+ measured at the beginning of an experiment (i.e., in A). *, At similar [K+]e, mean overshoot at 0.3 mM Ca2+ significantly different from that at 2.4 mM. †, At the same [K+]e and [Ca2+]e, mean overshoot of HyperKPP fibers significantly different that of wild type fibers. ¥, Significant effect of salbutamol. ANOVA and LSD, P < 0.05. No AP, no action potential.
Table 2. Mean resting EM of excitable fibers was not affected by lowering [Ca2+]e.
| Mouse | [K+]e (mM) | 2.4 mM Ca2+ | 0.3 mM Ca2+ |
|---|---|---|---|
| Wild type | 4.7 | −76.7 ± 0.9 (89/11) | −78.1 ± 1.2 (87/9) |
| 10.0 | −69.2 ± 1.8 (106/11) | −68.5 ± 3.5 (39/7) | |
| HyperKPP | 4.7 | −74.9 ± 1.4 (79/6) | −74.2 ± 1.0 (79/6) |
| 10.0 | −60.2 ± 1.5 (67/6) | — |
Excitable fibers were fibers that generated an action potential upon stimulation. No action potential could be recorded at 10 mM K+ and 0.3 mM Ca2+ in HyperKPP fibers. Mean resting EM values are expressed as mean ± SE (number of fibers/number of muscle). There was no significant difference in mean resting EM of excitable fibers between 0.3 and 2.4 mM Ca2. ANOVA, P > 0.05.
Replacing Ca2+ with Mg2+
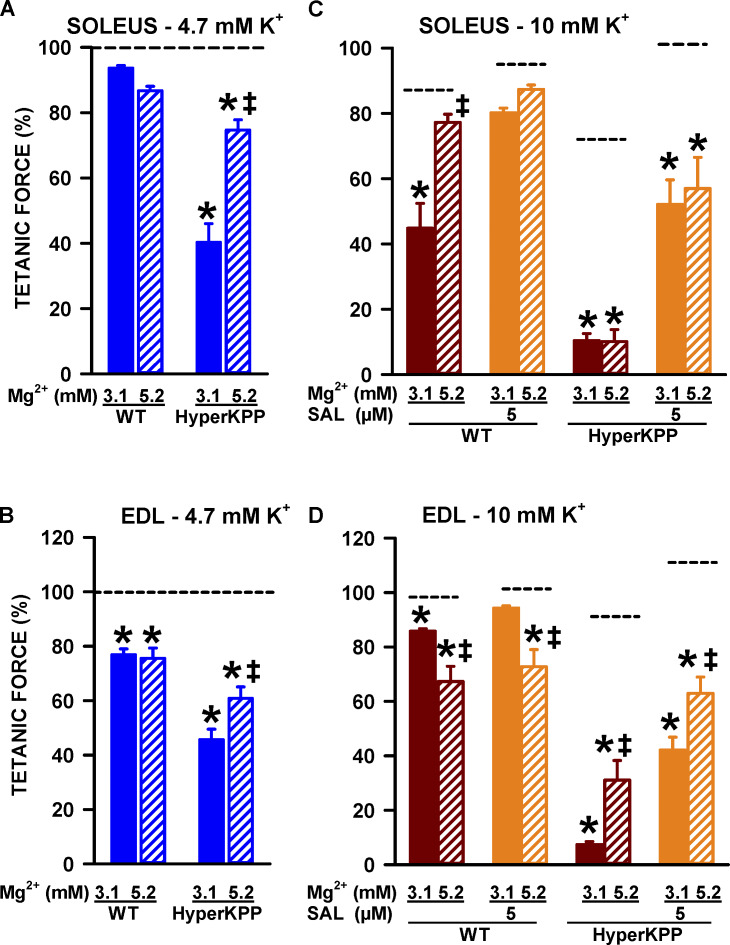
The experiments described so far were carried by only decreasing [Ca2+]e because in HyperKPP patients a decrease in Ca2+ is not necessarily associated with an increased Mg2+. According to the surface potential theory, lowering the concentration of a divalent cation, such as Ca2+, can set up a local negative potential at the surface of the cell membrane that can then bias the sensors of voltage-gated channels; this effect can be suppressed if Ca2+ is replaced by another divalent cation, such as Mg2+ (Hille, 2001). Therefore, experiments at 0.3 mM Ca2+ were repeated while [Mg2+]e was increased from 3.1 to 5.2 mM to maintain constant the divalent cation concentration. At 4.7 mM K+, the decreases in tetanic force of wild type soleus and EDL at 0.3 mM Ca2+ were not different between 3.1 and 5.2 mM Mg2+, whereas for the HyperKPP muscles the force decreases were less at 5.2 mM Mg2+ (Fig. 12, A and B). The K+-induced force depression at 10 mM K+ was significantly less at 5.2 mM than at 3.1 mM Mg2+ for wild type soleus and HyperKPP EDL (Fig. 12, C and D). Interestingly, this also significantly worsened the K+-induced force depression in wild type EDL.
Figure 12.
At 4.7 mM K+, replacing Ca2+ by Mg2+ significantly improved mean tetanic force of HyperKPP soleus and EDL, while at 10 mM K+, it improved force for wild type soleus and HyperKPP EDL. Changes in [K+]e and [Ca2+]e were as described in protocol 1, except that the 0.3 mM Ca2+ solution also contained 5.2 mM Mg2+. (A and B) Tetanic force measured at 4.7 mM K+ and 0.3 mM Ca2+ with either 3.1 or 5.2 mM Mg2+ and expressed as a percentage of the tetanic force at 4.7 mM K+ and 2.4 mM Ca2+. (C and D) Tetanic force measured at 10 mM K+ and 0.3 mM Ca2+ and expressed as a percentage of the force measured just before [K+]e was increased (i.e., 4.7 mM and 0.3 mM Ca2+). Horizontal dash black lines indicate the position of the mean values at 2.4 mM Ca2+. Vertical bars represent the SE of five muscles. ‡, Mean tetanic force at 5.2 mM Mg2+ was significantly different from that at 3.1 mM. *, Mean tetanic force at 0.3 mM Ca2+ was significantly different from that at 2.4 mM, ANOVA and LSD, P < 0.05.
Overall, an increase in [Mg2+]e had small effects, few being significant, on membrane excitability (Fig. 13 A), resting EM (Fig. 13 B), and action potential overshoot (Fig. 13 C). Notably, increasing [Mg2+]e to 5.2 mM significantly improved membrane excitability and overshoot of wild type soleus at 10 mM K+ in the absence and presence of salbutamol as well as all three parameters of HyperKPP soleus at 4.7 mM K+ and 10 mM K+ in the presence of salbutamol.
Figure 13.
Replacing Ca2+ by Mg2+ improved membrane excitability in wild type and HyperKPP soleus fibers at 0.3 mM Ca2+ but did not allow for full recovery toward values at 2.4 mM Ca2+. All measurements were performed while [Ca2+]e was 0.3 mM and [Mg2+]e either 3.1 or 5.2 mM. Horizontal black lines and arrows indicate the position of the mean values when [Ca2+]e was 2.4 mM Ca2+ at the indicated [K+]e. Vertical bars represent the SE of 77–107 fibers/6 muscles. ‡, Mean value at 5.2 mM Mg2+ was significantly different from that at 3.1 mM. *, Mean value at 0.3 mM Ca2+ was significantly different from that at 2.4 mM; ANOVA and LSD, P < 0.05.; ANOVA and LSD, P < 0.05.
The maximum rate of depolarization (dV/dt) has been used as a measure of the inward Na+ current during an action potential (Hodgkin and Katz, 1949). To have a better insight on how Ca2+ and Mg2+ affect Na+ channels, resting EM was separated in bins of 5 mV and resting EM and dV/dt were averaged for each bin. Lowering [Ca2+]e at 4.7 mM K+ caused a significant decrease in mean dV/dt of wild type soleus in the range of −75 and −60 mV (Fig. 14 A). Fitting the data to a sigmoidal curve demonstrates that lowering [Ca2+]e shifted the dV/dt–EM relationship toward more negative EM. Increasing [Mg2+]e from 3.1 to 5.2 mM while [Ca2+]e was 0.3 mM allowed for a full recovery of dV/dt to a level similar to that of 2.4 mM Ca2+. Furthermore, the dV/dt–EM relationships between the two conditions were basically the same. At 2.4 mM Ca2+, mean dV/dt of HyperKPP soleus did not decrease at resting EM between −70 and −60 mV and became significantly greater than the mean values for wild type soleus (Fig. 14 B). At 2.4 mM Ca2+, the dV/dt–EM relationship of HyperKPP occurred at less negative EM than for wild type. At similar EM, mean dV/dt values of HyperKPP fibers at resting EM between −75 and −65 mV were significantly higher than those for wild type, although the difference in the dV/dt–EM relationship was small (Fig. 14 B, inset). Increasing [Mg2+]e significantly improved mean dV/dt in HyperKPP soleus, and even became significantly greater than in wild type soleus.
Figure 14.
Replacing Ca2+ by Mg2+ improved the maximum rate of action potential depolarization in wild type soleus and HyperKPP soleus. Resting EM values were divided in bins of 5 mV and mean resting EM and maximum rate of depolarization were averaged. Lines were drawn from a sigmoidal nonlinear regression analysis (except for the 5.2 mM Mg2+ in A because a good fit could not be obtained). The inset in B is to illustrate the difference in the relationship at 0.3 mM Ca2+ between wild type and HyperKPP muscles. Horizontal and vertical bars represent the SE of 6–47 fibers/6 muscles. *, Mean maximum rate of depolarization significantly different from that at 2.4 mM Ca2+. †, Mean maximum rate of depolarization of HyperKPP soleus significantly different from that of wild type. ANOVA and LSD, P < 0.05.
Discussion
The major findings of this study were (1) in wild type muscles, a decrease in [Ca2+]e from 2.4 to 0.3 mM had little effect at 4.7 mM K+ on tetanic force and membrane excitability, whereas it significantly enhanced the K+-induced depression of force and membrane excitability; (2) in contrast, lowering [Ca2+]e negatively altered the capacity of HyperKPP muscles to generate tetanic force and action potentials at both 4.7 and 10 mM K+; (3) lowering [Ca2+]e reduced the capacity of both wild type and HyperKPP EDL to maintain a plateau phase during a tetanus; (4) lowering [Ca2+]e increased the generation of fast and transient contractures or a slow increase in unstimulated force when [K+]e was increased to 6–14 mM K+; and (5) replacing Ca2+ by an equivalent amount of Mg2+ improved mean tetanic force of HyperKPP muscles at 4.7 mM K+ and of wild type soleus and HyperKPP EDL at 10 mM K+.
Mechanism of action of lowering [Ca2+]e in wild type muscles
Tetanic force
As previously reported (Dulhunty and Gage, 1988; Cairns et al., 1998; Zhao et al., 2005; Cairns and Lindinger, 2008), lowering [Ca2+]e from 2.4 to 0.3 mM Ca2+ had little effect on the tetanic force of wild type soleus and EDL. Lowering [Ca2+]e, however, significantly shifted the tetanic force–[K+]e relationship to lower [K+]e increasing muscle sensitivity to the K+-induced force depression. Conversely, the depressive effect of low Ca2+ was small at 4.7 mM K+ and pronounced at high [K+]e. Lowering [Ca2+]e had no effect on resting EM at 4.7 mM K+, whereas it accentuated the K+-induced membrane depolarization by 6 mV at 10 mM K+ (Fig. 8). Membrane depolarization has also been reported in rat EDL and toad sartorius when [Ca2+]e is lowered (Ishiko and Sato, 1957; Ruff et al., 1988). Associated with the depolarization was a decrease in the number of excitable fibers (Fig. 7), as more fibers had resting EM less negative than −61 mV (Fig. 9), the mean resting EM of unexcitable fibers (Table 1). So, a first mechanism for the effect of low [Ca2+]e is greater membrane depolarization leading to more unexcitable fibers, which no longer generate force.
It is well established that a K+-induced membrane depolarization (Yensen et al., 2002) increases NaV1.4 channel inactivation (Hodgkin and Huxley, 1952), resulting in lower action potential amplitude (Yensen et al., 2002), and any decrease in action potential amplitude results in lower Ca2+ release (Zhu et al., 2014) and force (Godt and Nosek, 1989). Lowering [Ca2+]e from 2.4 to 0.3 mM Ca2+ resulted in smaller action potential amplitude of 8 and 17 mV at 4.7 and 10 mM K+, respectively. Lowering [Ca2+]e shifts the steady-state inactivation curve of NaV1.4 channels toward more negative EM (Frankenhaeuser and Hodgkin, 1957; Vogel, 1974; Hahin and Campbell, 1983). To our knowledge, no study has shown how Ca2+ between 0 and 2.5 mM affects mammalian muscle NaV1.4 channel inactivation. However, the maximum rate of depolarization (dV/dt) can provide an index of the inward Na+ current during an action potential (Hodgkin and Katz, 1949). More importantly, differences between wild type and HyperKPP muscles for the relationship between dV/dt and resting EM can be explained by differences in NaV1.4 channel steady-state slow inactivation properties.
At 2.4 mM Ca2+, dV/dt barely decreased in HyperKPP soleus when resting EM became less negative than −70 mV, whereas it decreased by more than threefold in wild type soleus. From −70 to −60 mV, steady-state slow inactivation of wild type NaV1.4 channels decreases from 0.69 to 0.52 (25% decline) compared with a change from 0.78 to 0.69 (12% decline) for the channel carrying the M1592V mutation (Hayward et al., 1997). The lack of large decrease in the dV/dt at resting EM less negative than −70 mV in HyperKPP muscles then most likely results from a greater Na+ inward current in the context of less slow inactivation. At 0.3 mM Ca2+, mean dV/dt values between resting EM of −75 and −65 mV were significantly greater in HyperKPP than in wild type soleus fibers, which would suggest a similar shift in the dV/dt–resting EM relationship toward less negative EM for HyperKPP muscles. However, fitting the data to a sigmoidal relationship demonstrated that such shift is smaller at 0.3 than at 2.4 mM Ca2+. Considering that lowering [Ca2+]e shifted the dV/dt–resting EM curve toward more negative EM, we therefore suggest that (1) the low Ca2+ effect on dV/dt is most likely related to a hyperpolarizing shift in steady-state inactivation of NaV1.4 channels, as shown in other excitable tissues; and (2) the decrease in action potential amplitude of wild type muscles at low [Ca2+]e involves greater NaV1.4 channel inactivation. The next two questions are (1) what is the relative contribution of the membrane depolarization and greater NaV1.4 channel inactivation to the decrease in excitability at low Ca2+? and (2) what causes the increase in inactivation?
At 2.4 mM Ca2+, resting EM changed from −75 to −67 mV when [K+]e is increased from 4.7 to 10 mM. Using the estimated sigmoidal dV/dt–EM relationship (Fig. 14 A), such change in resting EM reduces dV/dt to 68% of the value at 4.7 mM K+. Using the same resting EM of −67 mV but the dV/dt–EM curve at 0.3 mM Ca2+, the estimated dV/dt becomes 42%; i.e., the hyperpolarizing shift of the curve at 0.3 mM Ca2+ causes a further 26% decrease in dV/dt. Now, at 0.3 mM Ca2+ and 10 mM K+, resting EM was not −67 mV but −62 mV. For the latter EM, using the curve at 2.4 mM Ca2+, dV/dt is estimated at 43%; i.e., the low Ca2+-induced depolarization also causes a 25% decrease in dV/dt. If the low Ca2+–induced depolarization and hyperpolarizing shift in the dV/dt–EM curve are additive, then the estimated dV/dt at 0.3 mM Ca2+ and 10 mM K+ is 18% (0.42 × 0.43), a value close to the estimated value of 14% from the dV/dt–EM curve using a resting EM of −62 mV and the relation at 0.3 mM Ca2+. It is thus concluded that the larger decrease in tetanic force and fiber excitability from 2.4 to 0.3 mM Ca2+ (at 10 mM K+) is due to an additive effect of low Ca2+–induced depolarization and hyperpolarizing shift of the steady-state NaV1.4 channel inactivation.
Mean resting EM from all fibers (i.e., excitable and unexcitable fibers; Fig. 8) became less negative at low [Ca2+]e; this would suggest that the increased in Na+ channel inactivation would be due to the extra depolarization. However, mean resting EM of wild type excitable fibers at 4.7 and 10 mM K+ was −77 and −69 mV, respectively, regardless of [Ca2+]e (Table 2). This indicates that the increased channel inactivation at low [Ca2+]e is not due to greater membrane depolarization of the excitable fibers, at least not as measured with microelectrodes. Another factor contributing to inactivation stems from the surface charge potential theory (Hille, 2001). The cell membrane surface bears negative charges allowing Ca2+ binding. As [Ca2+]e is reduced and the number of bound Ca2+ decreases, a local negative surface potential influences the electrical field within the membrane, which becomes less than the measured potential and may bias voltage sensors of ion channels. Replacing Ca2+ by Mg2+ prevents this effect for NaV1.4 channels (Kostyuk et al., 1982; Hille, 2001). Furthermore, replacing Ca2+ by Mg2+ allowed for a full recovery of dV/dt. Thus, a second mechanism for the depressing effect of low Ca2+ involves an increased Na+ channel inactivation, possibly because of a change in surface charge potential, resulting in lower action potential amplitude and muscle force.
For wild type muscles, increasing [Mg2+]e to 5.2 mM when [Ca2+]e was 0.3 mM did not result in any tetanic force recovery at 4.7 mM K+, while at 10 mM K+ the tetanic force of soleus recovered from 45 to 80%, a 35% difference. Despite full recovery of dV/dt under those conditions, membrane excitability improved by only 11% and action potential peak from −25 to −18 mV. It therefore appears that the recovery of membrane excitability with Mg2+ is too small to fully explain the 35% force recovery. Membrane depolarization to 0 mV or removal of Ca2+ from the extracellular space inactivates CaV1.1, reducing the charge movement within CaV1.1 and abolishing Ca2+ release in frog muscles; normal charge movement and Ca2+ release is reestablished by repolarizing the membrane or adding Ca2+ in the extracellular space (Brum et al., 1988a; Dulhunty and Gage, 1988; Pizarro et al., 1989). Of significance are the effects of lowering [Ca2+]e while the cell membrane is partially depolarized. For example, half-maximum Ca2+ release occurs near −60 mV at 7.5 mM Ca2+ and −90 mV at 0.010 mM Ca2+ with no Ca2+ release at −60 mV (Brum et al., 1988b). While in normal [Ca2+]e and [K+]e none of the CaV1.1 are inactivated in frog muscles, there is evidence that they are partially inactivated in mouse muscle (Ferreira Gregorio et al., 2017); i.e., mouse muscles are expected to be more sensitive to changes in [Ca2+]e than frog muscles. Therefore, a third mechanism for the depressive effect of low [Ca2+]e on tetanic force may involve a hyperpolarizing shift for the inactivation of CaV1.1 and Ca2+ release as less Ca2+ is bound to CaV1.1.
It is unlikely that the surface charge potential is important for the inactivation of CaV1.1 and Ca2+ release for two reasons. First, lowering [Ca2+]e has little effect on the voltage dependence of activation, while it significantly shifts the voltage dependence of inactivation (Lüttgau and Spiecker, 1979; Brum et al., 1988b). Second, dimethonium, a cation capable of screening negative fixed charge on the surface of lipid bilayers, fails to prevent the inactivation observed when [Ca2+]e is lowered (Pizarro et al., 1989). Mg2+, on the other hand, has been shown to be effective at replacing Ca2+ at the Ca2+-binding site on CaV1.1, allowing a partial recovery of Ca2+ release (Pizarro et al., 1989). Considering that at 0.3 mM Ca2+ improvement of membrane excitability and action potential by Mg2+ cannot fully explain the increase in wild type soleus tetanic force at 10 mM K+, we suggest that decreases in Ca2+ release via an inactivation of CaV1.1 is an important mechanism for the force depression at low [Ca2+]e, especially when the membrane is depolarized.
Taken together, our results suggest three mechanisms to explain the enhanced K+-induced force depression at low [Ca2+]e: (1) a greater membrane depolarization that increases the number of unexcitable fibers; (2) a shift of the steady-state inactivation of NaV1.4 channels toward more negative EM, lowering action potential amplitude; and (3) a shift of the steady-state inactivation of CaV1.1 also toward more negative EM, resulting in lower Ca2+ release.
Unstimulated force
Another effect of low [Ca2+]e includes the force measured between contractions (i.e., unstimulated force). At 2.4 mM Ca2+, an increase in [K+]e rarely caused an increase in unstimulated force in wild type soleus and never caused an increase in EDL. At 0.3 mM Ca2+, all EDL and 30% of solei generated an unstimulated force. Intracellular [Ca2+] ([Ca2+]i) increases at elevated [K+]e (Quiñonez et al., 2010; Pedersen et al., 2019). Low [Ca2+]e causes a hyperpolarizing shift in the Na+ channel steady-state activation, resulting in a more negative threshold (Frankenhaeuser and Hodgkin, 1957; Costantin, 1968; Campbell and Hille, 1976; Kostyuk et al., 1982; Hahin and Campbell, 1983). This effect is actually a mechanism that causes myotonic discharge when blood [Ca2+] decreases in patients suffering of myotonia congenita (Skov et al., 2013, 2015). As wild type muscles are not, however, prone to myotonic discharge and none of them generated fast and transient contractures, it is unlikely that uncontrolled action potential generation was the cause for unstimulated force.
Another possibility is an activation of the Ca2+ release as the membrane is depolarized by increases in [K+]e and decreases in [Ca2+]e. Resting EM at which Ca2+ starts to be released is approximately −50 mV (Sandow et al., 1965). Low [Ca2+]e also shifts the inward Ca2+ current versus EM toward more negative potentials (Kostyuk et al., 1982), rendering the mechanical threshold even more negative. The number of fibers with resting EM near or less negative than −50 mV increased when [K+]e was raised to 10 mM while [Ca2+]e is 0.3 mM. Taken together, these results suggest that the increase in unstimulated force a high [K+]e and low [Ca2+]e is most likely related to a small activation of inward Ca2+ current that results in a small activation of Ca2+ release.
Salbutamol effects
As previously reported (Wang and Clausen, 1976; Clausen et al., 1980, 2011), exposing muscles to salbutamol allows for large force recovery at elevated [K+]e as it shifts the tetanic force–[K+]e relationship toward higher [K+]e. Exposing muscles to β2-adrenergic receptor agonists results in 4–10-mV hyperpolarization at normal [K+]e (Clausen and Flatman, 1977; Clausen and Flatman, 1980; van Mil et al., 1995). It has been proposed that the hyperpolarization is linked to an activation of the electrogenic Na+ K+ ATPase pump via the cAMP/PKA pathway (Clausen and Flatman, 1977). However, this study shows no hyperpolarization and no shift in the frequency distribution of resting EM by 5 µM salbutamol at 10 mM K+ and 2.4 mM Ca2+; this is in agreement with previous studies reporting hyperpolarization of just 1–2 mV at 10 mM K+(Kuba and Nohmi, 1987; Cairns et al., 1995; van Mil et al., 1995). Despite the lack of hyperpolarization, tetanic force largely increased upon the salbutamol addition. At 0.3 mM Ca2+, salbutamol exposure caused a 3-mV hyperpolarization and an increase in the number of fibers with resting EM between −65 and −70 mV. The hyperpolarization was associated with small increases in membrane excitability and action potential amplitude that are too small to explain the large force recovery. Activation of β2-adrenergic receptors also leads to CaV1.1 channel and ryanodine receptor phosphorylation (Curtis and Catterall, 1985; Suko et al., 1993; Cairns and Borrani, 2015), resulting in greater Ca2+ release (Cairns et al., 1993; Bruton et al., 1996; Liu et al., 1997; Rudolf et al., 2006; Cairns and Borrani, 2015). It is therefore most likely that most of the force recovery at high K+ in the presence of salbutamol is due to greater Ca2+ release and to a lesser extent to a hyperpolarization (at 0.3 mM Ca2+).
Ca2+ sensitivity of HyperKPP skeletal muscles
Tetanic force
The lower tetanic force, greater fiber inexcitability, less negative resting EM, and lower overshoot of HyperKPP muscles compared with their wild type counterpart reported here are similar to previous reports where the cause has been fully discussed (Hayward et al., 2008; Clausen et al., 2011; Lucas et al., 2014; Ammar et al., 2015; Khogali et al., 2015). This study now reveals the extent of which HyperKPP muscles are more Ca2+ sensitive than wild type muscles. At 4.7 mM K+, lowering [Ca2+]e from 2.4 to just 1.3 mM depressed tetanic force of HyperKPP muscles to a similar (EDL) or greater (soleus) extent than that observed for wild type muscles exposed to 0.3 mM. Similarly, the K+-induced force depression at 1.3 mM Ca2+ for HyperKPP muscles was the same or greater than at 0.3 mM Ca2+ for wild type muscles.
As explained in the Introduction, HyperKPP muscle fibers are depolarized compared with their wild type counterpart even when [K+]e is 4.7 mM K+ because of leaky M1592V NaV1.4 channels. For example at 1.3 mM Ca2+ (Ammar et al., 2015), the majority of wild type soleus muscle fibers (∼66%) have resting EM ranging between −85 and −70 mV, whereas for HyperKPP fibers, the majority (∼71%) ranges between −65 and −55 mV. We now show a similar situation at 2.4 mM Ca2+ (Fig. 9, A and B). As explained above for wild type muscles, depolarized fibers are more sensitive to the low Ca2+–induced membrane depolarization. Furthermore, while the number of wild type soleus fibers at 4.7 mM K+ with resting EM more negative than −70 mV is the same at 2.4 and 0.3 mM Ca2+ (i.e., 80% and 79%, respectively; Fig. 9 A); for HyperKPP fibers, the numbers were 54% and 33% (Fig. 9 B). Ca2+ is known to block Na+ channels with an increased magnitude as EM becomes more negative (Yamamoto et al., 1984; Armstrong and Cota, 1991; Santarelli et al., 2007). For example, Na+ current measured at −70 mV (measured immediately once the channels had been opened with a brief depolarization) increased by 36% when [Ca2+]e is reduced from 2 to 0.2 mM; the increase becomes 46% at −80 mV. A reduction in Ca2+ blocking at low [Ca2+]e is not expected to affect resting EM of wild type fibers because very few NaV1.4 channels are opened. HyperKPP fibers, on the other hand, have more NaV1.4 channels opened at rest as a result of the M1592V mutation. Therefore, when [Ca2+]e is lowered, fewer NaV1.4 channels are blocked by Ca2+, resulting in an increase in Na+ influx and further depolarization even at 4.7 mM K+. Thus, one mechanism as to why HyperKPP muscles are more sensitive to the low Ca2+-force depression is because of a greater number of depolarized fibers and further depolarization when [Ca2+]e decreases that enhances the degree of NaV1.4 channel inactivation.
Similar to wild type muscles, lowering [Ca2+]e shifted the dV/dt–EM relationship toward more negative EM, except that the shift was considerably more pronounced in HyperKPP than wild type soleus, suggesting a more Ca2+ sensitive steady-state inactivation for the M1592V than wild type NaV1.4 channel. At 10 mM K+ and 0.3 mM Ca2+, the estimated dV/dt of HyperKPP soleus at −60 mV was only 4% of that at 4.7 mM K+ and 2.4 mM Ca2+. It is therefore not surprising that no action potential could be measured at 10 mM K+ and 0.3 mM Ca2+. One may then ask the question as to how tetanic force at 10 mM K+ and 0.3 mM Ca2+ was 39% of the force under control conditions. At high [K+]e, some but not all stimulations during a train trigger action potentials; some fibers generate action potentials at the beginning of the train, while in other cases the first nine stimulations failed to trigger one, at least in frog muscle (Renaud and Light, 1992). That is, while twitch force is expected to be completely abolished at 10 mM K+ and 0.3 mM Ca2+, enough action potentials are generated during the stimulation train to allow some tetanic force to be generated.
Again as observed for wild type muscles, divalent replacement with Mg2+ at low [Ca2+]e was effective in reducing force loss, improving fiber excitability, and hyperpolarizing resting EM in HyperKPP soleus at 4.7 mM K+, which suggest that the mechanisms by which low Ca2+ affects membrane excitability and force are the same in wild type and HyperKPP muscles. Divalent replacement was, however, ineffective at 10 mM K+, probably because under those conditions soleus muscles are too severely affected, as noted with the lack of any action potential measurement and the very low estimated dV/dt.
Altogether, this study demonstrates that the mechanisms responsible for the low Ca2+–induced force depression in HyperKPP muscles are the same as those for wild type; that is, the low Ca2+ effect is an intrinsic property of mammalian muscle and not some unique feature of HyperKPP fibers. The apparent greater Ca2+ sensitivity of HyperKPP muscles is because HyperKPP fibers are more depolarized than normal, an effect enhanced by low Ca2+, and a more Ca2+-sensitive steady-state inactivation, at least for the M1592V mutation.
Unstimulated force
HyperKPP muscles generated slow increase in unstimulated force at low [Ca2+]e and high [K+]e, but unlike wild type muscles, they also generated fast transient contracture. It is very likely that the fast transient contractures are due to uncontrolled generation of action potentials because (1) HyperKPP muscle fibers are prone to myotonic discharge, as they are more depolarized and closer to threshold due to a hyperpolarizing shift of the steady-state activation of Na+ channel; and (2) the latter is further shifted by low Ca2+. On the other hand, the slow increase in unstimulated force is most likely related to a small inward Ca2+ current through CaV1.1 channels as discussed above for wild type muscles.
Perspectives for HyperKPP
The next two questions are (1) how relevant are the Ca2+ effects for HyperKPP patients, and (2) can they explain the alleviation of symptoms following treatment with calcium gluconate? Normal total blood Ca2+ ranges between 2.1 and 2.6 mM, while free ionized [Ca2+] ranges between 1.1 and 1.3 mM (Copstead and Banasik, 2013). Many case studies primarily report blood Na+ and K+ levels (for example, Streeten et al., 1971), while fewer report Ca2+ levels as normal (Gamstorp et al., 1957; Poskanzer and Kerr, 1961). One study (Mankodi et al., 2015) reported the case of one patient suffering of both hypomagnesemia (0.5 mM) and hypocalcemia (1.9 mM, total) due to cyclosporine treatments. Mg2+ supplementation alone allowed for a recovery of normal Mg2+ (0.8 mM) and Ca2+ (2.4 mM, total) levels associated with large decrease in paralytic attacks. In another study (Herman and McDowell, 1963), paralysis occurred while total blood Ca2+ was 2.6 mM; an intravenous 180 mg Ca2+ injection raised the blood Ca2+ to 3.1 mM, fully alleviating the paralysis within 10 min. Thus, while a small variation in Ca2+ serum levels is not expected to affect muscle force in wild type muscles, it does in HyperKPP patients between free [Ca2+]e of 1.3 and 2.4 mM, and it explains how small increases can alleviate symptoms. The case study on hypomagnesemia and hypocalcemia and this study suggest that increasing Mg2+ intake may be a potential treatment to alleviate HyperKPP symptoms.
Modulation of the salbutamol effects by Ca2+
Similar to the situation in wild type, the addition of salbutamol allowed for force recovery at elevated [K+]e. Furthermore, it reduced the Ca2+-depressing effect, especially for HyperKPP soleus. Finally, it reduced the slow increase in unstimulated force observed at elevated [K+]e. While hyperpolarization was a potentially small mechanism involved in the force recovery in wild type muscles, it is very likely to play a greater role in HyperKPP muscles in all three conditions (0.3, 1.3, and 2.4 mM Ca2+). As observed with wild type soleus at 0.3 mM Ca2+, the hyperpolarization was only 3–4 mV, but this hyperpolarization occurs in the most critical range of resting EM (−55/−60 mV) for which most of the decrease in force occurs, because mean resting EM at 10 mM K+ was −55/−57 mV and increased to −59/−63 mV upon addition of salbutamol. This hyperpolarization then improved membrane excitability and action potential amplitude. Of course, an effect on the Ca2+ release mechanism as discussed for wild type muscles is also expected to play a major role. Finally, hyperpolarization may also allow closure of CaV1.1 channels, resulting in a decrease in unstimulated force.
Another important aspect is how Ca2+ modulates the effectiveness of salbutamol. For example, at 10 mM K+ and 2.4 mM Ca2+, the mean tetanic force of HyperKPP EDL increased from 91.3% to 111.2% upon salbutamol addition. This gave rise to a force of 41 N/cm2, which is comparable to the mean tetanic force of wild type EDL (i.e., 38 N/cm2). At 1.3 mM Ca2+, HyperKPP EDL tetanic force increased from 52% to 88%, giving a force of 27 N/cm2, a value that is much lower than for wild type EDL. Therefore, Ca2+ can strongly impact the effectiveness of salbutamol in treating HyperKPP patients, considering that the normal range of blood free [Ca2+] is 1.1–1.4 mM. Replacing Ca2+ by Mg2+ had a major effect in HyperKPP EDL. Therefore, salbutamol, and likely other treatments as well, is expected to be more efficient in the setting of elevated blood Ca2+.
In conclusion, this study demonstrates that lowering [Ca2+]e has a major impact on force development in normal muscles when the cell membrane is depolarized. HyperKPP muscle fibers have a greater Ca2+ sensitivity not because there is a difference in the low Ca2+ mechanism of action compared with wild type but rather because their fibers are more depolarized than their wild type counterparts. Three major mechanisms are proposed for the Ca2+ effects: (1) a greater membrane depolarization increasing the number of unexcitable fibers, (2) a hyperpolarizing shift of the steady-state inactivation of NaV1.4 channels lowering action potential amplitude, and (3) a decrease Ca2+ release as CaV1.1 channels are inactivated at low Ca2+. Replacing Ca2+ by Mg2+ allows for some force recovery, but in most cases not to the level observed at 2.4 mM Ca2+. The effectiveness of salbutamol at allowing force recovery is seriously impaired by lowering [Ca2+]e to just 1.3 mM in HyperKPP muscles, a [Ca2+] concentration within the normal range of blood free [Ca2+]. It may be informative to monitor serum Ca2+ levels more closely in HyperKPP patients, especially during paralytic attacks. More clinical observations may help to establish whether Ca2+ (or Mg2+) supplement would help these patients.
Acknowledgments
Eduardo Ríos served as editor.
This study was supported by a Canadian Institutes of Health Research grant (#375151) to J.-M. Renaud.
The authors declare no competing financial interests.
Author contributions: F. Uwera: measurements of force; T. Ammar: measurements of force and membrane potential and writing of manuscript; C. McRae: measurements of force and membrnae potential and writing of manuscript; L.J. Hayward: generation of HyperKPP mouse model and writing of manuscript; J.M. Renaud: experimental design, statistical analyses, and writing the manuscript.
References
- Ammar T., Lin W., Higgins A., Hayward L.J., and Renaud J.M.. 2015. Understanding the physiology of the asymptomatic diaphragm of the M1592V hyperkalemic periodic paralysis mouse. J. Gen. Physiol. 146:509–525. 10.1085/jgp.201511476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C.M., and Cota G.. 1991. Calcium ion as a cofactor in Na channel gating. Proc. Natl. Acad. Sci. USA. 88:6528–6531. 10.1073/pnas.88.15.6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Fitts R., Pizarro G., and Ríos E.. 1988a Voltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling. J. Physiol. 398:475–505. 10.1113/jphysiol.1988.sp017053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Ríos E., and Stéfani E.. 1988b Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J. Physiol. 398:441–473. 10.1113/jphysiol.1988.sp017052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J.D., Lännergren J., and Westerblad H.. 1996. Effects of repetitive tetanic stimulation at long intervals on excitation-contraction coupling in frog skeletal muscle. J. Physiol. 495:15–22. 10.1113/jphysiol.1996.sp021570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns S.P., and Borrani F.. 2015. β-Adrenergic modulation of skeletal muscle contraction: key role of excitation-contraction coupling. J. Physiol. 593:4713–4727. 10.1113/JP270909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns S.P., and Lindinger M.I.. 2008. Do multiple ionic interactions contribute to skeletal muscle fatigue? J. Physiol. 586:4039–4054. 10.1113/jphysiol.2008.155424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns S.P., Westerblad H., and Allen D.G.. 1993. Changes of tension and [Ca2+]i during beta-adrenoceptor activation of single, intact fibres from mouse skeletal muscle. Pflugers Arch. 425:150–155. 10.1007/BF00374515 [DOI] [PubMed] [Google Scholar]
- Cairns S.P., Flatman J.A., and Clausen T.. 1995. Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+-K+ pump. Pflugers Arch. 430:909–915. 10.1007/BF01837404 [DOI] [PubMed] [Google Scholar]
- Cairns S.P., Hing W.A., Slack J.R., Mills R.G., and Loiselle D.S.. 1998. Role of extracellular [Ca2+] in fatigue of isolated mammalian skeletal muscle. J. Appl. Physiol. 84:1395–1406. 10.1152/jappl.1998.84.4.1395 [DOI] [PubMed] [Google Scholar]
- Cairns S.P., Leader J.P., Loiselle D.S., Higgins A., Lin W., and Renaud J.M.. 2015. Extracellular Ca2+-induced force restoration in K+-depressed skeletal muscle of the mouse involves an elevation of [K+]i: implications for fatigue. J. Appl. Physiol. 118:662–674. 10.1152/japplphysiol.00705.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D.T., and Hille B.. 1976. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J. Gen. Physiol. 67:309–323. 10.1085/jgp.67.3.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon S.C. 2006. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu. Rev. Neurosci. 29:387–415. 10.1146/annurev.neuro.29.051605.112815 [DOI] [PubMed] [Google Scholar]
- Cannon S.C., Brown R.H. Jr., and Corey D.P.. 1991. A sodium channel defect in hyperkalemic periodic paralysis: potassium-induced failure of inactivation. Neuron. 6:619–626. 10.1016/0896-6273(91)90064-7 [DOI] [PubMed] [Google Scholar]
- Charles G., Zheng C., Lehmann-Horn F., Jurkat-Rott K., and Levitt J.. 2013. Characterization of hyperkalemic periodic paralysis: a survey of genetically diagnosed individuals. J. Neurol. 260:2606–2613. 10.1007/s00415-013-7025-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., and Flatman J.A.. 1977. The effect of catecholamines on Na-K transport and membrane potential in rat soleus muscle. J. Physiol. 270:383–414. 10.1113/jphysiol.1977.sp011958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., and Flatman J.A.. 1980. Beta 2-adrenoceptors mediate the stimulating effect of adrenaline on active electrogenic Na-K-transport in rat soleus muscle. Br. J. Pharmacol. 68:749–755. 10.1111/j.1476-5381.1980.tb10868.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Wang P., Orskov H., and Kristensen O.. 1980. Hyperkalemic periodic paralysis. Relationships between changes in plasma water, electrolytes, insulin and catecholamines during attacks. Scand. J. Clin. Lab. Invest. 40:211–220. 10.3109/00365518009095569 [DOI] [PubMed] [Google Scholar]
- Clausen T., Nielsen O.B., Clausen J.D., Pedersen T.H., and Hayward L.J.. 2011. Na+,K+-pump stimulation improves contractility in isolated muscles of mice with hyperkalemic periodic paralysis. J. Gen. Physiol. 138:117–130. 10.1085/jgp.201010586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copstead L.E., and Banasik J.. 2013. Pathophysiology. 5th. Elsevier Saunders, Missouri, U.S.A. 1183 pp. [Google Scholar]
- Costantin L.L. 1968. The effect o f calcium on contraction and conductance thresholds in frog skeletal muscle. J. Physiol. 195:119–132. 10.1113/jphysiol.1968.sp008450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis B.M., and Catterall W.A.. 1985. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA. 82:2528–2532. 10.1073/pnas.82.8.2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A.F., and Gage P.W.. 1988. Effects of extracellular calcium concentration and dihydropyridines on contraction in mammalian skeletal muscle. J. Physiol. 399:63–80. 10.1113/jphysiol.1988.sp017068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyken M.L., and Timmons G.D.. 1963. Hyperkalemic Periodic Paralysis with Hypocalcemic Episode. Absence of an Autosomal Dominant Pedigree Pattern. Arch. Neurol. 9:508–517. 10.1001/archneur.1963.00460110076008 [DOI] [PubMed] [Google Scholar]
- Edwards J.N., Macdonald W.A., van der Poel C., and Stephenson D.G.. 2007. O2(*-) production at 37 ° C plays a critical role in depressing tetanic force of isolated rat and mouse skeletal muscle. Am. J. Physiol. Cell Physiol. 293:C650–C660. 10.1152/ajpcell.00037.2007 [DOI] [PubMed] [Google Scholar]
- Ferreira Gregorio J., Pequera G., Manno C., Ríos E., and Brum G.. 2017. The voltage sensor of excitation-contraction coupling in mammals: Inactivation and interaction with Ca2. J. Gen. Physiol. 149:1041–1058. 10.1085/jgp.201611725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeuser B., and Hodgkin A.L.. 1957. The action of calcium on the electrical properties of squid axons. J. Physiol. 137:218–244. 10.1113/jphysiol.1957.sp005808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeuser B., and Meves H.. 1958. The effect of magnesium and calcium on the frog myelinated nerve fibre. J. Physiol. 142:360–365. 10.1113/jphysiol.1958.sp006022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamstorp I., Hauge M., Helweglarsen H.F., Mjönes H., and Sagild U.. 1957. Adynamia episodica hereditaria: a disease clinically resembling familial periodic paralysis but characterized by increasing serum potassium during the paralytic attacks. Am. J. Med. 23:385–390. 10.1016/0002-9343(57)90318-2 [DOI] [PubMed] [Google Scholar]
- Godt R.E., and Nosek T.M.. 1989. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J. Physiol. 412:155–180. 10.1113/jphysiol.1989.sp017609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahin R., and Campbell D.T.. 1983. Simple shifts in the voltage dependence of sodium channel gating caused by divalent cations. J. Gen. Physiol. 82:785–805. 10.1085/jgp.82.6.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward L.J., Brown R.H. Jr., and Cannon S.C.. 1997. Slow inactivation differs among mutant Na channels associated with myotonia and periodic paralysis. Biophys. J. 72:1204–1219. 10.1016/S0006-3495(97)78768-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward L.J., Sandoval G.M., and Cannon S.C.. 1999. Defective slow inactivation of sodium channels contributes to familial periodic paralysis. Neurology. 52:1447–1453. 10.1212/WNL.52.7.1447 [DOI] [PubMed] [Google Scholar]
- Hayward L.J., Kim J.S., Lee M.Y., Zhou H., Kim J.W., Misra K., Salajegheh M., Wu F.F., Matsuda C., Reid V., et al. 2008. Targeted mutation of mouse skeletal muscle sodium channel produces myotonia and potassium-sensitive weakness. J. Clin. Invest. 118:1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R.H., and McDowell M.K.. 1963. Hyperkalemic Paralysis (Adynamia Episodica Hereditaria). Report of Four Cases and Clinical Studies. Am. J. Med. 35:749–767. 10.1016/0002-9343(63)90238-9 [DOI] [PubMed] [Google Scholar]
- Hille B. 2001. Ion channels of excitable membrane. 3rd edition. Sinauer Associates, Sunderland, MA. 814 pp. [Google Scholar]
- Hodgkin A.L., and Huxley A.F.. 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117:500–544. 10.1113/jphysiol.1952.sp004764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A.L., and Katz B.. 1949. The effect of sodium ions on the electrical activity of giant axon of the squid. J. Physiol. 108:37–77. 10.1113/jphysiol.1949.sp004310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiko N., and Sato M.. 1957. The effect of calcium ions on electrical properties of striated muscle fibres. Jpn. J. Physiol. 7:51–63. 10.2170/jjphysiol.7.51 [DOI] [PubMed] [Google Scholar]
- Khogali S., Lucas B., Ammar T., Dejong D., Barbalinardo M., Hayward L.J., and Renaud J.M.. 2015. Physiological basis for muscle stiffness and weakness in a knock-in M1592V mouse model of hyperkalemic periodic paralysis. Physiol. Rep. 3:e12656 10.14814/phy2.12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P.G., Mironov S.L., Doroshenko P.A., and Ponomarev V.N.. 1982. Surface Charges on the Outer Side of Mollusc Neuron Membrane. J. Membr. Biol. 70:171–179. 10.1007/BF01870560 [DOI] [Google Scholar]
- Kuba K., and Nohmi M.. 1987. Role of ion conductance changes and of the sodium-pump in adrenaline-induced hyperpolarization of rat diaphragm muscle fibres. Br. J. Pharmacol. 91:671–681. 10.1111/j.1476-5381.1987.tb11261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Horn F., Rüdel R., Ricker K., Lorković H., Dengler R., and Hopf H.C.. 1983. Two cases of adynamia episodica hereditaria: in vitro investigation of muscle cell membrane and contraction parameters. Muscle Nerve. 6:113–121. 10.1002/mus.880060206 [DOI] [PubMed] [Google Scholar]
- Liu Y., Kranias E.G., and Schneider M.F.. 1997. Regulation of Ca2+ handling by phosphorylation status in mouse fast- and slow-twitch skeletal muscle fibers. Am. J. Physiol. 273:C1915–C1924. 10.1152/ajpcell.1997.273.6.C1915 [DOI] [PubMed] [Google Scholar]
- Lucas B., Ammar T., Khogali S., DeJong D., Barbalinardo M., Nishi C., Hayward L.J., and Renaud J.M.. 2014. Contractile abnormalities of mouse muscles expressing hyperkalemic periodic paralysis mutant NaV1.4 channels do not correlate with Na+ influx or channel content. Physiol. Genomics. 46:385–397. 10.1152/physiolgenomics.00166.2013 [DOI] [PubMed] [Google Scholar]
- Lüttgau H.C., and Spiecker W.. 1979. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J. Physiol. 296:411–429. 10.1113/jphysiol.1979.sp013013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankodi A., Grunseich C., Skov M., Cook L., Aue G., Purev E., Bakar D., Lehky T., Jurkat-Rott K., Pedersen T.H., and Childs R.W.. 2015. Divalent cation-responsive myotonia and muscle paralysis in skeletal muscle sodium channelopathy. Neuromuscul. Disord. 25:908–912. 10.1016/j.nmd.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T.M., Dias da Silva M.R., Miller H.A., Kwiecinski H., Mendell J.R., Tawil R., McManis P., Griggs R.C., Angelini C., Servidei S., et al. 2004. Correlating phenotype and genotype in the periodic paralyses. Neurology. 63:1647–1655. 10.1212/01.WNL.0000143383.91137.00 [DOI] [PubMed] [Google Scholar]
- Pearson C.M. 1964. The periodic paralyses: differential features and pathological observations in permanent myopathic weakness. Brain. 87:341–354. 10.1093/brain/87.2.341 [DOI] [PubMed] [Google Scholar]
- Pedersen K.K., Cheng A.J., Westerblad H., Olesen J.H., and Overgaard K.. 2019. Moderately elevated extracellular [K(+)] potentiates submaximal force and power in skeletal muscle via increased [Ca(2+)]i during contractions. Am. J. Physiol. Cell Physiol. 317:C900–C909. 10.1152/ajpcell.00104.2019 [DOI] [PubMed] [Google Scholar]
- Pizarro G., Fitts R., Uribe I., and Ríos E.. 1989. The voltage sensor of excitation-contraction coupling in skeletal muscle. Ion dependence and selectivity. J. Gen. Physiol. 94:405–428. 10.1085/jgp.94.3.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer D.C., and Kerr D.N.. 1961. A third type of periodic paralysis, with normokalemia and favourable response to sodium chloride. Am. J. Med. 31:328–342. 10.1016/0002-9343(61)90122-X [DOI] [PubMed] [Google Scholar]
- Quiñonez M., González F., Morgado-Valle C., and DiFranco M.. 2010. Effects of membrane depolarization and changes in extracellular [K(+)] on the Ca (2+) transients of fast skeletal muscle fibers. Implications for muscle fatigue. J. Muscle Res. Cell Motil. 31:13–33. 10.1007/s10974-009-9195-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J.M., and Light P.. 1992. Effects of K+ on the twitch and tetanic contraction in the sartorius muscle of the frog, Rana pipiens. Implication for fatigue in vivo Can. J. Physiol. Pharmacol. 70:1236–1246. 10.1139/y92-172 [DOI] [PubMed] [Google Scholar]
- Ricker K., Camacho L.M., Grafe P., Lehmann-Horn F., and Rüdel R.. 1989. Adynamia episodica hereditaria: what causes the weakness? Muscle Nerve. 12:883–891. 10.1002/mus.880121103 [DOI] [PubMed] [Google Scholar]
- Rojas C.V., Neely A., Velasco-Loyden G., Palma V., and Kukuljan M.. 1999. Hyperkalemic periodic paralysis M1592V mutation modifies activation in human skeletal muscle Na+ channel. Am. J. Physiol. 276:C259–C266. 10.1152/ajpcell.1999.276.1.C259 [DOI] [PubMed] [Google Scholar]
- Rudolf R., Magalhães P.J., and Pozzan T.. 2006. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J. Cell Biol. 173:187–193. 10.1083/jcb.200601160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R.L., Simoncini L., and Stühmer W.. 1988. Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle Nerve. 11:502–510. 10.1002/mus.880110514 [DOI] [PubMed] [Google Scholar]
- Sandow A., Taylor S.R., and Preiser H.. 1965. Role of the action potential in excitation-contraction coupling. Fed. Proc. 24:1116–1123. [PubMed] [Google Scholar]
- Santarelli V.P., Eastwood A.L., Dougherty D.A., Ahern C.A., and Horn R.. 2007. Calcium block of single sodium channels: role of a pore-lining aromatic residue. Biophys. J. 93:2341–2349. 10.1529/biophysj.107.106856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov M., Riisager A., Fraser J.A., Nielsen O.B., and Pedersen T.H.. 2013. Extracellular magnesium and calcium reduce myotonia in ClC-1 inhibited rat muscle. Neuromuscul. Disord. 23:489–502. 10.1016/j.nmd.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Skov M., de Paoli F.V., Lausten J., Nielsen O.B., and Pedersen T.H.. 2015. Extracellular magnesium and calcium reduce myotonia in isolated ClC-1 chloride channel-inhibited human muscle. Muscle Nerve. 51:65–71. [DOI] [PubMed] [Google Scholar]
- Steel R.G.D., and Torrie J.H.. 1980. Principles and Procedures of Statistics: A Biometrical Approach. McGraw-Hill Book Company, New York. [Google Scholar]
- Streeten D.H.P., Dalakos T.G., and Fellerman H.. 1971. Studies on hyperkalemic periodic paralysis. Evidence of changes in plasma Na and Cl and induction of paralysis by adrenal glucocorticoids. J. Clin. Invest. 50:142–155. 10.1172/JCI106468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suko J., Maurer-Fogy I., Plank B., Bertel O., Wyskovsky W., Hohenegger M., and Hellmann G.. 1993. Phosphorylation of serine 2843 in ryanodine receptor-calcium release channel of skeletal muscle by cAMP-, cGMP- and CaM-dependent protein kinase. Biochim. Biophys. Acta. 1175:193–206. 10.1016/0167-4889(93)90023-I [DOI] [PubMed] [Google Scholar]
- van Mil H.G., Kerkhof C.J., and Siegenbeek van Heukelom J.. 1995. Modulation of the isoprenaline-induced membrane hyperpolarization of mouse skeletal muscle cells. Br. J. Pharmacol. 116:2881–2888. 10.1111/j.1476-5381.1995.tb15940.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W. 1974. Calcium and lanthanum effects at the nodal membrane. Pflugers Arch. 350:25–39. 10.1007/BF00586736 [DOI] [PubMed] [Google Scholar]
- Wang P., and Clausen T.. 1976. Treatment of attacks in hyperkalaemic familial periodic paralysis by inhalation of salbutamol. Lancet. 307:221–223. 10.1016/S0140-6736(76)91340-4 [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J.Z., and Narahashi T.. 1984. Voltage-dependent calcium block of normal and tetramethrin-modified single sodium channels. Biophys. J. 45:337–344. 10.1016/S0006-3495(84)84159-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yensen C., Matar W., and Renaud J.M.. 2002. K+-induced twitch potentiation is not due to longer action potential. Am. J. Physiol. Cell Physiol. 283:C169–C177. 10.1152/ajpcell.00549.2001 [DOI] [PubMed] [Google Scholar]
- Zhao X., Yoshida M., Brotto L., Takeshima H., Weisleder N., Hirata Y., Nosek T.M., Ma J., and Brotto M.. 2005. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol. Genomics. 23:72–78. 10.1152/physiolgenomics.00020.2005 [DOI] [PubMed] [Google Scholar]
- Zhu Z., Sierra A., Burnett C.M., Chen B., Subbotina E., Koganti S.R., Gao Z., Wu Y., Anderson M.E., Song L.S., et al. 2014. Sarcolemmal ATP-sensitive potassium channels modulate skeletal muscle function under low-intensity workloads. J. Gen. Physiol. 143:119–134. 10.1085/jgp.201311063 [DOI] [PMC free article] [PubMed] [Google Scholar]