Abstract
Objectives:
Subarachnoid hemorrhage (SAH) with negative angiographic findings has a heterogeneous nature with variations in clinical course and outcome as compared to the aneurysmal SAH. It makes up to 15% of the spontaneous SAH and is characterized by milder clinical presentation. The purpose of this study was the analyses of risk factors, clinical observations, radiologic characteristics, and outcome in patients with nonaneurysmal SAH (NA-SAH).
Patients and Methods:
In a retrospective design, 77 consecutive patients with NA-SAH were recruited from May 2008, to October 2018. All patients underwent conventional cerebral angiography. We stratified patients into two groups based on the distribution of blood on their CT scan into perimesencephalic (PM) and non-PM (NPM) SAH. We performed the Outcome using Glasgow Coma Scale (GCS) and modified Rankin scale (favorable mRS 0–2 vs. unfavorable mRS 3–6). Data were analyzed using IBM® SPSS® Statistics V22.0.
Results:
The mean age at presentation was 48.5 ± 8.4 years with male predominance (71.4%). About 76.7% of the patients had headache and vomiting, most of the patients (75.3%) presented with GCS 15 at initial clinical presentation (61.03%) had NPM versus (38.96%) with PM characters in computed tomography scans. Fourteen (17.9%) patients developed hydrocephalus and 12 (15.3%) needed external ventricular drain placement, while none of the patients needed permanent shunt placement. However, all patients had a favorable clinical and functional outcome at discharge and at late follow-up (up to 3 months).
Conclusions:
NA-SAH does not affect the short- and long-term prognosis. In our results, the pattern of bleeding affects the initial presentation, clinical course, and complications. The clinical and functional outcomes in the majority of our patients were comparable in both groups with good prognosis. Hypertension, smoking, and elevated venous pressure, such as a history of deep venous thrombosis and asthma might be considered as a risk factor.
Keywords: Intracranial aneurysm, nonaneurysmal subarachnoid hemorrhage, non perimesencephalic, perimesencephalic, subarachnoid hemorrhage
Introduction
Spontaneous nonaneurysmal subarachnoid hemorrhage (NA-SAH) is a heterogeneous disease with variations in clinical course and outcome as compared to the aneurysmal SAH.[1] NA-SAH is found in up to 15% of patients with spontaneous SAH,[1,2,3,4,5] whereas 20%–70% of SAH has a perimesencephalic (PM) location.[6] PM-SAH often shows a favorable prognosis, low risk of complications, and shorter hospital stay as compared to the aneurysmal SAH,[1,7] or non-PM (NPM) SAH,[1] respectively. However, both PM-SAH and NPM-SAH have better clinical outcome comparing to the aneurysmal SAH.[8]
The clinical course of the NA-SAH is not well defined in the literature. Few series exist to define the risk factors, clinical presentations, and outcomes in NA-SAH.[2,9,10,11,12,13] Therefore, we aimed in our study to identify the risk factors, clinical and neurological status at the initial presentation, radiographic appearance, complications, and outcome of the patients with spontaneous NA-SAH at the time of discharge and 3 months' follow-up. We conducted a retrospective study to investigate the clinical and functional outcomes of patients with spontaneous NA-SAH in short-term follow-up.
The objective of this study was the analyses of risk factors, clinical observations, radiologic characteristics, and outcome in patients with NA-SAH.
PM SAH is described as the entity in which the blood is inferior to Liliequist's membrane (LM) (i.e., PM and/or prepontine cisterns) extension into the suprasellar cistern is common. Significant amounts of blood penetrating LM to the chiasmatic, Sylvian, or interhemispheric cisterns should be viewed with suspicion as shown in Figure 1. While an NPM-SAH pattern has a more diffuse blood distribution that exceeds the previously mentioned regions.[7] that is, it might extend to areas beyond the cisterns such as the proximal part of Sylvian fissure either bilaterally or unilaterally as shown in Figure 2.
Figure 1.
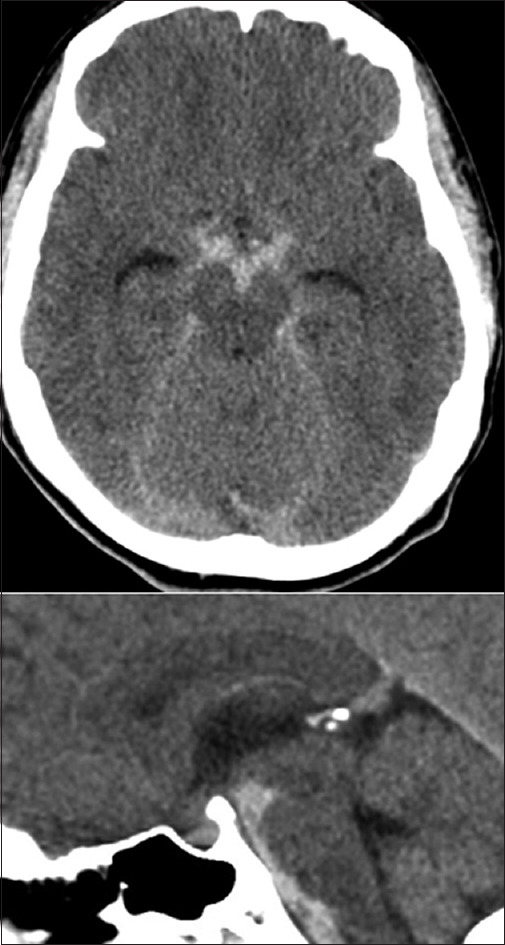
Peri mesencephalic subarachnoid hemorrhage
Figure 2.
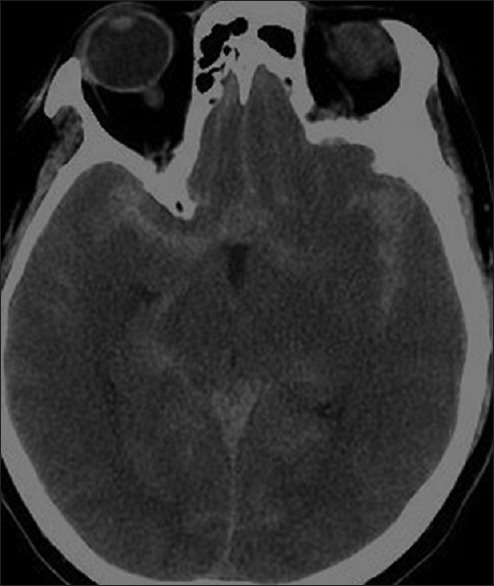
Non-Peri mesencephalic subarachnoid
Computed tomography (CT) scan images were reviewed by two experienced radiologist, for diagnosis and formulating a treatment plan, the mean interobserver kappa coefficient was 0.97
Patients and Methods
Inclusion criteria were as follows: (i) age over 18 years, (ii) spontaneous SAH, (iii) negative 2 subsequent cerebral angiography, (iv) no previous history of aneurysms, and (v) regardless of intraventricular hemorrhage (IVH). We collected retrospective data for the patients with NA-SAH admitted to Hamad General Hospital, Doha, Qatar, from May 2008 to September 2018. Ninety-two consecutive patients were identified from the medical database. CT scan confirmed SAH. 6-vessel angiography (digital subtraction angiography [DSA]) was used twice to rule out any intracranial vascular pathologies in the early and late stages. Fifteen patients were excluded; 6 had no late follow-up DSA, 6 cases had no follow-up after discharge, and 3 had incomplete medical records.
Patient demographics and radiological features
The patient characteristics, including medical identification number, age, gender, comorbidities, neurological status at the initial presentation, clinical course, radiographic or angiographic features, and clinical outcome were recorded.
Glasgow Coma Scale (GCS) and World Federation Neurosurgical Surgeon (WFNS) Score were used to evaluate the initial neurological status of the patients with NA-SAH. Patients were further dichotomized based on the location of blood on the CT scan into PM and NPM SAH. PM was defined if the SAH was limited mainly to the PM cisterns.[7,14] NPM was defined if the pattern of bleeding was extending to the Sylvian and interhemispheric cistern, and not limited to the PM cisterns.[7,14]
Prophylactic antiepileptic was given in most patients; calcium channel blocker (Nimodipine) was added to the patients from the 1st day of admission and stopped after negative second angiogram (late DSA). All patients underwent a second angiogram with a time frame of 7–10 days after onset.
Clinical and functional outcome measures
Primary outcome measures included the determination of clinical outcome at discharge using the modified rankin scale (mRS), as shown in Table 1. Secondary outcome includes any cardiopulmonary complications, vasospasm and/or electrolyte disturbance. The placement of the intracranial shunt system was also recorded.
Table 1.
Modified Rankin scale
| The mRS | |
|---|---|
| Score | Description |
| 0 | No symptoms |
| 1 | No significant disability. Able to carry out all usual activities, despite some symptoms |
| 2 | Slight disability. Able to look after own affairs without assistance, but unable to carry out all previous activities |
| 3 | Moderate disability. Requires some help, but able to walk unassisted |
| 4 | Moderately severe disability. Unable to attend to own bodily needs without assistance, and unable to walk unassisted |
| 5 | Severe disability. Requires constant nursing care and attention, bedridden, incontinent |
| 6 | Dead |
mRS – Modified Rankin Scale
The functional outcome of the patients was evaluated with modified Rankin scale (mRS) at 3 months after the presentation. The patients were followed up by reviewing the patient clinical data records from the outpatient clinic. Patients clinical status was further dichotomized into good and poor clinical, functional outcome based on these scales.
Complications
Delayed cerebral ischemia (DCI) was defined if the patient has deteriorated in the neurological status that cannot be defined by any other factor.[15] It was identified either by clinical presentation or CT scan perfusion.
Hydrocephalus was defined if the CT scan showed the temporal horns to be over 3 mm. Early hydrocephalus was defined from the initial presentation to 3 days, whereas late hydrocephalus was considered from 4 to 14 days. While Hyponatremia defined as serum sodium level less than 135 mmol/l.
Statistical analysis
Continuous variables are summarized by mean and standard deviations and were compared by performing the unpaired Student's t-test. Categorical data were expressed by absolute frequencies (n) and percentages (%) and were analyzed by Chi-square test or Fisher's exact test. We focused on clinical, functional outcome by dichotomizing it into favorable and poor clinical outcome. We performed all statistical analysis using IBM® SPSS® Statistics V22.0 (IBM, Chicago, Illinois, USA). A P = 0.05 or less showed a statistically significant difference.
Results
Statistic of the independent variable is shown in Tables 2 and 3.
Table 2.
Demography of all patients after nonaneurysmal subarachnoid hemorrhage
| Variables | Total |
|---|---|
| Demographics | |
| Age (years), mean±SD | 48.5±8.4 |
| Range (years) | 30-71 |
| Gender, n (%) | |
| Male | 55 (71.4) |
| Female | 22 (28.6) |
| Comorbidities, n (%) | |
| HTN | 12 (15.6) |
| DM | 12 (15.6) |
| Smoker | 5 (6.4) |
| Possible venous HTN (asthma, DVT, COPD) | 10 (13) |
| No | 43 (55.8) |
| GCS at presentation | 14.31 |
| WFNS at presentation, n (%) | |
| Grade 1 | 58 (75.3) |
| Grade 2 | 11 (14.3) |
| Grade 3 | 0 |
| Grade 4 | 8 (10.4) |
| Grade 5 | 0 |
| Clinical presentation, n (%) | |
| Headache | 73 (94.8) |
| Vomiting | 24 (31.2) |
| Low GCS (GCS≤12) | 8 (10.4) |
| Blurred vision, n (%) | 6 (8.2) |
| Radiological features, n (%) | |
| NPM | 47 (61.03) |
| PM | 30 (38.96) |
| Modified Fisher grading system, n (%) | |
| Grade 1 | 0 |
| Grade 2 | 0 |
| Grade 3 | 59 (76.6) |
| Grade 4 | 18 (23.4) |
| Complications, n (%) | |
| Seizure | 2 (2.5) |
| Hyponatremia | 2 (2.5) |
| IVH | 18 (23.4) |
| Hydrocephalus | 13 (16.9) |
| Vasospasm | 0 |
| EVD placement | 13 (16.9) |
| Shunt placement | 0 |
| Cardiopulmonary complications | 0 |
| Outcomes, n (%) | |
| GCS at discharge | 15 (100) |
| Modified Rankin scale at 3 months | 1 (100) |
| EGCOS at 3 months | 8 (100) |
PM – Perimesencephalic SAH; NPM – Non-PM SAH; EVD – External ventricular drainage; GCS – Glasgow Coma Scale; EGCOS – Extended Glasgow Coma Outcome Scale; SD – Standard deviation; HTN – Hypertension; DM – Diabetes mellitus; DVT – Deep vein thrombosis; COPD – Chronic obstructive pulmonary disease; WFNS – World Federation Neurosurgical Surgeon; IVH – Intraventricular hemorrhage
Table 3.
Statistical analysis of different features with the corresponding P value
| Features | NPM | PM | P |
|---|---|---|---|
| Frequency, n (%) | 47 (61) | 30 (39) | |
| Sex-female, n (%) | 9 (19.1) | 13 (43.3) | 0.037 |
| Hyponatremia, n (%) | 0 | 1 (3.33) | 0.39 |
| Seizures, n (%) | 1 (0) | 1 (3.33) | 0.39 |
| IVH, n (%) | 17 (36.1) | 8 (26.6) | 0.459 |
| Age, mean±SD | 48.09±7.77 | 49.17±9.51 | 0.587 |
| Diabetes, n (%) | 10 (21.27) | 2 (6.67) | 0.1539 |
| HTN, n (%) | 5 (10.63) | 7 (23.34) | 0.2413 |
| Possible venous HTN, n (%) | 7 (14.9) | 3 (10) | 0.7989 |
| GCS at presentation, mean±SD | 14.06±1.774 | 14.7±0.887 | 0.072 |
| GCS at 3 months, mean±SD | 15±0.00 | 15±0.00 |
PM – Perimesencephalic; NPM – Non-PM; IVH – Intraventricular hemorrhage; SD – Standard deviation; HTN – Hypertension; GCS – Glasgow Coma Scale
Gender
Twenty-two females and 55 males with a mean age of 48.5 ± 8.4 and a range of 30–71 years were identified.
Comorbidities
About 55.8% of the patients were previously healthy with no comorbidities, 15.6% had hypertension, while diabetes mellitus (DM) was found in 15.6% of the patients, 6.8% were smokers, 13% were asthmatic or had deep venous thrombosis or a history of chronic obstructive pulmonary disease (COPD).
Clinical presentation
Clinical presentation includes headache in 73 patients (94.8%), along with vomiting in 24 patients (31.6%) and low level of consciousness in 8 patients 10.4%, all (100%) of them were of NPM SAH subtype [Graph 1]. In our series WFNS score at presentation was 75.3%, 14.3% and 10.4% for Grade I, II and IV respectively as shown in Graph 2.
Graph 1.
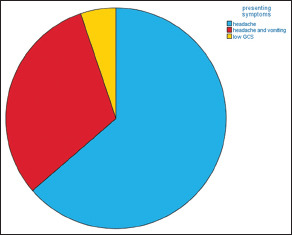
Clinical presentation, please notice that headache is the most common presenting symptoms in our series
Graph 2.
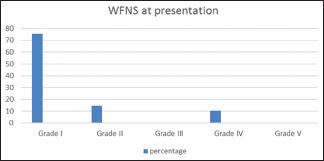
Initial World Federation Neurosurgical Surgeon grades at presentation. Grade I World Federation Neurosurgical Surgeon was the most common grade at presentation followed by Grade II and then Grade IV
Radiographic features
Forty-seven (61.03%) on CT scan had NPM SAH distribution, while 30 (38.96%) had PM SAH distribution. Eighteen patients (23.4%) of the patient had an IVH; however, 77% (14 patients) of these 18 were NPM SAH subtype. In addition to that 76.6% of the patients has Grade III on Modified fischer scale as shown in Graph 3.
Graph 3.
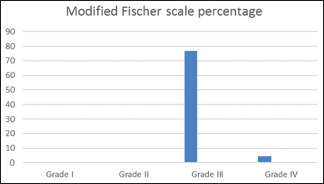
Modified Fischer grading System, Grade III was the most common grade at presentation, please notice the at Grade I and II were not seen in our series
Clinical outcome
All patients had GCS 15 at discharge with good clinical outcome functional outcome (mRS 1) at 3 months after discharge, as stated in Graph 4.
Graph 4.
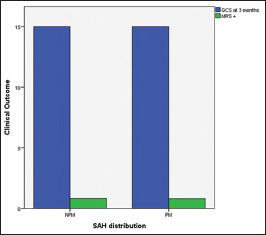
Functional outcome at 3 months, functional outcome was associated with good outcome in both groups of nonaneurysmal subarachnoid hemorrhage
Complications
During the stay in our hospital, only 2 patients had seizures and 2 patients had low Na+ (below 135 mmol). Eighteen patients had an IVH, Around 77% (14 patients of IVH patients) were NPM SAH subtype, and none of the patients had clinical or radiological evidence of vasospasm, and around 8 patients from patients who had IVH needed external ventricular drain (EVD) insertion, but none of them needed a permanent shunt as shown in Graph 5.
Graph 5.
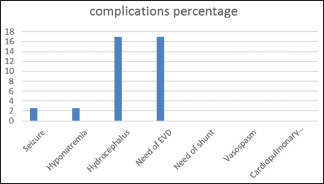
Complication, external ventricular drain need and hydrocephalus was common at presentation, but no patients need any permanent shunt
Thirteen patients developed hydrocephalus, and 13 patients needed EVD placement with no permanent shunt placement in our series of patients, 77% (10 patients out of 13 patients) who needed EVD have NPM SAH subtype.
Discussion
About 15% of patients with spontaneous SAH approximately have negative findings regarding the bleeding source.[1] Spontaneous SAH is not a homogenous disease. There are differences in the clinical course and outcome in patients with and without aneurysmal SAH.[1] PM-SAH makes up 21%–68% of negative DSA.[16] Even if the angiographic results are negative, repeated angiography reserves its essential to identify a potential source of bleeding after an acute phase.[17] In our cohort, all patients underwent a second angiogram within 7–10 days from presentation, around 17% of cases with spontaneous SAH, has negative angiogram, we take this percentage from a review done within our center.
Theories are controversial, but it can be assumed that the NA-SAH may be due to rupture of the perforating arteries or capillaries, arterial dissection, especially basilar, cavernous malformations, arteriovenous malformations, and superficial or deep vein thrombosis.[16,17,18,19,20,21] In our study, we have found 15.6% of the patients who had arterial hypertension, 15.6% had DM, 6.4% were smoker and 13% of the patients have other comorbidities (asthmatics, COPD, and deep venous thrombosis), these comorbidities share in the potential high venous pressure, Although none of these comorbidities has statistical significance (P > 0.05) [Table 3] which could be due to small study sample. This might be a risk factor for developing NA-SAH, but case–control study remains needed with large sample size.
It is different among various studies in identifying the incidence of NA-SAH among gender; it varies from female predominance,[6,22] to no predominance,[23] to male predominance.[8,2,23] In our study, 71.4% of males had NA-SAH. However, younger age and female sex have been considered as a risk factor for NA-SAH.[17,23] In our cohort, female sex reaches a statistical significance with P = 0.037. Male predominance in our cohort could be due to male predominance in Qatar. In 2015, male to female ratio for Qatar was 316.85 males per 100 females.[24]
Clinical presentation
As Konczalla et al.[25] 2014 reported, 85% of the patients with NA-SAH had WFNS Grade I–III at their initial presentation (P < 0.07). Our study also suggested that almost 58 (75.3%) of the patients had GCS 15 (WFNS Grade 1) at their initial presentation. PM-SAH has relatively favorable initial neurological status.[18,19,25] In our data, patients with NPM-SAH had initial severe symptoms with low GCS (GCS ≤12) as all patients with WFNS Grade IV had NPM SAH, and 3 of them had GCS <10 as compared to the PM-SAH.
Radiographic features
The percentage of patients with PM SAH (38.96%) in our cohort is lower than what reported by Van Calenbergh et al.[26] and Cánovas et al. (56%).[27] This is because SAH is relatively different percentages from regions to others. In our series, NPM-SAH had higher modified Fisher grade in comparison to PM SAH, as 77% of patients (14 Patients) with Grade IV Modified Fisher grade were NPM SAH subtype, while only 4 patients were with PM SAH.
Clinical outcome and complications
Compared to aneurysmal SAH, NA-SAH excellent clinical outcome and prognosis, with low risk of complications that include rebleeding, vasospasm, hydrocephalus, and DCI.[18,19] The rebleeding rate in PM SAH is lower than NPM-SAH,[25] and thus, it has a great impact on the clinical outcome of the patients with SAH.[20] In our retrospective study, no patient had rebleeding. Clinical vasospasm unrelated to angiography is rare,[27] and there is only 1 case reported by Sheehan et al.[26] with good clinical outcomes. In our series, none of the patients developed clinical or radiological vasospasm clear by the second angiogram.
Rinkel et al.[14] described hyponatremia in 29% of cases with NA-SAH (Na+ low than 135 mM). In our study, only two patients developed asymptomatic hyponatremia (Na dropped to <135) and were treated with salt supplementation and improved over the next days. This correlates with the findings in case series by Cánovas et al.[27] in which none of the patients developed hyponatremia.
Early hydrocephalus had a higher rate of incidence in patients with the PM-SAH as found in 1 series,[25] but the placement of permanent of the ventricular shunt system is 3%–13.5%.[6,8,16,21] As per Cánovas et al.,[27] only one patient with PM bleeding pattern had hydrocephalus that required the shunt placement. This correlates with our findings as stated in our study, 13 patients had initial EVD placement as shown in Graph 4, but none of the patients had a permanent shunt in place. However, it worth to mention here that 77% (10 patients out of 13 patients) who needed EVD where exclusively have NPM SAH subtype.
The prognosis of the patients with NA-SAH is much more favorable than aneurysmal SAH as clear by studies.[4,6,27] Our study also suggested that NA-SAH has a favorable outcome apart from whether it occurred, at PM or NPM location. Although NPM-SAH might have a worse initial presentation, higher Fisher grade, and more patients need EVD insertion, but the functional outcome at 3 months is the same.
Conclusions
NA-SAH does not affect the short- and long-term prognosis. In our results, the pattern of bleeding affects the initial presentation, clinical course, and complications. The clinical and functional outcomes in the majority of our patients were comparable in both groups with good prognosis. Hypertension, smoking, and elevated venous pressure like a history of deep venous thrombosis and asthma might be considered as a risk factor.
Limitations
A limitation of our monocentric study is the retrospective design and relatively small number of patients after NA-SAH. In addition, we based our data on the retrospective chart review of a detailed clinical outcome; therefore, there are potential errors in charting and individual history.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: Diagnosis, causes and management. Brain. 2001;124:249–78. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- 2.Gupta SK, Gupta R, Khosla VK, Mohindra S, Chhabra R, Khandelwal N, et al. Nonaneurysmal nonperimesencephalic subarachnoid hemorrhage: Is it a benign entity? Surg Neurol. 2009;71:566–71. doi: 10.1016/j.surneu.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Ruigrok YM, Rinkel GJ, Buskens E, Velthuis BK, van Gijn J. Perimesencephalic hemorrhage and CT angiography: A decision analysis. Stroke. 2000;31:2976–83. doi: 10.1161/01.str.31.12.2976. [DOI] [PubMed] [Google Scholar]
- 4.Bakker NA, Groen RJ, Foumani M, Uyttenboogaart M, Eshghi OS, Metzemaekers JD, et al. Repeat digital subtraction angiography after a negative baseline assessment in nonperimesencephalic subarachnoid hemorrhage: A pooled data meta-analysis. J Neurosurg. 2014;120:99–103. doi: 10.3171/2013.9.JNS131337. [DOI] [PubMed] [Google Scholar]
- 5.Delgado Almandoz JE, Jagadeesan BD, Refai D, Moran CJ, Cross DT, 3rd, Chicoine MR, et al. Diagnostic yield of repeat catheter angiography in patients with catheter and computed tomography angiography negative subarachnoid hemorrhage. Neurosurgery. 2012;70:1135–42. doi: 10.1227/NEU.0b013e318242575e. [DOI] [PubMed] [Google Scholar]
- 6.Ildan F, Tuna M, Erman T, Göçer AI, Cetinalp E. Prognosis and prognostic factors in nonaneurysmal perimesencephalic hemorrhage: A follow-up study in 29 patients. Surg Neurol. 2002;57:160–5. doi: 10.1016/s0090-3019(02)00630-4. [DOI] [PubMed] [Google Scholar]
- 7.van Gijn J, van Dongen KJ, Vermeulen M, Hijdra A. Perimesencephalic hemorrhage: A nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985;35:493–7. doi: 10.1212/wnl.35.4.493. [DOI] [PubMed] [Google Scholar]
- 8.Sandvei MS, Mathiesen EB, Vatten LJ, Müller TB, Lindekleiv H, Ingebrigtsen T, et al. Incidence and mortality of aneurysmal subarachnoid hemorrhage in two Norwegian cohorts, 1984-2007. Neurology. 2011;77:1833–9. doi: 10.1212/WNL.0b013e3182377de3. [DOI] [PubMed] [Google Scholar]
- 9.Beseoglu K, Pannes S, Steiger HJ, Hänggi D. Long-term outcome and quality of life after nonaneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 2010;152:409–16. doi: 10.1007/s00701-009-0518-8. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Goddeau RP, Jr, Selim MH, Thomas A, Schlaug G, Alhazzani A, et al. Atraumatic convexal subarachnoid hemorrhage: Clinical presentation, imaging patterns, and etiologies. Neurology. 2010;74:893–9. doi: 10.1212/WNL.0b013e3181d55efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang DH, Park J, Lee SH, Park SH, Kim YS, Hamm IS. Does non-perimesencephalic type non-aneurysmal subarachnoid hemorrhage have a benign prognosis? J Clin Neurosci. 2009;16:904–8. doi: 10.1016/j.jocn.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Hui FK, Tumialán LM, Tanaka T, Cawley CM, Zhang YJ. Clinical differences between angiographically negative, diffuse subarachnoid hemorrhage and perimesencephalic subarachnoid hemorrhage. Neurocrit Care. 2009;11:64–70. doi: 10.1007/s12028-009-9203-2. [DOI] [PubMed] [Google Scholar]
- 13.Renou P, Tourdias T, Fleury O, Debruxelles S, Rouanet F, Sibon I. Atraumatic nonaneurysmal sulcal subarachnoid hemorrhages: A diagnostic workup based on a case series. Cerebrovasc Dis. 2012;34:147–52. doi: 10.1159/000339685. [DOI] [PubMed] [Google Scholar]
- 14.Rinkel GJ, Wijdicks EF, Hasan D, Kienstra GE, Franke CL, Hageman LM, et al. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet. 1991;338:964–8. doi: 10.1016/0140-6736(91)91836-j. [DOI] [PubMed] [Google Scholar]
- 15.Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- 16.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–7. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 17.Kapadia A, Schweizer TA, Spears J, Cusimano M, Macdonald RL. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: Diagnosis, pathophysiology, clinical characteristics, and long-term outcome. World Neurosurg. 2014;82:1131–43. doi: 10.1016/j.wneu.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Kleinpeter G, Lehr S. Characterization of risk factor differences in perimesencephalic subarachnoid hemorrhage. Minim Invasive Neurosurg. 2003;46:142–8. doi: 10.1055/s-2003-40742. [DOI] [PubMed] [Google Scholar]
- 19.Kong Y, Zhang JH, Qin X. Perimesencephalic subarachnoid hemorrhage: Risk factors, clinical presentations, and outcome. Acta Neurochir Suppl. 2011;110:197–201. doi: 10.1007/978-3-7091-0353-1_34. [DOI] [PubMed] [Google Scholar]
- 20.Boswell S, Thorell W, Gogela S, Lyden E, Surdell D. Angiogram-negative subarachnoid hemorrhage: Outcomes data and review of the literature. J Stroke Cerebrovasc Dis. 2013;22:750–7. doi: 10.1016/j.jstrokecerebrovasdis.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Dalyai R, Chalouhi N, Theofanis T, Jabbour PM, Dumont AS, Gonzalez LF, et al. Subarachnoid hemorrhage with negative initial catheter angiography: A review of 254 cases evaluating patient clinical outcome and efficacy of short-and long-term repeat angiography. Neurosurgery. 2013;72:646–52. doi: 10.1227/NEU.0b013e3182846de8. [DOI] [PubMed] [Google Scholar]
- 22.Pyysalo LM, Niskakangas TT, Keski-Nisula LH, Kähärä VJ, Öhman JE. Long term outcome after subarachnoid haemorrhage of unknown aetiology. J Neurol Neurosurg Psychiatry. 2011;82:1264–6. doi: 10.1136/jnnp.2010.239335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canhão P, Ferro JM, Pinto AN, Melo TP, Campos JG. Perimesencephalic and nonperimesencephalic subarachnoid haemorrhages with negative angiograms. Acta Neurochir (Wien) 1995;132:14–9. doi: 10.1007/BF01404842. [DOI] [PubMed] [Google Scholar]
- 24. [Last accessed on 2019 Oct 22]. Available from: https://knoema.com/atlas/Qatar/topics/Demographics/Population/Male-to-female-ratio .
- 25.Konczalla J, Platz J, Schuss P, Vatter H, Seifert V, Güresir E. Non-aneurysmal non-traumatic subarachnoid hemorrhage: Patient characteristics, clinical outcome and prognostic factors based on a single-center experience in 125 patients. BMC Neurol. 2014;14:140. doi: 10.1186/1471-2377-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan JM, Cloft H, Kassell NF. Symptomatic delayed arterial spasm following non-aneurysmal perimesencephalic subarachnoid hemorrhage: A case report and review of the literature. Acta Neurochir (Wien) 2000;142:709–12. doi: 10.1007/s007010070117. [DOI] [PubMed] [Google Scholar]
- 27.Cánovas D, Gil A, Jato M, de Miquel M, Rubio F. Clinical outcome of spontaneous non-aneurysmal subarachnoid hemorrhage in 108 patients. Eur J Neurol. 2012;19:457–61. doi: 10.1111/j.1468-1331.2011.03542.x. [DOI] [PubMed] [Google Scholar]


