Abstract
Introduction:
The surgical strategies for clipping of paraclinoid aneurysms are diverse. These aneurysms are unique in their location, as they closely abut the anterior clinoid process (ACP) and the optic nerve. The ultimate goal of clipping encompasses the exposure of neck of the aneurysm which is seldom complete without the manipulation of optic nerve and the ACP. This manipulation may result in disturbances of vision postoperatively. We analyze our results of visual outcomes in the surgery for paraclinoid aneurysms in this retrospective study.
Materials and Methods:
All patients with paraclinoid aneurysms who underwent surgery from June 2014 to June 2019 were included in the study. Surgical procedure was uniform in all patients which included anterior clinoidectomy and clipping of aneurysms as per the Bantane protocol. Glasgow Outcome Scale as well as vision was assessed at discharge and at 1 month and 6 months.
Results:
There were 77 cases of paraclinoid aneurysms operated during the abovementioned period. All patients had no symptoms related to vision preoperatively. Visual deterioration was noted in two patients. All patients were discharged with a good outcome on the Glasgow Outcome Scale.
Conclusion:
Paraclinoid aneurysm has a good outcome when treated with surgery. The visual deterioration following surgery can be minimized with extradural anterior clinoidectomy and careful handling of the vessels and nerve.
Keywords: Clipping, paraclinoid aneurysms, visual outcomes
Introduction
Paraclinoid aneurysms originate from the internal carotid artery (ICA) between the distal dural ring and the origin of the posterior communicating artery.[1,2,3,4] These aneurysms arise from any wall of the ICA and classified by many authors into different types.[2,3,5,6] They may arise in relation to the ophthalmic or the superior hypophyseal branch, but more so often have no relation to either of them. Techniques for clipping these aneurysms once considered formidable have evolved through many modifications until today. Various methods have evolved from the times of Victor Horsley who advocated ligation of ICA to the present modern era of anterior clinoidectomy and clipping in the management of these aneurysms. The peculiar location of these aneurysms around the closely crowded space bounded by anterior clinoid process (ACP) and internal carotid along with its dural folds and the optic nerve makes it a daunting task for the surgeon to clip these aneurysms.
The sense of vision is closely related and hence deserves special mention when dealing with aneurysms in the paraclinoid region. Vision can be a presenting symptom of paraclinoid aneurysm as well as it can also be affected following surgery for paraclinoid aneurysms. It has been mentioned in the literature that visual deficits accompany paraclinoid aneurysms in the range of 16%–40%.[2,7,8,9,10,11,12,13] These visual deficits may improve, remain unchanged, or worsen after clipping surgery. Moreover, it has also been reported that the development of new-onset visual deficit can occur following clipping surgery.[2,12,14,15,16,17,18,19] The factors responsible for this visual decline are numerous ranging from mechanical injury, thermal injury, or vasospasm. In this retrospective analysis, we review our results of paraclinoid aneurysms emphasizing the visual outcomes managed with multimodality protocol performed at our institution.
Materials and Methods
In this report, we retrospectively analyze the outcomes of all paraclinoid aneurysms who underwent surgery from June 2014 to June 2019 at Banbuntane Hotokukai Hospital, Fujita Health University, Japan. We recommend to treat all aneurysms more than 7 mm in size. We also do not refuse treatment for apprehensive patients who are harboring an aneurysm of size <3–7 mm. All patients underwent surgery for paraclinoid aneurysms with multimodal strategy along with neuromonitoring known as the Bantane protocol. Medical records, imaging findings, surgical techniques, complications, and the final results of the surgery with emphasis on visual outcome were retrospectively reviewed.
Preoperative workup
The preoperative workup of all patients consisted of undergoing a four-vessel digital subtraction angiography. This was followed by computed tomography angiogram to know the details of the clinoid process in relation to the aneurysm. Fusion images were created in to know the relation of the optic nerve to the aneurysm in certain cases where it was anticipated that moderate handling of the optic nerve would occur during clipping of the aneurysm. All patients were physically examined for any neurologic deficit. Objective assessment of field of vision was performed by confrontation method.
Location of aneurysms
The location of the aneurysm was classified in relation to the parent ICA. Accordingly, they were classified into (1) superior (true ophthalmic aneurysm), (2) inferior (ventral aneurysms), (3) lateral aneurysms (subclinoid), and (4) medial (superior hypophyseal and carotid cave aneurysms) as described by Krisht and Hsu.[20]
Surgical technique
Bantane protocol consists of a series of steps that are orderly followed during the clipping of aneurysms. The protocol commences on starting all aneurysm surgery with neuromonitoring which is done by motor-evoked potential (MEP) and follows a series of steps outlined below. The decision to perform a clinoidectomy or not is taken during the planning of the procedure. The procedure of removal of clinoid is done by the method described by Dolenc. The dura of the temporal lobe is retracted back to expose the meningo-orbital band. This band is cut to retract the dura further backward to expose the attachments of the clinoid. The clinoid is removed extradurally in piecemeal by short-stout clinoidectomy rongeurs of various angulations (Fujita Medical Instruments Co., Ltd.). This is followed by opening the dura linearly straight down toward the junction of the optic nerve and ICA. This maneuver is done carefully under high magnification. The origin of the aneurysm in the region of the clinoid is verified before cutting the arachnoid around the ICA. After baring the ICA, careful dissection of the aneurysm neck is done using sharp dissection. This sometimes involves the cutting of the distal dural ring depending on the location of the aneurysm neck. The linear incision of the dura helps in performing this procedure with ease. After the aneurysm is exposed, careful dissection of the perforators is performed. The blind end of the aneurysm is further inspected with endoscope. This is followed by performing indocyanine green–videoangiography (ICG-VA) and dual injection videoangiography (DIVA)[21] to further delineate the perforators in real time. After this, proper clipping of the neck of the aneurysm is performed with appropriate clip. After clipping again, ICG-VA/DIVA is performed to check for the filling of the aneurysm. This procedure is followed by re-inspecting the site with the endoscope to check for the completeness of the clipping as well as status of perforators near the clip. Any changes in the MEP are verified before the procedure of closure begins. Arachnoidoplasty is done with Surgicel (Johnson & Johnson Co., Ltd.) upon which Fibrin glue is sprayed and finally the dura is closed.
There were few patients with large paraclinoid aneurysms who underwent surgical clipping through retrograde suction decompression (RSD) technique. In these patients, additional neck exploration and exposure of the ICA and the external carotid artery was performed. The superior thyroid artery was accessed by open technique described by Tamaki et al., and retrograde decompression was done thorough it after clamping the ICA in the neck as well as distal to the aneurysm site.
Results
There were a total of 77 cases of paraclinoid aneurysm operated by the abovementioned protocol during the period of June 2014 to June 2019. The distribution of aneurysms is summarized in Table 1. There were 72 females and 5 males in the study group. The mean age of the patients was 53.67 ± 13.09 years. The projection of the aneurysm dome was in the superior direction in 33 cases, inferior in 5 cases, medial in 30 cases, and lateral in 9 cases [Table 1]. The patients were all asymptomatic with respect to the aneurysm. The mean size of the aneurysm was 5.22 ± 2.28 mm. The largest size encountered was 15 mm. Extradural clinoidectomy was performed in all patients. Aneurysms in all patients except four were successfully clipped with various combinations of fenestrated and straight clips. There were six patients in whom successful clipping was performed through RSD approach. The four patients in whom the aneurysms could not be clipped underwent wrapping with Neoveil (Gunze, Medical Division, Japan). All these four aneurysms were directed medially. Among these four aneurysms, one case underwent attempted clipping through RSD. However, as there was intraoperative rupture during RSD, it could only be wrapped and could not be clipped. In one case, the aneurysm was found to be extradural in origin and hence was left alone.
Table 1.
Projection of the aneurysm in the study
| Aneurysm projection | n |
|---|---|
| Superior | 33 |
| Medial | 30 |
| Inferior | 5 |
| Lateral | 9 |
Outcome
All patients had a good outcome clinically with a Glasgow Outcome Scale of 5/5. Angiographic total occlusion was achieved in 72 cases, which was assessed by intraoperative ICG angiography. The remaining four cases in whom aneurysms could not be clipped and only coated were advised to undergo endovascular coiling for aneurysm obliteration. The only morbidity observed was in the form of visual decline, which was observed postoperatively in two patients. One patient had a total visual loss of one eye on one side and the other has inferior quadrantanopia. Both these aneurysms were medially directed. In the patient who had complete blindness, the deficit progressed from narrowing of visual field to total blindness in a span of 48 h. Following this complaint, hypertensive therapy was initiated as well as administration of steroids (dexamethasone 10 mg every 6 h). Specialist opinion from an ophthalmologist was sought, and compressive optic neuropathy was suspected. Magnetic resonance imaging (MRI) brain was performed which did not reveal any significant cause for the deficit. This deficit failed to improve in this patient until discharge. The second patient complained about partial loss of vision a day after the surgery. She too underwent ophthalmological evaluation, and MRI brain was taken which failed to detect any cause for her deficit. Steroid therapy was initiated along with induced hypertension without any improvement in her vision. At follow up of 1 month and 6 months, these visual deficits showed no improvement.
Illustrative cases
Case no 1
A 61-year-old female with headache was incidentally detected to have a left paraclinoid aneurysm [Figure 1a-d]. She underwent surgery for clipping of the aneurysm as per the above mentioned protocol. Postoperatively, she noticed diminution of vision in the left eye. This vision loss was initially peripheral to begin with but gradually progressed to total blindness in the left eye over the next 48 h. She was initiated on high-dose steroid dexamethasone MRI brain [Figure 2a, b] along with magnetic resonance angiogram did not show any focal injury to the optic nerve.
Figure 1.
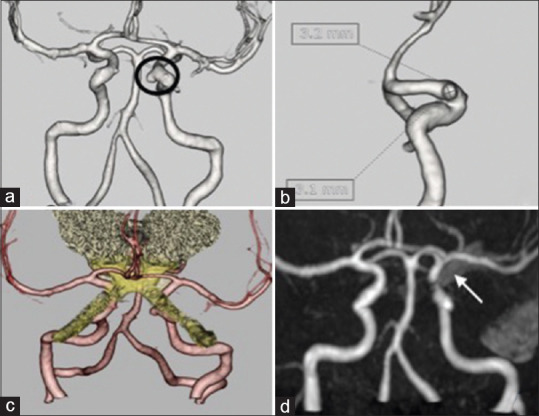
(a and b) Computed tomography angiogram revealed a saccular aneurysm of size 3.1 mm × 3.2 mm aneurysm in the left internal carotid artery. (c) Fusion image of magnetic resonance imaging with computed tomography angiogram shows the relation of the aneurysm as inferior and closely abutting the medial part of the left optic nerve. (d) Magnetic resonance angiography – postoperative magnetic resonance angiography showing total occlusion of the aneurysm with the aneurysm clip in situ (arrowhead)
Figure 2.
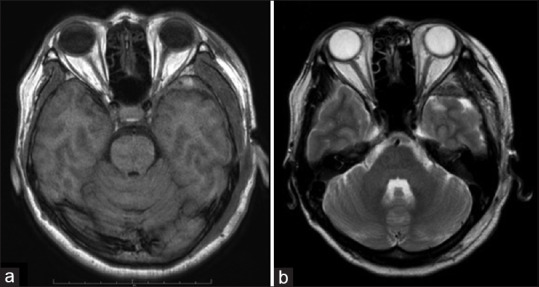
Postoperative scan (a) T1-weighted and (b) T2-weighted image showing normal and intact optic nerve
Case no 2
A 56-year-old female presented with headache and was incidentally detected to have a saccular aneurysm over the paraclinoid region of right Internal carotid artery [Figure 3]. She underwent clipping of aneurysm and on the second postoperative day noticed a partial visual loss (superior quadrantanopia) in her right eye A CT scan of the brain [Figure 4a] and MRI of the brain [Figure 4b, c] was taken which did not reveal any cause for the deficit. She was treated with high dose steroid but there was no improvement in the visual deficit.
Figure 3.
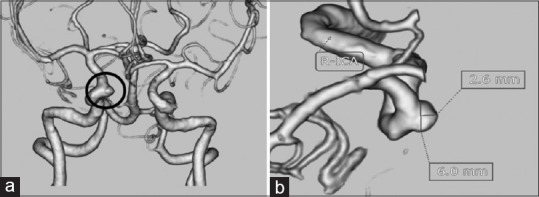
Computed tomography angiogram (a and b) paraclinoid aneurysm of size 2.6 mm × 6.0 mm in the right internal carotid artery
Figure 4.
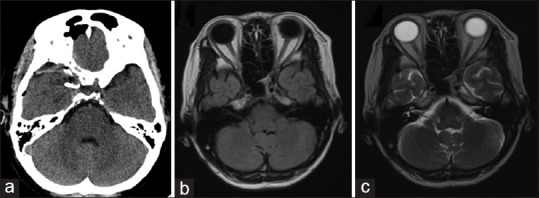
(a) Computed tomography scan brain plain – postoperative computed tomography scan showing normal brain. Right clinoidectomy can be visualized along with the clip in the right paraclinoid region (white arrow). There was no hematoma visualized in the orbit. (b and c) Magnetic resonance imaging brain plain – postoperative magnetic resonance imaging (a) T1-weighted and (b) T2-weighted images showing a normal study of the optic nerve and the brain
Discussion
Paraclinoid aneurysms once regarded as unclippable could be effectively clipped owing to the refinements in the microsurgical techniques over the years. These aneurysms arise in the narrow-crowded corridor as the ICA enters the subarachnoid space from the roof of the cavernous sinus. Due to technical challenges in handling these difficult aneurysms microsurgically, the focus has shifted toward endovascular management in recent times. However, owing to the increased incidence of angiographic recurrences as well as retreatments, many authors have advocated microsurgical clipping as the definitive treatment. However, microsurgical treatment has been associated with an increased incidence of visual decline in many patients.[22] Moreover, it has also been observed that these aneurysms also present with visual deficit preoperatively. Nonaka et al. noted that large aneurysms with intraluminal thrombosis and calcification are more frequently associated with visual deficits.[7] The outcomes of paraclinoid aneurysms with respect to vision are summarized in Table 2.
Table 2.
Summary of large series of paraclinoid aneurysms with visual outcome
| Author and years | Number of treated aneurysms | Cases with preoperative visual deficit | New postoperative visual deficit | |||
|---|---|---|---|---|---|---|
| Total | Improved | Unchanged | Worsened | |||
| Day, 1990 | 54 | 23 | 17 (73.9) | 6 (26.1) | 0 (0) | 3 (5.6) |
| Hoh et al., 2001[43] | 180 | 12 | 8 (66.7) | 3 (25.0) | 1 (8.3) | 5 (2.8) |
| Raco et al., 2008[14] | 108 | 26 | 13 (50.0) | 12 (46.2) | 1 (3.8) | 3 (2.8) |
| Fulkerson et al., 2009[15] | 126 | 27 | NR | NR | NR | 6 (4.8) |
| Nanda and Javalkar, 2011[16] | 86 | 15 | 10 (66.7) | 3 (20.0) | 2 (33.3) | 7 (8.1) |
| Kanagalingam et al., 2012[17] | 69 | 6 | 0 (0) | 1 (16.5) | 5 (83.3) | 30 (29.7) |
| Lai and Morgan, 2013[18] | 182 | 28 | 17 (57.1) | 9 (32.1) | 3 (10.7) | 6 (3.3) |
| Matsukawa et al., 2016[11] | 136 | 2 | 2 (100) | 0 (0) | 0 (0) | 23 (16.9) |
| Kikuta et al., 2016[19] | 38 | 18 | NR | NR | NR | 10 (26.3) |
| Pasqualin et al., 2016[10] | 66 | 20 | 3 (15.0) | 10 (50.0) | 7 (35.0) | 13 (19.7) |
| Matoano et al., 2016[44] | 127 | 2 | 1 (50) | 1 (50) | 0 (0) | 24 (18.9) |
| Kamide et al., 2018[12] | 207 | 17 | 9 (52.9) | 5 (29.4) | 3 (17.6) | 22 (10.6) |
| Otani et al., 2018[13] | 30 | 4 | 0 | 1 | 3 (10) | 0 |
| Sai Kiran et al., 2018[45] | 29 | 6 | 4 (66.6) | 2 (33.3) | 0 | 1 (3.4) |
| Present series | 77 | 0 | 0 | 0 | 0 | 2 (2.6) |
NR – Not reported.[12] Reproduced with modification from Kamide T,Tabani H, Safaee MM, Burkhardt JK, Lawton MT.Microsurgical Clipping of Ophthalmic artery aneurysms: Surgical results and Visual outcomes with 208 aneurysms. J Neurosurg 2018;129:1511-1521 with permission from JNSPG
These visual declines usually are thought to be a secondary phenomenon to the removal of the ACP[19] as well as handling of the optic nerve or delayed vasospasm. The ACP obscures the part of the ICA as it enters into SAC. The removal of ACP is the workhorse for open microsurgery for paraclinoid aneurysms. Pioneered by Drake et al., this was a first major step in the evolution of surgery for paraclinoid aneurysms.[23] Various methods of removal of ACP have been described in the literature. We follow the method of extradural anterior clinoidectomy described by Dolenc.[24] The orbitomeningeal band which tethers the temporal lobe is carefully severed to uncover the anterior part and tip of the clinoid process. After exposing the clinoid, it is removed in piecemeal by microbone rongeurs of various angulations. These aid to remove the clinoid process in total. Alternatively, this procedure can be done by opening the dura and intradural drilling of the clinoid. This procedure has limitation in that the length of the ICA exposed will marginally shorter than what it would be by extradural removal. Moreover, the chances of injury to vital structure such as optic nerve is high theoretically as there is no protection offered from dura as seen during extradural clinoidectomy.[25] There is also considerable concern about the possibility of thermal injury to the optic nerve as a result of the heat generated while drilling of the clinoid process.[26] This phenomenon has been studied thoroughly in the literature, and it has been observed that temperature in excess of 70°C can occur during the process of drilling.[27,28,29] This rise in temperatures is deemed hazardous and can lead to thermal injury of the optic nerve. It has been studied that irreversible electrophysiologic changes and permanent cell death occur in the optic nerve when exposed to temperatures of 47°C and 58°C within 1 or 2 min.[30] Some authors use ultrasonic bone curette instead of drill which performs clinoidectomy. However, it has been shown in the literature that ultrasonic bone curette can generate more heat compared to conventional drilling.[27,29] Copious irrigation with cold saline during the process of clinoidectomy either with ultrasonic curette or drill can reduce the heat generation and prevent subsequent thermal injury. To alleviate this concern, we generally advocate the removal of the clinoid by bone micro-rongeurs of various dimensions and angulations (Fujita Medical Instruments Co., Ltd.). With a small learning curve, this technique is relatively safe and exposes the optic nerve and the ICA without damaging them.
Matsukawa et al. analyzed risk factors for postoperative visual decline and noted that the plug-in method (plugging of muscle piece and sealant between the dura and ICA) at the end of surgery may alter the microcirculation to optic nerve and cause visual disturbances. They also postulated that the disturbances of flow in the superior ophthalmic vein as well as vasospasm of the superior hypophyseal arteries are contributing factors for visual loss.[11] Kamide et al. proposed that the aneurysm clips itself may be a cause of visual deterioration. The medial edge of the clips should be carefully applied so as not to include small perforators of the optic nerve under it. In a series of paraclinoid aneurysms, Shimizu et al. noted that preoperative visual acuity was the most important factor determining the visual outcome after clipping surgery. They noted that visual acuity less than 20/100 is most likely to worsen after surgery.[8] In another study, Date et al. noted that time interval between onset of visual symptom and surgery was the most important factor for visual outcome following surgery.[31]
In our institution as described above, endoscopic examination for perforators is a must before and after clipping, and hence, in none of the cases, we have had any deficits resulting from perforator injury. The selection of type of clip has been investigated to the visual outcome, and it was found that the usage of fenestrated clips was significantly associated with visual decline postoperatively.[12] Intraoperative visual-evoked potentials may contribute to early detection of visual impairments, but so far, it has found to be unreliable.[19,32,33,34,35] We did not use this modality of neuromonitoring in any of the cases.
Gentle handling of the optic nerve is “sine qua non” in the surgery for paraclinoid aneurysms. The arachnoid layer over the optic nerve should not be breached as far as possible to protect the delicate perforators supplying it. A “no-touch policy” toward optic nerve as advocated by Kamide et al. is essential while clipping of these aneurysms.[12] However, this policy may not always be feasible in some aneurysms, as illustrated in our first case above [Figure 1c]. The optic nerve was overlying the part of the base of the aneurysm, and it was impossible to clip the aneurysm without gentle handling of the optic nerve. Whether this handling led to the postoperative deficit, in this case, is not clearly understood.
The postoperative visual deficit following the clipping of paraclinoid aneurysms can be of immediate or delayed. The immediate visual deficit is always due to mechanical or thermal injury. The delayed visual deficits are a result of vasospasm causing perforator ischemia or vascular ischemia from mechanical pressure as that due to clip. These delayed deficits can be of benefit with the use of high-dose steroid therapy induced-hypertension and hypervolemia. Kamide et al. recommended this treatment in all patients during the first 48 h following clinoidectomy and clipping.[12] This prophylactic treatment has brought down the incidence of delayed visual deficits following surgery for paraclinoid aneurysms in their experience.
The visual apparatus is particularly at risk when clipping paraclinoid aneurysms by performing RSD technique. There have been reports of blindness following this technique which occurs as a result of retinal ischemia secondary to clamping of both the external and the ICAs.[36] Due to this clamping, the ophthalmic artery is deprived of its supply. In our series, there were 7 patients who underwent clipping with RSD, but none developed any postoperative visual deficits.
As of today, there is a rising trend for endovascular techniques in the management of paraclinoid aneurysms. Refinements in both the surgical techniques and endovascular advancements have reflected in better outcomes of patients. However, there have been growing reports of inadequate occlusion rates following endovascular methods. The occlusion rates of clipping have been superior to endovascular techniques. In an analysis of worsened vision following clipping, coiling, and flow diverters (FDs), it was noted that the rate of worsening of vision was 11% in clipping followed by 9% in coiling and 5% in FD group.[37] This applies to both clipping and endovascular intervention. In the same study, it was also found that the rate of iatrogenic visual impairment following clipping was 1%, whereas it was not seen in coiling or FD group. Other studies involving FDs noted a visual improvement in the range of 50%–84.2%.[38,39,40,41,42] There is growing concern regarding the possibility of compromised flow to the ophthalmic artery during the treatment of paraclinoid aneurysms with FDs. The results of treatment in large series involving FD have shown that the possibility of new-onset visual deficit is very small in the range of 1%–2%.[39,41]
The pattern of visual field impairment following coiling and clipping may vary. In their review of series, Shimizu et al. noted that superior field defects were more common in endovascular coiling, whereas inferior nasal quadrantanopia was more common in patients who underwent clipping.[8]
Limitations of the study
The limitation of this study is that all patients were not evaluated preoperatively objectively for quantifying visual loss. Since many a times, a patient with quadrantanopia may not be aware of any visual deficit. There might have been subclinical visual deficit which the patients were unaware and which could only be detected upon examination with perimetry. Hence, it cannot be ascertained to what extent these deficits improved postoperatively or did the visual deficits that were observed in two patients actually a worsening of deficits that were present preoperatively.
Conclusion
Microsurgical clipping of paraclinoid aneurysm is a challenging task. Maneuvers should be done with utmost care and minimal handling of the optic nerve. Copious drilling with saline should be done while drilling of clinoid. The possibility of visual loss should be always kept in mind while dealing with these difficult aneurysms. The patients should be made aware of the possibility of worsening of deficit or development of new deficit postoperatively.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.45
References
- 1.Heros RC, Nelson PB, Ojemann RG, Crowell RM, DeBrun G. Large and giant paraclinoid aneurysms: Surgical techniques, complications, and results. Neurosurgery. 1983;12:153–63. doi: 10.1227/00006123-198302000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Day AL. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. 1990;72:677–91. doi: 10.3171/jns.1990.72.5.0677. [DOI] [PubMed] [Google Scholar]
- 3.Batjer HH, Kopitnik TA, Giller CA, Samson DS. Surgery for paraclinoidal carotid artery aneurysms. J Neurosurg. 1994;80:650–8. doi: 10.3171/jns.1994.80.4.0650. [DOI] [PubMed] [Google Scholar]
- 4.Kim JM, Romano A, Sanan A, van Loveren HR, Keller JT. Microsurgical anatomic features and nomenclature of the paraclinoid region. Neurosurgery. 2000;46:670–80. doi: 10.1097/00006123-200003000-00029. [DOI] [PubMed] [Google Scholar]
- 5.De Jesús O, Sekhar LN, Riedel CJ. Clinoid and paraclinoid aneurysms: Surgical anatomy, operative techniques, and outcome. Surg Neurol. 1999;51:477–87. doi: 10.1016/s0090-3019(98)00137-2. [DOI] [PubMed] [Google Scholar]
- 6.Barami K, Hernandez VS, Diaz FG, Guthikonda M. Paraclinoid carotid aneurysms: Surgical management, complications, and outcome based on a new classification scheme. Skull Base. 2003;13:31–41. doi: 10.1055/s-2003-820555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nonaka T, Haraguchi K, Baba T, Koyanagi I, Houkin K. Clinical manifestations and surgical results for paraclinoid cerebral aneurysms presenting with visual symptoms. Surg Neurol. 2007;67:612–9. doi: 10.1016/j.surneu.2006.08.074. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu T, Naito I, Aihara M, Fujimaki H, Asakura K, Miyamoto N, et al. Visual outcomes of endovascular and microsurgical treatment for large or giant paraclinoid aneurysms. Acta Neurochir (Wien) 2015;157:13–20. doi: 10.1007/s00701-014-2251-1. [DOI] [PubMed] [Google Scholar]
- 9.Oh SY, Lee KS, Kim BS, Shin YS. Management strategy of surgical and endovascular treatment of unruptured paraclinoid aneurysms based on the location of aneurysms. Clin Neurol Neurosurg. 2015;128:72–7. doi: 10.1016/j.clineuro.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Pasqualin A, Meneghelli P, Cozzi F, Chioffi F. Outcome after surgical treatment of paraclinoid carotid aneurysms. Acta Neurochir Suppl. 2016;123:33–9. doi: 10.1007/978-3-319-29887-0_5. [DOI] [PubMed] [Google Scholar]
- 11.Matsukawa H, Tanikawa R, Kamiyama H, Tsuboi T, Noda K, Ota N, et al. Risk factors for visual impairments in patients with unruptured intradural paraclinoid aneurysms treated by neck clipping without bypass surgery. World Neurosurg. 2016;91:183–9. doi: 10.1016/j.wneu.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Kamide T, Tabani H, Safaee MM, Burkhardt JK, Lawton MT. Microsurgical clipping of ophthalmic artery aneurysms: Surgical results and visual outcomes with 208 aneurysms. J Neurosurg. 2018;129:1511–21. doi: 10.3171/2017.7.JNS17673. [DOI] [PubMed] [Google Scholar]
- 13.Otani N, Toyooka T, Takeuchi S, Tomiyama A, Nakao Y, Yamamoto T, et al. Less invasive modified extradural temporopolar approach for paraclinoid lesions: Operative technique and surgical results in 80 consecutive patients. J Neurol Surg B Skull Base. 2018;79:S347–55. doi: 10.1055/s-0038-1654703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raco A, Frati A, Santoro A, Vangelista T, Salvati M, Delfini R, et al. Long-term surgical results with aneurysms involving the ophthalmic segment of the carotid artery. J Neurosurg. 2008;108:1200–10. doi: 10.3171/JNS/2008/108/6/1200. [DOI] [PubMed] [Google Scholar]
- 15.Fulkerson DH, Horner TG, Payner TD, Leipzig TJ, Scott JA, DeNardo AJ, et al. Results, outcomes, and follow-up of remnants in the treatment of ophthalmic aneurysms: A 16-year experience of a combined neurosurgical and endovascular team. Neurosurgery. 2009;64:218–29. doi: 10.1227/01.NEU.0000337127.73667.80. [DOI] [PubMed] [Google Scholar]
- 16.Nanda A, Javalkar V. Microneurosurgical management of ophthalmic segment of the internal carotid artery aneurysms : single-surgeon operative experience from Louisiana State University. Neurosurgery. 2011;68:355–371. doi: 10.1227/NEU.0b013e3182039819. [DOI] [PubMed] [Google Scholar]
- 17.Kanagalingam S, Gailloud P, Tamargo RJ, Subramanian PS, Miller NR. Visual sequelae after consensus-based treatment of ophthalmic artery segment aneurysms: The Johns Hopkins experience. J Neuroophthalmol. 2012;32:27–32. doi: 10.1097/WNO.0b013e31823b6c60. [DOI] [PubMed] [Google Scholar]
- 18.Lai LT, Morgan MK. Outcomes for unruptured ophthalmic segment aneurysm surgery. J Clin Neurosci. 2013;20:1127–33. doi: 10.1016/j.jocn.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Kikuta K, Kitai R, Kodera T, Arishima H, Isozaki M, Hashimoto N, et al. Predictive factors for the occurrence of visual and ischemic complications after open surgery for paraclinoid aneurysms of the internal carotid artery. Acta Neurochir Suppl. 2016;123:41–9. doi: 10.1007/978-3-319-29887-0_6. [DOI] [PubMed] [Google Scholar]
- 20.Krisht A, Hsu SP. Paraclinoid aneurysms: Part 1: Superior (true ophthalmic) aneurysms. Contemp Neurosurg. 2008;30:1–5. [Google Scholar]
- 21.Sato T, Suzuki K, Sakuma J, Takatsu N, Kojima Y, Sugano T, et al. Development of a new high-resolution intraoperative imaging system (dual-image videoangiography, DIVA) to simultaneously visualize light and near-infrared fluorescence images of indocyanine green angiography. Acta Neurochir (Wien) 2015;157:1295–301. doi: 10.1007/s00701-015-2481-x. [DOI] [PubMed] [Google Scholar]
- 22.Asaid M, O’Neill AH, Bervini D, Chandra RV, Lai LT. Unruptured paraclinoid aneurysm treatment effects on visual function: Systematic review and meta-analysis. World Neurosurg. 2017;106:322–30. doi: 10.1016/j.wneu.2017.06.135. [DOI] [PubMed] [Google Scholar]
- 23.Drake CG, Vanderlinden RG, Amacher AL. Carotid-ophthalmic aneurysms. J Neurosurg. 1968;29:24–31. doi: 10.3171/jns.1968.29.1.0024. [DOI] [PubMed] [Google Scholar]
- 24.Dolenc V. Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983;58:824–31. doi: 10.3171/jns.1983.58.6.0824. [DOI] [PubMed] [Google Scholar]
- 25.Mishra S, Leão B, Rosito DM. Extradural anterior clinoidectomy: Technical nuances from a learner's perspective. Asian J Neurosurg. 2017;12:189–93. doi: 10.4103/1793-5482.145544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelstein C, Goldberg RA, Rubino G. Unilateral blindness after ipsilateral prophylactic transcranial optic canal decompression for fibrous dysplasia. Am J Ophthalmol. 1998;126:469–71. doi: 10.1016/s0002-9394(98)00118-4. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki K, Wanibuchi M, Minamida Y, Akiyama Y, Mikami T, Fujishige M, et al. Heat generation by ultrasonic bone curette comparing with high-speed drill. Acta Neurochir (Wien) 2018;160:721–5. doi: 10.1007/s00701-017-3445-0. [DOI] [PubMed] [Google Scholar]
- 28.Matthews LS, Hirsch C. Temperatures measured in human cortical bone when drilling. J Bone Joint Surg Am. 1972;54:297–308. [PubMed] [Google Scholar]
- 29.Chang JR, Gruener AM, Kum C, McCulley TJ. Temperature changes associated with bone drilling in an orbital model: Comparison of ultrasonic bone curette and conventional high-speed rotational drill. Orbit. 2019;38:376–82. doi: 10.1080/01676830.2018.1558267. [DOI] [PubMed] [Google Scholar]
- 30.Chang DJ. The “no-drill” technique of anterior clinoidectomy: A cranial base approach to the paraclinoid and parasellar region. Neurosurgery. 2009;64:ons96–105. doi: 10.1227/01.NEU.0000335172.68267.01. [DOI] [PubMed] [Google Scholar]
- 31.Date I, Asari S, Ohmoto T. Cerebral aneurysms causing visual symptoms: Their features and surgical outcome. Clin Neurol Neurosurg. 1998;100:259–67. doi: 10.1016/s0303-8467(98)00047-x. [DOI] [PubMed] [Google Scholar]
- 32.Gutzwiller EM, Cabrilo I, Radovanovic I, Schaller K, Boëx C. Intraoperative monitoring with visual evoked potentials for brain surgeries. J Neurosurg. 2018;130:654–60. doi: 10.3171/2017.8.JNS171168. [DOI] [PubMed] [Google Scholar]
- 33.Kamio Y, Sakai N, Sameshima T, Takahashi G, Koizumi S, Sugiyama K, et al. Usefulness of intraoperative monitoring of visual evoked potentials in transsphenoidal surgery. Neurol Med Chir (Tokyo) 2014;54:606–11. doi: 10.2176/nmc.oa.2014-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung SB, Park CW, Seo DW, Kong DS, Park SK. Intraoperative visual evoked potential has no association with postoperative visual outcomes in transsphenoidal surgery. Acta Neurochir (Wien) 2012;154:1505–10. doi: 10.1007/s00701-012-1426-x. [DOI] [PubMed] [Google Scholar]
- 35.Luo Y, Regli L, Bozinov O, Sarnthein J. Correction: Clinical utility and limitations of intraoperative monitoring of visual evoked potentials. PLoS One. 2015;10:e0133819. doi: 10.1371/journal.pone.0120525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattingly T, Kole MK, Nicolle D, Boulton M, Pelz D, Lownie SP. Visual outcomes for surgical treatment of large and giant carotid ophthalmic segment aneurysms: A case series utilizing retrograde suction decompression (the “Dallas technique”) J Neurosurg. 2013;118:937–46. doi: 10.3171/2013.2.JNS12735. [DOI] [PubMed] [Google Scholar]
- 37.Silva MA, See AP, Dasenbrock HH, Patel NJ, Aziz-Sultan MA. Vision outcomes in patients with paraclinoid aneurysms treated with clipping, coiling, or flow diversion: A systematic review and meta-analysis. Neurosurg Focus. 2017;42:E15. doi: 10.3171/2017.3.FOCUS1718. [DOI] [PubMed] [Google Scholar]
- 38.O’Kelly CJ, Spears J, Chow M, Wong J, Boulton M, Weill A, et al. Canadian experience with the pipeline embolization device for repair of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2013;34:381–7. doi: 10.3174/ajnr.A3224. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szikora I, Marosfoi M, Salomváry B, Berentei Z, Gubucz I. Resolution of mass effect and compression symptoms following endoluminal flow diversion for the treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2013;34:935–9. doi: 10.3174/ajnr.A3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanweer O, Raz E, Brunswick A, Zumofen D, Shapiro M, Riina HA, et al. Cavernous carotid aneurysms in the era of flow diversion: A need to revisit treatment paradigms. AJNR Am J Neuroradiol. 2014;35:2334–40. doi: 10.3174/ajnr.A4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahlein DH, Fouladvand M, Becske T, Saatci I, McDougall CG, Szikora I, et al. Neuroophthalmological outcomes associated with use of the pipeline embolization device: Analysis of the PUFS trial results. J Neurosurg. 2015;123:897–905. doi: 10.3171/2014.12.JNS141777. [DOI] [PubMed] [Google Scholar]
- 42.Zanaty M, Chalouhi N, Barros G, Schwartz EW, Saigh MP, Starke RM, et al. Flow-diversion for ophthalmic segment aneurysms. Neurosurgery. 2015;76:286–9. doi: 10.1227/NEU.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 43.Hoh BL, Carter BS, Budzik RF, Putman CM, Ogilvy CS. Results after surgical and endovascular treatment of paraclinoid aneurysms by a combined neurovascular team. Neurosurgery. 2001;48:78–89. doi: 10.1097/00006123-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Matano F, Tanikawa R, Kamiyama H, Ota N, Tsuboi T, Noda K, et al. Surgical Treatment of 127 paraclinoid aneurysms with multifarious strategy: Factors related with outcome. World Neurosurg. 2016;85:169–76. doi: 10.1016/j.wneu.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 45.Sai Kiran NA, Sivaraju L, Vidyasagar K, Raj V, Rao AS, Mohan D, et al. Intradural “limited drill” technique of anterior clinoidectomy and optic canal unroofing for microneurosurgical management of ophthalmic segment and PCOM aneurysms-review of surgical results [published online ahead of print, 2018 Nov 27] doi: 10.1007/s10143-018-1054-x. [DOI] [PubMed] [Google Scholar]


