Abstract
Background:
Clinical practice in postoperative bracing after posterior single-level lumbar spine fusion (PLF) is inconsistent between providers. This study seeks to assess the effect of bracing on short-term outcomes related to safety, quality of care, and direct costs.
Methods:
Retrospective cohort analyses of consecutive patients undergoing single-level PLF with or without bracing at a three-hospital urban academic medical center (2013–2017) were undertaken (n = 906). Patient demographics and comorbidities were analyzed. Test of independence, Mann–Whitney–Wilcoxon test, and logistic regression were used to assess differences in length of stay (LOS), discharge disposition/need for postacute care, quality-adjusted life year (QALY), surgical site infection (SSI), hospital cost, total cost, readmission within 30 days, and emergency room (ER) visits within 30 days.
Results:
Among the study population, 863 patients were braced and 43 were not braced. No difference was seen between the two groups in short-term outcomes from surgery including LOS (P = 0.836), discharge disposition (P = 0.226), readmission (P = 1.000), ER visits (P = 0.281), SSI (P = 1.000), and QALY gain (P = 0.319). However, the braced group incurred a significantly higher direct hospital cost (median increase of 41.43%, P < 0.001) compared to the unbraced cohort (bracing cost excluded). There was no difference in graft type (P = 0.145) or comorbidities (P = 0.20–1.00) such as obesity (P = 1.000), smoking (P = 1.000), chronic obstructive pulmonary disease (P = 1.000), hypertension (P = 0.805), coronary artery disease (P = 1.000), congestive heart failure (P = 1.000), and total number of comorbidities (P = 0.228).
Conclusion:
Short-term data suggest that removal of bracing from the postoperative regimen for PLF will not result in increased adverse outcomes but will reduce cost.
Keywords: Lumbar fusion, posterior lumbar spine fusion, single-level bracing
Introduction
Postoperative bracing is a commonly employed adjunct following posterior lumbar fusion (PLF), emerging from several early long-term studies of PLF using bracing as standard postoperative care.[1,2] Surveys of postoperative practices have shown that roughly half of the practitioners utilize bracing following PLF.[3,4] Theoretically, immobilization from an external brace should provide additional support against axial loading on an immature construct as well as limiting truncal motion, thereby improving fusion rates and decreasing pseudarthrosis. Some proponents of bracing additionally maintain that bracing can improve postoperative pain. However, the true utility of postoperative bracing following lumbar fusion is controversial. Conflicting data from both clinical outcomes and biomechanical perspective have led to a lack of guidelines regarding optimal postoperative practices.
Those in favor of bracing point to studies demonstrating an overall decrease in applied force to the spine with bracing[5] leading to decreased rates of pseudarthrosis.[6] Proponents of postoperative bracing also argue that the psychological or proprioceptive reminder of limiting movement is another important component of the utility of postoperative bracing. Practitioners who oppose bracing note that this purported benefit is difficult to measure objectively.[4] Arguments against bracing point to recent studies, which demonstrate no significant difference in quality of life or pain relief at short-term[7,8] and long-term follow-up[9] for braced and unbraced patients. In addition, custom-fit lumbar braces have an average cost of $2,471.04, which increases overall health-care dollars spent per surgical intervention.[10]
These disparate findings are representative of an overall lack of consensus regarding best practices. To more definitively outline the utility of bracing following PLF, in our present study, we sought to retrospectively evaluate short-term postoperative outcomes and cost in braced and unbraced cohorts following single-level PLF.
Methods
Study population
In this institutional review board-approved study, patients undergoing single-level PLF at a three-hospital, 1659-bed Urban University health system were enrolled retrospectively from July 1, 2013, to June 30, 2017. The Neurosurgery Quality Improvement Initiative (NQII) EpiLog tool provided prospective data acquisition on consecutive patients (n = 906). Briefly, the NQII EpiLog tool is a nonproprietary clinical research and quality improvement architecture that was built and overlaid onto the electronic health record system, which enables prospective data collection.[11]
All patients, over the age of 18, undergoing elective single-level PLF performed by neurosurgeons at the institution studied herein were included in the analysis. The study population was separated into braced and unbraced cohorts. The two study cohorts were separated based on the attending surgeon's practice – of the 20 neurosurgeons at the health system, a subset never braced patients for single-level PLF. Remaining cases were confirmed as elective with records of office visits 30 days before the surgery for evaluation and imaging. Intraoperative technique and instrumentation used were at each surgeon's discretion.
Data collection
Patient data were collected through the NQII EpiLog tool from the electronic health record. Patient age, gender, race, the American Society of Anesthesiologists score that rates perfect health as 1 and moribund as 5, and multiple medical comorbidities [Table 1] were recorded. Surgical site infection (SSI), length of stay (LOS), discharge disposition, emergency room (ER) visit within 30 days, and readmission within 30 days were also recorded. Of total 616 patients, 104 braced and 8 unbraced patients completed the EQ-5D-3L questionnaire, a validated measure of health outcomes for cost–utility analysis, to calculate quality-adjusted life year (QALY) for a small subset prospective pilot study. The total cost was calculated as all actual costs directly incurred by the hospital during the inpatient stay, retrieved from billing databases. All continuous variables were assessed with the Student t-test or Wilcoxon rank-sum test where appropriate. All categorical variables were analyzed with Pearson's Chi-square test or Fisher's exact test. Multivariate logistic regression analyses were used to determine disposition location based on the independent variable of bracing. Significant results were defined as P < 0.05. Averages are presented as mean ± standard deviation.
Table 1.
Demographics data for the study population separated by cohort
| Demographic data | |||
|---|---|---|---|
| Brace | No brace, n (%) | P | |
| Sex, n (%) | |||
| Male | 337 (40.26) | 17 (36.17) | 0.5774 |
| Female | 500 (59.74) | 30 (63.83) | |
| Diabetes, n (%) | 40 (5.35) | 1 (2.94) | 1.0000 |
| COPD, n (%) | 4 (0.54) | 0 (0.0) | 1.0000 |
| CAD, n (%) | 6 (0.80) | 0 (0.0) | 1.0000 |
| Obesity, n (%) | 12 (1.61) | 0 (0.0) | 1.0000 |
| CHF, n (%) | 1 (0.13) | 0 (0.0) | 1.0000 |
| HTN, n (%) | 126 (16.87) | 5 (14.71) | 1.0000 |
| Smoker, n (%) | 118 (14.22) | 5 (11.11) | 0.5594 |
| Race, n (%) | |||
| Asian | 14 (1.67) | 0 (0.0) | 0.5195 |
| Black | 107 (12.78) | 10 (21.28) | |
| White | 677 (80.88) | 37 (78.72) | |
| Hispanic/Latino | 11 (1.32) | 0 (0.0) | |
| Unknown | 17 (2.03) | 0 (0.0) | |
| Others | 7 (0.84) | 0 (0.0) | |
| Pacific Islands | 1 (0.12) | 0 (0.0) | |
| East Indian | 3 (0.36) | 0 (0.0) | |
| ASA grade, n (%) | |||
| ASA 1 | 28 (3.36) | 0 (0.0) | 0.0213 |
| ASA 2 | 539 (64.63) | 26 (55.32) | |
| ASA 3 | 266 (31.89) | 20 (42.55) | |
| ASA 4 | 1 (0.12) | 1 (2.13) | |
| Graft type, n (%) | |||
| Allograft | 34 (4.06) | 0 (0.0) | 0.0004 |
| Autograft | 7 (0.84) | 0 (0.0) | |
| Biomechanical | 400 (47.79) | 9 (19.15) | |
| Allograft + autograft | 0 (0.0) | 1 (2.13) | |
| Autograft + biomechanical | 5 (0.60) | 0 (0.0) | |
| Allograft + biomechanical | 23 (2.75) | 0 (0.0) | |
| None | 368 (43.97) | 37 (78.72) | |
| Pack years, mean (SD) | 19.12 (16.05) | 24.5 (26.55) | 0.5868 |
| Problem list number, mean (SD) | 5.74 (6.15) | 7.40 (7.34) | 0.2513 |
| BMI, mean (SD) | 29.35 (5.91) | 29.48 (5.16) | 0.8825 |
| Age, mean (SD) | 57.11 (13.64) | 60.15 (13.9) | 0.1373 |
COPD – Chronic obstructive pulmonary disease; CAD – Coronary artery disease; CHF – Congestive heart failure; HTN – Hypertension; ASA – American Society of Anesthesiologists; SD – Standard deviation; BMI – Body mass index
Results
Patient demographics [Table 1]
Among the study population, 863 patients were braced and 43 were not braced. There was no difference in graft type (P = 0.145) or comorbidities (P = 0.20–1.00) such as obesity (P = 1.000), smoking (P = 1.000), chronic obstructive pulmonary disease (P = 1.000), hypertension (P = 0.805), coronary artery disease (P = 1.000), congestive heart failure (P = 1.000), and problem list number (P = 0.228). The braced group incurred a significantly higher direct cost (median increase of 41.43%, P < 0.001) compared to the unbraced cohort (bracing cost excluded). No difference was seen between the two groups in LOS (P = 0.836), discharge disposition (P = 0.226), readmission (P = 1.000), ER visits (P = 0.281), SSI (P = 1.000), and QALY gain (P = 0.319). The follow-up was 244 days on average (median: 118 days).
Postoperative setting
No difference was seen between the braced and unbraced cohorts in LOS (P = 0.836), discharge disposition (P = 0.226), readmission (P = 1.000), or postoperative ER visits (0.281). In addition, there was no difference in number of SSI following surgery between the two groups (P = 1.000) [Table 2 and Figure 1].
Table 2.
Postoperative setting
| Postoperative variables and complications | ||||||
|---|---|---|---|---|---|---|
| Brace, n (%) | No brace, n (%) | P | OR | 95% low | 95% high | |
| SSI | 13 (1.55) | 1 (2.13) | 0.5373 | 0.7257 | 0.0929 | 5.6685 |
| ER visit within 30 days | 20 (2.39) | 2 (4.26) | 0.3286 | 0.5508 | 0.1249 | 2.4298 |
| Admission within 30 days | 63 (7.53) | 3 (6.38) | 1.0000 | 1.1938 | 0.3605 | 3.9532 |
| Discharge disposition | 562 (67.47) | 30 (63.83) | 0.6051 | 1.1752 | 0.637 | 2.168 |
Comparison of SSI, ER visits, readmissions, and discharge disposition between the two cohorts. OR – Odds ratio; SSI – Surgical site infection; ER – Emergency room
Figure 1.
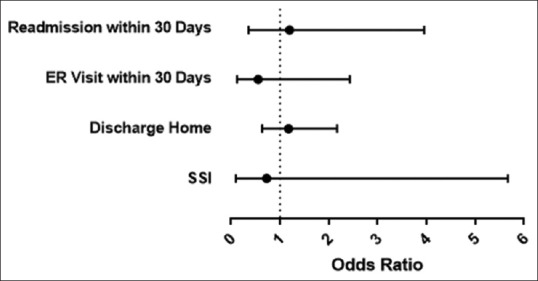
Odds ratios of short-term postoperative risk assessment
Cost analysis and quality
No significant difference was seen in QALY gain (P = 0.319). Assessment of the total direct cost of hospitalization revealed that the braced group incurred a significantly higher direct cost than the unbraced cohort (median increase of 41.43%, P < 0.0001) [Table 3].
Table 3.
Comparison of length of stay and cost for the two cohorts
| Length of stay and cost associated with bracing | |||
|---|---|---|---|
| Median (IQR) | P | ||
| Brace | No brace | ||
| LOS | 80 (33) | 79 (68) | 0.7474 |
| Total cost | 7754.21 (4779.1) | 4378.63 (4101.91) | <0.0001 |
| QALY | 0.04 (0.09) | 0.08 (0.56) | 0.3689 |
LOS – Length of stay; QALY – Quality-adjusted life year; IQR – Interquartile range
Discussion
Clinical decision-making about the application of postoperative bracing for PLF is variable. In this retrospective cohort study, no significant difference in postoperative course or early adverse events between bracing and not bracing following single-level PLF was noted. We assessed patients undergoing single-level PLF at our institution over 4 years, analyzing total cohorts of 863 braced and 43 unbraced. Furthermore, in cost analyses of these two postoperative populations, we show that those patients who were braced following PLF incurred higher direct costs when comparing the median increase of cost. Intraoperative costs are presumably similar for intraoperative costs by virtue of limiting the population to single-level operations. We hypothesized that those unbraced patients may be prone to increased resource utilization for fear of increased complications, but these results demonstrate that this was not the case, and the unbraced patients thereby had similar and less expensive hospital courses.
Prior studies of bracing following lumbar fusion show varied results. Major arguments for bracing are that it aids in postoperative pain control, improves integrity of and promotes fusion of hardware construct by decreasing load on the construct, and serves as a proprioceptive reminder of a recent surgery. With the exception of the latter, which is difficult to study, arguments against bracing are founded on studies that have attempted to disprove these arguments, including those describing the lack of difference in postoperative pain[9] and lack of significant biomechanical advantage to bracing.[12,13]
Differences in these measures would theoretically lead to a difference in adverse outcomes in the postoperative setting. We found no significant difference in LOS and return visits to the emergency department within 30 days. These factors can produce barriers to return to function and can be drivers for overall health-care cost increase. QALY is a more direct metric of disease burden, and we found no significant difference between the braced and unbraced cohorts in this measure in a small prospective pilot subgroup. Moreover, we show that there is an overall increase in direct costs for patients who underwent bracing postoperatively. Brace costs are not insignificant, with off-the-shelf thoraco-lumbo-sacral orthoses, a commonly utilized brace following PLF, costing over $1000, which is nearly doubled if a custom-fit brace is required.[10] These findings suggest that the addition of bracing in postoperative management does not provide added benefit and may also result in higher overall costs. Given findings in previous studies supporting the lack of efficacy of bracing across various presumed areas of benefit, and our findings here showing no major difference in multiple important short-term postoperative metrics, the elimination of postoperative bracing following single-level PLF may represent a safe, more cost-effective management strategy.
Limitations
There are important limitations to this study. Open PLF is an overarching category of spine surgery; there are different surgical approaches to achieve a PLF. These are dependent on operator preference and comfort. In this study, we have not separated approaches to lumbar fusion. As such, differing practices may yield different postoperative results that cloud the data. However, rather than restricting the study to a single type of instrumentation or operative technique, we believe that these results do reflect heterogeneity in operative techniques and thereby retain external validity. In addition, there is a multitude of braces available, which we did not standardize for in our retrospective study. Postoperative outcomes again may differ based on the brace type. Interestingly, despite this variation in bracing pattern, overall costs remained increased. Another important limitation is the statistical analysis in this study. We were limited by an imbalance in sample size between the two cohorts. The univariate analyses reported above are representative of the relationship between bracing and patient outcomes but are not able to incorporate preoperative variables in the analysis. The designation of being braced or unbraced was a feature of standardized surgeon-specific practice; some neurosurgeons braced all of their patients while others braced none of their patients. The dichotomous practices prevent overt selection bias on a case-by-case basis although there could still be biases on which cases each neurosurgeon elected to do. To this end, the cohort demographic analysis does not suggest that there is any significant difference between the braced and unbraced cohorts in this study. Further study will be needed to expand the population, focus on long-term outcomes, and potential for a randomized study.
Conclusion
In this retrospective study of bracing following open PLF, we describe short-term data that suggest that removal of bracing from the postoperative regimen for PLF will not result in increased adverse outcomes but will reduce cost. Long-term analysis of risk and fusion rates is necessary before the elimination of postoperative bracing from postoperative management strategies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the EpiLog project.
References
- 1.Fritzell P, Hägg O, Wessberg P, Nordwall A, Swedish Lumbar Spine Study Group Chronic low back pain and fusion: A comparison of three surgical techniques: A prospective multicenter randomized study from the Swedish lumbar spine study group. Spine (Phila PA 1976) 2002;27:1131–41. doi: 10.1097/00007632-200206010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bjarke Christensen F, Stender Hansen E, Laursen M, Thomsen K, Bünger CE. Long-term functional outcome of pedicle screw instrumentation as a support for posterolateral spinal fusion: Randomized clinical study with a 5-year follow-up. Spine (Phila PA 1976) 2002;27:1269–77. doi: 10.1097/00007632-200206150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Bible JE, Biswas D, Whang PG, Simpson AK, Rechtine GR, Grauer JN. Postoperative bracing after spine surgery for degenerative conditions: A questionnaire study. Spine J. 2009;9:309–16. doi: 10.1016/j.spinee.2008.06.453. [DOI] [PubMed] [Google Scholar]
- 4.Elsenbeck MJ, Wagner SC, Milby AH. Is routine bracing of benefit following posterior instrumented lumbar fusion for degenerative indications? Clin Spine Surg. 2018;31:363–5. doi: 10.1097/BSD.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 5.Nachemson A, Morris JM. In vivo measurements of intradiscal pressure.Discometry, a method for the determination of pressure in the lower lumbar discs. J Bone Joint Surg Am. 1964;46:1077–92. [PubMed] [Google Scholar]
- 6.Skoch J, Zoccali C, Zaninovich O, Martirosyan N, Walter CM, Maykowski P, et al. Bracing After surgical stabilization of thoracolumbar fractures: A systematic review of evidence, indications, and practices. World Neurosurg. 2016;93:221–8. doi: 10.1016/j.wneu.2016.05.067. [DOI] [PubMed] [Google Scholar]
- 7.Soliman HA, Barchi S, Parent S, Maurais G, Jodoin A, Mac-Thiong JM. Early Impact of postoperative bracing on pain and quality of life after posterior instrumented fusion for lumbar degenerative conditions: A randomized trial. Spine (Phila Pa 1976) 2018;43:155–60. doi: 10.1097/BRS.0000000000002292. [DOI] [PubMed] [Google Scholar]
- 8.Caplan I, Sinha S, Schuster J, Piazza M, Glauser G, Osiemo B, et al. The utility of cervical spine bracing as a postoperative adjunct to single-level anterior cervical spine surgery. Asian J Neurosurg. 2019;14:461–6. doi: 10.4103/ajns.AJNS_236_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yee AJ, Yoo JU, Marsolais EB, Carlson G, Poe-Kochert C, Bohlman HH, et al. Use of a postoperative lumbar corset after lumbar spinal arthrodesis for degenerative conditions of the spine. A prospective randomized trial. J Bone Joint Surg Am. 2008;90:2062–8. doi: 10.2106/JBJS.G.01093. [DOI] [PubMed] [Google Scholar]
- 10.Agabegi SS, Asghar FA, Herkowitz HN. Spinal orthoses. J Am Acad Orthop Surg. 2010;18:657–67. doi: 10.5435/00124635-201011000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Gawande AA. Why Doctors Hate Their Computers. The New Yorker: 2018. Nov 12, [Google Scholar]
- 12.Rohlmann A, Bergmann G, Graichen F, Neff G. Braces do not reduce loads on internal spinal fixation devices. Clin Biomech (Bristol, Avon) 1999;14:97–102. doi: 10.1016/s0268-0033(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 13.Jegede KA, Miller CP, Bible JE, Whang PG, Grauer JN. The effects of three different types of orthoses on the range of motion of the lumbar spine during 15 activities of daily living. Spine (Phila Pa 1976) 2011;36:2346–53. doi: 10.1097/BRS.0b013e31820921a5. [DOI] [PubMed] [Google Scholar]


