Abstract
Adenosine is important for local neuromodulation and rapid adenosine signaling can occur spontaneously or after mechanical stimulation, but little is known about how adenosine formed in the extracellular space for those stimulations. Here, we studied mechanically-stimulated and spontaneous adenosine to determine if rapid adenosine is formed by extracellular breakdown of ATP using mice globally deficient in extracellular breakdown enzymes, either CD39 (nucleoside triphosphate diphosphohydrolase 1, NTPDase1) or CD73 (ecto-5′-nucleotidase). CD39 knockout (KO) mice have a lower frequency of spontaneous adenosine events than wild-type (WT, C57BL/6). Surprisingly, CD73KO mice demonstrate sex differences in spontaneous adenosine; males maintain similar event frequencies as WT, but females have significantly fewer events and lower concentrations. Examining the mRNA expression of other enzymes that metabolize ATP revealed tissue nonspecific alkaline phosphatase (TNAP) was upregulated in male CD73KO mice, but not secreted prostatic acid phosphatase (PAP) or transmembrane PAP. Thus, TNAP upregulation compensates for CD73 loss in males but not in females. These sex differences highlight spontaneous adenosine is formed by metabolism of extracellular ATP by many enzymes. For mechanically-stimulated adenosine, CD39KO or CD73KO did not change stimulation frequency, concentration, or t1/2. Thus, the mechanism of formation for mechanically-stimulated adenosine is likely direct release of adenosine, different than spontaneous adenosine. Understanding these different mechanisms of rapid adenosine formation will help to develop pharmacological treatments that differentially target modes of rapid adenosine signaling, and all treatments should be studied in both sexes, given possible differences in extracellular ATP degradation.
Keywords: Adenosine, in vivo, Sex differences, Hippocampus, Mechanosensitive, Spontaneous adenosine, CD73KO, CD39KO, TNAP, PAP
Graphical Abstract
INTRODUCTION
Adenosine is an endogenous nucleoside that plays an important role in physiological and pathological processes including sleep,1 stroke,2 vasodilation,3 and inflammation.4 Recently, rapid changes in adenosine have been discovered using fast-scan cyclic voltammetry (FSCV).5–11 One mode of rapid adenosine signaling is spontaneous adenosine events (without stimulation), which lasts only about 2–3 seconds and occurs randomly, typically every 2–3 min.9 Another mode of rapid adenosine signaling is evoked by mechanical stimulation10 and lasts about 15–30 s. Both modes of transient adenosine signaling result in rapid neuromodulation, rapidly regulating dopamine neurotransmission12 and modulating local blood flow and oxygen levels.13,14 However, the mechanisms of extracellular formation of these two modes of rapid adenosine signaling have not been elucidated, and if they had different mechanisms of formation, it would allow them to be manipulated by different drugs.
Extracellular adenosine has two possible mechanisms of formation, it may be formed intracellularly and then released as adenosine or formed extracellularly via breakdown of extracellular ATP. For the intracellular pathway, adenosine is formed inside the cell via cytosolic 5′-nucleotidase, which dephosphorylates AMP,15 and then adenosine itself is released into the extracellular space via equilibrative adenosine transporters (ENTs) or vesicular release.16 For the extracellular pathway, adenosine is formed in the extracellular space after hydrolysis of ATP. ATP is released in the extracellular space by transporters or through exocytosis.17 The main enzymes that rapidly degrade extracellular ATP to adenosine are nucleoside triphosphate diphosphohydrolase (NTPDase; e.g., CD39, NTPDase-1) which degrades ATP or ADP to AMP and ecto-5′-nucleotidase (CD73), which degrades AMP to adenosine.18 However, adenosine is also formed by other enzymes extracellularly, such as tissue non-specific alkaline phosphatase (TNAP), which dephosphorylates ATP, ADP, and AMP,19 and prostatic acid phosphatase (PAP),11,19 which produces adenosine from AMP.11
Most studies probing the function of adenosine that is formed extracellularly use mice with global deletion of either CD73 (CD73KO) or CD39 (CD39KO).20,21 For example, deletion of CD73 leads to an exaggerated inflammatory response22 and CD73KO increases tumor growth through NF-κB activity via adenosine A2B receptor signaling.23 Decreasing CD39 expression is correlated with reduced adenosine concentrations,24,25 and during stroke, CD39KO mice exhibit larger cerebral infarct volumes and decreased post-ischemic perfusion.26 For rapid adenosine signaling, in lamina II of sagittal spinal cord slices, the concentration of spontaneous adenosine was decreased by CD73KO and PAPKO.11 Thus, we hypothesize that spontaneous adenosine signaling in the brain may also be due to extracellular breakdown of ATP. However, the mechanism of adenosine formation for mechanosensitive adenosine signaling is unknown. Thus, studies comparing spontaneous and mechanosensitive adenosine signaling in CD73KO or CD39KO mice would give insight into the extent that the mechanism of adenosine formation is conserved for these two discrete types of signaling and may lead to a better understanding of how to manipulate different modes of adenosine signaling for therapeutic purposes.
In addition, all previous studies of adenosine formation in CD73KO or CD39KO mice were performed in male mice or did not look for sex differences. Our recent studies show sex differences in the frequency and concentration of spontaneous adenosine release27 and sex differences have been elucidated in behaviors mediated by adenosine receptors,28,29 and in adenosine deaminase activity.30 Many other neuromodulators, such as dopamine, have well known sex differences in the concentration of stimulated release.31 Therefore, we examined sex differences in the mechanism of adenosine formation.
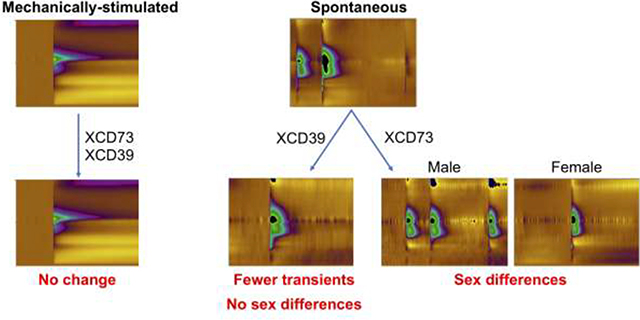
In this study, we investigated the effect of CD39 and CD73 on the formation of spontaneous and mechanically-stimulated adenosine by comparing adenosine in WT mice with mice with global deletions of CD73 or CD39. Adenosine was measured in the hippocampus, an area with a high frequency of spontaneous adenosine transients32 that is associated with memory and learning and adenosine neuromodulation of synaptic transmission during stroke.33–37 Mechanosensitive adenosine signaling is not affected by CD73KO or CD39KO but the frequency of spontaneous adenosine events was reduced in CD39KO and CD73KO mice. Sex differences in spontaneous adenosine events were evident in CD73KO mice and were due to upregulation of TNAP in male CD73KO mice which compensates for CD73 loss. Thus, spontaneous adenosine is formed by extracellular breakdown of ATP, while mechanically-stimulated adenosine is not and it may be released as adenosine per se. Understanding these different mechanisms of rapid adenosine formation will help to develop pharmacological treatments that differentially target modes of rapid adenosine signaling.
RESULTS
Deletion of CD39 or CD73 results in decreased spontaneous adenosine events
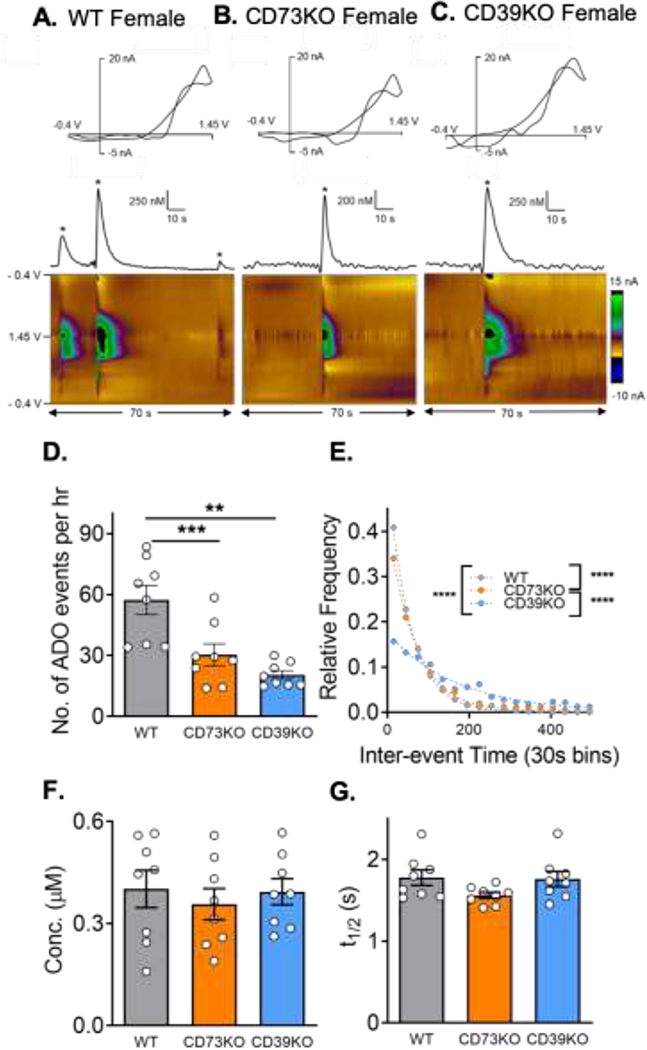
The goal of this study was to investigate the extent to which extracellular metabolism of ATP by CD39 or CD73 is involved in the formation of spontaneous and mechanically-stimulated adenosine in the hippocampus of both male and female mice. Adenosine events varied in WT, CD73KO, and CD39KO mice. In an example color plot of 70 s of data, there were 3 transient adenosine events in a WT female (Fig. 1A). However, there was only 1 event in the example CD73KO and CD39KO female mice (Fig. 1B and C). Example data from male mice are shown in Supplementary Fig. 1. The average number of adenosine events in all mice (males and females) per hour was 57 ± 7 in WT, 30 ± 5 in CD73KO, and 20 ± 2 in CD39KO. There was a significant overall effect of genotype on number of adenosine events (Fig. 1D, One-way ANOVA, n = 8 animals, 4M and 4F per genotype; p = 0.0002). The number of adenosine events in CD73KO and CD39KO mice were significantly lower than in WT (Tukey’s test, p = 0.0049 and p = 0.0002, respectively). To examine the frequency of spontaneous adenosine events, the inter-event time between two consecutive adenosine events was calculated (Fig. 1E). The average inter-event time was 63 ± 2 s in WT, 92 ± 3 s in CD73KO, and 174 ± 7 s in CD39KO. Therefore, transient adenosine events happened on average once a minute in WT mice while they occurred once every 3 minutes in CD39KO mice. There was an overall significant difference in the underlying distributions of inter-event time among all three genotypes (Kruskal-Wallis test, p < 0.0001). Post-tests revealed a significant difference between WT and CD73KO, WT and CD39KO, as well as CD73KO and CD39KO (Dunn’s test, all p < 0.0001). Thus, spontaneous adenosine events were most frequent in WT followed by CD73KO and CD39KO.
Figure 1. Spontaneous adenosine in the hippocampus.
Example adenosine events in females of (A) wild-type (B) CD73KO, and (C) CD39KO mice. Adenosine can be identified in cyclic voltammograms (top) by its primary oxidation peak at 1.3 V on the cathodic scan and secondary oxidation peak at 1.2 V on the anodic scan. Concentration vs. time traces (middle) were derived from corresponding 3-D color plots (bottom). Adenosine oxidations are the green/purple area in the middle of the color plot. (D) Number of adenosine events per hour (One-way ANOVA, n = 8 animals (4M/4F)/genotype, overall effect p = 0.0002, Tukey’s test, ** p<0.01, ***p < 0.001). (E) Inter-event time distributions. The underlying inter-event time distributions of adenosine were significantly different (Kruskal-Wallis test, overall effect p < 0.0001, Dunn’s test, **** p < 0.0001). (F) Mean concentrations of each adenosine event did not vary by geneotype (One-way ANOVA, n = 8 animals/genotype, p = 0.77). (G) Average t1/2 did not significantly differ by genotype (One-way ANOVA, n = 8 animals/genotype, p = 0.13).
The average concentration of adenosine events in each genotype was similar with 0.40 ± 0.05 μM in WT, 0.37 ± 0.05 μM in CD73KO, and 0.39 ± 0.04 μM in CD39KO. There was no significant overall effect of genotype on spontaneous adenosine concentration (Fig. 1F, one-way ANOVA, p = 0.77). The t1/2 was calculated from the width at the half peak height. The average t1/2 was 1.8 ± 0.1 s in WT, 1.6 ± 0.1 s in CD73KO, 1.8 ± 0.09 s in CD39KO, and they were not significantly different (Fig. 1G, one-way ANOVA, p = 0.13). Thus, CD73KO and CD39KO did not affect the concentration or t1/2, but significantly decreased the number and frequency of spontaneous adenosine events.
Deletion of CD73 or CD39 does not affect mechanically-stimulated adenosine in vivo
Mechanical stimulation of adenosine was performed by lowering a carbon-fiber microelectrode 0.1 μm after 4 hours data collection of spontaneous adenosine. Previous studies showed that this does not damage cells or cause apoptosis.10 The mechanical stimulation was repeated every 15 min for a total of 4 stimulations; new tissue was stimulated every time because the electrode was always moved down. Occasionally, a mechanical stimulation did not induce detectable adenosine concentrations. In both WT and CD39KO, 4 out of 32 stimulations did not evoke adenosine, while 2 out of 32 stimulations in CD73KO (n = 8 animals) did not evoke adenosine. Thus, there was no difference in frequency of mechanically-evoked adenosine events among the different genotypes.
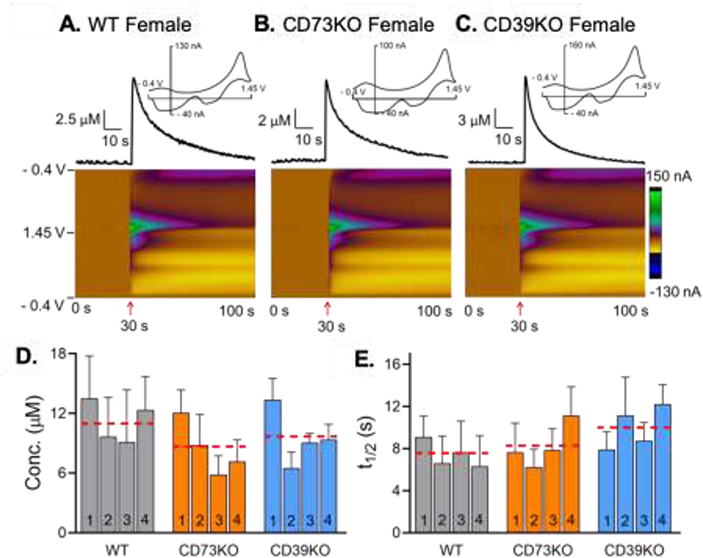
Fig. 2A–C show examples of mechanically-stimulated adenosine in female WT, CD73KO, and CD39KO mice after the electrode was lowered 0.1 mm (stimulation indicated by arrow under plot). The peak adenosine concentration for the example stimulations is 7 μM in WT, 6 μM in CD73KO, and 11 μM in CD39KO mice. Example color plots for male mice are shown in Supplemental Fig. 2. Fig. 2D and 2E show the average concentration and t1/2 of each successive mechanical stimulation as well as average for all stimulations (in combined male and female mice). The average concentration of adenosine per stimulation was slightly lower in CD73KO with a concentration of 8 ± 1 μM and compared to 10 ± 1 μM in CD39KO and 11 ± 1 μM in WT. However, there was no significant overall effect of genotype or stimulation number on the concentration of mechanically-evoked adenosine (Fig. 2D, two-way repeated measure ANOVA, n = 8 animals (4M and 4F)/genotype, F(2, 21) = 0.4428, p = 0.65 and F(3, 63) = 2.451, p = 0.072, respectively). The average t1/2 of mechanically-stimulated adenosine was 7 ± 1 s in WT, 8 ± 1 s in CD73KO, 10 ± 1 s in CD39KO, with no significant overall effect of genotype or stimulation number (Fig. 2E, two-way repeated measure ANOVA, F(2, 21) = 0.8810, p = 0.43 and F(3, 63) = 0.4586, p = 0.71, respectively). Overall, CD73KO and CD39KO did not affect the frequency of events, concentration, or t1/2 of mechanically-stimulated adenosine.
Figure 2. Mechanically-stimulated adenosine in the hippocampus.
Example of mechanically-stimulated adenosine in female (A) wild-type, (B) CD73KO, and (C) CD39KO mice. Electrode was lowered 0.1 mm at 30 s (arrow). Concentration vs. time traces show the peak concentration of adenosine. Four consecutive stimulations were performed every 15 min for totally 4 stimulations. (D) Concentration of mechanically-stimulated adenosine, shown by stimulation number. Red dashed lines are the average for all 4 stimulations for each genotype. There was no significant effect of genotypes or stimulation number on concentration of mechanically-stimulated adenosine (Two-way repeated measure ANOVA, n = 8 animals (4M and 4F)/genotype, p = 0.65 and p = 0.072, respectively). (E) t1/2 of mechanically-stimulated adenosine, by stimulation number. Red line is average for all stimulations. There was no significant effect of genotypes or stimulation number on t1/2 of mechanically-stimulated adenosine (Two-way repeated measure ANOVA, n=8 (4M/4F) p = 0.43 and p = 0.71, respectively).
Sex differences in spontaneous adenosine in CD73KO mice
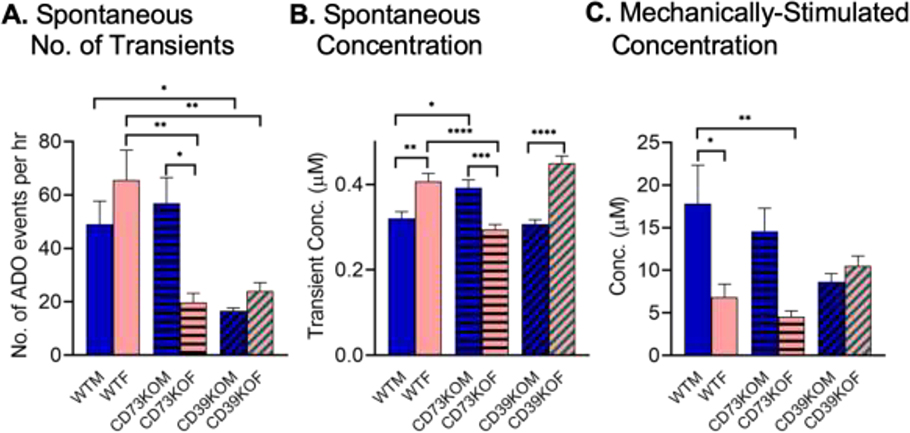
For each genotype, we performed experiments in 4 female and 4 male mice. Initial data was pooled (Fig. 1 and 2), but upon looking at the data by genotype, sex differences were apparent for the CD73KO mice for spontaneous adenosine. Figure 3 shows the data broken down by sex for both spontaneous and mechanically-stimulated adenosine. There was a significant overall effect of genotype (Fig. 3A, two-way ANOVA, F (2, 18) = 7.9, p=0.0003) but not sex (F(1, 18) = 0.5517, p=0.4672) on the number of adenosine events and a significant interaction (F (2, 18) = 13.16, p=0.0034). The average number of adenosine events per hour was 49 ± 9 in male and 66 ± 11 in female for WT mice, not statistically different (Tukey post-test, n = 4 animals/group, p=0.59). However, the number of events varies by sex in the CD73KO mice with an average number of adenosine events per hour of 57 ± 9 in male and 20 ± 3 in female (Tukey’s test, p=0.0196). The male CD73KO mice produce adenosine transients at the same frequency as WT males but the female CD73KO mice produce many fewer transients than WT females. The average number of adenosine events per hour was 17 ± 1 in male and 24 ± 3 in female for CD39KO and both males and females produce fewer transients than WT for the CD39KO mice (Tukey’s post-test, WTM vs. CD39KOM: p=0.049, WTF vs. CD39KOF: p=0.0079), so there were no sex differences for this genotype.
Figure 3. Sex differences in spontaneous (A-B) and mechanically-stimulated (C) adenosine.
(A) Number of spontaneous adenosine events per hour, broken down by sex in each genotype. There is a significant interaction of genotype and sex on number of adenosine events (Two-way ANOVA, n = 4 animals/group, F (2, 18) = 7.9, p = 0.0034, Tukey post- test data is marked for important differences and full post-test data is in Table S1). (B) Concentration of individual adenosine transients in each genotype. There is a significant interaction of genotype and sex on concentration of adenosine events (Two-way ANOVA, n = 200 transients/group, F (2, 1194) = 32, p < 0.0001. Tukey’s post-test data shown, with full post-test data given in Table S2). (C) Concentration of mechanically-stimulated adenosine. There is a significant interaction of genotype and sex on concentration of mechanically-stimulated adenosine (Two-way ANOVA, n = 4 animals/group, F (2, 18) =4.7, p = 0.023). Tukey’s post-test data is shown and full post-test results are in Table S3. * p<0.05 ** p<0.01, ***p < 0.001, ****p < 0.0001
For concentration of each spontaneous adenosine event, there were also sex differences for CD73KO mice. The concentrations of the first 50 adenosine events were taken from each animal to avoid bias from the differences in number of events per animal. A two-way ANOVA of the concentration data revealed a significant overall effect of sex (Fig. 3B, two-way ANOVA, F (2, 1194) =11.94, p=0.0006) but not genotype (F (2, 1194) = 2.51, p=0.081) and a significant interaction (F (2, 1194) = 32.12, p<0.0001). For WT mice, females on average had larger concentrations (0.41 ± 0.02 μM) than males (0.32 ± 0.01 μM) (Tukey’s test, n = 4 animals/group, p=0.0012). The pattern was the same for the CD39KO mice with an average concentration of 0.45 ± 0.02 μM in females and 0.31± 0.01 μM in males (Tukey’s test, p< 0.0001). However, the trend was the opposite for CD73KO mice, as males had larger concentrations (0.39 ± 0.02 μM) than females (0.29 ± 0.01 μM) (Tukey’s test, p=0.0002). When the data was pooled (Fig. 1), these differences averaged out to no effect, but when the sexes were separated, it was clear that the pattern of concentration changed in CD73KO mice.
For mechanically-stimulated adenosine, there is a significant overall effect of sex (Fig. 3C, two-way ANOVA, F(1, 18) = 11.21, p=0.0036) but not genotype (F(2, 18) = 0.9284, p=0.41) on concentration of adenosine and a significant interaction (F(2, 18) = 4.710, p=0.023). There were sex differences for WT mice, as females had lower concentrations (Tukey’s test, p=0.037). However, the patterns for sex differences were the same for WT and CD73KO, so the big sex differences that were observed with spontaneous adenosine for WT vs CD73KO are not evident for mechanically-stimulated adenosine. The average concentration of adenosine was 9 ± 1 μM in male and 11 ± 1 μM in female for CD39KO and no significant sex differences were observed.
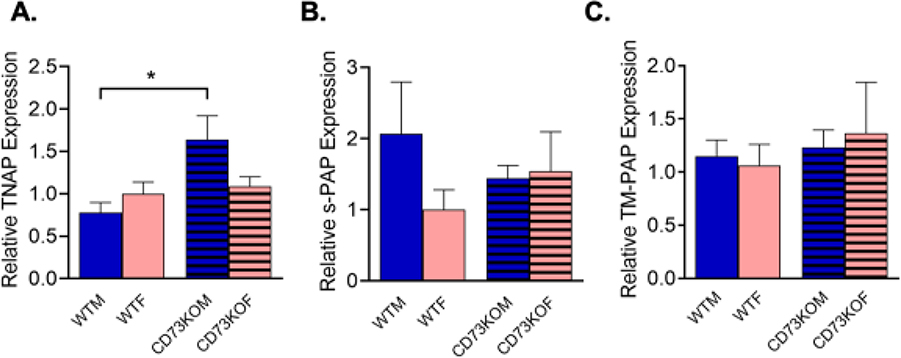
The hypothesis for the sex differences in spontaneous adenosine was that other compensatory enzymes were upregulated in male CD73KO mice compared to females. In order to understand the sex differences, quantitative PCR was performed to examine mRNA expression for some of the compensatory enzymes that can also hydrolyze AMP to adenosine. In particular, other studies have shown that both forms of PAP, secreted PAP (s-PAP) and transmembrane PAP (TM-PAP),38 as well as TNAP, also breakdown AMP to adenosine and thus affect adenosine transients in the spinal cord.19 Only WT and CD73KO mice were tested, since there were no large sex differences for the CD39KO mice. The results of the PCR are shown in Figure 4, with data normalized to the expression in WT females for each group. A two-way ANOVA revealed a significant overall effect of genotype (two-way ANOVA, F(1, 15) = 8.444, p=0.032) but not sex (F(1, 15) = 1.011, p=0.33) on TNAP expression and a significant interaction (Fig, 4A, F(1, 15) = 5.6, p = 0.032). TNAP was upregulated in males of CD39KO mice but not females (Tukey’s test, n = 5 animals/group, p = 0.015 and p = 0.92, respectively). Male CD73KO mice had about twice as much TNAP expression as female CD73KO mice. However, there were no effects sex or genotype for s-PAP (Fig. 4B, two-way ANOVA, F(1,15) = 0.87, p = 0.36 and F(1,15) = 0.0064, p = 0.94, respectively) or TM-PAP (Fig. 4C, Two-way ANOVA, F(1,15) = 0.002, p = 0.94 and F(1,15) = 0.17, p = 0.53, respectively).
Figure 4. mRNA expression of other ATP breakdown enzymes in the hippocampus of wild-type (WT) and CD73KO mice.
(A) The mRNA expression of TNAP in CD73KO males is significantly higher than in males of WT (2-way ANOVA, Tukey’s test, n = 5 animals/group, p=0.015), but TNAP is not upregulated in females (p=0.92). (B) For s-PAP, there are no significant differences by genotype or sex. (C) For TM-PAP, there are no significant differences by genotype or sex.
DISCUSSION
In this study, we investigated the effect of CD39 and CD73 on the formation of spontaneous and mechanically-stimulated adenosine in male and female mice. Our hypothesis was that in the CD73KO and CD39KO mice, the number and concentration of adenosine events in the hippocampus would decrease if the mechanism of adenosine formation was due to extracellular ATP breakdown. For mechanically-stimulated adenosine, the concentration and frequency of adenosine was not affected by global deletion of CD39 or CD73. For spontaneous adenosine, knocking out CD39 or CD73 reduced the frequency of spontaneous adenosine events. Therefore, spontaneous adenosine is formed through breakdown of extracellular ATP but mechanically-stimulated adenosine is not. These different mechanisms of formation for spontaneous and mechanically-stimulated adenosine could be important for future studies designing drugs to target different modes of adenosine for therapy.
CD73KO mice had interesting sex differences for spontaneous adenosine, where males produced adenosine events at the same frequency as WT males, but females had dramatically fewer transients than WT. The cause of these sex differences is upregulation of tissue nonspecific alkaline phosphatase (TNAP) in CD73KO males, while females had no changes. Thus, males produce more and larger adenosine transients because they compensate for CD73KO by upregulating TNAP, which also can produce adenosine from ATP. These sex differences confirm that spontaneous adenosine is a result of extracellular ATP breakdown and that many enzymes contribute to the process. Sex differences in CD73KO mice should be further explored for other types of adenosine as our studies indicate adenosine levels may not be low in male CD73KO mice.
Spontaneous adenosine is due to extracellular breakdown of ATP
The main research question of this study is how does global deletion of CD73 or CD39 affect spontaneous and mechanically-stimulated adenosine. The predominant enzyme responsible for the conversion of extracellular ATP or ADP to AMP is CD39 (NTPDase1) and for conversion of extracellular AMP to adenosine is CD73 (ecto-5’-nucleotidase).39 The conversion of adenine nucleotides to adenosine is very fast, as extracellular ATP is broken down to adenosine within 20 ms40 and thus on the time scale of FSCV, with data collected every 100 ms, adenosine concentrations rise quickly and conversion looks instantaneous. Previous studies have addressed questions of adenosine mechanism of formation with these mice11,19,21,41,42 and therefore, we examined spontaneous adenosine events in CD39KO and CD73KO mice with a hypothesis that they would have lower concentration and lower frequency of adenosine transients.11
Interestingly, for the aggregate data, concentration of each adenosine transient was not affected by CD73KO or CD39KO. However, female CD73KO mice had no compensation by other enzymes and thus had lower adenosine concentrations than WT, which suggests reducing the amount of enzyme present lowers concentration. Hydrolysis of ATP to adenosine is such an important pathway that there are redundant, alternative enzymes. For example, ATP and ADP are also hydrolyzed by NTPDase2 and 3.43 Tissue non-specific alkaline phosphatase (TNAP) dephosphorylates ATP, ADP, and AMP19 and prostatic acid phosphatase (PAP) produces adenosine from AMP.11 Thus, even when a major pathway of ATP metabolism is knocked out, adenosine is still formed by other enzymes. For example, double knock-out of PAP and CD73 reduced the frequency and concentration of spontaneous adenosine transients in spinal cord slices by more than 50%,11 but events were not fully eliminated until a drug was applied to block TNAP.19 Here, we did not check the CD39KO mice for compensation, because they had no sex differences, but they might have other upregulated enzymes that could increase the concentration. However, concentration is likely a proximity issue; if ATP is released near an enzyme that can convert it to adenosine, we see a full concentration transient, and if it is not (like in the KO mice), we see no event.
For spontaneous adenosine, the aggregate data (Fig. 2) showed a significant decrease in the number of adenosine transients in both CD73KO and CD39KO mice. CD39 and CD73 are both extracellular, membrane-bound enzymes,44 so knocking them out means that if ATP is released, it may not encounter an extracellular enzyme to be quickly metabolized to adenosine. Our electrode is 5-times more sensitive for adenosine than ATP detection, so ATP that is not metabolized to adenosine is likely not detected, and events are due to adenosine.45 Some ATP may be broken down by other enzymes, so adenosine formation is not eliminated, but fewer spontaneous events are detected, and some events might not be above the limit of detection (40 nM). This theory is validated by male CD73KO, which had higher spontaneous event frequencies, but also upregulated TNAP. There is also another possible explanation for lower frequencies of adenosine events: CD73KO or CD39KO may raise basal levels of ATP, which could feedback at ATP receptors to inhibit further events. For example, ATP receptors are located pre-synaptically on glutamate terminals and modulate glutamate release;40 if ATP is co-released with glutamate, this would affect the frequency of the events. Thus, we cannot rule out that CD73KO or CD39KO indirectly affects the frequency. Previous studies also revealed that spontaneous adenosine transients are regulated by frequency, not concentration. For example, A1 and A2a receptor antagonists and agonists change the event frequency dramatically but have little to no effect on concentration.9,13 Similarly, CD73KO and CD39KO regulate spontaneous adenosine mainly through frequency, rather than concentration.
Sex differences in spontaneous adenosine in CD73KO mice
The aggregate data that combined males and females suggested that the formation of spontaneous adenosine was due to ATP breakdown. However, on examining the data by sex, we noticed many sex differences for the CD73KO mice. In particular, male CD73KO mice had a similar frequency of transients as WT males, but female CD73KO had dramatically lower numbers of transients. Sex differences in concentration of transients were also observed, with higher concentration adenosine transients in females for WT and CD39KO mice, but lower concentration transients for females in CD73KO mice.
These sex differences led to the hypothesis that other enzymes that metabolize ATP or AMP may also have sex differences in CD73KO mice. PAP is made in the prostate (hence the word prostatic in its name) but tm-PAP is widely expressed in non-prostatic tissues,46 and females have it as well.47 However, the qPCR findings showed no sex differences in either form of PAP but instead in TNAP. TNAP is tissue non-specific, but highly expressed in the brain,48 and is a membrane-bound protein that regulates the amount of phosphorylated compounds. TNAP dephosphorylates tau protein, turning it into a protein suitable for aggregation and its activity is important for Alzheimer’s disease.49 Upregulation of TNAP has an important effect on spontaneous adenosine because male CD73KO mice have higher frequency and larger concentration adenosine events, demonstrating that twice the expression of TNAP can completely compensate for the lack of CD73. CD73 and TNAP are known to interact in the body. For example in the heart, extracellular adenosine produced by CD73 suppresses TNAP activity, which reduces levels of calcification, but blocking CD73 increases TNAP activity.50 In the brain, TNAP is important for producing increases in extracellular adenosine during ischemia in CD73KO mice, and could compensate almost completely for the loss of CD73.51 However, none of these studies examined female mice, and female CD73KO mice might have less neuroprotection via adenosine during stroke due to lower TNAP levels. The mechanism of sex specific compensation is not yet understood and should be examined in future studies. These data confirm that spontaneous adenosine is formed by extracellular metabolism of ATP, but there are other enzymes that play a role besides the CD73/CD39 pathway.
Mechanically-stimulated adenosine is not dependent on CD73 and CD39
We also directly investigated the effects of CD73 and CD39 on mechanically-stimulated adenosine in the same animals spontaneous adenosine events were measured. Our previous study in brain slices showed that insertion of an electrode and multiple stimulations by lowering electrode did not significantly damage the tissue.10 For mechanically-stimulated adenosine, CD73 and CD39 KO did not affect the frequency or concentration of adenosine events. The frequency of mechanical stimulation evoking adenosine was similar in all genotypes, with stimulation causing adenosine 88% of the time in WT, 94% in CD73KO, and 88% in CD39KO mice. The concentration of adenosine per event was also similar in all genotypes. Females had lower amounts of mechanically-stimulated adenosine in both WT and CD73KO mice, but the trends were the same between the genotypes and there were no striking sex differences like for the spontaneous adenosine events. CD39KO mice had similar concentrations of mechanically-stimulated adenosine for males and females, but the same average as the other genotypes. Therefore, mechanically stimulated adenosine is not affected by CD73 and CD39 KO and its mechanism of formation is different than that of spontaneous adenosine.
The mechanism of mechanically-stimulated adenosine could be direct release of adenosine, instead of ATP. This release of adenosine could be vesicular, as adenosine has been found in synaptic vesicles.16 Application of tetrodotoxin to block sodium channels or chelating calcium with EDTA decreased the concentration of mechanically-sensitive adenosine, suggesting mechanically-stimulated adenosine is activity dependent and vesicular.10 Another possibility is that adenosine is transported into the extracellular space through equilibrative nucleoside transporters (ENTs), but past studies showed that inhibiting ENTs with NBTI in rat brain slices did not affect the concentration of mechanically-stimulated adenosine.10 Finally, pannexin channels are gap junction proteins that are mechanosensitive and could directly allow adenosine to pass into the extracellular space.52 This mechanism could be investigated in further studies.
Implications of different mechanisms of formation for spontaneous and mechanically-stimulated adenosine
Rapid adenosine signaling is important for rapid modulation of both neurotransmission and blood flow.8,12–14,53 For example, spontaneous adenosine events increase during ischemia-reperfusion and provide fast, local neuroprotection.8,14 Therapies that upregulate CD73 or CD39 would provide even more local neuroprotection, by increasing spontaneous adenosine. However, they would do very little to protect the brain during trauma that might cause mechanically-stimulated adenosine events. There is the additional complication that any therapy that affects CD73 might also affect TNAP levels, which are also critical for maintaining spontaneous adenosine. In addition, any future studies should examine sex differences in these enzymes, as compensation may be different in males and females. Thus, understanding the mechanism of adenosine formation in both sexes is important for an understanding of how adenosine might be manipulated to provide therapeutic neuroprotection.
MATERIALS AND METHODS
Animals and surgery
Wild type C57BL/6J and CD73 deficient (CD73KO, with a C57BL/6 genetic background) male and female mice were purchased from Jackson Laboratory (Bar Harbor, ME). CD39 deficient (CD39KO) mice onto a C57BL/6 background were kindly provided by Dr. Bruce N. Cronstein and obtained from Taconic Biosciences (Rensselaer, NY).21 Previous studies have used the C57B/6J line as a WT comparison for both KO strains.21 All mice were used between 6–8 weeks of age. Animals were housed with food and water provided ad libitum on a standard 12:12 h light/dark cycle. Mice were anesthetized with 5% isoflurane in 100% oxygen in an anesthetic chamber and then transferred to a stereotaxic frame (Stoelting, Wood Dale, IL, USA). Anesthesia was maintained with 1.5–3% in 100% oxygen delivered via a facemask (Stoelting). Isoflurane levels were adjusted until loss of righting reflex was observed. A homeothermic blanket system (Stoelting, Wood Dale, IL, USA) was used to maintain body temperature around 37 °C. Bupivacaine (0.20 mL) (Sensorcaine® MPF; APP Pharmaceuticals, LLC, Schaumburg, IL, USA) was administered under the skin for local anesthesia and the surgical site was shaved. Holes were drilled to allow the placement of the electrode in the hippocampus (AP −2.5 mm, ML + 2.4 mm, and DV −1.8 mm) based on the atlas of Paxinos and Frankline.54 All experiments were approved by the Institutional Animal Care and Use Committee of the University of Virginia.
Chemicals
All reagents for the phosphate-buffered saline (PBS) solution were purchased from Fisher Scientific (Fair Lawn, NJ, USA) and used for electrode calibration, consisting of 3.0 mM KCl, 10.0 mM NaH2PO4, 2.0 mM MgCl2, 131.25 mM NaCl and 1.2 CaCl2, with pH adjusted to 7.4. Adenosine was purchased from Sigma Aldrich (Milwaukee, WI, USA). A 10.0 mM stock solution of adenosine was prepared in 0.1 mM HClO4 and stored at 4 oC. Adenosine was diluted daily in PBS solution to 1μM for calibration of the electrodes. All aqueous solutions were prepared using deionized water (Milli-Q Biocel; Millipore, Billerica, MA, USA).
Electrochemical detection of adenosine
Fabrication of carbon-fiber microelectrodes with T-650 carbon fibers was previously described.13 Cylinder electrodes were used with a 7 μm diameter and exposed length of 150–200 μm. Adenosine was detected with fast-scan cyclic voltammetry as previously described.13,55 The voltage was scanned from −0.4 V to 1. 45 V and back to −0.4 V at 400 V/s, which takes 9.25 ms. The voltage was then held at −0.4 V for 90.75 ms before the potential waveform was applied again, so the waveform frequency was 10 Hz. Therefore, adenosine could be detected electrochemically on the subsecond timescale.
Fast-scan cyclic voltammetry (FSCV) was used to detect and quantitate adenosine on a sub-second time scale.55–57 The FSCV waveform and data were collected through computer controlled HDCV software (University of North Carolina, Chapel Hill, NC, USA). A Dagan Chem Clamp potentiostat (Dagan Corporation, Minneapolis, MN, USA) with Pine headstage (Pine instruments, Durham, NC) was used to apply the potential. All adenosine FSCV data is background subtracted, by taking a background scan 5 seconds before an adenosine event and subtracting the current from that scan from the current when adenosine was present. Adenosine was identified with an oxidation peak at 1.3 V on the cathodic scan and 1.2 V on the anodic scan in the cyclic voltammograms (Fig. 1A–1C, top) and 3-D color plots (Fig. 1A–1C, bottom), where adenosine oxidation is the green/purple area in the middle of the color plot.16 Concentration vs. time traces (middle) were derived from corresponding 3-D color plots at the potential for the primary oxidation peak, which was converted to concentration using a calibration factor. An automated algorithm was used to identify adenosine transients, and confirmed adenosine events are starred on the concentration traces.58
Quantitative real-time polymerase chain reaction
The hippocampus was flash frozen after removal and stored at −80 °C prior to RNA extraction and RT-qPCR analyses. RNA was isolated from ~25 mg of mouse hippocampus tissue in 0.6 mL of TRIzol (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA was purified using the RNeasy Mini protocol (Qiagen, Hilden, Germany). Isolated RNA was quantified using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA), with samples selected only with A260/A280 ratios between 1.8 and 2.1. 700 micrograms of RNA was reverse transcribed using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA), according to the manufacturer’s instructions. The cDNA was stored at −80 °C until use.
Primers were designed using primer-BLAST (http://www.ncbi.nlm.nih.gov) (Table 1) or were taken from the literature (CD7359, actb60, TNAP19). All primer pairs spanned an exon to avoid amplification of genomic DNA. RT-qPCR was performed on a CFX Connect Detection System (Bio-Rad, Hercules, CA) using Sso Advanced Universal SYBR Green Mix (Bio-Rad, Hercules, CA). Reactions were carried out in 20 μL total volume, with 150 ng cDNA and 100 nM of each primer. The following amplification program was used in all RT-qPCRs: 95 °C for 2 min, and 40 cycles of 15 s at 95 °C and 15 s at 60 °C. The specificity of each amplified reaction was verified by a dissociation curve analysis after 40 cycles and by 2% agarose gel electrophoresis. Each sample was analyzed in quadruplicate wells and NTC “no-template controls” (without cDNA in the PCR) were included.
Table 1:
Primers for PCR experiments
| Gene ID | Accession Number | Forward Oligo Sequence 5’ – 3’ | Reverse Oligo Sequence 5’ – 3’ | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|
| CD73 | NM_011851 | AGTTCGAGGTGTGGACATCGTG | ATCATCTGCGGTGACTATGAATGG | 118 | Takedachi et al, 201259 |
| Actb | NM_007393 | CCTCCCTGGAGAAGAGCTATG | TTACGGATGTCAACGTCACAC | 157 | Thomas et al, 201460 |
| TNAP | NM_007431 | CTGACTGACCCTTCGCTCTC | TCATGATGTCCGTGGTCAAT | 116 | Street et al, 201319 |
| TM-PAP | NM_207668 | CCACCAAGTGCTGAGGGTTATC | TGAGAGACGTCCCCAAGGTC | 157 | |
| S-PAP | NM_207668 | CCACCAAGGACGGAATTAAAGC | TGGGGAAATCAGTCCTTCGC | 137 |
Serial 10-fold dilutions of each plasmid were used to generate a standard curve and amplification efficiencies (E) were determined based on the slope (M) of the log-linear portion of each standard curve (E = 10–1/M −1). Serial 2-fold dilutions were used to generate a standard curve, and amplification efficiencies (E) were determined based on the slope (M) of the log-linear portion of each standard curve (E = 10−1/M −1 * 100). The minimum information for publication of quantitative real-time PCR experiments (MIQE) guidelines (Bustin et al. 2009) was used to promote implementation of experimental consistency and to increase the integrity of our results.
Data Analysis and Statistics
Transient adenosine events were first identified and analyzed using a new automated algorithm,58 adenosine events were then confirmed by an analyst to exclude any signals that were not adenosine. The primary oxidation peaks of adenosine in Figure 1A–1C were filtered using a Fourier transform 1 Hz filter to reduce noise. All data were shown as mean ± SEM and all statistics were performed in GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA). One-way or two-way ANOVA with post hoc Tukey’s test or a Student’s unpaired t-test was performed on the number, concentration and t1/2 of transient adenosine to assess differences between knockout and wile type mice. The distribution of inter-event time of adenosine events was analyzed using Kruskal-Wallis test with post-hoc Dunn’s test. Some data points for adenosine inter-event time (longer times) were not shown in order to better show the lower inter-event time where changes were more obvious. However, all data for inter-event time were used for statistical analyses. Statistical significance was designated at p < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from NIH (R01NS076875, EB026497) to BJV. We thank Dr. Bruce N. Cronstein from University of New York School of Medicine for providing us with the CD39 KO mice.
Abbreviations
- WT
Wild-type
- KO
Knockout
- PAP
prostatic acid phosphatase
- FSCV
Fast-scan cyclic voltammetry
- CD39
triphosphate diphosphohydrolase 1
- CD73
ecto-5′-nucleotidase
- ENTs
equilibrative adenosine transporters
- NTPDase
triphosphate diphospholydrolase
Footnotes
SUPPORTING INFORMATION
Figure S1: Example color plots of spontaneous adenosine from male WT, CD73KO, and CD39KO mice. Figure S2: Example color plots from mechanically-stimulated adenosine from male WT, CD73KO, and CD39KO mice. Supplemental tables 1, 2, and 3 give the full statistical information of ANOVA post-tests for Figure 3 A, B, and C, respectively.
REFERENCES
- (1).Bjorness TE; Greene RW Adenosine and Sleep. Curr. Neuropharmacol. 2009, 7 (3), 238–245. 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Williams-Karnesky RL; Stenzel-Poore MP Adenosine and Stroke: Maximizing the Therapeutic Potential of Adenosine as a Prophylactic and Acute Neuroprotectant. Curr. Neuropharmacol. 2009, 7 (3), 217–227. 10.2174/157015909789152209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).O’Regan M Adenosine and the Regulation of Cerebral Blood Flow. Neurol. Res. 2005, 27 (2), 175–181. 10.1179/016164105X21931. [DOI] [PubMed] [Google Scholar]
- (4).Haskó G; Cronstein B Regulation of Inflammation by Adenosine. Front. Immunol. 2013, 4, 85 10.3389/fimmu.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Lee ST; Venton BJ Regional Variations of Spontaneous, Transient Adenosine Release in Brain Slices. ACS Chem. Neurosci. 2018, 9 (3), 505–513. 10.1021/acschemneuro.7b00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Nguyen MD; Venton BJ Fast-Scan Cyclic Voltammetry for the Characterization of Rapid Adenosine Release. Comput. Struct. Biotechnol. J. 2015, 13, 47–54. 10.1016/j.csbj.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ganesana M; Lee ST; Wang Y; Venton BJ Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods. Anal. Chem. 2017, 89 (1), 314–341. 10.1021/acs.analchem.6b04278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ganesana M; Venton BJ Early Changes in Transient Adenosine during Cerebral Ischemia and Reperfusion Injury. PLoS One 2018, 13 (5), e0196932 10.1371/journal.pone.0196932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Nguyen MD; Lee ST; Ross AE; Ryals M; Choudhry VI; Venton BJ Characterization of Spontaneous, Transient Adenosine Release in the Caudate-Putamen and Prefrontal Cortex. PLoS One 2014, 9 (1), e87165 10.1371/journal.pone.0087165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ross AE; Nguyen MD; Privman E; Venton BJ Mechanical Stimulation Evokes Rapid Increases in Extracellular Adenosine Concentration in the Prefrontal Cortex. J. Neurochem. 2014, 130 (1), 50–60. 10.1111/jnc.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Street SE; Walsh PL; Sowa NA; Taylor-Blake B; Guillot TS; Vihko P; Wightman RM; Zylka MJ PAP and NT5E Inhibit Nociceptive Neurotransmission by Rapidly Hydrolyzing Nucleotides to Adenosine. Mol. Pain 2011, 7 (27), 80 10.1186/1744-8069-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ross AE; Venton BJ Adenosine Transiently Modulates Stimulated Dopamine Release in the Caudate-Putamen via A1 Receptors. J. Neurochem. 2015, 132 (1), 51–60. 10.1111/jnc.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wang Y; Venton BJ Correlation of Transient Adenosine Release and Oxygen Changes in the Caudate-Putamen. J. Neurochem. 2017, 140 (1), 13–23. 10.1111/jnc.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Wang Y; Venton BJ Caffeine Modulates Spontaneous Adenosine and Oxygen Changes during Ischemia and Reperfusion. ACS Chem. Neurosci. 2019, 10 (4), 1941–1949. 10.1021/acschemneuro.8b00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).dos Santos-Rodrigues A; Pereira MR; Brito R; de Oliveira NA; Paes-de-Carvalho R Adenosine Transporters and Receptors: Key Elements for Retinal Function and Neuroprotection. Vitam. Horm. 2015, 98, 487–523. 10.1016/BS.VH.2014.12.014. [DOI] [PubMed] [Google Scholar]
- (16).Corti F; Cellai L; Melani A; Donati C; Bruni P; Pedata F Adenosine Is Present in Rat Brain Synaptic Vesicles. Neuroreport 2013, 24 (17), 982–987. 10.1097/WNR.0000000000000033. [DOI] [PubMed] [Google Scholar]
- (17).Westfall DP; Todorov LD; Mihaylova-Todorova ST ATP as a Cotransmitter in Sympathetic Nerves and Its Inactivation by Releasable Enzymes. J. Pharmacol. Exp. Ther. 2002, 303 (2), 439–444. 10.1124/jpet.102.035113. [DOI] [PubMed] [Google Scholar]
- (18).Latini S; Pedata F Adenosine in the Central Nervous System: Release Mechanisms and Extracellular Concentrations. J. Neurochem. 2001, 79 (3), 463–484. 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- (19).Street SE; Kramer NJ; Walsh PL; Taylor-Blake B; Yadav MC; King IF; Vihko P; Mark Wightman R; Millán JL; Zylka MJ Tissue-Nonspecific Alkaline Phosphatase Acts Redundantly with PAP and NT5E to Generate Adenosine in the Dorsal Spinal Cord. J. Neurosci. 2013, 33 (27), 11314–11322. 10.1523/JNEUROSCI.0133-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Augusto E; Matos M; Sévigny J; El-Tayeb A; Bynoe MS; Müller CE; Cunha RA; Chen J-F Ecto-5’-Nucleotidase (CD73)-Mediated Formation of Adenosine Is Critical for the Striatal Adenosine A2A Receptor Functions. J. Neurosci. 2013, 33 (28), 11390–11399. 10.1523/JNEUROSCI.5817-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Fernández P; Perez-Aso M; Smith G; Wilder T; Trzaska S; Chiriboga L; Franks A; Robson SC; Cronstein BN; Chan ESL Extracellular Generation of Adenosine by the Ectonucleotidases CD39 and CD73 Promotes Dermal Fibrosis. Am. J. Pathol. 2013, 183 (6), 1740–1746. 10.1016/j.ajpath.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yegutkin GG Enzymes Involved in Metabolism of Extracellular Nucleotides and Nucleosides: Functional Implications and Measurement of Activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49 (6), 473–497. 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- (23).Leclerc BG; Charlebois R; Chouinard G; Allard B; Pommey S; Saad F; Stagg J CD73 Expression Is an Independent Prognostic Factor in Prostate Cancer. Clin. Cancer Res. 2016, 22 (1), 158–166. 10.1158/1078-0432.CCR-15-1181. [DOI] [PubMed] [Google Scholar]
- (24).Enjyoji K; Sévigny J; Lin Y; Frenette PS; Christie PD; Esch JSA; Imai M; Edelberg JM; Rayburn H; Lech M; Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD Targeted Disruption of Cd39/ATP Diphosphohydrolase Results in Disordered Hemostasis and Thromboregulation. Nat. Med. 1999, 5 (9), 1010–1017. 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- (25).Lanser AJ; Rezende RM; Rubino S; Lorello PJ; Donnelly DJ; Xu H; Lau LA; Dulla CG; Caldarone BJ; Robson SC; Weiner HL Disruption of the ATP/Adenosine Balance in CD39 −/− Mice Is Associated with Handling-Induced Seizures. Immunology 2017, 152 (4), 589–601. 10.1111/imm.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Pinsky DJ; Broekman MJ; Peschon JJ; Stocking KL; Fujita T; Ramasamy R; Connolly ES; Huang J; Kiss S; Zhang Y; Choudhri TF, McTaggart RA, Liao H, Drosopoulos JHF, Price VL, Marcus AJ, Maliszewski CR Elucidation of the Thromboregulatory Role of CD39/Ectoapyrase in the Ischemic Brain. J. Clin. Invest. 2002, 109 (8), 1031–1040. 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Borgus JR; Puthongkham P; Venton BJ Complex Sex and Estrous Cycle Differences in Spontaneous Transient Adenosine. J. Neurochem. 2020, 152, e14981 10.1111/jnc.14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Doyle SE; Breslin FJ; Rieger JM; Beauglehole A; Lynch WJ Time and Sex-Dependent Effects of an Adenosine A2A/A1 Receptor Antagonist on Motivation to Self-Administer Cocaine in Rats. Pharmacol. Biochem. Behav. 2012, 102 (2), 257–263. 10.1016/j.pbb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Yang JN; Tiselius C; Daré E; Johansson B; Valen G; Fredholm BB Sex Differences in Mouse Heart Rate and Body Temperature and in Their Regulation by Adenosine A1 Receptors. Acta Physiol. 2007, 190 (1), 63–75. 10.1111/j.1365-201X.2007.01690.x. [DOI] [PubMed] [Google Scholar]
- (30).Tavilani H; Sheikh N; Vaisi-raygani A; Setarehbadi R Sex Differences in Adenosine Deaminase Activity of Stroke Patients. Clin. Chem. Lab. Med. 2008, 46, 506–509. 10.1515/CCLM.2008.108. [DOI] [PubMed] [Google Scholar]
- (31).Walker QD; Rooney MB; Wightman RM; Kuhn CM Dopamine Release and Uptake Are Greater in Female than Male Rat Striatum as Measured by Fast Cyclic Voltammetry. Neuroscience 2000, 95 (4), 1061–1070. 10.1016/S0306-4522(99)00500-X. [DOI] [PubMed] [Google Scholar]
- (32).Wang Y; Venton BJ Comparison of Spontaneous and Mechanically-Stimulated Adenosine Release in Mice. Neurochem. Int. 2019, 124, 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Fowler JC Changes in Extracellular Adenosine Levels and Population Spike Amplitude during Graded Hypoxia in the Rat Hippocampal Slice. Naunyn. Schmiedebergs. Arch. Pharmacol. 1993, 347 (1), 73–78. [DOI] [PubMed] [Google Scholar]
- (34).Fredholm BB; Dunwiddie T V; Bergman, B.; Lindström, K. Levels of Adenosine and Adenine Nucleotides in Slices of Rat Hippocampus. Brain Res. 1984, 295 (1), 127–136. [DOI] [PubMed] [Google Scholar]
- (35).Frenguelli BG; Llaudet E; Dale N High-Resolution Real-Time Recording with Microelectrode Biosensors Reveals Novel Aspects of Adenosine Release during Hypoxia in Rat Hippocampal Slices. J. Neurochem. 2003, 86 (6), 1506–1515. 10.1046/j.1471-4159.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- (36).Andiné P; Rudolphi KA; Fredholm BB; Hagberg H Effect of Propentofylline (HWA 285) on Extracellular Purines and Excitatory Amino Acids in CA1 of Rat Hippocampus during Transient Ischaemia. Br. J. Pharmacol. 1990, 100 (4), 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Frenguelli BG; Wigmore G; Llaudet E; Dale N Temporal and Mechanistic Dissociation of ATP and Adenosine Release during Ischaemia in the Mammalian Hippocampus. J. Neurochem. 2007, 101 (5), 1400–1413. 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Araujo CL; Quintero IB; Kipar A; Herrala AM; Pulkka AE; Saarinen L; Hautaniemi S; Vihko P Prostatic Acid Phosphatase Is the Main Acid Phosphatase with 5=-Ectonucleotidase Activity in the Male Mouse Saliva and Regulates Salivation. Am J Physiol Cell Physiol 2014, 306, 1017–1027. 10.1152/ajpcell.00062.2014. [DOI] [PubMed] [Google Scholar]
- (39).Reutershan J; Vollmer I; Stark S; Wagner R; Ngamsri K-C; Eltzschig HK Adenosine and Inflammation: CD39 and CD73 Are Critical Mediators in LPS-Induced PMN Trafficking into the Lungs. FASEB J. 2009, 23 (2), 473–482. 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- (40).Dunwiddie TV; Dlao L; Proctor WR Adenine Nucleotides Undergo Rapid, Quantitative Conversion to Adenosine in the Extracellular Space in Rat Hippocampus. J. Neurosci. 1997, 17 (20), 7673–7682. 10.1523/jneurosci.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wall MJ; Dale N Neuronal Transporter and Astrocytic ATP Exocytosis Underlie Activity-Dependent Adenosine Release in the Hippocampus. J. Physiol. 2013, 591 (Pt 16), 3853–3871. 10.1113/jphysiol.2013.253450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Klyuch BP; Dale N; Wall MJ Deletion of Ecto-5’-Nucleotidase (CD73) Reveals Direct Action Potential-Dependent Adenosine Release. J Neurosci 2012, 32 (11), 3842–3847. 10.1523/JNEUROSCI.6052-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Zimmermann H Extracellular Metabolism of ATP and Other Nucleotides. Naunyn. Schmiedebergs. Arch. Pharmacol. 2000, 362 (4–5), 299–309. 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- (44).Allard B; Longhi MS; Robson SC; Stagg J The Ectonucleotidases CD39 and CD73: Novel Checkpoint Inhibitor Targets. Immunol. Rev. 2017, 276 (1), 121–144. 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Xu Y; Venton BJ Microelectrode Sensing of Adenosine/Adenosine-5’-Triphosphate with Fast-Scan Cyclic Voltammetry. Electroanalysis 2010, 22 (11), 1167–1174. 10.1002/elan.200900559. [DOI] [Google Scholar]
- (46).Quintero IB; Araujo CL; Pulkka AE; Wirkkala RS; Herrala AM; Eskelinen E-L; Jokitalo E; Hellström PA; Tuominen HJ; Hirvikoski PP; Vihko PT Prostatic Acid Phosphatase Is Not a Prostate Specific Target. Cancer Res. 2007, 67 (14), 6549–6554. 10.1158/0008-5472.CAN-07-1651. [DOI] [PubMed] [Google Scholar]
- (47).Kamoshida S; Tsutsumi Y Extraprostatic Localization of Prostatic Acid Phosphatase and Prostate-Specific Antigen: Distribution in Cloacogenic Glandular Epithelium and Sex-Dependent Expression in Human Anal Grand. Hum. Pathol. 1990, 21 (11), 1108–1111. [DOI] [PubMed] [Google Scholar]
- (48).Sebastián-Serrano Á; de Diego-García L; Martínez-Frailes C; Ávila J; Zimmermann H; Millán JL; Miras-Portugal MT; Díaz-Hernández M Tissue-Nonspecific Alkaline Phosphatase Regulates Purinergic Transmission in the Central Nervous System During Development and Disease. Comput. Struct. Biotechnol. J. 2015, 13, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Díaz-Hernández M; Gómez-Ramos A; Rubio A; Gómez-Villafuertes R; Naranjo JR; Miras-Portugal MT; Avila J Tissue-Nonspecific Alkaline Phosphatase Promotes the Neurotoxicity Effect of Extracellular Tau. J. Biol. Chem. 2010, 285 (42), 32539–32548. 10.1074/jbc.M110.145003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Gan XT; Taniai S; Zhao G; Huang CX; Velenosi TJ; Xue J; Urquhart BL; Karmazyn M CD73-TNAP Crosstalk Regulates the Hypertrophic Response and Cardiomyocyte Calcification Due to Α1 Adrenoceptor Activation. Mol. Cell. Biochem. 2014, 394 (1–2), 237–246. 10.1007/s11010-014-2100-9. [DOI] [PubMed] [Google Scholar]
- (51).Zhang D; Xiong W; Chu S; Sun C; Albensi BC; Parkinson FE Inhibition of Hippocampal Synaptic Activity by ATP, Hypoxia or Oxygen-Glucose Deprivation Does Not Require CD73. PLoS One 2012, 7 (6), e39772 10.1371/journal.pone.0039772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Bao L; Locovei S; Dahl G Pannexin Membrane Channels Are Mechanosensitive Conduits for ATP. FEBS Lett. 2004, 572 (1–3), 65–68. 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- (53).Nguyen MD; Wang Y; Ganesana M; Venton BJ Transient Adenosine Release Is Modulated by NMDA and GABAB Receptors. ACS Chem. Neurosci. 2017, 8 (2), 376–385. 10.1021/acschemneuro.6b00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Watson C; Paxinos G Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, Fourth Edition; 2012. [Google Scholar]
- (55).Swamy BEK; Venton BJ Subsecond Detection of Physiological Adenosine Concentrations Using Fast-Scan Cyclic Voltammetry. Anal. Chem. 2007, 79 (2), 744–750. 10.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- (56).Venton BJ; Cao Q Fundamentals of Fast-Scan Cyclic Voltammetry for Dopamine Detection. Analyst 2020. 145, 1158–1168. 10.1039/C9AN01586H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Puthongkham P; Venton BJ Recent Advances in Fast-Scan Cyclic Voltammetry. Analyst 2020. 145, 1087–1102. 10.1039/C9AN01925A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Borman RP; Wang Y; Nguyen MD; Ganesana M; Lee ST; Venton BJ Automated Algorithm for Detection of Transient Adenosine Release. ACS Chem. Neurosci. 2017, 8 (2), 386–393. 10.1021/acschemneuro.6b00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Takedachi M; Oohara H; Smith BJ; Iyama M; Kobashi M; Maeda K; Long CL; Humphrey MB; Stoecker BJ; Toyosawa S; Thompson LF, Murakami S CD73-Generated Adenosine Promotes Osteoblast Differentiation. J. Cell. Physiol. 2012, 227 (6), 2622–2631. 10.1002/jcp.23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Thomas KC; Zheng XF; Garces Suarez F; Raftery JM; Quinlan KGR; Yang N; North KN; Houweling PJ Evidence Based Selection of Commonly Used RT-QPCR Reference Genes for the Analysis of Mouse Skeletal Muscle. PLoS One 2014, 9 (2), e88653 10.1371/journal.pone.0088653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.