Abstract
Since December 2019, COVID-19, the clinical syndrome associated with SARS-CoV-2 infection, has infected more than 6.2 million people and brought the function of the global community to a halt. As the number of patients recovered from COVID-19 rises and the world transitions toward reopening, the question of acquired immunity versus the possibility of reinfection are critical to anticipating future viral spread. Here, we present a case of a patient previously recovered from COVID-19 who re-presents with new respiratory, radiographical, laboratory, and real-time reverse transcriptase-polymerase chain reaction (RT-PCR) findings concerning for possible re-infection. We review this case in the context of the evolving discussion and theories surrounding dynamic RT-PCR results, prolonged viral shedding, and the possibility of developed immunity. Understanding how to interpret dynamic and late-positive SARS-CoV-2 RT-PCR results after primary infection will be critical for understanding disease prevalence and spread among communities worldwide.
Keywords: Coronavirus, Reinfection, COVID-19, Viral shedding
1. Introduction
First discovered in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated clinical syndrome (COVID-19) became a global pandemic over a few short months [1,2]. According to the World Health Organization recommendations, patients infected with COVID-19 may safely discontinue home isolation and are considered non-infectious after complete symptomatic recovery in addition to two negative real-time reverse-transcription polymerase chain reaction (RT-PCR) tests for SARS-CoV-2 drawn at least 24 h apart [3]. However, recent reports of patients re-testing positive even after resolved symptoms and negative testing raise questions about the possibility of reinfection [[4], [5], [6], [7]]. Here, we present a case of possible SARS-CoV-2 reinfection, and discuss this case in the context of the existing debate surrounding dynamic and late-positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR) results.
2. Case report
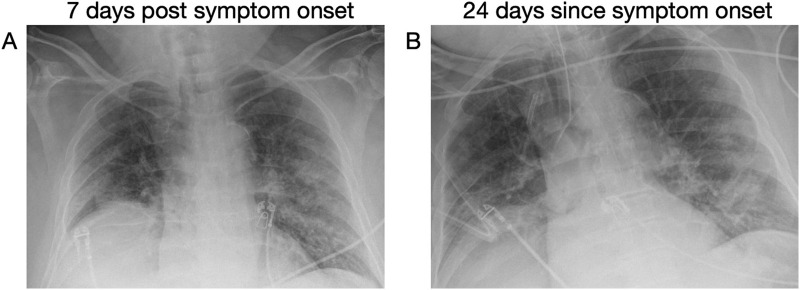
An 82-year-old male with a history of advanced Parkinson's disease, insulin-dependent diabetes, chronic kidney disease, and hypertension presented to the emergency department (ED) in early-April 2020 with one week of fever and shortness of breath. He was hemodynamically stable, but tachypneic, hypoxic to 89% on six liters of oxygen via nasal cannula, and febrile to 100.4 °F. Chest x-ray revealed peripheral and basilar patchy opacities concerning for COVID-19 (Fig. 1A). His respiratory status declined in the ED, and he was intubated for hypoxemic respiratory failure and admitted to the intensive care unit (ICU). An RT-PCR for SARS-CoV-2 sent from the ED resulted as positive. He remained intubated in the ICU for 28 days at which point he was successfully extubated and transferred to the medicine floor. He demonstrated clinical and radiographical improvement (Fig. 1B), and in early May 2020 two subsequent RT-PCRs for SARS-CoV-2 sent 24 h apart resulted as negative. On hospital day 39, he was discharged to a rehabilitation facility breathing comfortably on room air.
Fig. 1.
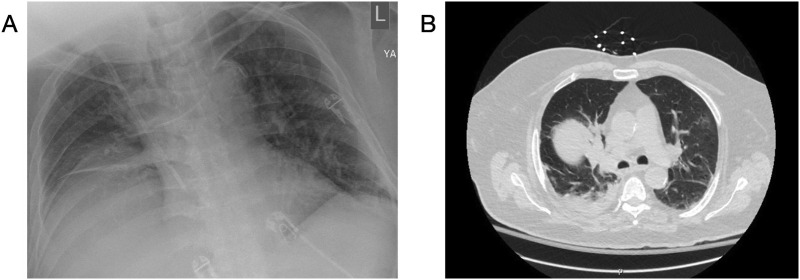
Ten days post-discharge (48 days after first presentation), he re-presented to the ED with fever and hypoxia. On arrival he was tachypneic, hypotensive to 70/40 mmHg, and tachycardic to 110 beats/min, with a temperature of 99.9 °F and oxygen saturations of 83% on room air which improved to 96% on eight liters via Oxymizer®. Chest x-ray (Fig. 2A) and computed tomography (CT) scan (Fig. 2B) demonstrated bilateral ground glass opacities again concerning for COVID-19, as well as unilateral focal consolidations concerning for bacterial pneumonia. RT-PCR for SARS-CoV-2 sent from the ED again resulted as positive. His code status of do not resuscitate/do not intubate was confirmed with family while the patient was in the ED. He was readmitted to the ICU, respiratory cultures later grew Corynebacterium, and antibiotics were continued. His ICU course was complicated by septic shock, delirium, and acute renal failure requiring continuous veno-venous hemofiltration. His oxygen requirement was weaned by hospital day five and he was transferred to the medical floor in stable condition on hospital day 7. He underwent repeat RT-PCR for SARS-CoV-2 on hospital days 11 and 12 which both resulted as negative. Given his stable yet severely deconditioned state he was discharge to an inpatient rehabilitation facility on hospital day 15.
Fig. 2.
3. Discussion
Here we present a case of a patient previously recovered from COVID-19 who demonstrated new respiratory symptoms and radiographical findings with newly positive RT-PCR, collectively concerning for possible SARS-CoV-2 reinfection. Though reinfection is possible in our case, alternative explanations for his presentation also exist. Dynamic RT-PCR results (i.e., oscillating positive/negative tests) have been described in COVID-19 patients with positive tests occurring after symptomatic and radiographic recovery and multiple negative tests [[4], [5], [6], [7]]. The most common alternative proposed explanations to true reinfection include prolonged viral shedding and inaccurate testing.
Unlike this case, in many cases of dynamic RT-PCR results patients are clinically improved at the time of repeat positive testing, calling in to question the likelihood of true reinfection. Many viruses demonstrate prolonged presence of genetic material in a host even after clearance of the live virus and symptomatic resolution [[8], [9], [10]]. Thus, detection of genetic material by RT-PCR alone does not necessarily correlate with the active infection or infectivity [11,12]. Observational data suggest SARS-CoV-2 viral shedding may last 20–22 days after symptom onset on average with some outlying cases exhibiting shedding as long as 44 days [6,7,13]. In one case series, asymptomatic patients had repeat positive RT-PCR 5- and 13-days post negative testing [7]. Both age and severity of initial infection may correlate with further prolonged shedding, with one case series describing a 71-year-old female with a severe case of COVID-19 demonstrating positive RT-PCR results for 60 days post-symptom onset and 36 days after complete symptom resolution [13,14]. Our patient had his first positive RT-PCR result on day 7 after symptom onset, then had two negative swabs on days 39 and 40 of illness in conjunction with symptomatic recovery. He re-presented and had repeat positive testing 55 days post-initial symptom onset making viral shedding somewhat likely given the prolonged timeframe.
Inaccurate or imprecise testing is another alternative explanation to recurrent infection. The sensitivity of RT-PCR for SARS-CoV-2 has been reported to be between 66 and 80%, depending on the instrument used [15]. In patients with early disease, symptoms and radiological findings can appear before RT-PCR becomes positive, suggesting the potential for false negative results [15,16]. Quantitative polymerase chain reaction (qPCR) tests such the SARS-CoV-2 RT-PCR often simultaneously assess for the presence of multiple different gene targets, some of which are very specific to SARS-CoV-2 infection, and some of which are quite sensitive to detection but may be common among many or all SARS-like coronaviruses. An RT-PCR can be considered positive if there is detection of sensitive targets but no detection of targets specific to SARS-CoV-2, which can be diagnostically misleading.
Though it is less likely our patient would have multiple false negative results at the end of his initial disease course, there was a chance his repeat positive test on representation may have been a false-positive. To further investigate the potential for reinfection versus viral shedding or a false positive test result, we re-analyzed the RT-PCR cycle thresholds of our patient's repeat positive test. Cycle thresholds are the number of PCR cycles required to amplify a particular gene target sufficiently to reach a set level of detection on a given instrument. The greater number of cycles required for gene detection, the fewer number of viral copies are present in a sample. These numbers are highly variable and depend largely on the particular instrument, assay, and sufficiency of the patient sample. For our patient, while his RT-PCR on re-presentation was in fact positive, the cycle threshold required for detection was relatively high suggesting a low viral load. This could be explained by either prolonged low-level viral shedding, or inadequacy of the submitted sample. Additionally, at the time of representation and repeat positive testing, further assessment of his results revealed that while the gene assessed which was very sensitive for infection but common among all SARS-coronaviruses was detected, the gene assessed which was specific to SARS-CoV-2 was not identified. Additional studies such as antibody testing or subsequent RT-PCR on varying instruments with newly collected samples may be helpful in clarifying his infection status.
While most existing data surrounding the possibility of SARS-CoV-2 reinfection are observational, recent work in an animal model suggests that macaque monkeys re-challenged with the same strain of SARS-CoV-2 after recovery from initial infection demonstrate no clinical, radiographical, or histopathological evidence of recurrent disease [17]. Additionally, the Korean CDC released a report in late-May 2020 investigating patients who re-tested positive for SARS-CoV-2 by RT-PCR after clinical recovery from COVID-19 [18]. Among this cohort, there were no cases in which complete SARC-CoV-2 virus could be isolated in cell culture, suggesting against these patients being actively infected. Though these early studies are reassuring against the possibility of reinfection, further work is needed particularly surrounding the possibility of reinfection with various viral strains.
4. Conclusion
Here, we discuss a case of possible COVID-19 reinfection in the context of the growing body of literature surrounding dynamic SARS-CoV-2 RT-PCR testing. For our patient, after a prolonged ICU stay and subsequent clinical and RT-PCR-verified recovery from confirmed COVID-19, one week later he returned acutely ill with recurrent clinical and radiological markers potentially concerning for COVID-19 in the setting of repeat positive RT-PCR. While alternative explanations for his dynamic RT-PCR testing exist, and his symptoms were likely secondary to an alternative infectious process such as bacterial pneumonia, a newly positive RT-PCR in conjunction with a new clinical picture consistent with COVID-19 raise suspicion for reinfection. There is large variability between instruments used for SARS-CoV-2 RT-PCR testing, and many of these results are largely open to interpretation. As in our case, interpreting cycle thresholds and understanding more about the targets of a particular instrument used for SARS-CoV-2 RT-PCR can be crucial for clinicians assessing for the possibility of true reinfection in their patients. As both the number of active cases and patients recovered from COVID-19 increases, clarity regarding the possibility of viral reinfection remains a high priority for predicting viral spread and pandemic trajectory.
Presentations
None.
Financial support
None.
Declaration of Competing Interest
The authors report no conflict of interest.
References
- 1.World Health Organization Rolling updates on coronavirus disease (COVID-19) 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen/ Website. [Accessed May 26, 2020]
- 2.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Clinical Management of severe acute respiratory infection when COVID-19 is suspected: Interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected 13 March 2020.Website.
- 4.Chen D., Xu W., Lei Z., et al. Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int J Infect Dis. 2020 Apr;93:297–299. doi: 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith J. South Korea reports more recovered coronavirus patients testing positive again. https://www.reuters.com/article/us-health-coronavirus-southkorea/south-korea-reports-more-recovered-coronavirus-patients-testing-positive-again-idUSKCN21V0JQ Retrieved May 24, 2020, from.
- 6.Xiao A.T., Tong Y.X., Gao C., et al. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol. 2020 Jun;127 doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan L., Xu D., Ya G., et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020 Feb 27;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paz-Bailey G., Rosenberg E.S., Sharp T.M. Persistence of Zika virus in body fluids - final report. N Engl J Med. 2019 Jan 10;380(2):198–199. doi: 10.1056/NEJMc1814416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh M.-D., Park W.B., Choe P.G., et al. Viral load kinetics of MERS coronavirus infection. N Engl J Med. 2016 Sep 29;375(13):1303–1305. doi: 10.1056/NEJMc1511695. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Guo Q., Yan Z., et al. Factors associated with prolonged viral shedding in patients with avian influenza a(H7N9) virus infection. J Infect Dis. 2018 May 5;217(11):1708–1717. doi: 10.1093/infdis/jiy115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkinson B., Petersen E. SARS-CoV-2 shedding and infectivity. Lancet. 2020 Apr 25;395(10233):1339–1340. doi: 10.1016/S0140-6736(20)30868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ip D.K.M., Lau L.L.H., Chan K.-H., et al. The dynamic relationship between clinical symptomatology and viral shedding in naturally acquired seasonal and pandemic influenza virus infections. Clin Infect Dis. 2016 Feb 15;62(4):431–437. doi: 10.1093/cid/civ909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Zhang L., Liu B., Song D. Case Report. Viral shedding for 60 days in a woman with novel coronavirus disease (COVID-19) Am J Trop Med Hyg. 2020 Apr 27 doi: 10.4269/ajtmh.20-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ai T., et al. Correlation of chest CT. And RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [Feb 26:200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie X., Zhong Z., Zhao W., et al. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [Feb 12;200343] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao L., Deng W., Gao H., et al. Lack of reinfection in rhesus macaques infected with SARS-CoV-2. BioRxiv. 2020 Mar 13;990226 [Preprint] [Google Scholar]
- 18.“Findings from investigation and analysis of re-positive cases”. Division of Risk assessment and International cooperation. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030 Updated 21 May 2020. Website.