Abstract
SARS-CoV-2 has been marked as a highly pathogenic coronavirus of COVID-19 disease into the human population, causing over 5.5 million confirmed cases worldwide. As COVID-19 has posed a global threat with significant human casualties and severe economic losses, there is a pressing demand to further understand the current situation and develop rational strategies to contain the drastic spread of the virus. Although there are no specific antiviral therapies that have proven effective in randomized clinical trials, currently, the rapid detection technology along with several promising therapeutics for COVID-19 have mitigated its drastic transmission. Besides, global institutions and corporations have commenced to parse out effective vaccines for the prevention of COVID-19. Herein, the present review will give exhaustive details of extensive researches concerning the drug discovery and therapeutic options for COVID-19 as well as some insightful discussions of the status of COVID-19.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Precise prevention and control, Drug discovery, Vaccine development
Graphical abstract
The possible viral entry and replication mechanism of SARS-CoV-2 and crucial targets for novel antiviral drug development.
1. Introduction
This century has witnessed the worldwide spread of several hitherto unknown coronaviruses. The rapid alteration of ecology and urbanization along with vulnerable public health systems have facilitated more frequent emerging of epidemics, which have become more and more intractable for us to prevent and contain. In December 2019, a new coronavirus (CoV) correlated with human respiratory disease was firstly reported causing pneumonia and death [1]. Soon afterward, the disease cases continued to expand and soared dramatically worldwide. The causative virus, named as severe acute respiratory syndrome CoV-2 (SARS-CoV-2), was identified as the pathogen leading to the CoV disease COVID-19. Infections with SARS-CoV-2 are now swift and violent, and as of 28 May 2020, over 5.5 million cases have been confirmed in more than 215 countries, with over 353, 334 deaths. To date, SARS-CoV-2 has most closest relation to SARS and relevant viruses that circulate in bats, as evidenced by viral genome analysis as well as the research probing into the proximal origin of such virus [2].
In general, CoVs, subfamily coronavirinae, are a cluster of highly diversified, enveloped, positive-sense and single-stranded viruses ((+)ssRNA virus) that can induce enteric, respiratory, hepatic as well as neurological disorders of discrepant severity in a wide range of animal species, encompassing humans [3,4]. As the largest RNA viruses ever discovered, CoVs can be categorized into α-, β-, δ- and γ-CoVs. Among these genera, the β group can be subdivided into A, B, C and D lineages [5]. In the past 17 years, three neoteric β-CoVs, SARS-CoV, Middle East respiratory syndrome CoV (MERS-CoV), along with SARS-CoV-2 have emerged, engendering severe human diseases. Although the origin of SARS-CoV-2 outbreak is not yet clear, recent studies have deduced that it might be transmitted through bats because it is highly similar to the bat SARS-CoV-like coronaviruses [6]. Later on, genomic and evolutionary proofs of the occurrence of Pangolin-CoV indicated that pangolin species might be the potential intermediate host for SARS-CoV-2 [7,8]. Unlike human CoVs, zoonotic viruses hold the capacity of infecting both animals and humans, leading to severe respiratory diseases (i.e., acute respiratory distress syndrome (ARDS) and pneumonia) [9,10]. Clinical data revealed that the COVID-19 symptoms are far more severe among the elders with comorbidities, while asthma, allergic illnesses, as well as chronic obstructive pulmonary disease are also risk factors [11,12]. Despite the continuous improvement of the prevention strategy and the disease surveillance system, the lack of efficacious drug treatment and correlated high morbidity cases of the SARS-CoV-2 along with its potentiality to induce pandemics, highlighting the urgent demand for neoteric drug discovery. In this review, we will first briefly describe the status of epidemiology, mechanism and diagnosis of COVID-19. In addition to the discussion of current management strategies for COVID-19, the emphasis has been given to the treatment options and drug discovery of COVID-19. We will refer to the drug screening progress and the development of vaccines for the COVID-19 therapy. Ultimately, we will delineate the overarching challenges in the clinical invention of new anti-coronavirus agents and offer some insights toward the prevention of such disease based on the understanding of its epidemic dynamics in real-time.
2. The epidemiology of COVID-19
Spread mainly through respiratory droplets or close contact, SARS-CoV-2 induced diseases has been growing dramatically in accordance with the published data from the World Health Organization (WHO) [13]. Although the number of confirmed cases in China has decreased a lot from late February 2020, and there is no report of COVID-19 deaths on 6th April, the confirmed cases of CoVs worldwide are still expanding with a vengeance. The spectrum of illness presentation or severity profile also affects triage and diagnostic decision-making, along with the therapeutic options and prognostic expectations [14]. Till now, the exact source of the current outbreak of COVID-19 remains unclear, but the dynamic model is similar to the classic zoonotic emergence to human-to-human transmission [15]. The mortality rate of SARS-CoV-2 (∼3.8%) is lower compared to that of MERS-CoV (37.1%) or SARS-CoV (10%), but the number of infections is more than ten times higher [16]. With respect to the lack of evidence that companion animals might be a source of infection, patients with COVID-19 are the prime source of infection, and those with severe conditions are more infectious than those with mild conditions. Intriguingly, asymptomatically infected persons or patients in incubation has also been demonstrated to shed the infectious virus, serving as a potential infection source to drive the transmission of the COVID-19 [17]. In addition, researches focused on the follow-up of recovered patients revealed that the tested samples of rehabilitees continuously showed a positive RT-PCR result, implicating that asymptomatic infection during incubation or recovery from COVID-19 may pose a daunting challenge to disease control and prevention [18].
The incubation period refers to the time between exposure to the virus and initial symptoms. A research report on the early propagation dynamics of COVID-19 unveiled that the average incubation period of COVID-19 was around 5 d, and its 95% distribution was 12.5 d [19]. Another study analyzing the travel history and symptoms in 88 confirmed cases revealed a similar average incubation period of around 6 days [20]. In addition, there was an unusual case with an incubation period of up to 19 d [21]. Although such a long incubation period may be a low probability event (the condition of 14 d was suggested by experts for quarantine), the longer incubation time indicates the adjustment of screening and control policies [22].
The early outbreak data of COVID-19 largely follow exponential growth. Disparate models based upon the clinical progression of the disease had been proposed to assess the basic reproductive ratio R0. A retrospective analysis of the first 425 identified cases demonstrated that in the early stages of COVID-19, the R0 was assessed to be 2.2 [19]. Nevertheless, deterministic compartmental models based upon the likelihood and a model analysis revealed that the control reproduction number Rc might be as high as 6.5 due to the estimation of four generations of viral transmission and serried social contacts [23]. In this regard, it is noteworthy that R0 estimates may vary in the light of numerous biologics, social behavior, and environmental factors [24]. In general, the basic R0 assessed by the majority of researches ranges between 2 and 4 [25]. According to the WHO data updated on May 28, 2020, more than 215 countries have reported 5593, 631 confirmed cases, including 353, 334 deaths (Fig. 1). The grand total case fatality rate of global cases outside China is 1.31% [26]. The US had also recorded the largest number of coronavirus deaths in a day, with more than 1810 deaths reported on April 7th, according to data from Johns Hopkins University. Given the condition that the population of all races and ages is generally susceptible, there is an urgent need to further implementing the timely diagnosis, along with efficient isolation of patients, to cut down the R0 of SARS-CoV-2 and control the epidemic outbreak.
Fig. 1.
Cumulatively identified cases of COVID-19 worldwide, as of 28 May 2020 [26]. As indicated in this figure, the overall data still presents a slow upward trend, suggesting that the epidemic has not been effectively alleviated.
3. Virological characteristics of SARS-CoV-2
As previously mentioned, SARS-CoV-2, belonging to the coronaviridae family, is the causative pathogen of COVID-19. Closely resembled other β-CoVs, the SARS-CoV-2 virion with a genome size of ∼3 kb has a nucleocapsid consisted of genomic RNA and phosphorylated nucleocapsid (N) protein [27]. Of note, the nucleocapsid is embedded in a phospholipid bilayer and is encased by two disparate types of spike proteins: spike glycoprotein trimmer present in all CoVs and the hemagglutinin-esterase shared merely in some CoVs. The spike (S) protein plays a pivotal role in binding to receptors and is the key to determine host tropism and transmission capacity (Fig. 2). The matrix protein (M) along with the viral envelop (E) are also located in the viral envelope [28]. Genome analysis showed that the SARS-CoV-2 possesses 5′ and 3′ terminal sequences, with a gene order 5′ -replicase open reading frame (ORF) 1ab-S-envelope(E)-membrane(M)-N-3′ [29]. The virus particles are 60–100 nm in diameter, and are round or oval [30]. It can be inactivated by ultraviolet light or heated at 56 °C for 30 min and is sensitive to most disinfectants (i.e., ether, 75% ethanol, peracetic acid, chlorine, as well as chloroform) [31].
Fig. 2.
Structure of SARS-CoV-2 S in the pre-fusion conformation and the genome, along with the crystal structure of the C-terminal domain of SARS-CoV-2 (SARS-CoV-2-CTD) S protein in complex with human ACE2. (A) Schematic of SARS-CoV-2 S primary structure colored by domain. SS, signal sequence; S2′, S2′ protease cleavage site; FP, fusion peptide; HR1, heptad repeat 1; CH, central helix; CD, connector domain; HR2, heptad repeat 2; TM, transmembrane domain; CT, cytoplasmic tail. Arrows denote protease cleavage sites. (B) Ribbon diagrams of the SARS-CoV-2 S ectodomain cryoEM structures. (C) The SARS-CoV-2 S1 subunits. (D) The SARS-CoV-2 S2 subunits. (E) A hACE2-binding mode of SARS-CoV-2. Reproduced with permission [32], [33], [34].
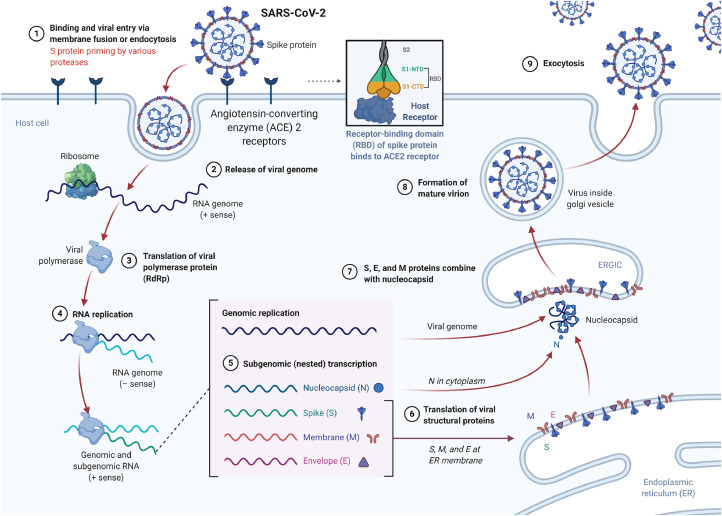
Accumulating evidence has revealed that SARS-CoV-2 and SARS-CoV have the same human cell receptor, the angiotensin-converting enzyme 2 (ACE2), while MERS-CoV hinges on dipeptidyl peptidase 4 for host cell entry [35]. ACE2 is a type I membrane protein expressed in the lungs, hearts, kidneys, and intestines, mainly associated with cardiovascular diseases [35]. A recent study analyzed the cryogenic electron microscopy structure of the SARS-CoV-2 S protein unveiled that it exhibits around 10 to 20-fold higher binding affinity to ACE2 in comparison with SARS-CoV [36]. The overall replication cycle of SARS-CoV-2 is depicted in Fig. 3.
Fig. 3.
The possible viral entry and replication mechanism of SARS-CoV-2. When the S protein of SARS-CoV-2 binds to the cellular receptor ACE2, it begins its life cycle. After the receptor is bound, the conformational change of the S protein helps the viral envelope to fuse with the cell membrane through the endosome pathway. Then, SARS-CoV-2 releases the RNA into the host cell. Genomic RNA is translated into viral replicase polyproteins pp1a and 1ab, which are then cleaved into small products by viral proteases. The polymerase generates a series of subgenomic mRNAs through discontinuous transcription, which is ultimately translated into related viral proteins. Viral proteins and genomic RNA are subsequently assembled into virions in the ER and Golgi, and then transported through vesicles and released from the cells. ERGIC, ER-Golgi intermediate compartment. Created with BioRender.com.
As for the phylogenetic network analysis of SARS-CoV-2 genomes, after analysis of the sample from across the world, researchers have found three central variants differentiated through amino acid alterations, denoted A, B, and C, with A being the ancestral type in line with the bat outgroup CoV. Intriguingly, types A and C account for a considerable proportion outside East Asia (Europe and the United States) [37]. By comparison, B is the most common type in East Asia, and its ancestors ’genomes do not appear to spread outside East Asia without first mutating into a derived type B. Hence, SARS-CoV-2 genomes were found to be closely correlated, and evolutionary selection took place in human hosts, sometimes with parallel evolutionary events, where the same viral mutation occurs in two disparate human hosts [37]. Owing to the erratic nature of RNA viruses as well as its high contagiousness, the continuous monitoring of SARS-CoV-2 from humans or animal species is of extreme significance for pandemic control.
4. Diagnosis and pathogenesis of SARS-CoV-2
4.1. Diagnostic testing for COVID-19
Rapid and accurate diagnosis of COVID-19 is of considerable significance for controlling outbreaks in the communities and hospitals [38]. Technologies such as polymerase chain reaction (PCR), reverse-transcription polymerase chain reaction (RT-PCR), real-time RT-PCR (rRT-PCR), and reverse transcription loop-mediated isothermal amplification (RT-LAMP) have been leveraged as ideal diagnostic tests for CoVs [39,40]. To date, the frontline reaction to the SARS-CoV-2 outbreak has been PCR testing. As the gold standard for diagnosing the source of infection, PCR holds the preponderance that the primers required for such assays can be generated relatively quickly once the viral sequence is identified (Fig. 4) [41]. Soon after the virus was identified, the first quantitative RT-PCR assays to detect SARS-CoV-2 were inaugurated and distributed in January 2020 by WHO. Nevertheless, this test protocol was complicated and high-priced, and is primarily applicable for large centralized diagnostic laboratories. As for the diagnostic criteria currently formulated by the China National Health Commission, nasopharyngeal cancer and oropharyngeal swab tests have ripened into the standard evaluation for the diagnosis of COVID-19 infection. So far, three new RT-PCR tests targeting the RNA-dependent RNA polymerase (RdRp)/helicase (Hel), nucleocapsid, and spike genes of SARS-CoV-2 had been inaugurated, with extremely lower detection limit in vitro [42]. The SARS-CoV E gene detection was superior to the RdRp gene test combined with the one-step RT-PCR system. The E gene PCR was adequate for diagnosing SARS-CoV-2 infection, but the RdRp protocol was endorsed to verify positive results [43]. Remarkably, a new FDA-authorized COVID-19 test using the Abbott ID NOW diagnostics platform has been developed, which can produce results in just 5 minutes cutting down on wait times both in terms of getting tested and receiving a diagnosis. As gene detection of SARS-CoV-2 might provide false negative results, it can be complemented by antibody detection, especially to better screen asymptomatic patients.
Fig. 4.
COVID-19 diagnostic test by RT-PCR. First, cotton swab is deployed to collect the secretion sample from the patient ’s nose or throat. The virus particles in the sample are then deactivated along with the separation of RNA strands. Then, the purified RNA strands are copied by utilizing reverse transcription and amplified by RT-PCR to detect the presence of virus-specific gene sequences. Created with BioRender.com.
Clinically, for those who are recently suffering from fever, fatigue, sore throat, cough or dyspnea due to exposure, the diagnosis of COVID-19 infection should be conducted with typical chest computerized tomography (CT) characteristics regardless of negative RT-PCR outcomes [44].
Most of the COVID-19 cases shared similar characteristics on CT images, presenting bilateral distribution of patchy shadows and ground-glass opacity, sometimes presenting a circular shape and peripheral lung distribution [45]. Some of the data published from China showed that in 21 primal chest CT scans, a large proportion of patients (86%) developed frosted glass opacity, affecting more than one lung lobe (71%) (bilateral involvement) [46]. It is also worth noting that lung cavitation, pleural effusions, discrete pulmonary nodules, along with lymphadenopathy were absent [46]. In addition to imaging technology, a recent study displayed that the Cas13-based SHERLOCK (specific high-sensitivity enzymatic reporter unlocking) platform can be harnessed for diagnosis of SARS-CoV-2 [47]. In this system, RNA-targeting Cas13 enzyme is deployed to identify specific genetic targets. The Cas13 can cleave nearby RNAs, a ‘collateral’ feature useful for amplifying a reporter signal in the diagnostic test. However, such a system needs to be further verified in clinical tests. Overall, combined with immunochromatography, colloidal gold, and other biotechnologies, associative detection strategies have been progressed swiftly.
4.2. Pathogenesis of SARS-CoV-2
With regard to the transmission of SARS-CoV-2, primary viral replication is assumed to occur in the mucosal epithelium of the upper respiratory tract and further multiplicated in the lower respiratory tract and gastrointestinal mucosa, inducing a mild viremia [48]. At this point, very few infections are under control and remain asymptomatic. Some patients may also suffer non-respiratory symptoms (i.e., acute liver and heart injury, renal failure, diarrhea), suggesting multiple organ involvement [49,50]. Since ACE2 is extensively expressed in the nasal mucosa, bronchus, lung, heart, and kidney, etc., many human organs are vulnerable to SARS-CoV-2 [51]. Specifically, the S protein plays a critical role in determining the cell tropism and hence interspecies transmission of SARS-CoV-2 since it hitches the virus to a cellular receptor and subsequently prompts virus entry via membrane fusion [32]. After binding to the receptor, the spike protein can catalyze the viral fusion process, allowing the viral genome to enter the cytoplasm. A prerequisite for this procedure is the division of S to subunits, a process known as priming (Fig. 3). The work of Hoffmann et al. unraveled that SARS-CoV-2 utilizes the ACE2 receptor for entry and the serine protease TMPRSS2 for S protein priming [52]. Hence, TMPRSS2 inhibitors approved for clinical use may block the entrance and may give rise to an underlying treatment option. Notably, the ability that S can readily obtain new protease cleavage sites and the fact that miscellaneous proteases can perform the same task suggests that this virus can easily adapt to the proliferation in several cell types [33]. Further, a panel of murine monoclonal antibodies (mAbs) and polyclonal antibodies (pAbs) against SARS-CoV-S1/receptor-binding domain (RBD) had been reported to be unable to interplay with S protein, implicating conspicuous discrepancies in antigenicity between SARS-CoV-2 and SARS-CoV [34].
According to the pathological findings, the first report on the pathological results of severe COVID-19 revealed that diffuse alveolar injury on both sides of the lung was accompanied by cellular fibromyxoid exudates [53]. The right lung displayed significant lung cell shedding and hyaline membrane formation, suggesting ARDS. Moreover, the left lung tissue showed pulmonary edema and hyaline membrane formation, implying early ARDS. Interstitial mononuclear inflammatory infiltrates, dominated by lymphocytes, were found in both lungs. Another study reported that acute kidney injury and proteinuria might also occur during the progression of COVID-19 disease. ACE2 was seen to be upregulated in COVID-19 patients, and immunostaining with the SARS-CoV nucleoprotein antibody was positive in tubules [54]. Additionally, only a few interstitial mononuclear inflammatory infiltrates were found in the heart tissue, meaning that this virus may not directly induce heart impairment [53]. Aside from the acute respiratory distress syndrome, exuberant inflammatory responses during the infection process were also observed in clinical, giving rise to unrestrained pulmonary inflammation. Of note, the virus-induced ACE2 downregulation, rapid virus replication and cell damage, and antibody-dependent enhancement may lead to aggressive inflammation aroused by SARS-CoV-2 [55]. The initial stage of rapid viral replication would induce a large number of epithelial and endothelial cell death, thereby facilitating the generation of raging pro-inflammatory cytokines and chemokines (Fig. 5) [56]. Intriguingly, a recent research compared the transcriptional responses of SARS-CoV-2 with other respiratory viruses to identify the transcriptional features that might form the biological basis of COVID-19 [57]. Their work demonstrated that the overall transcriptional induction of SARS-CoV-2 is abnormal. Despite viral replication, the host's response to SARS-CoV-2 failed to initiate powerful responses of type I and III interferons (IFN-I and -III), and at the same time induced high levels of chemokines required to recruit effector cells. In other words, this unique inflammatory response was defined by low levels of IFN-I and -III juxtaposed to elevated chemokines and high expression of IL-6. The reduced innate antiviral defenses coupled with exuberant inflammatory cytokine production may be the defining and driving features of COVID-19 [57]. Moreover, because the weakened immune response will further enable sustained viral replication, this critical finding may explain why severe cases of COVID-19 are more frequently observed in patients with comorbidities [58].
Fig. 5.
The potential mechanism of SARS-CoV-2 inducing cytokine storm. Increased cytokine levels (IL-6, IL-10, and TNF-α) are associated with severe COVID-19. ISGs, IFN-stimulated genes. Parts of this figure created with BioRender.com.
In addition to the cytokine storm, several experimentations had unraveled that lymphopenia is a customary characteristic of COVID-19, which may also accounts for severity and mortality [49,57].
5. Treatment strategy of COVID-19
5.1. Crucial SARS-CoV-2 targets for novel drug development
The preceding overview of the virology of SARS-CoV-2, as well as the sundry potential mechanisms of damage to the host, lay the foundation for developing specific targeted treatment and prevention. A general idea of pivotal targets for drug discovery is shown in Fig. 6. In consideration of the role of the surface structural S in the virus-cell receptor interplay, it is of particular interest for the antiviral development. mAbs against the S1 subunit RBD and fusion inhibitors targeting the S2 subunit possess potential anti-SARS-CoV-2 capacity in vitro or in vivo [59]. Besides, since ACE2 is the key functional host receptor of SARS-CoV-2 to determine the pathogenicity, mAbs, or molecules targeting the host receptor are effective anti- SARS-CoV-2 drugs, as long as they do not elicit immunopathological effects in animal models [4]. A recent study also probed into the COVID-19 S protein-binding site to the cell-surface receptor (known as Glucose Regulated Protein 78 (GRP78)). Their outcomes unveiled that the binding between regions III and IV of the S protein model and GRP78 was more favorable. In this regard, region IV is the major tractive force for GRP78 binding, and these 9 residues can be leveraged to design therapeutics specific against this disease [60]. Of note, although inhibitors of the proteases that prime S for fusion possess antiviral activity, multiple inhibitors are needed because S can utilize a variety of proteases for priming [61]. In addition, agents directly targeting the highly conserved S2 subunit may be potential treatment candidates.
Fig. 6.
Crucial SARS-CoV-2 targets for novel antiviral drug development. ACE2 receptor, receptor-binding domain along with the main protease Mpro can be leveraged as antiviral targets. Created with BioRender.com.
For SARS-CoV-2, the large replicase polyprotein 1a (pp1a) and pp1ab encoded by the ORF1a/b are subjected to two viral proteases, papain-like protease (PLpro) and 3C cleavage-like protease (3CLpro) (also known as Mpro), for producing non-structural proteins (i.e., RdRp, helicases) which are correlated to viral transcription and replication (Fig. 3) [4]. Therefore, enzyme inhibitors targeting these proteins may exhibit anti-SARS-CoV-2 activity in vitro. A recent study has uncovered that the Mpro of SARS-CoV-2, which is the translated polyproteins of ORF 1a/b, is a crucial enzyme that mediates viral replication and transcription [62]. Specifically, a Gln-residue almost always requires a substrate at P1 (an amino acid in substrates). There is currently no human homologous for Mpro, which makes it a promising antiviral target (Fig. 6) [63]. Dai and coworkers had conducted the structure-based design of antiviral agents targeting this protease by parsing out the substrate-binding pocket of Mpro [62]. In this regard, further study targeting such a protease may give rise to certain antiviral drug candidates.
Another notable drug target may be the cellular enzymes that attach fatty acids to a cluster of cysteines in the cytoplasmic tail of S due to the fact that fatty acids are essential for the fusion of host cell and assembly of the virus, like the description of other S proteins, such as hemagglutinin for influenza viruses. The enzyme that connects the acyl chain to S has not yet been discovered, but the cellular protein will undergo acetylation by the members from the ZDHHC family with unique, only partially overlapping substrate specificities. If a few of them may be acetylated in the airway cells of the lungs, their blockage may suppress the viral replication, and the acylation of cellular proteins will rarely be damaged. In this regard, targeting acyltransferases may be promising, because the cysteine group is existed in all CoV genus S, in spite of their source [64]. However, in consideration of the palmitoylation of crucial proteins in the innate immunity, if the proteins of the innate immune response are modified with the same enzymes as viral proteins, the acylation inhibitor may be limited.
In addition, Bojkova et al. recently identified the host cell pathways modulated by SARS-CoV-2 infection and revealed that suppression of these pathways may prevent viral replication in human cells [65]. Of note, SARS-CoV-2 infection profile was determined by translatome3 and proteome proteomics at different times after infection, suggesting that this virus could reshape central cellular pathways (e.g., translation, splicing, carbon metabolism and nucleic acid metabolism) [65]. Accordingly, spliceosome inhibitors and glycolysis inhibitors may also be the potential targets.
The last point of view is the application of small interfering RNAs (siRNAs). SARS-CoV-2 can disintegrate in host cells, releasing the nucleocapsid and viral RNA into the cytoplasm, and then translate ORF1a/b into pp1a and pp1ab for genomic RNA replication [66]. Hence, siRNAs targeting structural genes may play a role in the SARS-CoV-2 infections, and further optimizing the delivery of siRNAs in vivo may make them clinically valuable.
5.2. Drug discovery approaches toward anti-SARS-CoV-2 drug screening
The drug discovery strategy bears the brunt of the COVID-19 outbreak is the test of existing broad-spectrum antiviral agents that have been harnessed to cure other viral infections via utilizing standard tests for measuring the effects of drugs on the cytopathy, virus production along with plaque generation in live and pseudotyped CoVs [67]. Drug discovery utilizing this method encompass interferon α, interferon β, interferon γ, arbidol, ribavirin, along with cyclophilin inhibitors [65,66,68]. These drugs possess the distinct preponderance of easy access to known pharmacokinetic and pharmacodynamic features, dose regimens, and adverse effects [69]. Nevertheless, they have no specific anti-SARS-CoV-2 effect and may be correlated with severe untoward effects.
In addition to the test of existing broad-spectrum antiviral agents, another anti-SARS-CoV-2 drug discovery approach might be the chemical libraries screening, which involves a good deal of existing compounds or databases with the information on transcriptional signatures in disparate cell lines [68,69]. Such a method holds the potential of offering a prompt, high-throughput screening of many off-the-shelf composites, which can thereby be further assessed via antiviral detection test. Importantly, a variety of different types of agents have been discovered among these agent repurposing programs, incorporating many that with significant physiological and immune effects (i.e., those affecting the lipid or sterol metabolism, regulation of neurotransmitters, kinase signaling, estrogen receptors, and DNA synthesis/repair) [70], [71], [72], [73]. It is noteworthy that this method has the main drawback that most agents are not clinically useful in virtue of their underlying immunosuppressive effects or anti-SARS-CoV-2 half-maximal effective concentration values that significantly surpass the peak serum concentration levels that are obtainable at the therapeutic dose [74]. An interesting exception is the anti-HIV protease inhibitor lopinavir-ritonavir, which has been reported to be effective for SARS-CoV in both non-human primate models and in non-randomized clinical trials [75]. In addition, nelfinavir, a selective inhibitor of HIV protease, which has been demonstrated to possess a sound suppresion of SARS-CoV, suggestive of a potential drug candidate for COVID-19 [76].
The most critical method for anti-SARS-CoV-2 drug discovery includes the de novo development of new, specific drugs based upon the genomic and biophysical understanding of this virus. For instance, the determination of key SARS-CoV-2 targets may bring about the production of siRNA molecules or inhibitors against specific viral enzymes correlated with viral replication. Additionally, mAb targeting host receptors, inhibitors of host cell proteases, host cell endocytosis viruses, along with humanized mAb targeting the RBD, and antiviral peptides targeting the S2 subunit offer various methodology and options for the design and development of possible therapeutics. With the emerging outbreak of the COVID-19 pandemic, the above-mentioned methods are critical for identifying candidate drug composites that can be widely categorized into virus-based and host-based therapy options.
5.3. Therapeutics options for SARS-CoV-2 in clinical
During the outbreak of the pandemic COVID-19, considerable efforts are underway to discover novel therapeutic drugs for CoV infections. A wide variety of agents has been selected as therapeutic options for SARS-CoV-2 in clinical trials (Table 1).
Table 1.
List of candidate therapeutic drugs for SARS-CoV-2 therapy in clinical trials.
| Category | Candidate therapeutics | Modality | Manufacturer | Status of clinical trials |
|---|---|---|---|---|
| Nucleoside Analogs | Pegylated interferon with ribavirin [77] | / | Valeant | Under clinical trial for COVID-19 (ChiCTR2000029387) |
| Favipiravir (T-705) [78] | A guanine analog for the treatment of influenza virus infections | Toyama | Under clinical trial for COVID-19 (ChiCTR2000029548) | |
| Remdesivir [79] (GS-5734) | An adenine analog with a similar chemical structure to the approved HIV reverse transcriptase inhibitor tenofovir alafenamide. | Gilead | Phase II clinical trial for Ebola (NCT03719586); Under phase III clinical trials for COVID-19 (NCT04252664) |
|
| Type I interferons | IFN-β1 [80] | Used as a treatment for multiple sclerosis | Multiple companies | Treatment of COVID-19 in the early stage |
| Protease inhibitors | Lopinavir and ritonavir [76] | Protease inhibitors approved as anti-HIV drugs | Abbott | Under clinical trials for SARS; Under clinical trial for COVID-19 (ChiCTR2000029539) |
| Chloroquine | Chloroquine and hydroxychloroquine [81] | Oral prescription drugs for treatment of malaria and certain inflammatory conditions | Multiple companies | Under investigation in clinical trials for pre-exposure or post-exposure prophylaxis of SARS-CoV-2 infection |
| Antibodies | CR3022 [82] | A SARS Cov-specific human monoclonal antibody | Multiple companies | Experimental phase |
5.3.1. Antiviral agents
-
(I)
Nucleoside Analogs
Nucleoside analogs can interfere with cellular nucleotide synthesis pathways and terminate viral genome replication through accumulating mutations and cutting off the entry of natural nucleotides [83]. Since nucleosides and nucleotides are the basic components of viral nucleic acids, nucleoside analogs serve as viral RNA synthesis inhibitors in a wide spectrum of RNA viruses. With the view of the targeting ability toward RdRp, nucleoside analogs are responsible for viral RNA replication [84].
Ribavirin is a guanine analog among approved nucleoside analogs used to treat hepatitis c virus and respiratory syncytial virus infections and has been harnessed to cure patients with SARS [85]. Ribavirin was extensively leveraged for patients with or without concomitant usage of steroids during the SARS outbreak in 2003 [86]. When combined with IFN-β, it can exert the synergistical inhibition effect on SARS-associated CoV replication in vitro [87]. However, the efficacy and safety of this agent remain uncertain, and it may arouse adverse reactions like anemia in high doses [77]. In COVID-19 therapy, ribavirin was utilized with pegylated interferon for stimulating the innate antiviral reaction at a relatively lower dose to curtail side effects.
In addition, another promising guanine analog is favipiravir (T-705), which has been approved in Japan for the therapy of influenza virus infections and has also been demonstrated to suppress the replication of Ebola, yellow fever, enterovirus and norovirus [78]. Recently, Wang and coworkers suggested that favipiravir may also be a potential candidate for COVID-19 therapy, which showed effective antiviral activity in Vero E6 cells with an EC50 value of around 61 μM. To improve the condition of COVID-19 patients, favipiravir was utilized with other antiviral drugs like baloxavir marboxil [88].
As an adenine analogue with a similar chemical structure to the approved HIV reverse transcriptase inhibitor tenofovir alafenamide, remdesivir (GS-5734) exhibits broad-spectrum antiviral activity against several RNA viruses and has the capacity of competing with RdRp (Fig. 7) [89]. It also possesses outstanding in vitro antiviral activity compared with lopinavir and ritonavir [90]. In the United States, the first case of SARS-CoV-2 infection was reported, and remdesivir was administered. The patient's clinical condition improved after only one day of remdesivir treatment [79]. A newly released research offered remdesivir for COVID-19 inpatients on the basis of sympathy. In the cohort of patients admitted for treatment of severe COVID-19, patients treated with sympathetic remdesivir achieved relatively good clinical improvement (36 of 53 patients (68%)) [91]. However, recently, leaked data from a crucial remdesivir investigation suggests this potent CoV agent may not be effective. Accordingly, the chief medical officer of Gilead Sciences said that the summary post online might include inappropriate characterizations of the study and it was terminated early due to low enrollment [92]. Paradoxically, the drug's maker announced that in its own trial, more than half of 400 participants with severe COVID-19 had recovered from their illness within two weeks of receiving treatment [93]. However, this study lacked a placebo-controlled arm. Moreover, the outcomes from the National Institute of Allergy and Infectious Diseases (NIAID) further demonstrated that this drug can stop some patients from becoming critically ill [93]. In this regard, these findings suggest that differences in races and differences in clinical trials may lead to disparate experimental outcomes. The real anti-SARS-CoV-2 activity of remdesivir need to be further studied in the long term.
-
(II)
Type I interferons
Fig. 7.
Potential antiviral mechanism of remdesivir against SARS-CoV-2. The active molecule metabolized from remdesivir prodrug (GS-441524) may intercept RdRp early in viral replication, thereby interfering with the downstream steps of the SARS-CoV-2 replication cycle. Created with BioRender.com.
Type I interferons designate a cluster of antiviral cytokines consisting of the omnipresent α and β subtypes, along with the ω, ε and κ subtypes, inducing large numbers of proteins that can undermine viral replication in host cells [94]. Former researches have revealed that IFN-β was superior against SARS-CoV in comparison with IFN-α [95]. As for the clinical trial of type I interferons, in China, therapy guidelines for COVID-19 recommend the management of 5 million U of IFN-α via vapor inhalation in patients twice daily with ribavirin as combined therapy [96]. In the detailed study of Sallard et al., they reported that IFN-β1 might constitute a safe and easy to upscale therapy against COVID-19 in the early stages of the disease. Besides, in vitro data indicated that this virus might be significantly more sensitive to IFN-I than other CoVs [80].
-
(III)
Protease inhibitors
Protease inhibitorsare promising candidates for antiviral agents, and can block the replication of viral genes via binding to enzymes that are responsible for proteolysis [97]. Lopinavir and ritonavir, both protease inhibitors, have been approved as HIV medicines and have been found to possess antiviral activity against SARS and MERS. For the sake of destroying the SARS-CoV-2, clinical trials had begun to parse out the antiviral property of HIV protease inhibitors among patients. Notwithstanding, the antiviral effect of such inhibitors in coronavirus proteases remains controversial. Notably, a study regarding the comparison of the efficacy of prophylactic remdesivir and therapeutic remdesivir in combination with lopinavir, ritonavir, and interferon β against MERS-CoV unveiled that remdesivir was more effective than the combination therapy in reducing viral load and improving the degree of pathological changes in lung tissue. Aside from the gastrointestinal adverse reactions aroused by lopinavir-ritonavir, it is worth noting that lopinavir-ritonavir treatment alone may fail to offer benefits in comparison with standard care alone. The median time for clinical improvement was 16 d, and the reduction in viral RNA load among patients with severe SARS-CoV-2 did not appear to differ in both cases [98]. Despite the disheartening outcomes, a marginally lower number of deaths were observed among the patients with lopinavir-ritonavir treatment in the late stage of this disease in contrast to the standard-care group. Further, Baden and Rubin unraveled that lopinavir simply was not particularly potent against SARS-CoV-2. The concentration neccessary to suppress viral replication is relatively high as compared with the serum levels found in patients treated with lopinavir-ritonavir [99]. Besides, nelfinavir, which is a selective inhibitor of HIV protease, has also been displayed to possess a robust suppression of SARS-CoV, indicating an alternative therapeutic option for COVID-19 [76].
5.3.2. Chloroquine and hydroxychloroquine
Chloroquine, as a drug extensively utilized in anti-malaria and autoimmune diseases, has been found to be a potential broad-spectrum antiviral agent [100]. It can prevent viral infections via elevating the endosomal pH needed for virus-cell fusion and disturbing the glycosylation of SARS-CoV cell receptors [101]. Gao et al. revealed that chloroquine was effective in the therapy of COVID-19-associated pneumonia [102]. Wang and coworkers also conducted in vitro study, and they found that it is an ideal candidate antiviral drug against SARS-CoV-2 infection in Vero E6 cells with EC50 value of around 1 μM [88]. Although several trials had verified that chloroquine suppresses the exacerbation of COVID-19, the optimal dosage of chloroquine will require to be evaluated in future trials [102].
Hydroxychloroquine is an analog of chloroquine, and there are few studies on its interaction [103]. In previous SARS outbreaks, hydroxychloroquine was found to possess anti-SARS-CoV capacity in vitro [104]. In line with the research of Yao et al. by applying a physiologically based pharmacokinetic model, they found that hydroxychloroquine is more effective than chloroquine in Vero cells infected with SARS-CoV-2 [105]. Of note, it has been revealed that cytokines IL-6 and IL-10 are elevated in response to SARS-CoV-2 infection, which may induce the cytokine storm (Fig. 5), which in turn cause multiple organ failure and death [49]. Both chloroquine and hydroxychloroquine possess immunomodulatory effects and can inhibit such immune responses [106]. Hence, Chinese hospitals and Oxford University had initiated 21 clinical studies to evaluate the efficacy of these drugs in COVID-19 infection. The further crucial study may relate with the determination of whether the benefit of chloroquine treatment is hinged on the age of the patients as well as the clinical manifestations [107]. Recently, a multinational registry analysis of the application of hydroxychloroquine or chloroquine unraveled that there might be an increased frequency of ventricular arrhythmias during the treatment of COVID-19 diseases [108]. The WHO has also suspended hydroxychloroquine treatment in COVID-19 solidarity trial in terms of the outcomes of in-hospital mortality and de-novo ventricular arrhythmias [109].
5.3.3. Monoclonal or polyclonal antibodies
Monoclonal or polyclonal antibodies have been recommended as tools for preventing and treating viral infections. Considering the relatively high RBD in SARS-CoV-2, the cross-reactivity of anti-SARS-CoV antibodies with the COVID-19 S protein was evaluated. Tian et al. determined the potent binding of S protein via a SARS CoV-specific human mAb CR3022 [82]. Unfortunately, other SARS-CoV RBD directed mAbs (i.e., 230, m396, and 80R) cannot bind to the COVID-19 RBD [32]. In this respect, CR3022 may be a promising therapeutic candidate, either alone or in combination with other neutralizing mAbs, for the therapy of COVID-19 disease. In addition, tocilizumab is a monoclonal antibody for the therapy of RA exacerbation. It was designed to suppress the binding of IL-6 to its receptors, hence mitigating cytokine release syndrome. At present, it is also being trailed for the COVID-19 therapy [110]. Interestingly, Shi et al. reported a human neutralizing antibody targets the receptor binding site of SARS-CoV-2 [111]. In their work, they successfully isolated 2 specific human mAbs (named as CA1 and CB6) from a convalescent COVID-19 patient. CA1 and CB6 demonstrated potent SARS-CoV2-specifc neutralization activity in vitro against SARS-CoV-2. Moreover, CB6 could suppress SARS-CoV-2 infection in rhesus monkeys at both prophylactic and treatment settings. The following structural studies unraveled that CB6 could recognize an epitope that overlaps with ACE2-binding sites in SARS-CoV-2 receptor binding domain, thus interfering with the virus/receptor interactions by both steric hindrance and direct interface-residue competition [111]. Another recent study revealed the isolation and characterization of 206 RBD-specific mAbs derived from single B cells of eight SARS-CoV-2 infected individuals [112]. The researchers identified antibodies with potent anti-SARS-CoV-2 neutralization capacity that correlated with their competitive capacity with ACE2 for RBD binding. Of note, crystal structure analysis of RBD-bound antibody uncovered steric hindrance that suppressed viral engagement with ACE2 and thereby blocked viral entry [112]. These findings implicate that anti-RBD antibodies may be viral species-specific inhibitors.
Besides, most patients with severe COVID-19 suffered a lot from the cytokine storm (Fig. 5) [53]. So, neutralizing antibodies against other pro-inflammatory cytokines may be another promising strategy to dampen the inflammatory responses, thus responding well to treat the COVID-19. In a clinical trial conducted in Anhui, China, IL-6 receptor-targeted mAb tocilizumab was utilized to treat 21 patients with severe COVID-19. Clinical data showed quick fever control and improved respiratory function [113]. Overall, the development of COVID-19-specific antibodies takes a long time, meaning the difficulty in applying antibodies for neoteric pathogens to clinical practice in a brief period.
5.3.4. Corticosteroids
As a class of drug that lowers inflammation in the body, corticosteroids have been utilized in several serious viral respiratory infections such as SARS-CoV and MERS-CoV with limited benefits. However, in some cases, there is proof of delayed viral clearance and increased rates of secondary infection and mortality [114]. In a study of 41 COVID-19 patients, 22% were given corticosteroids, which inhibit inflammation in the lungs [49]. However, according to the current WHO interim guidance, glucocorticoids are not recommended for routine treatment unless otherwise indicated, as other coronaviruses and influenza studies have identified possible injury and increased risk of death from glucocorticoid therapy [115]. Despite the potential antiviral activity toward COVID-19, corticosteroids should not be given principally, and corticosteroid pulse therapy should be conducted with caution. In addition, corticosteroid therapy for the SARS therapy yielded adverse effects such as psychosis, diabetes, and avascular necrosis [116]. In general, the treatment with corticosteroids may be harmful, but such agents can be prescribed to the right patient at the proper time.
5.3.5. Convalescent plasma transfusion
Convalescent plasma has also been utilized as a last resort to improve the survival in patients with miscellaneous viral infections (i.e., SARS, H5N1, H1N1, and Ebola virus infection) [117]. The theoretical basis for the therapeutic effect of plasma in the recovery phase is that immunoglobulin Abs in the plasma of patients recovering from the viral infection may inhibit viremia. To date, in the previous SARS therapy, convalescent plasma given early after the onset of symptoms reduced overall mortality after treatment compared to placebo or no treatment [118]. For the test of convalescent plasma effect on COVID-19 infection, Zhou et al. found that the SARS-CoV-2 isolated from bronchoalveolar lavage fluid in a severe patient could be neutralized by the serum of several patients [6]. Additionally, another study of Shen et al. showed that improvements in clinical conditions were observed following plasma transfusions, improvements included normalization of body temperature within three days, a decrease in Sequential Organ Failure Assessment score, resolution of ARDS, and decline in viral loads [119]. During the emerging of COVID-19 infection in China, the National Health Commission of China also called on convalescent patients to donate blood for COVID – 19 therapy. Despite the difficulty in collecting the plasma, convalescent plasma therapy showed great therapeutic potential for the COVID-19 therapy.
5.3.6. Herbal medications
Based on data mining, the Traditional Chinese Medicine was also regarded as an alternative method for the treatment of COVID-19 disease in high-risk populations. During the COVID-19 outbreak in China, Chinese herbal drugs such as Astragali Radix, Glycyrrhizae Radix Et Rhizoma, Atractylodis Macrocephalae Rhizoma, Saposhnikoviae Radix, Lonicerae Japonicae Flos and Fructus forsythia were commonly harnessed for improving the treatment effect of COVID-19 infections. Herbal medications can offer a more effective and personalized treatment by adjusting the specific medicine for each patient based on the disparate syndromes [120]. Previous studies also indicated that the patients with SARS-CoV infection have benefited from TCM treatment [121], involving amelioration of adverse effects of tranditional therapeutics. Some TCM such as Lianhuaqingwen capsules exert anti-viral replication and anti-inflammatory activity against SARS-CoV-2 in vitro [122]. However, the experimental study on the efficacy of herbal medicines lags behind the clinical application of traditional Chinese medicine in the COVID-19 treatment [123]. Hence, rigorous clinical trials on large populations should be carried out to identify the potential therapeutic efficacy of herbal medications.
5.3.7. Mesenchymal stem cell therapy
Stem cell therapy is also making its way into COVID-19 disease treatment. Recently, some researches have demonstrated that the intravenous transplantation of mesenchymal stem cells (MSCs) was safe and efficient for COVID-19 pneumonia, especially for critically ill patients [124]. As mentioned, COVID-19 infection may induce uncontrolled inflammatory innate reactions along with undermined adaptive immune reactions, subsequently leading to detrimental tissue damage. MSC-based immunomodulation treatment is able to counteract the cytokine storm aroused by the immune system and foster endogenous repair via reparative attributes of the stem cells [125]. As it is unveiled by the recent study of China, seven COVID-19 patients (1 critically ill patient, 4 critically ill patients, and 2 patients with mild symptoms) received intravenous injection of bone marrow MSCs. In all cases, the patients were cured, while the 3 patients in the placebo control group all suffered from severe illness, 1 died, 1 developed ARDS, and 1 was in stable condition [126]. This study uncovered that MSCs could reclaim the lung microenvironment, protect the alveolar epithelial cells, block pulmonary fibrosis, and treat pulmonary dysfunction [124]. Notably, the FDA has also opened the way to the compassionate use of MSCs intravenous infusions in patients with COVID-19 ARDS and very dismal prognosis [127]. Although such a kind of treatment is an ideal option for COVID-19 therapy, it has limitations regarding the supply of clinical-grade MSCs and the rate of preparation for subsequent clinical use.
5.3.8. Other therapies
Hydrogen peroxide (H2O2) appears to be another potential therapeutic option for COVID-19. Of note, health experts have said that this compound could help prevent the virus from spreading across the body and from causing damage [128]. A recent study illustrated that even just 0.5% of H2O2 could kill human CoVs, such as those that caused SARS and MERS [129]. Inhaling the vapor with a nebulizer has been the most convenient way to receive H2O2 to fight viral infections. The microscopic mist can easily penetrate the nostrils, sinuses and lungs, which are commonly affected by respiratory diseases like COVID-19. Besides, molecular hydrogen has been verified to favorably modulate the generation of both O2− and NO through influencing NADPH oxidase, and the NOS enzymes [130]. According to the National Health Commission of China, the conditional use of mixed inhalation of hydrogen and oxygen (H2/O2: 66.6%/33.3%) treatment may improve the symptoms [131].
Lung transplantation can be performed in advanced patients with respiratory failure owing to COVID-19-related pulmonary fibrosis. As it is reported in a clinical study, lung transplantation may offer the ultimate treatment option for severe patients to avoid certain deaths, while at the same time protecting transplantation doctors and medical staff appropriately [132].
6. Development of SARS-CoV-2 vaccines
In terms of controlling the epidemic aroused by emerging viruses, rapid diagnosis and effective vaccines serve as a complementation to antiviral therapy. Preventive and therapeutic SARS-CoV-2 vaccines will be of fundamental value as the most conspicuous way to mitigate the pandemic crisis [133]. Fortunately, published data on the SARS-CoV-2 genetic sequence has sparked a global campaign to inaugurate a vaccine against the infections. The scope of the impact of the COVID-19 pandemic on humanitarianism and the economy is also prompting the assessment of the next-generation vaccine technology platform through new paradigms. On March 16th, 2020, the first trial of COVID-19 vaccine candidate was launched in record speed. Besides, recently, Chen and her team developed and assessed the safety, tolerability and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine [134]. Their clinical data further revealed that the Ad5 vectored COVID-19 vaccine was tolerable and immunogenic at 28 days post-vaccination [134]. Moreover, the Coalition for Epidemic Preparedness Innovations (CEPI) is also combining efforts to espouse the development of vaccines against COVID-19. Fig. 8 summarizes the current cross-sectional COVID-19 vaccine candidates which have entered the clinical phase.
Fig. 8.
Clinical phase vaccine candidates for COVID-19. aAPC, artificial antigen-presenting cell; MHC, major histocompatibility complex class; VLP, virus-like particle; DC, dendritic cell; LV, lentiviral vector; CTLs, cytotoxic T lymphocytes; HLA-A, a group of human leukocyte antigens (HLA) that are coded for by the HLA-A locus. Created with BioRender.com.
As for the vaccine development of SARS-CoV-2, the pivotal and tangible avenues can be divided into four aspects: (1) Selection of antigen epitope; (2) Overcoming the antibody-dependent enhancement (ADE) issue; (3) Weighing humoral immunity and cellular immunity; (4) Selection of technical route befittingly. Up till now, structural epitope mapping by homology modeling has uncovered the immunoreactive antigen epitopes of SARS-CoV-2 [135]. The mainstream of the vaccine development is based upon the S protein in virtue of its essential role in the viral infectivity. Other subsequent developments can constrain focus on other viral proteins (i.e., the N protein, and E protein). Further, the titers of neutralizing antibodies that were variable among different patients were associated with the spike-binding Abs targeting S1, RBD, and S2 regions [136]. In this regard, we should also pay more attention to the titers of neutralizing antibodies.
In addition, researchers need to know whether the vaccine will induce the same type of immune system failures that have been observed previously. In some cases, the vaccine-primed immune system seems to initiate a shoddy response to natural infections [137]. Additionally, allergic inflammation aroused by Th2 immunopathology should be taken into consideration, according to theCoV experts [137]. Therefore, animal and human clinical trials of COVID-19 candidate vaccines should encompass a rigorous assessment of possible immune complications before putting into use.
According to the previous study on SARS-CoV, SARS-specific IgG Ab may ultimately fade away, and the peripheral memory B cell response cannot be detected in recovered SARS patients. In stark contrast, the memory response of specific T cells lasted at least six years, implicating the significance of cellular immunity for preventing the recurrence epidemics [138]. With regard to the technical routes, we can see efforts to espouse ‘quick-fix’ programs for the purpose of developing vaccines against COVID-19 worldwide [139]. There is a desperate need for selecting effective technical routes to develop different kinds of vaccines (i.e., live-attenuated vaccines, inactivated vaccines, nucleic acid vaccines, subunit, recombinant, polysaccharide and conjugate vaccines) in a quicker and safer manner [140].
As announced by the WHO, there are now more than 70 potential vaccines under development, with three already in clinical trials [141]. The following section will describe the status of vaccine development against this crisis by miscellaneous methods. The potential vaccine candidates for COVID-19 are summarized in Table 2.
Table 2.
The potential vaccine candidates for COVID-19 [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145].
| Candidate | Vaccine characteristics | Lead developer/sponsor | Current status | |
|---|---|---|---|---|
| Clinical-phase | INO-4800 | DNA plasmid encoding S protein delivered via electroporation | Inovio Pharmaceuticals | Phase I (NCT04336410) |
| mRNA-1273 | LNP-encapsulated mRNA vaccine encoding S protein | Moderna | Phase I (NCT04283461) | |
| LV-SMENP-DC | DCs modified with lentiviral vector expressing synthetic minigene based upon domains of selected viral proteins; | Shenzhen Geno-Immune Medical Institute | Phase I (NCT04276896) | |
| / | Recombinant novel coronavirus (2019-CoV) vaccine (adenoviral vector) | Institute of Military Medicine under the Academy of Military Sciences of the People's Liberation Army of China | Phase II (ChiCTR2000030906) | |
| / | Inactivated vaccine | Beijing Institute of Biological Products/Wuhan Institute of Biological Products | Phase I (ChiCTR2000031809) |
|
| ChAdOx1 | Attenuated adenovirus capable of producing the S protein of SARS-CoV-2 | University of Oxford | Phase I/II (NCT04324606) |
|
| Ad5-nCoV | Adenovirus type 5 vector that expresses S protein | CanSino Biologicals | Phase I (NCT04313127) | |
| Pathogen-specific aAPC | aAPCs modified with lentiviral vector expressing synthetic minigene based upon domains of selected viral proteins | Shenzhen Geno-Immune Medical Institute | Phase I (NCT04299724) | |
| Experimental-phase | / | Single-dose intranasal replication-defective adenovirus vector vaccine incorporating the SARS-CoV-2 S protein | Altimmune | Phase 1 trial planned for mid-August. |
| BNT162 | mRNA vaccine expressing codon-optimized undisclosed SARS-CoV-2 proteins | BioNTech | Clinical testing to begin late April | |
| STARR | Self-transcribing and replicating RNA vaccine expressing undisclosed epitopes | Arcturus | Manufacturing stage | |
| / | Protamine-complexed mRNA-based vaccine expressing undisclosed SARS-CoV-2 protein(s) | CureVac | Phase 1 planned in June or July | |
| / | Undisclosed SARS-CoV-2-derived synthetic peptide conjugated to the key moiety of the MHC II–associated invariant chain | Generex Biotechnology | Human trials planned in June | |
| / | Modified vaccinia Ankara VLP vaccine based upon Wuhan strain of SARS-CoV-2 | GeoVax | Candidates in animal studies | |
| / | Electroporated linear DNA vaccine based on S protein and selected epitopes | LineaRx | Four candidates for testing by the beginning of May or June | |
| / | Undisclosed recombinant SARS-CoV-2 protein VLP produced in tobacco | Medicago | preclinical testing ongoing with clinical trials to begin summer 2020 | |
| / | Recombinant subunit vaccine of SARS-CoV-2 S protein locked in prefusion conformation by polypeptide moiety (molecular clamp) | University of Queensland | Preclinical as of mid-March | |
| / | Oral recombinant adenovirus 5 vector vaccine of undisclosed SARS-CoV-2 proteins for mucosal immune response | Vaxart | Preclinical as of mid-March |
aAPC, artificial antigen-presenting cell; MHC, major histocompatibility complex class; VLP, virus-like particle; DC, dendritic cell.
6.1. DNA-based vaccines
DNA vaccines provide a precise and flexible tactic to deliver antigens to the immune system and can contain additional sequences of coding molecules to manipulate the results [147]. So far, a variety of DNA vaccine platforms have been exploited to enhance the vaccine efficacy through electroporation to deliver plasmids and addition of adjuvants, yielding improved the immune responses [148]. Inovio Pharmaceuticals have begun pre-clinical trials of a DNA vaccine (named as INO-4800) against COVID-19 [149]. This vaccine can induce T cell activation via transferring DNA plasmids that express the SARS-CoV-2 S proteins [150]. This vaccine platform holds the preponderance of producing therapeutic antibodies and activating immune cells to deliver the vaccine to patients through the skin. Recently, Yu and colleagues developed a series of DNA vaccine candidates expressing different forms of SARS-CoV-2 S protein and assessed them in 35 rhesus macaques [151]. Vaccinated animals developed humoral and cellular immune responses, including neutralizing antibody titers in comparison with those found in convalescent humans and macaques infected with SARS-CoV-2. Surprisingly, all animals were challenged with SARS-CoV-2 after vaccination, and the vaccine encoding the full-length S protein resulted in over 3.1 and over 3.7 log10 reductions in median viral loads in bronchoalveolar lavage and nasal mucosa, respectively, as compared with sham controls [151]. Their data indicated vaccine protection against SARS-CoV-2 in nonhuman primates [152]. Hence, in the near future, with the support of CEPI, these findings will boost the vaccine development and the corporation is making provision for the first phase of trials in the United States and China [153].
6.2. mRNA-based vaccines
The mRNA vaccine is a hopeful alternative to traditional vaccine methods in virtue of its high efficiency, rapid development capabilities, and the potential for low-cost manufacturing [154]. Recently, Moderna, Inc. has launched phase I clinical trials for mRNA-1273, which encodes S protein of SARS-CoV-2. This mRNA-vaccine was fabricated with the cooperation of the National Institute of Allergy and Infectious Diseases [155]. mRNA-1273, which encodes a prefusion-stabilized form of the SARS-CoV-2 spike, is under test for a broad dosing range (25—250 micrograms) during its phase 1 submission [143]. The firm further announced that although commercial vaccines are unlikely to be marketed in at least 12 to 18 months, in an emergency, possibly in the fall of 2020, some people, including medical professionals, may obtain the vaccine [146,147].
6.3. Recombinant subunit vaccines
Subunit vaccines are superior to other types of vaccines since they are highly safe and possess fewer adverse effects via eliciting the immune system without drawing into any infectious viruses [9]. It has also been reported that the enhancement of T cell responses and generation of high titer neutralizing Abs were observed in vivo during the vaccine development researches [157]. Clover Biopharmaceuticals was pre-clinically testing a recombinant subunit vaccine in the light of the S-Trimer of the SARS-CoV-2 [158]. The researchers detected the antigen-specific neutralizing Abs in the sera of fully recovered patients [158]. Besides, GlaxoSmithKline (GSK) disclosed a vaccine which can elicit a protective immune response against SARS. The vaccine contains an S protein immunogen, which was combined with the emulsion adjuvant, GSK2, yielding elevated level of anti-SARS-CoV IgG2a/IgG2b Ab responses. Recently, GSK and Clover Biopharmaceuticals announced a partnership to enhance immune response via introducing GSK's adjuvant system to S-Trimer [159]. In addition, the team of the University of Queensland is also designing subunit vaccines via the transformative technology, known as “molecular clamp” [160]. Molecular clamps are peptides that stabilize surface proteins, improve the recognition of correct antigens, and lead to a more robust immune response. This vaccine platform can be easily applied to a variety of enveloped viruses and rapidly manufacture their products [160].
Further, patent application US20060002947 disclosed the fabrication of hybrid peptides comprised of three elements: (1) invariant chain; (Ii) essential peptide; (2) chemical structure linking the Ii to the antigenic epitope; (3) antigen epitope binding MHC II molecule. This theory was harnessed to produce Ii-Key/MHC II SARS hybrids. In this respect, Generex reported that they will employ its Ii-Key immune system activation biotechnology to generate a COVID-19 viral peptide vaccine for human clinical practice [161].
6.4. Other vaccine approaches
Aside from the aforementioned approaches, Genexine Inc. is exploring a new vaccine via utilizing the Hyleukin-7 platform technology [162]. Such a platform may improve the immune responses fusing IL-7 with hyFc, aiming to hybridize IgD and IgG4 for the long-term effect of Fc fusion proteins [163], [164], [165]. Specifically, IgD possesses a flexible hinge structure and can maximize the biological activity [153,154]. IgG4 possesses an unexposed junction site that mitigates immunogenicity via preventing antibody-dependent cellular cytotoxicity [155,156]. This corporation reported enhanced vaccine efficacy, lung T cell accumulation, and plasmacytoid dendritic cell growth after Fc-fused IL-7 treatment in the virus infection models [166].
7. Conclusions and inspirations
Despite prodigious global efforts made to contain SARS-CoV-2, the current COVID-19 epidemic has expanded into a full-blown pandemic, yielding outright panic and economic slowdown. Health-care systems in many regions are overburdened and under-resourced, impelling medical workers and governors to make formerly unthinkable judgments concerning the allotment of medical care. In the wake of such a severe situation, a number of researches have trailed strategies to tackling the direct impact of COVID-19, either by modeling studies of the viral activity or via parsing out potential therapeutic options and finding a vaccine to end the coronavirus pandemic. Although the development of therapeutics and vaccines for the COVID-19 therapy is still in its middle stage, some marked advances have been made from complete genome sequencing of SARS-CoV-2 to the clinical practice of COVID-19 vaccines.
Rapid genome-wide association of SARS-CoV-2, along with international sharing of information, enabled us to generate rapider and more proper diagnostic tools. As set forth, the major fashioned diagnostic tools are qRT-PCR-based approaches, requiring a long time for specimen preparation and analysis, thus putting off the imperative actions for COVID-19 infections. Of note, in acute respiratory infections, RT-PCR is commonly utilized for detecting pathogenic viruses in respiratory secretions. The positive rate of PCR from oropharyngeal swabs is not very high, implicating the requirement of more swab testing are need to confirm the diagnosis. With the continuous spread of SARS-CoV-2, there is a desperate demand to develop rapid diagnostic methods that can be tested more efficiently.
As the report goes, certain drugs are known to effectively treat patients with COVID-19. Nevertheless, the lack of clinical data may render the clinical prognosis difficult to predict. In addition to the judicious design of novel therapeutics that target viral replication or immunopathology, currently, rapid screening of therapeutic agents to repurpose FDA-approved and well-characterized agents might be a more workable method. Considering the severity of the recent zoonotic coronavirus outbreaks, therapeutic drugs for pancoronavirus should be carried out to cope with future outbreaks.
With respect to the detailed exploitation of COVID-19 vaccines, several pharmaceutical corporations and institutions have also launched the project for vaccine development. Notwithstanding, the commercial market for vaccines, especially vaccines for emerging infectious diseases, is confined by the high cost and time required for vaccine development along with the uncertainty of profitability. Accordingly, CEPI is combining efforts to encourage the progress of vaccines against COVID-19. Besides, in the near future, the production of a lot of vaccines may bring about the challenge in scaling up manufacturing quickly, because the infrastructure needed will differ in virtue of the vaccine type. Another concern regarding the vaccine development may be the antibody-dependent enhancement issue.
In addition, this global epidemic crisis indicated that host-species expansion or interspecies transmission of neoteric coronavirus to humans might be unavoidable. Clinicians and researchers should combine efforts to swiftly evolve our perception of all facets of SARS-CoV-2 infections and fill in the gaps concerning the emergence of this virus. Ultimately, we can also learn from this epidemic that we need to improve our disease monitoring and surveillance system to prevent such a serious disease outbreak. Digital technologies such as big-data analytics, artificial intelligence, and blockchain technology may also need to be fully exploited and developed, thereby enabling real-time data collection at scale and modeling risk associations immediately to contain the pathophoresis.
Overall, the challenges posed by 21st century epidemics are real and changing: future epidemics will be fueled by various internal and external causes. In our response, we must view pandemics as interconnected cycles, not isolated events, and while we cannot forecast specific outbreaks, we can make provision for them.
Conflict of interest
The authors assert that there is no conflict of interests concerning the publication of this review.
References
- 1.Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat Med. 2020:1–3. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J.F.W., Lau S.K.P., Woo P.C.Y. The emerging novel Middle East respiratory syndrome coronavirus: the “knowns” and “unknowns.”. J Formos Med Assoc. 2013;112:372–381. doi: 10.1016/j.jfma.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.Y. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020:1–13. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cyranoski D. Did pangolins spread the China coronavirus to people. Nature. 2020 doi: 10.1038/d41586-020-00364-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. 2020;30:1346–1351. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11:836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J., Dong X., Cao Y., Yuan Y., Yang Y., Yan Y. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020;00:1–12. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 13.Organization W.H.Coronavirus disease 2019 (COVID-19): situation report, 722020. https://apps.who.int/iris/bitstream/handle/10665/331685/nCoVsitrep01Apr2020-eng.pdf.
- 14.Wu J.T., Leung K., Bushman M., Kishore N., Niehus R., de Salazar P.M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020:1–5. doi: 10.1038/s41591-020-0822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metcalf C.J.E., Lessler J. Opportunities and challenges in modeling emerging infectious diseases. Science. 2017;357:149–152. doi: 10.1126/science.aam8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn D.G., Shin H.J., Kim M.H., Lee S., Kim H.S., Myoung J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30:313. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoehl S., Rabenau H., Berger A., Kortenbusch M., Cinatl J., Bojkova D. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382:1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Eurosurveillance. 2020;25(5) doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X., Rayner S., Luo M. Does SARS‐CoV‐2 has a longer incubation period than SARS and MERS? J Med Virol. 2020;92:476–478. doi: 10.1002/jmv.25708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang B., Wang X., Li Q., Bragazzi N.L., Tang S., Xiao Y. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J Clin Med. 2020;9:462. doi: 10.3390/jcm9020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delamater P.L., Street E.J., Leslie T.F., Yang Y.T., Jacobsen K.H. Complexity of the basic reproduction number (R0) Emerg Infect Dis. 2019;25:1. doi: 10.3201/eid2501.171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coronavirus (COVID-19)Last updated: 2020/5/28, 2:00am CEST. https://covid19.who.int/.
- 27.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Hu Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS‐CoV‐2 infection. J Med Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kratzel A., Todt D., V'kovski P., Steiner S., Gultom M.L., Thao T.T.N. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020;26:1592–1595. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walls A.C., Park Y.-.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q.H., Zhang Y.F., Wu L.L., Niu S., Song C.L., Zhang Z.Y. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127. doi: 10.1128/JVI.00127-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry J.D., Jones S., Drebot M.A., Andonov A., Sabara M., Yuan X.Y. Development and characterisation of neutralising monoclonal antibody to the SARS-coronavirus. J Virol Methods. 2004;120:87–96. doi: 10.1016/j.jviromet.2004.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci. 2020;117(17) doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.To K.K.W., Tsang O.T.Y., Yip C.C.Y., Chan K.H., Wu T.C., Chan J.M. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020:ciaa149. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan J.F.W., Choi G.K.Y., Tsang A.K.L., Tee K.M., Lam H.Y., Yip C.C.Y. Development and evaluation of novel real-time reverse transcription-PCR assays with locked nucleic acid probes targeting leader sequences of human-pathogenic coronaviruses. J Clin Microbiol. 2015;53:2722–2726. doi: 10.1128/JCM.01224-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhadra S., Jiang Y.S., Kumar M.R., Johnson R.F., Hensley L.E., Ellington A.D. Real-time sequence-validated loop-mediated isothermal amplification assays for detection of Middle East respiratory syndrome coronavirus (MERS-CoV) PLoS One. 2015;(4) doi: 10.1371/journal.pone.0123126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheridan C. Fast, portable tests come online to curb coronavirus pandemic. Nat Biotechnol. 2020;38:515–518. doi: 10.1038/d41587-020-00010-2. [DOI] [PubMed] [Google Scholar]
- 42.Chan J.F.W., Yip C.C.Y., To K.K.W., Tang T.H.C., Wong S.C.Y., Leung K.H. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58(5):e00310–e00320. doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. pii=2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanne J.P.Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist2020;295:16–7 [DOI] [PMC free article] [PubMed]
- 46.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broughton JP, Deng W, Fasching CL, Singh J, Chiu CY, Chen JS. A protocol for rapid detection of the 2019 novel coronavirus SARS-CoV-2 using CRISPR diagnostics: SARS-CoV-22020. https://mammoth.bio/wp-content/uploads/2020/04/200423-A-protocol-for-rapid-detection-of-SARS-CoV-2-using-CRISPR-diagnostics_3.pdf.
- 48.Xiao F., Tang M., Zheng X., Li C., He J., Hong Z. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020:1–8. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–277. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020:1–6. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang M.Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. https://ssrn.com/abstract=3527420or http://dx.doi.org/10.2139/ssrn.3527420.
- 57.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan K., Yi L., Chen J., Qu X., Qing T., Rao X. Suppression of SARS-CoV entry by peptides corresponding to heptad regions on spike glycoprotein. Biochem Biophys Res Commun. 2004;319:746–752. doi: 10.1016/j.bbrc.2004.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibrahim I.M., Abdelmalek D.H., Elshahat M.E., Elfiky A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80:554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun J., He W.T., Wang L., Lai A., Ji X., Zhai X. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol Med. 2020;26(5):483–495. doi: 10.1016/j.molmed.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai W.H., Zhang B., Jiang X.M., Su H.X., Li J., Zhao Y. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368(6497):1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang H., Xie W., Xue X., Yang K., Ma J., Liang W. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3(11):e428. doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gadalla M.R., Veit M. Toward the identification of ZDHHC enzymes required for palmitoylation of viral protein as potential drug targets. Expert Opin Drug Discov. 2020;15:159–177. doi: 10.1080/17460441.2020.1696306. [DOI] [PubMed] [Google Scholar]
- 65.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020 doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3:e00412–e00473. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng V.C.C., Lau S.K.P., Woo P.C.Y., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khamitov R.A., Si Loginova, Shchukina V.N., Borisevich S.V., Maksimov V.A., Shuster A.M. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol. 2008;53:9–13. [PubMed] [Google Scholar]
- 69.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elshabrawy H.A., Fan J., Haddad C.S., Ratia K., Broder C.C., Caffrey M. Identification of a broad-spectrum antiviral small molecule against severe acute respiratory syndrome coronavirus and Ebola, Hendra, and Nipah viruses by using a novel high-throughput screening assay. J Virol. 2014;88:4353–4365. doi: 10.1128/JVI.03050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Que T.L., Wong V.C.W., Yuen K.Y. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. [PubMed] [Google Scholar]
- 75.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto N., Yang R., Yoshinaka Y., Amari S., Nakano T., Cinatl J. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun. 2004;318:719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Tawfiq J.A., Momattin H., Dib J., Memish Z.A. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Clercq E. New Nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem Asian J. 2019;14:3962–3968. doi: 10.1002/asia.201900841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holshue M.L., De Bolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sallard E., Lescure F.X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.CDC. Information for clinicians on therapeutic options for patients with COVID-192020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html.
- 82.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y., Wang W., Xu L., Zhou X., Shokrollahi E., Felczak K. Cross talk between nucleotide synthesis pathways with cellular immunity in constraining hepatitis E virus replication. Antimicrob Agents Chemother. 2016;60:2834–2848. doi: 10.1128/AAC.02700-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Debing Y., Emerson S.U., Wang Y., Pan Q., Balzarini J., Dallmeier K. Ribavirin inhibits in vitro hepatitis E virus replication through depletion of cellular GTP pools and is moderately synergistic with alpha interferon. Antimicrob Agents Chemother. 2014;58:267–273. doi: 10.1128/AAC.01795-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.So L.K.Y., Lau A.C.W., Yam L.Y.C., Cheung T.M.T., Poon E., Yung R.W.H. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet. 2003;361:1615–1617. doi: 10.1016/S0140-6736(03)13265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wenzel R.P., Edmond M.B. Managing SARS amidst uncertainty. N Engl J Med. 2003;348:1947–1948. doi: 10.1056/NEJMp030072. [DOI] [PubMed] [Google Scholar]
- 87.Morgenstern B., Michaelis M., Baer P.C., Doerr H.W. Ribavirin and interferon-β synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326:905–908. doi: 10.1016/j.bbrc.2004.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park A.Leaked data from a key remdesivir study suggest the potential coronavirus drug is not effective2020. https://time.com/5826618/remdesivir-leaked-data-who-website/.
- 93.Ledford H.Hopes rise for coronavirus drug remdesivir2020. Doi: 10.1038/d41586-020-01295-8. [DOI] [PubMed]
- 94.Samuel C.E. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scagnolari C., Vicenzi E., Bellomi F., Stillitano M.G., Pinna D., Poli G. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antivir Ther. 2004;9:1003–1011. [PubMed] [Google Scholar]
- 96.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 97.Wu C.Y., Jan J.T., Ma S.H., Kuo C.J., Juan H.F., Cheng Y.S.E. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baden L.R., Rubin E.J. Covid-19-the search for effective therapy. N Engl J Med. 2020;382:1851–1852. doi: 10.1056/NEJMe2005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;4(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 103.Jallouli M., Galicier L., Zahr N., Aumaitre O., Frances C., Le Guern V. Determinants of hydroxychloroquine blood concentration variations in systemic lupus erythematosus. Arthritis Rheumatol. 2015;67:2176–2184. doi: 10.1002/art.39194. [DOI] [PubMed] [Google Scholar]
- 104.Biot C., Daher W., Chavain N., Fandeur T., Khalife J., Dive D. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem. 2006;49:2845–2849. doi: 10.1021/jm0601856. [DOI] [PubMed] [Google Scholar]
- 105.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020:ciaa237. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 107.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Kunzmann K.A.WHO suspends hydroxychloroquine treatment in COVID-19 solidarity trial2020. https://www.contagionlive.com/news/who-hydroxychloroquine-treatment-covid-19-solidarity-trial.
- 110.Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53:436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shi R., Shan C., Duan X., Chen Z., Liu P., Song J. A human neutralizing antibody targets the receptor binding site of SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- 112.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020 doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 113.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou M., Zhang X., Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front Med. 2020;14(2):126–135. doi: 10.1007/s11684-020-0767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee N., Chan K.C.A., Hui D.S., Ng E.K.O., Wu A., Chiu R.W.K. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.-.M., Lim W.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Du H.Z., Hou X.Y., Miao Y.H., Huang B.S., Liu D.H. Traditional chinese medicine: an effective treatment for 2019 novel coronavirus pneumonia (NCP) Chin J Nat Med. 2020;18:206–210. doi: 10.1016/S1875-5364(20)30022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X., Zhang M., He L., Li Y. Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS) Cochrane Database Syst Rev. 2012;25(1) doi: 10.1002/14651858.CD004882.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li R.F., Hou Y.L., Huang J.C., Pan W.Q., Ma Q.H., Shi Y.X. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16:1708. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev Rep. 2020;16(3):427–433. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Williams R.Are mesenchymal stem cells a promising treatment for COVID-19?2020. https://www.the-scientist.com/news-opinion/are-mesenchymal-stem-cells-a-promising-treatment-for-covid-19–67402.
- 127.Stem cell therapy: a promising treatment for COVID-19?. https://www.technologynetworks.com/biopharma/product-news/stem-cell-therapy-a-promising-treatment-for-covid-19-333498.
- 128.Malicdem D.Hydrogen peroxide as treatment for coronavirus infection: does it work?https://www.medicaldaily.com/hydrogen-peroxide-treatment-coronavirus-infection-does-work-451710.
- 129.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.LeBaron T.W., McCullough M.L., Ruppman Sr K.H. A novel functional beverage for COVID-19 and other conditions: hypothesis and preliminary data, increased blood flow, and wound healing. J Transl Sci. 2020;6:1–6. [Google Scholar]
- 131.Hydrogen molecular biomedicine promotes new coronary pneumonia diagnosis and treatment plan. https://www.sohu.com/a/375239106_99929695
- 132.Chen J.Y., Qiao K., Liu F., Wu B., Xu X., Jiao G.Q. Lung transplantation as therapeutic option in acute respiratory distress syndrome for COVID-19-related pulmonary fibrosis. Chin Med J. 2020;133:1390–1396. doi: 10.1097/CM9.0000000000000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.André F.E. The future of vaccines, immunisation concepts and practice. Vaccine. 2001;19:2206–2209. doi: 10.1016/s0264-410x(00)00546-6. [DOI] [PubMed] [Google Scholar]
- 134.Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tilocca B., Soggiu A., Sanguinetti M., Musella V., Britti D., Bonizzi L. Comparative computational analysis of SARS-CoV-2 nucleocapsid protein epitopes in taxonomically related coronaviruses. Microbes Infect. 2020;22(4–5):188–194. doi: 10.1016/j.micinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. MedRxiv. 2020 doi: 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 137.Peeples L. News feature: avoiding pitfalls in the pursuit of a COVID-19 vaccine. Proc Natl Acad Sci. 2020;117(15) doi: 10.1073/pnas.2005456117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tang F., Quan Y., Xin Z.T., Wrammert J., Ma M.J., Lv H. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186:7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 139.Jiang S.B.Don't rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees2020. Doi: 10.1038/d41586-020-00751-9. [DOI] [PubMed]
- 140.Vaccine Types2020. https://www.vaccines.gov/basics/types.
- 141.Keown A.WHO: more than 70 COVID-19 vaccines are in development, three in clinical trials2020. https://www.biospace.com/article/more-than-70-vaccines-for-covid-19-are-in-development-for-covid-19-who-says/.
- 142.Le T.T., Andreadakis Z., Kumar A., Roman R.G., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 143.Hodgson J.The pandemic pipeline Nature Biotechnology2020;38(5):523–32. [DOI] [PubMed]
- 144.Times T.E.Chinese research firm attached to Army first to start 2nd clinical trial for coronavirus vaccine. https://health.economictimes.indiatimes.com/news/diagnostics/chinese-research-firm-attached-to-pla-first-to-start-2nd-clinical-trial-for-coronavirus-vaccine/75152489.
- 145.UK researchers to trial Covid-19 vaccine and plasma therapy. https://www.clinicaltrialsarena.com/news/uk-covid-vaccine-plasma-therapy/.
- 146.Chinese clinical trial registry2020. http://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2000031809.
- 147.Rice J., Ottensmeier C.H., Stevenson F.K. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 148.Sardesai N.Y., Weiner D.B. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23:421–429. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Inovio accelerates timeline for COVID-19DNA vaccine INO-4800. https://www.prnewswire.com/news-releases/inovio-accelerates-timeline-for-covid-19-dna-vaccine-ino-4800-301015031.html.
- 150.Inovio's Product Pipeline2020. https://pipelinereview.com/index.php/2020040674247/Vaccines/INOVIO-Initiates-Phase-1-Clinical-Trial-Of-Its-COVID-19-Vaccine-and-Plans-First-Dose-Today.html.
- 151.Yu J.Y., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020:eabc6284. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chandrashekar A., Liu J., Martinot A.J., McMahan K., Mercado N.B., Peter L. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020:eabc4776. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Inovio's DNA-encoded monoclonal antibody (dMAbtm) platform leaps forward with first-in-human trial. https://mail.lelezard.com/en/news-18575477.html.
- 154.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moderna's work on a potential vaccine against COVID-19. https://www.modernatx.com/modernas-work-potential-vaccine-against-covid-19.
- 156.mRNA platform: enabling drug discovery&development. https://www.modernatx.com/mrna-technology/mrna-platform-enabling-drug-discovery-development.
- 157.Okba N.M.A., Raj V.S., Haagmans B.L. Middle East respiratory syndrome coronavirus vaccines: current status and novel approaches. Curr Opin Virol. 2017;23:49–58. doi: 10.1016/j.coviro.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Clover Biopharmaceuticals vaccines programs. http://www.cloverbiopharma.com/index.php?m=content&c=index&a=lists&catid=42.
- 159.GSK. Clover and GSK announce research collaboration to evaluate coronavirus (COVID-19) vaccine candidate with pandemic adjuvant system. https://www.gsk.com/en-gb/media/press-releases/clover-and-gsk-announce-research-collaboration-to-evaluate-coronavirus-covid-19-vaccine-candidate-with-pandemic-adjuvant-system/.
- 160.University of Queensland. ‘Significant step’ in COVID-19 vaccine quest. https://www.uq.edu.au/news/article/2020/02/significant-step%E2%80%99-covid-19-vaccine-quest.
- 161.Generex. Update:, Generex Provides Coronavirus Develop, Generex Receives Contract from Chinese Partners to Vaccines, a COVID-19 Vaccine Using Ii-Key Peptide. https://pipelinereview.com/index.php/2020022973919/Vaccines/Generex-Provides-Coronavirus-Update-Generex-Receives-Contract-from-Chinese-Partners-to-Develop-a-COVID-19-Vaccine-Using-Ii-Key-Peptide-Vaccines.html.
- 162.Genexine. hyFc® Platform. http://www.genexine.com/m21.php.
- 163.Seo Y.B., Im S.J., Namkoong H., Kim S.W., Choi Y.W., Kang M.C. Crucial roles of interleukin-7 in the development of T follicular helper cells and in the induction of humoral immunity. J Virol. 2014;88:8998–9009. doi: 10.1128/JVI.00534-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Løset G.Å., Roux K.H., Zhu P., Michaelsen T.E., Sandlie I. Differential segmental flexibility and reach dictate the antigen binding mode of chimeric IgD and IgM: implications for the function of the B cell receptor. J Immunol. 2004;172:2925–2934. doi: 10.4049/jimmunol.172.5.2925. [DOI] [PubMed] [Google Scholar]
- 165.Lee J.H., Cho J.H., Yeo J., Lee S.H., Yang S.H., Sung Y.C. The pharmacology study of a new recombinant TNF receptor-hyFc fusion protein. Biologicals. 2013;41:77–83. doi: 10.1016/j.biologicals.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 166.Kang M.C., Park H.W., Choi D.H., Choi Y.W., Park Y., Sung Y.C. Plasmacytoid dendritic cells contribute to the protective immunity induced by intranasal treatment with Fc-fused interleukin-7 against lethal influenza virus infection. Immune Netw. 2017;17:343–351. doi: 10.4110/in.2017.17.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]