Abstract
Background.
Low-dose computed tomography (LDCT) screening for lung cancer is a preference-sensitive intervention that should ideally be individualized according to patients’ likelihood of benefit and personal values. Personalized cancer risk information (PCRI) may facilitate this goal, but its effects are unknown.
Objective.
To evaluate the effects of providing PCRI to patients referred for LDCT screening.
Design.
Mixed-methods, pre-post study using surveys administered to patients before and after provision of PCRI—calculated by the PLCOm2012 risk prediction model—in shared decision-making consultations, and postvisit qualitative interviews.
Setting.
Centralized specialty-based LDCT screening program at a tertiary care hospital.
Participants.
Convenience sample of eligible patients referred for LDCT screening.
Measurements.
Pre- and postvisit surveys assessed patients’ 1) perceived lung cancer risk, 2) uncertainty about their risk, 3) minimum risk threshold for wanting screening, 4) interest in LDCT screening, and 5) interest in smoking cessation. Qualitative interviews explored patients’ perceptions of the value of PCRI. Screening uptake was assessed by chart review.
Results.
Sixty of 70 (86%) patients received PCRI and completed pre-post surveys, and 17 patients (28%) completed qualitative interviews. Perceived lung cancer risk decreased from 52% previsit to 31% postvisit (P < 0.0001). However, patients’ minimum risk thresholds for screening decreased, their screening interest increased, and all patients completed screening. Qualitative interviews corroborated these effects, suggesting that patients discount and interpret PCRI according to preexisting beliefs and attitudes.
Limitations.
The study population was a relatively small, single-institution sample of patients referred for screening.
Conclusions.
Personalized cancer risk information decreases cancer risk perceptions of patients referred for LDCT screening, but has complex effects on screening-related judgments and decisions. The value of PCRI for patients considering LDCT screening requires further investigation.
Keywords: lung cancer, risk perceptions, risk prediction, screening
Lung cancer is the leading cause of cancer mortality in the United States and a malignancy for which an effective new screening test—low-dose computed tomography (LDCT)—currently exists. In 2011, the National Lung Screening Trial (NLST) demonstrated that LDCT screening reduced lung cancer mortality among patients aged 55 to 74 with a ≥30 pack-year smoking history and who currently smoke or have quit within ≤15 years.1 LDCT screening has been recommended by professional organizations, including the US Preventive Services Task Force,2 and the Centers for Medicare & Medicaid Services (CMS) have authorized coverage of this service.3
Yet several aspects of LDCT screening make it a preference-sensitive intervention. The absolute mortality risk reduction from screening is arguably modest, averaging <1% over a 6- to 7-year period among patients who meet NLST eligibility criteria. LDCT screening also has potential harms, including false-positive test results, overdiagnosis, and radiation exposure. Professional organizations have thus recommended—and CMS has required—that patients be informed about the benefits, harms, and uncertainties of LDCT, through a process of shared decision making (SDM), before undergoing screening.
These concerns have also fueled growing interest in an alternative implementation strategy: “risk-based,”4 “personalized,”5,6 or “precision”7 screening based on personalized cancer risk information (PCRI)—that is, evidence-based estimates of an individual’s risk of cancer.2,8–10 Personalized lung cancer risk information is now obtainable from several validated clinical prediction models and may improve LDCT screening in 2 main ways. First, PCRI may help maximize the net benefits of LDCT screening from a population perspective by identifying high-risk individuals who are most likely to benefit and enabling screening to be targeted toward them.4,11–15 Secondary analyses of data from the NLST and the US Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) Screening Trial have suggested that risk-based screening would result in fewer individuals being screened, more lung cancers identified, fewer false positives, and fewer false negatives.16,17 A recent analysis, however, has shown that risk-based screening may not improve the cost-effectiveness of screening because higher-risk patients are more costly to screen and have a lower life expectancy if they survive lung cancer.18
Nevertheless, PCRI may also improve LDCT screening from an individual, clinical perspective by enhancing informed and shared decision making.4,8,9,11–13,19–22 In theory, PCRI enables patients to determine for themselves whether their own disease risks are sufficiently high to justify undertaking risk-reducing action.23 Individual patients could compare their PCRI with their own personal risk thresholds to make this determination. Supporting this individual model of use of PCRI is a recent simulation study, which showed that patients’ perceptions of the net benefits and harms of lung cancer screening influence screening decisions at particular cancer risk thresholds.9 At least 2 currently available decision aids for LDCT screening include risk calculators and could be used to integrate PCRI in the SDM process.9,24,25
Yet several unanswered questions remain regarding the use of PCRI in LDCT screening. One important question is how PCRI affects patients’ screening-related judgments and decisions. Patients and laypersons are known to overestimate their cancer risks,26–29 and PCRI suggesting a lower-than-expected personal cancer risk might correct these misperceptions.30 In doing so, however, PCRI might diminish interest in screening, given that elevated risk perceptions motivate health-protective behaviors.29,31,32 However, we lack evidence about these potential effects. A 2013 Cochrane review analyzed 6 randomized controlled trials involving the provision of personalized breast cancer risk information and found improvements in patient knowledge but inconsistent effects on cancer screening interest and uptake.33 Recent studies of risk communication in colorectal cancer screening have also examined only nonquantitative (categorical)34–36 or nonpersonalized37 risk information. To our knowledge, prior studies have not examined how PCRI affects patients considering lung cancer screening with LDCT.
To begin to address this question, we conducted a mixed-methods pre-post intervention study to evaluate the effects of PCRI on patients referred to a centralized specialty-based LDCT screening program for prescreening SDM consultations. We used qualitative interviews to explore patients’ perceptions of the value of PCRI and pre- and postvisit surveys to examine whether the provision of PCRI affected patients’ perceptions of their lung cancer risk, their personal risk thresholds for wanting screening, their interest in LDCT screening, their actual screening behavior, and—among patients who currently smoked—their interest in smoking cessation.
Methods
The study was conducted between October 2015 and May 2017 and approved by the Maine Medical Center Institutional Review Board.
Study Population and Setting
The population consisted of all screening-eligible patients (aged 55–80 with ≥30-pack-year smoking history, who either currently smoked or quit ≤15 years ago) referred to a centralized, specialty-based LDCT screening program affiliated with a 606-bed tertiary care hospital and staffed by board-certified pulmonary medicine physicians. Patients were referred from community primary care practices, and the program assumed responsibility for all screening tasks, including prescreening SDM counseling, which was conducted during separately scheduled appointments. At the time of the study, the program received approximately 0 to 4 patient referrals weekly; all referred patients were approached to participate in the study.
Study Intervention
The program’s prescreening SDM counseling was provided by 2 pulmonary physicians during 40-minute consultation visits, guided by a brief 1-page decision aid (DA) (online Appendix A) developed with input from national experts (Acknowledgments). The DA was modeled on the “Option Grid” approach of Elwyn et al.38 and utilized a “Frequently Asked Questions” format designed to structure conversations about the potential benefits, harms, and uncertainties of LDCT screening.
For the current study, the SDM counseling protocol was modified to include provision of PCRI produced by the PLCOm2012, a prediction model that estimates individuals’ 6-year risk of developing lung cancer based on smoking history and other variables (age, race/ethnicity, education, body mass index [BMI], personal history of cancer, chronic obstructive pulmonary disease, family history of lung cancer).16,39 Developed and externally validated by Tammemagi et al.39 using data from the PLCO trial,40 this model has shown good discrimination and calibration12,22 and is publicly available as a Microsoft Excel-based calculator (http://www.brock-u.ca/lung-cancer-risk-calculator). We developed a simple user interface (online Appendix B) to display model estimates both textually and visually.
For all study participants, the counseling clinician first introduced the topic of LDCT screening and the purpose of the visit. The clinician then explained the nature of the PLCOm2012, informing patients that it was developed using data from a large, well-done national study and produces accurate estimates of an individual’s risk of developing lung cancer. The clinician then entered the patient’s risk factor information using the software interface and communicated the patient’s risk estimate using standardized language (e.g., “Your estimated risk of developing lung cancer in the next 6 years is 2%”). Because our aim was to assess the effects of PCRI itself on judgment and decision making, no additional support in interpreting the risk estimate was provided. At the conclusion of the visit, the clinician provided patients with a printout of their risk estimate.
Measures
Both quantitative (pre- and postvisit surveys) and qualitative (individual in-depth interviews) data were collected to assess various potential outcomes of PCRI.
Pre- and postvisit surveys.
Immediately before and after the counseling visit, surveys (Table 1) were administered to assess several outcomes: 1) perceived lung cancer risk, measured using a question, adapted from prior research,41 assessing participants’ perceptions of their lifetime risk of lung cancer using quantitative percentages; 2) perceived uncertainty about lung cancer risk, measured using a question, adapted from prior research,42 assessing participants’ level of certainty about their lung cancer risk; 3) interest in lung cancer screening; 4) interest in smoking cessation (for current smokers only); and 5) personal risk threshold for screening, measured by a newly developed question assessing patients’ minimum level of lung cancer risk for wanting screening. Lung cancer screening uptake was measured by review of medical records documenting completion of LDCT screening within 3 months.
Table 1.
Pre- and Postvisit Survey Measures
| Perceived lung cancer risk |
| • On a scale from 0% to 100%, what do you believe is the chance that you will develop lung cancer? (21-point Likert scale: 0%–100% in 5% increments) |
| Perceived uncertainty about lung cancer risk |
| • How certain do you feel about the opinions you just gave regarding your risk of developing lung cancer? (5-point Likert scale: 1 = not at all certain, 5 = extremely certain) |
| Interest in lung cancer screening |
| • How interested are you in having a lung CT scan to test for lung cancer? (5-point Likert scale: 1 = not interested, 5 = extremely interested) |
| Interest in smoking cessation |
| • How interested are you in quitting smoking? (10-point Likert scale: 1 = not interested, 10 = extremely interested) |
| Personal risk threshold for lung cancer screening |
| • On a scale from 0% to 100%, how high would your lifetime lung cancer risk have to be to make you want to have a lung cancer screening test? (21-point Likert scale: 0%–100% in 5% increments) |
CT, computed tomography.
Qualitative interviews.
Patients referred for screening were approached by telephone within 2 weeks of the counseling visit to participate in additional individual in-depth, 30-minute qualitative interviews. Interviews were conducted by telephone by an experienced qualitative researcher (CG), using a semistructured guide designed to explore patients’ perceptions of the value of PCRI and its impact on their screening decisions (online Appendix C). Interviews were audiorecorded, then transcribed verbatim and anonymized by a professional transcription service.
Data Analysis
Descriptive statistics were computed for all study variables. To assess the change in the primary and secondary outcomes following the counseling visit, we fit repeated-measures analysis of variance (ANOVA) models including age, sex, time (pre- or postvisit), and a random patient effect using the lme4 function in the lmer package in R (version 3.5.1). To assess the potential moderating effect of objective (model-estimated) risk on the study outcomes, we fit additional models that included objective risk and its interaction with time. To assess the magnitude of the potential effects of the pre-post visit change in lung cancer risk perceptions on the change in both interest in screening and personal risk threshold for screening, we fit analysis of covariance (ANCOVA) models with the change in perceived risk (Δ) as the independent variable and the change in screening interest and personal risk threshold (Δ) as dependent variables, adjusting for age and sex. To account for missing data, we conducted multiple imputation using the R package mice with 5 imputed data sets and the pmm (predictive mean matching) method and implemented the Rubin method to compute standard errors and confidence intervals.43
Interview data were analyzed using inductive qualitative methods. Line-by-line software-assisted coding of anonymized interview transcripts was conducted using the program MaxQDA. Analysis used a constant comparative method and inductive “grounded theory” approach in which the investigators strove to minimize preconceptions, allowing important themes to emerge.44–47 Two investigators conducted initial analysis and “open coding” of 3 transcripts to identify themes and develop a preliminary codebook, which the research team iteratively revised. Three investigators then applied the revised code-book to the remaining transcripts and categorized text passages within an overall conceptual schema. Coding decisions were compared, harmonized, and validated through further team discussions.
Results
Seventy consecutive patients were approached to participate, and all completed SDM counseling and pre-post surveys; 60 of 70 (86%) received PCRI, and 17 of 60 (28%) completed qualitative interviews. The quantitative analytic sample (N = 60) was 59% male and 41% female with a mean (SD) age of 63.2 (5.2) years and a mean (SD) 6-year model-estimated lung cancer risk of 4.55% (4.78; range, 1.0%–30.0%); 51% were current smokers, indicated by completed responses to the smoking cessation interest item. Missing data for individual items ranged from 5% (perceived risk) to 21% (screening interest). The qualitative analytic sample (N = 17) was 65% male and 35% female with a mean (SD) age of 64.9 (4.0) years and a mean (SD) 6-year model-estimated lung cancer risk of 4.08% (2.31); 35% were current smokers.
Pre- and Postvisit Survey Outcomes
Multivariate analyses, comparing pre- and postvisit primary outcomes and adjusted for age and sex, are shown in Table 2 (detailed parameter estimates provided in online Appendix D). There was a significant decrease in lung cancer risk perceptions (from a previsit model-adjusted mean of 52.1% to 32.8% postvisit; P < 0.0001) but no significant change in perceived uncertainty about lung cancer risk (P = 0.062). Meanwhile, interest in lung cancer screening paradoxically increased, from a mean previsit value of 4.37 (on a 5-point scale) to 4.70 postvisit (P < 0.001). This effect, however, was not clearly attributable to PCRI; change in interest in lung cancer screening was not associated with change in perceived lung cancer risk (P = 0.09). All patients (100%) scheduled and completed LDCT screening. There was no significant change in interest in smoking cessation among current smokers (P = 0.61).
Table 2.
Pre-Post Changes in Study Outcomes following Receipt of Personalized Cancer Risk Informationa
| Outcome | Pre | Post | Difference | P |
|---|---|---|---|---|
| Perceived lung cancer risk (quantitative)b | 52.10 (45.59, 58.60) | 32.84 (26.37, 39.31) | −19.26 (−24.80, −13.72) | <0.0001 |
| Perceived uncertainty about lung cancer riskc | 3.25 (2.99, 3.51) | 3.56 (3.28, 3.84) | 0.31 (−0.02, 0.63) | 0.062 |
| Interest in lung cancer screeningc | 4.37 (4.20, 4.53) | 4.70 (4.53, 4.88) | 0.34 (0.15,0.53) | <0.001 |
| Interest in smoking cessationd | 3.69 (3.00, 4.38) | 3.82 (3.32, 4.33) | 0.13 (−0.39, 0.65) | 0.611 |
| Personal risk threshold for screeningb | 47.71 (40.37, 55.04) | 35.48 (28.12, 42.84) | −12.23 (−18.36, −6.09) | <0.001 |
Model-adjusted means (95% CI) estimated by repeated-measures analysis of variance models controlling for participant age and sex.
Interval scale ranging from 0% to 100% in 5% increments.
Ordinal scale ranging from 1 to 5.
Ordinal scale ranging from 1 to 5, converted from original 10-point ordinal scale.
Objective, model-estimated lung cancer risk was found to moderate the effect of PCRI on patients’ risk perceptions (P = 0.005, for objective risk × time interaction). Figure 1 demonstrates an interactive dose-response effect, in which higher model-estimated risk was associated with a smaller change in perceived risk.
Figure 1.
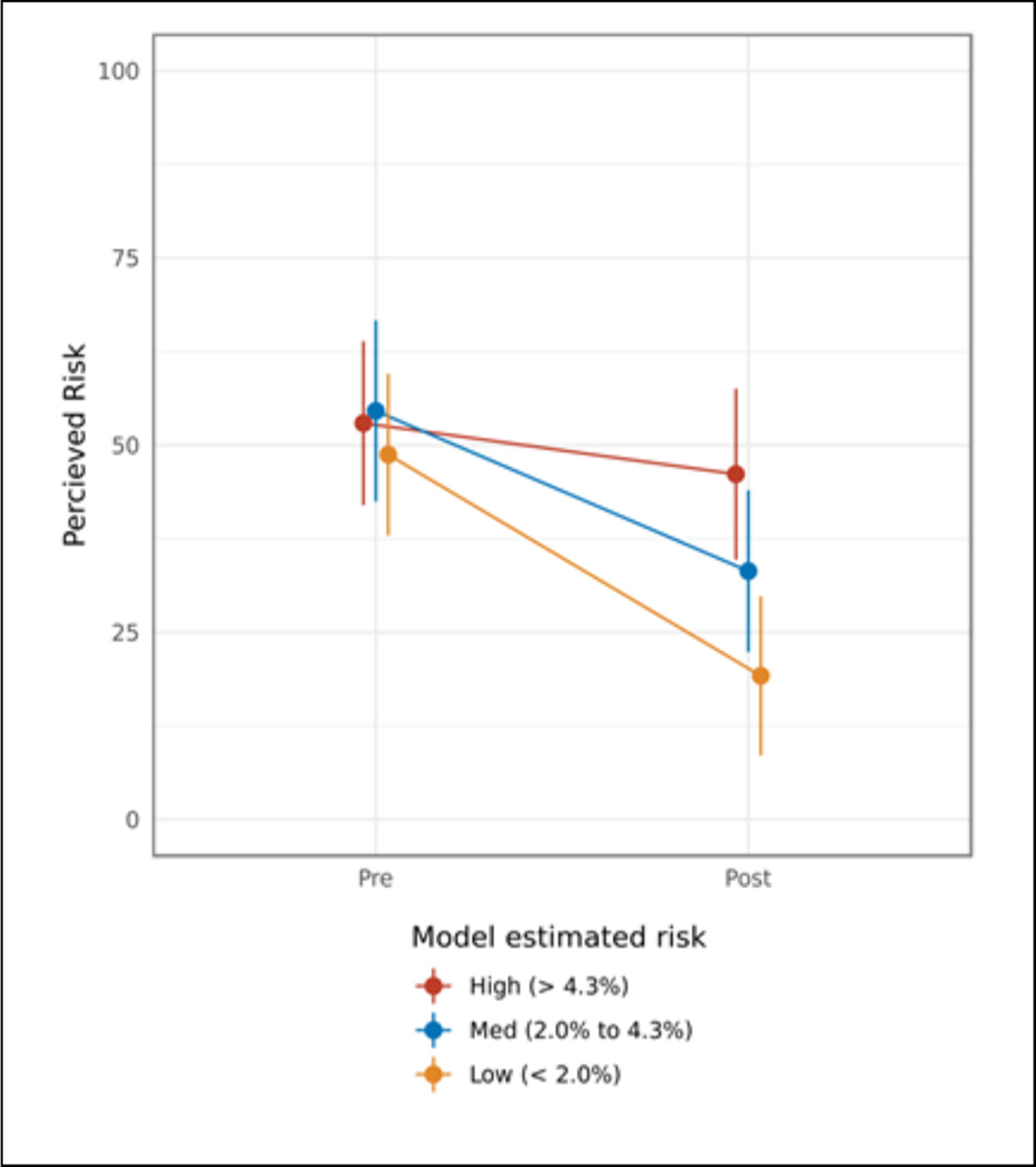
Pre- and postvisit perceived lung cancer risk, stratified by participants’ model-estimated lung cancer risk.
Analyses comparing pre- and postvisit personal risk threshold for screening showed a significant decrease in participants’ minimum level of lung cancer risk for wanting lung cancer screening. Prior to the provision of PCRI, patients’ model-adjusted mean minimum risk threshold was 47.7%, which decreased to 35.5% postvisit (P < 0.0001). Furthermore, change in perceived lung cancer risk was positively associated with change in personal risk threshold (P < 0.001) (Figure 2).
Figure 2.
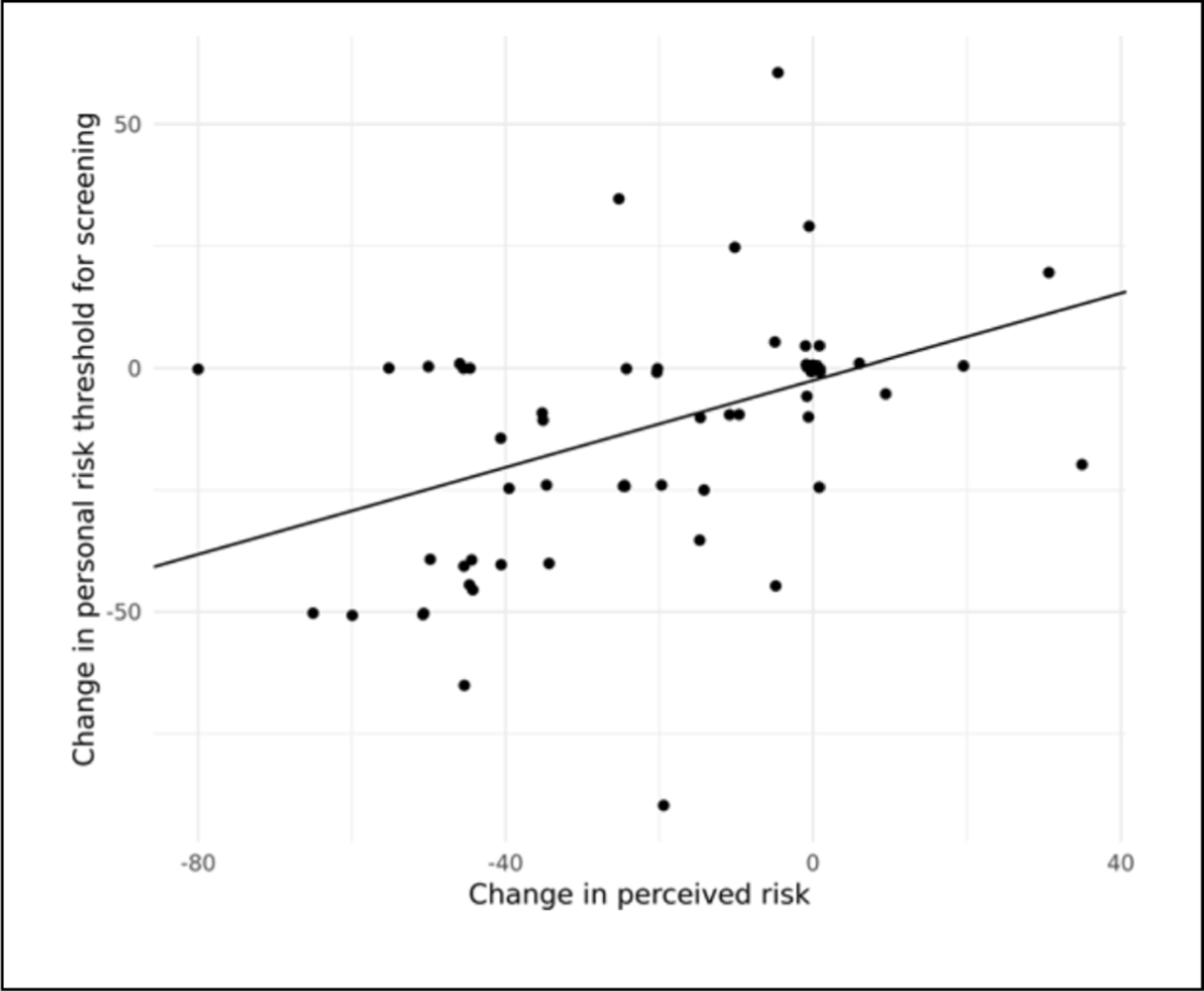
Association between change in personal risk threshold and change in perceived lung cancer risk.
Qualitative Interview Findings
Qualitative analysis of patient interviews revealed several dominant themes, which corroborated and illuminated the survey findings.
Disbelief of personalized cancer risk information was a sentiment expressed by all participants, who reported that their model-estimated lung cancer risks were much lower than they expected. Patient 2, for example, initially believed his risk to be “about 60% to 70%” and was “wicked surprised” to learn that it was approximately 5%. The discrepancy between patients’ subjective, perceived risks and their model-estimated risks, furthermore, promoted disbelief in the latter. “Intuitively I thought, that’s crazy,” reported Patient 69 in response to being given a risk estimate of 1% to 2%. Patient 1 asserted, “To me, it’s the best information they have that they do profiles on and I fell in the lower profile but I don’t really believe it either.” Similarly, Patient 24 reported, “Oh, did I trust it? Let’s just say that I thought it was, oh what’s the word I’m looking for here? Optimistic.” Patients identified 2 main causes of their dis-belief. One was a belief that their own smoking history and other unique characteristics overrode any other assessments of their cancer risk. Patient 11, for example, explained that as “a person who had pneumonia when she was a small baby …. and a couple of times as an adult,” she could not believe her relatively low model-estimated risk.
Uncertainty about personalized cancer risk information was the other major cause of patients’ disbelief of PCRI. Most participants expressed uncertainty about the validity of objective, model-estimated risks, which they traced to various sources (Table 3). One was the fundamental unpredictability of single events—an inherent limitation of all risk estimates.23 As patient 5 related, “You can’t assume just because the number says you won’t get it that you won’t.” The other major cause of uncertainty was inadequate information or scientific knowledge.23 Participants identified several specific sources of this problem, including limitations in available statistical methods, scientific knowledge, risk factor evidence, and the lung cancer risk model itself (Table 3).
Table 3.
Qualitative Findings on Reasons for Disbelief of Personalized Cancer Risk Information: Sources of Uncertainty
| Unpredictability of single events | |
| Patient 15 | Well, what’s the reliability of something like that? You know it’s hard to know. You could be the odd man out with this thing and you know just because you’re in a 4% risk category doesn’t necessarily mean that you’re not going to have it… after you think about it, it’s like, well there’s no guarantee. |
| Limitations of statistical methods | |
| Patient 27 | Patient: I actually told them pretty directly that I think statistics are a bunch of ballyhoo. You can make numbers do anything that you want them to… . Percentages mean nothing to me. I mean, I think that it’s a nifty tool, but you can look at it, if it says there’s a 10% chance of this, that means there’s a 90% chance of it not, or the opposite, depending on how you look at what’s going into the risk calculator. I can’t remember what it was, it was some something or other that was on there that I just laughed… . Because studies have been done and studies have shown that depending on who is doing this study, have found that, “Ooh, look at this, there’s a large percentage of people, a much larger percentage of people who didn’t go to college who have developed lung cancer.” And so that kind of gets thrown in there because it seems to be statistically sound. |
| Interviewer: And so are you saying that if that is the case, that then you don’t trust those studies or— | |
| Patient: Oh, and it wouldn’t just be this. It would be any study like that because it’s a human being that’s deciding what are the criteria in this study. And so I would tend to make my decisions far more based on a lot of human beings standing in front of me telling me, such as a doctor, here’s my experience, than I would be a mathematician who’s off somewhere pulling together a statistical study… . And maybe that’s because I did go to college and studied some statistics that I am that way. That’s just me. But I look at that and go, Oohhh. It’s like multiple-choice questions. You know, whoever designed the test is the one who is actually skewing it whatever way it might be skewed because they’re determining what the multiple-choice answers are. | |
| Limitations of scientific knowledge | |
| Patient 5 | I liked it [personalized risk information]; I thought it was helpful. But for me, it almost provided a sense of, what would be the word, a false sense of security because even though we can use these calculators, in the end I don’t think we know yet so I’m hoping that I’m adding to that pool of data and knowledge. |
| Patient 1 | I don’t think there’s enough information on why some people can smoke a long time with no issues and other people smoke a very short time and have lots of issues. |
| Limitations of risk factor information | |
| Patient 1 | Just basically that you know they were using the best information that they could; it’s just a factor, a risk factor; it certainly could not, you know it could be way off. You know I kind of took it with a grain of salt because I still think you know that it can be much higher. |
| Limitations of lung cancer risk model | |
| Patient 25 | Well from what I can recall talking to her initially, is it’s a young program, relatively new, if you know what I mean. So the information that had been gathered at that point was relatively small so they were working on a small base, I guess, as far as numbers of people that have been screened. So that part of it, there was no concern. But I think as the screening process grows, and if it continues, and she said it was supposed to keep continue, because I guess they got funding or grants or something like that to continue the program, that the numbers might start changing as the screening numbers get higher, if you understand what I’m saying. |
Lack of influence of personalized risk information on patients’ interest in lung cancer screening and risk reducing behavior was the final important theme, which participants explained in various ways (Table 4). Patients held strong beliefs in both their at-risk status and in the inherent value of early detection, both of which led them to discount their model-estimated risks. Some patients reported reassurance from PCRI; specifically, the receipt of a lower-than-expected risk estimate diminished the threat of receiving bad news from screening. Other patients remained interested in screening despite their low model-estimated risks because of a need for definitive evidence, rather than mere probabilities, that they were free of cancer. Several patients reported remaining interested in smoking cessation despite their low estimated lung cancer risks.
Table 4.
Qualitative Findings on Lack of Influence of Personalized Cancer Risk Information on Interest in Lung Cancer Screening and Risk Reduction
| Inherent value of early detection | |
| Patient 1 | Patient: No, no, no that really didn’t affect it; I still wanted to be screened personally and be part of the program. |
| Interviewer: Uh huh. And is that just because you had already made up your mind or do you think that that number would sway you one way or the other if it were a different number? | |
| Patient: Not in my case, no. No, I think that you know I feel I’m at risk and that early detection is a good thing no matter you know if it benefits me or benefits the future so I’m just kind of committed to it I guess regardless of the, what you know range I fall in personally. | |
| Reassuring effect of risk information | |
| Patient 26 | Interviewer: Right, okay. So when you got a number that was lower than 50/50, we’ll assume, we don’t know what it was, but it was lower. Was it helpful to get that number, or what? |
| Patient: Very, yes. | |
| Interviewer: Tell me why. | |
| Patient: Well, just the apprehension of having a CT scan that, it gave me a feeling that it’s not necessarily going to be bad news from the CT scan type of thing. | |
| Patient 27 | Interviewer: Okay. And do you think, you know I completely appreciate everything you’re saying about these models. Do you think that there’s any advantage to sharing them with patients? |
| Patient: I think there is. I think that I’m probably an exception, an anomaly that a lot of people would cling very hopefully to those. And I think the idea is that you want to get as many people as possible to be tested and not to feel there’s no point. Or, I’m too scared, or whatever. So I think it’s probably very good for probably the majority of people. | |
| Interviewer: Okay, meaning that if it’s a lower number it will make them less anxious and able to proceed. Is that what you’re saying? | |
| Patient: Right, and if it’s a higher number, “God, maybe I’d better do something about this now.” And then that’s where the doctor would come in with counseling, so … | |
| Need for certainty | |
| Patient 8 | Interviewer: So seeing that 8% when you had in your head that it was going to be 75% or even more, did that affect your decision to proceed with getting scanned or tell me about that thinking. |
| Patient: No, no, you know I wasn’t looking for a way out of getting a scan. | |
| Interviewer: Uh huh. | |
| Patient: I wanted the scan. I mean I trust; I mean figures are one thing but I wanted them to get in there and look at my lungs. | |
| Patient 10 | Oh because again, I just, I was, I made a decision to do it and I was curious is probably not quite the right word but you know I wanted facts … and so whatever the probability would’ve been, I think I still would’ve done it. |
| Patient 15 | Interviewer: So you’re making a good point. Given that there’s no guarantee as you were saying, do you feel like it’s helpful to even get a number like that or how would you assess that? |
| Patient: I think that’s, that could lead to the false-positive kind of feeling that you know if they gave you a number, where’s the number coming from you know. It’s, medicine is risky at best so I don’t see how that they could absolutely say, oh don’t worry Mr. X, you’re going to be fine or no sorry, you’ve got lung cancer and we don’t even have to look. So you know I don’t really want to go there in terms of having somebody say that you’re safe. | |
| Interviewer: Yeah. So I guess I would say given your feeling about that, is it helpful to you to even get a number or do you feel like you’d rather not you know even have that information? | |
| Patient: Well I, you know I don’t know why they even come up with the number; it’s just, to me it’s not an important thing to have. Well maybe it is but to some people it might be. I didn’t want any reassurances from anybody until I know for sure and hopefully this CT scan will give me a much better idea of what my risks are. | |
| Patient 2 | Patient: No, it didn’t change my thinking of the screening because I wanted to go ahead with it and have it done anyhow because I’d want to know if I did have lung cancer. |
| Interviewer: Yeah. So and it, am I correct in saying that it didn’t really matter what the number was because you would proceed either way? | |
| Patient: Correct, yeah, it wouldn’t have mattered. | |
| Patient 24 | Interviewer: Okay and given that, that 3% that seemed optimistic, did that give you any pause in proceeding with the scan or make you think maybe you didn’t need the scan, or how did that affect your thinking if at all? |
| Patient: Oh, absolutely not. No, it was, if anything it was, it encouraged me to take the scan. | |
| Interviewer: And tell me why that is. | |
| Patient: Good question. Let’s see if the empirical data shows, is consistent with the computer-generated simulation guesswork. | |
| Interviewer: Okay. So is that like a curiosity factor then or— | |
| Patient: Well it’s a combination of both a curiosity factor and I tend to, I felt more comfortable in getting empirical data. The fact of the matter, we start with the understanding that medical science is not an exact science, even with testing. But better to have the test than not, and simply rely on computer models. | |
| Continued interest in smoking cessation | |
| Patient 1 | Well I, it wouldn’t, the 4% was low but it doesn’t make me not want to quit; you know it doesn’t make me think that I’m going to live forever, that smoking’s good for me or any of that so I, if I really believed it, I’d probably have no desire to quit. |
Discussion
This mixed-methods study of patients referred to a centralized LDCT screening program evaluated the effects of providing PCRI as part of prescreening SDM consultations. To our knowledge, it is the first study to specifically assess how PCRI affects patients considering LDCT screening, and its findings have several implications for future efforts to implement risk-based cancer screening.
First, our study demonstrated that PCRI influences risk perceptions. The provision of objective risk estimates during SDM consultations decreased patients’ perceived lifetime lung cancer risk, from 52% to 31%. We cannot draw firm conclusions about the accuracy of these perceptions, given that we ascertained patients’ perceived lifetime cancer risks, whereas the PLCOm2012 model produced estimates of their 6-year risks. However, a previsit perceived cancer risk of 52% is unrealistically high for either time frame; therefore, the observed postvisit decrease in perceived risk arguably represented a move toward greater accuracy. Our findings thus support the notion that PCRI may facilitate better-informed decisions about cancer screening.33
However, our study also demonstrated that PCRI has paradoxically limited influence on screening preferences and decisions. Both social cognitive theories of health behavior and behavioral economic theories of decision making predict that lowering risk perceptions will result in lower risk-reducing intentions and behaviors.31,48 Our study, however, showed that although patients’ perceived risk of lung cancer decreased after receipt of PCRI, patients’ interest in LDCT screening increased, and all patients proceeded with screening. Of course, this effect cannot be attributed solely to PCRI; patients received other information during SDM counseling, and our study also showed that change in interest was not associated with change in risk perceptions. Our qualitative interviews, furthermore, identified several reasons why PCRI may have had either no effect or a positive effect on screening judgments and decisions. Many patients dis-believed their model-estimated risks due to various uncertainties about the reliability, credibility, or adequacy of PCRI (Table 3). Consistent with these findings, our pre- and postvisit surveys demonstrated that PCRI did not reduce patients’ uncertainty about their lung cancer risk (Table 2). Our qualitative interviews also revealed strong patient beliefs in both early detection—beliefs shared by the general public49,50—and in their own at-risk status, which led them to discount their model-estimated risks. Similar beliefs have been identified in other qualitative studies of patients considering LDCT screening.51,52 Our quantitative data further suggested that this discounting effect was more pronounced for patients at higher objective risk (Figure 1), perhaps reflecting their past exposure to cancer risk messages.49 Finally, our qualitative interviews revealed how PCRI can have a positive, potentiating effect on screening interest; specifically, lower-than-expected risk estimates decreased some patients’ worry about being diagnosed with cancer and correspondingly increased their willingness to be screened.
Finally, our study demonstrated that PCRI influences patients’ personal risk thresholds for screening. Specifically, the provision of PCRI resulted in a change in patients’ risk thresholds and in a direction favoring screening; furthermore, the change in thresholds was significantly associated with the pre-post change in risk perceptions. These findings—to our knowledge not previously reported—suggest that risk thresholds, like other personal values and preferences, are not stable and simply elicited and retrieved on demand, but unstable and constructed—that is, sensitive to context and created in response to new information.53–57 They also suggest that risk thresholds may not simply be causes of patients’ preferences for or against screening, but consequences—that is, post hoc rationalizations—of preexisting preferences. In response to lower-than-expected personal cancer risk estimates, patients in our study reduced not their interest in screening—as rational theories of decision making would predict—but their thresholds for action, suggesting that their interest was being driven by underlying prior preferences favoring screening. This observed reduction in personal risk thresholds in response to PCRI may be an instance of “motivated reasoning” aimed at minimizing cognitive dissonance and maintaining consistency between patients’ values and underlying preferences.58,59
From a practical standpoint, these findings suggest that implementing risk-based cancer screening requires more than simply providing PCRI to patients and expecting them to compare their personal cancer risks to some supposedly stable, preexisting threshold for action. It requires actively helping patients construct new risk perceptions and action thresholds, based both on PCRI as well as their own deeper values and preferences. More research is needed to determine how best to accomplish these tasks and to manage the added complexity of integrating PCRI in cancer screening decisions.
From an ethical standpoint, our study findings raise important questions about the appropriate use of PCRI in LDCT screening. The overall lack of a negative effect of PCRI on screening interest and uptake may be reassuring to screening advocates. However, patients’ tendencies to discount and use PCRI in a motivated, nonrational manner—to simply justify prior screening preferences—raise questions about the goals and value of providing PCRI to patients in the first place. If the goal is to enable patients to seriously consider objective risk estimates and to incorporate this information in screening decisions, then our findings suggest the need to provide additional support to help patients better understand the value of PCRI, as well as its uncertainties and limitations.23 They also suggest the need to overcome patients’ implicit biases—perhaps fostered by the medical profession itself49,60—about their own cancer risks and the benefits of screening. Yet one can also question whether the added precision of personalized risk information is necessary for individuals to make informed decisions about cancer screening.61,62 The more appropriate model of use of PCRI may therefore not be to facilitate SDM at the individual clinical level, but to establish screening thresholds at the population, public health level.10 The difficult ethical question this raises, however, is who ought to establish these thresholds—health professionals or patients—and how. Their malleable nature suggests the need for some sort of co-creation process.
Our study had several limitations that qualify its findings. It was a single-institution pilot study involving a relatively small patient sample; our findings thus need to be replicated in larger, diverse populations. Various patient characteristics that may have influenced their understanding of and responses to PCRI—including education level, health literacy, and health numeracy—were not assessed and need to be explored in future research. Furthermore, participants in our study had already been referred for screening and identified by their referring physicians as being at high risk for lung cancer, which may have made their screening preferences more resistant to change.63 More research is needed to evaluate the provision of PCRI earlier in the LDCT screening process, within primary care settings, and to understand its effects upon patients who are at lower risk of lung cancer or otherwise more ambivalent about screening. Our study was also not a randomized clinical trial; thus, we cannot quantify the independent effects of PCRI v. other components of SDM counseling. Nevertheless, our study provides seminal data on the overall magnitude of effect of integrating PCRI within SDM consultations, and hypothesis-generating qualitative data on its independent effects and the thought processes patients use to incorporate PCRI in decision making. In our study, PCRI was also provided to patients in unembellished fashion, without support in interpreting this information. Although intentional given our study aims, this strategy may have predisposed patients to discount the validity of PCRI. Finally, our study focused exclusively on personalized estimates of the risk of developing lung cancer, but other risks (e.g., cancer mortality, false-positive screening results) may be important in screening decisions and require further investigation.
Despite these limitations, our study provides new evidence to guide future efforts to implement risk-based screening for lung cancer and other malignancies. PCRI affects patients in complex ways that raise important questions about its clinical value and appropriate use, and call for further research.
Supplementary Material
Acknowledgments
The authors thank Margaret Byrne, PhD, Michael Gould, MD, MS, and Jamie Studts, PhD, for their input on the development of the decision support tool used in this study; Alexandra McCown, Lindsay McFarren, and Peter Michalakes for data analytic assistance; Celeste Sartor for data management assistance; and Chest Medicine Associates, Portland, ME, for logistical support.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided by an Outcomes Research Grant from the Maine Cancer Foundation to PKJH. PKJH, CD, AB, CG, and JY received additional support from the Maine Lung Cancer Coalition, an initiative jointly supported by the Bristol Myers Squibb Foundation, Maine Cancer Foundation, and Maine Economic Improvement Fund. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
Supplementary material for this article is available on the Medical Decision Making Web site at http://journals.sagepub.com/home/mdm.
References
- 1.National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyer VA US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–8. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Medicare and Medicaid Services decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274 (2015, accessed 25 June 2019).
- 4.Sakoda LC, Henderson LM, Caverly TJ, Wernli KJ, Katki HA. Applying risk prediction models to optimize lung cancer screening: current knowledge, challenges, and future directions. Curr Epidemiol Rep. 2017;4(4):307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onega T, Beaber EF, Sprague BL, et al. Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level. Cancer. 2014;120(19):2955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shieh Y, Eklund M, Madlensky L, et al. Breast cancer screening in the precision medicine era: risk-based screening in a population-based trial. J Natl Cancer Inst. 2017;109(5). [DOI] [PubMed] [Google Scholar]
- 7.Marcus PM, Pashayan N, Church TR, et al. Population-based precision cancer screening: a symposium on evidence, epidemiology, and next steps. Cancer Epidemiol Bio-markers Prev. 2016;25(11):1449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caverly TJ, Cao P, Hayward RA, Meza R. Identifying patients for whom lung cancer screening is preference-sensitive: a microsimulation study. Ann Intern Med. 2018;169(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tammemagi MC. Selecting lung cancer screenees using risk prediction models: where do we go from here? Transl Lung Cancer Res. 2018;7(3):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung LC, Katki HA, Chaturvedi AK, Jemal A, Berg CD. Preventing lung cancer mortality by computed tomography screening: the effect of risk-based versus U.S. Preventive Services Task Force Eligibility Criteria, 2005–2015. Ann Intern Med. 2018;168(3):229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katki HA, Kovalchik SA, Petito LC, et al. Implications of nine risk prediction models for selecting ever-smokers for computed tomography lung cancer screening. Ann Intern Med. 2018;169(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hocking WG, Tammemagi MC, Commins J, et al. Diagnostic evaluation following a positive lung screening chest radiograph in the Prostate, Lung, Colorectal, Ovarian (PLCO) Cancer Screening Trial. Lung Cancer. 2013;82(2): 238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15): 1685–92. [DOI] [PubMed] [Google Scholar]
- 16.Tammemagi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med. 2014;11(12):e1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;315(21):2300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar V, Cohen JT, van Klaveren D, et al. Risk-targeted lung cancer screening: a cost-effectiveness analysis. Ann Intern Med. 2018;168(3):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bach PB, Elkin EB, Pastorino U, et al. Benchmarking lung cancer mortality rates in current and former smokers. Chest. 2004;126(6):1742–9. [DOI] [PubMed] [Google Scholar]
- 20.Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Ann Intern Med. 2012;157(8):571–3. [DOI] [PubMed] [Google Scholar]
- 21.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ten Haaf K, Jeon J, Tammemagi MC, et al. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med. 2017; 14(4):e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han PK. Conceptual, methodological, and ethical problems in communicating uncertainty in clinical evidence. Med Care Res Rev. 2013;70(1 Suppl):14S–36S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau YK, Caverly TJ, Cao P, et al. Evaluation of a personalized, web-based decision aid for lung cancer screening. Am J Prev Med. 2015;49(6):e125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau YK, Caverly TJ, Cherng ST, et al. Development and validation of a personalized, web-based decision aid for lung cancer screening using mixed methods: a study protocol. JMIR Res Protoc. 2014;3(4):e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black WC, Nease RF Jr, Tosteson AN. Perceptions of breast cancer risk and screening effectiveness in women younger than 50 years of age. J Natl Cancer Inst. 1995; 87(10):720–31. [DOI] [PubMed] [Google Scholar]
- 27.Davids SL, Schapira MM, McAuliffe TL, Nattinger AB. Predictors of pessimistic breast cancer risk perceptions in a primary care population. J Gen Intern Med. 2004;19(4): 310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstein ND, Atwood K, Puleo E, Fletcher R, Colditz G, Emmons K. Colon cancer: risk perceptions and risk communication. J Health Commun. 2004;9(1):53–65. [DOI] [PubMed] [Google Scholar]
- 29.Klein WM, Stefanek ME. Cancer risk elicitation and communication: lessons from the psychology of risk perception. CA Cancer J Clin. 2007;57(3):147–67. [DOI] [PubMed] [Google Scholar]
- 30.Han PK, Duarte CW, Daggett S, et al. Effects of personalized colorectal cancer risk information on laypersons’ interest in colorectal cancer screening: the importance of individual differences. Patient Educ Couns. 2015;98(10): 1280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connor M, Norman P, eds. Predicting Health Behavior: Research and Practice with Social Cognition Models. Buckingham, UK: Open University Press; 2001. [Google Scholar]
- 32.Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26(2):136–45. [DOI] [PubMed] [Google Scholar]
- 33.Edwards AG, Naik G, Ahmed H, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev. 2013;2:CD001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sequist TD, Zaslavsky AM, Colditz GA, Ayanian JZ. Electronic patient messages to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2011;171(7):636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroy PC 3rd, Emmons KM, Peters E, et al. Aid-assisted decision making and colorectal cancer screening: a randomized controlled trial. Am J Prev Med. 2012;43(6):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkins T, Gillies RA, Panchal P, Patel M, Warren P, Schade RR. Colorectal cancer risk information presented by a nonphysician assistant does not increase screening rates. Can Fam Physician. 2014;60(8):731–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz PH, Perkins SM, Schmidt KK, Muriello PF, Alt-house S, Rawl SM. Providing quantitative information and a nudge to undergo stool testing in a colorectal cancer screening decision aid: a randomized clinical trial. Med Decis Making. 2017;37(6):688–702. [DOI] [PubMed] [Google Scholar]
- 38.Elwyn G, Lloyd A, Joseph-Williams N, et al. Option Grids: shared decision making made easier. Patient Educ Couns. 2013;90(2):207–12. [DOI] [PubMed] [Google Scholar]
- 39.Tammemagi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21(6 Suppl):251S–72S. [DOI] [PubMed] [Google Scholar]
- 41.Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making. 2008;28(6):917–25. [DOI] [PubMed] [Google Scholar]
- 42.Han PK, Klein WM, Killam B, Lehman T, Massett H, Freedman AN. Representing randomness in the communication of individualized cancer risk estimates: effects on cancer risk perceptions, worry, and subjective uncertainty about risk. Patient Educ Counsel. 2012;86(1):106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–89. [Google Scholar]
- 44.Boyatzis R Transforming Qualitative Information: Thematic Analysis and Code Development. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- 45.Glaser BG. The constant comparative method of qualitative analysis. Social Problems. 1965;12(4):436–45. [Google Scholar]
- 46.Sofaer S Qualitative research methods. Int J Qual Health Care. 2002;14(4):329–36. [DOI] [PubMed] [Google Scholar]
- 47.Strauss AL, Corbin J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. 2nd ed. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- 48.Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211:453–8. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71–8. [DOI] [PubMed] [Google Scholar]
- 50.Scherer LD, Valentine KD, Patel N, Baker SG, Fagerlin A. A bias for action in cancer screening? J Exp Psychol Appl. 2019;25(2):149–61. [DOI] [PubMed] [Google Scholar]
- 51.Carter-Harris L, Ceppa DP, Hanna N, Rawl SM. Lung cancer screening: what do long-term smokers know and believe? Health Expect. 2017;20(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roth JA, Carter-Harris L, Brandzel S, Buist DSM, Wernli KJ. A qualitative study exploring patient motivations for screening for lung cancer. PLoS One. 2018;13(7):e0196758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warren C, McGraw AP, Van Boven L. Values and preferences: defining preference construction. Cogn Sci. 2011;2: 193–205. [DOI] [PubMed] [Google Scholar]
- 54.Fischhoff B Value elicitation: is there anything in there? Am Psychol. 1991;46:835–47. [Google Scholar]
- 55.Nelson WL, Han PK, Fagerlin A, Stefanek M, Ubel PA. Rethinking the objectives of decision aids: a call for conceptual clarity. Med Decis Making. 2007;27(5):609–18. [DOI] [PubMed] [Google Scholar]
- 56.Lichtenstein S, Slovic P, eds. The Construction of Preference. New York: Cambridge University Press; 2006. [Google Scholar]
- 57.Amir O, Levav J. Choice construction versus preference construction: the instability of preferences learned in context. J Marketing Research. 2008;45:145–58. [Google Scholar]
- 58.Klein WM, Kunda Z. Motivated person perception: constructing justifications for desired beliefs. J Exp Soc Psych. 1992;28:145–68. [Google Scholar]
- 59.Kunda Z The case for motivated reasoning. Psychol Bull. 1990;108:480–98. [DOI] [PubMed] [Google Scholar]
- 60.Woloshin S, Schwartz LM, Byram SJ, Sox HC, Fischhoff B, Welch HG. Women’s understanding of the mammography screening debate. Arch Intern Med. 2000;160(10):1434–40. [DOI] [PubMed] [Google Scholar]
- 61.Zikmund-Fisher BJ. The right tool is what they need, not what we have: a taxonomy of appropriate levels of precision in patient risk communication. Med Care Res Rev. 2013;70(1 Suppl):37S–49S. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz PH. Questioning the quantitative imperative: decision aids, prevention, and the ethics of disclosure. Hastings Cent Rep. 2011;41(2):30–9. [DOI] [PubMed] [Google Scholar]
- 63.Mazzone PJ, Tenenbaum A, Seeley M, et al. Impact of a lung cancer screening counseling and shared decision-making visit. Chest. 2017;151(3):572–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


