Abstract
Objective
To examine predictors of spirometry use at a tertiary academic health system and association between receipt of spirometry and outcomes.
Patients and Methods
We conducted a retrospective cohort study of adult patients with an ICD-9 CM diagnostic code for asthma and a 2014 outpatient visit in either a community health center or private practice associated with a tertiary academic medical center. The main outcome was receipt of spirometry during a 2007–2015 “exposure period.” We secondarily examined future hospitalizations and emergency department (ED) visits during a follow-up period (2016–2019).
Results
In a sample of 394 patients, the majority were white (48%; n=188) and female (72%; n=284). Mean (SD) age was 52 years. Approximately half (185, 47%) of the patients received spirometry and 25% (n=97) saw a specialist during the exposure period. Nearly, 88% (n=85) of patients who saw a specialist received spirometry. More than half of the cohort (220/394, 56%) had an ED visit or admission during the follow-up period. Of these, 168 (76.4%) had not seen a specialist and 111 (50.5%) had not received spirometry within the exposure period. We saw no association between spirometry in the exposure window and future ED visit or hospitalization.
Conclusion
In a cohort of patients at a tertiary medical center, spirometry was underused. We observed a strong association between seeing a specialist and use of spirometry, suggesting a need to better incorporate spirometry into routine primary care for patients with asthma. Among 220 patients who had an asthma-related hospitalization or ED visit in 2016–2019, the majority had no record of receiving spirometry and no documentation indicating a prior specialist visit.
Keywords: asthma, spirometry, health care delivery, quality of care, population health
Introduction
Asthma, a chronic inflammatory disorder of the airways that is characterized by reversible airflow obstruction, bronchial hyperresponsiveness and underlying inflammation, affects an estimated 39.5 million people in the United States.1 From 2008–2013, the estimated total cost of asthma was $81.9 billion, including $50.3 billion in medical expenses, $29 billion due to asthma-related mortality, and $3 billion in losses due to missed work and school days.2
Although a typical patient who presents with asthma reports episodes of wheezing, dyspnea, chest tightness and a cough that worsens in the night or early morning, according to the American Academy of Allergy, Asthma, and Immunology (AAAAI) history alone cannot be used to reliably diagnose asthma.3 Instead, most experts (including the National Asthma Education and Prevention Program (NAEPP), Global Initiative for Asthma (GINA), National Institute for Health and Care Excellence (NICE), and the Joint Task Force on Practice Parameters representing the AAAAI) recommend spirometry for all patients in whom the diagnosis of asthma is being considered or monitored.4–7 Spirometry is a basic lung function test that measures the volume, time, and flow of air throughout inspiration and expiration.8
Failure to use spirometry increases the risk of either a missed diagnosis or a false diagnosis of asthma9–12 which, in turn, increases the risk of inappropriate medication prescriptions.1,13 In addition, objectively assessing lung function at follow-up is an important element of monitoring improvement as well as risk of adverse future outcomes for patients with asthma.5,14 Evidence suggests that a Forced Expiratory Volume in One second (FEV1) <60% predicted is a potentially modifiable independent risk factor for exacerbations and fixed airflow limitation. Therefore assessing FEV1 may help identify patients who need medication adjustments.15–18
Yet prior research suggests that spirometry is widely underutilized in the diagnosis of asthma.19–22 Prior retrospective cohort studies that have examined pulmonary function testing (PFT) in asthma, show that an appointment with a specialist and higher income were associated with receiving spirometry.21,22 Therefore, we aimed to describe use of spirometry in a population of patients receiving primary care in a large health system that included both community health centers and private practices. We examined use of spirometry for both diagnosis and follow up and identified predictors of spirometry use to assess the effect of patient income as well as care by a specialist. As a secondary, exploratory analysis, we evaluated whether spirometry use was associated with future emergency department visits or hospitalizations.23
Patients and Methods
Design and Study Population
We conducted a retrospective study of adults who had a diagnosis of asthma and an outpatient appointment in a private practice or community health center during the year of 2014. As transition from pediatric to adult medical care usually occurs around 21 years of age, we utilized 28 years of age as a minimum age cutoff to allow for a similar observation opportunity for our entire sample (eg these patients were 21 years old in 2007, the beginning of our observation window). We used the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), to identify a range of asthma diagnoses (codes 493, 490.0, 493.00, 493.01, 493.1, 493.10, 493.11, 493.12, 493.2, 493.20, 439.21, 493.22, 493.8, 493.81, 493.82, 493.9, 493.90, 493.91, 493.92).22 We excluded patients diagnosed with neuromuscular conditions (eg myasthenia gravis, diaphragmatic paralysis, paraplegia, muscular dystrophy, poliomyelitis, amyotrophic lateral sclerosis, etc) and any history of pneumonectomy.
We used random number generation to identify a subset of 400 patients from the total list of extracted patients. In order to evaluate the influence of practice types, 50% of the selected patients were taken from private practices and 50% from a population of patients served by a community health center. The community health centers included in the study consist of three clinics in a medically underserved city that are directly affiliated with an academic health center. The private practices were composed of nine distinct clinics representing various towns and neighborhoods within the county.
Data Collection
Three physicians reviewed clinical notes in the electronic health record (EHR). After confirming an asthma diagnosis, they collected patient demographics, laboratory data, spirometry results, and evidence of subspecialist visits. This data was manually entered into a REDCap database.24 Efforts were taken to standardize chart extraction and reduce inter-observer variability. All three physicians interrogated approximately 10 of the same patients records with similar results. Any differences were resolved via discussion and the reviewers extracted the remaining charts independently.
Additionally, a billing database was used to collect ICD-9 codes for comorbidities at baseline and to identify ED visits and hospitalizations in the 4 years following extraction of the medical record.
Outcomes
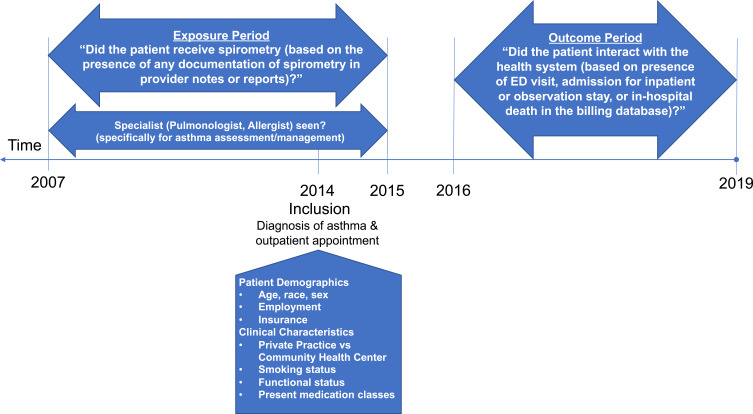
We defined the presence or absence of spirometry based on documentation of this procedure within seven years prior to the sampled visit date or up to one year after (eg January 2007 - December 2015 study window or “exposure period”) (Figure 1). To assess this, patient records were manually reviewed for the presence of any documentation of spirometry in provider notes or reports. Among those who received spirometry, we summarized the indications for spirometry, the total occurrences within the observation window, and attempted to categorize whether the spirometry was obtained for incident asthma or follow-up. However, for many of the patients, we were unable to reliably discern from the medical record whether spirometry was ordered for diagnostic or follow-up purposes.
Figure 1.
Study timeline.
In order to conduct an exploratory analysis of longer-term outcomes, we examined health system billing databases for the years January 2016 through March 2019. We identified visits to the emergency department (ED), admissions for inpatient or observation stays, and in-hospital deaths (Figure 1). We determined whether visits were related to asthma (principal diagnoses including respiratory failure/dyspnea, chest pain, chronic obstructive pulmonary disease (COPD) or pneumonia, or upper respiratory infections25–29 (ICD-10-CM diagnoses (J45.9xx, J45.xx, J96.01, J96.21, R06.00, R07.9, R07.89, J44.1, J44.9, J18.9, J69.0, J15.9, J12.1, J85.1, J21.1, J06.9).
Predictors
We recorded several demographic and clinical characteristics at the 2014 index visit, including: age, race, sex, employment and insurance type. Clinical characteristics included: private practice vs public community health center type, smoking status, functional status (according to the proportion of Activities of Daily Living (ADL) and Instrumental Activities of Daily Living Requiring Assistance (IADL)) and total number of medication classes used to control asthma. In addition, we assessed whether subjects had ever seen a specialist (pulmonologist and/or allergist) specifically for asthma assessment or management. We also captured comorbidities, based on ICD-9 coding as defined by Elixhauser, at the time of this 2014 visit.30
Statistical Analysis
We cross-tabulated each predictor with the presence of spirometry using frequencies and percentages as well as chi-squared or Fisher exact tests. We identified predictors of receiving spirometry using logistic regression models to estimate the crude and multivariable odds ratios and 95% confidence intervals (CI). We estimated that with 185 spirometry events, our model had sufficient stability to assess up to 18 predictors.31 Therefore, all predictors were included in both models in order to estimate effect sizes. We also created a similar model to evaluate predictors of a specialist visit.
We estimated that in the case of a single binary predictor logistic model with a sample size of 400 observations (of which 50% are in each group) we would achieve 92% power at a 0.05 significance level to detect an odds ratio of 2.0.
As observation time could vary, we were concerned about inadvertently capturing patients with a short amount of follow-up time in the system. These patients may have received asthma care elsewhere yet contributed to our denominator of patients. Therefore, we conducted sensitivity analyses reducing the exposure window from nine years (2007–2015) to four years (2012–2015). Analyses were performed using Stata 15.1. (College Station, TX). The study protocol was approved by Institutional Review Board of BMC.
Results
Among 400 randomly selected subjects, 394 met eligibility criteria. Of these, 197 patients were seen in community health centers and 197 from private practices (Table 1). The mean (SD) age was 5213 years, with a large proportion of the sample being white (48%; n=188) and female (72%; n=284). Comorbidities were relatively balanced between groups with the most common being hypertension (22%; n=87). Most patients (n=297, 75%) did not have a visit with a pulmonologist or allergist during the exposure period. A minority (185, 47%) received spirometry at any time during the exposure period, and a majority (209, 53%) did not. Of these, we determined that 64% (n=119) were for incident asthma, 12% (n=23) were for follow-up and 23% (n=43) were unknown. Dyspnea was the most common indication (35%, n=65) followed by history of asthma (33%, n=61).
Table 1.
Patient Characteristics
| Overall | No Spirometry n (col %) | Yes Spirometry n (col%) | Probability of Spirometry | p-value | |
|---|---|---|---|---|---|
| Overall n (%) | 394 (100.0) | 209 (53.0) | 185 (47.0) | 47.0% | |
| Age Group, n (%) | 0.04 | ||||
| 28–39 | 77 (19.5) | 52 (24.9) | 25 (13.5) | 32.5% | |
| 40–49 | 97 (24.6) | 50 (23.9) | 47 (25.4) | 48.5% | |
| 50–59 | 105 (26.6) | 55 (26.3) | 50 (27.0) | 47.6% | |
| 60–69 | 72 (18.3) | 35 (16.7) | 37 (20.0) | 51.4% | |
| 70–95 | 43 (10.9) | 17 (8.1) | 26 (14.1) | 60.5% | |
| Number of Present Medication Classes, n (%) | <0.01 | ||||
| 0 | 26 (6.6) | 19 (9.1) | 7 (3.8) | 26.9% | |
| 1–2 | 122 (31.0) | 79 (37.8) | 43 (23.2) | 35.2% | |
| 3–6 | 246 (62.4) | 111 (53.1) | 135 (73.0) | 54.9% | |
| Race, n (%) | 0.01 | ||||
| White | 188 (47.7) | 106 (50.7) | 82 (44.3) | 43.6% | |
| Black | 43 (10.9) | 20 (9.6) | 23 (12.4) | 53.5% | |
| Hispanic | 154 (39.1) | 74 (35.4) | 80 (43.2) | 51.9% | |
| Other | 9 (2.3) | 9 (4.3) | 0 (0.0) | – | |
| Gender, n (%) | 0.05 | ||||
| Female | 284 (72.1) | 142 (67.9) | 142 (76.8) | 50.0% | |
| Male | 110 (27.9) | 67 (32.1) | 43 (23.2) | 39.1% | |
| Insurance, n (%) | 0.06 | ||||
| Private | 144 (36.5) | 82 (39.2) | 62 (33.5) | 43.1% | |
| Medicare | 84 (21.3) | 39 (18.7) | 45 (24.3) | 53.6% | |
| Medicaid | 135 (34.3) | 66 (31.6) | 69 (37.3) | 51.1% | |
| None | 31 (7.9) | 22 (10.5) | 9 (4.9) | 29.0% | |
| Clinic Type, n (%) | <0.01 | ||||
| Community Health Center | 197 (50.0) | 89 (42.6) | 108 (58.4) | 54.8% | |
| Private Practice | 197 (50.0) | 120 (57.4) | 77 (41.6) | 39.1% | |
| Employment, n (%) | 0.09 | ||||
| Unemployed | 95 (24.1) | 43 (20.6) | 52 (28.1) | 54.7% | |
| Employed | 153 (38.8) | 90 (43.1) | 63 (34.1) | 41.2% | |
| Retired | 42 (10.7) | 20 (9.6) | 22 (11.9) | 52.4% | |
| Disabled | 62 (15.7) | 29 (13.9) | 33 (17.8) | 53.2% | |
| Not Documented | 42 (10.7) | 27 (12.9) | 15 (8.1) | 35.7% | |
| Smoking Status, n (%) | 0.36 | ||||
| Unknown | 4 (1.0) | 3 (1.4) | 1 (0.5) | 25.0% | |
| Current | 83 (21.1) | 49 (23.4) | 34 (18.4) | 41.0% | |
| Never | 208 (52.8) | 110 (52.6) | 98 (53.0) | 47.1% | |
| Former | 99 (25.1) | 47 (22.5) | 52 (28.1) | 52.5% | |
| Functional Status, n (%) | 0.76 | ||||
| Unknown | 3 (0.8) | 2 (1.0) | 1 (0.5) | 33.3% | |
| Independent | 376 (95.4) | 198 (94.7) | 178 (96.2) | 47.3% | |
| Dependent | 3 (0.8) | 2 (1.0) | 1 (0.5) | 40.0% | |
| Visit with Specialist, n (%) | <0.01 | ||||
| No | 297 (75.4) | 195 (94.2) | 102 (54.5) | 33.8% | |
| Yes | 97 (24.6) | 12 (5.8) | 85 (45.5) | 88.4% | |
| Comorbidities**, n(%) | |||||
| Chronic Pulmonary Disease | 0.14 | ||||
| No | 39 (9.9) | 25 (12.0) | 14 (7.6) | 35.9% | |
| Yes | 355 (90.1) | 184 (88.0) | 171 (92.4) | 48.2% | |
| Hypertension, Uncomplicated | 0.24 | ||||
| No | 307 (77.9) | 158 (75.6) | 149 (80.5) | 48.5% | |
| Yes | 87 (22.1) | 51 (24.4) | 36 (19.5) | 41.4% | |
| Obesity | 0.88 | ||||
| No | 344 (87.3) | 182 (87.1) | 162 (87.6) | 47.1% | |
| Yes | 50 (12.7) | 27 (12.9) | 23 (12.4) | 46.0% | |
| Depression | 0.30 | ||||
| No | 351 (89.1) | 183 (87.6) | 168 (90.8) | 47.9% | |
| Yes | 43 (10.9) | 26 (12.4) | 17 (9.2) | 39.5% | |
| Diabetes, Uncomplicated | 0.89 | ||||
| No | 346 (87.8) | 184 (88.0) | 162 (87.6) | 46.8% | |
| Yes | 48 (12.2) | 25 (12.0) | 23 (12.4) | 47.9% | |
| Hypothyroidism | 0.47 | ||||
| No | 369 (93.7) | 194 (92.8) | 175 (94.6) | 47.4% | |
| Yes | 25 (6.3) | 15 (7.2) | 10 (5.4) | 40.0% |
Note: **Elixhauser comorbidities <5% not shown.
In our multivariable model, patients aged 70–95 were more likely to have spirometry (compared to those 28–39 years old, adjusted OR (aOR) = 4.76; 95% confidence interval (CI) 1.35–16.13) (Table 2). In addition, patients seen in the private practice were less likely to have spirometry compared to a community health center (aOR = 0.31; 95% CI = 0.12–0.77). However, the strongest predictor, by far, of receiving spirometry was a visit with a specialist (aOR 20.44; 95% CI = 9.36–44.63).
Table 2.
Unadjusted and Multivariable Model for Predictors of Spirometry
| Unadjusted | Multivariable | |||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Age Group | ||||
| 28–39 | Referent | Referent | ||
| 40–49 | 1.96 | 1.05–3.64 | 2.48 | 1.15–5.32 |
| 50–59 | 1.89 | 1.03–3.49 | 2.17 | 1.02–4.64 |
| 60–69 | 2.20 | 1.13–4.27 | 2.48 | 1.03–5.95 |
| 70–95 | 3.18 | 1.46–6.91 | 4.76 | 1.35–16.13 |
| Number of Present Medication Classes | ||||
| 0 | Referent | Referent | ||
| 1–2 | 1.48 | 0.58–3.79 | 1.80 | 0.57–5.66 |
| 3–6 | 3.30 | 1.34–8.14 | 2.36 | 0.78–7.07 |
| Race | ||||
| White | Referent | Referent | ||
| Black | 1.49 | 0.76–2.89 | 0.81 | 0.30–2.18 |
| Hispanic | 1.40 | 0.91–2.14 | 0.69 | 0.28–1.71 |
| Other | – | – | – | – |
| Sex | ||||
| Female | Referent | Referent | ||
| Male | 0.64 | 0.41 −1.00 | 0.57 | 0.32–1.02 |
| Insurance | ||||
| Private | Referent | Referent | ||
| Medicare | 1.53 | 0.89–2.62 | 1.13 | 0.54–2.35 |
| Medicaid | 1.38 | 0.86–2.22 | 1.13 | 0.59–2.18 |
| None | 0.54 | 0.23–1.26 | 0.65 | 0.21–2.04 |
| Clinic Type | ||||
| Community Health Center | Referent | Referent | ||
| Private Practice | 0.53 | 0.35–0.79 | 0.31 | 0.12–0.77 |
| Employment | ||||
| Unemployed | 1.73 | 1.03 −2.90 | 1.02 | 0.49–2.11 |
| Employed | Referent | Referent | ||
| Retired | 1.57 | 0.79–3.12 | 0.31 | 0.09–1.02 |
| Disabled | 1.63 | 0.90–2.94 | 1.19 | 0.54–2.60 |
| Not Documented | 0.79 | 0.39–1.61 | 0.73 | 0.31–1.74 |
| Smoking Status | ||||
| Unknown | 0.37 | 0.04–3.66 | 1.96 | 0.16–23.78 |
| Current | 0.78 | 0.47–1.30 | 0.85 | 0.44–1.62 |
| Never | Referent | Referent | ||
| Former | 1.24 | 0.77–2.01 | 1.03 | 0.56–1.87 |
| Functional Status | ||||
| Unknown | 0.56 | 0.05–6.19 | 0.77 | 0.04–15.20 |
| Independent | Referent | Referent | ||
| Dependent | 0.74 | 0.26–2.12 | 0.29 | 0.07–1.27 |
| Visit with Specialist | ||||
| No | Referent | Referent | ||
| Yes | 14.97 | 7.6–29.33 | 20.44 | 9.36–44.63 |
| Chronic Pulmonary Disease | ||||
| No | Referent | Referent | ||
| Yes | 1.66 | 0.84–3.30 | 0.75 | 0.30–1.86 |
| Hypertension, Uncomplicated | ||||
| No | Referent | Referent | ||
| Yes | 0.75 | 0.46–1.21 | 0.50 | 0.25–0.99 |
| Obesity | ||||
| No | Referent | Referent | ||
| Yes | 0.96 | 0.53–1.74 | 1.70 | 0.77–3.74 |
| Depression | ||||
| No | Referent | Referent | ||
| Yes | 0.71 | 0.37–1.36 | 0.72 | 0.31–1.69 |
| Diabetes, Uncomplicated | ||||
| No | Referent | Referent | ||
| Yes | 1.04 | 0.57–1.91 | 0.96 | 0.42–2.18 |
| Hypothyroidism | ||||
| No | Referent | Referent | ||
| Yes | 0.74 | 0.32–1.69 | 0.29 | 0.09–0.99 |
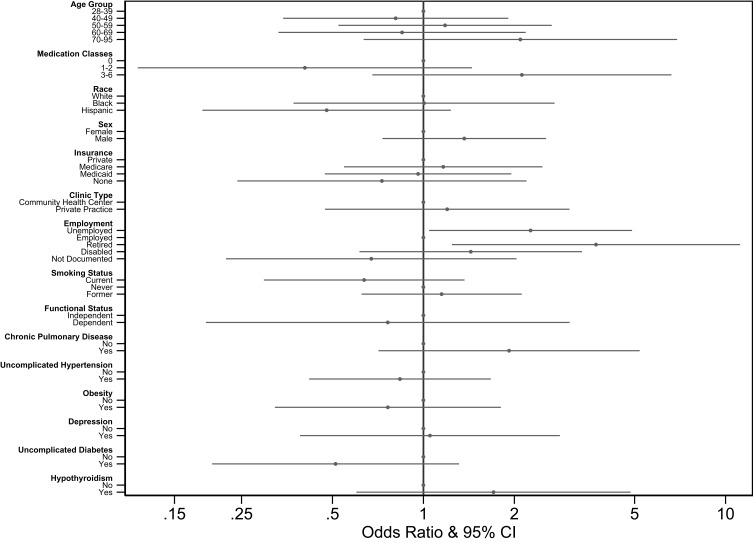
Because seeing a specialist was the dominant predictor in these models, we constructed an additional multivariable model assessing predictors of seeing a specialist (Figure 2). In this model, being retired or unemployed (vs employed) was the only significant predictor of seeing a specialist (aOR 3.73; 95% CI = 1.24–11.17 and aOR 2.26; 95% CI = 1.04–4.90, respectively).
Figure 2.
Multivariable odds ratios for seeing a specialist as derived by multivariate logistic regression modeling of patient characteristics.
A total of 374 (95%) patients had at least one inpatient or outpatient visit in our health system between 2016–2019 (“follow-up” period). The median follow up time was 38 months (out of a maximum observation time of 39 months). Among those not lost to follow-up, 185 (49.5%) had a subsequent ED visit, 122 (32.6%) had an inpatient hospitalization and 7 (1.9%) visits ended in an in-hospital death (Table 3). Of all patients with follow up visits (n= 220), approximately 47% (103) were likely asthma-related (principal diagnosis of respiratory failure, asthma, COPD, chest pain, or upper respiratory infection). Most patients with ED visits and hospitalizations had never seen a specialist (168, 76.4%) and most (111, 50.5%) had not received spirometry during the exposure period. The unadjusted association between prior spirometry and future asthma-related hospital admissions was not statistically significant (OR = 1.73; 95% CI=0.88–3.42).
Table 3.
Follow-Up Outcomes
| Overall N=374 | No Spirometry n=199 (53.2%) | Spirometry n=175 (46.8%) | Crude OR & 95% CI | |
|---|---|---|---|---|
| Visit Type: n(%) | ||||
| All Types of Visits* | 220 (58.8%) | 111 (55.8%) | 109 (62.3%) | 1.31 (0.87–1.98) |
| Emergency Department | 185 (49.5%) | 92 (46.2%) | 93 (53.1) | 1.32 (0.88–1.98) |
| Inpatient | 122 (32.6%) | 57 (28.6%) | 65 (37.1%) | 1.47 (0.95–2.27) |
| In-Hospital Death | 7 (1.9%) | 2 (1.0%) | 5 (2.9%) | 2.90 (0.55–15.12) |
| Principal Discharge Diagnosis among those with “all visits” (n=220): n(%) | ||||
| Asthma Related** | 43 (19.6%) | 17 (15.3%) | 26 (23.9%) | 1.73 (0.88–3.42) |
| Chest Pain | 32 (14.6%) | 15 (13.5%) | 17 (15.6%) | 1.18 (0.56–2.51) |
| COPD or Pneumonia | 16 (7.3%) | 3 (2.7%) | 13 (11.9%) | 4.87 (1.35–17.62) |
| Upper Respiratory Infection | 12 (5.6%) | 9 (8.1%) | 3 (2.8%) | 0.32 (0.08–1.22) |
Notes: *Emergency department, inpatient/observation or in-hospital death. **Includes asthma diagnoses, respiratory failure or dyspnea.
We also conducted a sensitivity analysis using a shorter observation window (2012–2015). Spirometry use was less common when using this window (31%, n=121). In regard to predictors of spirometry, the only factor that continued to have an association with spirometry was a visit with a specialist. When assessing future visit types and primary diagnoses, associations remained similar.
Discussion
Despite guidelines4–7 suggesting use of spirometry in all initial diagnoses and in the follow-up care for asthma, we found that spirometry is underutilized in the evaluation of asthma. The probability of receiving spirometry was strongly associated with seeing a specialist; 88% of those who had a specialist visit received spirometry, compared to 34% of those who did not have a specialist visit. In addition, patients who received spirometry tended to be older, which suggests a potential need to evaluate the practice of incorporating spirometry into the care of younger patients with asthma. Among patients who had a diagnosis of asthma in 2014 and an asthma-related hospitalization or ED visit in 2016–2019, the majority had never had spirometry performed in the 2007–2015 study window and had never seen a specialist. However, we saw no association between receipt of spirometry and the likelihood of being hospitalized for an asthma-related diagnosis.
Prior studies have demonstrated rates of spirometry utilization around 42.7% for incident asthma and 47.6% respectively within 1 year of diagnosis.21,22 Our study attempted to look at use of spirometry over a longer period of time and incorporated spirometry used for both diagnosis and monitoring. Our finding that 47% of our sample had ever received spirometry within a nine-year observation window is consistent with prior work and affirms that spirometry is underutilized in the diagnosis or ongoing management of patients with asthma.
Similar to our results, a previous study completed by Sokol et al, showed that patients cared for by specialists were more likely to receive spirometry than those cared for by primary care physicians (80.1% vs 23.3%, respectively).22 Given the retrospective nature of our study, we were unable to determine whether the specialist or the primary care physician ordered the test. However, we strongly suspect that specialists are more likely to order the test than primary care physicians. These findings suggest that education is needed to help primary care physicians understand the importance of ordering spirometry and relaying its value to young patients with respiratory symptoms.
The reasons for the low rates of spirometry utilization that others and we have observed may be related to barriers to routine spirometry that have been previously described.19,21,22 For example, some clinicians may hold attitudes and beliefs about asthma diagnosis that differ from the NAEPP guidelines while others may not be aware of the existence of the guidelines.21,32 Other clinicians may fail to order the study because of cost, lack of time, lack of access to in-office spirometry, lack of proper training, limited staff and other organizational structures that do not permit easy ordering of the test.19,20,32-34 Patient factors such as disinterest in learning about a diagnosis or inability to perform the test may play a role as well.20
Although we could not identify the reasons that the test was not ordered, it is notable that most of our included patients had insurance, all had a primary care physician, and our health system has spirometry readily available at a nearby outpatient facility. Future work will focus on qualitative studies to further characterize the specific barriers that primary care clinicians face in ordering spirometry within our health system.
Unlike Gershon et al, our study found that older adult patients were more likely to receive spirometry.21 This difference may be due to these authors evaluating patients with incident asthma in a large sample (nearly 20,000 patients age 70 or greater) and an outcome of PFTs 1–2.5 years following diagnosis. Our sample was much smaller and our outcome was evaluated over a 9-year window. In addition, there may be regional variation in care delivery and insurance coverage that contributed to the observed differences (eg, greater prevalence of high deductible insurance plans in younger populations of some states).
Racial disparities in asthma diagnosis and treatment have also been well documented, but we did not find a statistically or clinically significant association between race and receipt of spirometry.35–40 We hypothesize that this is due to the racial makeup of our community health center patients which are majority non-white whereas the private practices tend to be majority white. Patients in the community health centers, in this study, received spirometry more frequently. We hypothesize that this is because these centers are teaching practices and are highly focused on evidence-based care.
There is mixed evidence about spirometry and asthma-related outcomes. Our finding, like one prior study, showed that there was no association between receipt of spirometry and outcomes.41 However, at least one other study suggested that integration of spirometry into intensive management with a specialist is a strong predictor of improved outcomes in severe asthma.42 Additionally, spirometry in the context of an in-home asthma disease management program may also reduce hospitalizations, in-patient days, cost and other health-quality outcomes for patients with asthma.43 Taken in the context of prior studies, our findings suggest a need for future work evaluating the benefits of spirometry for patients with mild vs severe asthma and across care delivery models (eg, intensive vs usual disease management).
Our study is novel because it focuses on a primary care population of adult patients and includes distinct primary care models (private practices vs community health centers). In addition, we further characterized the relationship between receipt of spirometry and future asthma-related ED visits and admissions.23 Our study also adds to the literature because it offers a longer snapshot around the time interval during which patients received spirometry and it further validates the role of the specialist as the main determinant for receipt of spirometry.21,22
This study also has limitations. First, it was a retrospective chart review and was subject to the limitations of data in the medical record. Further, the included length of time varied for each patient to allow the maximum time period for any patient to have completed spirometry. It is notable that despite being very inclusive, we still had a rate of less than 50% of patients receiving spirometry at any time. In addition, we did not gather data about the chronology of when a patient saw a specialist or received spirometry. This is a limitation as it does not allow us to say with certainty if patients are seeing the specialist as a result of poor spirometry outcomes or vice versa. We were also unable to determine the timing of asthma diagnosis in some cases, so for some patients we could not determine whether the spirometry was used for diagnostic or follow-up purposes. Our sample size was small, resulting in wide confidence intervals. We were unable to determine whether patients received spirometry or had ED visits or hospitalizations outside of our health system, which further limits the generalizability of the findings. Nevertheless, our health system is large with few competitors, meaning that most patients receive nearly all of their care within the system.
Conclusion
In conclusion, more than half of our patients with asthma never received spirometry. In a subset of patients with future hospitalizations or ED visits, spirometry was also used infrequently in the years prior to the event. This suggests an urgent need for education for primary care physicians to increase the use of this guideline-recommended care that can reduce misdiagnoses in patients with cough and wheezing, improve asthma control, and ultimately improve population health.
Institutional Review Board Approval
This study met approval from the IRB of Baystate Health. The IRB determined that subjects’ informed consent was not required in order to complete the research due to the retrospective nature of the study and the precautions taken to protect patient confidentiality. A Waiver of HIPAA authorization was obtained, and approved precautions were taken to protect patient confidentiality. All procedures were performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Disclosure
Portions of this study were funded by Grant UL1 TR001064, an Institutional Clinical and Translational Science Award (CTSA) from the NIH awarded to the Tufts University School of Medicine. Dr. Lagu reports grants from and was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL114745. Dr. Lagu has served as a consultant for and received personal fees from the Yale Center for Outcomes Research and Evaluation, under contract to the Centers for Medicare and Medicaid Services, for which she has provided clinical and methodological expertise and input on the development, reevaluation, and implementation of hospital outcome and efficiency measures, outside the submitted work. Dr. Lindenauer is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number 1K24HL132008. He has also served as a consultant for the Yale Center for Outcomes Research and Evaluation, under contract to the Centers for Medicare and Medicaid Services, for which he has provided clinical and methodological expertise and input on the development, reevaluation, and implementation of hospital outcome and efficiency measures. The views expressed in this manuscript do not necessarily reflect those of the Yale Center for Outcomes Research and Evaluation or the Centers for Medicare and Medicaid Services. The authors declare no conflicts of interest in this work.
References
- 1.Prevention C. Asthma facts CDC’s national asthma control program grantees In: Services U, editor. Atlanta. GA; 2013. [Google Scholar]
- 2.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc. 2018;15(3):348–356. doi: 10.1513/AnnalsATS.201703-259OC [DOI] [PubMed] [Google Scholar]
- 3.Medicine A. Choosing wisely In: An Initiative of the ABIM Foundation. 2013. [Google Scholar]
- 4.National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. UDoHaHS NIH, editor. Washington DC: National Heart, Lung, and Blood Institute; 2007. [Google Scholar]
- 5.Global Initiative for Asthma. National Heart Lung and Blood Institute. Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. Bethsda, ed. U.S: Dept. of Health and Human Services, Public Health Service; 2014. viii, 132. [Google Scholar]
- 6.Green T. Use spirometry first to improve diagnosis of asthma, says NICE. Nurs Stand. 2015;29(23):11. doi: 10.7748/ns.29.23.11.s12 [DOI] [PubMed] [Google Scholar]
- 7.Spector SL, Nicklas R. Practice parameters for the diagnosis and treatment of asthma. J Allergy Clin Immunol. 1995;96. [DOI] [PubMed] [Google Scholar]
- 8.Moore VC. Spirometry: step by step. Breathe. 2012;8(3):232–240. doi: 10.1183/20734735.0021711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce DP, Chapman KR, Kesten S. Prior diagnosis and treatment of patients with normal results of methacholine challenge and unexplained respiratory symptoms. Chest. 1996;109(3):697–701. doi: 10.1378/chest.109.3.697 [DOI] [PubMed] [Google Scholar]
- 10.Cowie RL, Underwood MF, Field SK. Asthma symptoms do not predict spirometry. Can Respir J. 2007;14(6):339–342. doi: 10.1155/2007/816132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yawn BP, Enright PL, Lemanske RF Jr, et al. Spirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPD. Chest. 2007;132(4):1162–1168. doi: 10.1378/chest.06-2722 [DOI] [PubMed] [Google Scholar]
- 12.Heffler E, Crimi C, Mancuso S, et al. Misdiagnosis of asthma and COPD and underuse of spirometry in primary care unselected patients. Respir Med. 2018;142:48–52. doi: 10.1016/j.rmed.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 13.Lenhardt RO, Catrambone CD, Walter J, McDermott MF, Weiss KB. The asthma emergency department visit: treating a crisis in the midst of uncontrolled disease. Ann Allergy Asthma Immunol. 2008;100(3):237–243. doi: 10.1016/S1081-1206(10)60448-6 [DOI] [PubMed] [Google Scholar]
- 14.Celli BR. The importance of spirometry in COPD and asthma: effect on approach to management. Chest. 2000;117(2Suppl):15S–9S. doi: 10.1378/chest.117.2_suppl.15S [DOI] [PubMed] [Google Scholar]
- 15.Osborne ML, Pedula KL, O’Hollaren M, et al. Assessing future need for acute care in adult asthmatics: the profile of Asthma risk study: a prospective health maintenance organization-based study. Chest. 2007;132(4):1151–1161. doi: 10.1378/chest.05-3084 [DOI] [PubMed] [Google Scholar]
- 16.Ulrik CS. Outcome of asthma: longitudinal changes in lung function. Eur Respir J. 1999;13(4):904–918. doi: 10.1034/j.1399-3003.1999.13d35.x [DOI] [PubMed] [Google Scholar]
- 17.Buzoianu E, Moiceanu M, Plesca DA. asthma control assessment in children: correlation between Asthma control test and peak expiratory flow. Maedica. 2014;9(4):338–343. [PMC free article] [PubMed] [Google Scholar]
- 18.Kotses H, Harver A, Humphries CT. Home monitoring in asthma self-management. J Asthma. 2006;43(9):649–655. doi: 10.1080/02770900600701309 [DOI] [PubMed] [Google Scholar]
- 19.Grant EN, Moy JN, Turner-Roan K, Daugherty SR, Weiss KB. Asthma care practices, perceptions, and beliefs of chicago-area primary-care physicians. Chest. 1999;116:145S–54S. doi: 10.1378/chest.116.suppl_2.145S [DOI] [PubMed] [Google Scholar]
- 20.Dennis SM, Price JF, Vickers MR, Frost CD, Levy ML, Barnes PJ. The management of newly identified asthma in primary care in England. Primary Care Respir J. 2002;11:120. doi: 10.1038/pcrj.2002.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gershon AS, Victor JC, Guan J, Aaron SD, To T. Pulmonary function testing in the diagnosis of asthma: a population study. Chest. 2012;141(5):1190–1196. doi: 10.1378/chest.11-0831 [DOI] [PubMed] [Google Scholar]
- 22.Sokol KC, Sharma G, Lin YL, Goldblum RM. Choosing wisely: adherence by physicians to recommended use of spirometry in the diagnosis and management of adult asthma. Am J Med. 2015;128(5):502–508. doi: 10.1016/j.amjmed.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 23.Snyder DA, Thomas OW, Gleeson SP, et al. Reducing emergency department visits utilizing a primary care asthma specialty clinic in a high-risk patient population. J Asthma. 2018;55(7):785–794. doi: 10.1080/02770903.2017.1369989 [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mergoni M, Rossi A. Fisiopatologia dell'insufficienza respiratoria acuta nella BPCO e nell'asma [Physiopathology of acute respiratory failure in COPD and asthma]. Minerva Anestesiol. 2001;67(4):198–205. Italian. [PubMed] [Google Scholar]
- 26.Edmondstone WM. Chest pain and non-respiratory symptoms in acute asthma. Postgrad Med J. 2000;76(897):413–414. doi: 10.1136/pmj.76.897.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung JM, Sin DD. Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. BMJ. 2017;358:j3772. doi: 10.1136/bmj.j3772 [DOI] [PubMed] [Google Scholar]
- 28.Tomita K, Sano H, Iwanaga T, et al. Association between episodes of upper respiratory infection and exacerbations in adult patients with asthma. J Asthma. 2012;49(3):253–259. doi: 10.3109/02770903.2012.661009 [DOI] [PubMed] [Google Scholar]
- 29.Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352(20):2082–2090. doi: 10.1056/NEJMoa044113 [DOI] [PubMed] [Google Scholar]
- 30.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 31.Van Belle G. Statistical Rules of Thumb. 2nd ed. Oxford: Wiley-Blackwell; 2008. 270. [Google Scholar]
- 32.Wiener-Ogilvie S, Huby G, Pinnock H, Gillies J, Sheikh A. Practice organisational characteristics can impact on compliance with the BTS/SIGN asthma guideline: qualitative comparative case study in primary care. BMC Fam Pract. 2008;9(1):32. doi: 10.1186/1471-2296-9-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Dowd LC, Fife D, Tenhave T, Panettieri RA. Attitudes of physicians toward objective measures of airway function in asthma. Am J Med. 2003;114(5):391–396. doi: 10.1016/S0002-9343(03)00007-X [DOI] [PubMed] [Google Scholar]
- 34.Eaton T, Withy S, Garrett JE, Whitlock RML, Rea HH, Mercer J. Spirometry in primary care practice: the importance of quality assurance and the impact of spirometry workshops. Chest. 1999;116(2):416–423. doi: 10.1378/chest.116.2.416 [DOI] [PubMed] [Google Scholar]
- 35.Anarella JP, Wagner VL, McCauley SG, Mane JB, Waniewski PA. Eliminating disparities in asthma care: identifying broad challenges in quality improvement. Am J Med Qual. 2017;32(6):598–604. doi: 10.1177/1062860616682587 [DOI] [PubMed] [Google Scholar]
- 36.Fitzpatrick AM, Gillespie SE, Mauger DT, et al. Racial disparities in asthma-related health care use in the national heart, lung, and blood institute’s severe asthma research program. J Allergy Clin Immunol. 2019;143(6):2052–2061. doi: 10.1016/j.jaci.2018.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haselkorn T, Lee JH, Mink DR, Weiss ST, Group TS. Racial disparities in asthma-related health outcomes in severe or difficult-to-treat asthma. Ann Allergy, Asthma Immunol. 2008;101(3):256–263. doi: 10.1016/S1081-1206(10)60490-5 [DOI] [PubMed] [Google Scholar]
- 38.Canino G, McQuaid EL, Rand CS. Addressing asthma health disparities: a multilevel challenge. J Allergy Clin Immunol. 2009;123(6):1209–17; quiz 18–9. doi: 10.1016/j.jaci.2009.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta RS, Springston EE, Weiss KB. Eliminating asthma disparities: is there evidence of progress? Curr Opin Pulm Med. 2009;15(1):72–78. doi: 10.1097/MCP.0b013e32831da911 [DOI] [PubMed] [Google Scholar]
- 40.Curtis LM, Wolf MS, Weiss KB, Grammer LC. The impact of health literacy and socioeconomic status on asthma disparities. J Asthma. 2012;49(2):178–183. doi: 10.3109/02770903.2011.648297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abramson MJ, Schattner RL, Sulaiman ND, et al. Do spirometry and regular follow-up improve health outcomes in general practice patients with asthma or COPD? A cluster randomised controlled trial. Med J Australia. 2010;193(2):104–109. doi: 10.5694/j.1326-5377.2010.tb03817.x [DOI] [PubMed] [Google Scholar]
- 42.Jain VV, Allison R, Beck SJ, et al. Impact of an integrated disease management program in reducing exacerbations in patients with severe asthma and COPD. Respir Med. 2014;108(12):1794–1800. doi: 10.1016/j.rmed.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 43.Shelledy DC, Legrand TS, Gardner DD, Peters JI. A randomized, controlled study to evaluate the role of an in-home asthma disease management program provided by respiratory therapists in improving outcomes and reducing the cost of care. J Asthma. 2009;46(2):194–201. doi: 10.1080/02770900802610068 [DOI] [PubMed] [Google Scholar]