Abstract
Biomechanical changes caused by structural foot deformities predispose patients to plantar ulceration. Plantar ulcer recurrence often leads to osteomyelitis, which is more commonly observed in patients with diabetes. Once the infection of diabetic foot ulcer (DFU) spreads and is complicated by osteomyelitis, treatment becomes more complicated and difficult. Osteomyelitis treatment remains challenging because of low drug concentration within the tissue caused by poor circulation and inadequate localized nutrition. Moreover, tissues around plantar ulcers are fewer and are thin, making the formation of granulation tissues difficult due to elevated plantar pressure. Furthermore, the skin around the wound is excessively keratinized, and the epidermis is hard to regenerate. Meanwhile, skin grafting at that site is often not successful due to poor blood circulation. Therefore, it is technically challenging to manage diabetic pressure plantar ulcer with osteomyelitis and prevent its recurrence. Here, we present a case of chronic DFU complicated by osteomyelitis due to foot deformity. The ulcer was successfully healed using advanced wound repair technology comprising of surgical bone resection, vancomycin-loaded bone cement implant, negative-pressure wound therapy, and autologous platelet-rich gel. Subsequently, preventive foot care with custom-made offloading footwear was prescribed. The plantar ulcer did not recur and improvement in biomechanical parameters was observed after the intervention. This case represents an effective and comprehensive management strategy for limb salvage and prevention in patients with complicated foot conditions.
Keywords: diabetic foot ulcer, osteomyelitis, antibiotic-loaded bone cement, autologous platelet-rich gel, negative-pressure wound therapy, offloading footwear
Introduction
Diabetic foot ulcer (DFU) is one of the most common, complex, and costly complications affecting the lower extremities in people with diabetes. DFU is usually associated with high morbidity and mortality and considerable financial costs.1 The incidence of DFU in people with diabetes is estimated to be as high as 19–34% during their lifetime.2 Peripheral arterial disease independently increases the risk of DFU and often leads to poor healing, infection, and amputation.2,3 A majority of patients with diabetes-related chronic limb-threatening ischemia present non-healing ischemic ulcers or with gangrene (Fontaine stage IV) in real-world settings.4 The mechanism of DFU pathogenesis involves diabetic neuropathy, peripheral vascular disease, tissue loss, infection, and other high-risk factors including foot deformity or minor trauma.
In patients with foot deformity, continual ambulation over high plantar pressure areas may injure the localized areas of deformity, resulting in chronic non-healing ulceration. The ulcers can be divided into two types: superficial lesions that are limited within the skin, and deep ulcers combined with tissue loss or infection that are deeper than the skin.5 Moreover, pressure ulcers on the sole of the foot are caused by repetitive weight-loaded pressure, shear force, or friction and usually damage both the skin and underlying tissue. Commonly, the prognosis for patients with plantar ulcers and deep ulcers is poor. Together, these multi-factorial etiologies lead to non-healing ulcers and amputation. In particular, infection commonly aggravates DFU and causes a poor clinical outcome, which is usually the first step to lower extremity amputation. Osteomyelitis is the most frequent infection in patients with DFU, an important factor for amputation, and prolongs antibiotic treatment and hospital admission. Diabetic foot osteomyelitis (DFO) is associated with more than 20% of moderate infections and 50–60% of severe infections of DFU.6 It is estimated that once infection involves the bone, the risk of amputation increases up to fourfold.7 However, there are currently no widely accepted guidelines available for the diagnosis and treatment of DFO. Recently, guidelines published by the International Working Group on the Diabetic Foot have provided some appropriate recommendations on treatments for diabetic foot infections including antimicrobial therapy and surgical treatment approaches.8 In clinical practice, the treatment for patients with DFO occurring in areas such as the plantar sole, with co-morbidities including peripheral arterial disease, typically involves revascularization, resection of infected bone, and offloading modalities. Presently, there are no clear evidence-based recommendations for such a management approach.
Recent guidance suggests that pharmacotherapy and surgical resection are often used in the treatment of DFO under different circumstances. The assumed major benefits of surgical intervention are that it can remove infected soft tissues and bone, resect bony prominences, drain pus collection, and reduce bioburden. Nonetheless, this will leave patients with residual structural deformity, altering their dynamic pattern, and increase the risk of lesion transfer if the bone has been removed inappropriately. Instead of surgical intervention, conservative pharmacotherapy treatment of DFO with antibiotics can be used to protect the integrity and stability of the foot. Moreover, the key to pharmacotherapy for DFO treatment is to ensure optimal antibiotic concentration at the cellular level, especially in patients with poor microcirculation. Anecdotal evidence has demonstrated poor DFO outcomes are associated with delayed diagnosis and referral, or the use of ill-indicated treatments.6,9
Therefore, with the view of reducing the risk of limb loss, it is important to promptly manage DFO infection to promote wound healing and prevent its recurrence by using an appropriate treatment strategy. We herein present a patient with chronic DFU complicated by osteomyelitis enrolled in the Footwear and Offloading Optimum Therapy (FOOT) study. A combination of treatments was used to facilitate wound healing and provide the patient with successful limb salvage and to minimize the risk of recurrence.
Case Report
A 76-year-old female admitted to hospital with a two-year medical history of type 2 diabetes mellitus, hypertension, and a diabetic foot ulcer on her left mid-foot that had not healed in over 4 months. The patient sustained a snake bite on the left midfoot at 6 years of age, resulting in a severe foot infection. She recovered from the infection but acquired a residual foot deformity. She has been suffering from numbness and pain in the left mid-foot for many years. To cope with the left foot pain, she attempted to bear more weight in the right foot but reduced activity than the right foot. Two years ago, she was diagnosed with type 2 diabetes mellitus. Subsequently, a recurrent chronic ulcer appeared at the site of the snakebite and for more than 4 months was difficult to heal.
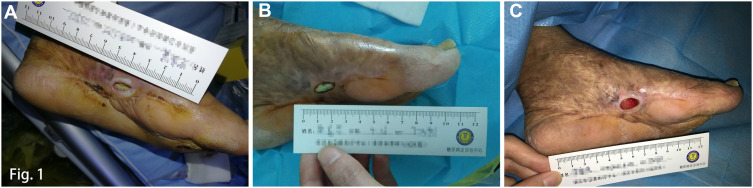
Physical examination revealed an ulcer of approximately 1.5cm×0.5cm on the left medial midfoot with purulent discharge (Figure 1A). The ulcer had a 0.8cm deep cavity and a portion of bone was observed at the wound bed with a positive probe-to-bone test. The surrounding tissue was pale. There was no obvious pain and bleeding on the wound surface. The left foot was hypoesthesic and slightly swollen. Light touch sensation was intact in the left foot. The distal pulses, including pedal and posterior tibial pulses, of both feet were palpable.
Figure 1.
Wound status after debridement, vancomycin-loaded bone cement implant, and negative pressure wound therapy installment. (A) After sharp debridement; (B) After resection of non-viable bone and application of the vancomycin-loaded bone cement implant; (C) After negative pressure wound therapy installment.
Laboratory investigation showed elevated random blood glucose of 14.3 mmol/L and hemoglobin A1c of 7%. The C-reactive protein level was 1.8 mg/L. The erythrocyte sedimentation rate was 17 mm/h. The ankle-brachial index greater than 1.3 in both legs suggested posterior tibial and dorsalis arteriosclerosis. Vibration perception threshold testing indicated severe peripheral neuropathy. Radiographic imaging of the left foot revealed changes in the distal part of the first and second metatarsal bones and abnormal bone morphology. MRI showed multiple bone marrow edema of metatarsal, navicular, cuboid, medial, and lateral cuneiform bones from the first to the fourth toes, indicating infectious disease. Doppler ultrasound results showed that the femoral, popliteal, anterior tibial, and posterior tibial arteries of both limbs were sclerotic, and that the veins were normal.
The patient was diagnosed as having diabetic peripheral neuropathy and diabetic peripheral vascular disease. Based on the University of Texas (UT) Diabetic Foot Ulcer Classification System, the classification of the wound on the patient’s left foot was UT Ш D. According to the International Working Group on the Diabetic Foot classification guidelines for diabetic foot wounds, the diabetic foot infection perfusion, extent, depth, infection and sensation grade was Grade 3. The Society for Vascular Surgery Wound, Ischemia, and foot Infection score was: wound 3, ischemia 0, foot infection 2, correlating to clinical stage 3. A series of standard medical treatments including anti-hypertensive treatment and antibiotics were administered, blood glucose control was optimized and peripheral circulation was improved. In addition, the wound was thoroughly debrided, including wound cleaning and removal of all infected and nonviable (necrotic or dead) tissue. Local dressing was performed simultaneously. Intravenous administration of vancomycin was commenced based on microbial culture and drug sensitivity test results from a wound secretion sample.
After treatment for 6-weeks, there was increased exudation from the wound, new granulation formation slowed, and bleeding was minimal, all of which probably suggest lower limb ischemia. Since no individual medical specialty is able to manage all aspects of DFU, a multidisciplinary team in our hospital was launched to manage the patient. The infected dead bone was resected and inflammatory granulation tissues were surgically debrided until healthy granulation wound bed was observed. The space left by bone resection was filled with antibiotic-loaded bone cement (Figure 1B). The antibiotic-loaded bone cement was prepared by mixing 10 g of Palacos G Bone Cement powder (PALACOS® R+G, Heraeus Medical GmbH, Germany) and 0.4 g of vancomycin. Pathological diagnosis showed that nonviable skin, fiber, and bone tissue with a large number of necrotic bones was surgically removed. Eight days later, the vancomycin bone cement bead was removed. Fresh granulation tissue was present on the ulcer surface. The overlying fibrin tissue and excess hardened epithelized tissue were removed, and negative pressure wound therapy (NPWT) with intermittent pressure (−50 to −125 mmHg) was commenced to promote wound healing.
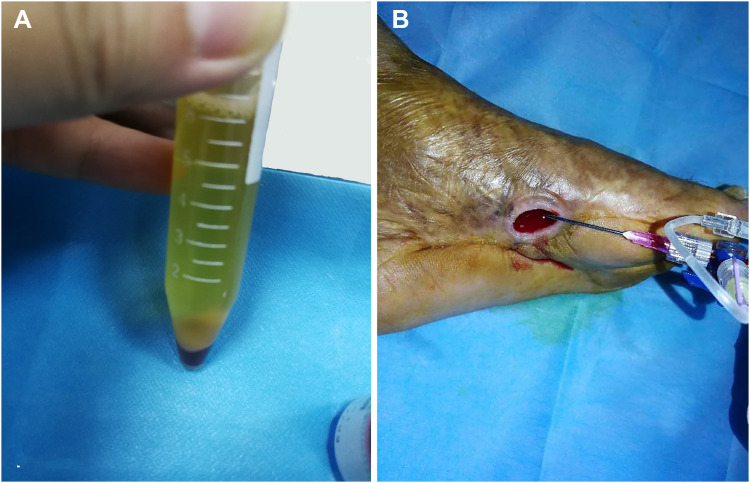
After 5 days, wound bleeding could be observed on the ulcer surface. The appearance of fresh granulation tissues at the bottom of the cleaned wound was minimal. The exposed bone had been successfully closed although there was no significant reduction in the size of ulceration (Figure 1C). After full evaluation and informed consent was obtained, autologous platelet-rich gel (APG) was prepared as described in our previous study10 (Figure 2A) and the gel was administered to the surface of the wound (Figure 2B). After 10 days, granulation tissues gradually grew in the ulcer cavity. However, considering the new granulation tissues were not enough to fill the ulcer cavity, the patient had to receive APG treatment once more. The wound was significantly improved after twice administrations for the treatment of APG (Figure 3A).
Figure 2.
Autologous platelet-rich gel (APG) preparation and the topical administration over the wound bed. (A) APG was prepared after evaluation and informed consent obtained; (B) The gel was administered onto the surface of the wound.
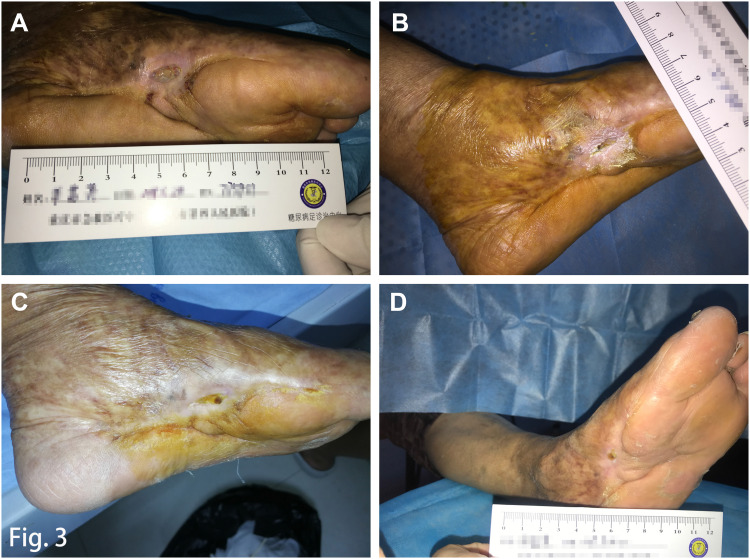
Figure 3.
Ulcer healing and follow-up. (A) Wound status after the second autologous platelet-rich gel treatment; (B) Complete wound closure was observed after a treatment period of nearly 5 months; (C) Foot condition at the 1 month follow-up; (D) After the intervention, and 1 year after she left the hospital, the patient was without ulcer recurrence.
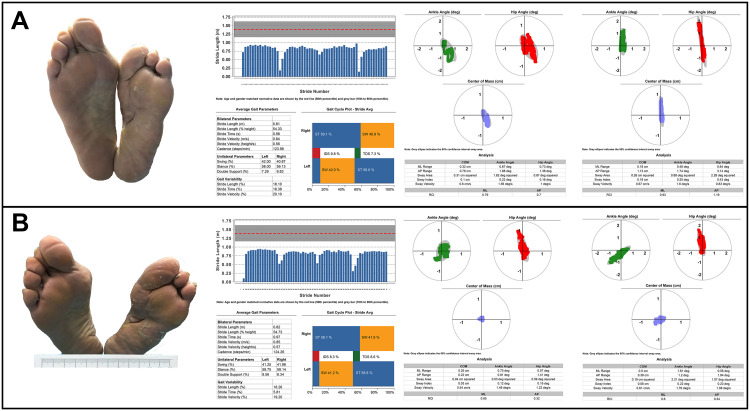
Complete wound closure was achieved nearly 5 months after a combination of treatments (Figure 3B). The patient reported no side effects, and no complications were observed during the entire therapy period. There was no sign of ulcer recurrence at follow-up 1-month (Figure 3C) and one-year (Figure 3D) after wound closure. Considering her severe left foot deformity, the protection of epithelized, yet fragile, tissue is paramount to reducing the risk of recurrence. After a comprehensive foot assessment, we prescribed therapeutic footwear that had a demonstrated effect on the relief of plantar pressure. Biomechanical parameters, including gait and balance, were assessed before and after therapeutic offloading footwear were worn (Figure 4) (DaveMed LLC, Chongqing, China). After the next 6-month post intervention follow-up, the ulcer had not recurred.
Figure 4.
Biomechanical parameter improvement following treatment with offloading therapeutic footwear. (A) Biomechanical parameters including ankle angle, hip angle, center of mass, gait, and balance before wearing the custom-made footwear; (B) Changes in biomechanical parameters after wearing the custom-made footwear.
Discussion
Recent studies indicate that foot deformities are associated with ulcer occurrence and recurrence.11,12 Intrinsic factors of biomechanical changes, including sensory impairment, fragile plantar tissue, and excessive plantar pressure, combined with extrinsic factors such as inappropriate footwear and non-standard self-management, eventually lead to the occurrence of plantar ulcers.13–15 The common pathogenic factor of DFO, an important cause of admission and amputation, is infection of repetitive plantar pressure ulcers that extends to involve bone. With our clinical experience, appropriate management for DFO should take into consideration its complicated clinical condition.
During the treatment period, many clinical difficulties need to be overcome. Firstly, DFU located on the plantar aspect of the foot has reduced peripheral perfusion, which may reduce the delivery of antibiotic agents into the bone tissue and limit pharmacotherapy efficacy. Secondly, migration and proliferation for granulation tissues are compromised because the viable tissues around plantar ulcers are reduced and thin. Thirdly, the epidermis is difficult to regenerate because the plantar sole is usually thickened and excessively keratinized, and skin grafting from donor sites may not provide similar tensile strength as that of the epidermis of the plantar sole. Lastly, the ability to effectively prevent the DFU recurrence in a structurally deformed foot is an additional problem that should be considered.
In this case, the patient with DFO had plantar foot ulcer which was not improved despite receiving conventional wound therapy and antibiotic treatment. We speculated that this related to the low tissue penetration of antibiotics, resulting in a poor outcome. Debridement is considered to be an effective intervention in the treatment of DFU and hydrogel has been suggested to increase the healing rate of DFU.16,17 However, when DFU is with osteomyelitis, these approaches are less successful. Therefore, in the patient presented here, a combination of advanced wound therapy, including removal of necrotic bones, implantation of vancomycin-loaded bone cement, NPWT, and APG was consecutively applied and limb salvage was eventually achieved.
There is evidence to suggest that the prognosis of more than half of patients with DFO was amputation, either minor or major ones.18 As was the case for the patient presented here, the risk of amputation increases significantly once the bone margin is exposed. This is further compounded by the presence of limb ischemia and tissue infection. Senneville and colleagues reviewed the treatment of DFO.19 DFO treatment generally involves prolonged antibiotic drug use, surgical intervention if indicated, and management of the patient’s comorbidities. Whether or not remove infected or necrotic bone by surgical excision at an early stage in patients with DFO remains controversial. Surgical treatment is not the only available treatment choice for DFO. Non-surgical treatment of DFO is frequently feasible, particularly in patients without limb-threatening infection.20 However, another study has shown that non-surgical treatment can achieve a cure rate of 60–80%, but does require patients to undergo long-term broad-spectrum antibiotic treatment, which increases the risk of antibiotic-associated diarrhea or the emergence of multidrug-resistant organisms.21 Thus, DFO treatment is tailored to the patient by physicians and surgeons following a comprehensive assessment.
Many previous studies have suggested that antibiotic beads could offer the benefit of managing dead zones by allowing high local antibiotic concentrations and maintaining low systemic antibiotic levels.22,23 The material of the antibiotic cement delivery system can be classified as biodegradable and non-biodegradable. The use of Cerament Bone Void Filler, a unique biocompatible and biodegradable material, as antibiotic beads have been reported in DFO treatment.24 Using this material, the subsequent stage of removing the antibiotic beats was not necessary. Heat-stable vancomycin could be used as a common antibiotic and incorporated into the topical wound therapy system.
Recently, APG has been applied to the clinical treatment of DFU. Platelet-rich plasma (PRP) contains fibrin and a high concentration of growth factors and studies suggested that autologous PRP has the potential to improve DFU healing of promoting cell proliferation.25 The safety and effectiveness of APG have been confirmed in the treatment of diabetic chronic refractory cutaneous ulcers.26 Moreover, PRP also demonstrated antibacterial and anti-inflammatory roles in diabetic wounds.27–30 In this case, the topical application of APG accelerated wound healing. Combined with the application of NPWT, bilayered acellular matrix grafting, and split-thickness skin grafting, APG shows obvious effectiveness in foot necrotizing fasciitis and gaseous gangrene.10 NPWT could facilitate wound healing by promoting tissue perfusion and providing a moist local environment for wound coverage.31,32
Additionally, wound healing is not the ultimate goal of DFU management. How to prevent ulcer recurrence is an important issue for clinicians and patients. Recent evidence suggests that the relative risk of ulceration in patients with a history of DFU is 10 times higher than in patients without such history.33 Clinical evidence suggests that offloading footwear has a protective effect in patients with DFU and can effectively prevent ulcer recurrence.34,35 Moreover, nearly half of DFUs occur on the plantar surface of the foot.36 However, the importance of offloading management for pressure ulcers has not been adequately emphasized in the literature. Offloading, as an integral part of diabetic foot management, has been recommended in the updated International Working Group on the Diabetic Foot guidelines (2019) which outlines clear offloading treatment suggestions to heal or prevent a neuropathic plantar ulcer.37
To the best of our knowledge, this is the first case report related to both the treatment and prevention of DFU with osteomyelitis. Furthermore, our pilot FOOT study on custom-made offloading footwear has shown some beneficial effects through the study of quantitative dynamic gait and balance data in patients with DFU. Suitable offloading footwear could improve dynamic gait as well as relieve foot plantar pressure to prevent the recurrence of ulcers.
Conclusion
When treating patients with DFU, clinicians must pay more attention to all contributing issues because some common foot problems could rapidly worsen and lead to serious complications. Once the presence of DFO is confirmed, multi-advanced wound therapeutic technology should be considered for limb salvage as early as possible. When the wound is healed, preventing foot ulcers among individuals with risk factors is the key factor to reduce ulcer recurrence. DFU located on the plantar side of the foot is usually associated with elevated foot pressure. Therefore, offloading with custom-made footwear to prevent DFU recurrence should be an integral part of the multidisciplinary standards of care in patients with pressure ulcers.
Funding Statement
This study was supported by the Fundamental Research Funds for the Central Universities at Chongqing University (Grant No.2019CDYGYB020) and the Joint Medical Research Programs for Chongqing Municipal Science and Technology Bureau and Health Commission (Grant No.2019MSXM028) awarded to Dr. Wuquan Deng and Xiaoyan Jiang.
Abbreviation
DFU, diabetic foot ulcer; DFO, diabetic foot osteomyelitis; NPWT, negative pressure wound therapy; APG, autologous platelet-rich gel; PRP, platelet-rich plasma.
Ethics Consent for Publication
Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. A formal ethical review by an institutional review board was not required because this is a case report.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. New Engl J Med. 2017;376(24):2367–2375. doi: 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 3.Mills JL Sr, Conte MS, Armstrong DG, et al. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220–34.e1–2. doi: 10.1016/j.jvs.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 4.Takahara M, Iida O, Fujita Y, Haneda M. Clinical characteristics of Japanese diabetic patients with critical limb ischemia presenting Fontaine stage IV. Diabetol Int. 2018;10:231–235. doi: 10.1007/s13340-018-0387-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker K, Apelqvist J, Schaper NC. International Working Group on Diabetic Foot Editorial Board. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28(Suppl 1):225–231. doi: 10.1002/dmrr.2253 [DOI] [PubMed] [Google Scholar]
- 6.Lazaro Martinez JL, Garcia Alvarez Y, Tardaguila-Garcia A, Garcia Morales E. Optimal management of diabetic foot osteomyelitis: challenges and solutions. Diabetes Metab Syndr Obes. 2019;12:947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson MJ, Shumway N, Bivins M, Bessesen MT. Outcomes of limb-sparing surgery for osteomyelitis in the diabetic foot: importance of the histopathologic margin. Open Forum Infect Dis. 2019;6(10):ofz382. doi: 10.1093/ofid/ofz382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lissky BA, Senneville E, Abbas ZG, et al; on behalf of the International Working Group on the Diabetic Foot (IWGDF). IWGDF Guideline on the diagnosis and treatment of foot infection in persons with diabetes. Part of the 2019 IWGDF Guidelines on the prevention and management of diabetic foot disease. Available from: https://iwgdfguidelines.org/. Accessed June15, 2020.
- 9.Wukich DK, Hobizal KB, Sambenedetto TL, Kirby K, Rosario BL. Outcomes of osteomyelitis in patients hospitalized with diabetic foot infections. Foot Ankle Int. 2016;37(12):1285–1291. doi: 10.1177/1071100716664364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng W, Boey J, Chen B, et al. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J Wound Care. 2016;25(7):393–397. doi: 10.12968/jowc.2016.25.7.393 [DOI] [PubMed] [Google Scholar]
- 11.Ledoux WR, Shofer JB, Smith DG, et al. Relationship between foot type, foot deformity, and ulcer occurrence in the high-risk diabetic foot. J Rehabil Res Dev. 2005;42(5):665–671. doi: 10.1682/JRRD.2004.11.0144 [DOI] [PubMed] [Google Scholar]
- 12.Allan J, Munro W, Figgins E. Foot deformities within the diabetic foot and their influence on biomechanics: a review of the literature. Prosthet Orthot Int. 2016;40(2):182–192. [DOI] [PubMed] [Google Scholar]
- 13.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361(9368):1545–1551. doi: 10.1016/S0140-6736(03)13169-8 [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald RH, Vedpathak A. Plantar pressure distribution in a hyperpronatedfoot before and after intervention with an extraosseous talotarsalstabilization device-a retrospective study. J Foot Ankle Surg. 2013;52(4):432–443. doi: 10.1053/j.jfas.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 15.Andersen H. Motor dysfunction in diabetes. Diabetes-Metab Res. 2012;28:89–92. doi: 10.1002/dmrr.2257 [DOI] [PubMed] [Google Scholar]
- 16.Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2010;1:CD003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumville JC, O’Meara S, Deshpande S, Speak K. Hydrogel dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013;7:CD009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aragon-Sanchez FJ, Cabrera-Galvan JJ, Quintana-Marrero Y, et al. Outcomes of surgical treatment of diabetic foot osteomyelitis: a series of 185 patients with histopathological confirmation of bone involvement. Diabetologia. 2008;51(11):1962–1970. doi: 10.1007/s00125-008-1131-8 [DOI] [PubMed] [Google Scholar]
- 19.Senneville E, Robineau O. Treatment options for diabetic foot osteomyelitis. Expert Opin Pharmacother. 2017;18(8):759–765. doi: 10.1080/14656566.2017.1316375 [DOI] [PubMed] [Google Scholar]
- 20.Mutluoglu M, Lipsky BA. Non-surgical treatment of diabetic foot osteomyelitis. Lancet Diabetes Endocrinol. 2017;5(8):668. doi: 10.1016/S2213-8587(16)30141-3 [DOI] [PubMed] [Google Scholar]
- 21.Game F. Management of osteomyelitis of the foot in diabetes mellitus. Nat Rev Endocrinol. 2010;6(1):43–47. doi: 10.1038/nrendo.2009.243 [DOI] [PubMed] [Google Scholar]
- 22.Wahlig H, Dingeldein E, Bergmann R, Rruss K. The release of gentamicin from polymethylmethacrylate beads: an experimental and pharmacokinetic study. J Bone Joint Surg Br. 1979;60(2):270–275. [DOI] [PubMed] [Google Scholar]
- 23.Haydon RC, Blaha JD, Mancinelli C, Koike K. Audiometric thresholds in osteomyelitis patients treated with gentamicin-impregnated methylmethacrylate beads (Septopal). Clin Orthop Relat Res. 1993;295:43–46. [PubMed] [Google Scholar]
- 24.Karr JC. Management in the wound-care center outpatient setting of a diabetic patient with forefoot osteomyelitis using Cerament Bone Void Filler impregnated with vancomycin: off-label use. J Am Podiatr Med Assoc. 2011;101(3):259–264. doi: 10.7547/1010259 [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;5:CD006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Chen D, Wang C, et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: a prospective, randomized clinical trial. Wound Repair Regen. 2015;23(4):495–505. doi: 10.1111/wrr.12294 [DOI] [PubMed] [Google Scholar]
- 27.Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg. 2017;38:206–211. doi: 10.1016/j.avsg.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 28.Li T, Ma Y, Wang M, et al. Platelet-rich plasma plays an antibacterial, anti-inflammatory and cell proliferation-promoting role in an in vitro model for diabetic infected wounds. Infect Drug Resist. 2019;12:297–309. doi: 10.2147/IDR.S186651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Guo Y, Kuss M, et al. Platelet-rich plasma for the treatment of tissue infection: preparation and clinical evaluation. Tissue Eng Part B Rev. 2019;25(3):225–236. doi: 10.1089/ten.teb.2018.0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariani E, Filardo G, Canella V, et al. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy. 2014;16(9):1294–1304. doi: 10.1016/j.jcyt.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 31.Borys S, Hohendorff J, Koblik T, et al. Negative-pressure wound therapy for management of chronic neuropathic noninfected diabetic foot ulcerations - short-term efficacy and long-term outcomes. Endocrine. 2018;62(3):611–616. doi: 10.1007/s12020-018-1707-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wynn M, Freeman S. The efficacy of negative pressure wound therapy for diabetic foot ulcers: a systematised review. J Tissue Viability. 2019;28(3):152–160. doi: 10.1016/j.jtv.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 33.Abbott CA, Carrington AL, Ashe H, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19(5):377–384. doi: 10.1046/j.1464-5491.2002.00698.x [DOI] [PubMed] [Google Scholar]
- 34.Bus SA, Valk GD, van Deursen RW, et al. The effectiveness of footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in diabetes: a systematic review. Diabetes Metab Res Rev. 2008;24(Suppl.1):S162–S180. doi: 10.1002/dmrr.850 [DOI] [PubMed] [Google Scholar]
- 35.Maciejewski ML, Reiber GE, Smith DG, Wallace C, Hayes S, Boyko EJ. Effectiveness of diabetic therapeutic footwear in preventing reulceration. Diabetes Care. 2004;27:1774–1782. doi: 10.2337/diacare.27.7.1774 [DOI] [PubMed] [Google Scholar]
- 36.Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18–25. doi: 10.1007/s00125-006-0491-1 [DOI] [PubMed] [Google Scholar]
- 37.Bus SA, Armstrong DG, Gooday C, et al; on behalf of the International Working Group on the Diabetic Foot (IWGDF). IWGDF Guideline on the offloading foot ulcers in persons with diabetes. Part of the 2019 IWGDF Guidelines on the prevention and management of diabetic foot disease. Available from: https://iwgdfguidelines.org/. Accessed June15, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lissky BA, Senneville E, Abbas ZG, et al; on behalf of the International Working Group on the Diabetic Foot (IWGDF). IWGDF Guideline on the diagnosis and treatment of foot infection in persons with diabetes. Part of the 2019 IWGDF Guidelines on the prevention and management of diabetic foot disease. Available from: https://iwgdfguidelines.org/. Accessed June15, 2020.
- Bus SA, Armstrong DG, Gooday C, et al; on behalf of the International Working Group on the Diabetic Foot (IWGDF). IWGDF Guideline on the offloading foot ulcers in persons with diabetes. Part of the 2019 IWGDF Guidelines on the prevention and management of diabetic foot disease. Available from: https://iwgdfguidelines.org/. Accessed June15, 2020.