Abstract
Background
Subcutaneous nerve stimulation (ScNS) remodels the stellate ganglion and reduces stellate ganglion nerve activity (SGNA) in dogs. Acute myocardial infarction (MI) increases SGNA through nerve sprouting.
Objective
To test the hypothesis that ScNS remodels the stellate ganglion and reduces SGNA in ambulatory dogs with acute MI.
Methods
In the experimental group, a radiotransmitter was implanted during the first sterile surgery to record nerve activity and an electrocardiogram, followed by a second sterile surgery to create MI. The dogs then underwent ScNS for two months. The average SGNA (aSGNA) was compared with a historical control group (N=9) with acute MI monitored for two months without ScNS.
Results
In the experimental group, the baseline aSGNA and heart rate were 4.08±0.35 μV and 98±12 bpm, respectively. They increased within one week after MI to 6.91±1.91 μV (p=0.007) and 107±10 bpm (p=0.028), respectively, compared with baseline. ScNS reduced aSGNA to 3.46±0.44 μV (p<0.039) and 2.14±0.50 μV (p<0.001) at 4 and 8 weeks, respectively, after MI. In comparison, the aSGNA at 4 and 8 weeks in dogs with MI but no ScNS were 8.26±6.31 μV (p=0.005) and 10.82±7.86 μV (p=0.002), respectively. Immunostaining showed confluent areas of remodeling in bilateral stellate ganglia and high percentage of tyrosine hydroxylase-negative ganglion cells. Terminal deoxynucleotidyl transferase dUTP nick end labeling was positive in 26.61±11.54% of ganglion cells in the left and 15.94±3.62% ganglion cells in the right stellate ganglion.
Conclusion:
ScNS remodels the stellate ganglion, reduces stellate ganglion nerve activity and suppresses cardiac nerve sprouting after acute MI.
Keywords: Animal Models of Human Disease, Autonomic Nervous System, Arrhythmias, Cardiac nerve sprouting, Electrical stimulation, Immunostaining, Sudden Cardiac Death
Acute myocardial infarction (MI) increases sympathetic tone and the risk of life threatening ventricular tachyarrhythmias (VT). Studies in canine models showed that MI results in the immediate elevation of the transcardiac nerve growth factor (NGF) concentration, followed by the upregulation of both cardiac and left stellate ganglion (SG) NGF and growth associated protein 43 (GAP43) expression.1 These changes are associated with progressively increased left stellate ganglion nerve activity (SGNA) for at least 2 months after MI.2 Cardiac sympathetic denervation can reduce the risk of sudden death in post MI patients.3 In addition to sympathetic denervation, vagal nerve stimulation is also known to reduce ischemia-induced ventricular fibrillation in dogs with healed MI by antagonizing sympathetic activity.4 Our recent studies confirmed that vagal nerve stimulation can reduce sympathetic nerve activity in ambulatory dogs5, 6 and in humans.7 However, because implanting vagal nerve stimulating electrodes is technically challenging, it has not been widely used to reduce arrhythmias in patients with MI. The skin of the dogs is well innervated by sympathetic nerves.8, 9 We showed that skin sympathetic nerve activity and subcutaneous nerve activity can be used to estimate SGNA in ambulatory dogs.8 Because of the direct connection between skin sympathetic nerves and stellate ganglion, we next tested the hypothesis that subcutaneous nerve stimulation (ScNS) can rapidly activate the stellate ganglion and cause stellate ganglion remodeling. The latter hypothesis was confirmed in a recent study from our laboratory in normal ambulatory dogs.7 However, because MI causes nerve sprouting and sympathetic hyperinnervation,1, 10 whether or not ScNS can be used to reduce sympathetic tone after MI remains unknown. The purpose of the present study was to test the hypothesis that ScNS can remodel stellate ganglion and reduce SGNA in dogs with acute MI.
Methods
The animal protocol was approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine and the Methodist Research Institute, Indianapolis, IN, and conformed to the Guide for Care and Use of Laboratory animals.
Experimental protocol
Six mongrel dogs (23-30 kg) underwent isoflurane inhalation general anesthesia and sterile left lateral thoracotomy through the third intercostal space. A radiotransmitter (D70EEE, Data Sciences International, St. Paul, MN) was implanted to record SGNA and vagal nerve activity (VNA) according to methods reported elsewhere.11 The skin incision was then extended to the ventral side to reach the left lateral thoracic nerve (LTN), which was used both for ScNS and for recording of the subcutaneous nerve activity (left lateral thoracic nerve activity, or LTNA). A Model 304 Cyberonics (Houston, TX) bipolar vagal nerve stimulation lead was implanted with electrodes wrapped around LTN for ScNS and then connected to a subcutaneously positioned Cyberonics Demipulse neurostimulator. A third pair of the bipolar recording electrodes was placed in the subcutaneous tissue to record from the nerve being stimulated, with the two electrodes bracketing the point of stimulation. The latter bipolar leads have an interelectrode distance of 4 cm. The chest was then closed. The DSI radiotransmitter was turned on two weeks after surgery to record baseline electrocardiogram (ECG) and nerve activities (Figure 1). Dexmedetomidine (2.5 μg/kg), a highly selective α2-adrenergic agonist with central sympatholytic actions,12, 13 was injected into four ambulatory unsedated dogs between 10 AM to 11 AM in a quiet room. Significant suppression of SGNA by dexmedetomidine supports the validity of SGNA recordings as a measure of sympathetic tone.
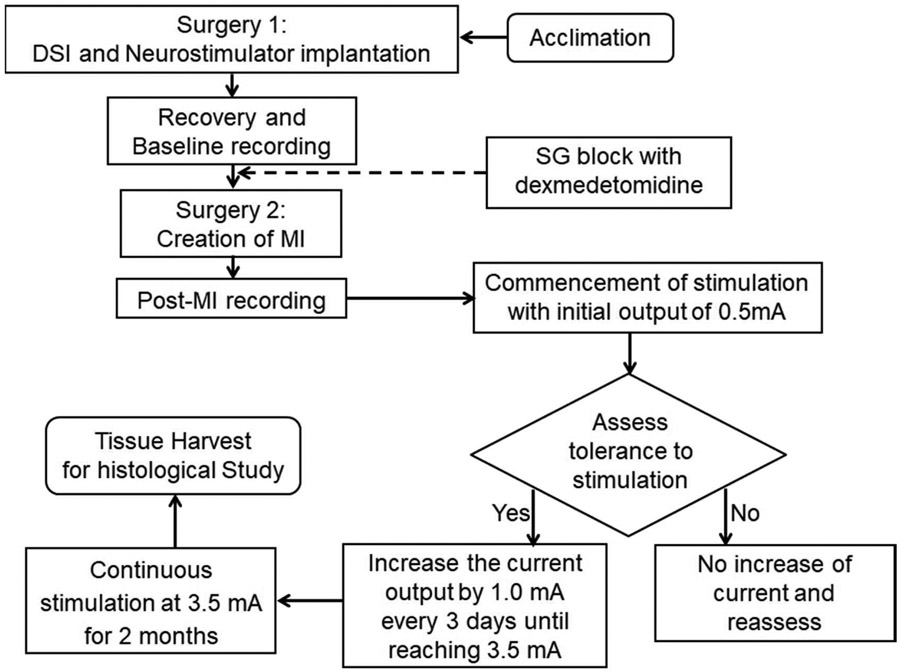
Figure 1.
Schematic of the study protocol. The baseline nerve activity and ECG were recorded two weeks after radiotransmitter implantation. Dexmedetomidine (2.5 μg/kg) injection was performed in 4 dogs to determine the effects of ganglionic blockade on nerve activity. A second sterile surgery was then performed approximately 4 weeks after the first surgery to create MI. After post-MI recording, neurostimulator was turned on and programmed to 14-s ON (10 Hz, 500 μs pulse duration) and 66-s OFF. The output current was increased gradually from 0.5 mA to 3.5 mA in 2 weeks if there were no changes of appetite of behavior. After an additional 8 weeks of stimulation, the dogs were euthanized.
A second sterile left lateral thoracotomy was performed through the fifth intercostal space under isoflurane inhalation general anesthesia 4 weeks after the first surgery. A branch of left circumflex coronary artery was ligated by 6-0 polypropylene suture without reflow to create an acute MI. The radiotransmitter was turned on after two days of recovery to record post-MI ECG and nerve activity. At one week after MI, the neurostimulator was turned on and programmed to 14-s ON and 1.1-min OFF (10 Hz, 500 μs pulse duration) for ScNS.14 The initial output current (mA) was 0.5 mA. The strength of stimulation was progressively increased to 3.5 mA over 2 weeks during which the animals were observed for signs of discomfort or reduced appetite. We found that the dogs tolerated 3.5 mA stimulation without losing appetite or showing signs of discomfort. The average body weight increased from 25.8±1.1 Kg at baseline to 29.1±3.4 at the end of the study (p=0.025). The dogs were euthanized after 10 weeks of ScNS. The stellate ganglion and heart were harvested for immunohistochemical studies.
Controls
We2 have previously performed a study in ambulatory dogs using the same recording methods. The results showed continuous elevation of SGNA up to 2 months after MI. The data from those dogs were used for comparison. In addition, the tissues harvested from 6 normal dogs were also studied.
Immunohistochemical Studies
Triphenyltetrazolium chloride staining was performed in all hearts to determine the size of MI. Both stellate ganglion of all dogs were fixed in 4% formalin for 45-60 min, followed by storage in 70% alcohol for at least 48 hours.10 The tissues were processed routinely, paraffin embedded and cut into 5-μm thick sections. Immunohistochemical staining was performed with antibodies against tyrosine hydroxylase (TH) using mouse monoclonal anti-TH (Accurate Chemical, Westbury, NY) and antibody against GAP43 (Millipore Sigma, Burlington, MA). For comparison, we retrieved the paraffin-embedded stellate ganglion blocks from a prior study for the same immunohistochemical analyses.2 In that study, the dogs had MI but no ScNS. Slides were examined to determine if there were regions of remodeling, characterized by decreased ganglionic cell density, pyknotic cell bodies, decreased TH staining, increased fibrosis and hypereosinophilia on Masson’s trichrome staining. Digital photographs were taken from five roughly even spaced fields per slide with 20X objective. The mean percentage of TH-negative ganglion cells was calculated manually in both stellate ganglion using the same methods reported elsewhere.15 Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed to probe for cell death. A Leica Microsystems model TCS SP8 confocal microscope was used to detect TUNEL-positive cells. For quantitative analyses, we randomly selected 5 high power (40X objective) fields for image acquisition. We then manually counted the TUNEL-positive ganglion cells in each high power fields for analyses. Masson’s trichrome staining was performed in all stellate ganglion to show fibrosis remodeling. GAP43 staining was performed to document myocardial nerve sprouting.10
Data Analyses
The canine SGNA is highly sensitive to emotional and auditory stimulation. Because the human activities in the vivarium during weekdays are highly variable, we analyzed only the data recorded during the weekends. The electrical signals recorded during the stimulator OFF-time were manually analyzed using custom-written software to determine the temporal relationship between nerve activities and heart rate (HR) changes. Data from 3 recording electrodes were high-pass filtered at 150 Hz to obtain nerve activities (SGNA, VNA and LTNA). Nerve activities were quantified by integrating the absolute value of the filtered signal over 20-s windows. The integrated nerve activities were then divided by the total number of samples in each window (i.e., the product of sampling rate and 20) to calculate the average SGNA (aSGNA), VNA (aVNA) and LTNA (aLTNA). To quantify the hourly nerve activities over a 24 hour period, we selected for manual analyses 2-min of data at the beginning, 20 min, and 40 min past each hour during ScNS-OFF time. Stimulating artifacts or noises during that 2-min period were excluded from analyses. Nerve activity and HR were compared between baseline and different time points of the experiment. We also compared the number of paroxysmal atrial tachyarrhythmia (PAT) episodes over 24 hours between baseline and different time points of the experiment. PAT was defined as an abrupt (>50 bpm/s) onset or offset of the tachycardia with atrial rate reaching 200 bpm and persisted for at least 5 s.16
Statistical Analyses
The data were reported as mean ± Standard deviation (SD). Paired t test or signed-rank test were performed to compare the differences between HR, integrated nerve activities and the number of PAT episodes at different stages of experiments. Because paired t and signed rank reach similar p-values, only the paired t result is reported. The statistics were computed using the PASW Statistic (version 22; SPSS Inc, Chicago, IL). A two-sided p value of ≤0.05 was considered statistically significant.
Results
The effects of left lateral thoracic nerve stimulation
Effects on SGNA, VNA, LTNA and HR
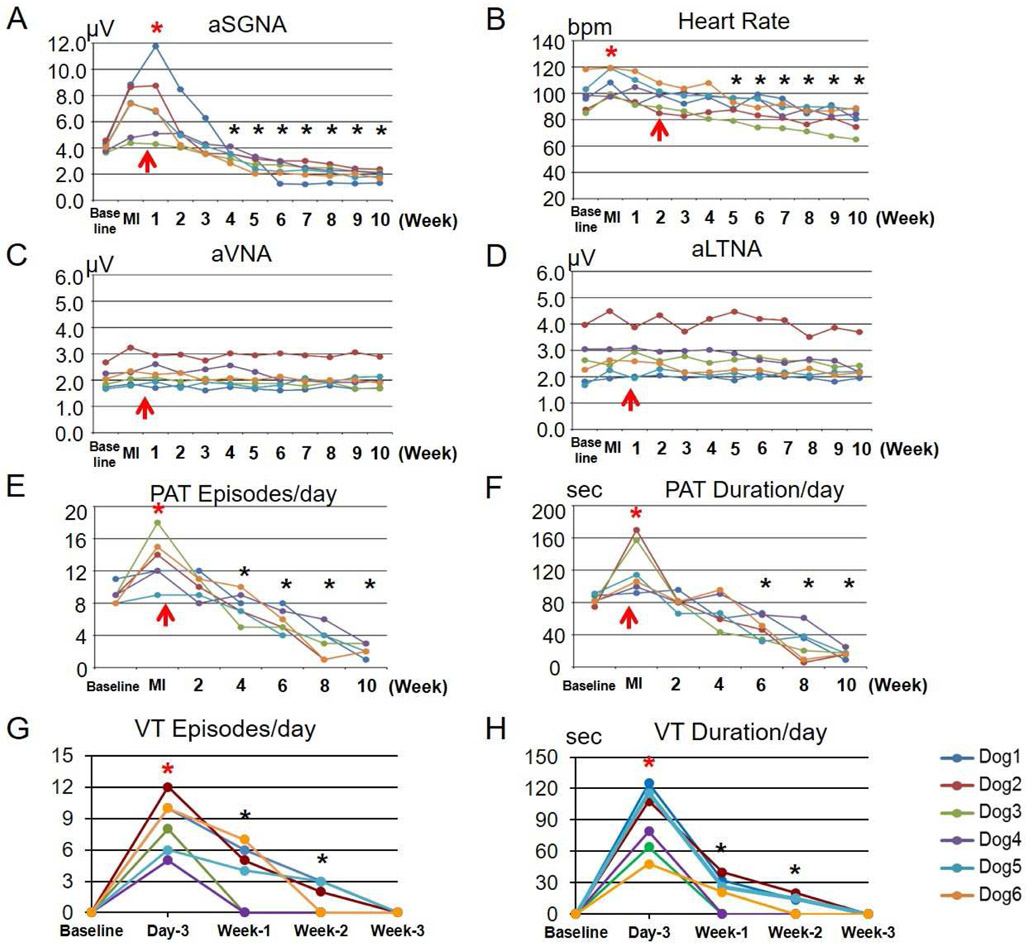
Figures 2A shows baseline respiratory HR response when there were nerve activities recorded. Figure 2B shows that co-firing of SGNA and LTNA is associated with HR elevation before MI. The infarct size ranges from 1-6% of all dogs studied. Premature ventricular contractions and VT were observed for an average of 9±4 days after MI in the experimental group (Figure 2C and 2D). After the commencement of ScNS, SGNA gradually reduced. Bradycardia were often observed during the stimulation ON-time along with elevated VNA and reduced HR variability (Figure 3). There were no significant changes of T wave morphologies. Weekly aSGNA analyses showed that aSGNA increased to 6.91 ±1.91 μV 3 days after MI, significantly higher than baseline (4.08±0.35 μV, p=0.007). After the commencement of ScNS, aSGNA was significantly reduced to 3.46±0.44 μV (p=0.039) and 2.14±0.50 μV (p=0.001) 4 and 8 weeks after MI, respectively. In the final week of study, the aSGNA was further reduced to 1.89±0.37 μV (p<0.001 compared to baseline) (Figure 4A). The mean HR was 98±12 bpm at baseline and 107±10 bpm after acute MI (p=0.028). HR significantly decreased to 94±10 bpm (p=0.084) and 83±7 bpm (p=0.006) at 4 and 8 weeks, respectively and persistently reduced afterwards. At the final week of ScNS, HR decreased further to 80±9 bpm (p<0.001) (Figure 4B). The aVNA (Figure 4C) and aLTNA (Figure 4D) did not change significantly during the study. A previous study in our laboratory followed the dogs with MI for two months without ScNS.2 In those dogs, we reported the continued elevation of aSGNA after MI for up to 8 weeks. Compared with the ScNS group, the aSGNA in the control group at 4 weeks and at 8 weeks were 8.26±6.31 μV (p=0,024) and 10.82±7.86 μV (p=0.003), respectively. Figure 4E shows episodes of VT increased significantly after MI, but reduced afterwards.
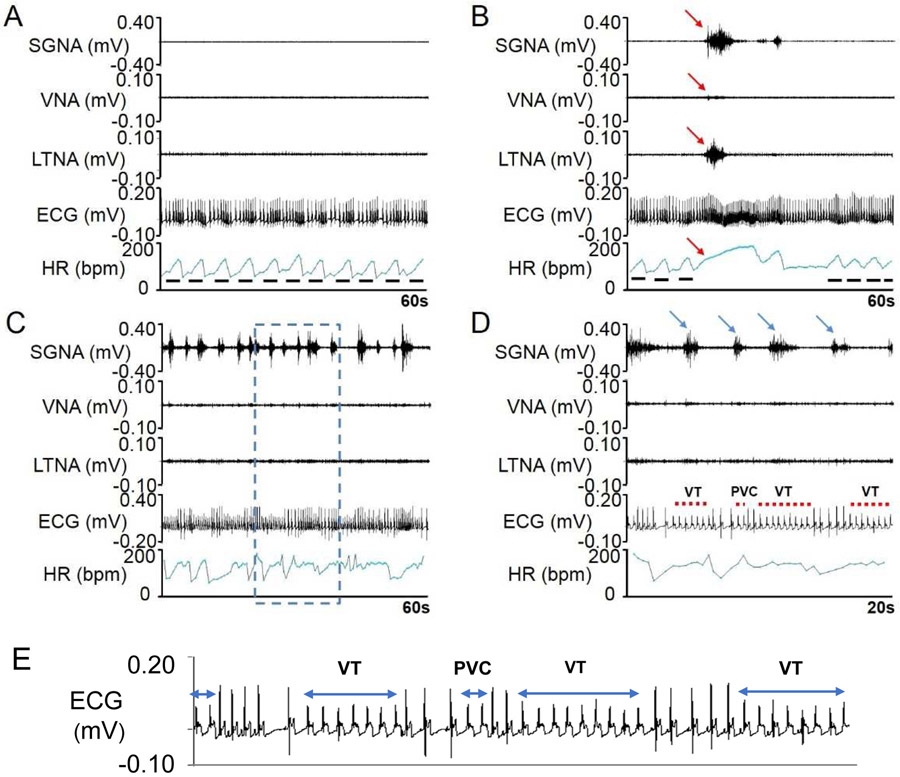
Figure 2.
Typical examples of nerve activity and HR before and after MI. Before MI, the nerve activities are often quiescent (A) with irregular HR due to respiratory HR responses (solid line segments). When SGNA and LTNA co-firing occurred (B), the HR is elevated and the respiratory HR responses are transiently suppressed (red arrows). C shows bursts of SGNA 3 days after acute MI. These bursts were associated with short episodes of ventricular tachyarrhythmias. D shows zoom-in of blue dotted box in panel C. The highly active SGNA was associated with premature ventricular contractions and nonsustained VT (red dots). E shows further enlarged ECG of Panel D.
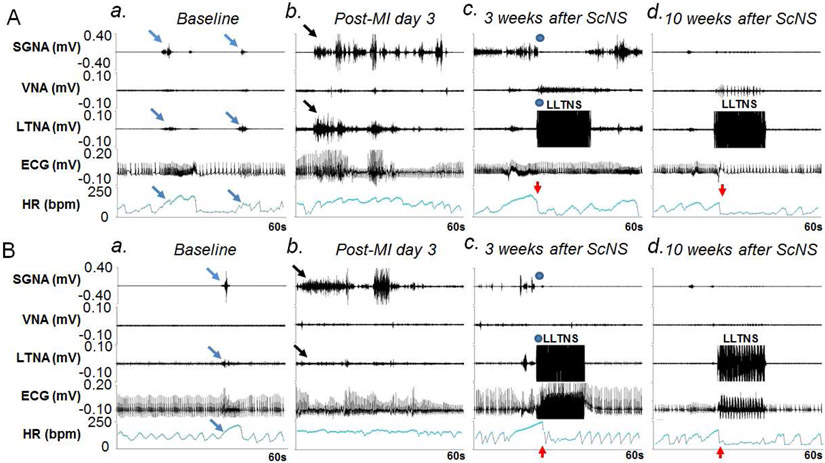
Figure 3.
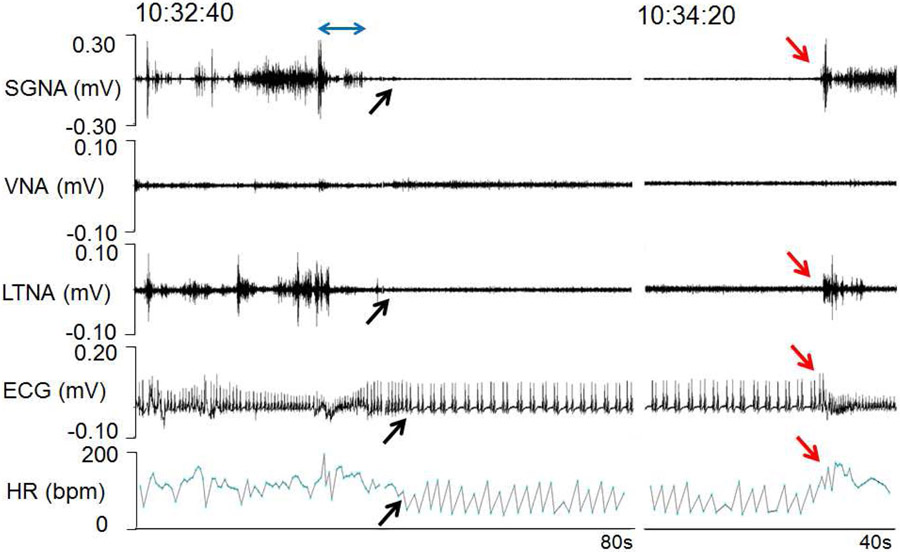
Effects of ScNS on SGNA, VNA, LTNA and HR. Panels A and B are from two different dogs. Both dogs showed similar patterns of the nerve activity. a: Baseline. Brief nerve activities are associated with HR elevation (arrows). b: Nerve activities (black arrows) significantly increased post-MI, associated with high HR. c: There was an abrupt (blue dots) reduction of SGNA and HR (red arrows) during ScNS ON-time at 3 weeks after the commencement of 3.5-mA ScNS. In Panel Ac but not in Bc was there elevation of VNA during and return of SGNA immediately at the cessation of ScNS. Note that there was increased VNA during the stimulation, along with abrupt reduction of HR (red arrows). d: After 10 weeks of 3.5-mA ScNS, SGNA reduced significantly compared to post-MI period. Onset of ScNS (red arrows) abruptly reduced SGNA, HR and respiratory HR responses. Bradycardia was observed during ScNS ON-time. (ScNS= Left lateral thoracic nerve stimulation, SGNA= stellate ganglion nerve activity, VNA= vagal nerve activity, LTNA= lateral thoracic nerve activity, MI= myocardial infarction).
Figure 4.
Changes of nerve activities, HR and VT episodes in MI dogs with ScNS. A: aSGNA increased after MI and kept increasing after the first week of ScNS (upward red arrow), and then reduced gradually. The reduction was statistically significant compared to baseline (asterisks) after 4 or more weeks of ScNS. Note that the strength of ScNS reached 3.5 mA at 2 weeks after commencement of ScNS. B: Heart rate slightly increased after MI and gradually reduced after ScNS onset (upward red arrow). The HR reduction was statistically significant compared to baseline at or after 5 weeks of ScNS (3 weeks of 3.5-mA ScNS). C and D: Average VNA (aVNA) and average LTNA (aLTNA) did not change significantly by ScNS. Episodes of VT reduced significantly after 4 weeks of stimulation (E), along with a reduction of the total duration of VT (sec/day) (F). The VT episodes (G) and duration (H) increased after MI and then gradually reduced afterwards. The time course of PAT and VT reduction corresponds to the changes of aSKNA (A).
Effects on arrhythmias
Figure 4E and 4F show the PAT episodes at baseline averaged 9±1 episodes per day, which was reduced to 6±2 (p=0.010) after 6 weeks of ScNS, along with a reduction of total duration from 83.21±5.85 s/day to 49.06±13.50 s/day (p=0.004). During the final week of the study, the PAT was reduced to 2±1 (p<0.001) episodes per day, with the total duration of 16.71±5.07 s/day (p<0.001). Figure 4G and 4H show the VT episodes increased to 8±3/day 3 days after MI and decreased to 4±3 after 1 weeks of ScNS along with a reduction of the total duration of VT (90±31 sec/day to 20±17 sec/day). Pearson correlation coefficients between daily aSGNA and VT episodes was 0.669 (p=0.146), between aSGNA and VT duration was 0.824 (p=0.044). However, because no ventricular arrhythmias were detected after the immediate post-MI period in the control group either, we cannot conclude that ScNS reduces post-MI ventricular arrhythmias.
The effects of dexmedetomidine on nerve activity
Dexmedetomidine suppressed the SGNA and LTNA, associated with HR reduction (black arrows, Figure 5). These responses to dexmedetomidine indicate that the high frequency signals recorded by SGNA and LTNA channels include sympathetic nerve activities. For all 4 dogs studied, dexmedetomidine reduced the HR from 131±31 to 59±3 bpm (p<0.001), the aSGNA from 7.33±3.82 to 1.33±0.17 μV (p<0.001) and the aLTNA from 6.12±3.55 to 2.76±0.76 μV (p=0.008). There was also a small but significant reduction of VNA from 1.81±1.04 to 1.05±0.08 μV (p=0.031) after dexmedetomidine injection.
Figure 5.
Effects of dexmedetomidine on nerve activity. The dogs are generally excited when investigators are present, resulting in continuous stellate ganglion nerve activity (SGNA) and left thoracic nerve activity (LTNA). A double headed arrow marks the time of low dose dexmedetomidine (2.5 μg/kg) injection through the antecubital vein. The dog was conscious and the injection was associated with transient elevation of SGNA and HR. SGNA and LTNA were then suppressed by the drug (black arrows). There were reduced HR and atrial bigeminy. SGNA, LTNA and HR recovered in about 2 min (red arrows) after injection. These responses to dexmedetomidine are consistent with sympathetic suppression.
ScNS remodels both stellate ganglia
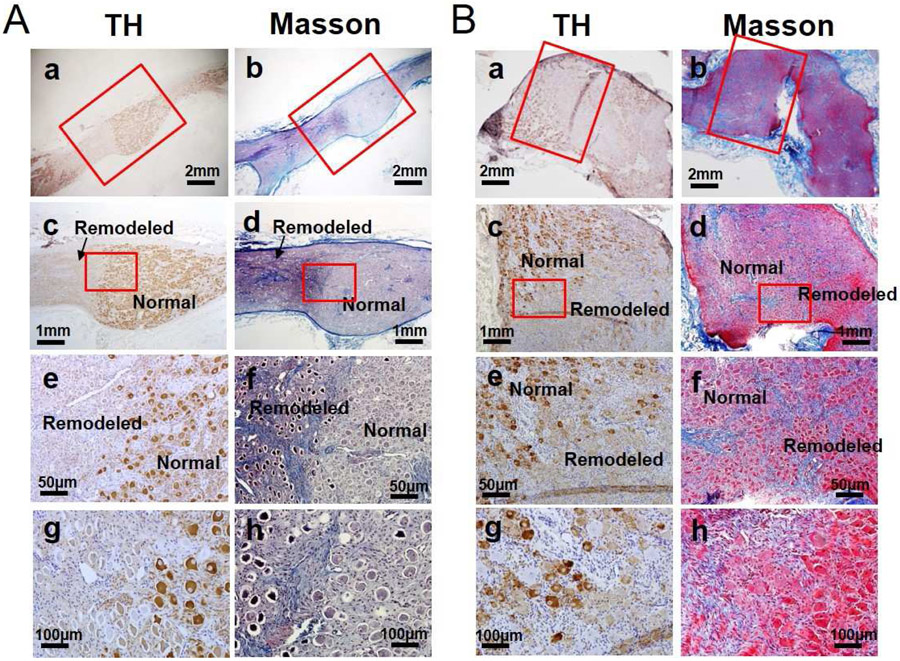
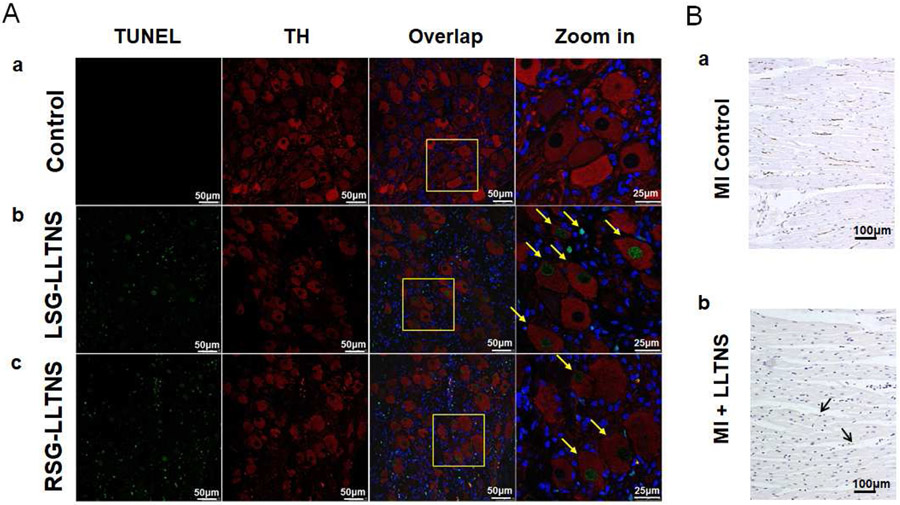
All left stellate ganglion (LSG) and four right stellate ganglion (RSG) were successfully harvested for analyses. There were large areas of remodeling, characterized by reduced or negative TH staining. Masson’s trichrome staining showed hypereosinophilia, pyknotic nuclei and shrinkage of cytoplasm in all LSG and RSG studied (Figure 6). These remodeled regions could be either confluent or multifocal. Within the remodeled region, the percentage of TH-negative ganglion cells was 18.04±5.13% in LSG and 15.92±3.99% in RSG, both significantly higher than that of the normal regions (9.50±2.11%, p=0.004, and 9.48±3.04%, p=0.011, respectively). The overall mean percentage of the TH negative ganglion cell was 15.59±2.72% in LSG and 12.70±3.44% in RSG (p=0.001). Tissue sections from the same specimens were then double stained for TH and TUNEL. Normal SG stained negative for TUNEL (Figure 7A-a). The ganglion cells in normal region of the ScNS dogs mostly stained positive for TH (Red) and negative for TUNEL. Abundant TUNEL positive ganglion cells (green) could be found in the remodeled regions of both specimens. In addition, non-ganglion cells were also found to be TUNEL positive in the same region (Figure 7A-b, 7A-c). The mean percentage of TUNEL-positive ganglion cells was 26.61±9.99% in LSG and 15.94±3.13% in RSG. GAP43 staining showed the density of GAP43-positive fibers was 500±200 μm2/mm2 in LV (Figure 7B), significantly lower than that reported for MI dogs without ScNS (4,761 ± 1,523 μm2/mm2, p<0.001).2
Figure 6.
Typical examples of tyrosine hydroxylase (TH) staining and Masson’s trichrome staining in LSG (A) and RSG (B) after ScNS. Low power view shows the presence of both remodeled region and normal region in the same LSG (a) and RSG (b). High power view of the normal regions show normal morphology and rare TH-negative cells (c & d). In comparison, the ganglion cells in the remodeled region stained lightly with TH, had reduced cytoplasm and pyknotic nuclei (e) and (f). Many cells stained negatively or weakly for TH. Masson’s trichrome staining (g and h) showed increased fibrosis in the remodeled region (blue). (LSG = left stellate ganglion, RSG = right stellate ganglion, ScNS = left lateral thoracic nerve stimulation).
Figure 7.
Effects of ScNS on stellate ganglion and cardiac nerves. A: Confocal microscope images of immunofluorescent Tyrosine hydroxylase (TH, red) and TUNEL (green) double staining in a dog with ScNS. Blue is the DAPI stain of the nuclei. a: Normal stellate ganglion showed no TUNEL positive staining. b and c: Left and right stellate ganglions after ScNS, respectively. Arrows point to ganglion cells that stained positive for TUNEL. Those cells could be either TH-positive or TH-negative (Calibration bar=50 μm). B: Immunostaining of the left ventricle. a. Cardiac nerve sprouting (brown GAP43-positive nerve twigs) are abundantly present in a dog with MI but no ScNS from a previous study.2 In comparison, GAP43-positive nerve fibers (arrows) are rare in the hearts from the present study (b). LLTNS = subcutaneous nerve stimulation using left lateral thoracic nerve. Calibration bar=100 μm.
Discussion
The primary finding of this study is that ScNS can remodel the stellate ganglion, reduce SGNA and prevent myocardial nerve sprouting/sympathetic hyperinnervation in a canine model of acute MI.
Sympathetic hyperinnervation after MI
Heterogeneous cardiac nerve sprouting and sympathetic hyperinnervation play important roles in arrhythmogenesis and sudden cardiac death after MI.10, 17 The neural remodeling is not limited to the heart, but extend to extracardiac nerve structures such as the stellate ganglion.2, 18 Selective left cardiac sympathetic denervation is effective in reducing the risk of sudden cardiac death after MI.3 Further studies are warranted to determine if ScNS can also be used for sudden death prevention after MI.
Autonomic nerve activity in ambulatory dogs
Spontaneous SGNA can be recorded using chronically implanted electrodes in ambulatory dogs.19 The magnitudes of SGNA are significantly increased in heart failure and after MI.2, 11 Cryoablation of the stellate ganglia reduces SGNA and helps control atrial fibrillation.20 In the present study, we further validated the methods of nerve recording by dexmedetomidine injection. Because the electrodes have been implanted for at least two weeks prior to injection, the sudden loss of high frequency signals is unlikely to be caused by electrode movement. Rather, the best explanation is that those high frequency signals were nerve activities. We recently compared the effects of dexmedetomidine with other anesthetic agents on SGNA in canine models. Among several drugs tested, dexmedetomidine had the largest effects on SGNA suppression. In addition to suppressing the SGNA and LTNA, we also showed that there is a small but significant reduction of VNA. The vagal nerves in both dogs and humans contain 1-5% of the sympathetic nerve fibers in the cross sectional area.21, 22. While rare, VNA activity could be associated with heart rate elevation.21 It is possible that dexmedetomidine reduced VNA through reducing the sympathetic nerve activity inside that nerve.
Bilateral stellate ganglion remodeling
We showed in this and other studies that ScNS on the left side causes remodeling both stellate ganglia.14, 23 Direct ambulatory recordings show that the left and right stellate ganglia in dogs usually activate together.19 Electrical stimulation of one stellate ganglion can activate the contralateral stellate ganglion.24 Because of the connections between the two stellate ganglia, ScNS may indirectly excite the contralateral stellate ganglia to cause remodeling. An alternative possibility is that the stimulating site receive innervation from both stellate ganglia. However, that hypothesis is not supported by previous tracer studies.9
Clinical implications
Increased sympathetic tone after MI may provoke ventricular arrhythmia both in the animal models and human patients, leading to increased risk of sudden cardiac death. While left cardiac sympathetic denervation and vagal nerve stimulation both can potentially be used to reduce the incidence of sudden death after MI,3, 4 the surgical complexity and potential complications prevented their wide spread use in clinical practice. In comparison, the nerves under the skin are easily accessible. It is possible that future clinical trials can be performed to determine the effects of ScNS on cardiac arrhythmia control in patients with MI.
Limitations of the study
We do not have long term follow up to document the persistent efficacy of ScNS in suppressing sympathetic discharges. It is also unclear if stellate ganglion remodeling is reversible after stopping stimulation. It is still unclear how long will take ScNS to work and what current output is necessary to achieve antiarrhythmic effects. Due to the complexity and severity of the canine model, we did not include a contemporary control group. Rather, we used the increased SGNA of a previously published study as control.2 In addition to that study, others have also demonstrated that MI is followed by sustained increase of sympathetic nerve activity.25, 26 Based on those studies as well as the histological findings of stellate ganglion remodeling, we propose that the intervention (ScNS) is responsible for reduced SGNA observed in the present study. Dogs with MI without nerve growth factor infusion to the left stellate ganglion do not have ventricular arrhythmias after the immediate post-MI period.10 Therefore, we were not able to directly determine the effects of ScNS on ventricular arrhythmias or sudden cardiac death.
Acknowledgement
We thank Nicole Courtney, Christopher Corr and David Adams for their assistance. We also thank Bruce KenKnight, Jason Begnaud, and Imad Libbus of Cyberonics Inc for donating research equipment used in this study.
Sources of funding
National Institutes of Health Grants R42DA043391, R56HL71140, TR002208-01, R01 HL139829, American Heart Association Grant #16POST30760002 and #18TPA34170284/ZC/2018a, a Medtronic-Zipes Endowment of the Indiana University and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhou S, Chen LS, Miyauchi Y, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. [DOI] [PubMed] [Google Scholar]
- 2.Han S, Kobayashi K, Joung B, et al. Electroanatomic remodeling of the left stellate ganglion after myocardial infarction. J Am Coll Cardiol. 2012;59:954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Motolese M, Pollavini G, et al. Prevention of sudden cardiac death after a first myocardial infarction by pharmacologic or surgical antiadrenergic interventions. J Cardiovasc Electrophysiol. 1992;3:2–16. [Google Scholar]
- 4.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ. Res 1991;68:1471–1481. [DOI] [PubMed] [Google Scholar]
- 5.Chinda K, Tsai WC, Chan YH, et al. Intermittent Left Cervical Vagal Nerve Stimulation Damages the Stellate Ganglia and Reduces Ventricular Rate During Sustained Atrial Fibrillation in Ambulatory Dogs. Heart Rhythm. 2016;13:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123:2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Y, Hassel JL, Doytchinova A, et al. Left cervical vagal nerve stimulation reduces skin sympathetic nerve activity in patients with drug resistant epilepsy. Heart Rhythm. 2017;12:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson EA, Rhee KS, Doytchinova A, et al. Estimating sympathetic tone by recording subcutaneous nerve activity in ambulatory dogs. J Cardiovasc Electrophysiol. 2015;26:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniguchi T, Morimoto M, Taniguchi Y, Takasaki M, Totoki T Cutaneous distribution of sympathetic postganglionic fibers from stellate ganglion: A retrograde axonal tracing study using wheat germ agglutinin conjugated with horseradish peroxidase. J Anesth. 1994;8:441–449. [DOI] [PubMed] [Google Scholar]
- 10.Cao JM, Chen LS, KenKnight BH, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–821. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. [DOI] [PubMed] [Google Scholar]
- 12.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–268; discussion 269-270. [DOI] [PubMed] [Google Scholar]
- 13.Kontak AC, Victor RG, Vongpatanasin W. Dexmedetomidine as a novel countermeasure for cocaine-induced central sympathoexcitation in cocaine-addicted humans. Hypertension. 2013;61:388–394. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Y, Jiang Z, Zhao Y, et al. Long-term intermittent high-amplitude subcutaneous nerve stimulation reduces sympathetic tone in ambulatory dogs. Heart Rhythm. 2018;15:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen MJ, Hao-Che Chang X, Park HW, et al. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm. 2013;10:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi EK, Shen MJ, Han S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao JM, Fishbein MC, Han JB, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–1969. [DOI] [PubMed] [Google Scholar]
- 18.Ajijola OA, Yagishita D, Reddy NK, et al. Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: Neuropeptide and morphologic changes. Heart Rhythm. 2015;12:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung BC, Dave AS, Tan AY, et al. Circadian variations of stellate ganglion nerve activity in ambulatory dogs. Heart Rhythm. 2006;3:78–85. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara K, Tanaka H, Mani H, Nakagami T, Takamatsu T Burst Emergence of Intracellular Ca2+ Waves Evokes Arrhythmogenic Oscillatory Depolarization via the Na+− Ca2+ Exchanger Simultaneous Confocal Recording of Membrane Potential and Intracellular Ca2+ in the Heart. Circ.Res. 2008;103:509–518. [DOI] [PubMed] [Google Scholar]
- 21.Onkka P, Maskoun W, Rhee KS, et al. Sympathetic nerve fibers and ganglia in canine cervical vagus nerves: Localization and quantitation. Heart Rhythm. 2013;10:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seki A, Green HR, Lee TD, et al. Sympathetic nerve fibers in human cervical and thoracic vagus nerves. Heart Rhythm. 2014;11:1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan J, Chen M, Yuan Y, et al. Antiarrhythmic and proarrhythmic effects of subcutaneous nerve stimulation in ambulatory dogs. Heart Rhythm. 2019;16:1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan AY, Zhou S, Jung BC, et al. Ectopic atrial arrhythmias arising from canine thoracic veins during in vivo stellate ganglia stimulation. Am J Physiol Heart Circ Physiol. 2008;295:H691–H698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jardine DL, Charles CJ, Ashton RK, et al. Increased cardiac sympathetic nerve activity following acute myocardial infarction in a sheep model. J.Physiol 2005;565:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham LN, Smith PA, Stoker JB, Mackintosh AF, Mary DA. Time course of sympathetic neural hyperactivity after uncomplicated acute myocardial infarction. Circulation. 2002;106:793–797. [DOI] [PubMed] [Google Scholar]