Abstract
Objective:
Given significant focus on improving survival for “high-risk” congenital diaphragmatic hernia (CDH), there is the potential to overlook the need to identify risk factors for suboptimal outcomes in “low-risk” CDH cases. We hypothesized that early cardiac dysfunction or severe pulmonary hypertension (PH) were predictors of adverse outcomes in this “low-risk” CDH population.
Design:
This is a retrospective cohort study using data from the CDH Study Group (CDHSG) registry. “Low-risk” CDH was defined as CDHSG defect size A/B without structural cardiac and chromosomal anomalies. Examined risk factors included left ventricular dysfunction (LVD), right ventricular dysfunction (RVD), and severe PH on the first postnatal echocardiogram. The primary outcome was composite adverse events, defined as either death, extracorporeal membrane oxygenation (ECMO) utilization, oxygen requirement on day 30 of life, or hospitalization ≥ 8 weeks. Multivariable adjustment was performed with logistic regression and inverse probability weighting.
Setting:
Neonatal index hospitalization for CDH
Patients:
“Low-risk” CDH infants born between January 2015 and December 2018
Interventions:
First postnatal echocardiogram performed within 24 hours from birth
Measurements and Main Results:
778 patients were identified as “low-risk” CDH. LVD, RVD, and severe PH were present in 10.8%, 20.5%, and 57.5%, respectively. The primary outcome occurred in 21.3%. Death occurred in 3.0% and 9.1% utilized ECMO. On unadjusted analysis, all 3 risk factors were associated with the primary outcome. On all multivariable adjustment methods, LVD and severe PH remained significant predictors of adverse outcomes while RVD no longer demonstrated any effect.
Conclusions:
Early left ventricular dysfunction and severe pulmonary hypertension are independent predictors of adverse outcomes among “low-risk” CDH infants. Early recognition may lead to interventions that can improve outcome in this at-risk cohort.
Keywords: Congenital Diaphragmatic Hernia, Ventricular Dysfunction, Pulmonary Hypertension, Extracorporeal Membrane Oxygenation
Introduction:
Congenital diaphragmatic hernia (CDH) is a complex disease that affects 1 in every 2000–3000 live births per year (1) and remains one of the most costly and challenging pediatric surgical conditions (2–4). Collaborative efforts have led to a better understanding of the disease and risk stratification that could guide management and prognostication (5), one of which was the development of the CDH Study Group (CDHSG) staging system (6). According to this staging system, CDH severity was graded based on the size of the diaphragmatic defect, which ranges from “A” (small defects mostly surrounded by diaphragmatic muscle) to “D” (agenesis or near complete absence of the diaphragm) (6). While much effort and attention has been paid to optimizing the outcomes in patients with severe CDH, such as those with defect size C and D on the CDHSG Staging System, there is a potential risk of overlooking those with “low-risk” CDH. Identifying risk factors for adverse events in infants with favorable CDH characteristics can both improve clinical outcome and provide further insight into the pathophysiology of this disease.
While pulmonary hypoplasia and pulmonary hypertension have long been considered the central features of CDH (7), cardiac dysfunction has recently emerged as another important marker of disease severity and outcome prediction (8). Evidence of cardiac impairment and adaptation in the setting of CDH has been observed on pathological examination of both animal and human tissues (9, 10). Early assessment of postnatal cardiac function has been proposed as a valuable adjunct to disease risk stratification and an important tool to guide targeted therapy (11). We hypothesized that cardiac dysfunction diagnosed on early postnatal echocardiogram was an important risk factor for adverse outcomes in infants with “low-risk” CDH, defined in this study as having an A or B CDHSG defect without major structural cardiac disease or a chromosomal abnormality.
In this study, we investigated the effect of abnormal findings on early postnatal echocardiograms on outcomes in “low-risk” CDH. The independent effect of left ventricular dysfunction (LVD), right ventricular dysfunction (RVD), and severe pulmonary hypertension (PH) on adverse outcomes was examined with different statistical methods to adjust for confounders, including traditional multivariable regression as well as inverse probability weight.
Materials and Methods:
Inclusion/Exclusion Criteria:
This study included patients in the CDHSG registry who were born between January 2015 – December 2018. CDHSG data collection forms have evolved over 4 versions to collect data intended to answer specific and clinically relevant questions, and 2015 was the first year when assessment of cardiac function on postnatal echocardiograms was recorded (version 4). Only patients with defect size A and B were included. Exclusion criteria were chromosomal and major structural cardiac anomalies, which included cyanotic congenital heart defects and diagnoses such as hypoplastic left heart syndrome, coarctation of the aorta, and double outlet right ventricle. Patients were followed from the time of birth until discharge from the index hospitalization. The study was approved by the Institutional Review Board at Boston Children’s Hospital (#M05–10-244) and the University of Texas, Houston (#HSC-MS-03–223).
Exposures and Endpoints:
The three primary exposures of this study were LVD, RVD, and severe PH diagnosed on the first postnatal echocardiogram, performed within 24 hours from birth. LVD and RVD were dichotomous variables and qualitatively determined by an experienced echocardiographer (cardiologists, accredited echocardiographers or neonatologists with expertise in functional echocardiography) at each institution, using combined qualitative and quantitative measures in accordance with recommended practice (12). Cardiac dysfunction included both systolic and diastolic dysfunction. Severe PH was defined as peak pulmonary artery pressure of 2/3 systemic systolic pressure or greater (13).
The primary endpoint was defined as meeting one of the following outcomes: death, extracorporeal membrane oxygenation (ECMO) utilization, oxygen requirement at 30 days of age, or hospitalization duration of at least 8 weeks. An 8-week duration for hospitalization was included as an adverse outcome because this far exceeded the median length of stay in this cohort (26 days; interquartile range: 17–43 days). Secondary endpoints were death and ECMO utilization. All endpoints had to occur after the first postnatal echocardiogram.
Baseline Variables:
Information collected at birth included sex, race, inborn status, estimated gestational age (EGA), birth weight (BW), and Apgar score. Disease-specific variables included prenatal diagnosis of CDH, defect side, and liver position. Variables that pertained to postnatal management were comprised of cardiopulmonary resuscitation after birth, surfactant administration, usage of patch for surgical correction, thoracoscopic repair, and need for pulmonary vasodilators (i.e. inhaled nitric oxide (iNO), sildenafil, prostacyclin, alprostadil, milrinone, and endothelial receptor blockade among others). Center-specific information included patient volume status. High-volume was defined as ≥ 10 patients per year.
Statistical Analysis:
The effects of primary exposures on outcomes were examined with multivariable regression and inverse probability weighting.
Multivariable Regression:
Variables of interest included LVD, RVD, PH, sex, EGA, BW, inborn status, Apgar score at 5 minutes (Apgar5), prenatal diagnosis, hernia side, liver position, and center volume status. EGA, BW, and Apgar5 were dichotomized at 37 weeks, 3 kg, and 8, respectively. Selection of variables into the multivariable regression model started with univariable screen of the variables of interest. Variables that achieved a significance level of 0.2 were selected; LVD, RVD, and PH were forced to stay in the model regardless of their significance level. Subsequently, a backward selection with a significance level threshold of 0.1 was used to identify the next set of significant variables. Variables with significance level of > 0.2 on the univariable screen were then added back to the model through a forward selection with a significance level threshold of 0.05. Final “pruning” of the model was performed with a stepwise selection process. Due to a lower number of endpoints, multivariable regression was not performed for secondary outcomes due to the concern for overfitting.
Inverse Probability Weighting:
First, a multivariable logistic regression model was fitted to predict the probability of having the exposure given other baseline variables, also known as propensity score (PS). Baseline variables used in the PS model included sex, EGA, BW, inborn status, Apgar5, prenatal diagnosis, hernia side, liver position, and center volume status. In addition, the model also adjusted for confounding from other echocardiographic findings. For example, the PS model for LVD also included RVD and PH as covariates. A PS model was separately fitted for each of the three primary exposures. This PS was subsequently used to derive the inverse probability weight (IPW). As an example, for patients with LVD:
where Pr (LVD) is the proportion of patients with LVD. For those who did not have LVD:
where Pr (No LVD) is the proportion of patients without LVD. These weights effectively created a pseudo-population where accounted confounding variables were balanced between those with and without the exposure. The IPW was subsequently used in logistic regression models to estimate the adjusted effect of each exposure on outcomes. This statistical approach allowed for analyses of both primary and secondary outcomes.
Sensitivity Analysis:
The effect of early echocardiographic findings on outcomes was re-examined in a different cohort of “low-risk” CDH patients. In this sensitivity analysis, “low-risk” was defined as a score of 2 or lower according to the CDH mortality risk model by Brindle et al (14). This model used a combination of birth weight, Apgar5, pulmonary hypertension status, major cardiac anomaly, and chromosomal anomaly to risk stratify infants with CDH without the use of defect size, which could only be obtained at the time of surgery. IPW was again used to determine the adjusted effect of primary exposures on outcomes.
All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC). Throughout the study, a two-sided alpha level of 0.05 was accepted as the threshold for statistical significance.
Results:
There were 2095 infants in the CDHSG registry who were born during the study period. 778 met the inclusion/exclusion criteria. Of these patients, 758 patients had measurement of ventricular function or pulmonary pressure on the first postnatal echocardiogram (Figure 1). The majority of the infants in the study were male, white, and treated at a high-volume center (Table 1). Almost 90% had a left-sided hernia. CDH was diagnosed prenatally in 62.4% of the patients and 23.8% had liver herniation into the thoracic cavity. Pulmonary vasodilators were utilized in almost half of the patients. LVD was diagnosed on the first postnatal echocardiogram in 10.8% of patients, RVD in 20.5%, and severe PH in 57.5%.
Figure 1. Study Cohort.
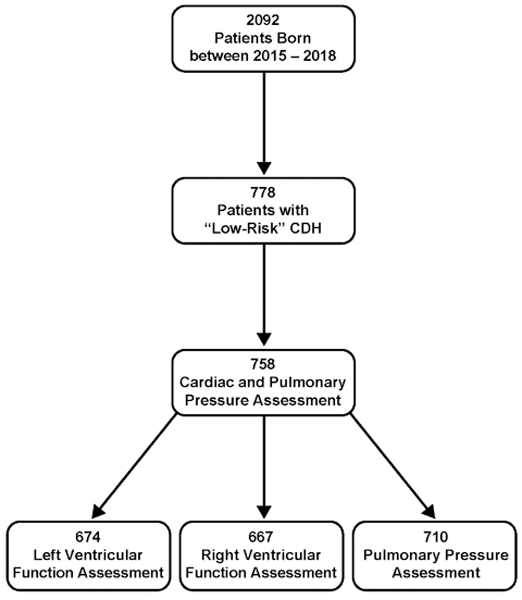
Flow chart of the study population as derived from the CDH Study Group registry.
Table 1. Baseline Characteristics of the Study Population.
Demographic and perinatal characteristics as well as findings on the first postnatal echocardiogram were described for the entire cohort. Dichotomous variables are expressed as number and percentage. Continuous variables are expressed as mean and standard deviation (SD). EGA: estimated gestational age; BW: birth weight; CPR: cardiopulmonary resuscitation; LVD: left ventricular dysfunction; RVD: right ventricular dysfunction; PH: pulmonary hypertension. Severe PH was defined as peak pulmonary pressure of at least 2/3 systemic systolic pressure.
| Variable | N (%) or mean (SD) |
|---|---|
| Demographic and Perinatal Characteristics | |
| Male | 440 (58.2%) |
| White race | 478 (65.0%) |
| High-volume center | 486 (64.1%) |
| Inborn | 384 (50.7%) |
| EGA (wk) | 38.0 (1.9) |
| BW (kg) | 3.1 (0.5) |
| APGAR at 1 minute | 5.9 (2.4) |
| APGAR at 5 minutes | 7.5 (1.8) |
| Left-sided hernia | 674 (88.9%) |
| Prenatal diagnosis | 473 (62.4%) |
| Liver-up | 180 (23.8%) |
| CPR at birth | 47 (7.0%) |
| Surfactant | 45 (6.0%) |
| Patch repair | 163 (21.5%) |
| Thoracoscopic repair | 199 (26.3%) |
| Pulmonary vasodilator | 356 (47.0%) |
| First Postnatal Echocardiogram | |
| LVD | 73 (10.8%) |
| RVD | 137 (20.5%) |
| Severe PH | 408 (57.5%) |
The primary composite endpoint occurred in 21.3% of patients in the study. 3.0% of patients died before discharge and 9.1% utilized ECMO support. Among those who experienced the primary outcome, 85.8% had severe PH, 39.7% had RVD, and 26.4% had LVD. The unadjusted risks of experiencing composite adverse outcome, death, and ECMO utilization given different exposures are displayed in Table 2. Of note, when LVD, RVD, and severe PH were all present on the first postnatal echocardiogram, the risk of composite outcome, death, and ECMO utilization was 61.5% (95% confidence interval (CI): 46.3%, 76.8%), 7.7% (95% CI: 0.0%, 16.1%), and 35.9% (95% CI: 20.8%, 51.0%), respectively. In contrast, among infants who demonstrated none of the three exposures, only 6.5% (95% CI: 3.3%, 9.6%) experienced the composite adverse outcome; and the risk of death and ECMO utilization was negligible, 0.4% (95% CI: 0.0%, 1.3%) and 1.3% (95% CI: 0.0%, 2.8%), respectively.
Table 2. Unadjusted Risk of Outcomes with Exposures.
The unadjusted risks of primary and secondary outcomes were calculated in the presence of left ventricular dysfunction (LVD), right ventricular dysfunction (RVD), severe pulmonary hypertension (PH), all three exposures, or none of the exposures. Shown are risks and their 95% confidence interval. ECMO: extracorporeal membrane oxygenation.
| Exposure | Composite Outcome | Death | ECMO Utilization |
|---|---|---|---|
| LVD | |||
| Yes | 52.0% (40.6%, 63.5%) | 6.8% (1.0%, 12.6%) | 30.1% (19.6%, 40.7%) |
| No | 17.6% (14.6%, 20.7%) | 2.0% (0.9%, 3.1%) | 6.7% (4.7%, 8.6%) |
| RVD | |||
| Yes | 42.3% (34.1%, 50.6%) | 6.6% (2.4%, 10.7%) | 20.4% (13.7%, 27.2%) |
| No | 16.6% (13.4%, 19.8%) | 1.9% (0.7%, 3.0%) | 6.4% (4.3%, 8.5%) |
| Severe PH | |||
| Yes | 31.1% (26.6%, 35.6%) | 3.9% (2.0%, 5.8%) | 14.2% (10.8%, 17.6%) |
| No | 7.0% (4.1%, 9.8%) | 0.3% (0.0%, 1.0%) | 1.7% (0.2%, 3.1%) |
| LVD + RVD + Severe PH | |||
| All | 61.5% (46.3%, 76.8%) | 7.7% (0.0%, 16.1%) | 35.9% (20.8%, 51.0%) |
| None | 6.5% (3.3%, 9.6%) | 0.4% (0.0%, 1.3%) | 1.3% (0.0%, 2.8%) |
Identification of Independent Predictors of Primary Outcome:
On univariable logistic regression analyses, most tested baseline variables were associated with the primary endpoint, including LVD, RVD, and PH (Figure 2A). After variable selection with multivariable logistic regression, only LVD (P = 0.014), PH (P < 0.001), liver position (P < 0.001), Apgar5 (P < 0.001), EGA (P = 0.048), and prenatal diagnosis (P < 0.024) remained independent predictors of the primary outcome (Figure 2B). RVD was no longer a significant predictor (P = 0.062) despite being forced into the model.
Figure 2. Univariable and Multivariable Logistic Regression for the Primary Outcome.
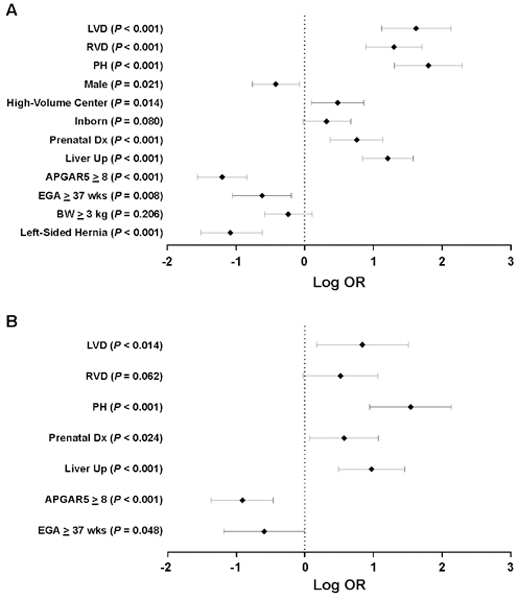
The association between different baseline variables and primary outcome was first tested with univariable (A), followed by multivariable logistic regression (B). Shown are logarithmic transformation of odds ratio (OR), their 95% confidence interval, and P values. LVD: left ventricular dysfunction; RVD: right ventricular dysfunction; PH: pulmonary hypertension; Apgar5: Apgar score at 5 minutes; EGA: estimated gestational age; BW: birth weight.
Effects of LVD on Outcomes:
There was a high degree of correlation between LVD and other abnormalities on echocardiography. Patients with LVD also had a significantly higher risk of RVD (71.2% vs 13.8%; P < 0.001) and severe PH (89.1% vs 55.3%; P < 0.001). Patients with LVD also utilized more pulmonary vasodilators (79.4% vs 43.6%; P < 0.001), likely a result of the association between LVD and PH.
On unadjusted analysis, LVD increased the odds of primary composite outcome 5-fold (odds ratio (OR): 5.06; 95% CI: 3.05, 8.38; P < 0.001). On multivariable regression, LVD still increased the odds of experiencing the primary outcome more than 2-fold (OR: 2.31; 95% CI: 1.18, 4.52; P = 0.014) (Table 3).
Table 3. Determination of the Effect of Each Exposure on Outcomes with Three Different Statistical Methods.
The adjusted effect of left ventricular dysfunction (LVD), right ventricular dysfunction (RVD), and severe pulmonary hypertension (PH) on both primary and secondary outcomes was determined with multivariable logistic regression and inverse probability weight. Shown are odds ratios (OR), their 95% confidence interval, and P values. ECMO: extracorporeal membrane oxygenation
| Exposure | Unadjusted Analysis | Multivariable Regression | Inverse Probability Weight | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |||||||||||
| LVD | |||||||||||||||||||
| Composite Outcome | 5.06 | (3.05, 8.38) | < 0.001 | 2.31 | (1.18, 4.52) | 0.01 | 2.26 | (1.08, 4.74) | 0.03 | ||||||||||
| Death | 3.60 | (1.23, 10.54) | 0.03 | -- | -- | -- | 0.61 | (0.15, 2.40) | 0.48 | ||||||||||
| ECMO Utilization | 5.06 | (3.05, 8.38) | < 0.001 | -- | -- | -- | 2.74 | (1.15, 6.52) | 0.02 | ||||||||||
| RVD | |||||||||||||||||||
| Composite Outcome | 3.68 | (2.44, 5.54) | < 0.001 | 1.68 | (0.97, 2.90) | 0.06 | 1.32 | (0.78, 2.24) | 0.31 | ||||||||||
| Death | 3.65 | (1.45, 9.17) | 0.007 | -- | -- | -- | 2.50 | (0.77, 8.13) | 0.13 | ||||||||||
| ECMO Utilization | 3.68 | (2.44, 5.54) | < 0.001 | -- | -- | -- | 1.59 | (0.79, 3.19) | 0.19 | ||||||||||
| Severe PH | |||||||||||||||||||
| Composite Outcome | 6.03 | (3.69, 9.84) | < 0.001 | 4.68 | (2.58, 8.48) | < 0.001 | 3.92 | (1.95, 7.87) | < 0.001 | ||||||||||
| Death | 12.24 | (1.61, 92.85) | 0.002 | -- | -- | -- | 9.08 | (1.16, 70.83) | 0.04 | ||||||||||
| ECMO Utilization | 6.03 | (3.69, 9.84) | < 0.001 | -- | -- | -- | 4.86 | (1.25, 18.90) | 0.02 | ||||||||||
After adjustment with IPW, LVD remained a significant risk factor for the primary outcome (OR: 2.26; 95% CI: 1.08, 4.74; P = 0.030) (Table 3). Again, LVD increased the odds of ECMO utilization (OR: 2.74; 95% CI: 1.15, 6.52; P = 0.022) but had no effect on mortality (Table 3).
Effects of RVD on Outcomes:
Again, there was a high degree of correlation among the positive findings on echocardiogram. Patients with RVD were more likely to also have LVD (39.4% vs 4.0%, P < 0.001) and PH (82.8% vs 52.1%, P < 0.001).
The presence of RVD on the first postnatal echocardiogram was a significant predictor of the primary outcome on unadjusted analysis (OR: 3.68; 95% CI: 2.44, 5.54; P < 0.001) (Table 3). Interestingly, when adjustment was performed with multivariable regression or IPW, RVD no longer displayed any effect on the primary or secondary outcomes (Table 3).
Effects of PH on Outcomes:
Patients with severe PH were more frequently treated with pulmonary vasodilators (63.2% vs 25.2%, P < 0.001). On echocardiogram study, they were also more likely to have LVD (15.4% vs 2.7%, P < 0.001) or RVD (29.2% vs 8.5%, P < 0.001).
On unadjusted analysis, severe PH similarly showed a strong association with the primary outcome (OR: 6.03; 95% CI: 3.69, 9.84; P < 0.001) (Table 3). On multivariable logistic regression, PH increased the odds of composite adverse outcome more than 4-fold (OR: 4.68; 95% CI: 2.58, 8.48; P < 0.001).
With IPW adjustment, PH remained a significant predictor of the primary outcome (OR: 3.92; 95% CI: 1.95, 7.87; P < 0.001) (Table 3). In addition, it also showed a significant effect on both secondary outcomes, death (OR: 9.08; 95% CI: 1.16, 70.83; P = 0.035) and ECMO utilization (OR: 4.86; 95% CI: 1.25, 18.90; P = 0.022). Of note, death only happened to one of 302 patients without PH (0.3%). This low number of outcome resulted in an unstable estimate of the association between PH and death, which was accompanied by a wide CI (Table 2).
Sensitivity Analysis:
When the CDH mortality risk model (14) was used for risk stratification, 1561 patients recorded a score of 2 or lower and met the criteria for “low-risk”. On unadjusted analysis, LVD, RVD, and severe PH were all associated with primary and secondary outcomes (Table 4). With IPW adjustment, there was a 2-fold increase in odds of primary composite outcome with both LVD (OR: 2.23; 95% CI: 1.33, 3.75; P = 0.002) and severe PH (OR: 2.23; 95% CI: 1.49, 3.35; P < 0.001) (Table 4). Although with a smaller magnitude, RVD also demonstrated an association with the primary outcome (OR: 1.41; 95% CI: 1.00, 1.99; P = 0.05) in this new “low-risk” cohort. As for the secondary outcomes, all 3 exposures were significantly associated with ECMO utilization, while none of them showed an effect on mortality (Table 4).
Table 4. Repeat Analysis Using a Different Definition of “Low-Risk”.
“Low-Risk” in this analysis was defined as a score of 2 or lower on the CDH mortality risk model (Brindle et al). The adjusted effect of left ventricular dysfunction (LVD), right ventricular dysfunction (RVD), and severe pulmonary hypertension (PH) on both primary and secondary outcomes was determined with inverse probability weight. Shown are odds ratios (OR), their 95% confidence interval, and P values. ECMO: extracorporeal membrane oxygenation.
| Exposure | Unadjusted Analysis | Inverse Probability Weight | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| LVD | ||||||
| Composite Outcome | 4.53 | (3.33, 6.16) | < 0.001 | 2.23 | (1.33, 3.75) | 0.002 |
| Death | 3.26 | (2.37, 4.48) | < 0.001 | 1.52 | (0.82, 2.84) | 0.19 |
| ECMO Utilization | 4.51 | (3.34, 6.09) | < 0.001 | 2.43 | (1.48, 3.97) | < 0.001 |
| RVD | ||||||
| Composite Outcome | 3.29 | (2.57, 4.21) | < 0.001 | 1.41 | (1.00, 1.99) | 0.05 |
| Death | 2.74 | (2.06, 3.64) | < 0.001 | 1.46 | (0.88, 2.42) | 0.14 |
| ECMO Utilization | 3.10 | (2.39, 4.03) | < 0.001 | 1.68 | (1.15, 2.45) | 0.007 |
| Severe PH | ||||||
| Composite Outcome | 3.83 | (2.90, 5.05) | < 0.001 | 2.23 | (1.49, 3.35) | < 0.001 |
| Death | 2.95 | (2.05, 4.25) | < 0.001 | 1.54 | (0.81, 2.92) | 0.18 |
| ECMO Utilization | 5.29 | (3.68, 7.62) | < 0.001 | 2.14 | (1.29, 3.54) | 0.003 |
Discussion:
In this study, we demonstrated the value of early postnatal echocardiography in the risk-stratification of “low-risk” CDH infants. Specifically, LVD and severe PH on the first postnatal echocardiogram were independent risk factors for adverse outcomes after adjustment for baseline confounders and associated echocardiogram findings. Interestingly, RVD showed no effect on outcomes after multivariable adjustments. These results suggested that even for CDH infants with favorable disease characteristics, pulmonary hypertension status and left ventricular function on the first postnatal echocardiogram are major determinants of clinical outcomes.
In recent years, LVD has been increasingly recognized as an important component of CDH management (15, 16). Evidence of disturbances in cardiac development can be seen in utero, although correlating indices of fetal echocardiography with clinical outcomes remains challenging and unreliable (17, 18). Postnatally, infants with CDH demonstrate biventricular dysfunction and impaired cardiac output compared to age-matched controls on early echocardiography (19). The presence of LVD, and resulting pulmonary venous hypertension, may be a major culprit behind treatment failure of pulmonary vasodilators, such as iNO (20–22). As such, early LVD has been shown in other studies to correlate with adverse outcomes, such as the utilization of ECMO support (23, 24). However, these studies were limited by small sample size and lack of control for confounding variables. Coupled with the observation that biventricular interactions may play a role in causing LVD (19), delineating the effects of different echocardiographic findings on clinical outcomes has therefore been challenging. With this study, we demonstrated for the first time that LVD, and not RVD, is an independent predictor of adverse outcomes, including the risk for ECMO utilization.
Perhaps one of the most surprising findings in this study was the lack of association between RVD and adverse outcomes after adjustment for baseline confounders, including PH and LVD. While this result seems contradictory with previous studies that correlated indices of RVD on early echocardiography with poor prognosis (25, 26), it should be noted that these studies were limited by small sample size and, more importantly, did not adjust for the effect of LVD. Interestingly, in another study that examined the myocardial performance and cardiac output index of both ventricles, only parameters pertaining to the left ventricle remained independent predictors of mortality on multivariable regression (27). Implicit in all of these studies is the interaction between the two ventricles, which probably plays a major role in the progression from RVD to LVD (28, 29). However, there is also evidence that LVD in CDH can be separate from RVD and a secondary result of direct compression from herniated abdominal organs (30). Regardless of the pathophysiology of LVD in this population of “low-risk” CDH, it appears that LVD, and not RVD, signifies more severe cardiac disturbance and portends much worse prognosis, likely due to systemic hypotension and poor organ perfusion (31).
In this study, we again observed an association between PH and clinical outcomes. Although the rate of PH in this cohort of “low-risk” CDH (57.5%) was less than the 70% rate reported in the general CDH population (22), the effect of PH on adverse outcomes remained strong and significant. However, there were several unique implications to be gleaned from the results of our study. In contrast to other studies that have linked persisted PH to poor clinical outcomes (32, 33), our results demonstrated that severe PH as early as the first 24 hours of life was an important prognostic indicator for “low-risk” CDH patients, a result that has been observed in the general CDH population (22). Most importantly, it should be noted that this association persisted even after adjustment for cardiac dysfunction. This suggested that in patients with less severe CDH, PH could also impact clinical outcomes through other extra-cardiac physiologic effects, such as hypoxic right-to-left shunting. However, as PH is known to continually evolve throughout the postnatal period (13, 32), the lack of serial pulmonary artery pressure measurements in this study certainly poses an important limitation.
The results of our study should be interpreted in the context of its design. As previously mentioned, one of the major limitations of this study was the lack of serial assessment of echocardiographic findings. While serial postnatal echocardiograms were captured in the CDHSG registry, a high degree of missing data on later studies limited our ability to carry out a longitudinal analysis. There is also potential variation in the qualitative classification of cardiac function between submitting centers. This reflects current clinical practice and international guidelines which recommend combined interpretation of qualitative and quantitative measures by an experienced echocardiographer. Submitting centers are known to use similar echocardiographic techniques; however, we are now working to create formal consensus to define standardized measures of cardiac function in CDH, which can be incorporated in future versions of the CDHSG Registry. Due to transitional changes in the circulation, variation in timing of echocardiograms may have also influenced results. The precise timing of echocardiograms within the first 24 hours of life was not recorded; however, most submitting centers reported that scans were performed after 4–6 hours of age, and therefore after the immediate transitional period. Within this cohort it was not possible to determine the potential effect of concurrent cardiovascular therapies. To minimize this possible confounding effect, analysis was limited to echocardiograms in the first 24 hours of life. Due to the nature of a multi-institutional registry, there was also a lack of uniformity in the type of echocardiography performed as this varied among different institutions. Furthermore, the registry did not distinguish between diastolic and systolic LVD, which could provide more insight into the pathophysiology of cardiac dysfunction in this population. Finally, due to the retrospective nature of the study and limitation in the number of baseline variables that could be reliably captured in the registry, there was a potential for residual and unmeasured confounders.
Conclusions:
Our study demonstrated that LVD and severe PH diagnosed on the first postnatal echocardiogram were important prognostic indicators of adverse outcomes in “low-risk” CDH infants. The results of this study should call more attention to this often-overlooked aspect of CDH management, and future studies are warranted to further investigate the natural history of cardiac dysfunction and its effect among “high-risk” CDH patients. Early postnatal echocardiography therefore is an important component of clinical assessment and risk stratification. Early interventions to optimize cardiac function and pulmonary vasculature hemodynamics may result in improved outcomes among CDH infants with favorable disease characteristics.
Acknowledgements:
The authors acknowledge Mrs. Kristin Johnson (Vascular Biology Program, Boston Children’s Hospital) for her work in preparation of the figures.
Copyright form disclosure: Dr. Dao received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Appendix.
Contributing Centers to the CDH Study Group Registry
| Center | City | State/Province | Country |
|---|---|---|---|
| Alberta Children’s Hospital | Calgary | AB | Canada |
| Arkansas Children’s Hospital | Little Rock | AR | |
| Astrid Lindgren Children’s Hospital | Stockholm | Sweden | |
| BC Children’s & Women’s Health Centre | Vancouver | BC | Canada |
| Cairo University Pediatric Hospital (Aboul Reesh) | Cairo | Egypt | |
| Children’s Hospital Colorado | Aurora | CO | |
| Children’s Hospital at Skanes University Hospital | Lund | Sweden | |
| Children’s Hospital Boston | Boston | MA | |
| Children’s Hospital of Akron | Akron | OH | |
| Children’s Hospital of Georgia - AU Health | Augusta | GA | |
| Children’s Hospital of Illinois at OSF St. Francis Med Center | Peoria | IL | |
| Children’s Hospital of Los Angeles | Los Angeles | CA | |
| Children’s Hospital of Orange County | Orange | CA | |
| Children’s Hospital of Wisconsin | Milwaukee | WI | |
| Children’s Hospital Omaha | Omaha | NE | |
| Children’s Hospital, University Bonn | Bonn | Germany | |
| Children’s Hospitals and Clinics (Minneapolis) | Minneapolis | MN | |
| Children’s Memorial Hermann Hospital | Houston | TX | |
| Children’s of Alabama | Birmingham | AL | |
| Cincinnati Children’s Hospital Medical Center | Cincinnati | OH | |
| Dell Children’s Medical Center of Central Texas | Austin | TX | |
| Duke University Medical Center | Durham | NC | |
| Golisano Children’s Hospital at Strong | Rochester | NY | |
| Hospital Clinico Universidad Católica de Chile | Santiago | RM | Chile |
| IRCCS Fondazione Ca’ Granda Ospedale Maggiore Policlinico | Milano | Italy | |
| James Whitcomb Riley Children’s Hospital | Indianapolis | IN | |
| Johns Hopkins All Children’s Hospital | St Petersburg | FL | |
| Johns Hopkins Hospital | Baltimore | MD | |
| Juan P. Garrahan Children Hospital | Buenos Aires | Argentina | |
| La Paz University Hospital | Madrid | Spain | |
| Le Bonheur Children’s Medical Center | Memphis | TN | |
| Loma Linda University Children’s Hospital | Loma Linda | CA | |
| Lucile Salter Packard Children’s Hospital | Palo Alto | CA | |
| Mattel Children’s Hospital at UCLA | Los Angeles | CA | |
| NICU Health Sciences Centre | Winnipeg | MB | Canada |
| Norton Children’s Hospital | Louisville | KY | |
| Ospedale Pediatrico Bambino Gesù | Rome | Italy | |
| Palmetto Health Richland | Columbia | SC | |
| Phoenix Children’s Hospital | Phoenix | AZ | |
| Polish Mother’s Memorial Hospital Research Institute | Lodz | Poland | |
| Primary Children’s Hospital | Salt Lake City | UT | |
| Radboud University Nijmegen Medical Centre | Nijmegen | The Netherlands | |
| Rady Children’s Hospital | San Diego | CA | |
| Research Institute at Nationwide Children’s Hospital | Columbus | OH | |
| Royal Children’s Hospital | Parkville | Victoria | Australia |
| Royal Hospital for Sick Children | Glasgow | Scotland | |
| Shands Children’s Hospital/University of Florida | Gainesville | FL | |
| Sophia Children’s Hospital | Rotterdam | The Netherlands | |
| St. Francis Children’s Hospital | Tulsa | OK | |
| St. Louis Children’s Hospital | St. Louis | MO | |
| St. Louis Univ School of Medicine at SSM Health Cardinal Glennon Children’s Hospital | St. Louis | MO | |
| Stollery Children’s Hospital | Edmonton | AB | Canada |
| Sydney Children’s Hospital | Randwick | NSW | Australia |
| The Children’s Hospital at OU Medical Center | Oklahoma City | OK | |
| The Children’s Hospital of Pittsburgh of UPMC | Pittsburgh | PA | |
| The Queen Silvia Children’s Hospital SU/Östra | Gothenburg | Sweden | |
| Tufts Medical Center | Boston | MA | |
| UNC School of Medicine | Chapel Hill | NC | |
| University Children’s Hospital | Uppsala | Sweden | |
| University Malaya Medical Centre | Kuala Lumpur | Malaysia | |
| University of Michigan, C.S. Mott Children’s Hospital | Ann Arbor | MI | |
| University of Padua | Padua | Italy | |
| University of Texas Medical Branch at Galveston | Galveston | TX | |
| University of Virginia Medical School | Charlottesville | VA | |
| Vanderbilt Children’s Hospital | Nashville | TN | |
| Vladivostok State Medical University | Vladivostok | Russia | |
| Winnie Palmer Hospital for Women & Babies | Orlando | FL | |
| Yale New Haven Children’s Hospital | New Haven | CT |
References:
- 1.Morini F, Lally P, Lally K, et al. : The Congenital Diaphragmatic Hernia Study Group Registry. Eur J Pediatr Surg 2015; 25:488–496 [DOI] [PubMed] [Google Scholar]
- 2.Cameron DB, Graham DA, Milliren CE, et al. : Quantifying the Burden of Interhospital Cost Variation in Pediatric Surgery. JAMA Pediatr 2017; 171:e163926 [DOI] [PubMed] [Google Scholar]
- 3.Barrière F, Michel F, Loundou AD, et al. : One-Year Outcome for Congenital Diaphragmatic Hernia: Results From the French National Register. J Pediatr 2018; 193:204–210 [DOI] [PubMed] [Google Scholar]
- 4.Harting MT, Hollinger L, Tsao K, et al. : Aggressive Surgical Management of Congenital Diaphragmatic Hernia: Worth the Effort?: A Multicenter, Prospective, Cohort Study. Ann Surg 2017; 267:1. [DOI] [PubMed] [Google Scholar]
- 5.Lally PA, Skarsgard ED: Congenital diaphragmatic hernia: The role of multi-institutional collaboration and patient registries in supporting best practice. Semin Pediatr Surg 2017; 26:129–135 [DOI] [PubMed] [Google Scholar]
- 6.Lally KP, Lasky RE, Lally PA, et al. : Standardized reporting for congenital diaphragmatic hernia--an international consensus. J Pediatr Surg 2013; 48:2408–15 [DOI] [PubMed] [Google Scholar]
- 7.Montalva L, Antounians L, Zani A: Pulmonary hypertension secondary to congenital diaphragmatic hernia: factors and pathways involved in pulmonary vascular remodeling. Pediatr Res 2019; 85:754–768 [DOI] [PubMed] [Google Scholar]
- 8.Kinsella JP, Steinhorn RH, Mullen MP, et al. : The Left Ventricle in Congenital Diaphragmatic Hernia: Implications for the Management of Pulmonary Hypertension. J Pediatr 2018; 197:17–22 [DOI] [PubMed] [Google Scholar]
- 9.Pelizzo G, Calcaterra V, Lombardi C, et al. : Fetal Cardiac Impairment in Nitrofen-Induced Congenital Diaphragmatic Hernia: Postmortem Microcomputed Tomography Imaging Study. Fetal Pediatr Pathol 2017; 36:282–293 [DOI] [PubMed] [Google Scholar]
- 10.Pelizzo G, Bussani R, Zandonà L, et al. : Cardiac Adaptation to Severe Congenital Diaphragmatic Hernia. Fetal Pediatr Pathol 2016; 35:10–20 [DOI] [PubMed] [Google Scholar]
- 11.Patel N, Massolo AC, Paria A, et al. : Early Postnatal Ventricular Dysfunction Is Associated with Disease Severity in Patients with Congenital Diaphragmatic Hernia. J Pediatr 2018; 203:400–407.e1 [DOI] [PubMed] [Google Scholar]
- 12.Lai WW, Geva T, Shirali GS, et al. : Guidelines and Standards for Performance of a Pediatric Echocardiogram: A Report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 2006; 19:1413–1430 [DOI] [PubMed] [Google Scholar]
- 13.Harting MT: Congenital diaphragmatic hernia-associated pulmonary hypertension. Semin Pediatr Surg 2017; 26:147–153 [DOI] [PubMed] [Google Scholar]
- 14.Brindle ME, Cook EF, Tibboel D, et al. : A clinical prediction rule for the severity of congenital diaphragmatic hernias in newborns. Pediatrics 2014; 134:e413–9 [DOI] [PubMed] [Google Scholar]
- 15.Abman SH, Hansmann G, Archer SL, et al. : Pediatric pulmonary hypertension: Guidelines from the American Heart Association and American Thoracic Society. Circulation 2015; 132:2037–2099 [DOI] [PubMed] [Google Scholar]
- 16.Patel N, Lally PA, Kipfmueller F, et al. : Ventricular dysfunction is a critical determinant of mortality in congenital diaphragmatic hernia. [in submission] [DOI] [PubMed] [Google Scholar]
- 17.Yamoto M, Tanaka Y, Fukumoto K, et al. : Cardiac fetal ultrasonographic parameters for predicting outcomes of isolated left-sided congenital diaphragmatic hernia. J Pediatr Surg 2015; 50:2019–2024 [DOI] [PubMed] [Google Scholar]
- 18.Kailin JA, Dhillon GS, Maskatia SA, et al. : Fetal left-sided cardiac structural dimensions in left-sided congenital diaphragmatic hernia - association with severity and impact on postnatal outcomes. Prenat Diagn 2017; 37:502–509 [DOI] [PubMed] [Google Scholar]
- 19.Altit G, Bhombal S, Van Meurs K, et al. : Diminished Cardiac Performance and Left Ventricular Dimensions in Neonates with Congenital Diaphragmatic Hernia. Pediatr Cardiol 2018; 39:993–1000 [DOI] [PubMed] [Google Scholar]
- 20.Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Pediatrics 1997; 99:838–45 [DOI] [PubMed] [Google Scholar]
- 21.Loh E, Stamler JS, Hare JM, et al. : Cardiovascular effects of inhaled nitric oxide in patients with left ventricular dysfunction. Circulation 1994; 90:2780–5 [DOI] [PubMed] [Google Scholar]
- 22.Putnam LR, Tsao K, Morini F, et al. : Evaluation of Variability in Inhaled Nitric Oxide Use and Pulmonary Hypertension in Patients With Congenital Diaphragmatic Hernia. JAMA Pediatr 2016; 170:1188. [DOI] [PubMed] [Google Scholar]
- 23.Altit G, Bhombal S, Van Meurs K, et al. : Ventricular Performance is Associated with Need for Extracorporeal Membrane Oxygenation in Newborns with Congenital Diaphragmatic Hernia. J Pediatr 2017; 191:28–34.e1 [DOI] [PubMed] [Google Scholar]
- 24.Gaffar S, Ellini AR, Ahmad I, et al. : Left ventricular cardiac output is a reliable predictor of extracorporeal life support in neonates with congenital diaphragmatic hernia. J Perinatol 2019; [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal S, Stockman PT, Klein MD, et al. : The right ventricular systolic to diastolic duration ratio: a simple prognostic marker in congenital diaphragmatic hernia? Acta Paediatr 2011; 100:1315–8 [DOI] [PubMed] [Google Scholar]
- 26.Moenkemeyer F, Patel N: Right Ventricular Diastolic Function Measured by Tissue Doppler Imaging Predicts Early Outcome in Congenital Diaphragmatic Hernia. Pediatr Crit Care Med 2014; 15:49–55 [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal S, Stockmann P, Klein MD, et al. : Echocardiographic measures of ventricular function and pulmonary artery size: prognostic markers of congenital diaphragmatic hernia? J Perinatol 2011; 31:561–6 [DOI] [PubMed] [Google Scholar]
- 28.Burkett DA, Slorach C, Patel SS, et al. : Left Ventricular Myocardial Function in Children With Pulmonary Hypertension. Circ Cardiovasc Imaging 2015; 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burkett DA, Slorach C, Patel SS, et al. : Impact of Pulmonary Hemodynamics and Ventricular Interdependence on Left Ventricular Diastolic Function in Children With Pulmonary Hypertension. Circ Cardiovasc Imaging 2016; 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz-Lemini M, Valenzuela-Alcaraz B, Granados-Montiel J, et al. : Characterizing cardiac dysfunction in fetuses with left congenital diaphragmatic hernia. Prenat Diagn 2018; 38:422–427 [DOI] [PubMed] [Google Scholar]
- 31.Patel N, Kipfmueller F: Cardiac dysfunction in congenital diaphragmatic hernia: Pathophysiology, clinical assessment, and management. Semin Pediatr Surg 2017; 26:154–158 [DOI] [PubMed] [Google Scholar]
- 32.Dillon PW, Cilley RE, Mauger D, et al. : The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg 2004; 39:307–12; discussion 307–12 [DOI] [PubMed] [Google Scholar]
- 33.Wynn J, Krishnan U, Aspelund G, et al. : Outcomes of congenital diaphragmatic hernia in the modern era of management. J Pediatr 2013; 163:114–9.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]


