Abstract
Background
An early report of recipient heart transplantation outcomes under the new US heart allocation system introduced in late 2018 demonstrated a lower post-transplant survival rate compared with the prior system.
Objectives
We sought to examine recipient survival under the new system using an updated dataset.
Methods
The 2015–2019 United Network for Organ Sharing (UNOS) registry was queried for adult heart transplant recipients, stratified by whether they were listed and transplanted before or after October 18, 2018 when the new allocation system was implemented. The association between allocation system and recipient mortality was analyzed using the Kaplan-Meier method and multivariable Cox proportional hazards regression.
Results
7,119 recipients met inclusion criteria including 6,004 (84%) and 1,115 (16%) listed and transplanted in the old and new allocation systems, respectively. This registry update included 576 new-system recipients, over double the amount previously analyzed. Recipients from the new system were more likely to be bridged to transplant with temporary mechanical circulatory support (MCS) devices instead of durable left ventricular assist devices (LVADs) and had longer graft ischemic times. After adjustment, the new system was not associated with poorer survival on Kaplan-Meier survival analysis (logrank p=0.075) or multivariable Cox Proportional Hazards modeling (aHR 1.23, 95% CI 0.88–1.71).
Conclusions
The short-term survival of recipients listed and transplanted under the old and new allocation systems appears to be comparable. The allocation system change has resulted in several changes to the clinical profiles of transplanted patients that must be closely monitored in the coming years.
Keywords: Heart transplantation, Organ allocation, recipient outcomes, mechanical circulatory support
Introduction
In October of 2018, the United States heart transplant organ allocation system was modified by the Organ Procurement and Transplantation Network (OPTN) Thoracic Organ Transplantation Committee to promote an allocation scheme that would direct organs to the most critically ill waitlisted patients. A major goal was to further decrease the waitlist mortality on the heart transplant list after the last revision in the early 2000s (1). This was partially in response to an increasing number of recipients supported with durable mechanical circulatory support devices that stabilized hemodynamics but allowed patients to wait outside the hospital but with a relatively high status. (2). Given this disparate risk among patients listed at the highest status, the prior system suffered from long waitlist times among candidates listed at the most urgent status with a high chance of dying while awaiting a suitable cardiac allograft (3–7). The prior system also promoted regional disparities in access to donor allografts (1,8).
After the first wave of transplants were performed under the new allocation scheme, Cogswell and colleagues made a valuable effort to assess the preliminary impact of the new allocation system (9). In comparing recipient outcomes between the new and old allocation systems using data from the United Network for Organ Sharing (UNOS) heart registry, they found decreasing waitlist mortality but surprisingly poor post-transplant survival rates compared with the prior system: 90- and 180-day survival of 87.6% and 77.9% in the new system, respectively compared with 94.5% and 93.4% in the prior system (9). This apparent doubling of 90-day and tripling of 180-day post-transplant mortality was unsettling, although these findings rested on a relatively small sample size of new allocation system recipients with a median follow-up time of only 22 days (IQR 12–48).
We sought to build on Cogswell and colleagues’ thoughtful analysis to determine whether this decrease in recipient survival would persist in a larger data set ending in June 2019. Furthermore, given the substantial differences observed in donor and recipient characteristics between the two allocation systems found by Cogswell et al., we aimed to examine whether the new allocation system was associated with worsened recipient survival using a multivariable Cox Proportional Hazards regression analysis.
Methods
Study Population
The United Network for Organ Sharing (UNOS) provided deidentified donor and recipient transplant data from October 1987 through June 2019 with follow-up information through September 2019. The database includes prospectively collected data for all organ transplants performed in the US during this period. The registry was queried for all adults (age ≥ 18) undergoing first-time single-organ heart transplantation. As in the analysis by Cogswell et al., patients were stratified into two primary cohorts: those that were listed and transplanted between October 18, 2015 and prior to when the allocation system changed on October 18, 2018, and those that were listed and transplanted after this date (9). Patients listed prior to but transplanted after the allocation system change were excluded (Figure 1).
Figure 1. Study inclusion and exclusion criteria.
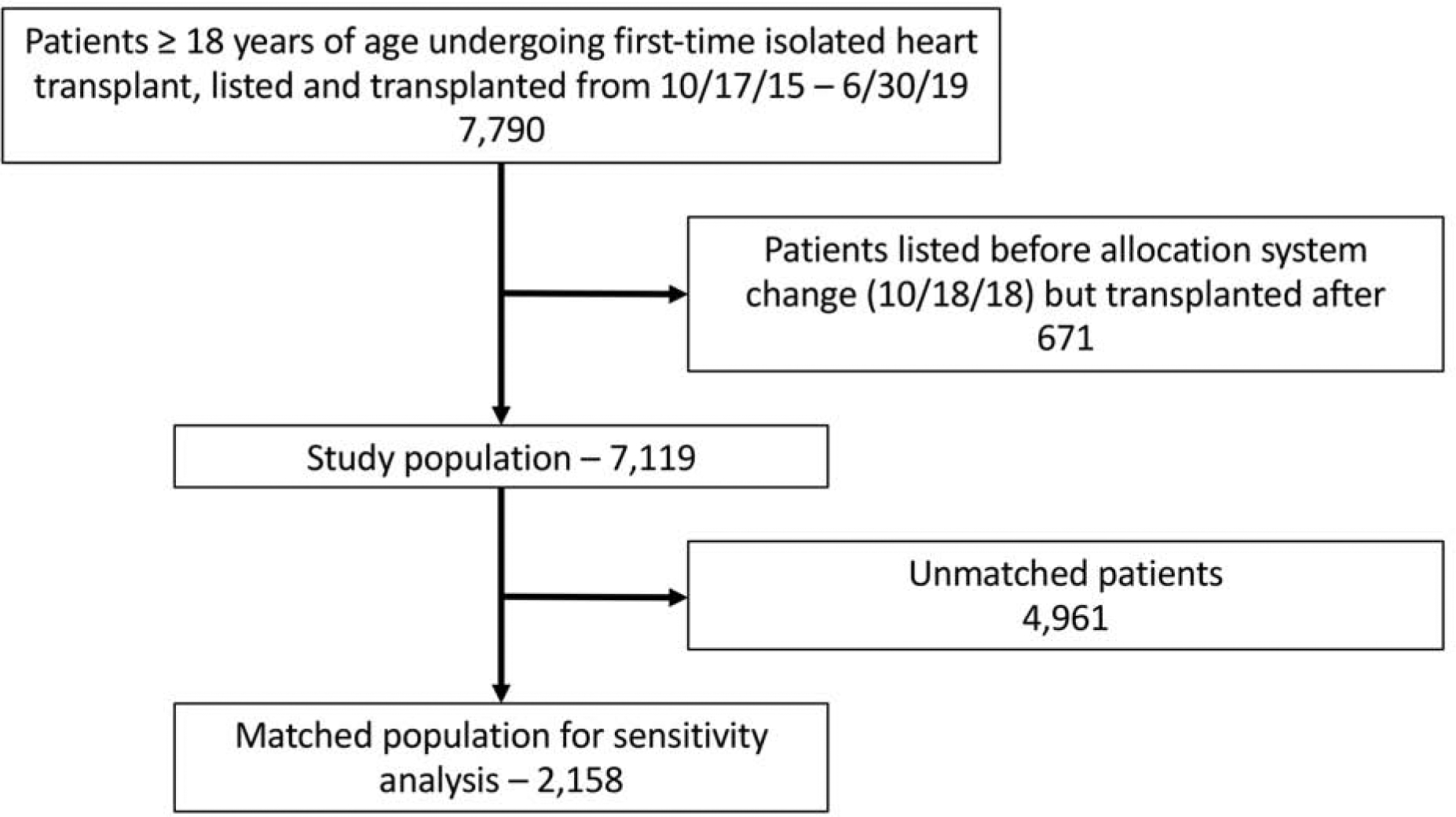
After application of inclusion and exclusion criteria, 7,119 heart transplant recipients and their associated donors were analyzed. A propensity-matched sensitivity analysis was performed, which included 2,158 recipients.
Data analysis
Unadjusted descriptive analysis of baseline recipient and donor characteristics was performed, stratified by allocation system. Baseline demographic and clinical characteristics are presented as median (interquartile range) for continuous variables and percent (count) for categorical variables, unless otherwise specified. Unadjusted comparisons between cohorts were performed using the Wilcoxon rank sum test for continuous variables and the Pearson χ2 test for categorical variables. Unadjusted freedom from death or re-transplantation was analyzed using the Kaplan-Meier method, with differences between cohorts assessed using the log-rank test.
Due to imbalances in baseline characteristics between the two groups, multivariable regression was performed to determine the independent association between allocation system and recipient survival. Adjusted post-transplant survival was modeled using multivariable Cox Proportional Hazards regression, with covariates chosen a priori based upon clinically relevant factors available within the dataset. In addition to old/new allocation system, included covariates consisted of donor age and recipient age, sex, ethnicity, donor/recipient sex mismatch, heart failure etiology, durable LVAD support, ECMO support, temporary VAD support, IABP support, prior cardiac surgery, graft ischemic time, and annualized heart transplant center volume. Linearity of continuous variables with the hazard of the outcome was modeled using restricted cubic splines with 4 pre-specified knots based upon each variable’s distribution. In the final model, continuous variables were modeled as linear functions or piecewise linear splines for ease of interpretation, where appropriate.
In addition to multivariable regression utilizing the entire cohort, a 1:1 propensity score matching sensitivity analysis was performed comprised of recipients transplanted in the new allocation system and a cohort of patients with the most similar baseline characteristics from the old system. A nearest neighbor algorithm was used that matches patients based on a series of logistic regressions and a caliper width of 0.1 (10). Patients were matched based on donor and recipient covariates used in the Cogswell study, as well as other potential confounders available in the dataset. These included donor age, ethnicity, cause of death, diabetes, and cocaine use as well as pre-transplant recipient variables including age, sex, donor/recipient sex mismatch (female donor and male recipient), ethnicity, heart failure etiology, medical condition, diabetes, IV antibiotic usage in the preceding 2 weeks, use of IV inotropes, durable LVAD support, temporary mechanical circulatory support, extracorporeal membrane oxygenation (ECMO) support, ventilator requirement, and graft ischemic time. Comparisons between post-matching cohorts were performed by examining standardized mean differences (SMD).
Two-sided p-values ≤ 0.05 were considered statistically significant. Multivariable modeling was performed as a complete case analysis. All statistical analyses were performed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). This analysis was deemed exempt by the Duke University Institutional Review Board.
Results
Recipient and donor characteristics
In total, 7,119 recipients met inclusion criteria including 6,004 (84%) and 1,115 (16%) listed and transplanted in the old and new allocation systems, respectively. Median follow-up time was 376 days (IQR 207–730) overall, and 51 days (IQR 18–179) for patients listed and transplanted in the new system. Recipient baseline demographic and clinical characteristics, stratified by allocation system, are presented in Table 1. Recipients listed and transplanted in the new system were less likely to have diabetes, ischemic cardiomyopathy, and be bridged to transplant with a durable LVAD. They were more likely; however, to be supported with temporary MCS devices including intraaortic balloon pumps (IABP), ECMO, and temporary VADs. This trend was also observed for all candidates listed for heart transplant under the two allocation systems, regardless of whether transplant occurred (Supplemental Table 1). New allocation system recipients spent a significantly shorter amount of time on the waitlist (median 15 vs 68 days, p<0.001) and graft ischemic time was longer (median 3.5 vs 3.0 hours, p<0.001) with a longer distance between donor and recipient centers (median 296 vs. 90 miles, p<0.001).
Table 1.
Demographic and clinical characteristics of heart transplant recipients stratified by allocation system pre- and post-October 2018.
| Allocation System | |||
|---|---|---|---|
| Variable | Old | New | p-value |
| (n=6,004) | (n=1,115) | ||
| Male sex | 4,319 (71.9%) | 776 (69.6%) | 0.120 |
| Sex mismatch | 852 (14.2%) | 133 (11.9%) | 0.050 |
| Age (median years, IQR) | 57 (47–64) | 56 (45–63) | 0.047 |
| BMI (median kg/m2, IQR) | 27.2 (23.840.0) | 26.3 (22.9–30.6) | <0.001 |
| Ethnicity | 0.320 | ||
| White | 3,859 (64.3%) | 730 (65.5%) | |
| Black | 1,314 (21.9%) | 218 (19.6%) | |
| Hispanic | 540 (9.0%) | 110 (9.9%) | |
| Other | 291 (4.8%) | 57 (5.1%) | |
| Recipient history | |||
| Diabetes | 1,691 (28.2%) | 274 (24.7%) | 0.018 |
| Malignancy | 570 (9.5%) | 95 (8.5%) | 0.332 |
| Cerebrovascular disease | 366 (6.1%) | 72 (6.5%) | 0.694 |
| Heart failure etiology | 0.003 | ||
| Ischemic | 1,694 (28.2%) | 268 (24.0%) | |
| Non-ischemic dilated | 3,319 (55.3%) | 629 (56.4%) | |
| Other | 991 (16.5%) | 218 (19.6%) | |
| Recipient creatinine (median mg/dL, IQR) | 1.2 (0.9–1.4) | 1.1 (0.9–1.4) | <0.001 |
| Recipient bilirubin (median mg/dL, IQR) | 0.7 (0.4–1.0) | 0.7 (0.5–1.1) | 0.007 |
| Pre-transplant status | <0.001 | ||
| Intensive care unit | 1,863 (31.0%) | 676 (61.7%) | |
| Hospitalized (non-ICU) | 964 (16.1%) | 146 (13.3%) | |
| Not hospitalized | 3,177 (52.9%) | 273 (24.9%) | |
| Medical therapy | |||
| IV antibiotics in two weeks before transplant | 500 (8.3%) | 116 (10.4%) | 0.027 |
| IV inotropes prior to transplant | 2,311 (38.5%) | 465 (41.7%) | 0.047 |
| Ventilator support prior to transplant | 53 (0.9%) | 39 (3.5%) | <0.001 |
| Durable LVAD support prior to transplant | 2,511 (41.8%) | 236 (21.2%) | <0.001 |
| Temporary MCS prior to transplant | 809 (13.5%) | 496 (44.5%) | <0.001 |
| IABP | 518 (8.6%) | 368 (33.0%) | <0.001 |
| ECMO | 91 (1.5%) | 72 (6.5%) | <0.001 |
| Temporary VAD | 115 (1.9%) | 66 (5.9%) | <0.001 |
| ABO blood type | 0.221 | ||
| A | 2,509 (41.8%) | 457 (41.0%) | |
| B | 936 (15.6%) | 194 (17.4%) | |
| AB | 379 (6.3%) | 81 (7.3%) | |
| O | 2,180 (36.3%) | 383 (34.3%) | |
| Days on waitlist (median days, IQR) | 68 (22–178) | 15 (6–44) | <0.001 |
| Waitlist status at transplant | - | ||
| Old 1A | 4,124 (68.7%) | - | |
| Old 1B | 1,708 (28.4%) | - | |
| Old 2 | 172 (2.9%) | - | |
| New 1 | - | 110 (9.9%) | |
| New 2 | - | 564 (50.6%) | |
| New 3 | - | 251 (22.5%) | |
| New 4 | - | 145 (13.0%) | |
| New 5 | - | - | |
| New 6 | - | 36 (3.2%) | |
| Graft ischemic time (median hours, IQR) | 3.0 (2.3–3.7) | 3.5 (2.8–4.0) | <0.001 |
| Ex vivo perfusion | 56 (0.9%) | 9 (0.8%) | 0.816 |
| Distance from donor hospital to transplant center (median miles, IQR) | 90 (14–284) | 296 (99–492) | <0.001 |
IQR, interquartile range; BMI, body mass index; ICU, intensive care unit; IV, intravenous; LVAD, left ventricular assist device; MCS, mechanical circulatory support; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device
Donor characteristics stratified by allocation system are presented in Table 2. Overall, donor characteristics were comparable between the two groups although new allocation system donors were more likely to have a history of cocaine use and a cause of death listed as anoxia and were less likely to have a cause of death listed as head trauma.
Table 2.
Characteristics of donors stratified by donation before or after introduction of a new allocation system in October 2018.
| Allocation System | |||
|---|---|---|---|
| Variable | Old | New | p-value |
| (n=6,004) | (n=1,115) | ||
| Male gender | 4,059 (67.6%) | 769 (69.0%) | 0.390 |
| Donor age (median years, IQR) | 31 (23–40) | 32 (24–41) | 0.030 |
| Donor BMI (median kg/m2, IQR) | 26.4 (23.2–30.7) | 26.6 (23.6–30.6) | 0.241 |
| Donor ethnicity | 0.634 | ||
| White | 3,862 (64.3%) | 710 (63.7%) | |
| Black | 982 (16.4%) | 173 (15.5%) | |
| Hispanic | 957 (15.9%) | 194 (17.4%) | |
| Other | 203 (3.4%) | 38 (3.4%) | |
| Donor history | |||
| Cigarette use | 674 (11.2%) | 115 (10.3%) | 0.402 |
| Cocaine use | 1,509 (25.1%) | 324 (29.1%) | 0.007 |
| Alcohol abuse | 1,045 (17.4%) | 201 (18.0%) | 0.646 |
| Diabetes | 224 (3.7%) | 36 (3.2%) | 0.463 |
| Hypertension | 975 (16.2%) | 176 (15.8%) | 0.738 |
| Cancer | 88 (1.5%) | 17 (1.5%) | 0.988 |
| Donor creatinine (median mg/dL, IQR) | 1.0 (0.8–1.5) | 1.0 (0.8–1.6) | 0.025 |
| Donor bilirubin (median mg/dL, IQR) | 0.7 (0.5–1.2) | 0.7 (0.5–1.1) | 1.000 |
| Donor cause of death | 0.008 | ||
| Anoxia | 2,265 (37.7%) | 459 (41.2%) | |
| Cerebrovascular/stroke | 925 (15.4%) | 174 (15.6%) | |
| Head trauma | 2,655 (44.2%) | 440 (39.5%) | |
| CNS tumor | 30 (0.5%) | 5 (0.4%) | |
| Other | 127 (2.1%) | 37 (3.3%) | |
| ABO blood type | 0.233 | ||
| A | 2,296 (38.2%) | 400 (35.9%) | |
| B | 678 (11.3%) | 129 (11.6%) | |
| AB | 149 (2.5%) | 37 (3.3%) | |
| O | 2,881 (48.0%) | 549 (49.2%) | |
IQR, interquartile range; CNS, central nervous system
Unadjusted analysis of survival
On unadjusted Kaplan-Meier survival analysis of freedom from death or retransplantation, the new allocation system was associated with a minimally decreased survival among heart transplant recipients overall (Figure 2a, logrank p=0.009) and candidates transplanted as urgent status 1A (old system) or 1/2/3 (new system) (Figure 2b, log rank p=0.002). Specifically, 90- and 180-day survival was estimated as 93.0% (95% CI 91.2–94.9) and 90.6% (95% CI 88.3–92.9), respectively, under the new allocation system compared with 94.4% (95% CI 93.9–95.0) and 93.3% (95% CI 92.7–93.9) under the old system. These 90- and 180-day survival estimates, in addition to the estimates presented in the early report by Cogswell et al., are presented in Figure 2c.
Figure 2. Unadjusted Kaplan-Meier analysis of freedom from death or re-transplantation.
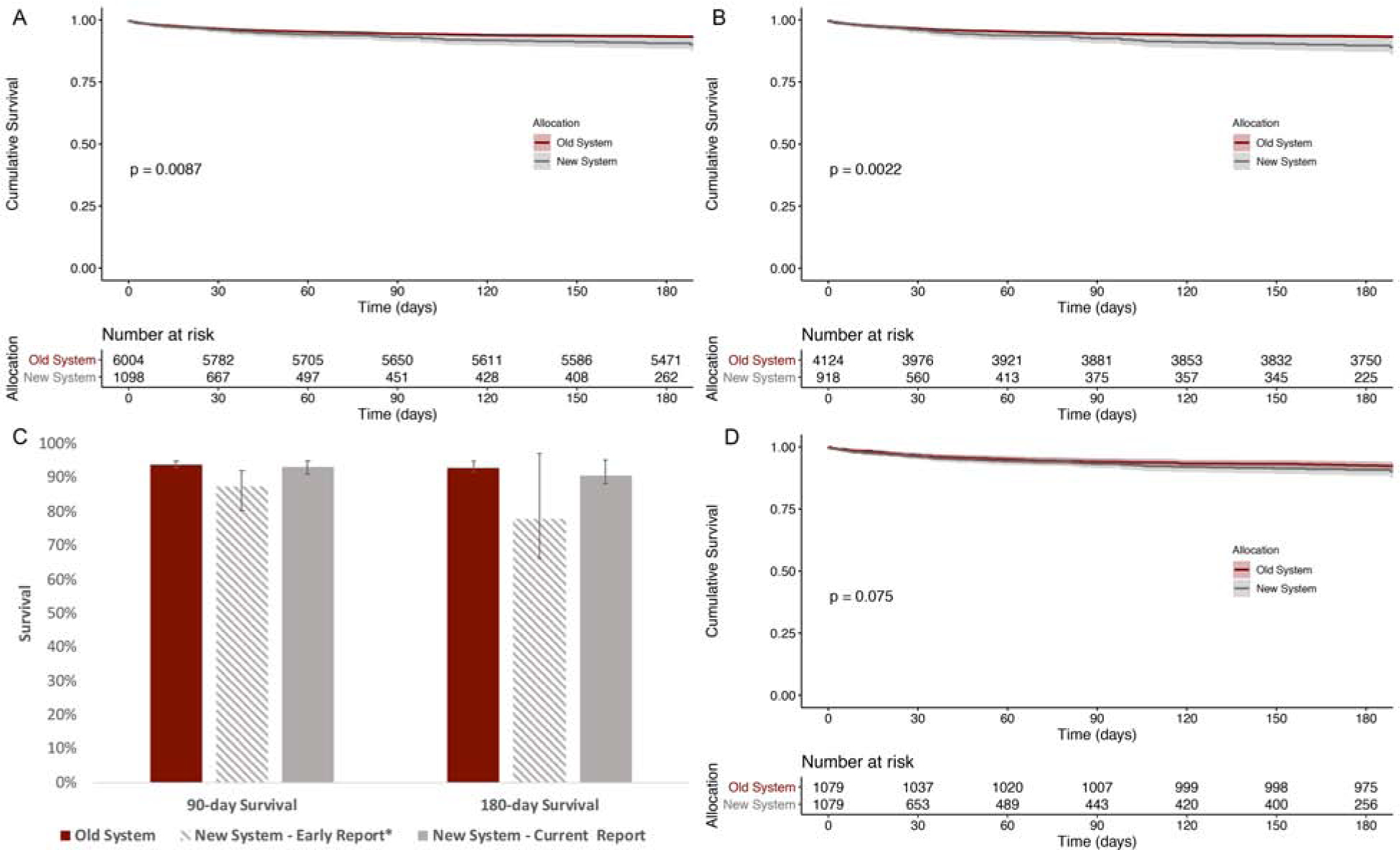
Unadjusted Kaplan-Meier analysis of freedom from death or re-transplantation of (A) all recipients, and (B) those listed as most urgent (status 1A in old system or 1/2/3 in new system) stratified by organ allocation system. Panel (C) presents survival estimates at 90- and 180-days based upon the current report as well as the *early report by Cogswell et al. Panel (D) presents Kaplan-Meier analysis of propensity score matched cohort stratified by organ allocation system. P-values calculated using log-rank statistic.
Adjusted analysis of survival
Multivariable adjusted Cox Proportional Hazards regression was performed to determine the independent association between allocation system and recipient survival (Table 3). After adjustment, the new allocation system was not associated with significantly worse recipient survival compared with the older allocation system (aHR 1.18, 95% CI 0.90–1.55). Identified factors independently associated with lower survival included increasing donor age (aHR 1.09 per 5 years, 95% CI 1.05–1.12), increasing recipient age (aHR 1.05 per 5 years, 95% CI 1.01–1.08), pre-transplant ECMO support (aHR 2.97, 95% CI 2.06–4.28), prior cardiac surgery (aHR 1.29, 95% CI 1.10–1.51), increasing graft ischemic time (aHR 1.20 per hour, 95% CI 1.12–1.28), and increasing annualized center volume above 25 (aHR 1.19 per 5 transplants, 95% CI 1.09–1.30). Factors associated with improved recipient survival included non-ischemic dilated cardiomyopathy (vs ischemic; aHR 0.92, 95% CI 0.69–0.98) and increasing annualized center volume below 25 (aHR 0.87, 95% CI 0.82–0.93).
Table 3.
Multivariable Cox Proportional Hazards model of freedom from post-heart transplant death or re-transplantation
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| New allocation system | 1.18 | 0.90 | 1.55 | 0.228 |
| Donor age (per 5 years) | 1.09 | 1.05 | 1.12 | <0.001 |
| Recipient age (per 5 years) | 1.05 | 1.01 | 1.08 | 0.004 |
| Recipient male sex | 0.93 | 0.78 | 1.10 | 0.379 |
| Recipient ethnicity (reference: White) | ||||
| Black | 1.03 | 0.85 | 1.24 | 0.766 |
| Hispanic | 1.18 | 0.92 | 1.52 | 0.181 |
| Other | 0.91 | 0.64 | 1.30 | 0.616 |
| Donor/recipient sex mismatch | 1.09 | 0.88 | 1.34 | 0.432 |
| Recipient heart failure etiology (reference: ischemic) | ||||
| Non-ischemic dilated | 0.92 | 0.69 | 0.98 | 0.026 |
| Other | 0.92 | 0.74 | 1.16 | 0.494 |
| Recipient durable LVAD support | 1.12 | 0.95 | 1.34 | 0.186 |
| Recipient ECMO support | 2.97 | 2.06 | 4.28 | <0.001 |
| Recipient temporary VAD support | 1.10 | 0.71 | 1.73 | 0.667 |
| Recipient IABP support | 1.07 | 0.83 | 1.38 | 0.616 |
| Recipient prior cardiac surgery | 1.29 | 1.10 | 1.51 | 0.002 |
| Graft ischemic time (per hour) | 1.20 | 1.12 | 1.28 | <0.001 |
| Transplant center annualized volume | ||||
| <25 (per 5 transplants) | 0.87 | 0.82 | 0.93 | <0.001 |
| >25 (per 5 transplants) | 1.19 | 1.09 | 1.30 | <0.001 |
LVAD, left ventricular assist device; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device; IABP, intraaortic balloon pump
A propensity score matching sensitivity analysis was performed to identify a subgroup of recipients transplanted under the old allocation system with similar baseline demographic and clinical characteristics as those transplanted under the new system. Using 1:1 matching identified 1,079 recipients transplanted under each allocation system with similar baseline recipient and donor characteristics (Supplemental Tables 2 & 3). Kaplan-Meier survival analysis estimated similar survival between propensity score matched groups (Figure 2d, logrank p=0.08).
Discussion
Comparable recipient survival across allocation systems
In this retrospective analysis of the UNOS heart transplant registry, we compared the survival of recipients who underwent transplantation in the final years of the old allocation system to recipients listed and transplanted in the first 8 months of the new allocation system. In addition, as a result of significant imbalances in patient baseline characteristics between the two eras, we performed a propensity score matched analysis with a group of recipients listed and transplanted before and after the allocation system change with similar characteristics. On unadjusted analysis, we demonstrated slightly worsened recipient survival under the new allocation system, however the difference in survival estimates between the old and new systems was smaller than the rates presented in the earlier report by Cogswell and colleagues (9). Furthermore, after matching was performed, there was no difference in recipient survival observed between the old and new allocation systems on Kaplan-Meier survival analysis or multivariable Cox Proportional Hazards regression.
There are several factors that likely account for the difference in estimated recipient survival estimates under the new allocation system presented in the current report and the prior report by Cogswell and colleagues. First, the additional 3 months of transplant data in the current report permitted an analysis of an additional 576 recipients transplanted in the new system, which is more than double the population size of the prior study with a longer follow-up time. The greater precision is reflected by the significantly narrower confidence intervals associated with the survival estimates presented in this updated analysis. Second, the design of the UNOS Registry, namely the ascertainment of recipient death through linkage with the Social Security Death Master File, may have introduced a degree of event ascertainment bias among recipients very recently transplanted: recipients who were still living at the time of the most recent database harvest may not have had adequate time to follow up (11). This is reflected by the median 55-day follow up of censored patients in the present analysis compared with 23 days in the earlier cohort. Lastly, the Cogswell analysis likely overlooked the non-informative censoring assumption of the Kaplan-Meier estimator and did not include ECMO use as an adjustment variable in their final model, likely due to being underpowered, which may have influenced the results (12).
MCS bridging practices
Although the allocation system change itself does not appear to be associated with worse than expected post-transplant recipient mortality, it has clearly impacted the clinical profile of patients receiving donor allografts. Compared with recipients transplanted under the prior system, new allocation system recipients were approximately four times more likely to have been supported with temporary MCS devices including IABP, ECMO, and temporary VADs and fewer recipients were bridged to transplant with a durable LVAD. While this change is likely being driven primarily by the prioritization of these sicker patients in the new allocation system, and may reflect other trends in planning LVAD therapy as destination or bridge therapy, it is conceivable that some programs are modifying their practices in favor of temporary mechanical support in an effort to elevate candidate status and reduce wait time. Indeed, when examining candidates listed for transplant regardless of subsequent transplantation, rates of temporary MCS use have approximately doubled in the era of the new allocation system (Supplemental Table 1). Due to the lower status assigned to patients supported with durable LVAD, some degree of practice modification was expected. However, at the individual program level these practices should be closely monitored to ensure that a deviation toward use of temporary MCS is balanced with the well-established risk of post-transplant mortality when using such devices (13,14). Indeed, the multivariable adjusted analyses suggest that if a significant change in bridging practice was not instituted in the new allocation scheme then post-transplant outcomes remain comparable (15).
Graft ischemic time
In addition to a change in MCS bridging practices, it is clear that the new allocation system has had an impact on graft ischemic times, which have increased significantly compared with the prior system. This is likely related in part to increased distances between donor and recipient centers, which increased from a median of 90 miles in the old system to almost 300 miles, consistent with overt goals of the new system to promote sharing over greater distances. From an allocation prospective the broader regional sharing of organs based on recipient need is an important priority. However, given the known association between ischemic time and recipient mortality (aHR 1.19 per hour in this study), it will be important to ensure that these increased ischemic times do not impact post-transplant mortality to an unacceptable degree (16,17). Furthermore, an examination of the economic impact of broader sharing will be necessary with regard to costs associated with transportation of organs across greater distances. In addition, the expanding use of ex vivo perfusion systems may represent a way for centers to mitigate the risk of longer ischemic times under the new system, however very few centers have thus far adopted this technology (18). As mentioned, monitoring the program-specific median ischemic time in an era of expanded regional organ sharing is an important method of quality assurance to provide the most beneficial outcomes to heart transplant recipients.
Limitations
There are several limitations associated with this analysis worth noting. First, as a retrospective analysis of registry data, the quality of the data as well as the availability of necessary predictor variables can be an issue. Our multivariable data suggests that recipient survival improves with increasing annualized transplant center volume until a threshold of approximately 25 annually, after which it begins to decline. These findings may be a result of unmeasured confounders, where recipients transplanted at higher volume centers have higher baseline risk profiles than documented in the UNOS registry. Second, while this analysis included greater than double the number of recipients transplanted under the new allocation system as the earlier report, it is still a relatively small sample size with less than a year of follow-up. Thus, we cannot make any conclusions about the impact of the new allocation system on longer-term survival. It will take several years of data reporting to fully understand its clinical implications. Lastly, registry data cannot provide robust insight regarding clinical decision-making surrounding bridging candidates to transplant with various forms of mechanical circulatory support. Thus, while we can make inferences about the impact of the new allocation system on decision making in a broad sense, we can only indirectly analyze the management of heart failure within individual transplant programs. As a result, our analysis is likely limited by bias from unmeasured confounders.
Conclusions
In conclusion, by examining short-term outcomes of over 1,000 adults listed and undergoing heart transplantation in the new allocation system since October 2018, we found that the short-term post-transplant survival of this group is comparable to that of recipients who were transplanted in the final years of the old allocation system. Initial data on long-term outcomes and more data on 90-day and 180-day outcomes will continue to inform the evaluation of the new system. The new system has already resulted in several important changes to the clinical profile of transplanted patients, including increased temporary MCS support and decreased durable LVAD support as well as increased ischemic times, that should be closely monitored over the coming years. Furthermore, the impact of the new allocation system on waitlist outcomes should be examined as more data become available.
Supplementary Material
Central Illustration. Post-heart transplant recipient survival estimates.
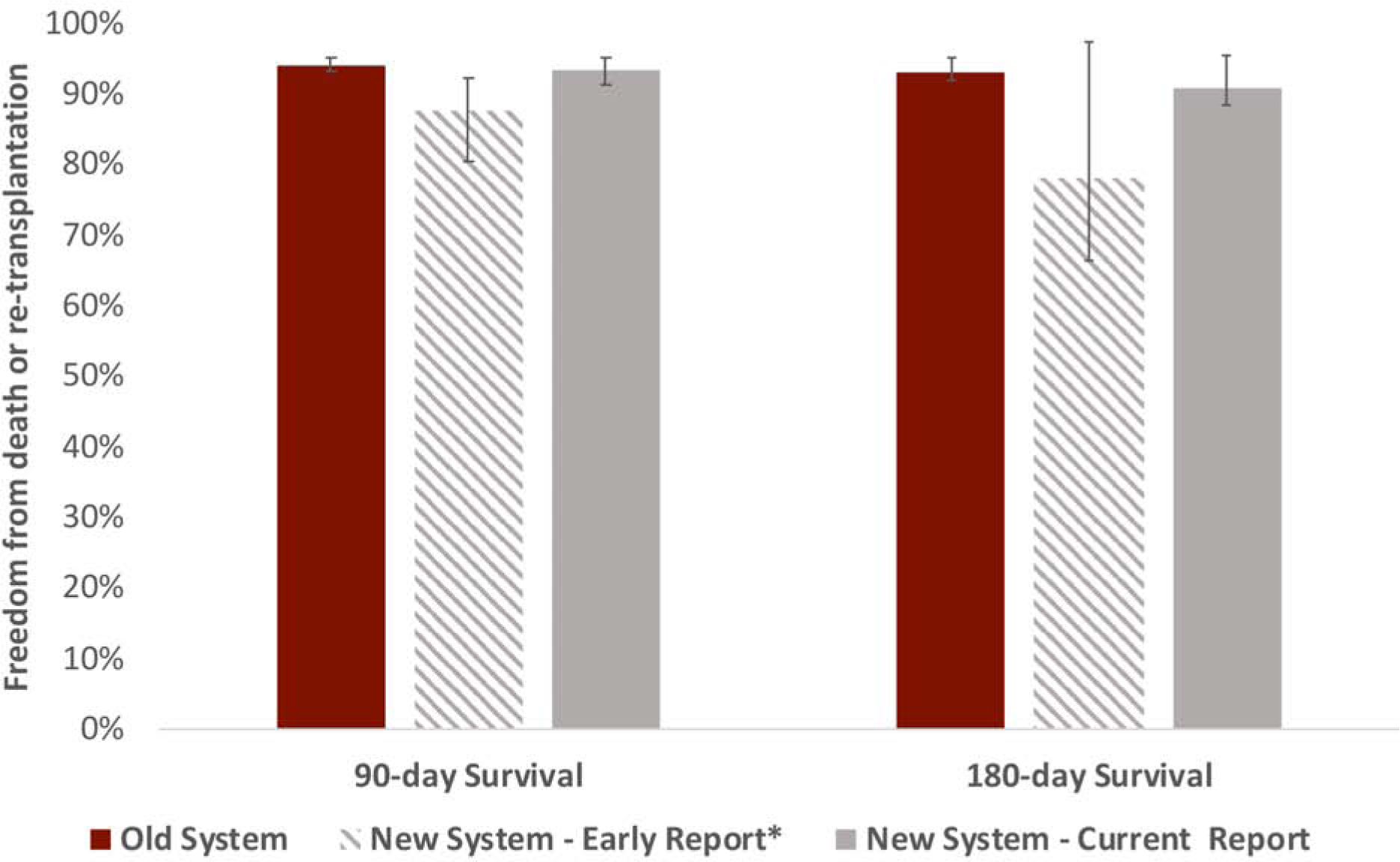
Post-heart transplant recipient survival estimates at 90- and 180-days under old and new US heart allocation systems based upon the current updated report as well as the *early report by Cogswell et al.
Clinical Perspectives.
Competencies in Medical Knowledge
The modification to the US heart transplant allocation system implemented in 2018 has resulted in substantial changes to the clinical profile of transplanted patients, including an increased use of temporary MCS and decreased use of durable LVADs as well as increased ischemic times. Despite an early report of significantly worsened recipient mortality under the new allocation system, an analysis of the updated UNOS registry suggests that the short-term post-transplant survival of this group is comparable to that of recipients who were transplanted in the final years of the old allocation system.
Translational Outlook
Recipient outcomes, waitlist mortality, graft ischemic times, and MCS bridging practices must be closely monitored over the coming years as additional data from the new heart transplant allocation system becomes available.
Acknowledgements
Dr. Jawitz was supported by a NIH T-32 grant 5T32HL069749 and Dr. Raman was supported by NIH T-32 grant 5T32CA093245. The authors have no disclosures relevant to the presented work. This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Funding for this study was provided by NIH T-32 grants 5T32HL069749 (Jawitz) and 5T32CA093245 (Raman)
Abbreviations:
- UNOS
United Network for Organ Sharing
- MCS
mechanical circulatory support
- LVAD
left ventricular assist device
- OPTN
Organ Procurement and Transplantation Network
- ECMO
extracorporeal membrane oxygenation
- IABP
intraaortic balloon pump
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no relevant disclosures
References
- 1.Schulze PC, Kitada S, Clerkin K, Jin Z, Mancini DM. Regional differences in recipient waitlist time and pre- and post-transplant mortality after the 2006 United Network for Organ Sharing policy changes in the donor heart allocation algorithm. JACC Heart Fail 2014;2:166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer DM, Rogers JG, Edwards LB et al. The future direction of the adult heart allocation system in the United States. Am J Transplant 2015;15:44–54. [DOI] [PubMed] [Google Scholar]
- 3.Rogers JG. Changes in United States heart allocation: A community energized to improve policy. J Thorac Cardiovasc Surg 2016;152:1484–1486. [DOI] [PubMed] [Google Scholar]
- 4.Mancini DM, Schulze PC. Heart transplant allocation: in desperate need of revision. J Am Coll Cardiol 2014;63:1179–1181. [DOI] [PubMed] [Google Scholar]
- 5.Kobashigawa JA, Johnson M, Rogers J et al. Report from a forum on US heart allocation policy. Am J Transplant 2015;15:55–63. [DOI] [PubMed] [Google Scholar]
- 6.Dardas T, Mokadam NA, Pagani F, Aaronson K, Levy WC. Transplant registrants with implanted left ventricular assist devices have insufficient risk to justify elective organ procurement and transplantation network status 1A time. J Am Coll Cardiol 2012;60:36–43. [DOI] [PubMed] [Google Scholar]
- 7.Pinney SP. Timing isn’t everything: donor heart allocation in the present LVAD era. J Am Coll Cardiol 2012;60:52–3. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson LW, Kormos RL, Young JB, Kirklin JK, Hunt SA. Major advantages and critical challenge for the proposed United States heart allocation system. J Heart Lung Transplant 2016;35:547–9. [DOI] [PubMed] [Google Scholar]
- 9.Cogswell R, John R, Estep JD et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant 2019. [DOI] [PubMed] [Google Scholar]
- 10.Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw 2011;42. [Google Scholar]
- 11.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant 2014;14:1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker WF, Churpek MM, Anderson AS. Is it too early to investigate survival outcomes of the new US heart allocation system? J Heart Lung Transplant 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin MY, Wever-Pinzon O, Mehra MR et al. Post-transplant outcome in patients bridged to transplant with temporary mechanical circulatory support devices. J Heart Lung Transplant 2019;38:858–869. [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara S, Takeda K, Kurlansky PA, Naka Y, Takayama H. Extracorporeal membrane oxygenation as a direct bridge to heart transplantation in adults. J Thorac Cardiovasc Surg 2018;155:1607–1618 e6. [DOI] [PubMed] [Google Scholar]
- 15.Bernhardt AM. The new tiered allocation system for heart transplantation in the United States-a Faustian bargain. J Heart Lung Transplant 2019;38:870–871. [DOI] [PubMed] [Google Scholar]
- 16.Lund LH, Khush KK, Cherikh WS et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1037–1046. [DOI] [PubMed] [Google Scholar]
- 17.Segovia J, Cosio MD, Barcelo JM et al. RADIAL: a novel primary graft failure risk score in heart transplantation. J Heart Lung Transplant 2011;30:644–51. [DOI] [PubMed] [Google Scholar]
- 18.Ardehali A, Esmailian F, Deng M et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015;385:2577–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


