Abstract
Despite major advancements in genomic medicine, research to optimize the design and communication of genetically-informed interventions in behavioral health has lagged. The goal of this study was to engage potential end-users in participatory co-design of a personalized genetically-informed risk tool to intervene on high-risk health behaviors. We used structured interviews to examine end-user attitudes and interest in personalized genetics, qualitative interviews to guide iterative design of a genetically-informed tool, and questionnaires to assess acceptability and potential utility of the tool. Participants expressed strong demand for using personal genetics to inform smoking and alcohol-related disease risk and guide treatment (78–95% agreed). Via iterative design feedback, we co-created a genetically-informed risk profile featuring (1) explanation of genetic and phenotypic markers used to construct a risk algorithm, (2) personalized risks and benefits of healthy behavior change, and (3) recommended actions with referral to freely available resources. Participants demonstrated sufficient understanding and cited motivating behavior change as the most useful purpose of the tool. In three phases, we confirmed strong desire for personalized genetics on high-risk health behaviors, co-designed a genetically-informed profile with potential end-users, and found high acceptability, comprehensibility, and perceived usefulness of the profile. As scientific discovery of genomic medicine advances in behavioral health, we must develop the tools to communicate these discoveries to consumers who stand to benefit. The potential of genomic medicine to engage populations and personalize behavioral health treatment depends in part on preparatory studies to design for the future implementation of genetically-informed interventions.
Keywords: Participatory Design, Health Behaviors, Risk Communication, Genetics, Precision Medicine
INTRODUCTION
The Precision Medicine Initiative sparked a new era of medicine in which data on genomics and patient preferences can be used to better engage consumers and personalize treatment for a wide range of diseases (1–4). Large strides toward routine implementation of genomic applications have been made in medical fields that specialize in diagnosing and treating chronic and rare disorders, such as oncology, pharmacology, and infectious disease (5–7). These efforts have resulted in a growing infrastructure on which to base clinical and translational studies that involve the practical application of genomic information. However, behavioral health disorders, including tobacco and alcohol use disorders, have been overlooked in such studies despite their high morbidity and mortality (8), as well as scientific advancements on the potential role of genetics as a prognostic indicator of these disorders.
Emerging genomic discoveries in behavioral health include the ability to predict modifiable disease risk and treatment responsiveness. For instance, unequivocal evidence shows that genetic variation in nicotinic receptor subunits and nicotine metabolism genes drives differences in smoking behaviors and risk of smoking-related diseases (9–14). Genetic information that highlights personalized risks of smoking, benefits of quitting, and advice on treatment approach may soon be primed for implementation into community and routine care settings. Other scientific advances involving alcohol- and obesity-related disease risk and personalized treatment will likely follow closely behind (15).
Recent studies indicate strong consumer demand for receiving genetic susceptibility test results related to smoking and other high-risk health behaviors (16–19). Unfortunately, there is a shortage of trained genetic counselors in the U.S. (20), with gaps particularly problematic in southern (21) and rural (22) areas. Furthermore, relatively few physicians have the time, knowledge, or resources to be able to counsel patients effectively in the context of behavioral genomics (23). One solution is to create scalable communication tools that reduce or eliminate the need for additional burden on genetic counselors and other healthcare professionals while also delivering valuable information to individuals in community settings. Indeed, many cancer risk assessment and communication tools are freely and widely available on the internet (24). This study presents a tool designed by content experts in health communication, visual design, genetics of addiction, and behavioral health intervention research to provide clinically valid genetic information on high-risk behavioral health disorders to individuals regardless of the availability of in-person professional guidance.
The evidence on behavioral effects of receiving genetic results has been mixed. Whereas one meta-analysis revealed little to no behavioral change following the return of genetic risk estimates for long-term health outcomes (e.g., one’s genetic risk for lung cancer) (25), another found increases in healthy behaviors >6 months after return of results among carriers of high-risk genotypes (26). Importantly, neither of these meta-analyses found evidence to support prevailing concerns about negative behavioral or psychological impacts (e.g., sense of fatalism, risk compensation, adverse effects on depression or anxiety) following return of results. Relatedly, a study among college students found that receipt of genetic susceptibility results on nicotine dependence reduced smoking without producing detrimental effects (27).
A key limitation of prior research that may account for the mixed findings is that many studies did not also intervene on health cognitions that are known to promote behavior change, such as self-efficacy, response efficacy, and self-regulation and problem solving (28,29). In contrast, the Your Disease Risk tool (www.yourdiseaserisk.org), as an example, was designed to target constructs most likely to motivate behavior change. When compared to a static list of risk factors, this tool elicited more health-promoting cognitions such as self-efficacy, response efficacy, and behavioral intentions (30).
Research is also needed to examine the extent to which incorporating genetic information as part of an evidence-based intervention may enhance behavioral activation through more personally engaging, salient, and targeted messaging. Thus, a key research direction moving forward is to optimize the design of genetically-informed behavioral health interventions that can then be rigorously evaluated for the potential to motivate positive behavior change and increase use of tailored treatments to reduce disease risk (31,32). The genetic risk tool presented here builds on these key insights, aiming to design a scalable communication tool that enhances evidence-based interventions and activates behavior change to address high-risk behavioral health disorders.
A long line of research has sought to identify the best strategies for communicating genetic risks related to cancer (33). Risk communication scientists have combined knowledge from the genetic risk communication literature and the general risk communication literature to develop an overarching set of principles for the effective communication of probabilistic risk estimates (e.g., use visual displays, provide recommendations for reducing risk (34,35). However, the genetics of behavioral health disorders has unique features that must be accommodated. In qualitative work (36–38), researchers found that some smokers are skeptical of a genetic basis for nicotine addiction, a concept which may reduce perceptions of agency in quitting smoking. However, many smokers strongly endorsed the benefits of using genetic information to inform treatment (e.g., use of smoking cessation medications), a process which may regain one’s sense of agency. Thus, any behavioral health intervention that includes genetic information should also emphasize that individuals still have the power to change their high-risk health behaviors.
As we develop the scientific evidence base for genetics in behavioral health, we must also develop the tools to communicate the application of this science to consumers, referred to herein as end-users, who stand to benefit from this information. However, too little research has focused on the design and communication of genetically-informed interventions, which are necessary components to maximize the personal utility of these tools. The goal of this study was to engage potential end-users in participatory co-design of a personalized genetically-informed risk tool to intervene on high-risk health behaviors such as smoking, harmful alcohol use, and poor diet. This three-step process involved (1) quantitative examination of end-user attitudes toward and interest in receiving personalized genetic results related to high-risk health behaviors, (2) qualitative interviews to iteratively design a genetically-informed behavioral health intervention, and (3) quantitative assessment of end-user acceptability and usability of the intervention. Advancing our understanding of these three pieces of information can fill current knowledge gaps on the role of genetically-informed feedback to guide behavioral health decision-making and develop more personalized interventions moving forward.
MATERIALS AND METHODS
This three-step study was conducted after approval by the institutional review board in the Human Research Protection Office at Washington University in St. Louis (IRB ID: 201704049). In Step One, all participants provided written informed consent prior to enrollment. In Steps Two and Three, all participants provided verbal consent upon reviewing an IRB-approved information sheet. This study was conducted in accordance with recognized ethical guidelines (e.g., Declaration of Helsinki, CIOMS, Belmont Report, and U.S. Common Rule).
Step One: Attitudes and Interest in Genetic Results for Behavioral Health (Quantitative)
Sample.
Participants included adults aged 21 or older (N=111) who were current smokers of tobacco from the Greater St. Louis, MO region. Participants were recruited from existing institutional registries of individuals who had previously reported to be current smokers and who also indicated interest in being contacted for clinical research studies. An initial postal mail recruitment letter was sent, followed by up to three phone calls or emails. Participants were also recruited through word-of-mouth, posted flyers, and online ads on recruitment websites. This study was approved by an institutional review board in the Human Research Protection Office at Washington University in St. Louis (IRB ID: 201704049). Recruited participants were screened for self-reported past month smoking prior to enrollment. The sample was broadly diverse across demographics, including sex (59% female), race (59% Caucasian, 36% African-American), age (range 21 to 82; mean=47.8), and education level (30% completing a 4-year college degree).
Design and Measures.
Via in-person structured interviews in a research laboratory setting, participants responded to a 2-item measure of their current smoking (frequency and heaviness of smoking in past 30 days), 3-item measure of alcohol use (frequency, heaviness, and binge drinking in past 30 days), 9-item measure of attitudes toward receiving, using and sharing genetic risk results for smoking and alcohol-related diseases (5-point Likert-type scale: Strongly Disagree=1; Strongly Agree=5), and demographic information. Questions stemmed from the Patient Baseline Knowledge and Attitudes of Genetic Testing Survey published in the Spark Toolbox for the Implementing Genomics in Practice (IGNITE) project (https://gmkb.org/) (39); this survey provided the following sample item on which our attitudinal items were based: ‘It is a good idea to xxxxx [e.g., get genetic testing] to find out whether yyyyy [e.g., you are at risk for getting a common disease…]’. Gaining this understanding of attitudes and interest in genetic results for behavioral health disorders was critical before embarking on the design of a genetic risk tool in collaboration with end-users (Step Two).
Step Two: Iterative Design of Genetic Risk Report (Qualitative)
Sample.
Participants were a convenience sample of adults (N=100) commuting on active pedestrian thoroughfares on the local hospital, college, and medical school campuses. They included healthcare professionals, academicians, students, patients, or other members of the public, recruited at various times of day over a 5-week period. A subset of hospitalized patients who smoke (n=8) were purposefully recruited from a smoking area immediately outside the hospital, as this group had not been reached via convenience sampling. Participants self-identified as medical professionals (43%) and non-medical audience members (57%).
Design and Measures.
The purpose of designing a genetic report for high-risk health behaviors was to effectively communicate one’s personalized risks, benefits, and recommended courses of action, as informed by an algorithm of raw genotyped data from a direct-to-consumer genotyping service (e.g., 23andMe). The goal of this step was to garner feedback on evolving versions of a potential genetically-informed intervention tool, named “Your Genetic Risk Profile”—for smoking, alcohol, and obesity. To achieve this goal, we utilized a design thinking framework (40) that prioritized understanding of end-user needs and desires to approach intervention development in a more informed and context-sensitive manner. Consistent with this framework, we incorporated principles of rapid prototyping (i.e., testing multiple rough versions in quick succession) and a “doing-to-think” approach (i.e., immediate hands-on testing with very little priming or introduction) to accelerate discovery of unforeseen reactions to the report (40). We employed a specific type of rapid prototyping—contextual prototyping—comprised of activities (described in next paragraph) to refine our understanding of potential end-users’ ecological experience of the genetic risk report by testing prototypes within their daily environment. A key feature of contextual prototyping is to avoid explaining how the prototype is ‘supposed’ to work, instead allowing the end-user to experience the tool (i.e., genetic risk report) with little or no direction and then observing the outcome (e.g., questions asked, reactions expressed, suggestions offered). Two members of the research team (AD and MZ) conducted brief semi-structured interviews with participants, using contextual prototyping to anchor the participatory design process.
Via contextual prototyping, participants were asked to reflect on iterative versions of the sample report as if it were a personalized report and to state their perception of the primary message being communicated by the report. Each participant reviewed only one version of the report. Building on prior stakeholder-engaged intervention design (41), participants were given the opportunity to provide open-ended feedback on and suggested changes to the content (e.g., usefulness, meaningfulness) and format (e.g., clarity, conciseness) of the report. If participants had difficulty generating feedback, interviewers offered prompts to examine the potential usefulness (e.g., “How do you envision this tool being used?”), meaningfulness (e.g., “How might this tool be personally valuable?”), clarity (e.g., “How can the language or visuals be modified to make the key messages more obvious?”), and conciseness (e.g., “What elements are not useful that we should consider removing?”).
This step was complete when design feedback tapered, indicating saturation of themes regarding content and format of the report. The collective research team comprised of interdisciplinary content experts in health communication, visual design, genetics of addiction, and behavioral health intervention research conducted iterative analysis of the design-oriented feedback to inform development of subsequent versions. Following the conclusion of this iterative design and feedback process, the final step was to quantitatively assess the acceptability and potential utility of the report among end-users (Step Three).
Step Three: Acceptability and Usefulness of the Report (Quantitative)
Sample.
Similar to the qualitative design interviews, participants included a convenience sample of adults (N=100) recruited from active pedestrian thoroughfares on the local hospital, college, and medical school campuses. They included healthcare professionals, academicians, students, patients, or other members of the public, recruited at various times of day over a 2-week period. Participants self-identified as medical professionals (47%) and non-medical audience members (53%).
Design and Measures.
Approximately one-third (n=34) of the sample was randomly assigned to view the smoking-related report, one-third (n=33) to view the alcohol-related report, and one-third (n=33) to view the weight-related report. Two members of the research team (AD and MZ) administered a brief 12-item questionnaire regarding the sample report. Participants were asked about their interest in learning their genetic risk for the phenotypes of (1) smoking and related diseases, (2) high-risk drinking and related diseases, and (3) high-risk weight and related diseases, and (4) receiving a personalized genetic risk report similar to the prototype report they were assigned to review (each with 5-point scale: Not at all, Somewhat, Moderately, Very, Extremely). Participants were also asked to endorse (i.e., check all that apply) whether they found their assigned genetic risk report to be useful across various purposes, including (5) to learn something new about myself/my health, (6) to reduce shame/stigma associated with certain health behaviors (e.g., weight, smoking), (7) to help motivate behavior change, (8) to advise specific actions to take based on the results, as well as (9) which of these purposes is the most useful aspect of this report. Finally, participants were asked to (10) report via free response the perceived key messages communicated in the report, (11) select their preference between the original and final versions of the genetic risk report, and (12) identify as either a professional in the medical field or as a non-medical member.
RESULTS
Step One: Attitudes and Interest in Genetic Results for High-Risk Health Behavior (Quantitative)
Approximately 83% of participants smoked every day over the past 30 days and had smoked an average of 13.3 cigarettes per day. Additionally, 77% of participants drank alcohol at least once over the past 30 days (averaging 1.1 drinks per day), and among these participants, 53% had engaged in binge drinking at least once over the past 30 days. As shown in Figure 1, a clear majority of participants agreed or strongly agreed that it is important to get genetic testing to find out one’s likelihood of the following outcomes: developing smoking-related diseases like lung cancer and emphysema (90%), responding to smoking cessation medications (87%), becoming addicted to nicotine (80%), developing alcohol-related diseases like liver disease or esophageal cancer (93%), becoming addicted to alcohol (89%), and getting common diseases (86%).
Figure 1.
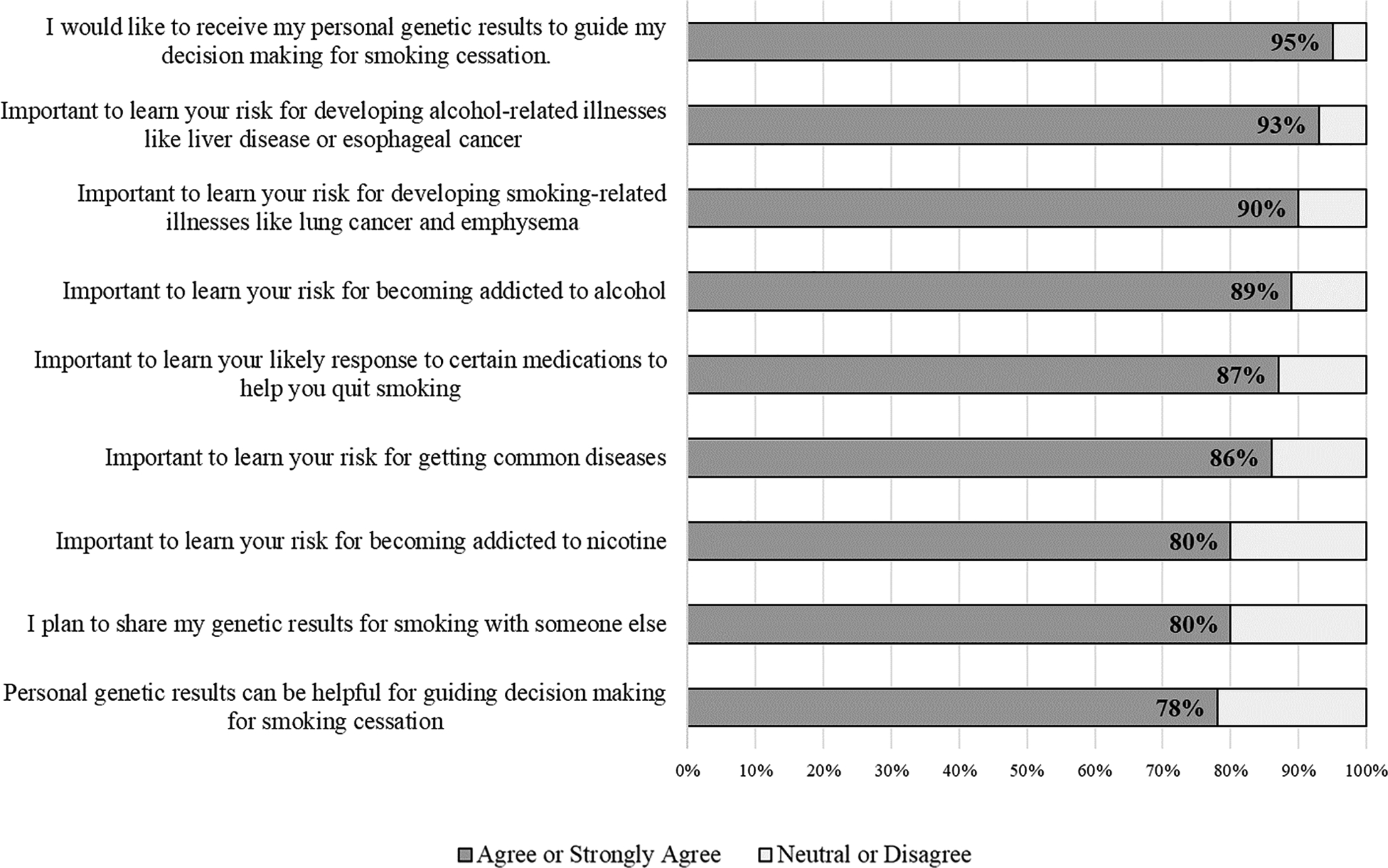
Favorable attitudes toward receiving personalized genetic risk results for smoking and alcohol (N=111).
Most participants agreed or strongly agreed that they would like to receive their personal genetic results to guide decision making for smoking cessation (95%), that they plan to share their genetic results for smoking with someone else (80%), and that personal genetic results can be helpful for guiding decision making for smoking cessation (78%). Participants reported an intent to share genetic results most commonly with siblings (56%), spouse or partner (46%), friends (45%), parents (43%), children (39%), and a doctor or other healthcare professional (24%). The positive attitudes and interest in receiving personalized genetic risk results motivated efforts to design a genetic risk tool collaboratively with a sample of potential end-users.
Step Two: Iterative Design of Genetic Risk Report (Qualitative)
Through qualitative semi-structured interviews in response to evolving prototypes of the genetic risk report, thematic analysis identified several key themes. These included the perceived key messages communicated, positive and negative issues related to content and format, and suggested changes to the report. Participants perceived the key messages to be (1) “I am at/high/very high genetic risk for these diseases”, (2) “I can reduce my risk by changing my behavior”, and (3) “Medications and other treatment options are recommended”. Participants identified a number of positive features about the content (e.g., “This isn’t sugar-coating it, which is a good thing”) and the formatting from the early versions (e.g., “The ‘What to Do’ section is direct and straightforward”), which served as anchor points during the iterative design.
Participants also had negative remarks about content (e.g., “Some people will have questions about the meaning of genetic markers”) and formatting (e.g., “Too much repetitive text; don’t like the color scheme”; “don’t like the name ‘Report Card’ because it associates it with grades”), which guided targeted design changes to the genetic risk profile. In addition, participants gave very clear recommendations for specific changes throughout the iterative design process (e.g., “add background information about genetics”; “show risk along a continuum”; “add info-graphics for resources and treatment options at the end”; “make it into a brochure so that you can expand on content and guide the participant through the risk profile”).
As a tangible example of how this collective feedback was integrated, the original one-page “Report Card” was ultimately transformed into a tri-fold brochure with (1) a front page intended to be visually appealing, (2) an inner flap that offers a brief explanation of the chromosome regions examined and the process of using an individual’s genetic (e.g., single nucleotide polymorphisms) and phenotypic data to create a risk algorithm, (3) the inside of the brochure which depicts risks along a continuum and provides actionable information about the benefits of healthy behavior changes, and (4) a back page that refers the individual to specific “take-home” resources that are freely available and provides treatment recommendations to facilitate healthy behavior changes. Following the rapid and iterative design of the report using qualitative interview data, the next logical aim was to examine the acceptability and perceived usefulness of the tool among a new sample reflecting a broad array of potential end-users.
Step Three: Acceptability and Usefulness of the Report (Quantitative)
Altogether, we tested four iterative versions of the sample report. Versions 1 (n=42), 2 (n=40), and 3 (n=18) were evaluated in Step Two. Based on the collective feedback from Step Two, version 4 was created and tested quantitatively in Step Three (n=100). A clear majority (79%) of participants preferred the newest version of the genetic risk report over the original version (Figure 2). After viewing the template genetic risk report to which they were assigned, participants indicated high interest in learning about genetic risk results for several high-risk behaviors (75–89%) as well as in receiving their own personalized genetic risk tool (86%) (Figure 3). Of note, 32 of the 34 participants (94%) who viewed the sample smoking-related genetic risk report expressed desire to receive a similar personalized report. Similarly, 28 of the 33 participants (85%) who viewed the sample alcohol-related report, and 26 of the 33 participants (79%) who viewed the sample obesity-related report, expressed desire to receive a similar personalized report.
Figure 2.

Evolving design toward the “Genetics and Smoking Risk Profile” in brochure format.
Figure 3.
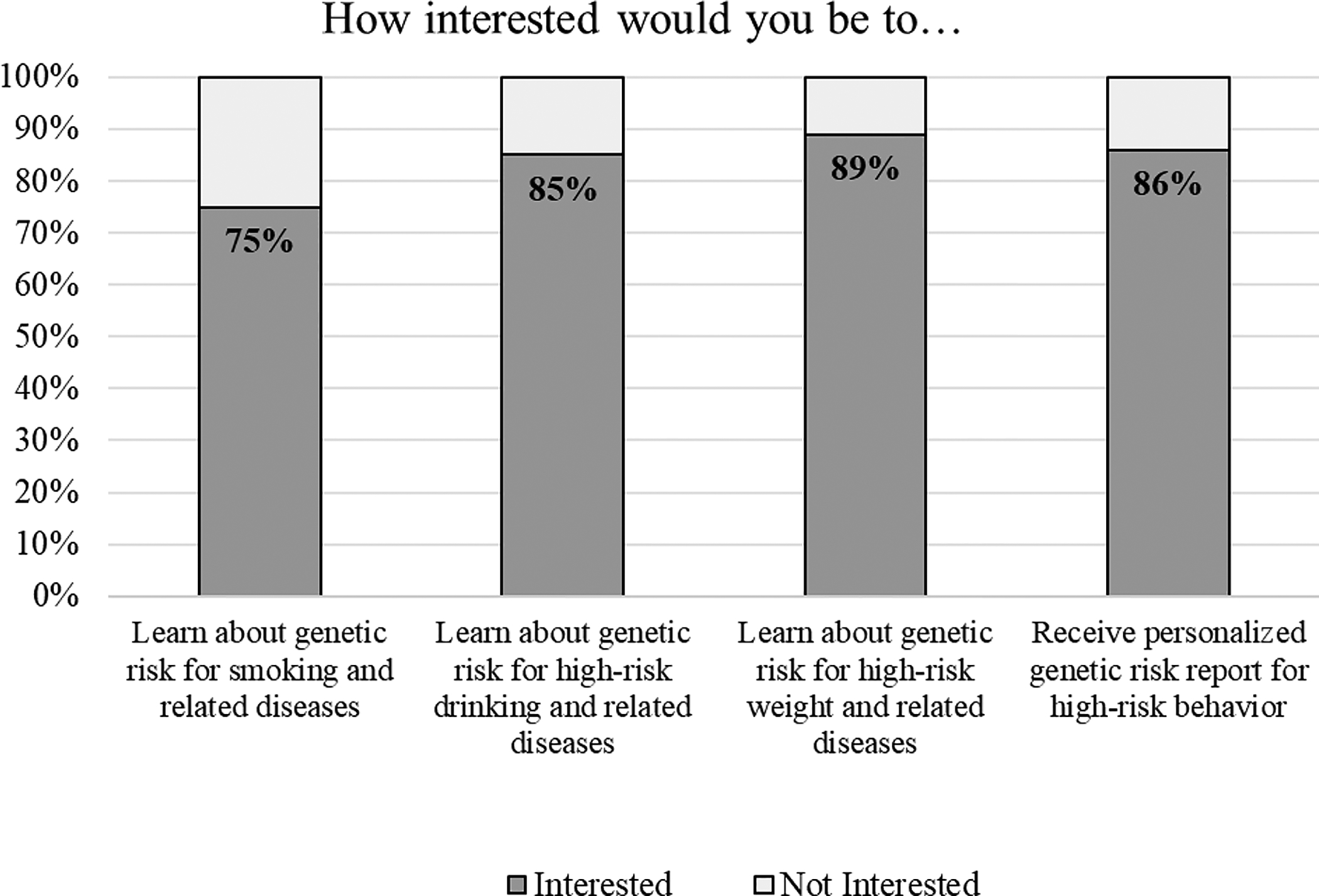
High interest for a personalized tool to communicate genetic risk results for several high-risk behaviors (N=100).
Participants also reported that the genetic risk tool would be highly useful for learning something new about their health (83%), followed by motivating behavior change (81%), advising specific actions to take (78%), and reducing shame or stigma associated with addiction (27%). When asked about the most useful purpose of the tool, participants cited motivating behavior change (42%), followed by advising specific actions to take (27%) (Figure 4). Finally, 88% demonstrated sufficient understanding of the genetic risk report and accurately reported key messages communicated in the report (i.e., level of hypothetical genetic risk being conveyed, ways to reduce risks through behavior change, medications/treatments recommended). For all variables, there were no significant differences between medical professionals and non-medical audiences (all ps ≥ .154).
Figure 4.
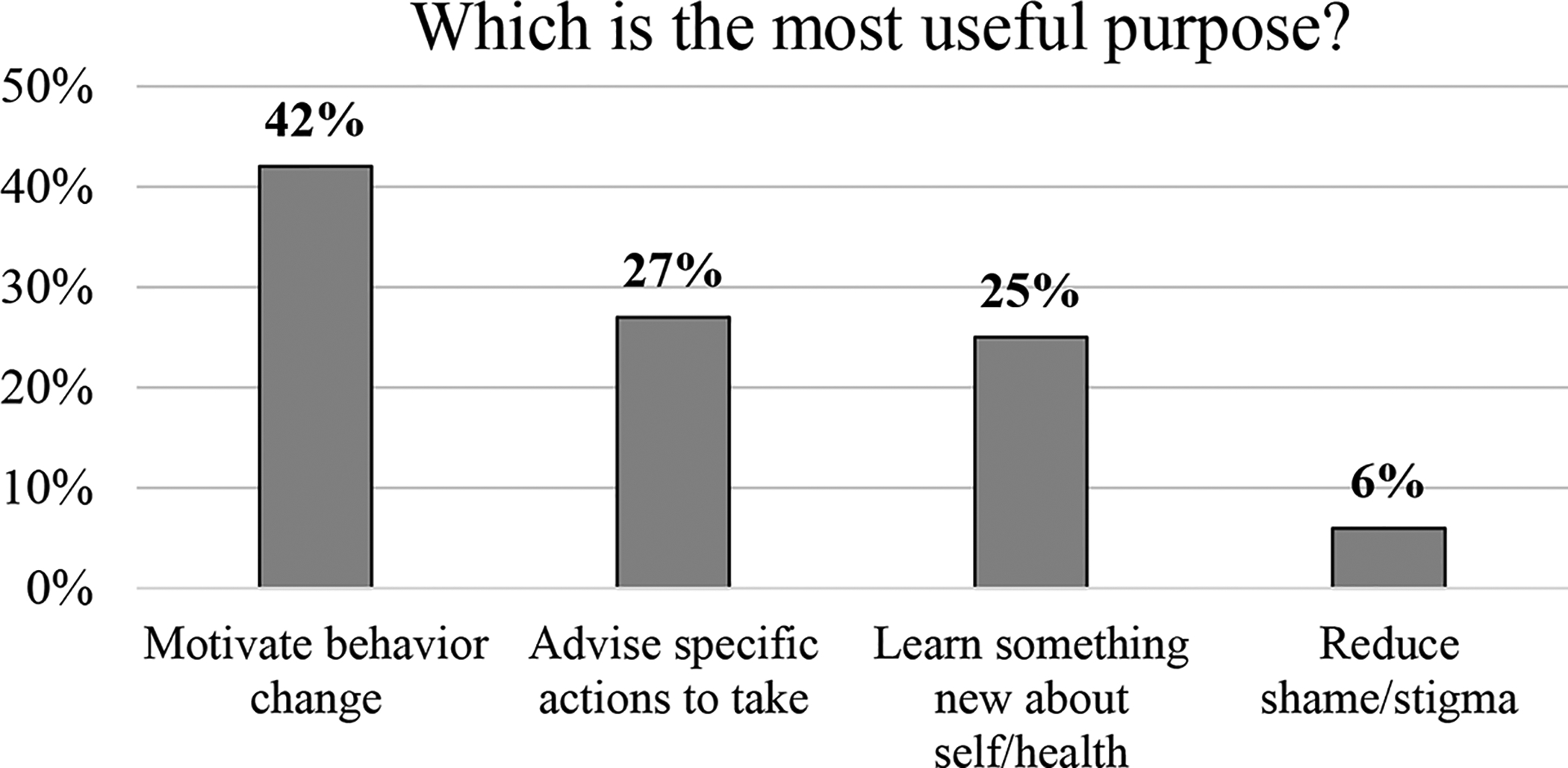
High perceived usefulness of genetic risk tool to motivate behavior change and advise specific actions (N=100).
Of note, we also evaluated the readability and accessibility of the final genetic risk report by using the well-validated Flesch-Kincaid Grade Level Index (42,43) as applied by the built-in Microsoft Word tool. Despite the inherently complex nature of communicating genetic information, the Flesch-Kincaid Grade Level Index for the genetic risk tool was 8.1, which is consistent with efforts to provide health education materials that are accessible to low literacy and marginal literacy levels (44). This grade level index of 8.1 also compares favorably with prior research findings that diabetes education materials were written at the 10th grade level (45) and breast cancer risk assessment tools were written at the 12th grade level (46). As we continue to refine the genetic risk profile in collaboration with specific intended audiences (e.g., current smokers, patients with risky alcohol use, individuals with varying health literacy levels), we expect to identify additional opportunities to enhance the readability and accessibility of the profile.
DISCUSSION
This three-part study yielded a genetically-informed risk profile that, among a broad sample of potential end-users, demonstrated promise for supporting interventions on risky health behaviors. We first confirmed findings from prior research (18,19) demonstrating strong demand and interest in receiving personalized genetic results for risky health behaviors among individuals who smoke. We then co-designed a personalized genetic risk profile with potential end-users and subsequently found high acceptability, comprehensibility, and perceived usefulness of the personalized genetic risk profile. Of note, the absence of group differences between medical and non-medical audiences’ understanding of and interest in the profile highlights the potential for wide appeal of the tool to scientific and lay audiences alike.
By incorporating personalized genetic information and end-user participatory design feedback into the development of a behavioral health intervention, this study advances toward the goal of enhancing health behavior decision making through more personally engaging, salient, and targeted messaging (47). Although prior research on motivational and behavioral effects of returning genetic results has been mixed (25–27), studies have only begun to explore ways to optimally communicate this information, recognizing the need to include sufficient resources to support end-users as they bridge the intention-behavior gap (28,29). Participatory approaches that are central to design thinking and human-centered design processes offer a promising step toward effectively incorporating genetic information into existing behavioral health intervention (40,48). Furthermore, interdisciplinary collaborations comprised of expertise in genetics, health communication, visual arts and design, and implementation science, among others, are likely to yield well-designed and compelling genetically-informed behavioral health interventions (47).
The integration of genomic medicine into practice is still in its infancy. Countless scientific discoveries will continue to advance and refine our understanding of the role of genomic data in clinical care (49). However, the unprecedented potential of genomic data to engage populations and personalize treatment approaches depends in part on preparatory studies to design for the future implementation of personalized genetic risk tools for behavioral health disorders (47,50). As scientific discovery in genomic medicine progresses, we must develop the tools to communicate these discoveries to affected individuals. Enhancing the rigor and real-world relevance of tools to communicate genomic results for high-risk health behaviors in multiple contexts—clinical, community, and direct-to-consumer—will generate long-range, translatable findings that will greatly benefit the field as genomic medicine continues to advance.
This study has several limitations. In examining multiple high-risk health behaviors, our participatory co-design samples included but did not focus on populations with characteristics of current smoking, high-risk drinking, or high-risk weight. In fact, we did not collect data on demographic or substance use in these samples for two key reasons: (1) the unique time constraints of convenience sampling on busy pedestrian thoroughfares, which demanded expediency among uncompensated participants, and (2) our decision to conduct efficient, rapid prototyping with a relatively large sample to improve the tool. Although we prioritized rapid feedback from many potential end-users, our convenience sample from hospital, college, and medical school campuses can be expected to have behavioral and socioeconomic differences from a sample that meets specific criteria for smoking, drinking, and weight. For instance, it is possible that even the non-medical professionals sampled within our rapid prototyping process may have been more highly educated than the intended audience of the risk profile.
We also acknowledge that the sample risk reports to which participants were exposed only indicated high levels of risk. Although we purposefully opted to limit the number of variables to test in our iterative design process and to explore the potential utility of the tool to motivate behavior change, we encourage others to examine lower-risk communication using this and other tools in future research. Although we expect that most aspects of the tool are fairly robust and applicable across sectors of the population, certain aspects of the tool would need to be adapted for individuals with lower risk, such as replacing cessation advice and resources with reinforcing messages and resources to remain smoke-free within the “What To Do” section. In addition, the sizable potential impact of this tool is currently limited by our knowledge of its effectiveness in real-world settings as well as its potential scalability through digital (web and mobile) health tools. Even at this early stage, however, the demonstrated high demand for health-related genetic risk information followed by considerable efforts with a broad array of potential end-users to co-design an acceptable personalized genetically-informed risk profile, bodes well for the potential utility of this tool.
This study provides proof of concept and a useful starting point for development of a genetically-informed intervention—a formative step that must be validated and, more appropriately, adapted to fit the needs of various target populations for the tool moving forward. Building on these data and materials, future studies can further refine the development and adaptation of the genetically-informed risk profile. Moving forward, efficacy and effectiveness testing is needed in which investigators return genetic results on high-risk health behaviors using this tool and then assess the extent to which individuals make behavioral changes including, for example, increased cessation attempts and use of medications and other interventions to quit or reduce smoking. This genetically-informed risk profile must undergo rigorous intervention testing in clinical, direct-to-consumer, or other community-based settings to demonstrate its potential for engaging high-risk individuals and driving behavior change to reduce disease risk.
ACKNOWLEDGMENTS
A.T. Ramsey was supported by the National Institute on Drug Abuse (NIDA) grant K12DA041449. J.L. Bourdon was supported by NIDA grant T32DA015035. L-S. Chen was supported by the National Cancer Institute (NCI) grant P30CA091842-16S2 and NIDA grant R01DA038076. L.J. Bierut was supported by NIDA grant R01DA036583, National Center for Advancing Translational Sciences grant UL1TR002345, and NCI grant P30CA091842.
Footnotes
Conflict of Interest: L.J. Bierut is listed as an inventor on Issued U.S. Patent 8,080,371 “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction, and served as a consultant for the pharmaceutical company Pfizer Inc. (New York City, New York, USA) in 2008. The remaining authors declare no potential conflicts of interest.
REFERENCES
- 1.Collins FS, Varmus H. A New Initiative on Precision Medicine. N Engl J Med. 2015. February 26;372(9):793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley EA. The precision medicine initiative: A new national effort. JAMA. 2015. June 2;313(21):2119–20. [DOI] [PubMed] [Google Scholar]
- 3.Allenby CE, Boylan KA, Lerman C, Falcone M. Precision Medicine for Tobacco Dependence: Development and Validation of the Nicotine Metabolite Ratio. J Neuroimmune Pharmacol. 2016. February 12;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley WT, Nilsen WJ, Manolio TA, Masys DR, Lauer M. News from the NIH: potential contributions of the behavioral and social sciences to the precision medicine initiative. Transl Behav Med. 2015. September;5(3):243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts MC, Kennedy AE, Chambers DA, Khoury MJ. The current state of implementation science in genomic medicine: opportunities for improvement. Genet Med. 2017. August;19(8):858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson JA, Weitzel KW. Advancing Pharmacogenomics as a Component of Precision Medicine: How, Where, and Who? Clin Pharmacol Ther. 2016;99(2):154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury MJ, Iademarco MF, Riley WT. Precision Public Health for the Era of Precision Medicine. Am J Prev Med. 2016. March;50(3):398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García MC. Potentially Preventable Deaths Among the Five Leading Causes of Death — United States, 2010 and 2014. MMWR Morb Mortal Wkly Rep [Internet]. 2016. [cited 2019 Dec 27];65 Available from: https://www.cdc.gov/mmwr/volumes/65/wr/mm6545a1.htm [DOI] [PubMed] [Google Scholar]
- 9.Bierut LJ, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007. January 1;16(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008. April 3;452(7187):638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008. May;40(5):616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saccone NL, Culverhouse RC, Schwantes-An T-H, Cannon DS, Chen X, Cichon S, et al. Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: a Meta-Analysis and Comparison with Lung Cancer and COPD. PLOS Genet. 2010. August 5;6(8):e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L-S, Hung RJ, Baker T, Horton A, Culverhouse R, Saccone N, et al. CHRNA5 Risk Variant Predicts Delayed Smoking Cessation and Earlier Lung Cancer Diagnosis—A Meta-Analysis. J Natl Cancer Inst. 2015. May 1;107(5):djv100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L-S, Baker T, Hung RJ, Horton A, Culverhouse R, Hartz S, et al. Genetic Risk Can Be Decreased: Quitting Smoking Decreases and Delays Lung Cancer for Smokers With High and Low CHRNA5 Risk Genotypes - A Meta-Analysis. EBioMedicine. 2016. September;11:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera AV, Chaffin M, Wade KH, Zahid S, Brancale J, Xia R, et al. Polygenic Prediction of Weight and Obesity Trajectories from Birth to Adulthood. Cell. 2019. April 18;177(3):587–596.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartz SM, Olfson E, Culverhouse R, Cavazos-Rehg P, Chen L-S, DuBois J, et al. Return of individual genetic results in a high-risk sample: enthusiasm and positive behavioral change. Genet Med. 2015. May;17(5):374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olfson E, Hartz S, Carere DA, Green RC, Roberts JS, Bierut LJ, et al. Implications of Personal Genomic Testing for Health Behaviors: The Case of Smoking. Nicotine Tob Res. 2016. July 12;ntw168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu A, Hartz S, Smock N, Chen J, Qazi A, Onyeador J, et al. Most Current Smokers Desire Genetic Susceptibility Testing and Genetically-Efficacious Medication. J Neuroimmune Pharmacol. 2018. December 1;13(4):430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senft N, Sanderson M, Selove R, Blot WJ, King S, Gilliam K, et al. Attitudes towards precision treatment of smoking in the Southern Community Cohort Study. Cancer Epidemiol Prev Biomark [Internet]. 2019. January 1 [cited 2019 Nov 11]; Available from: https://cebp.aacrjournals.org/content/early/2019/06/01/1055-9965.EPI-19-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg J, Hoskovec J, Hashmi SS, McCarthy Veach P, Ownby A, Singletary CN. Relieving the Bottleneck: An Investigation of Barriers to Expansion of Supervision Networks at Genetic Counseling Training Programs. J Genet Couns. 2018;27(1):241–51. [DOI] [PubMed] [Google Scholar]
- 21.Villegas C, Haga SB. Access to Genetic Counselors in the Southern United States. J Pers Med. 2019. July 1;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penon-Portmann M, Chang J, Cheng M, Shieh JT. Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med Off J Am Coll Med Genet. 2020;22(1):227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubanovich CK, Cheung C, Mandel J, Bloss CS. Physician preparedness for big genomic data: a review of genomic medicine education initiatives in the United States. Hum Mol Genet. 2018. August 1;27(R2):R250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waters EA, Sullivan HW, Nelson W, Hesse BW. What is my cancer risk? How internet-based cancer risk assessment tools communicate individualized risk estimates to the public: content analysis. J Med Internet Res. 2009. July 31;11(3):e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollands GJ, French DP, Griffin SJ, Prevost AT, Sutton S, King S, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ [Internet]. 2016. March 15 [cited 2019 Dec 16];352 Available from: https://www.bmj.com/content/352/bmj.i1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frieser MJ, Wilson S, Vrieze S. Behavioral impact of return of genetic test results for complex disease: Systematic review and meta-analysis. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2018. December;37(12):1134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipkus IM, Schwartz-Bloom R, Kelley MJ, Pan W. A Preliminary Exploration of College Smokers’ Reactions to Nicotine Dependence Genetic Susceptibility Feedback. Nicotine Tob Res. 2015. March 1;17(3):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C-Q, Zhang R, Schwarzer R, Hagger MS. A meta-analysis of the health action process approach. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2019. July;38(7):623–37. [DOI] [PubMed] [Google Scholar]
- 29.Moller AC, Merchant G, Conroy DE, West R, Hekler EB, Kugler KC, et al. Applying and advancing behavior change theories and techniques in the context of a digital health revolution: Proposals for more effectively realizing untapped potential. J Behav Med. 2017. February;40(1):85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler SL, Klein WMP, Ball L, McGuire J, Colditz GA, Waters EA. Using an Internet-Based Breast Cancer Risk Assessment Tool to Improve Social-Cognitive Precursors of Physical Activity. Med Decis Mak Int J Soc Med Decis Mak. 2017;37(6):657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsey AT, Chen L-S, Hartz SM, Saccone NL, Fisher SL, Proctor EK, et al. Toward the implementation of genomic applications for smoking cessation and smoking-related diseases. Transl Behav Med. 2018. January 29;8(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bierut LJ, Tyndale RF. Preparing the Way: Exploiting Genomic Medicine to Stop Smoking. Trends Mol Med. 2018. February 1;24(2):187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welkenhuysen M, Evers-Kiebooms G, d’Ydewalle G. The language of uncertainty in genetic risk communication: framing and verbal versus numerical information. Patient Educ Couns. 2001. May;43(2):179–87. [DOI] [PubMed] [Google Scholar]
- 34.Trevena LJ, Zikmund-Fisher BJ, Edwards A, Gaissmaier W, Galesic M, Han PKJ, et al. Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zikmund-Fisher BJ. The right tool is what they need, not what we have: a taxonomy of appropriate levels of precision in patient risk communication. Med Care Res Rev MCRR. 2013. February;70(1 Suppl):37S–49S. [DOI] [PubMed] [Google Scholar]
- 36.Philpott SE, Gehlert S, Waters EA. Smokers’ unprompted comments on cigarette additives during conversations about the genetic basis for nicotine addiction: a focus group study. BMC Public Health. 2018. 13;18(1):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waters EA, Ball L, Carter K, Gehlert S. Smokers’ beliefs about the tobacco control potential of “a gene for smoking”: a focus group study. BMC Public Health. 2014. November 25;14:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waters EA, Ball L, Gehlert S. “I don’t believe it.” Acceptance and skepticism of genetic health information among African-American and White smokers. Soc Sci Med 1982. 2017;184:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orlando LA, Sperber NR, Voils C, Nichols M, Myers RA, Wu RR, et al. Developing a common framework for evaluating the implementation of genomic medicine interventions in clinical care: the IGNITE Network’s Common Measures Working Group. Genet Med. 2018. June;20(6):655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts JP, Fisher TR, Trowbridge MJ, Bent C. A design thinking framework for healthcare management and innovation. Healthcare. 2016. March 1;4(1):11–4. [DOI] [PubMed] [Google Scholar]
- 41.Lennox L, Doyle C, Reed JE, Bell D. What makes a sustainability tool valuable, practical and useful in real-world healthcare practice? A mixed-methods study on the development of the Long Term Success Tool in Northwest London. BMJ Open [Internet]. 2017. September 1 [cited 2019 Dec 27];7(9). Available from: https://bmjopen.bmj.com/content/7/9/e014417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flesch R. A new readability yardstick. J Appl Psychol. 1948;32(3):221–33. [DOI] [PubMed] [Google Scholar]
- 43.Kincaid J, Fishburne R, Rogers R, Chissom B. Derivation Of New Readability Formulas (Automated Readability Index, Fog Count And Flesch Reading Ease Formula) For Navy Enlisted Personnel. Inst Simul Train [Internet]. 1975. January 1; Available from: https://stars.library.ucf.edu/istlibrary/56 [Google Scholar]
- 44.Cotugna N, Vickery CE, Carpenter-Haefele KM. Evaluation of literacy level of patient education pages in health-related journals. J Community Health. 2005. June;30(3):213–9. [DOI] [PubMed] [Google Scholar]
- 45.Hosey GM, Freeman WL, Stracqualursi F, Gohdes D. Designing and Evaluating Diabetes Education Material for American Indians. Diabetes Educ. 1990. October 1;16(5):407–14. [DOI] [PubMed] [Google Scholar]
- 46.Cortez S, Milbrandt M, Kaphingst K, James A, Colditz G. The readability of online breast cancer risk assessment tools. Breast Cancer Res Treat. 2015. November;154(1):191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein WMP, McBride CM, Allen CG, Arredondo EM, Bloss CS, Kaphingst KA, et al. Optimal Integration of Behavioral Medicine into Clinical Genetics and Genomics. Am J Hum Genet. 2019. February 7;104(2):193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matheson GO, Pacione C, Shultz RK, Klügl M. Leveraging Human-Centered Design in Chronic Disease Prevention. Am J Prev Med. 2015. April 1;48(4):472–9. [DOI] [PubMed] [Google Scholar]
- 49.Chambers DA, Feero W, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: A new model for biomedical research. JAMA. 2016. May 10;315(18):1941–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brownson RC, Colditz GA, Proctor EK. Dissemination and Implementation Research in Health: Translating Science to Practice. Oxford University Press; 2017. 545 p. [Google Scholar]


