Abstract
The purpose of this study was to identify critical pathways promoting survival of tamoxifen-tolerant, estrogen receptor α positive (ER+) breast cancer cells, which contribute to therapy resistance and disease recurrence. Gene expression profiling and pathway analysis was performed in ER+ breast tumors of patients before and after neo-adjuvant tamoxifen treatment and demonstrated activation of the NFκB pathway and an enrichment of EMT/stemness features. Exposure of ER+ breast cancer cell lines to tamoxifen, in vitro and in vivo, gives rise to a tamoxifen-tolerant population with similar NFκB activity and EMT/stemness characteristics. Small molecule inhibitors and CRISPR/Cas9 knock out were used to assess the role of the nuclear factor κB (NFκB) pathway and demonstrated that survival of tamoxifen-tolerant cells requires NFκB activity. Moreover, this pathway was essential for tumor recurrence following tamoxifen withdrawal. These findings establish that elevated NFκB activity is observed in breast cancer cell lines under selective pressure with tamoxifen in vitro and in vivo, as well as in patient tumors treated with neo-adjuvant tamoxifen therapy. This pathway is essential for survival and regrowth of tamoxifen-tolerant cells, and, as such, NFκB inhibition offers a promising approach to prevent recurrence of ER+ tumors following tamoxifen exposure.
Keywords: ER+ breast cancer, tamoxifen tolerance, tumor recurrence, NFκB pathway
Introduction
Over 70% of breast tumors express estrogen receptor α (ER) and are typically treated with endocrine therapies, such as tamoxifen or aromatase inhibitors. Despite the relative success of endocrine agents, resistance to treatment is common, resulting in metastatic relapse for which there is no cure. In fact, over 50% of recurrences and 2 out of every 3 deaths from ER+ breast cancer will occur after completing 5 years of adjuvant endocrine therapy (1, 2). One hypothesis to explain these late recurrences in ER+ disease, is that a small population of tumor cells “persist” in the presence of tamoxifen, for years to even decades, and eventually grow out as a recurrent tumor. These recurrent tumors are frequently therapy resistant, metastatic and lethal. Thus, one way to prevent lethal recurrence is to eradicate the population of persister cells. However, little is known about the nature of these cells in ER+ breast cancers or how they eventually contribute to tamoxifen resistance and disease recurrence.
The notion of a drug-tolerant persister cell population was first introduced in lung cancers treated with EGFR inhibitors (3) and subsequently described in other cancers (Reviewed in (4)). A drug-tolerant phenotype was shown to be transiently acquired via an altered chromatin state to protect the population from eradication by targeted therapeutics. Tolerant cells can display considerable plasticity with the ability to revert back to drug sensitivity once the drug is removed or to develop complete drug resistance (Reviewed in (5)). However, in breast cancer and particularly in ER+ breast cancer, the concept of drug tolerance is less well developed. For ER-negative breast cancers, some examples of treatment-induced drug tolerance have been reported. These include HER2-amplified breast cancer cells treated with lapatinib, which acquire dependency on the lipid hydroperoxidase GPX4 (6). In triple negative breast cancer, residual tumor cells remaining after treatment with doxorubicin and cyclophosphamide chemotherapies adopt a reversible drug-tolerant state that does not involve clonal selection, and pharmacologic inhibition of oxidative phosphorylation delayed residual tumor regrowth (7).
In ER+ breast cancers, traditional approaches to model therapy failure have largely focused on prolonged exposure of ER+ breast cancer cells to tamoxifen or estrogen deprivation. This approach is necessary to reach a stable resistant phenotype but may fail to elucidate early cellular adaptations that occur under therapeutic selection and that can contribute to the eventual development of resistance. While it is known that diverse mechanisms that give rise to endocrine therapy resistance, including perturbations to ER signaling complex (8, 9), crosstalk between ER and growth factor receptor signaling (10), and/or mutations in ESR1 gene (11, 12), it is not clear how these various mechanisms arise. Importantly, it was shown in lung cancer that multiple clones, each with different resistance mechanisms, can arise from a drug-tolerant/persister population, suggesting that drug-tolerant tumor cells are not limited in their evolution; rather, they may serve as a latent reservoir of cells capable of giving rise to multiple resistance mechanisms (13). Whether this occurs in ER+ breast cancer is unknown. Thus, the purpose of this study was to examine early changes in ER+ breast cancer cells and tumors under the selection pressure of tamoxifen and identify mechanisms that may contribute to their therapy tolerance. Clearly, eradication of a tamoxifen-tolerant tumor cell population would represent a highly promising new therapeutic strategy in the treatment of ER+ breast cancer.
In this report, we demonstrate that tamoxifen-tolerant persister cells can be identified in ER+ breast cancer cell lines, xenograft tumors, and samples from breast cancer patients. In each of the studied settings, activation of the pro-inflammatory NFκB pathway and a gain of epithelial-to mesenchymal (EMT)/stem-like features are observed. The NFκB pathway is required for survival and regrowth of tamoxifen-tolerant cells, as well as tumor recurrence. Our findings provide compelling evidence that pharmacological targeting of the NFκB pathway can be exploited to eradicate tamoxifen-tolerant cells to prevent disease recurrence.
Materials and Methods
Reagents
4-hydroxy-tamoxifen (4OHT) and dimethyl fumarate (DMF) were purchased from Sigma. IKK7 was purchased from Selleck Chemicals. TNFα was purchased from R&D Systems. The antibodies for ERα (8644), p52 (37359), c-Rel (4727), RelB (4922), and TBP (8515) were purchased from Cell Signaling. The antibodies for p65 (sc-372) and p50 (sc-8414) were purchased from Santa Cruz. The antibody for β-actin (A5441) was purchased from Sigma.
Gene Expression Analysis in Patient Tumor Samples
Tumor gene expression data was obtained from breast cancer patients (details in Supplementary Table 1) enrolled in a pre-operative window trial (ClinicalTrials.gov Identifier: NCT00738777), in which patients received an oral loading dose of 40 mg of tamoxifen twice daily for the first seven days, followed by a daily dose of 20 mg until surgery. The clinical trial protocol was approved by the local medical ethical authorities, in accordance with appropriate international ethical guidelines, and written informed consent was obtained from all patients. A core needle biopsy of the tumor was taken prior to treatment (n=62) and post-treatment tumor material (n=40) was obtained during surgery 20.7 (±9.6) days later. Paired material was available for 27/74 patients. RNA was isolated and hybridized to a custom full genome array by Agendia as previously described (14). Feature Extraction software v11.5.1.1 was used to quantify fluorescent intensities and those were normalized using DataPrint software v1.15. Missing values were imputed with knn=10, data was batch corrected for date of RNA extraction using ComBat from the R package sva, and the median value was used in case multiple probes mapped to a single gene. Expression data are available through Gene Expression Omnibus (GEO accession number GSE147271). Differential analysis between pre- and post-tamoxifen samples was performed with Limma v.3.37.3. Expression changes were considered significant if FC<−1 or FC>1 and adj.P.Val < 0.005 (Supplementary Table 2). Results were interpreted with GSEA software with MsigDBv7.0 Hallmark gene sets on default settings (15) (Supplementary Table 3). GSEA enrichment plots were generated with a custom r script by Dr. Thomas Kuilman (https://github.com/PeeperLab/Rtoolbox/blob/master/R/ReplotGSEA.R).
Cell Lines, Culture Conditions and Drug Treatments
The human ER+ breast cancer cell lines MCF-7, T47D, ZR75–1, and BT474, were obtained from Dr. Debra Tonetti (University of Illinois at Chicago). HCC1428 cells were purchased from ATCC. These cells were routinely maintained in RPMI 1640 media (Invitrogen Life Technologies) with phenol red supplemented with 10% FBS, 1% non-essential amino acids, 2 mmol/L L-glutamine, 1% antibiotics penicillin-streptomycin, and 6 ng/mL insulin. NFκB-RE-GFP cells were obtained from Dr. Elaine T. Alarid (University of Wisconsin-Madison). These cells were generated from MCF-7 cells stably transfected with 3X-κB reporter, which has three enhancer elements (Igκ, IκBα and the palindromic consensus sequence) upstream of the thymidine kinase promoter driving green fluorescent protein (GFP) expression, as described in (16). All cell lines are routinely authenticated by short tandem repeat analysis and tested for mycoplasma using LookOut Mycoplasma PCR Detection Kit (Sigma).
Clonogenic Assay
Cells were seeded at clonogenic density of 1,000 cells per well in 6-well plates in phenol red-proficient media with 10% complete FBS. After overnight attachment, cells were treated as indicated. Media was changed and fresh treatment added every 3–4 days for 2 weeks. After 2 weeks, colonies were stained with 1% crystal violet in methanol and water (1:4) and imaged using ImageJ software. Colony area was quantified automatically using the ColonyArea ImageJ plugin (17). Alternatively, plates were scanned with Celigo Imaging Cytometer (Nexcelom Bioscience). Confluence ratio is calculated in brightfield using the confluence application. For GFP+ cell, GFP expression is measured in green fluorescence using the confluence application.
RT-Quantitative PCR (QPCR)
Total RNA was isolated using Trizol and RT-QPCR performed as previously described (18). Fold change was calculated using the ΔΔCt method with 36B4 serving as the internal control. All QPCR primers used were validated and previously reported (18).
FACS Analysis
Clonogenic cells were trypsinized, washed and re-suspended in Hank’s Balanced Salt Solution buffer + 2% FBS. Flow cytometry of live cells was performed to quantify GFP+ cells using a Fortessa instrument (BD Biosciences). Sorting of live cells to isolate the GFP+ population was run using a MoFlo cell sorter (Beckman Coulter).
Western Blot
Whole cell extracts were prepared using the M-PER reagent (Thermo Scientific). Nuclear lysates were collected using NE-PER kit (Thermo Scientific). Proteins were separated by SDS-PAGE (Bio-Rad Laboratories), transferred to nitrocellulose membranes (Thermo Scientific), blocked for 1 hour in buffer containing 5% nonfat dry milk (Lab Scientific) or 5% bovine serum albumin, and incubated with the appropriate primary antibody overnight. The next day, secondary antibody was applied and the signal visualized on a Molecular Imager ChemidocXRS (Bio-Rad Laboratories) using the Pierce Supersignal West Pico chemiluminescent substrate (Thermo Scientific). Images were obtained using Quantity One software (Bio-Rad Laboratories).
Aldefluor Assay
ALDH1 activity assay (Stem Cell Technologies) and FACS analysis were conducted according to manufacturer’s instructions.
Mammosphere (MS) assay
Breast cancer cells were seeded at single cell density on low attachment plates in media described by Dontu et al. (19), supplemented with 1% methyl cellulose to prevent cellular aggregation. After 7 days, the number of MS ≥75μm in diameter was determined using a Celigo imaging cytometer, and MS forming efficiency (MFE) was calculated.
Immunofluorescence and Microscopy
Cells were seeded on non-coated glass coverslips and treated according to the clonogenic assay protocol. After treatment, cells were fixed with 4% paraformaldehyde for 15 minutes and permeabilized using 0.2% Triton X-100 for 20 minutes. Cells were then blocked with 1x casein for 1 hour and incubated with the primary antibodies for 1 hour. After washing with TBS, coverslips were incubated with the secondary antibodies for 1 hour: Alexa Fluor 594 (Thermo Scientific #A21207) and Alexa Fluor 488 (Thermo Scientific #A-11001). Cells were then washed with TBS and mounted with ProLong Gold anti-fade reagent (Life Technologies). Images were acquired at ×63 magnification using a Leica DMi8 microscope (Leica).
In vivo Studies
All mouse experiments were carried out at the University of Illinois at Chicago animal facility, and conducted in accordance with institutional procedures and guidelines, and after prior approval from the Institutional Animal Care and Use Committee. Female athymic nude mice (nu/nu), aged 5 week-old, were purchased from Harlan. Five million MCF-7 cells were injected orthotopically into the thoracic mammary glands and supplemented with estrogen pellets. Tumor formation was monitored by palpitation and once tumors were detected (~0.1 cm2 in size), mice were randomized into either vehicle control or treatment groups. To study NFκB pathway activation, mice were treated with either vehicle control or tamoxifen (500 μg/animal s.c., in peanut oil) for 5 days/week for 2 weeks (n=3 tumors per group). To study tumor recurrence, mice were treated with vehicle, DMF (30mg/kg, oral gavage suspended, in 0.8% methyl cellulose), tamoxifen (500 μg/animal s.c., in peanut oil), or the combination of DMF with tamoxifen daily 5 days/week for 4 weeks (n=10 tumors per group). Tumor sizes were measured daily with an electronic caliper and tumor area was calculated as length/2 × width × π.
Statistical Analysis
Data are presented as mean ± SEM from at least three independent determinations. Statistical analysis consisted of 1- or 2-way ANOVA followed by Tukey posttest, or t-test, as appropriate.
Results
Early changes in pathway activation status in ER+ tumors from patients treated with neo-adjuvant tamoxifen therapy
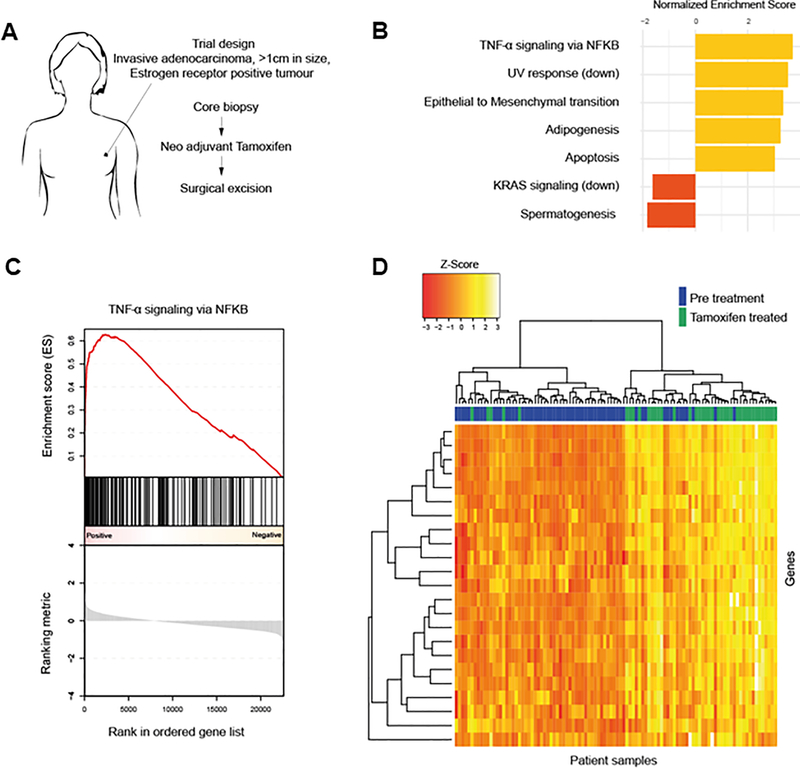
Data from a neo-adjuvant window trial allowed us to investigate early tamoxifen-induced effects in ER+ breast tumors (Fig. 1A). Patients underwent a core needle biopsy of the tumor prior to treatment and subsequently received tamoxifen until the date of routine surgery at an average of 21 days later. Patient characteristics are described in Supplementary Table 1. Gene expression was generated using tumor tissues isolated before and after treatment. GSEA analysis identified a number of pathways regulated by tamoxifen treatment including tumor necrosis factor (TNF) signaling via the pro-inflammatory NFκB pathway (Fig. 1B–D, Supplementary Table 3, Supplementary Fig. 1). Although the NFκB pathway has previously been implicated in endocrine resistance (20–22), these findings in patient tumors suggest that even short-term tamoxifen treatment may enhance NFκB signaling in ER+ tumors. We examined whether this change may be the result of an increase in immune cell infiltration into the tumors following TAM treatment. However, no significant difference in the percentage of tumor infiltrating lymphocytes or specific immune cell subset (CD4, CD8, and CD68) was observed after TAM treatment (Supplementary Fig. 2), suggesting altered NFκB signaling in the tumor cells.
Figure 1.
NFκB pathway is active in patients treated with neo-adjuvant tamoxifen therapy. A, Setup of a neo-adjuvant trial in which patients with small ER+ breast tumors received tamoxifen for several weeks. Pre-treatment biopsies were compared to surgical specimens taken several weeks later. B, Top five enriched gene sets (FDR q-value < 0.05) following tamoxifen treatment when comparing surgical specimens to pre-treatment biopsies. C, GSEA enrichment plot of the ‘TNFα signaling via NFκB’ geneset. D, Heatmap of genes in the ‘TNFα signaling via NFκB’ geneset that were found to be significantly differentially expressed when comparing surgical specimens to pre-treatment biopsies.
Tamoxifen exposure results in NFκB activation in cell lines
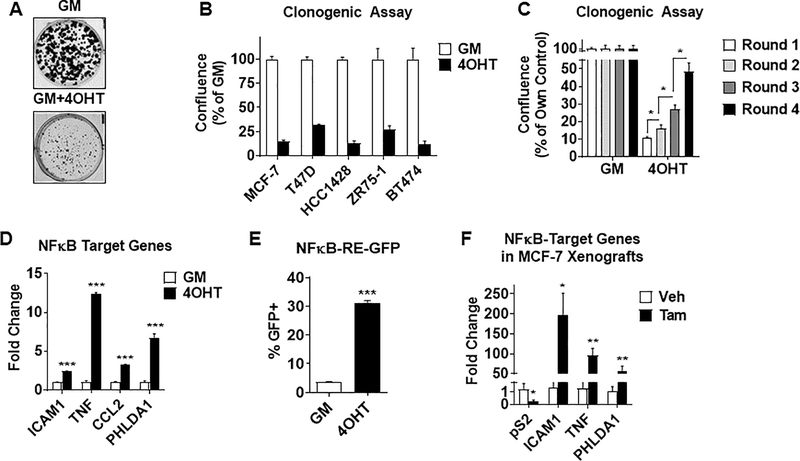
To determine if changes observed in tumors from patients treated with tamoxifen can be modeled in vitro, clonogenic assays were performed using ER+ breast cancer cell lines cultured in growth media (phenol-red proficient + 10% FBS) in the presence or absence of 4-hydroxytamoxifen (4OHT). As shown in Fig. 2A and Fig. 2B, 4OHT caused an overall suppression of clonogenic growth, as expected. However, in each cell line tested, a number of tamoxifen-tolerant clones were able to grow under the selective pressure of 4OHT. Multiple rounds of clonogenic assays were performed and demonstrated that cells become increasingly more refractory to 4OHT exposure (Fig. 2C), suggesting that the tamoxifen-tolerant population may be a precursor to the development of resistance.
Figure 2.
Tamoxifen-tolerant cell populations display elevated NFκB activity in vitro and in vivo. A-B, Clonogenic growth was conducted in ER+ breast cancer cell lines cultured in growth media (GM) in the presence or absence of 1μM 4-hydroxytamoxifen (4OHT). Dissociated single cells were seeded at clonogenic density and re-treated twice weekly for 2 weeks. Colonies were then fixed and stained with crystal violet. Representative pictures of MCF-7 cells are shown (A). Colony confluence (area covered by colonies) was quantified with Celigo imager (B). Data for each cell line is normalized to the GM control, which was set to 100%. C, Clonogenic growth following multiple rounds of 2-week treatments with 4OHT as described in (A) were quantified in MCF-7 cells. Data is normalized to GM control. *P<0.05. D, MCF-7 cells were seeded and treated as in (A) for 2 weeks. NFκB-target genes are measured by RT-QPCR. E, MCF-7 cells stably transfected with 3x κB-RE-GFP reporter are treated as in (A) with GM −/+ 1μM 4OHT for 2 weeks. The percentage of GFP+ cells was determined by FACS. F, NFκB-target genes were measured in MCF-7 xenograft tumors. Mice were randomized into treatment groups of either vehicle control or tamoxifen (500 μg/animal s.c. in peanut oil) and treated for 2 weeks. After treatments, tumors were excised and RNA isolated. pS2 was used as a control ER-target gene. *P<0.01, **P<0.005, ***P<0.001.
The unsupervised discovery of NFκB as the top-enriched pathway in tumors from patients treated with neo-adjuvant tamoxifen therapy (Fig. 1) motivated us to probe this pathway in tamoxifen-tolerant cells. Indeed, several approaches revealed that the NFκB pathway is activated in tamoxifen-tolerant cells in vitro, similar to the activation observed in patient samples. RT-QPCR analysis showed elevated NFκB-target gene (23–25) expression in both MCF-7 (Fig. 2D) and T47D cells (Supplementary Fig. 3A). In addition, a cell line stably expressing an NFκB-RE driven GFP reporter demonstrated an increase in the percentage of GFP+ cells in response to 4OHT, implying enrichment of NFκB-active cells upon continuous 4OHT exposure (Fig. 2E). Finally, MCF-7 xenograft tumors in mice exposed to tamoxifen also demonstrated an increase in NFκB-target gene expression (Fig. 2F). Together, these findings suggest that tamoxifen exposure gives rise to a tamoxifen-tolerant population, and that increased NFκB signaling is an early event occurring in these cells both in vitro and in vivo, as was observed in human tumors.
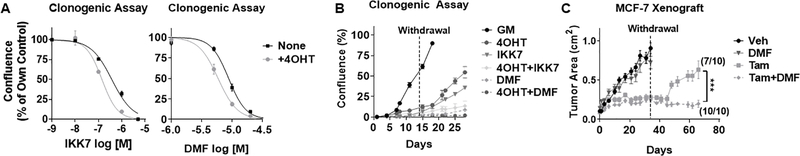
Tamoxifen-tolerant cells require NFκB signaling for their survival and regrowth
To understand the role of NFκB activation in tamoxifen-tolerant cells, we utilized two NFκB pathway inhibitors – IKK7 (an IKKα/β kinase inhibitor (26)) and DMF (an inhibitor of p65 nuclear translocation and DNA binding (27)). Both inhibitors significantly reduced the outgrowth of colonies, and in particular of tamoxifen-tolerant colonies (Fig. 3A). Similar results were observed in T47D cells (Supplementary Fig. 3B). Given that NFκB inhibitors block colony outgrowth, we hypothesized that this inhibition would be most-relevant in preventing cell and tumor regrowth following cessation of tamoxifen treatment. To test this, tamoxifen withdrawal studies were conducted both in vitro and in vivo. The in vitro approach demonstrated that regrowth of cells following withdrawal of tamoxifen was substantially attenuated by IKK7 and DMF (Fig. 3B). Similarly, MCF-7 xenograft tumor growth in vivo following tamoxifen withdrawal was examined. Seven out of 10 xenograft tumors displayed regrowth within 30 days, whereas 0 out of 10 tumors recurred if DMF and tamoxifen were administered together (Fig. 3C). Importantly, DMF monotherapy had no effect on tumor growth, suggesting that i) there is no indication of NFκB-dependence in growing tumors in the absence of tamoxifen, and ii) the presence of tamoxifen is necessary for the induction of NFκB activity and dependence, thereby allowing for the growth inhibition by DMF. These findings strongly suggest that the activation of NFκB following tamoxifen exposure is required for the survival of a cell population that is capable of giving rise to a recurrent tumor.
Figure 3.
NFκB pathway is required for the survival and regrowth of tamoxifen-tolerant cells in vitro and in vivo. A, NFκB pathway inhibitors (IKK7 and DMF) alone or in combination with 4OHT were added to MCF-7 cells treated as described in Fig. 1A. Data was normalized to own controls, each set to 100%. B, MCF-7 cells were treated with 4OHT, IKK7 (1μM), or DMF (20μM) alone or in combination for 2 weeks. After 2 weeks, all drugs were withdrawn and regrowth (% confluence) was monitored by Celigo cell imager. C, MCF-7 xenograft tumor growth curves are shown for mice treated with: vehicle (Veh), DMF, tamoxifen (Tam), and Tam+DMF for 4 weeks, and then monitored for an additional 6 weeks after all therapies were withdrawn. Tumor recurrence between Tam vs Tam+DMF is significantly different, p=0.0015 using a Fisher’s exact test analysis. End point comparison of mean tumor area (length/2 × width × π) for Tam vs Tam+DMF is indicated. ***P<0.001.
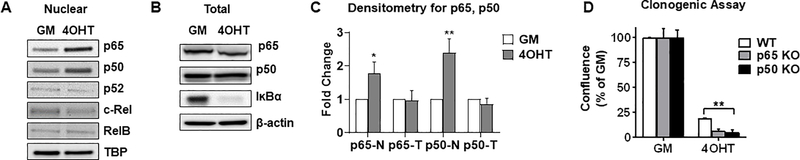
NFκB family members p65 and p50 are required for tamoxifen-tolerant cell survival
We next investigated which NFκB transcription factor family members may be involved in the survival of tamoxifen-tolerant cells. Western blot analysis revealed that nuclear p65 and p50 levels are increased after 2 weeks of exposure to 4OHT, whereas total levels were unchanged (Fig. 4A–4C). Similar results were observed in T47D cells (Supplementary Fig. 3C). This elevation in nuclear p65 and p50 is accompanied by reduced protein expression of the NFκB signaling inhibitor IκBα (Fig. 4B), which acts to sequester inactive p65 and p50 in the cytoplasm, suggesting a mechanism by which this pathway is activated. To investigate possible causal involvement of p65 and p50, these genes were knocked out individually in MCF-7 cells using CRISPR-Cas9 (Supplementary Fig. 4). A significant reduction in the ability of tamoxifen-tolerant clones to survive was observed (Fig. 4D). These findings suggest that p65 and p50 are critical drivers of tamoxifen-tolerance in ER+ breast cancer cells.
Figure 4.
Nuclear p65 and p50 are required for the survival of tamoxifen-tolerant cells. A-C, Western blot analysis of nuclear (A) or whole cell lysates (B) of MCF-7 cells treated as in Fig. 1A are shown. TBP or β-actin served as loading controls. Densitometry quantitation is shown (C). N-Nuclear, T-total. D, Clonogenic assay was conducted in MCF-7 parental wild type (WT) or in p65 or p50 CRISPR/Cas9 knock out clones. *P<0.01, **P<0.005.
NFκB-positive cell retain ER but are insensitive to tamoxifen
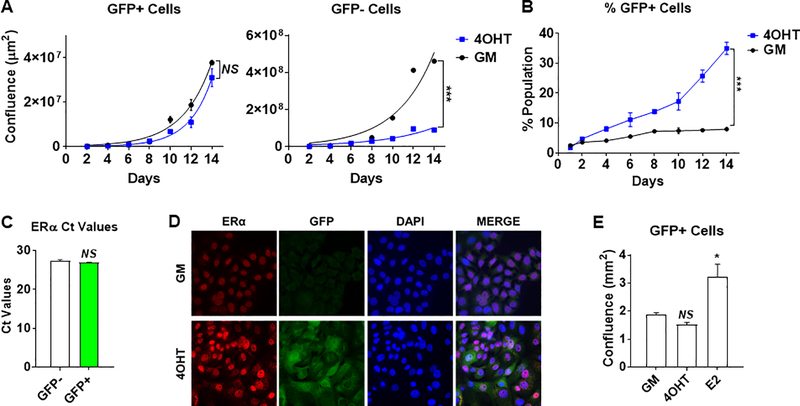
To understand how the NFκB pathway is activated upon tamoxifen exposure, we first asked whether 4OHT could directly affect the pathway. Short-term exposure of MCF-7 cells to 4OHT suppressed ER-target genes, as expected, but did not directly stimulate endogenous NFκB-target genes or exogenous NFκB-RE-GFP reporter activity (Supplementary Fig. 5), suggesting that the effect is not direct. Rather it appears that NFκB-positive cells, based on elevated NFκB-RE-driven GFP expression, grow at a similar rate in the presence or absence of 4OHT, whereas NFκB-negative cells are growth suppressed by 4OHT (Fig. 5A). As a result, the NFκB-positive cell population expands over time with 4OHT treatment (Fig. 5B). Are the tamoxifen-insensitive NFκB-positive cells still ER+? To address this, we examined ER expression by QPCR (Fig. 5C), and found comparable ER mRNA levels between the NFκB-positive and NFκB-negative cell populations. These results were confirmed on the protein level using immunofluorescence (IF) for ER and GFP, showing that NFκB-positive cells readily express ER protein (Fig. 5D). Furthermore, although NFκB-positive cells failed to respond to 4OHT, they do proliferate upon estrogen (E2) treatment, suggesting they retain functional ER signaling while being insensitive to tamoxifen (Fig. 5E).
Figure 5.
Uninhibited outgrowth of NFκB+ cells underlies tamoxifen tolerance. A-B, MCF-7-κB-RE-GFP reporter cells were treated in GM −/+ 1μM 4OHT for 2 weeks. The outgrowth (confluence) of GFP+ cells (left panel) and GFP- (right panel) was monitored and quantified using a Celigo imager. B, The ratio of GFP+/total cells was calculated over the treatment period. C, ER expression was measured by RT-QPCR in sorted MCF-7-κB-RE-GFP reporter cells treated with 1μM 4OHT for 2 weeks. D, Expression of ER (red) and GFP (green) was examined by immunofluorescence. DAPI staining of nuclei is shown in blue. E, The GFP+ cell population of MCF-7-κB-RE-GFP reporter cell line was quantified in response to treatments with 1μM 4OHT and 10nM E2 for 2 weeks. NS, not significant, *P<0.01, **P<0.005, ***P<0.001.
Tamoxifen-tolerant cells display EMT/stem-like features
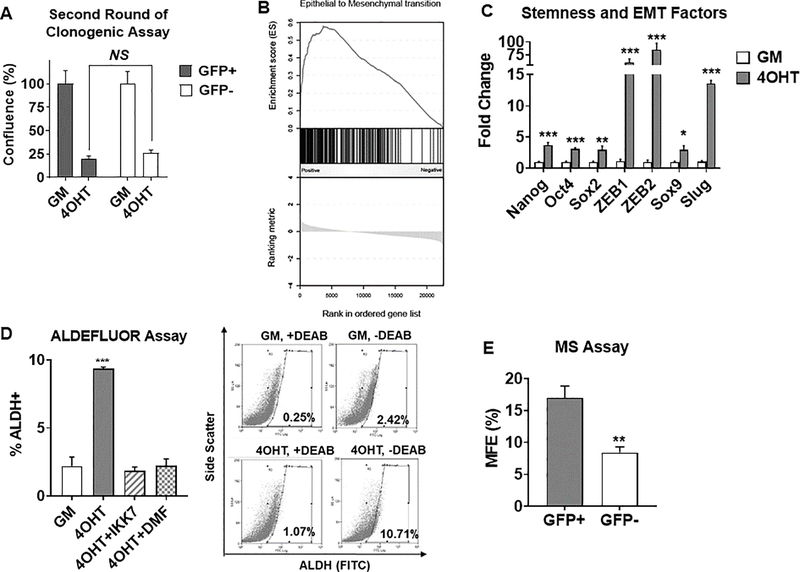
To examine whether all NFκB-positive cells give rise to tamoxifen tolerance, we sorted GFP-positive and –negative populations after two weeks of 4OHT treatment. We found that both populations give rise to a similar number of tamoxifen-tolerant colonies (Fig. 6A), suggesting that each population reverts back to the naive population’s response to 4OHT. Moreover, a similar level of NFκB activity was observed in the secondary clones, indicating that GFP-negative cells give rise to GFP-positive cells, and vice versa, following a second round of selection (Supplementary Fig. 6). This data suggests that cell plasticity may be an underlying feature of tamoxifen-tolerance. Both EMT and stemness have overlapping de-differentiation features and display significant plasticity (28–31). Given that EMT and stemness-associated genes were also elevated in samples from patients treated with tamoxifen (Fig. 1B, 6B), we decided to investigate the EMT and stem-like properties of tamoxifen-tolerant cells. We confirmed that expression of multiple factors associated with EMT and stemness were increased following 4OHT exposure (Fig. 6C). This observation was confirmed in tamoxifen-tolerant T47D cells (Supplementary Fig. 3D). Moreover, ALDH1 activity, a marker of stemness in breast cancer (32), was elevated in tamoxifen-tolerant tumor cells in an NFκB-dependent manner (Fig. 6D). In addition, NFκB-positive cells were more capable of forming mammospheres than NFκB-negative cells (Fig. 6E). Together, these findings suggest that a distinct EMT/stem-like cell population expands in an NFκB-dependent manner in response to tamoxifen exposure, and that the plasticity of this population may contribute the development of tamoxifen resistance through various mechanisms.
Figure 6.
Tamoxifen-tolerant cells have EMT/stemness features controlled by the NFκB pathway. A, Sorted NFκB+ vs NFκB− tamoxifen-tolerant cells based on GFP expression were reseeded for another round of clonogenic assay and quantified. NS, not significant. B, EMT pathway was enriched in patients treated with neo-adjuvant tamoxifen therapy. C, Expression of EMT and stemness factors were measured by RT-QPCR in MCF-7 clonogenic cells treated with GM −/+ 1μM 4OHT for 2 weeks. D, ALDH+ population measured by FACS in MCF-7 cells clonogenic cells treated with 1μM 4OHT for 2 weeks. Representative histograms for GM vs 4OHT are shown (right panel). Inhibitors, IKK7, 1μM, or DMF, 20μM, were added for the last week in culture. E, Mammosphere (MS) formation was quantified in sorted NFκB+ vs NFκB− tamoxifen-tolerant cells. MS > 75μm in diameter were quantified and MS forming efficiency (MFE) was calculated. *P<0.01, **P<0.005, ***P<0.001.
Discussion
This study provides novel mechanistic insights into tamoxifen-tolerance in ER+ breast cancer. We identified a tolerant cell population, driven by the NFκB pathway, in all biological systems studied (ER+ breast cancer cells, xenograft tumors, and patient samples). We also identified a mechanism underlying the observed tolerance to tamoxifen – the expansion of an NFκB-active, EMT/stem-like population of cells that express functional ER but are insensitive to tamoxifen. Rather, these cells are reliant on NFκB transcription factors (p65 and p50) for survival and growth. The expansion of a tamoxifen-tolerant population under the selective pressure of tamoxifen is highly clinically relevant, as we have demonstrated that these cells become increasingly more refractory to growth suppression by tamoxifen, and retain their regrowth capability in vitro and in vivo to support tumor recurrence.
In other fields of oncology, drug-tolerant persister cells have been reported previously. Some of the reported strategies that these cells utilize for survival include (as reviewed in (4)): negligible growth and low cell cycling, altered metabolism, epigenetic reprogramming, stem-like properties, low immunogenicity, and modulation of their microenvironment. Here, we reveal a novel feature of drug-tolerant persister cells in a breast cancer-specific context: the expansion of an NFκB+ ER+ population with short-term selective pressure from tamoxifen treatment. The elevated NFκB activity in these cells does not appear to be directly stimulated by tamoxifen, since short term treatment with 4OHT does not up-regulate NFκB target genes. Rather, it appears that tamoxifen allows a subpopulation of the tumor cells with NFκB activity to expand. The reliance on canonical NFκB signaling as an initial survival strategy employed by ER+ tamoxifen-tolerant cells is in line with prior studies reporting that primary ER+ tumors with higher levels of constitutive NFκB activation are more likely to acquire treatment resistance and metastasize, and are associated with poor outcome (20–22). Also of interest is our finding that NFκB+, tamoxifen-tolerant cells retain functional ER, which is stabilized in the nucleus upon tamoxifen treatment (in line with previous reports (33)), but is insensitive to tamoxifen antagonism. Several potential mechanisms could explain the lack of ER response to tamoxifen. For example, post-translational modifications of ER, such as S305 phosphorylation or K303 acetylation, could explain the loss of tamoxifen antagonism (34–36). Alternatively, these cells may gain a reliance on NFκB signaling, thereby masking the growth inhibitory effect of tamoxifen.
Current thinking in the field of endocrine therapy resistance suggests that resistance may occur de novo (i.e. prior to exposure) or can be acquired over the course of therapy, which typically lasts for 5–10 years. In acquired resistance, it is thought that tamoxifen initially acts as an ER-antagonist in breast cancer to switch off growth, but during the years of exposure, residual cancer cells can mutate and adapt to grow in a tamoxifen environment (37). We propose two possible scenarios by which can NFκB-driven, stem-like/plastic, tamoxifen-tolerant cell population could contribute to resistance. First, there may an intrinsic subpopulation of cells in some ER+ tumors that have active NFκB, expand rapidly in response to tamoxifen treatment, and contribute to de novo resistance. A similar selection mechanism was recently proposed for aromatase inhibitor tolerance/resistance, where an enrichment of cells bearing ESR1 mutations could rapidly be detected in tumors of women treated with aromatase inhibitors (38). Alternatively, tolerance may be an early step in the development of acquired endocrine therapy resistance. The long-term survival of an NFκB-driven, tamoxifen-tolerant cell population that no longer relies on ER for growth and survival could allow for adaptation, the development of other resistance mechanisms, and tumor recurrence.
Similar to other reports of a transient and reversible drug-tolerant state in other tumor types (3), and consistent with its stem-like features, the tamoxifen-tolerant phenotype in breast cancer displays significant cellular plasticity. Importantly, NFκB is driving this plastic stemness state as indicated by ALDH1 activity and mammosphere forming capacity. This is consistent with other reports of NFκB promoting stem-like phenotypes in breast cancer (39, 40), and cancer stem cells’ reliance on inflammatory cytokines and networks (41, 42). It is becoming clearer that cancer stem cells display remarkable genetic and phenotypic heterogeneity, and a metastable EMT program bestows this plasticity (43). In tamoxifen-tolerance, we identified an ER+ EMT/stem-like population driven by the NFκB pathway that gives rise to tumor recurrence. It should be noted that our findings appear to indicate a partial EMT since E-cadherin levels were not altered in tamoxifen-tolerant cells (data not shown). This phenomenon has been referred to as ‘partial EMT’ where cells are characterized by a hybrid epithelial/mesenchymal state, where they maintain cell-cell adhesion and expression of proteins such as E-cadherin, but also express several EMT associated genes (44, 45). Additionally, recent clinical studies demonstrated partial EMT in carcinoma cells allows for a rapid and transient adaptation to cytotoxic or molecularly targeted therapy (46).
One important implication of our findings is that targeting NFκB in addition to standard ER-blocking endocrine drugs could be a beneficial therapeutic strategy to prevent the development of resistance and eventual recurrence. Unfortunately, targeting NFκB in the clinic has proved challenging. While multiple NFκB inhibitors have been investigated, most have failed in the clinic due to the innate immune system’s reliance on NFκB, as well as considerable toxic side effects (47). This raises the issue of how to safely and effectively inhibit the NFκB pathway. One option is to use DMF (Tecfidera®), an orally bioavailable drug approved by the FDA in 2013 to treat multiple sclerosis. DMF is safe in humans and shows none of the immune-suppressive side effects (48). Alternatively, a water soluble parthenolide analog has gained some attention as a safe and effective NFκB inhibitor in other types of cancer, such as prostate, bladder and lung (49, 50) and may be potentially useful in targeting tamoxifen-tolerant cells.
In summary, the findings reported here describe a novel tamoxifen-tolerant, ER+, NFκB-dependent cell population with EMT/stem-like features that emerges in ER+ breast cancer cell lines, xenograft tumors and patient tumors upon tamoxifen treatment. Targeting these cells using NFκB inhibitors may address the urgent unmet need of how to prevent the development of endocrine resistance and metastatic relapse in tamoxifen-treated breast cancer patients.
Supplementary Material
Implications.
Understanding initial changes that enable survival of tamoxifen-tolerant cells, as mediated by NFκB pathway, may translate into therapeutic interventions to prevent resistance and relapse, which remain major causes of breast cancer lethality.
Acknowledgements
We thank the UIC flow cytometry core researchers, Drs. Balaji Ganesh and Suresh Ramasamy, for their assistance. We thank Jermya Buckley for assistance in preparing the manuscript. We thank Rutger Koornstra and Annelot van Rossum for clinical trial management, Tesa Severson, Agendia and the NKI CFMPB for assisting in generating gene expression data, NKI CFMPB for multiplex immunofluorescence and patients for participating in the trial. This work is supported by NIH R01 CA200669 funding to JF and GG, DOD award W81XWH19-1-0108 to IK, a Sister’s Hope Grant to SCL and WZ, and by the Dutch Cancer Foundation award NKI-2014-7140 to WZ.
Financial Support: This work was supported by NIH R01 CA200669 to JF and GG, DOD award W81XWH19-1-0108 to IK, a Sister’s Hope Grant to SCL and WZ, and by the Dutch Cancer Foundation NKI-2014-7140 to WZ.
Footnotes
Conflict of Interest Disclosure Statement: The authors have no financial conflicts to disclose.
References
- 1.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–46. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. [DOI] [PubMed] [Google Scholar]
- 3.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallette FM, Olivier C, Lezot F, Oliver L, Cochonneau D, Lalier L, et al. Dormant, quiescent, tolerant and persister cells: Four synonyms for the same target in cancer. Biochem Pharmacol. 2019;162:169–76. [DOI] [PubMed] [Google Scholar]
- 5.Boumahdi S, de Sauvage FJ. The great escape: tumour cell plasticity in resistance to targeted therapy. Nature Reviews Drug Discovery. 2019. [DOI] [PubMed] [Google Scholar]
- 6.Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echeverria GV, Ge Z, Seth S, Zhang X, Jeter-Jones S, Zhou X, et al. Resistance to neoadjuvant chemotherapy in triple-negative breast cancer mediated by a reversible drug-tolerant state. Sci Transl Med. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiff R, Massarweh S, Shou J, Osborne CK. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res. 2003;9:447S–54S. [PubMed] [Google Scholar]
- 9.Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. [DOI] [PubMed] [Google Scholar]
- 10.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–35. [DOI] [PubMed] [Google Scholar]
- 11.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez M, Rajaram S, Steininger RJ, Osipchuk D, Roth MA, Morinishi LS, et al. Diverse drug-resistance mechanisms can emerge from drug-tolerant cancer persister cells. Nat Commun. 2016;7:10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Severson TM, Nevedomskaya E, Peeters J, Kuilman T, Krijgsman O, van Rossum A, et al. Neoadjuvant tamoxifen synchronizes ERalpha binding and gene expression profiles related to outcome and proliferation. Oncotarget. 2016;7:33901–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wuerzberger-Davis SM, Chang PY, Berchtold C, Miyamoto S. Enhanced G2-M arrest by nuclear factor-{kappa}B-dependent p21waf1/cip1 induction. Mol Cancer Res. 2005;3:345–53. [DOI] [PubMed] [Google Scholar]
- 17.Guzman C, Bagga M, Kaur A, Westermarck J, Abankwa D. ColonyArea: an ImageJ plugin to automatically quantify colony formation in clonogenic assays. PLoS One. 2014;9:e92444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–74. [DOI] [PubMed] [Google Scholar]
- 19.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Eppenberger-Castori S, Eppenberger U, Benz CC. The NFkappaB pathway and endocrine-resistant breast cancer. Endocr Relat Cancer. 2005;12 Suppl 1:S37–46. [DOI] [PubMed] [Google Scholar]
- 21.Frasor J, El-Shennawy L, Stender JD, Kastrati I. NFkappaB affects estrogen receptor expression and activity in breast cancer through multiple mechanisms. Mol Cell Endocrinol. 2015;418 Pt 3:235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Eppenberger-Castori S, Marx C, Yau C, Scott GK, Eppenberger U, et al. Activation of nuclear factor-kappaB (NFkappaB) identifies a high-risk subset of hormone-dependent breast cancers. Int J Biochem Cell Biol. 2005;37:1130–44. [DOI] [PubMed] [Google Scholar]
- 23.Stratowa C, Audette M. Transcriptional regulation of the human intercellular adhesion molecule-1 gene: a short overview. Immunobiology. 1995;193:293–304. [DOI] [PubMed] [Google Scholar]
- 24.Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. [DOI] [PubMed] [Google Scholar]
- 25.Kastrati I, Canestrari E, Frasor J. PHLDA1 expression is controlled by an estrogen receptor-NFkappaB-miR-181 regulatory loop and is essential for formation of ER+ mammospheres. Oncogene. 2015;34:2309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waelchli R, Bollbuck B, Bruns C, Buhl T, Eder J, Feifel R, et al. Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorg Med Chem Lett. 2006;16:108–12. [DOI] [PubMed] [Google Scholar]
- 27.Kastrati I, Siklos MI, Calderon-Gierszal EL, El-Shennawy L, Georgieva G, Thayer EN, et al. Dimethyl Fumarate Inhibits the Nuclear Factor kappaB Pathway in Breast Cancer Cells by Covalent Modification of p65 Protein. J Biol Chem. 2016;291:3639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan NV, Johnson GL, Abell AN. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle. 2011;10:2865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granit RZ, Slyper M, Ben-Porath I. Axes of differentiation in breast cancer: untangling stemness, lineage identity, and the epithelial to mesenchymal transition. Wiley Interdiscip Rev Syst Biol Med. 2014;6:93–106. [DOI] [PubMed] [Google Scholar]
- 30.Liao TT, Yang MH. Revisiting epithelial-mesenchymal transition in cancer metastasis: the connection between epithelial plasticity and stemness. Mol Oncol. 2017;11:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kocanova S, Mazaheri M, Caze-Subra S, Bystricky K. Ligands specify estrogen receptor alpha nuclear localization and degradation. BMC Cell Biol. 2010;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giordano C, Cui Y, Barone I, Ando S, Mancini MA, Berno V, et al. Growth factor-induced resistance to tamoxifen is associated with a mutation of estrogen receptor alpha and its phosphorylation at serine 305. Breast Cancer Res Treat. 2010;119:71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, et al. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5:597–605. [DOI] [PubMed] [Google Scholar]
- 36.Stender JD, Nwachukwu JC, Kastrati I, Kim Y, Strid T, Yakir M, et al. Structural and Molecular Mechanisms of Cytokine-Mediated Endocrine Resistance in Human Breast Cancer Cells. Mol Cell. 2017;65:1122–35 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan P, Craig Jordan V. Acquired resistance to selective estrogen receptor modulators (SERMs) in clinical practice (tamoxifen & raloxifene) by selection pressure in breast cancer cell populations. Steroids. 2014;90:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leal MF, Haynes BP, Schuster EF, Yeo B, Afentakis M, Zabaglo L, et al. Early enrichment of ESR1 mutations and the impact on gene expression in pre-surgical primary breast cancer treated with aromatase inhibitors. Clin Cancer Res. 2019. [DOI] [PubMed] [Google Scholar]
- 39.Hinohara K, Kobayashi S, Kanauchi H, Shimizu S, Nishioka K, Tsuji E, et al. ErbB receptor tyrosine kinase/NF-kappaB signaling controls mammosphere formation in human breast cancer. Proc Natl Acad Sci U S A. 2012;109:6584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Shennawy L, Dubrovskyi O, Kastrati I, Danes JM, Zhang Y, Whiteley HE, et al. Coactivation of Estrogen Receptor and IKKbeta Induces a Dormant Metastatic Phenotype in ER-Positive Breast Cancer. Cancer Res. 2018;78:974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer’s inflammatory roots. Clin Cancer Res. 2011;17:6125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo M, Brooks M, Wicha MS. Epithelial-mesenchymal plasticity of breast cancer stem cells: implications for metastasis and therapeutic resistance. Curr Pharm Des. 2015;21:1301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jolly MK, Boareto M, Huang B, Jia D, Lu M, Ben-Jacob E, et al. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front Oncol. 2015;5:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navas T, Kinders RJ, Lawrence SM, Ferry-Galow KV, Borgel S, Hollingshead MG, et al. Clinical Evolution of Epithelial-Mesenchymal Transition in Human Carcinomas. Cancer Research. 2019:canres.3539.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garber K The second wave in kinase cancer drugs. Nat Biotechnol. 2006;24:127–30. [DOI] [PubMed] [Google Scholar]
- 48.Hoefnagel JJ, Thio HB, Willemze R, Bouwes Bavinck JN. Long-term safety aspects of systemic therapy with fumaric acid esters in severe psoriasis. Br J Dermatol. 2003;149:363–9. [DOI] [PubMed] [Google Scholar]
- 49.Shanmugam R, Kusumanchi P, Appaiah H, Cheng L, Crooks P, Neelakantan S, et al. A water soluble parthenolide analog suppresses in vivo tumor growth of two tobacco-associated cancers, lung and bladder cancer, by targeting NF-kappaB and generating reactive oxygen species. Int J Cancer. 2011;128:2481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shanmugam R, Kusumanchi P, Cheng L, Crooks P, Neelakantan S, Matthews W, et al. A water-soluble parthenolide analogue suppresses in vivo prostate cancer growth by targeting NFkappaB and generating reactive oxygen species. Prostate. 2010;70:1074–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.