Abstract
Multiple myeloma (MM) is a plasma cell malignancy that grows in the bone marrow (BM). The major population of cells in the BM is represented by neutrophils and they can form neutrophil extracellular traps (NETs). Here, we investigated whether MM cells induce NET formation and whether targeting this process would delay MM progression. We demonstrated that murine and human MM cells stimulate citrullination of histone H3 and NET formation by neutrophils and that this process is abrogated by pharmacological targeting of peptidylarginine deiminase 4 (PAD4) with a novel specific small molecule inhibitor BMS-P5. Administration of BMS-P5 to MM-bearing mice delays appearance of symptoms and disease progression. Taken together, our data demonstrate that targeting PAD4 may be beneficial for treatment of MM.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy that grows preferentially in the bone marrow (BM) (1). Crosstalk of MM cells with the BM microenvironment plays a critical role in promoting MM cell survival and growth (2,3). Although several mechanisms within the BM niche have been implicated in disease progression, our understanding of the contribution of interactions between tumor cells and their microenvironment remains scarce.
Neutrophils represent one of the predominant cell types in the BM and are essential effector cells of the innate immune system. Neutrophils serve as the first line of defense against a wide range of pathogens including bacteria, fungi, and protozoa, by utilizing two major mechanisms: phagocytosis and degranulation (4). Recently, formation of neutrophil extracellular traps (NETs) has been described as another host defense mechanism of neutrophils against pathogens (5,6). NETs are extracellular web-like structures composed of chromatin backbone with peptides and proteins assembled on it. Proteins and anti-microbial peptides that are normally found in neutrophil granules, cytoplasm, or nucleus, including neutrophil elastase and myeloperoxidase, are found in NETs (5,7). NETs are formed via a novel cell death pathway called NETosis. During NETosis, disintegration of the nuclear envelope allows mixing of chromatin with cytoplasmic and granular proteins followed by their release outside the cell (7). A number of stimuli have been described to prime neutrophils to undergo NETosis including lipopolysaccharide (LPS), interleukin 8, granulocyte colony stimulating factor, transforming growth factor β among others, as well as artificial stimuli such as phorbol myristate acetate and calcium ionophore (8).
The occurrence of NETosis is not limited to infection; the presence of NETs has recently been documented in other pathologies including solid and blood cancers (8,9) and was associated with promoting metastasis and poor prognosis (10–12). NETs were shown to trap circulating lung carcinoma cells (10), to stimulate cancer cell adhesion, migration, and invasion in vitro (13,14). In addition, NETs promoted cancer-associated thrombosis (15,16) and had pro-coagulant activity in patients with gastric cancer (17).
The mechanism of NETosis is not entirely understood. However, it has been postulated that one of the steps in NET formation involves decondensation of chromatin and that citrullination of histones, specifically histone H3, is necessary for chromatin decondensation (18). Citrullination is a process of converting arginine residues into citrulline and is mediated by a family of enzymes called peptidylarginine deiminases (PADs) (19). PAD4, a member of this family, is responsible for the citrullination of histone H3 at arginine residues 2, 8, and 17 that leads to loss of the positive charge of chromatin and consequently weakens histone-DNA binding facilitating chromatin decondensation during NET formation (20). PAD4 knockout (KO) mice have shown reduced bacterial killing by NETs in a mouse infectious disease model of necrotizing fasciitis (21), reduced NET-dependent inflammatory and procoagulant host response to endotoxin in a polymicrobial sepsis model (22), and protection from NET-dependent cardiac fibrosis (23).
To date, several compounds inhibiting PAD activity have been reported. Those include Cl-amidine and related compounds that are covalent non-selective irreversible PAD inhibitors (24,25) and GSK-484, a reversible selective PAD4 inhibitor (26). GSK-484 blocked calcium ionophore-induced NET formation by mouse neutrophils in vitro (26) and through blocking LPS-induced NET generation prevented awakening of dormant cancer cells in an in vivo breast cancer model (27). Although GSK-484 provided a proof-of-concept that pharmacological targeting of PAD4 blocks NET formation, this compound does not possess a strong potency as it required micromolar concentrations to be effective and thus, it is not suitable for clinical development. Therefore, a need for development of more potent PAD4 inhibitors exists.
Although the majority of patients with MM respond well to the first line of treatment, they inevitably develop therapy-resistance. Novel agents that are effective in refractory MM as a monotherapy or in combination with commonly used treatments are needed. Here, we investigated whether MM cells can stimulate NET formation and if so, whether interruption of this process would delay disease progression. We utilized the novel PAD4-specific inhibitor BMS-P5 developed by Bristol-Myers Squibb. Our data provide evidence that pharmacologically targeting PAD4 with BMS-P5 blocks MM-induced NET formation and delays progression of MM in a syngeneic mouse model.
Materials and methods
Cell lines and reagents
Human MM RPMI-8226 and MM1.S cell lines were obtained from ATCC (Manassas, VA). Mouse MM DP42 cell line was a gift from Dr. Van Ness (University of Minnesota); 5TGM1 cell line was provided by Dr. Oyajobi (UT Health San Antonio). All MM cells were cultured in RPMI-1640 medium supplemented with L-glutamine, 10% fetal bovine serum (FBS), and 1% antibiotic-antimycotic. DP42 cells were also supplemented with 0.5 ng/mL murine IL-6 (Cat.#406-ML, R&D Systems). Cell lines were Mycoplasma negative as detected by PCR. Cells were maintained in culture for a maximum of 6 weeks.
BMS-P5, ((cis)-5-Amino-2-methylpiperidin-1-yl)(2-(1-(cyclopropylmethyl)-1H-pyrrolo[2,3-b]pyridine-2-yl)-7-methoxy-1-methyl-1H-benzo[d]imidazole-5-yl)methanone, hydrochloride) (Example 24, patent US9127003B2) (28) was provided by Bristol-Myers Squibb (BMS, Princeton, NJ). Cl-amidine (Cl-A, Cat.#10599) and GSK-484 (Cat.#17488) were purchased from Cayman Chemical (Ann Arbor, MI) and Calcium ionophore (CaIo, Cat.#A23187) was obtained from Sigma-Aldrich (St.Louis, MO).
PAD Enzyme Assays
Measurements of the inhibitory potency of BMS-P5 against PAD enzymes (PAD4, PAD1, PAD2 or PAD3) employed recombinant human proteins with recombinant histone H3 as a substrate in 100 mM Tris, pH 7.5, containing 2 mM DTT and 0.65 mM CaCl2. The citrullinated histone H3 was detected by either immunoblot with LiCor quantitation or an ELISA-based assay using anti-citrullinated histone H3 rabbit polyclonal antibody (Abcam ab5103) with either IRDye 800CW-conjugated or HRP-conjugated donkey anti-rabbit IgG secondary detection antibodies (Licor 926–32213 or Invitrogen A16029).
Mouse model and treatment
All animal experiments were carried out in accordance with institutional guidelines and were approved by the Institutional Animal Care and Use Committee of the Wistar Institute. C57BL/6 and FVB/N mice were purchased from Charles River Laboratories and were crossed to obtain F1 progeny of mixed C57BL/6×FVB/N background. In these mice, MM tumors were established by i.v. inoculation into the tail vein of DP42 cells (5×103). Six to ten week old male and female mice were used for experiments. For in vivo treatment studies, DP42-bearing mice were split into 2 groups and treated with BMS-P5 or vehicle control (VC, 0.5% Methocel A4M and 0.1% polysorbate 80 in 100 mM sodium acetate pH 4.6). BMS-P5 was given at a dose of 50 mg/kg and administered by oral gavage twice a day beginning on day 3 after tumor cell injection. Onset of symptoms (paralysis and hunched posture) were monitored. Survival of mice was determined as they were euthanized at the humane endpoint.
PAD4 flox mice (PAD4fl/fl, B6(Cg)-Padi4tm1.2Kmow/J, stock no.026708) and Mrp8CreTg mice (B6.Cg-Tg (S100A8-cre,-EGFP)1Ilw/J, stock no.021614) were purchased from the Jackson Laboratory and crossed to obtain PAD4fl/flMrp8Cre+ mice (further designated as PAD4 KO) and their PAD4fl/flMrp8Cre- littermates control.
Isolation of mouse neutrophils
Mouse neutrophils were isolated from the BM of tumor-free mice using magnetic cell separation technique. Briefly, BM cells were labeled with biotin-Ly6G antibody followed by incubation with streptavidin conjugated microbeads and positive selection using LS columns (all from Miltenyi Biotech). The purity of isolated Ly6G+ cells was more than 98% as detected by flow cytometry.
Isolation of human primary cells
Collection of BM samples from patients with MM at the Abramson Cancer Center was approved by the Institutional Review Board (IRB) of the University of Pennsylvania. All patients signed IRB-approved consent forms. Collection of peripheral blood samples from healthy donors was approved by the Wistar IRB. Neutrophils were isolated using Percoll gradient from cells pelleted following Ficoll-Paque gradient centrifugation. Briefly, pelleted cells were resuspended in PBS and were layered over 63%/72% Percoll and centrifuged at 700×g for 30 minutes. Neutrophils were collected from the interface between 72% (bottom) and 63% (top) layers. Primary MM cells were isolated from the BM mononuclear fraction by positive selection of CD138 cells using CD138 MicroBeads and MACS columns (all from Miltenyi Biotec).
Preparation of conditioned medium (CM)
DP42 or 5TGM1 cells were plated at 2×106 cells/mL in phenol-free RPMI 1640 supplemented with L-glutamine and 2% FBS. Cells were collected after 24 hours and centrifuged for 5 minutes at 300×g. Supernatants were collected and frozen.
Detection and quantitation of NETs
Neutrophils were placed in 24-well flat bottom plates and cultured for 8h with or without MM cells separated by Transwell insert or DP42 or 5TGM1 CM followed by fixation of neutrophils with 500 μL of 4% paraformaldehyde and staining with Sytox green nucleic acid stain (Cat. #S7020, ThermoFisher Scientific, final concentration of 0.25 μM). NETs were visualized with a TE300 inverted microscope (Nikon) equipped with a motorized XY stage. An average of 25 z-stacked images with random location (consistent pattern set-up) were acquired using a 20x objective and analyzed with the digital image analysis NIS-Elements software (Nikon). Data presented as NET area per cell (total NET area measured across 25 images divided by the number of neutrophils).
Western blotting
Neutrophils were plated in phenol-free RPMI-1640 medium containing 2% FBS at a density of 2×106 cells/mL and cultured alone or in the presence of DP42 or 5TGM1 cells separated by Transwell insert, DP42 or 5TGM1 CM, or 5 μM calcium ionophore for the indicated time periods. In some experiments, neutrophils were pre-treated for 1h with PAD inhibitors Cl-Amidine, GSK-484, or BMS-P5.
Neutrophils were lysed using RIPA buffer supplemented with 1 mM EDTA and 1x Halt protease and phosphatase inhibitor (Cat. #1861281, ThermoFisher Scientific). Membranes were blocked with 5% nonfat dry milk in TBS-T and incubated with anti-histone H3 (citrulline R2+R8+R17) (Cat. #5103, Abcam) or PAD4 antibodies (Cat. #ABIN2856939, Antibodies Online) followed by incubation with secondary HRP-conjugated antibodies. Membranes were re-probed with anti-histone H3 antibody (Cat. #9715s, Cell Signaling Technology), and then re-probed with antibody against β-actin (Cat. #sc-47778, Santa Cruz Biotechnology).
Trichloroacetic acid (TCA) protein precipitation
Femurs and tibias were collected from mice and flushed with 500 μL of cold PBS. Cold acetone (30 μL) was added to 120 μL of sample, vortexed, and incubated for 30 minutes on ice. Tubes were then centrifuged at 14,000×g at 4°C for 10 minutes. The supernatant was discarded, and the protein pellet was then rinsed with 1 mL 100% ethanol prior to centrifugation at 14,000×g at 4°C for 10 minutes. This step was repeated followed by drying the pellet. The protein pellet was dissolved in 100 μL of 15 mM Tris-HCl pH8.8 and 6X loading buffer (Cat # BP-111R, Boston BioProducts) was added per tube and heated for 5 minutes at 95°C. TCA precipitated samples were subjected to Western blotting.
Flow cytometry
BM cells were labeled with CD138-APC antibody (Cat. #347207, BD Biosciences), washed with ice cold PBS, and resuspended in PBS containing DAPI. Apoptosis of MM cells was evaluated using Annexin V binding assay. Briefly, cells were washed twice with ice cold PBS and once with binding buffer followed by staining with Annexin V-FITC (BioLegend) and DAPI (Life Technologies). At least 10,000 events were acquired using LSR II flow cytometer (BD). Data were analyzed with FlowJo software (TreeStar).
Qubit dsDNA HS assay
The level of DNA present in the serum from MM-bearing mice was determined using the Qubit dsDNA HS assay (Cat # Q32851, Invitrogen) as per the manufacturer’s protocol.
Statistical analysis
Data are presented as mean ± SD. Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software Inc). A two-tailed Student’s t test was used to detect significant differences (p<0.05) between treatment groups. A log-rank test was used for the comparison of survival curves. A p value of less than 0.05 was considered statistically significant.
Results
MM cells stimulate NET formation
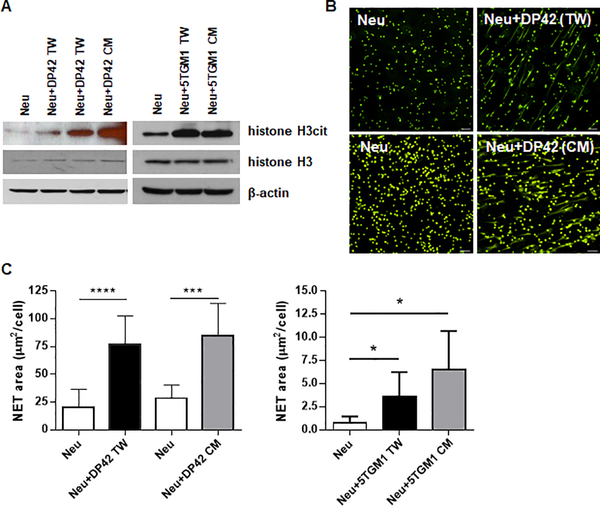
We evaluated the ability of MM cells to induce citrullination of histone H3 and formation of NETs by mouse BM neutrophils. Co-culturing of neutrophils with murine MM DP42 or 5TGM1 cells separated by Transwell inserts resulted in increased histone H3 citrullination in neutrophils. Similar results were obtained when neutrophils were kept in the presence of DP42 or 5TGM1 cell conditioned medium (CM) (Fig. 1A). To evaluate NETosis, BM neutrophils were cultured with murine MM cells or MM cell CM followed by staining with SYTOX Green Nuclear Acid Stain, a cell-impermeable fluorescent dye, and assessment of NET formation by fluorescent microscopy. The presence of MM cells or MM cell CM equally and significantly up-regulated NET production by neutrophils (Fig. 1B,C).
Figure 1. Myeloma cells induce NET formation.
Neutrophils (Neu) were isolated from the BM of tumor-free mice and cultured in vitro alone (Neu), in the presence of mouse myeloma DP42 or 5TGM1 cells separated by Transwell insert (TW), or in the presence of DP42 or 5TGM1 conditioned medium (CM). (A) Western blot analysis of citrullinated histone H3 (H3cit) and total histone H3 in cell lysates prepared from neutrophils cultured for 4h. Experiment was repeated at least three times with similar results. (B,C) NET formation was evaluated using fluorescent microscopy. Representative images of NETs induced by DP42 cells or DP42 cell CM (magnification 20x, scale bar: 50μm) (B) and quantitation of NETs induced by DP42 and 5TGM1 cells (C). Mean and SD values are shown. Each experiment was performed using neutrophils isolated from at least 3 individual mice. * - p<0.05; *** - p=0.0005; **** - p<0.0001 in Student’s t test.
PAD4 is required for MM-induced NET formation
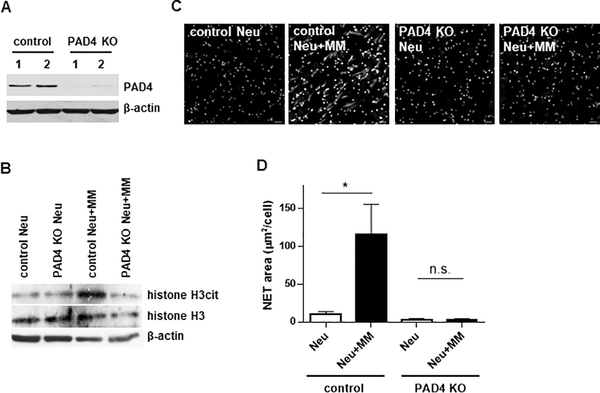
PAD4 is required for citrullination of R2, R8, and R17 residues of histone H3. We utilized PAD4 KO mice (Fig. 2A) to investigate whether PAD4 is involved in MM-induced NET formation. Citrullination of histone H3 was significantly increased in control neutrophils upon addition of MM cells. In contrast, PAD4-deficient neutrophils were not able to upregulate citrullination of histone H3 in response to MM cells (Fig. 2B). In line with these data, MM cells were not able to induce NETosis in PAD4-deficient neutrophils in contrast to control neutrophils (Fig. 2C,D).
Figure 2. PAD4 is required for myeloma-induced NET formation.
(A) Western blot analysis of PAD4 levels in neutrophils isolated from PADfl+/+Cre+ (PAD4 KO) mice or their PAD4fl+/+Cre- littermates (control). (B-D) Neutrophils were isolated from the BM of PAD4 KO mice or their control littermates and cultured in vitro with or without myeloma DP42 cells separated by Transwell insert. (B) Level of citrullinated histone H3 was detected by western blotting. Experiment was performed twice with similar results and representative western blot is shown. (C,D) NET formation was evaluated by microscopy (n=3 mice per group). Representative images (magnification 20x) (C) and quantitation (D) are shown. Mean values and SD obtained using neutrophils isolated from 3 individual mice are shown. * - p< 0.05.
Effect of BMS-P5 on MM-induced NET formation
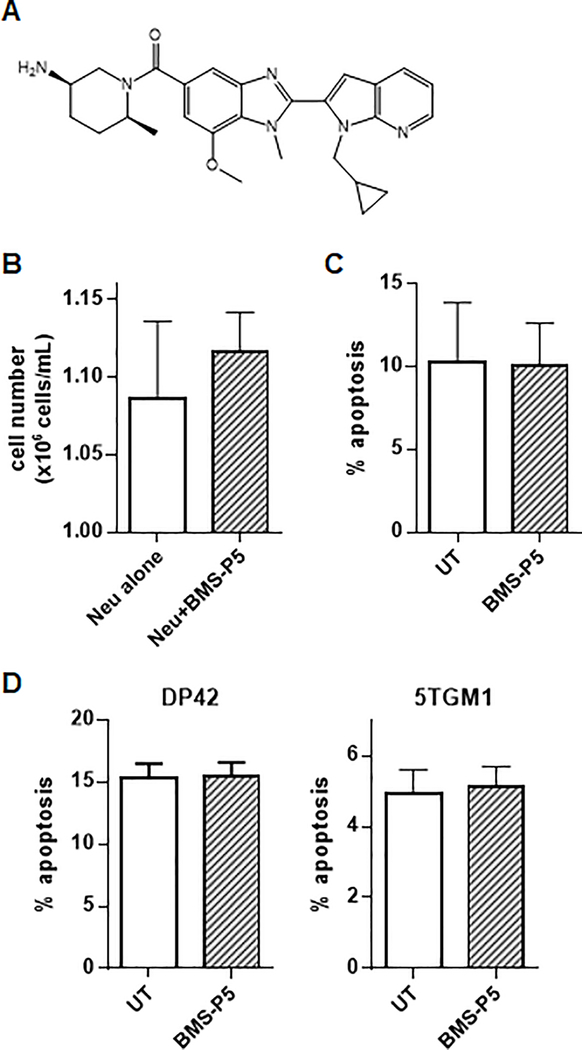
BMS-P5 (Fig. 3A) is a potent selective pharmacological inhibitor of PAD4 (Supplementary Table 1) and was used to evaluate the effect of PAD4 inhibition on MM-induced NET formation. Our preliminary experiments demonstrated a lack of cytotoxicity of BMS-P5 on neutrophils (Fig. 3B, C) and myeloma cells (Fig. 3D).
Figure 3. Cytotoxicity of BMS-P5.
(A) Structure of BMS-P5, a selective PAD4 inhibitor. (B,C) Neutrophils isolated from the BM of 3 individual mice were cultured for 6h with or without 1 μM BMS-P5. Neutrophil viability (B) and apoptosis (C) were determined. (D) Indicated mouse myeloma cells were treated with BMS-P5 for 24h followed by detection of apoptosis. Mean and SD values are shown.
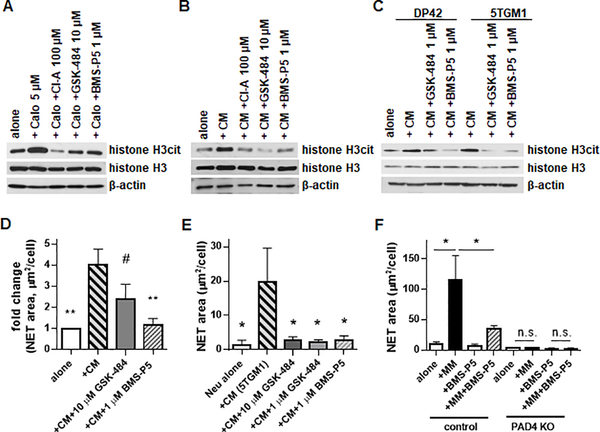
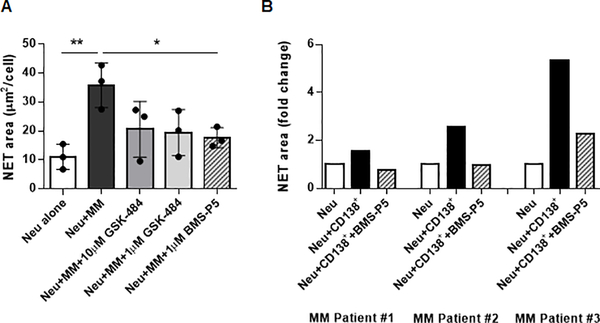
Initially, the ability of BMS-P5 to inhibit NETosis was compared with that of known PAD inhibitors: non-selective inhibitor Cl-A and selective PAD4 inhibitor GSK-484. Both inhibitors were used at the previously reported doses of 100 μM and 10 μM, respectively. All three compounds blocked calcium ionophore-induced citrullination of histone H3 (Fig. 4A). Next, we evaluated whether BMS-P5 could prevent MM-induced NET formation. Neutrophils were pre-treated with 1 μM BMS-P5, 1 μM or 10 μM GSK-484, or 100 μM Cl-A for 30 min followed by addition of DP42 or 5TGM1 CM. As anticipated, both DP42 and 5TGM1 CM induced citrullination of histone H3 (Fig. 4B,C) and formation of NETs (Fig. 4D,E). Addition of all three PAD inhibitors significantly reduced MM-induced citrullination of histone H3 (Fig. 4B,C) and NET formation (Fig. 4D,E). In the absence of PAD4, no induction of NET formation by MM cells was observed, and BMS-P5 did not have any effect (Fig. 4F).
Figure 4. BMS-P5 inhibits PAD4 and prevents mouse myeloma-induced NET formation.
Mouse BM neutrophils were pre-treated with indicated PAD inhibitors for 30 min followed by stimulation with calcium ionophore (CaIo) for 45 min (A), or DP42 (B,C,D) or 5TGM1 (C,E) cell CM for 8h. Level of citrullinated histone H3 was determined by western blotting (A-C) and NET formation was evaluated by fluorescent microscopy and quantitated (D-E). # - p=0.0501; *- p < 0.05; ** - p < 0.01 in Student’s t test between indicated treatment condition and neutrophils cultured with CM (+CM group). (F) BM neutrophils isolated from PADfl+/+Cre+ (PAD4 KO) mice or their PADfl+/+Cre- littermates (control) (n꞊3 per each group) were cultured in the presence or absence of DP42 cells separated by Transwell insert with or without addition of 1 μM BMS-P5. NET formation was evaluated by fluorescent microscopy and quantitated. Mean and SD values are shown. *- p < 0.05 in Student’s t test.
Next, we investigated the effect of PAD4 inhibition with BMS-P5 on NET formation by human neutrophils. Neutrophils were isolated from peripheral blood of healthy donors and cultured in the presence or absence of human MM cell lines with or without addition of 1 μM BMS-P5. In parallel, cells were treated with 1 μM or 10 μM GSK-484. As anticipated, both RPMI-8226 and MM1.S MM cell lines induced NET formation (Fig. 5A and Supplementary Fig. S1). Both PAD4 inhibitors reduced NETosis stimulated by MM cells; however, the effect of BMS-P5 was more substantial (Fig. 5A and Supplementary Fig. S1). BMS-P5 was also able to inhibit formation of NETs induced by primary MM cells isolated from the BM of patients with MM (Fig. 5B)
Figure 5. BMS-P5 prevents NETosis induced by human myeloma cells.
Human neutrophils were cultured for 4h in the presence or absence of human MM RPMI-8226 cells (A) or primary CD138 MM cells isolated from the BM of patients with MM (B) with or without addition of indicated PAD4 inhibitors. NET formation was evaluated by fluorescent microscopy and quantitated. Individual results, mean, and SD values (A) or individual results for each patient (B) are shown. *- p < 0.05 in Student’s t test.
Effect of BMS-P5 in syngeneic mouse model of MM
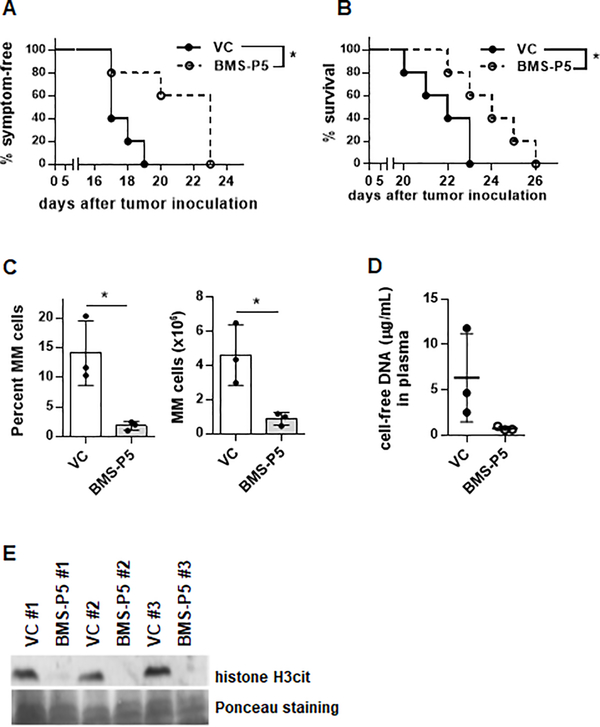
We next investigated whether pharmacological targeting of PAD4 with BMS-P5 could have an anti-tumor effect in a syngeneic mouse model of MM. DP42-bearing mice were treated with BMS-P5 or vehicle control and the onset of disease symptoms (paralysis and hunched posture) and mice survival were evaluated. Administration of BMS-P5 significantly delayed development of symptoms (Fig. 6A) and significantly prolonged survival of MM-bearing mice (Fig. 6B). In parallel, groups of BMS-P5 and vehicle control-treated mice were euthanized 13 days after tumor cell inoculation for analysis. Selection of this time point was based on known kinetics of tumor growth when a substantial proportion of DP42 cells is already present in the BM microenvironment while other hematopoietic cells still represented the majority of the BM cells. The proportion and absolute number of MM cells were significantly reduced in BMS-P5-treated mice as compared to vehicle control-treated mice (Fig. 6C). DNA represents one of the main NET components and the presence of citrullinated histone H3 has been associated with NETosis. Therefore, we measured levels of cell-free DNA and histone H3cit in the BM plasma. Administration of PAD4 inhibitor BMS-P5 resulted in significantly reduced plasma levels of cell-free DNA (Fig. 6D) and BM level of cell-free citrullinated histone H3 (Fig. 6E). Taken together, our data suggest that pharmacological targeting of PAD4 could be beneficial for the treatment of MM.
Figure 6. Anti-tumor effect of BMS-P5 in a syngeneic mouse model of multiple myeloma.
MM tumors were established by i.v. inoculation of DP42 cells into syngeneic mice. Treatment with PAD4 inhibitor BMS-P5 or vehicle control (VC) started on day 3 after tumor cell injection (n=5 mice per group). Onset of paralysis (A) and survival (B) were evaluated. * - p< 0.05 in log-rank test. (C-E) BMS-P5 or VC-treated DP42-bearing mice were euthanized on day 13 after tumor cell injection (n=3 mice per group). (C) Proportion and absolute number of CD138+ MM cells in the BM was detected by flow cytometry. Individual values, mean and SD are shown. * - p< 0.05 in Student’s t test. (D) Blood was collected, and levels of cell-free DNA was determined in plasma using Qubit dsDNA HS assay. (E) Femur bones were collected and flushed. Presence of citrullinated histone H3 was detected by western blotting.
Discussion
The overall objective of the present study was to evaluate whether MM cells can stimulate neutrophils to form NETs and whether pharmacological blocking of NET formation would delay MM progression. Our results, for the first time, demonstrated that MM cells induce NETosis and that PAD4 is required for MM-induced NET formation. We further reported a novel small molecule PAD4-specific inhibitor BMS-P5 (Bristol-Myers Squibb) and demonstrated that this compound was able to block MM-induced NET formation. Administration of BMS-P5 in vivo in MM-bearing mice delayed appearance of symptoms and prolonged mice survival in a syngeneic model of this disease.
Although the presence of NETs has been reported for several cancer types, their involvement in the pathogenesis of MM has not yet been demonstrated. We observed an increase in citrullination of histone H3 associated with an increase in NET formation in neutrophils stimulated by MM cells. These effects were abrogated when MM cells were co-cultured with PAD4-deficient neutrophils indicating that PAD4 is indeed required for MM-induced NET formation. Our data are consistent with previous studies in other tumor types demonstrating that tumor cells can induce PAD4-depenedent NETosis (12,13,15,29–36).
Histone H3 citrullination plays an important role in NET formation and is mediated by the PAD4 enzyme. PAD4 is receiving increased interest in the pharmaceutical field due to its implication in the biology of cancer and other pathologies. In the present study, we aimed to understand the in vitro and in vivo effects of selective PAD4 inhibition in MM. Several previously reported PAD inhibitors have been shown to prevent NETosis (12,26,27,37); however, only one of them, GSK-484, possesses selectivity for PAD4 (26). Our findings demonstrated that, in vitro, BMS-P5 significantly reduced MM-stimulated PAD4-dependent NET formation by mouse BM neutrophils and human neutrophils isolated from peripheral blood of healthy donors. Similar results were obtained with GSK-484 confirming robustness of these data. In vivo, administration of BMS-P5 significantly improved survival of MM-bearing mice. Ex vivo data demonstrated that the proportion and absolute number of MM cells in MM-bearing mice treated with BMS-P5 was significantly reduced as compared to vehicle control-treated mice. In addition, the level of citrullinated histone H3 was reduced in the BM flushes from BMS-P5-treated mice as compared to control mice. There is a possibility that mechanisms other than blocking NETosis are responsible for the anti-MM effect of BMS-P5. Identification of these mechanisms could represent a subject of future studies.
The pro-tumorigenic effect of NETs may be due to many mechanisms. NETs contain a number of proteins including high mobility group box 1 (HMGB1), neutrophil elastase (NE), and matrix metalloproteinase 9 (MMP9) among others. HMGB1 has been implicated in the angiogenesis, invasion, progression, metastasis, and drug resistance of cancers (38–40). Li et al. showed that HMGB1 can cause immunosuppression by stimulating myeloid-derived suppressor cells and promote peritoneal spread of colon cancer cells after surgical resection (41). NE and MMP9 have also been implicated in tumor progression (42,43). A study by Albrengues et al. demonstrated that NET-associated NE and MMP9 induced proteolytic cleavage of laminin which drove awakening of cancer cells and caused metastasis in mice (27). In the context of MM, the accumulation of NET-derived DNA scaffold whereby a lot of proteases are concentrated could also alter the tumor microenvironment and provide a growth advantage to MM cells. Therefore, by targeting the process of NET formation, BMS-P5 may attenuate the presence of pro-tumorigenic proteins in the tumor microenvironment, and thus delay tumor progression.
Taken together, our data for the first time demonstrate that MM cells may induce NETosis associated with PAD4 activation and that disruption of the NET formation process by pharmacological inhibition of PAD4 has anti-tumor effects in a syngeneic model of MM. These data suggest that targeting PAD4 could modify the tumor microenvironment and may be beneficial for the treatment of patients with MM.
Supplementary Material
Acknowledgments
This work was supported by NIH/NCI grant R01CA196788 (to Y.N.). Support for the Shared Resources utilized in this study was provided by Cancer Center Support Grant P30CA010815 to The Wistar Institute.
Abbreviation list
- BM
bone marrow
- CM
conditioned medium
- HMGB1
high mobility group box 1
- KO
knockout
- LPS
lipopolysaccharide
- MM
multiple myeloma
- MMP9
matrix metalloproteinase 9
- NE
neutrophil elastase
- NETs
neutrophil extracellular traps
- PAD
peptidylarginine deiminase
Footnotes
Conflict of interest: M. Li, J. Strnad, and J. Burke were employed by BMS. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple myeloma. Nat Rev Dis Primers 2017;3:17046 doi 10.1038/nrdp.2017.46. [DOI] [PubMed] [Google Scholar]
- 2.Shain KH, Dalton WS. Environmental-mediated drug resistance: a target for multiple myeloma therapy. Expert Rev Hematol 2009;2(6):649–62 doi 10.1586/ehm.09.55. [DOI] [PubMed] [Google Scholar]
- 3.Kawano Y, Moschetta M, Manier S, Glavey S, Gorgun GT, Roccaro AM, et al. Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev 2015;263(1):160–72 doi 10.1111/imr.12233. [DOI] [PubMed] [Google Scholar]
- 4.Neutrophils Borregaard N., from marrow to microbes. Immunity 2010;33(5):657–70 doi 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss D, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303(5663):1532–5. [DOI] [PubMed] [Google Scholar]
- 6.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 2014;15(11):1017–25 doi 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007;176(2):231–41 doi 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cools-Lartigue J, Spicer J, Najmeh S, Ferri L. Neutrophil extracellular traps in cancer progression. Cell Mol Life Sci 2014;71(21):4179–94 doi 10.1007/s00018-014-1683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decker AS, Pylaeva E, Brenzel A, Spyra I, Droege F, Hussain T, et al. Prognostic Role of Blood NETosis in the Progression of Head and Neck Cancer. Cells 2019;8(9) doi 10.3390/cells8090946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 2013;123(8):3446–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger-Achituv S, Brinkmann V, Abed U, Kühn L, Ben-Ezra J, Elhasid R, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol 2013;4(48) doi 10.3389/fimmu.2013.00048. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res 2016;76(6):1367–80 doi 10.1158/0008-5472.Can-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 2016;8(361):361ra138 doi 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Haidari AA, Algethami N, Lepsenyi M, Rahman M, Syk I, Thorlacius H. Neutrophil extracellular traps promote peritoneal metastasis of colon cancer cells. Oncotarget 2019;10(12):1238–49 doi 10.18632/oncotarget.26664. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A 2012;109(32):13076–81 doi 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdol Razak N, Elaskalani O, Metharom P. Pancreatic Cancer-Induced Neutrophil Extracellular Traps: A Potential Contributor to Cancer-Associated Thrombosis. Int J Mol Sci 2017;18(3) doi 10.3390/ijms18030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C, Sun W, Cui W, Li X, Yao J, Jia X, et al. Procoagulant role of neutrophil extracellular traps in patients with gastric cancer. Int J Clin Exp Pathol 2015;8(11):14075–86. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009;184(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilvawala R, Thompson PR. Peptidyl arginine deiminases: detection and functional analysis of protein citrullination. Curr Opin Struct Biol 2019. doi 10.1016/j.sbi.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 2008;180(3):1895–902 doi 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 2010;207(9):1853–62 doi 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 2015;125(12):1948–56 doi 10.1182/blood-2014-07-587709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinod K, Witsch T, Erpenbeck L, Savchenko A, Hayashi H, Cherpokova D, et al. Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J Exp Med 2017;214(2):439–58 doi 10.1084/jem.20160530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Causey CP, Jones JE, Slack JL, Kamei D, Jones LE, Subramanian V, et al. The development of N-alpha-(2-carboxyl)benzoyl-N(5)-(2-fluoro-1-iminoethyl)-l-ornithine amide (o-F-amidine) and N-alpha-(2-carboxyl)benzoyl-N(5)-(2-chloro-1-iminoethyl)-l-ornithine amide (o-Cl-amidine) as second generation protein arginine deiminase (PAD) inhibitors. J Med Chem 2011;54(19):6919–35 doi 10.1021/jm2008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knuckley B, Causey CP, Jones JE, Bhatia M, Dreyton CJ, Osborne TC, et al. Substrate specificity and kinetic studies of PADs 1, 3, and 4 identify potent and selective inhibitors of protein arginine deiminase 3. Biochemistry 2010;49(23):4852–63 doi 10.1021/bi100363t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol 2015;11(3):189–91 doi 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018;361(6409) doi 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atkinson S, Barker M, Campbell M, Diallo H, Douault C, Garton N, et al. ; Glaxo Group Ltd, assignee. 2-(azaindol-2-yl)benzimidazoles as PAD4 inhibitors. USA: 2015. [Google Scholar]
- 29.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 2013. doi 10.1172/jci67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berger-Achituv S, Brinkmann V, Abed UA, Kuhn LI, Ben-Ezra J, Elhasid R, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol 2013;4:48 doi 10.3389/fimmu.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guglietta S, Chiavelli A, Zagato E, Krieg C, Gandini S, Ravenda PS, et al. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat Commun 2016;7:11037 doi 10.1038/ncomms11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin W, Xu HX, Zhang SR, Li H, Wang WQ, Gao HL, et al. Tumor-Infiltrating NETs Predict Postsurgical Survival in Patients with Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 2019;26(2):635–43 doi 10.1245/s10434-018-6941-4. [DOI] [PubMed] [Google Scholar]
- 33.Cedervall J, Zhang Y, Olsson AK. Tumor-Induced NETosis as a Risk Factor for Metastasis and Organ Failure. Cancer Res 2016;76(15):4311–5 doi 10.1158/0008-5472.Can-15-3051. [DOI] [PubMed] [Google Scholar]
- 34.Richardson JJR, Hendrickse C, Gao-Smith F, Thickett DR. Neutrophil Extracellular Trap Production in Patients with Colorectal Cancer In Vitro. Int J Inflam 2017;2017:4915062 doi 10.1155/2017/4915062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boone B, Orlichenko L, Schapiro N, Loughran P, Gianfrate G, Ellis J, et al. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Therapy 2015; 22:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, et al. Gradient Infiltration of Neutrophil Extracellular Traps in Colon Cancer and Evidence for Their Involvement in Tumour Growth. PLoS One 2016;11(5):e0154484 doi 10.1371/journal.pone.0154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cedervall J, Dragomir A, Saupe F, Zhang Y, Arnlov J, Larsson E, et al. Pharmacological targeting of peptidylarginine deiminase 4 prevents cancer-associated kidney injury in mice. Oncoimmunology 2017;6(8):e1320009 doi 10.1080/2162402x.2017.1320009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venereau E, De Leo F, Mezzapelle R, Careccia G, Musco G, Bianchi ME. HMGB1 as biomarker and drug target. Pharmacol Res 2016;111:534–44 doi 10.1016/j.phrs.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 39.Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res 2007;13(10):2836–48 doi 10.1158/1078-0432.Ccr-06-1953. [DOI] [PubMed] [Google Scholar]
- 40.Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet 2015;385(9983):2197–208 doi 10.1016/s0140-6736(14)60493-1. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Wu K, Zhao E, Shi L, Li R, Zhang P, et al. HMGB1 recruits myeloid derived suppressor cells to promote peritoneal dissemination of colon cancer after resection. Biochem Biophys Res Commun 2013;436(2):156–61 doi 10.1016/j.bbrc.2013.04.109. [DOI] [PubMed] [Google Scholar]
- 42.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med 2010;16(2):219–23 doi 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000;103(3):481–90 doi 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.