Abstract
Fractional exhaled nitric oxide (FENO50), a marker of allergic airway inflammation, is used in respiratory research and asthma clinical care; however, its trajectory with increasing age during childhood has not been well characterized. We examined FENO50 longitudinally during a period of important somatic growth to describe trajectories across childhood and adolescence in healthy participants and evaluate clinical factors as potential determinants of trajectories.
FENO50 was collected at six visits over eight years in a population-based cohort of 1791 schoolchildren without asthma (median age at entry 8.4). Smooth sex-specific FENO50 trajectories were estimated using generalized additive mixed models, with participant-level random effects. We evaluated whether sex-specific trajectories were influenced by race/ethnicity, BMI percentile, allergic rhinitis, or puberty.
Different FENO50 patterns were observed by sex in later childhood and several factors were associated with either FENO50 level or change in FENO50 as participants aged. FENO50-age trajectories were similar by sex until age ~11.5, after which males had greater FENO50 change than females. This divergence in FENO50-age trajectories coincides with puberty. Males with higher starting BMI percentile had attenuated FENO50-age slopes. Among males, FENO50 levels were lower in non-Hispanic Whites. Among both sexes, participants with rhinitis had higher FENO50. FENO50 levels within individuals tracked over time; however, there was considerable variation in FENO50 patterns across participants.
FENO50 trajectories from longitudinal data provide evidence of sex differences coinciding with puberty, suggesting potential hormone link. Improved understanding of determinants of FENO50 trajectories is needed to realize the potential for using individualized predicted FENO50 trajectories.
Keywords: Adolescence, breath test, FENO50, pediatric, reference value
Introduction
There is increasing evidence of the utility of fractional exhaled nitric oxide (FENO50)—a noninvasive biomarker of allergic airway inflammation—for research, patient management of asthma treatment and in providing supporting evidence for asthma diagnosis [1–4]. Examining trends with age in FENO50 among healthy children during this critical period of growth as well as the impact of clinical factors such as sex, race/ethnicity, and allergic disease may enhance the utility of FENO50 in clinical and research settings.
Prior studies of the distribution and determinants of FENO50 in healthy pediatric populations were based on single cross-sectional measurements among children across a range of ages [5–11] which may have missed important patterns in FENO50 change as children grow. Serial measurements within the same set of children as they age is likely to be more informative for understanding trajectory of FENO50 across childhood and adolescence as well as potential determinants of this evolution. Furthermore, during childhood and adolescence there is an important epidemiological shift in the rates of allergic diseases, including asthma and allergic rhinitis, with higher rates among males during childhood, then in adolescence rates are higher among females—a reversal that maybe puberty related [12–14]. A better understanding of FENO50 trajectories coinciding with the timing of this transition is needed for improved use of FENO50 in research and clinical medicine.
The aim of this study was to examine FENO50 trajectories across childhood and adolescences in a unique data resource of healthy children from the Southern California Children’s Health Study (CHS) with serial FENO50 measurements during a period of rapid somatic growth, ages 8–16 years. We investigate sex-specific FENO50 trajectories as a function of age and height, and evaluate potential determinants of FENO50 trajectories including race/ethnicity, body mass index (BMI), allergic rhinitis, and puberty.
Material and methods
Study design and study population
This study was performed within a cohort of the population-based CHS, described in detail previously [15]. Briefly, in 2002–2003, a prospective cohort of children primarily aged 6–7 years was enrolled from kindergarten and first grade public school classrooms from 13 communities. Follow-up for FENO50 began in study year 3. Ethical approval of the protocol was obtained from the University of Southern California Institutional Review Board (approval # HS-13–00150, continuing review date April 17, 2019). Informed consent was obtained from a parent or guardian at first and follow-up visits through 2007–2008, and informed assent was obtained from each child at visits after 2008.
Because the study aim was to examine FENO50 trajectories in healthy children, we excluded participants with any history of asthma based on all study questionnaire data (n=618). We sequentially excluded FENO50 measurements conducted when height was not recorded (29 observations), or the participant had reported respiratory infection within the past 3 days (389 observations), or the participant had reported personal smoking within the last month (56 observations). These last eligibility criteria excluded that year’s FENO50 measurements and not the participants themselves, who could contribute FENO50 measurements in years when they met eligibility criteria for FENO50 measurements. The final study population consisted of 1791 healthy children with 7185 FENO50 measurements.
Measurement of Exhaled Nitric Oxide
FENO50 assessment in the CHS has been described in detail previously [16–18]. Briefly, FENO50 was collected at schools using an offline breath collection technique in years 3–4 at 100 mL/s flow using Sievers sampling kits and analyzed with a Sievers Model 280i NO analyzer, and online using ECO MEDICS CLD-88-SP instruments (with DeNOx) at 50mL/s flow during years 5, 6, 8, and 10 following manufacturers’ instructions and professional societies guidelines [19, 20] (Table 1; additional details in eMethods). Offline FENO50 values were converted to online values at 50mL/s using a prediction model developed internally (R2 = 0.94) from a pilot study including concurrent offline and online measurements among 362 schoolchildren in 2006–2007 [17]. Prediction model inputs included offline FENO50, ambient NO, and lag time before analysis [17]. Model-predicted online FENO50 estimates produced results similar to observed online FENO50 measures in analysis of school and age differences [17].
Table 1.
Study design information including type and number of FENO50 measurements and average participant age over the study period. Over the entire study period 1791 children contributed FENO50 measurements.
| Study Year | 1 | 2 | 3* | 4 | 5 | 6 | 8 | 10 |
|---|---|---|---|---|---|---|---|---|
| Calendar Years | 2002–03 | 2003–04 | 2004–05 | 2005–06 | 2006–07 | 2007–08 | 2009–10 | 2011–12 |
| FENO50 measure type | None | Offline | Online | |||||
| Participants with FENO50 data (N) | 1429 | 1498 | 1364 | 1100 | 938 | 856 | ||
| Mean age (years) | 8.4 | 9.3 | 10.3 | 11.4 | 13.5 | 15.3 | ||
No FENO50 data collected study years 1 and 2. No follow-up, FENO50 or otherwise, conducted in study years 7 (2008–09) or 9 (2010–11)
The start of follow up for the current study
Covariate assessment
Parents/guardians completed written questionnaires at first and follow-up visits (children responded beginning year 8 when they were aged 13.5 years (standard deviation (SD):0.63) providing information on child’s sex, race/ethnicity, date of birth, allergic rhinitis, and history of doctor-diagnosed asthma. Ever reported allergic rhinitis at year 3 was defined based on responses to the questions “has your child ever/in the past 12 months had a problem with sneezing or a runny or blocked nose, when he/she did not have a cold or the ‘flu?’” and “in the past 12 months, has this nose problem been accompanied by itchy-watery eyes?” We categorized a positive response to both questions as “allergic rhinitis.” Children were classified as having asthma if it was reported on any questionnaire that a doctor had “ever diagnosed this child as having asthma.” Reported respiratory infection within the past 3 days was based on questionnaire data from parents (through year 6) or participants (years 8 and 10). Starting year 6, children were asked about personal smoking (cigarettes or anything else) [15, 18]. Height and weight measurements were used to calculate age- and sex-specific BMI percentiles (BMI%) from the Centers for Disease Control and Prevention growth charts [21]. Height velocity was calculated as the difference in height divided by the difference in age between consecutive annual study visits. Age at peak height velocity—a proxy for puberty [22]—was then determined and subsequently categorized as “early” or “late” based on sex-specific median.
Statistical analysis
FENO50 values are right skewed. Therefore, unless otherwise specified, we performed analyses with natural log-transformed FENO50 (logFENO50), with results presented using back-transformed values. Analyses were stratified by sex a priori, based on reported differences in FENO50 between male and female children [5].
Descriptive analyses present study population characteristics and describe sex-specific associations of these characteristics with year 3 FENO50 in the subset of n=1429 participants with available FENO50 and with percent change in FENO50 from year 3 (median age 8.4 years [interquartile range [IQR]: 7.9–8.9) to year 10 (median age 15.3 years [IQR:14.7–15.8]) in the subset of n=681 children with FENO50 measured in both years. Percent change relates the geometric mean of FENO50 at year 10 to the geometric mean of FENO50 at year 3 (i.e., exponentiated mean difference in logFENO50 from year 3 to 10). We evaluated statistical significance of differences between categorical covariates for year 3 FENO50 using one-way analysis of variance and for year 3 to 10 FENO50 percent change using a non-parametric Kruskal-Wallis test. To quantify the correlation of repeated, longitudinal measurements of logFENO50 in participants over the 8-year period, we calculated intraclass correlation coefficients (ICC).
Sex-specific FENO50 trajectories as function of age and/or height were modeled by fitting generalized additive mixed models [23, 24], with a participant-level random intercept and random slope on age (or on height for height-only model), to all available data on the 1791 participants using the R package ‘mgcv’ [24]. Smooth functions of only height or only age were fitted using a penalized regression spline [24]. A joint smooth function of age and height was fitted using a penalized tensor product smooth [24]. Next, using data on 1717 participants with complete year 3 BMI%, we evaluated whether sex-specific age trajectories were influenced by: (a) year 3 BMI%, (b) race/ethnicity (Hispanic, White/non-Hispanic, other (includes missing)), or (c) year 3 ever reported allergic rhinitis (yes, no, missing), using parameterizations that allowed for quantification of age interactions (see online supplement eMethods). Based on statistical significance in bivariate analyses, final models for FENO50-age trajectories were then developed. Lastly, we stratified final models by dichotomized (early or late) age at peak height velocity as a proxy for puberty. Sensitivity analysis included analyses: (i) with height instead of age trajectories, (ii) with time-varying BMI% collected over follow-up instead of year 3 BMI%, (iii) restricted to 1062 children who never reported allergic rhinitis through year 10, (iv) restricted to 1526 children who never reported wheeze or whistling in the chest through year 10, and (v) restricted to online FENO50 measurements only. All statistical analyses were performed using R 3.5.1 [25].
Results
Participants’ mean age was 8.4 years (SD: 0.65) in study year 3, the initial year of FENO50 follow up. In year 3 (n=1429), geometric mean FENO50 was 10.6 parts per billion (ppb) (geometric SD: 1.84), with no difference by sex (p=0.90). Median follow-up time from first to last FENO50 measurement was 5.2 years (interquartile range: 2.0–6.8) and 46.6% of participants had 5 or 6 FENO50 measurements (mean: 4.0; Table E1). See Figure E1 for example trajectories. The ICC was 0.57 for males and 0.59 for females, indicating moderately strong within-participant correlation of FENO50. FENO50 generally increased from year 3 to 10. Percent change in FENO50 from year 3 to 10 in the n=681 children with available data, was higher in males than females (53.2% vs 14.4%, respectively, p<0.001). There were no statistically significant differences in covariate distributions between participants with and without year 10 data (Table E1).
In descriptive analyses, determinants of year 3 FENO50 were different from determinants of FENO50 change from year 3 to 10 as shown in Tables 2a (males) and Table 2b (females). In year 3, FENO50 was statistically significantly different by race/ethnicity for males and by age and allergic rhinitis for females. From year 3 to 10, there was modest evidence for differences in percent change in FENO50 by year 3 BMI% in males (p=0.07), with an attenuated increase in FENO50 for those with higher year 3 BMI%. Among females, percent change in FENO50 differed by age and height in year 3, with larger FENO50 change among those younger or shorter in year 3.
Table 2a.
For males, participant characteristics at year 3 and associations with fractional exhaled nitric oxide (FENO50) or percent change in FENO50 over approximately 7 years.
| Year 3 Characteristic | Year 3 FENO50 (N=672) | % Change in FENO50, year 3 to 10 (N=318) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | (%) | Geometric mean | p-value* | N | (%) | Estimate | p-value† | |
| Age, years | 0.178 | 0.749 | ||||||
| 7 | 48 | (7%) | 9.2 | 25 | (8%) | 60.4 | ||
| 8 | 313 | (47%) | 10.3 | 144 | (45%) | 52.1 | ||
| 9 | 276 | (41%) | 11.0 | 133 | (42%) | 56.5 | ||
| 10 | 35 | (5%) | 11.6 | 16 | (5%) | 26.8 | ||
| Height, cm | 0.140 | 0.816 | ||||||
| ≤125 | 129 | (19%) | 9.9 | 50 | (16%) | 56.7 | ||
| (125, 130] | 168 | (25%) | 10.9 | 73 | (23%) | 46.1 | ||
| (130, 135] | 205 | (31%) | 10.1 | 114 | (36%) | 60.7 | ||
| >135 | 170 | (25%) | 11.4 | 81 | (25%) | 47.2 | ||
| BMI percentile | 0.957 | 0.067 | ||||||
| 0–15 | 41 | (6%) | 10.4 | 22 | (7%) | 45.6 | ||
| 15–85 | 359 | (53%) | 10.7 | 169 | (53%) | 68.2 | ||
| 85–95 | 122 | (18%) | 10.6 | 61 | (19%) | 44.5 | ||
| 95+ | 150 | (22%) | 10.4 | 66 | (21%) | 29.3 | ||
| Race/ethnicity | <0.001 | 0.468 | ||||||
| Hispanic | 386 | (57%) | 11.0 | 177 | (56%) | 62.9 | ||
| White/non-Hispanic | 226 | (34%) | 9.5 | 115 | (36%) | 48.2 | ||
| Black | 12 | (2%) | 10.4 | 4 | (1%) | 67.0 | ||
| Asian | 27 | (4%) | 18.1 | 11 | (3%) | 28.4 | ||
| Other | 19 | (3%) | 8.0 | 10 | (3%) | 72.2 | ||
| Missing | 2 | (0.3%) | 17.1 | 1 | (0.3%) | −35.4 | ||
| Ever reported allergic rhinitis | 0.109 | 0.779 | ||||||
| No | 380 | (57%) | 10.2 | 180 | (57%) | 51.2 | ||
| Yes | 193 | (29%) | 11.5 | 94 | (30%) | 57.7 | ||
| Missing | 99 | (15%) | 10.3 | 44 | (14%) | 51.5 | ||
Analysis of variance (ANOVA) F-test (excluding missing categories, if applicable)
Kruskal-Wallis test, a non-parametric alternative to ANOVA (excluding missing categories, if applicable)
Table 2b.
For females, participant characteristics at year 3 and associations with fractional exhaled nitric oxide (FENO50) or percent change in FENO50 over approximately 7 years.
| Year 3 Characteristic | Year 3 FENO50 (N=757) | % Change in FENO50, year 3 to 10 (N=363) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | (%) | Geometric mean | p-value* | N | (%) | Estimate | p-value† | |
| Age, years | 0.001 | 0.069 | ||||||
| 7 | 65 | (9%) | 8.7 | 33 | (9%) | 34.5 | ||
| 8 | 381 | (50%) | 10.3 | 179 | (49%) | 18.6 | ||
| 9 | 288 | (38%) | 11.7 | 136 | (37%) | 4.3 | ||
| 10 | 23 | (3%) | 10.0 | 15 | (4%) | 22.1 | ||
| Height, cm | 0.166 | 0.040 | ||||||
| ≤125 | 202 | (27%) | 10.2 | 92 | (25%) | 31.4 | ||
| (125, 130] | 143 | (19%) | 11.6 | 68 | (19%) | 2.1 | ||
| (130, 135] | 236 | (31%) | 10.3 | 117 | (32%) | 16.4 | ||
| >135 | 176 | (23%) | 10.8 | 86 | (24%) | 5.6 | ||
| BMI percentile | 0.962 | 0.445 | ||||||
| 0–15 | 58 | (8%) | 10.5 | 21 | (6%) | 30.3 | ||
| 15–85 | 452 | (60%) | 10.5 | 223 | (61%) | 16.1 | ||
| 85–95 | 127 | (17%) | 10.8 | 64 | (18%) | 13.5 | ||
| 95+ | 120 | (16%) | 10.8 | 55 | (15%) | 3.8 | ||
| Race/ethnicity | 0.131 | 0.394 | ||||||
| Hispanic | 449 | (59%) | 11.1 | 203 | (56%) | 14.2 | ||
| White/non-Hispanic | 219 | (29%) | 9.8 | 114 | (31%) | 11.4 | ||
| Black | 14 | (2%) | 13.0 | 6 | (2%) | 8.0 | ||
| Asian | 31 | (4%) | 10.6 | 14 | (4%) | 44.9 | ||
| Other | 41 | (5%) | 9.6 | 25 | (7%) | 24.9 | ||
| Missing | 3 | (0.4%) | 10.7 | 1 | (0.3%) | 102.2 | ||
| Ever reported allergic rhinitis | 0.022 | 0.122 | ||||||
| No | 467 | (62%) | 10.2 | 238 | (66%) | 14.7 | ||
| Yes | 206 | (27%) | 11.7 | 84 | (23%) | 20.6 | ||
| Missing | 84 | (11%) | 10.7 | 41 | (11%) | 1.4 | ||
Analysis of variance (ANOVA) F-test (excluding missing categories, if applicable)
Kruskal-Wallis test, a non-parametric alternative to ANOVA (excluding missing categories, if applicable)
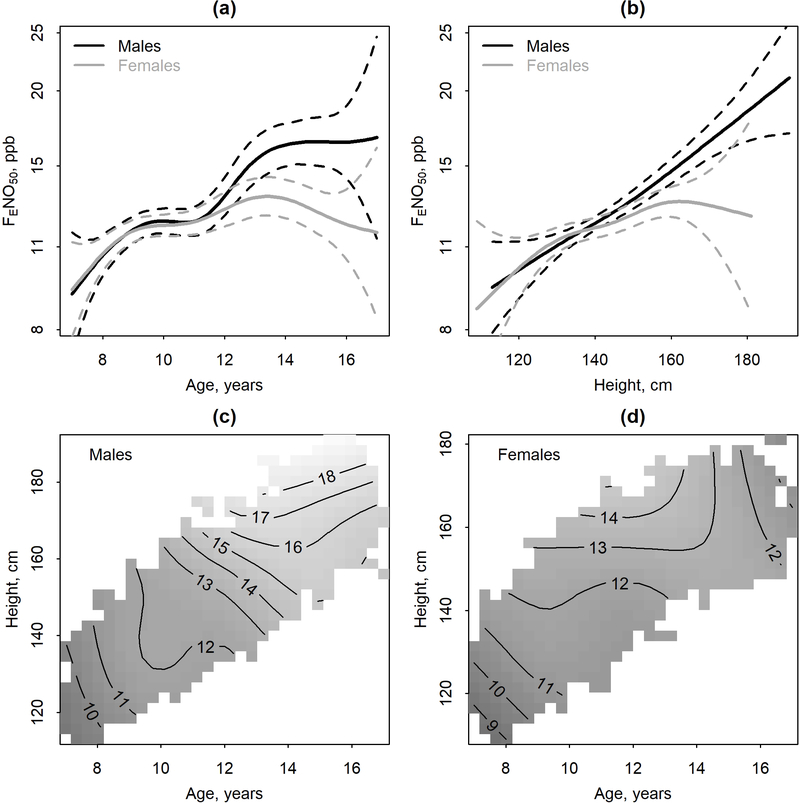
Estimates of population-averaged sex-specific trajectories of FENO50 by age and/or height (n=1791) are displayed in Figure 1 and summarized in Table E2. As a function of age, estimated geometric mean FENO50 at age 8 was similar by sex (10.6 and 10.7 ppb for males and females, respectively) and the nonlinear FENO50 trajectory was similar by sex through approximately age 11.5. After age 11.5, males tended to have a larger increase in FENO50 than females. By age 15, predicted geometric mean FENO50 for an average male was 16.5 (95%CI: 15.4, 17.6) ppb and for an average female 12.6 (11.9, 13.4) ppb. This translates to an increase from age 8 to 15 of 54.9% (44.8, 65.8) for the average male and 17.8% (11.0, 25.0) for the average female. As a function of height, FENO50 trajectory for an average child was nearly linear for males and nonlinear, with a shallower slope for females. When considering joint smooths of age and height, there was evidence of an interaction (Figure 1c–d, Table E2). For example, for females (Figure 1d) the positive association between age and FENO50 was attenuated for taller younger females (approximately height>140 cm, age < 13) and negative for taller older females (approximately height>140 cm, age ≥13).
Figure 1.
Population-averaged sex-specific trajectories of fractional exhaled nitric oxide (FENO50) (solid lines) (N=851 males, N=940 females) and 95% Confidence Intervals (dashed lines) modeled as a: (a) smooth function of age only, (b) a smooth function of height only, or a joint smooth of age and height for males (c) or females (d). In panels (c-d), the contour lines labels refer to predicted geometric mean FENO50 in ppb. Analyses performed using logFENO50 with results here presented using back-transformed values on an axis scaled using natural log.
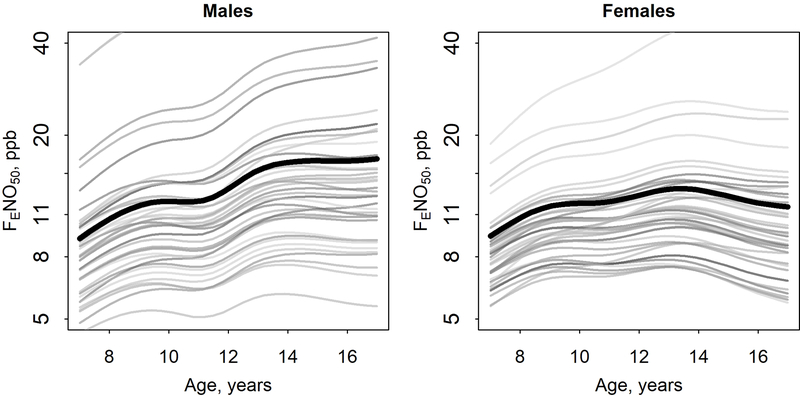
There was considerable between-participant heterogeneity (i.e., nonuniformity) as shown in the participant-specific predicted trajectories (Figure 2). To put estimated random intercept SDs into context (males: 0.50; females: 0.47 (logFENO50 scale)), estimated average FENO50 for males at age 8 was 10.6 ppb, but with a 95% CI that ranged from 4.0 ppb to 28.2 ppb (exp(2.36±1.96*0.50)). Subsequent “tracking” of participant-specific predicted trajectories is apparent in Figure 2, with participants with high (or low) FENO50 at start of follow up generally having high (or low) FENO50 through end of follow up. There was also considerable heterogeneity in the random slopes on age (SD: males: 0.051; females: 0.052). For context, linear mixed effects models assuming a linear (rather than smooth) trajectory of logFENO50 in age had population average slopes on age of 0.061 for males and 0.022 for females.
Figure 2.
Participant-specific predicted trajectories of fractional exhaled nitric oxide (FENO50), modeled as a smooth function of age, for 40 randomly selected males and 40 randomly selected females. Analyses performed using logFENO50 with results here presented using back-transformed values on an axis scaled using natural log. The population average predictions (identical to Figure 1a) are plotted with a heavy black line.
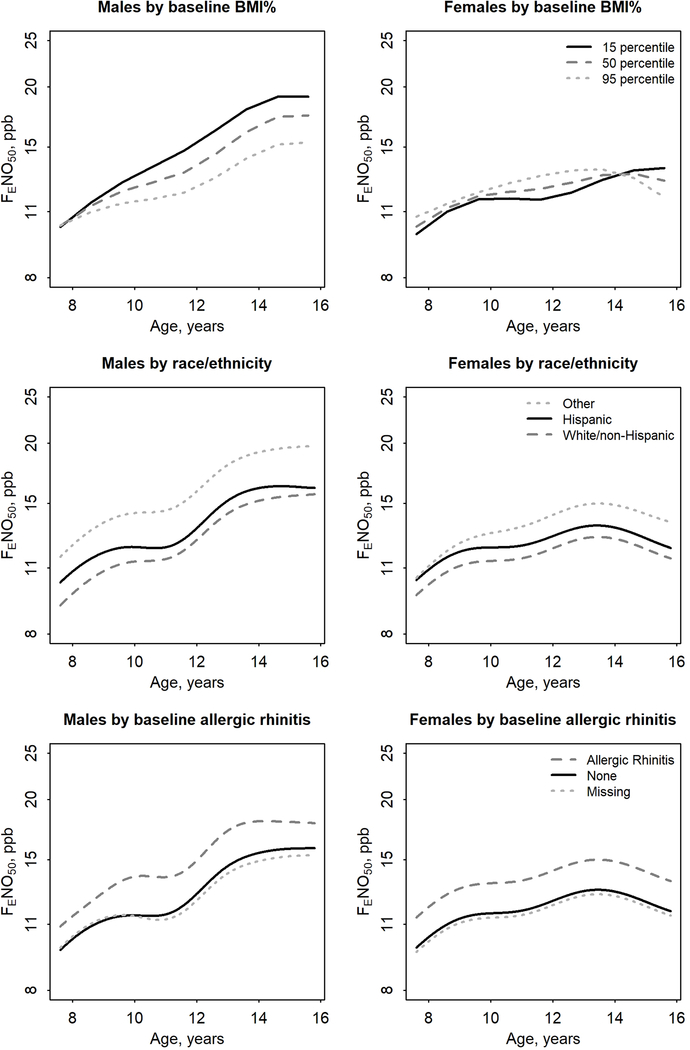
The estimated sex-specific age trajectories of FENO50 by categories of sex, race/ethnicity, and allergic rhinitis, and year 3 BMI% (n=1717) are displayed in Figure 3 and summarized in Tables 3 and E3. The only covariate that significantly modified the average FENO50-age trajectory (i.e., FENO50 change from age 8 to 16 years) was year 3 BMI% in males. Males with higher year 3 BMI% had a lower rate of FENO50 increase than males with lower BMI (interaction p=0.009) as demonstrated in Figures 3 and E3c–d. Year 3 allergic rhinitis in both sexes and race/ethnicity in males shifted the intercept of, but did not modify, FENO50-age trajectories. At any given age, children reporting allergic rhinitis ever in year 3 had higher FENO50 (p<0.001) and FENO50 was higher for males in the “Other” race/ethnicity group (composed of Black, Asian, other and missing) than in the Hispanic group (p=0.02).
Figure 3.
Modeled sex-specific trajectory of fractional exhaled nitric oxide (FENO50) (N=809 males, N=908 females) as a joint smooth of age and year 3 body mass index percentile or as smooths of age that vary by race/ethnicity or ever reported allergic rhinitis in year 3. Analyses performed using logFENO50 with results here presented using back-transformed values on an axis scaled using natural log.
Table 3.
Summary of fitted Generalized Additive Mixed Models that model longitudinal log fractional exhaled nitric oxide (logFENO50) trajectories as smooth functions of time-varying age and a time-constant covariate plus a participant-level random intercept and random slope on age.
| Males (N=809) |
Females (N=908) |
||||||
|---|---|---|---|---|---|---|---|
| Model | Parameter* | Regression coefficient** | EDF† | p-value | Regression coefficient** | EDF† | p-value |
| Age only | s(age) | 5.6 | <0.0001 | 5.1 | <0.0001 | ||
| Age and year 3 BMI % | te(age, bmi %) | 8.4 | <0.0001 | 7.6 | <0.0001 | ||
| ti(age) | 3.8 | <0.0001 | 3.6 | <0.0001 | |||
| ti(bmi %) | 1.0 | 0.02 | 1.0 | 0.43 | |||
| ti(age, bmi %) | 1.7 | 0.009 | 2.7 | 0.07 | |||
| Age and race/ethnicity‡ | Intercept | 2.55 | <0.0001 | 2.49 | <0.0001 | ||
| White/non-Hispanic | −0.07 | 0.11 | −0.06 | 0.13 | |||
| Other | 0.16 | 0.02 | 0.07 | 0.21 | |||
| s(age) | 5.4 | <0.0001 | 5.1 | <0.0001 | |||
| s(age:Hispanic) | 1.6 | 0.13 | 0.5 | 0.23 | |||
| s(age:White/non-Hisp) | <0.1 | 0.31 | <0.1 | 0.51 | |||
| s(age:Other) | <0.1 | 0.70 | 1.2 | 0.11 | |||
| Age and ever reported allergic rhinitis at year 3 | Intercept | 2.50 | <0.0001 | 2.45 | <0.0001 | ||
| Allergic Rhinitis | 0.15 | 0.001 | 0.14 | 0.0005 | |||
| Missing | −0.01 | 0.83 | −0.02 | 0.70 | |||
| s(age) | 5.5 | <0.0001 | 5.0 | <0.0001 | |||
| s(age:None) | <0.1 | 0.40 | <0.1 | 0.62 | |||
| s(age:AllergicRhinitis) | 2.7 | 0.07 | <0.1 | 0.51 | |||
| s(age: Missing) | 1.0 | 0.16 | <0.1 | 0.74 | |||
| Full model | Intercept | 2.52 | <0.0001 | 2.46 | <0.0001 | ||
| White/non-Hispanic | −0.10 | 0.02 | −0.07 | 0.09 | |||
| Other | 0.13 | 0.07 | 0.05 | 0.35 | |||
| Allergic Rhinitis | 0.15 | 0.001 | 0.15 | 0.0004 | |||
| Missing | −0.02 | 0.79 | −0.03 | 0.56 | |||
| te(age, bmi %) | 8.4 | <0.0001 | 7.6 | <0.0001 | |||
| Intercept | 2.52 | <0.0001 | 2.46 | <0.0001 | |||
| White/non-Hispanic | −0.10 | 0.02 | −0.07 | 0.10 | |||
| Other | 0.13 | 0.07 | 0.05 | 0.35 | |||
| Allergic Rhinitis | 0.15 | 0.001 | 0.15 | 0.0004 | |||
| Missing | −0.02 | 0.79 | −0.03 | 0.57 | |||
| ti(age) | 3.8 | <0.0001 | 3.6 | <0.0001 | |||
| ti(bmi %) | 1.0 | 0.01 | 1.0 | 0.40 | |||
| ti(age, bmi %) | 1.5 | 0.02 | 2.7 | 0.07 | |||
Smooth terms definitions: s(): penalized regression spline; ti():tensor product interaction; te():tensor product smooth.
For categorical variables; on logFENO50 scale
Effective degrees of freedom (EDF) for the smooth term; a value of 1 indicates a linear effect, higher values indicate a higher degree of non-linearity
k=5 specified in the s(age, by=race, m=1, k=5) term for improved model convergence
Finally, we estimated sex-specific models including the joint smooth function of age and year 3 BMI% and main effects of race/ethnicity and year 3 allergic rhinitis. Associations were similar to those reported in models including one covariate at a time (Table 3). Predictions for participants with selected covariate values are provided in Table E4 to demonstrate the range of FENO50 values and rates of change over time. Despite statistically significant evidence for covariate associations with FENO50 levels and trajectories, inclusion of the three covariates in the final model did not considerably reduce the SD of the random intercept (males: 0.49; females: 0.47) or random slope (males: 0.049; females: 0.052), nor did it improve model fit based on Bayesian Information Criterion (BIC) (Table E3).
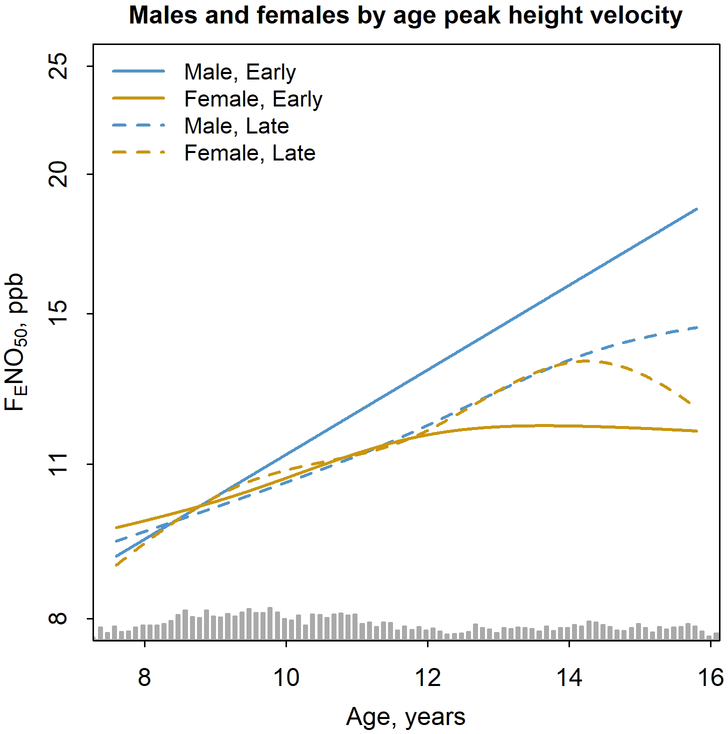
Analyses stratifying FENO50 trajectories by age at peak height velocity reduced the sample size to 611 participants due to insufficient height data needed to identify age at peak height velocity; sex-specific FENO50-age trajectories, however, were similar in this subset compared with the full study population (Figure E2). Separation in FENO50 trajectories between males and females occurred at younger ages among children with early age at peak height velocity and at older ages among children with late age at peak height velocity (Figure 4 and Table E5).
Figure 4.
Modeled sex-specific trajectory of fractional exhaled nitric oxide (FENO50) (N=288 males, N=323 females) as a joint smooth of age that vary by early (solid line) or late (dashed line) age at peak height velocity for males (blue) and females (yellow). Sex-specific models including a joint smooth of age and year 3 body mass index percentile by category of age at peak height velocity and adjustments for race/ethnicity and year 3 allergic rhinitis status. Grey bars at bottom is histogram of age.
The main findings of diverging FENO50 trajectories between males and females in later childhood and attenuated FENO50 trajectory slopes for males with higher year 3 BMI% were robust in sensitivity analyses (trajectories in height rather than age, Table E6 and Figure E3; trajectories in age using time-varying BMI% instead of year 3 BMI%, Figure E4; trajectories in age in children without allergic rhinitis, Figures E5–E6; trajectories in age in children without wheeze or whistling in the chest, Figures E7–E8; and restricting to online FENO50 measurements, Figures E9–E10). Although year 3 BMI% did not reach statistical significance as a modifier of FENO50 trajectories when restricting to online FENO50 measurements, the patterns observed by year 3 BMI% was similar for the overlapping ages in the two analyses (~10 to ~16). Additionally, we further excluded FENO50 observations with reported respiratory infection in the last month and results were not markedly different (data not shown).
Discussion
Using repeated FENO50 measurements from a large, population-based sample of healthy children during a period of prepubescent/pubescent growth (median starting age 8.4 years through median age at end of follow up 15.3 years), we report longitudinal evidence that on average FENO50 increased nonlinearly with age, with larger increases in males than females at older ages. Interestingly, when stratified by early or late age at peak height velocity, this departure in FENO50 trajectories between the sexes shifted to younger or older ages coinciding with early/later estimated timing of puberty. Additionally, males with higher year 3 BMI% had attenuated FENO50 slopes in age and race/ethnicity in males and year 3 allergic rhinitis in both sexes affected FENO50 level but did not have impact on FENO50 trajectories. We also observed considerable unexplained variation in FENO50 level and FENO50 change across age between children (large SD of random intercept and slope).
Longitudinal trends in FENO50 among children as they age observed in this study confirm reports of age association from previous cross-sectional studies in children and adolescents [5, 7, 10, 26]. Combining both sexes, linear increases in FENO50 were reported by Buchvald et al. over ages 4–17 [7] and Yao et al. over ages 5–18 [26], while Jacinto et al. reported linear increases in FENO50 through 14.1 years old in females and 15.7 years in males, after which point FENO50 levels plateaued until late middle age [5]. Brody et al. reported no difference in FENO50 levels for males and females ages 6–11 years, but at ages 12–19 females had lower FENO50 levels than males [10]. The FENO50 levels reported here are slightly larger compared with these earlier studies, but this may be due in part to the large percentage (69%) of non-White participants (see discussion below regarding race/ethnicity and FENO50). Overall, these earlier studies are generally consistent with our findings that males and females have similar FENO50 trajectories until early adolescence, after which males exhibit continued increases while females appear to plateau. Somatic growth and the increase in anatomic dead space volume may be a biological rationale for increases in FENO50 with age and differences between males and females in later childhood [5]. Development and growth of the bronchial tree leads to larger total airway mucosal surface area available for NO diffusion, thus higher FENO50 [5]. Potential impacts of alveolar growth on FENO50 trajectories could be investigated in future studies of alveolar versus airway NO concentrations [27, 28]. Although we did not have data on pubertal staging for participants, we were able to use a proxy for puberty—age at peak height velocity—to assign children as either early or late “maturers.” Timing of divergence between the sexes in FENO50 trajectories corresponded with this estimate of early versus late onset of puberty. Sex hormones have been suggested by animal studies to be involved in mechanisms of lung inflammation and asthma [29], with ovarian hormones found to increase and testosterone found to decrease airway inflammation in asthma; however, there are discrepancies between experimental and epidemiologic studies [14]. The mechanism by which puberty may affect FENO50 trajectories is unclear but may shed light on sex differences trends in asthma and other allergic diseases.
With these longitudinal data, we were able to observe differences in FENO50 slopes by BMI% among male children, with participants with higher year 3 BMI% having attenuated FENO50 slopes which persisted after controlling for race/ethnicity and year 3 allergic rhinitis. Differences in slope are primarily observed in earlier ages (8–10 years). This pattern was observed both for year 3 BMI% and time-varying BMI%. Previous studies have found no association between FENO50 in children and weight [6–8], body surface area [8], or BMI [7, 10, 30], with exception of a reported negative association with BMI z-score, primarily in atopic children [31] and weak positive correlation for body surface area [7]. BMI%, which is considered in the present study, may better represent being under/over-weight at a given age and sex. Furthermore, the longitudinal associations we examined—the impact of prepubescent BMI% on FENO50 trajectory during adolescence—may differ from cross-sectional associations. A recent study found individuals with higher BMI to have greater adipose tissue area within airway walls and greater airway wall thickness [32]. This structural change in the airways could potentially impact airway NO production/diffusion as well as FENO50 measured at the mouth.
We also observed an association between allergic rhinitis and FENO50, which is supported by the literature [7, 8, 33, 34], although some studies report no association [6]. Some studies of FENO50 in healthy children exclude those with reported rhinitis [34], but results were similar in our sensitivity analyses excluding children with allergic rhinitis.
Non-Hispanic white males had lower FENO50 levels across age compared with Hispanic and all other race males. Similar patterns were observed for females, but results did not reach statistical significance. Evidence from cross-sectional studies supports an association between race/ethnicity and FENO50. A 2017 review reported that among eight studies with children, seven found race/ethnicity to be a significant influencing factor for FENO50, with six observing higher FENO50 levels in the non-Caucasian ethnic groups (including African American, Hispanic, and Asian) compared with Caucasian levels [35]. Higher FENO50 has been reported in non-Hispanic blacks and Hispanics compared to non-Hispanic whites among children in the U.S. aged 6–19 years [10]. In a separate study, non-Caucasian subjects (African American, Asian, and Hispanic subjects) had higher FENO50 compared with Caucasian subjects, but this difference was only borderline significant after controlling for age, sex, and self-reported atopy [7]. Whether differences are driven by genetic versus contextual factors is unclear and warrants further investigation.
This study has limitations. First, the age range is limited thus it is unclear whether differences between males and females persist into early adulthood. Second, due to budgetary constraints FENO50 assessments were conducted every two years in years 6–10; however, because there was a range of ages at study entry inference regarding FENO50 trajectory in early and mid-teenage years are well-supported by the data. Third, the first two years of FENO50 data (years 3 and 4) were collected using offline methods, while FENO50 data for the remainder of the study (years 5–10) were collected using online methods. Although offline FENO50 measurement were converted to online values based on a published internally-developed prediction model with an R2 = 0.94 [17], there remains a possibility that this change in breath collection technique during the study could influence FENO50 trajectories in age, especially for estimated trajectories around ages 9–10 years when the change in collection method occurred which coincides with an apparent attenuate in FENO50 slope. However, in sensitivity analyses restricted to online FENO50 measurements, patterns in FENO50 trajectories, including the divergence between the sexes in later childhood, were similar to those in the main analysis, although the attenuation in slope around ages 9–11 years was less evident and remained only for males. The only other difference was in the findings for year 3 BMI%, which is likely due to lack of data in younger ages—the age period when FENO50 slope was most different by BMI% in the main analysis. Fourth, guidelines have been updated after the conclusion of data collection, but these would not have substantively impacted our FENO50 collection protocol [36]. Last, an objective measure of atopy (e.g., skin prick test, IgE sensitization) was not available and we instead relied on questionnaire assessment of allergic rhinitis. IgE sensitization may be present without obvious symptoms and may still cause increased FENO50 but would not have been captured by our classification of allergic rhinitis. Without such data we were unable to investigate the potential for effect modification by atopy.
This study has several strengths. First, we leveraged a large unique longitudinal data resource to be the first study to use serial measurements to examine FENO50-age trajectories in healthy children. Second, FENO50 trajectories are investigated during an important period of growth for children. Last, the study population is diverse, with over 50% Hispanic, allowing for identification of racial/ethnic differences in FENO50-age trajectories.
In conclusion, we reported novel longitudinal evidence for nonlinear trajectories of FENO50 in healthy children followed from ages ~8 to ~15 years, with differences in trajectories by sex coinciding with puberty and prepubescent BMI%, and differences in level by allergic rhinitis and race/ethnicity. The considerable remaining unexplained between-child variability in trajectories highlights the limitations of fixed FENO50 reference values. As such, our results are not intended as reference growth curves, but to provide information on population average FENO50 trajectories in healthy children during a period of growth. However, the moderately strong within-participant correlation of FENO50 over an 8-year period suggests that tracking personalized FENO50 measurements over time (to exploit this high within-participant correlation) may maximize the clinical utility of FENO50 measurements [37, 38].
Supplementary Material
Acknowledgements
We thank the participating students and their families, the school staff and administrators, and the study field team for their efforts.
Funding
This work was supported by the National Heart, Lung and Blood Institute (grants R01HL61768 and R01HL76647); the Southern California Environmental Health Sciences Center (grant P30ES007048) funded by the National Institute of Environmental Health Sciences; the Children’s Environmental Health Center (grants P01ES009581, R826708-01 and RD831861-01) funded by the National Institute of Environmental Health Sciences and the Environmental Protection Agency; the National Institute of Environmental Health Sciences (grants P01ES011627, R01ES023262, R01ES027860, and T32ES013678); the James H. Zumberge Research and Innovation Fund, and the Hastings Foundation.
Footnotes
Take home message of paper
Longitudinal FENO50 trajectories in healthy children ages 8–16 displayed a similar upward trend in males and females until age 11.5, after which males had higher FENO50. Males with higher starting BMI percentile had attenuated FENO50 slopes in age.
References
- 1.Berry MA, Shaw DE, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy 2005: 35(9): 1175–1179. [DOI] [PubMed] [Google Scholar]
- 2.Mahr TA, Malka J, Spahn JD. Inflammometry in pediatric asthma: a review of fractional exhaled nitric oxide in clinical practice. Allergy Asthma Proc 2013: 34(3): 210–219. [DOI] [PubMed] [Google Scholar]
- 3.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR, American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels for Clinical A. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011: 184(5): 602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005: 171(8): 912–930. [DOI] [PubMed] [Google Scholar]
- 5.Jacinto T, Malinovschi A, Janson C, Fonseca J, Alving K. Evolution of exhaled nitric oxide levels throughout development and aging of healthy humans. J Breath Res 2015: 9(3): 036005. [DOI] [PubMed] [Google Scholar]
- 6.Mallol J, Aguirre V, Cordova P, Cortez E, Gallardo A, Riquelme C. Fraction of exhaled nitric oxide in healthy Chilean schoolchildren aged 8–15 years. Allergol Immunopathol (Madr) 2015: 43(6): 528–532. [DOI] [PubMed] [Google Scholar]
- 7.Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol 2005: 115(6): 1130–1136. [DOI] [PubMed] [Google Scholar]
- 8.Malmberg LP, Petays T, Haahtela T, Laatikainen T, Jousilahti P, Vartiainen E, Makela MJ. Exhaled nitric oxide in healthy nonatopic school-age children: determinants and height-adjusted reference values. Pediatr Pulmonol 2006: 41(7): 635–642. [DOI] [PubMed] [Google Scholar]
- 9.Rouatbi S, Alqodwa A, Ben Mdella S, Ben Saad H. Fraction of exhaled nitric oxide (FeNO ) norms in healthy North African children 5–16 years old. Pediatr Pulmonol 2013: 48(10): 981–995. [DOI] [PubMed] [Google Scholar]
- 10.Brody DJ, Zhang X, Kit BK, Dillon CF. Reference values and factors associated with exhaled nitric oxide: U.S. youth and adults. Respir Med 2013: 107(11): 1682–1691. [DOI] [PubMed] [Google Scholar]
- 11.Hogman M, Thornadtsson A, Liv P, Hua-Huy T, Dinh-Xuan AT, Tufvesson E, Dressel H, Janson C, Koskela K, Oksa P, Sauni R, Uitti J, Moilanen E, Lehtimaki L. Effects of growth and aging on the reference values of pulmonary nitric oxide dynamics in healthy subjects. J Breath Res 2017: 11(4): 047103. [DOI] [PubMed] [Google Scholar]
- 12.Almqvist C, Worm M, Leynaert B, working group of GALENWPG. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy 2008: 63(1): 47–57. [DOI] [PubMed] [Google Scholar]
- 13.Pinart M, Keller T, Reich A, Frohlich M, Cabieses B, Hohmann C, Postma DS, Bousquet J, Anto JM, Keil T. Sex-Related Allergic Rhinitis Prevalence Switch from Childhood to Adulthood: A Systematic Review and Meta-Analysis. Int Arch Allergy Immunol 2017: 172(4): 224–235. [DOI] [PubMed] [Google Scholar]
- 14.Naeem A, Silveyra P. Sex Differences in Paediatric and Adult Asthma. Eur Med J (Chelmsf) 2019: 4(2): 27–35. [PMC free article] [PubMed] [Google Scholar]
- 15.McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Kunzli N, Gauderman J, Avol E, Thomas D, Peters J. Traffic, susceptibility, and childhood asthma. Environ Health Perspect 2006: 114(5): 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastain TM, Islam T, Berhane KT, McConnell RS, Rappaport EB, Salam MT, Linn WS, Avol EL, Zhang Y, Gilliland FD. Exhaled nitric oxide, susceptibility and new-onset asthma in the Children’s Health Study. Eur Respir J 2011: 37(3): 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linn WS, Berhane KT, Rappaport EB, Bastain TM, Avol EL, Gilliland FD. Relationships of online exhaled, offline exhaled, and ambient nitric oxide in an epidemiologic survey of schoolchildren. J Expo Sci Environ Epidemiol 2009: 19(7): 674–681. [DOI] [PubMed] [Google Scholar]
- 18.Linn WS, Rappaport EB, Berhane KT, Bastain TM, Avol EL, Gilliland FD. Exhaled nitric oxide in a population-based study of southern California schoolchildren. Respir Res 2009: 10: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ATS (American Thoracic Society). Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 1999: 160(6): 2104–2117. [DOI] [PubMed] [Google Scholar]
- 20.ATS/ERS (American Thoracic Society and the European Respiratory Society). ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005: 171(8): 912–930. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC). A SAS program for the CDC growth charts. 2009.
- 22.Kelly A, Winer KK, Kalkwarf H, Oberfield SE, Lappe J, Gilsanz V, Zemel BS. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab 2014: 99(6): 2104–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. Journal of the American Statistical Association 2004: 99(467): 673–686. [Google Scholar]
- 24.Wood SN. Generalized additive models: an introduction with R. CRC press, 2017. [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2018. [Google Scholar]
- 26.Yao TC, Lee WI, Ou LS, Chen LC, Yeh KW, Huang JL, Group PS. Reference values of exhaled nitric oxide in healthy Asian children aged 5 to 18 years. Eur Respir J 2012: 39(2): 378–384. [DOI] [PubMed] [Google Scholar]
- 27.George SC, Hogman M, Permutt S, Silkoff PE. Modeling pulmonary nitric oxide exchange. J Appl Physiol (1985) 2004: 96(3): 831–839. [DOI] [PubMed] [Google Scholar]
- 28.Hogman M Extended NO analysis in health and disease. J Breath Res 2012: 6(4): 047103. [DOI] [PubMed] [Google Scholar]
- 29.Yung JA, Fuseini H, Newcomb DC. Hormones, sex, and asthma. Ann Allergy Asthma Immunol 2018: 120(5): 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovesi T, Kulka R, Dales R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest 2008: 133(1): 169–175. [DOI] [PubMed] [Google Scholar]
- 31.Yao TC, Tsai HJ, Chang SW, Chung RH, Hsu JY, Tsai MH, Liao SL, Hua MC, Lai SH, Chen LC, Yeh KW, Tseng YL, Lin WC, Chang SC, Huang JL, Prediction of Allergies in Taiwanese Children Study G. Obesity disproportionately impacts lung volumes, airflow and exhaled nitric oxide in children. PLoS One 2017: 12(4): e0174691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliot JG, Donovan GM, Wang KCW, Green FHY, James AL, Noble PB. Fatty Airways: Implications for Obstructive Disease. Eur Respir J 2019. [DOI] [PubMed] [Google Scholar]
- 33.Jouaville LF, Annesi-Maesano I, Nguyen LT, Bocage AS, Bedu M, Caillaud D. Interrelationships among asthma, atopy, rhinitis and exhaled nitric oxide in a population-based sample of children. Clin Exp Allergy 2003: 33(11): 1506–1511. [DOI] [PubMed] [Google Scholar]
- 34.Jacinto T, Alving K, Correia R, Costa-Pereira A, Fonseca J. Setting reference values for exhaled nitric oxide: a systematic review. Clin Respir J 2013: 7(2): 113–120. [DOI] [PubMed] [Google Scholar]
- 35.Blake TL, Chang AB, Chatfield MD, Petsky HL, Rodwell LT, Brown MG, Hill DC, McElrea MS. Does Ethnicity Influence Fractional Exhaled Nitric Oxide in Healthy Individuals?: A Systematic Review. Chest 2017: 152(1): 40–50. [DOI] [PubMed] [Google Scholar]
- 36.Horvath I, Barnes PJ, Loukides S, Sterk PJ, Hogman M, Olin AC, Amann A, Antus B, Baraldi E, Bikov A, Boots AW, Bos LD, Brinkman P, Bucca C, Carpagnano GE, Corradi M, Cristescu S, de Jongste JC, Dinh-Xuan AT, Dompeling E, Fens N, Fowler S, Hohlfeld JM, Holz O, Jobsis Q, Van De Kant K, Knobel HH, Kostikas K, Lehtimaki L, Lundberg J, Montuschi P, Van Muylem A, Pennazza G, Reinhold P, Ricciardolo FLM, Rosias P, Santonico M, van der Schee MP, van Schooten FJ, Spanevello A, Tonia T, Vink TJ. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J 2017: 49(4). [DOI] [PubMed] [Google Scholar]
- 37.Ferrante G, Malizia V, Antona R, Corsello G, Grutta S. The value of FeNO measurement in childhood asthma: uncertainties and perspectives. Multidiscip Respir Med 2013: 8(1): 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holz O, Magnussen H. Cutoff values for FENO-guided asthma management. Am J Respir Crit Care Med 2009: 180(3): 281–282; author reply 282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.