Abstract
Background
Trimethylamine N-oxide (TMAO) is a microbiome- and diet-derived metabolite implicated in adverse cardiovascular outcomes. To date, studies of plasma TMAO concentrations have largely focused on individuals with metabolic disease. As such, data on TMAO concentrations in population settings and parent–child dyads are lacking.
Objectives
This study aimed to investigate parent–child concordance, age, and sex effects on plasma concentrations of TMAO and its precursors [l-carnitine, choline, betaine, and dimethylglycine (DMG)]. Associations between concentrations of TMAO and its precursors and self-reported dietary intakes of animal protein (i.e., red meat, meat products, chicken, fish, milk products, and cheese) and fast-food meals were also investigated.
Methods
A total of 1166 children (mean ± SD age: 11 ± 0.5 y, 51% female) and 1324 parents (mean ± SD age: 44 ± 5.1 y, 87% female) had a biomedical assessment as part of Growing Up in Australia's Child Health Checkpoint. Plasma TMAO and precursor concentrations were quantified using ultra-high-pressure LC coupled with tandem MS.
Results
Familial dyads significantly contributed to plasma TMAO and precursor concentrations (P < 0.0001), explaining 37% of variance for TMAO concentrations. Least-square mean ± SE plasma TMAO was lower in children (0.79 ± 0.02 µM on the log-scale) than in adults (1.22 ± 0.02 µM). By contrast, children's betaine (40.30 ± 0.34 µM) and DMG concentrations (1.02 ± 0.01 µM on the log-scale) were higher than adults’ betaine (37.50 ± 0.32 µM) and DMG concentrations (0.80 ± 0.01 µM) (P < 0.0001). Mean values of all metabolites, except adult TMAO, were higher in males than in females (P < 0.001). Greater reported intake of red meat and fish was associated with higher TMAO concentrations in both children [estimates (95% CIs) for red meat: 0.06 (0.01, 0.10); fish: 0.11 (0.06, 0.17)] and adults [red meat: 0.13 (0.08, 0.17); meat products: 0.07 (0.03, 0.12); and fish: 0.09 (0.04, 0.14)].
Conclusions
Age, sex, and shared family factors, including diet, contribute to variation in plasma concentrations of TMAO and its precursors.
Keywords: trimethylamine N-oxide, l-carnitine, choline, betaine, dimethylglycine, epidemiology, children, adults, Growing Up in Australia, Longitudinal Study of Australian Children
Age, sex, and shared family factors characterize the concentrations of plasma TMAO and those of its precursors in Growing Up in Australia's CheckPoint Study of children and adults.
Introduction
Trimethylamine N-oxide (TMAO) has gained increasing attention over the past decade owing to its potential links to metabolic syndrome and cardiovascular disease (1–8). TMAO concentrations, and those of its precursors, have prognostic utility in predicting cardiovascular events (4). Despite this, links between TMAO concentrations and cardiovascular disease have been inconsistent (9, 2, 3–7,10–16), with stronger relations found in diseased patients (4) than in “healthy” population settings (11). Varying results may also, in part, be due to baseline concentrations of TMAO, with a U-shaped curve of association with cardiovascular outcomes being reported (17). Studies on TMAO and related metabolites have generally focused on individuals with existing metabolic conditions (2,3, 9, 18, 19), with few population-wide studies (11, 13, 15, 20).
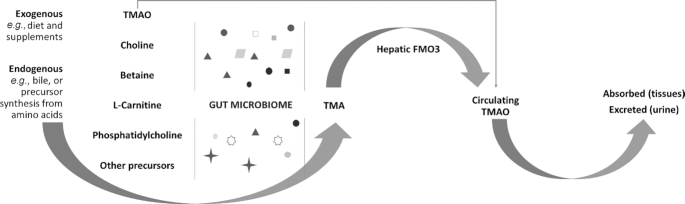
TMAO concentrations vary with diet and pharmacological interventions (5, 21, 22). TMAO can be directly ingested in its preformed state from fish (1, 12, 22–26). This ingested form of TMAO is more rapidly metabolized. TMAO can also be synthesized directly or indirectly from l-carnitine, choline, betaine, dimethylglycine (DMG), and their precursors (e.g., phosphatidylcholine, crono-betaine, γ-butyrobetaine) (1, 12, 22–26) (Figure 1). These are obtained from a variety of dietary or supplemental sources, endogenous biological fluids (e.g., bile), or by synthesis from other amino acids (1, 12, 22–26). Precursors are metabolized by trimethylamine (TMA)-producing bacteria in the gut microbiota to form TMA, which is subsequently oxidized by hepatic flavin-containing monooxygenase 3 (FMO3) enzymes into TMAO (1, 12, 22–26). TMAO circulates in the blood, is absorbed by peripheral tissues, and is almost fully (>90%) excreted in the urine in healthy subjects (22, 23,24, 27–29). However, the dietary contributions to plasma TMAO concentrations have been inconsistent in the literature (11, 22, 23, 24, 29, 30, 31, 32).
FIGURE 1.
Simplified graphical representation of TMAO metabolism. Preformed TMAO and its dietary precursors provided from exogenous or endogenous sources are metabolized by TMA-producing bacteria in the gut microbiota to form TMA, which is then oxygenated by hepatic enzymes to form TMAO. FMO3, flavin monooxygenase 3; TMA, trimethylamine; TMAO, trimethylamine N-oxide.
Cardiovascular disease is one of the leading causes of death worldwide (33). Measures of TMAO and of its precursors have shown utility in the prediction and prognosis of cardiovascular disease (2, 4), and these metabolites’ concentrations can be altered by the diet (5, 21, 22). Although cardiometabolic conditions have their origins in early life (34), baseline reference values of TMAO concentrations in healthy populations of children, and across generations, are generally lacking (11, 35, 36). Therefore, we aimed at characterizing homeostatic plasma concentrations of TMAO and of its precursors l-carnitine, choline, betaine, and DMG in 11- to 12-y-olds and their parents from a population-derived cohort study. We analyzed familial concordance (i.e., differences between parent–child dyads), absolute differences between generations (i.e., children compared with adults) and sexes, as well as associations with self-reported dietary animal protein intake in children and adults.
Methods
Ethical approval, consent, and sample collection
The Child Health CheckPoint study was approved by The Royal Children's Hospital (Melbourne, Australia) Human Research Ethics Committee (33225D) and the Australian Institute of Family Studies Ethics Committee. A total of 1874 parent–child dyads participated in a biomedical assessment, Child Health CheckPoint (CheckPoint), nested between waves 6 and 7 of the B-cohort of the Longitudinal Study of Australian Children (LSAC) (37, 38) (Supplemental Figure 1). Parents or caregivers provided consent for themselves and their child to participate in CheckPoint and for the collection of their blood samples (39).
Procedures
Venous blood was collected from parents and children after on average a 4-h fast (39). Venous blood was collected into EDTA-coated tubes, processed within 1 h into plasma, and frozen at −80°C. For the current analysis, a total of 2490 plasma samples (1166 children and 1324 parents) were shipped, on dry ice in thermally monitored boxes, to the Liggins Institute, New Zealand. Samples were received in plates containing 96 FluidXTM tubes in no specific order except for keeping parent–child pairs on the same plate. For the purpose of our assay, only 74 samples could be run per plate because the remaining positions were required for blanks, quality controls (QCs), and the calibration curve. All 96 samples were firstly sorted by the family barcode to ensure that parent–child pairs were kept on the same plate after randomization. After filtering by family, the last 22 samples were moved to the following plate. The RAND() formula was then used in Microsoft Excel to assign a random number for the remaining 74 samples on each plate. The list of numbers generated by the RAND() formula was then ascendingly sorted to randomly distribute all 74 samples on the randomization plate. Samples were randomly distributed onto 34 different 96-well plates on dry ice, keeping all parent–child pairs (1121 pairs) on the same plate, and stored at −80°C before both ultra-high-performance liquid chromatography (UHPLC)/tandem mass spectrometry (MS-MS) assays.
Sample preparation and UHPLC/MS-MS analysis
All compounds were measured from a single plasma aliquot (100 µL, thawed to room temperature) using a Vanquish UHPLC+ system coupled with a TSQ Quantiva triple quadrupole mass spectrometer (Thermo Scientific). The protein precipitation was conducted using an Eppendorf robot fitted with a thermal mixer and vacuum manifold (EpMotion 5075vt).
Two analytical methods were used. The assay measuring precursor concentrations (l-carnitine, choline, betaine, and DMG) is described in detail elsewhere (40). TMAO concentrations were profiled as part of an analytical panel quantifying B vitamers, the results of which were analyzed separately (under review). Briefly, 300 µL of the protein precipitation mixture 0.3% (vol:vol) acetic acid and 2.5% (vol:vol) MilliQ® H2O in methanol was added to either 1) 100 µL calibration curve standards (Supplemental Table 1); 2) 100 µL plasma sample; 3) 100 µL of a 4% (wt:vol) solution of BSA in PBS; or 4) triplicate QC samples [located at 3 different positions of a 96-well IMPACT protein precipitation plate (Phenomenex®)]. Then, 10 µL of an internal standard solution (detailed in Supplemental Table 2) was added to all wells, and the plate was agitated on the epMotion 5075 robot thermomixer (room temperature; 800 rpm; 5 min). The protein precipitate eluent was then vacuum filtrated into the collection plate (250 mbar, 5 min). The filtrate was dried (40°C, 3 h, 0.03 bars of pressure) in a SpeedVac concentrator (Savant SC250EXP, Thermo Scientific) coupled to a refrigerated vapor trap (Savant RVT4104, Thermo Scientific). The dried residue was suspended in a reconstitution solution [200 µL; 1% (wt:vol) ascorbic acid] mixed with the mobile phase [5% acetic acid, 0.2% (wt:vol) heptafluorobutyric acid (HFBA) in deionized Millipore H2O (MilliQ®)]. The plate was agitated (epMotion 5075 robot thermomixer; room temperature; 800 rpm; 5 min) and placed in the UHPLC autosampler for analysis. Chromatographic separation was achieved using a Kinetex® 2.6 µm F5 100 Å 150 × 2.1 mm column (Phenomenex®) coupled with a Krudkatcher (Phenomenex®) precolumn filter, with a mobile phase consisting of 5% acetic acid, 0.2% (wt:vol) HFBA in Millipore H2O (MilliQ®). The source consisted of a heated electrospray ionization in positive ionization mode. A 200 µL/min flow was applied starting at 4% acetonitrile and 96% mobile phase. The analytical run time was 14 min/sample. QCs were used to monitor inter- and intra-assay reproducibility (with ≤20% variation between days being accepted). One plate failed the 20% QC cutoff and was reanalyzed.
Dietary data
Adults and children filled out a 26-item brief food-frequency survey of usual intakes of several foods, adapted from the Australian National Secondary Students’ Diet and Activity survey, as well as the ISCOLE (International Survey of Childhood Obesity, Lifestyle and Environment) study (39, 41, 42). The food groups analyzed in relation to TMAO concentrations and its precursors included habitual intakes of red meat (e.g., beef, lamb, steaks, chops, roasts, mince, stir fries, and casserole, meat products (e.g., hot dogs, sausages, ham, sausage rolls, salami, meat pies, chicken nuggets, or bacon), chicken, fish and canned fish, fast-food meals and snacks (e.g., pizzas, burgers, chips), cheese, and dairy products (e.g., yogurt, chocolate milk, pudding). Intake was reported as follows: never, less than once a week, ∼1–2 times/wk, ∼3–4 times/wk, ∼5–6 times/wk, or every day.
Statistical analysis
All statistical analyses were performed in the R programming environment, version 3.6.1 (43). TMAO and DMG concentrations were positively skewed after accounting for plate effects and were therefore log transformed before use in mixed modeling. Choline, betaine, and l-carnitine were normally distributed after adjustment for plate effects, and therefore were not transformed (Supplemental Figure 2).
Two sets of mixed models were used to identify the effects of generation (parents compared with children) and within-family concordance using the lme4 package on R (44), after controlling for plate effects. Likelihood ratio tests were conducted between models to compare the fit (i.e., log likelihood) of models with and without the factor tested (i.e., family or generation). Log likelihoods were compared between models that contained both family (as a random effect) and generation (as a fixed effect), and those excluding one or the other. Family effect sizes were calculated as the ratio of family variance divided by the total variance of each adjusted variable. Pearson's correlations adjusted for multiple testing using the Holm method in R were also conducted within parent–child dyads to confirm familial correlations.
Two sets of linear models were fitted for each plate-adjusted/log-transformed variable to determine the effect of 1) sex in children and adults; and 2) age within the adult subgroup (28–71 y). Given that females exhibit fluctuations in TMA oxidation (and therefore TMAO production) throughout their menstrual cycle, a sensitivity analysis, using t tests, was conducted to assess differences in TMAO concentrations between menstruating (on the day of blood collection) and non-menstruating females in the child and adult groups separately. ANOVAs were computed to evaluate associations between the reported intakes of the listed foods and the concentrations of metabolites of interest in both children and adults (45).
Results
Population characteristics
The sample analyzed in this study consisted of 2490 individuals (1121 parent–child pairs), including 1166 children (601 girls; 565 boys; mean ± SD age: 11 ± 0.5 y) and 1324 adults (1150 women; 174 men; mean ± SD age: 44 ± 5.1 y) (Table 1).
TABLE 1.
Sample characteristics1
| Children | Adults | |||||
|---|---|---|---|---|---|---|
| Characteristic | All | Boys | Girls | All | Men | Women |
| n | 1166 | 565 | 601 | 1324 | 174 | 1150 |
| Age, y | 11.4 ± 0.5 | 11.4 ± 0.5 | 11.5 ± 0.5 | 43.9 ± 5.1 | 46.2 ± 6.4 | 43.6 ± 4.8 |
| BMI rounded, kg/m2 | ||||||
| Median [IQR] | 18.4 [16.8, 20.6] | 18.1 [16.7, 20.2] | 18.8 [17.0, 21.1] | 26.5 [23.4, 31.0] | 27.4 [25.2, 31.1] | 26.3 [23.1, 31.0] |
| BMI z scores | 0.3 ± 0.9 | 0.3 ± 0.9 | 0.3 ± 0.9 | N/A | N/A | N/A |
| Biological parent of child, n | N/A | N/A | N/A | 1313 | 172 | 1141 |
| Australian state of current residence, n | New South Wales (359); Victoria (261); Queensland (221);South Australia (92); West Australia (139); Tasmania (40);Northern Territory (17); Australian Capital Territory (38) | New South Wales (391); Victoria (311); Queensland (240);South Australia (108); West Australia (164); Tasmania (46);Northern Territory (18); Australian Capital Territory (47) | ||||
| Socio-Economic Indexes for Areas (SEIFA) disadvantage quintile, n | Most disadvantaged (83); second most (171); middle (199);second least (272); least disadvantaged (442) | Most disadvantaged (94); second most (193); middle (233);second least (304); least disadvantaged (501) | ||||
1Values are medians [IQRs] for skewed variables and means ± SDs for normally distributed variables.
Measures of TMAO and precursor concentrations were concordant between children and parents
Both likelihood ratio tests (P < 0.0001) and Pearson's correlations between dyads highlighted the strongest family effect for TMAO (correlation coefficient: 0.39; CI adjusted for multiple testing: 0.34, 0.44), followed by DMG (0.38; CI: 0.33, 0.43), betaine (0.22; CI: 0.17, 0.28), and l-carnitine (0.21; CI: 0.15, 0.26), with the weakest correlation for choline (0.13; CI: 0.08, 0.19). Family effects explained 37% of the variability for TMAO, 36% for DMG, 26% for l- carnitine, 22% for betaine, and finally 13% for choline (Table 2).
TABLE 2.
Variances and effect sizes of family on compound concentrations1
| Compound, µM | Effect of dyad (or family) on mixed model2 | Family (dyad) effect variance | Plate-adjusted compound variance | Effect size of family on compound concentrations,3 % |
|---|---|---|---|---|
| TMAO4 |
P < 0.0001
|
0.25 | 0.67 | 37 |
| l-Carnitine |
P < 0.0001
|
63.92 | 249.62 | 26 |
| DMG4 |
P < 0.0001
|
0.05 | 0.12 | 36 |
| Betaine |
P < 0.0001
|
29.86 | 136.01 | 22 |
| Choline |
P < 0.0001
|
0.90 | 7.07 | 13 |
1DMG, dimethylglycine; TMAO, trimethylamine N-oxide.
Log likelihoods were derived from mixed models including generation as a fixed effect, with or without family as a random effect.
Calculated as the family (dyad) effect variance divided by the plate-adjusted compound variance × 100.
Log-transformed variable.
Plasma TMAO, betaine, and DMG concentrations were age-dependent
Plasma TMAO concentrations were higher (P < 0.0001) in adults (least-square mean ± SE: 1.22 ± 0.02 µM on the log scale) than in children (0.79 ± 0.02 µM) (Table 3). By contrast, adult betaine (37.50 ± 0.32 µM) and DMG concentrations (0.80 ± 0.01 µM on the log scale) were lower than children's betaine (40.30 ± 0.34 µM) and DMG concentrations (1.02 ± 0.01 µM on the log scale) (P < 0.0001 for both). No intergenerational differences were identified for plasma choline and l-carnitine (Table 3).
TABLE 3.
LS-means, SEs, and mixed models’ results for TMAO and its precursors in children and parents1
| Compound, µM | Parent LS-mean ± SE | Child LS-mean ± SE | Effect of generation on mixed model2 (children, adults) |
|---|---|---|---|
| TMAO3 | 1.22 ± 0.02 | 0.79 ± 0.02 |
P < 0.0001
|
| l-Carnitine | 47.60 ± 0.43 | 48.30 ± 0.46 |
P = 0.16
|
| DMG3 | 0.80 ± 0.01 | 1.02 ± 0.01 |
P < 0.0001
|
| Trimethylglycine (betaine) | 37.50 ± 0.32 | 40.30 ± 0.34 | *P < 0.0001
|
| Choline | 11.00 ± 0.07 | 10.80 ± 0.08 |
P = 0.14
|
1DMG, dimethylglycine; LS-mean, least-square mean; TMAO, trimethylamine N-oxide.
Log likelihoods were derived from mixed models including family as a random effect, with or without generation as a fixed effect.
LS-means ± SEs are computed from log-transformed variables.
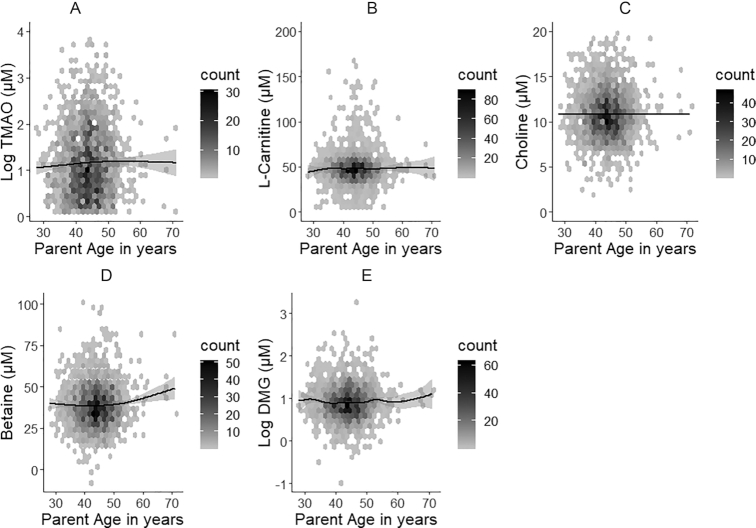
Given the narrow age distribution in children (11–12 y), we characterized age-specific differences within the adults exclusively (28–71 y as a continuous scale). Only TMAO and betaine plasma concentrations showed weak positive increases with ascending age (Figure 2, Supplemental Table 3).
FIGURE 2.
Hexagonal plots of TMAO (A), l-carnitine (B), choline (C), betaine (D), and DMG (E) across the adult age range (n = 1324). Plasma concentrations of TMAO and betaine are weakly positively associated with age (P ≤ 0.05). DMG, dimethylglycine; TMAO, trimethylamine N-oxide.
Plasma concentrations of TMAO and its precursors were higher in males
Sex-specific differences were identified in the plasma concentrations of all tested compounds. Plasma concentrations were higher in boys for TMAO (least-square means ± SEs: 0.86 ± 0.03 in boys; 0.72 ± 0.03 µM in girls on the log-scale; P = 0.003), l-carnitine (50.30 ± 0.65 µM in boys; 46.50 ± 0.63 µM in girls; P < 0.0001), DMG (1.09 ± 0.01 µM in boys; 0.96 ± 0.01 µM in girls on the log scale; P < 0.0001), betaine (42.00 ± 0.46 µM in boys; 38.70 ± 0.44 µM in girls; P = 0.02), and choline (11.20 ± 0.11 µM in boys; 10.50 ± 0.11 µM in girls; P < 0.0001). Similarly, the concentrations of l-carnitine (53.10 ± 1.20 µM in men; 46.70 ± 0.47 µM in women; P < 0.0001), DMG (0.98 ± 0.03 µM in men; 0.78 ± 0.01 µM in women on the log-scale; P < 0.0001), betaine (45.10 ± 0.89 µM in men; 36.40 ± 0.35 µM in women; P < 0.0001), and choline (12.00 ± 0.20 µM in men; 10.80 ± 0.08 µM in women; P < 0.0001) were higher in men than women in the adult subgroup. However, TMAO concentrations did not differ by sex in adults (Table 4).
TABLE 4.
LS-means, SEs, and linear model results of TMAO concentrations by sex and generation1
| Children | Adults | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Girls, LS-means ± SEs | Boys, LS-means ± SEs | Adjusted R2 of linear model | P value | Women, LS-means ± SEs | Men, LS-means ± SEs | Adjusted R2 of linear model | P value |
| TMAO,2 µM | 0.72 ± 0.03 | 0.86 ± 0.03 | 0.01 | 0.003 | 1.21 ± 0.02 | 1.32 ± 0.06 | 0.001 | 0.09 |
| l-Carnitine, µM | 46.50 ± 0.63 | 50.30 ± 0.65 | 0.01 | <0.0001 | 46.70 ± 0.47 | 53.10 ± 1.20 | 0.02 | <0.0001 |
| DMG,2 µM | 0.96 ± 0.01 | 1.09 ± 0.01 | 0.04 | <0.0001 | 0.78 ± 0.01 | 0.98 ± 0.03 | 0.04 | <0.0001 |
| Betaine, µM | 38.70 ± 0.44 | 42.00 ± 0.46 | 0.02 | <0.0001 | 36.40 ± 0.35 | 45.10 ± 0.89 | 0.06 | <0.0001 |
| Choline, µM | 10.50 ± 0.11 | 11.20 ± 0.11 | 0.02 | <0.0001 | 10.80 ± 0.08 | 12.00 ± 0.20 | 0.02 | <0.0001 |
1DMG, dimethylglycine; LS-mean, least-square mean; TMAO, trimethylamine N-oxide.
2LS-means and SEs are computed from log-transformed variables.
A sensitivity analysis did not identify marked differences in the plasma concentrations of TMAO and of its precursors (P > 0.05 for all metabolites) between females who reported that they were menstruating (20 children and 175 women) and those who reported that they were not (95 postpubertal children and 953 women) on the day of blood collection (Supplemental Table 4). By contrast, within the adults, plasma concentrations of both betaine and l-carnitine were present at higher concentrations in women who were menstruating on the day of blood collection.
TMAO correlated with l-carnitine and choline but not with DMG or betaine
l-carnitine (r = 0.053, P = 0.03) and choline (r = 0.076, P = 0.0006) showed small correlations with TMAO concentrations. However, DMG and betaine were not correlated with TMAO concentrations (r = −0.004, P = 0.9; and r = 0.02, P = 0.6, respectively) (Supplemental Table 5).
Fish and red meat consumption was positively associated with plasma TMAO
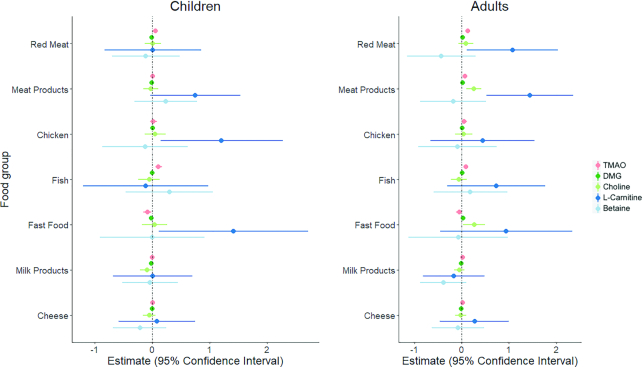
In children, consumption of fish (estimate: 0.11; 95% CI: 0.06, 0.17; P < 0.0001) and red meat (0.06; 95% CI: 0.01, 0.10; P = 0.01) was positively associated with TMAO concentrations (Figure 3, Supplemental Table 6). The consumption of fast-food meals and snacks (e.g., pizzas, chips, burgers) (−0.08; 95% CI: −0.15, −0.01; P = 0.02) was negatively associated with TMAO concentrations in children. Most children (n = 1106, 96%) consumed fast-food meals ≤3 times/wk with only 3 children reportedly consuming fast-food meals 5–7 d/wk (Supplemental Table 7). TMAO concentrations were also positively associated with regular intakes of fish (0.09; 95% CI: 0.04, 0.14; P = 0.001), red meat (0.13; 95% CI: 0.08, 0.17; P < 0.0001), meat products (0.07; 95% CI: 0.03, 0.12; P = 0.001), and chicken (0.05; 95% CI: 0.002, 0.11; P = 0.04) in adults. Choline and DMG concentrations were not associated with any of the foods assessed in children (P > 0.05 for all). In adults, choline concentrations were positively associated with meat products (0.25; 95% CI: 0.10, 0.41; P = 0.001) and fast-food meals and snacks (0.26; 95% CI: 0.03, 0.49; P = 0.03), yet DMG concentrations were weakly associated with red meat products and fast-food meals and snacks (Figure 3). l-carnitine concentrations were positively associated with habitual intakes of chicken (1.21; 95% CI: 0.15, 2.28; P = 0.03) and fast-food meals and snacks (1.42; 95% CI: 0.12, 2.72; P = 0.03) in children only, and positively associated with red meat intake (1.07; 95% CI: 0.11, 2.03; P = 0.03) and meat product intake (1.44; 95% CI: 0.52, 2.36; P = 0.002) in adults only. Plasma betaine concentrations were not associated with any of the food groups tested in either generation. The CIs for both l-carnitine and betaine were very large (Figure 3), therefore assertions about these associations must be made cautiously. Finally, reported intakes of milk products and cheese were not associated with any of the measured metabolites in either generation (Figure 3, Supplemental Table 6).
FIGURE 3.
Forest plots of associations between reported intakes of animal protein sources and fast food and the concentrations of TMAO and its precursors in children (n = 1166) and adults (n = 1324). Plasma TMAO concentrations are positively associated with the reported consumption of fish (estimate: 0.11; 95% CI: 0.06, 0.17; P < 0.0001) and red meat (0.06; 95% CI: 0.01, 0.10; P = 0.01), and negatively associated with the reported consumption of fast-food meals and snacks (−0.08; 95% CI: −0.15, −0.01; P = 0.02) in children. TMAO concentrations were positively associated with reported regular intakes of fish (0.09; 95% CI: 0.04, 0.14; P = 0.001), red meat (0.13; 95% CI: 0.08, 0.17; P < 0.0001), meat products (0.07; 95% CI: 0.03, 0.12; P = 0.001), and chicken (0.05; 95% CI: 0.001, 0.11; P = 0.04) in adults. DMG, dimethylglycine; TMAO, trimethylamine N-oxide.
Discussion
This is the first study that we know of to report plasma concentrations of TMAO and its precursors in a population-based sample of children and parents. The proportion of variability explained by familial relationships (i.e., a shared gene–environment setting) ranged from 13% for choline concentrations to 37% for TMAO concentrations. Intergenerational differences were evident for plasma TMAO, betaine, and DMG concentrations, with higher concentrations of TMAO and lower concentrations for betaine and DMG in adults than in children. Finally, all compounds were higher in males in both children and adults, except for TMAO in adults. We identified family, age, and sex as important factors potentially characterizing plasma concentrations of TMAO and its precursors.
Strong family effects were expected because genetic and dietary factors are known to be important in TMAO synthesis (1, 23, 24, 46, 47, 48–50). Reported heritability estimates based on family studies for TMAO have identified a moderate genetic contribution to its circulating concentrations (∼27%) (50). The proportion of variability explained by familial relationships for TMAO was higher in our study (37%) but was not calculated from genetic data. Familial concordance in nutritional metabolites is supported by previous work on our cohort, with marked concordance reported for amino acid and fatty acid profiles between children and their parents (51).
Mutations and polymorphisms that inactivate or change the activity of the hepatic FMO3 enzyme have been associated with the accumulation of TMA (known as trimethylaminuria or “fish odor” syndrome) and concomitantly lower plasma TMAO concentrations (1). The hepatic expression of FMO3 becomes evident between birth and 2 y of age, gradually increasing to intermediate expression around 11–12 y of age and peaking in adulthood (47). Age-dependent changes in FMO3 expression may explain the intergenerational differences in TMAO concentrations observed here between middle-aged adults and their 11- to 12-y-old children. Consistent with this and previous studies (11, 29, 52), we identified a positive association for TMAO with increased age within the adult subgroup (28–71 y), although lower and upper age ranges were underrepresented (mean ± SD age: 44 ± 5.1 y). Although age-specific increases in FMO3 expression have been reported (47), the exact adult age at which FMO3 expression peaks is unknown. Therefore, studies looking at age-dependent changes of FMO3 gene expression, enzymatic activity of FMO3, and their impact on circulating and urinary concentrations of TMAO in a large cohort of healthy adults with a more consistent age distribution as well as a balanced ratio of males to females are needed.
Although concentrations of TMAO were higher in adults than in children, those of its precursors DMG and betaine were lower, which may reflect increased rates of TMAO synthesis. However, the concentrations of betaine and DMG were not correlated with those of TMAO, consistent (for betaine) with previous reports (29). The oral administration of betaine (a DMG precursor) has been reported to result in only a modest increase in urinary TMAO compared with administration of l-carnitine, choline, or preformed TMAO (53), which may explain the lack of a strong correlation. Nevertheless, the cross-sectional design of our study coupled with a single plasma measurement may have limited our ability to detect strong correlations between these compounds at 1 specific time point. Therefore, our results should be regarded as preliminary and further longitudinal studies are needed to determine the relations between lower plasma concentrations of betaine and DMG and higher TMAO concentrations in healthy populations.
In both generations, l-carnitine, DMG, betaine, and choline concentrations were markedly higher in males than in their female counterparts. TMAO concentrations were also higher in boys with weak evidence for higher concentrations in men. Previous reports on sex-specific differences in plasma concentrations of these metabolites have been inconsistent (9, 11, 13, 15, 20, 54, 55), which may be due in part to modest sample sizes in some studies. For example, TMAO serum concentrations were similar between adult males (n = 90) and females (n = 130) in a German cohort with a sample size 10 times smaller than ours (56). Sex differences in TMAO and precursor concentrations have been proposed to be mediated by sex-specific hormonal effects on TMAO metabolism (57–59) For example, females exhibit fluctuations in TMA oxidation throughout their menstrual cycles, with higher TMA and concomitantly lower urinary TMAO concentrations around the time of menstruation (60), explaining the exacerbation of malodor symptoms of females with trimethylaminuria during that phase of the cycle (61). Theoretically, these differences can be mediated by hormonal suppression of FMO3 expression and enzymatic activity (62, 57–60) . Notably, our results did not identify marked differences for TMAO concentrations by menstruation in either adult or young females. Conversely, both betaine and l-carnitine exhibited differences between menstruating and nonmenstruating adult women. Previous work identified menstrual metabolic rhythmicity for several metabolites (e.g., acylcarnitines, amino acids) involved in key metabolic pathways and proposed that these differences reflect fluctuations in anabolic requirements in response to menstrual hormonal changes (e.g., variations in demands for β-oxidation and energy utilization) (63). Nevertheless, we had limited numbers of females reporting menstruation status and larger studies are needed to determine the effect of menstruation on TMAO concentrations. Moreover, given the reported hormonal influences on TMAO concentrations, it may be important to account for states of hormonal fluctuations (e.g., menstruation, menopause) when characterizing the concentrations of TMAO and of its precursors in future studies.
Higher plasma concentrations of TMAO and its metabolites in males may be associated with higher protein intake (22,23, 64,65). Notably, protein intakes of twice the RDAs have been shown to increase circulating TMAO concentrations in an intervention study of healthy older adult males (65). However, associations between the diet and TMAO concentrations have been largely inconsistent (11, 22, 23, 29–32). These differences may partly be attributed to disparities in dietary assessment methods between studies, and/or differences between observational and interventional study designs (11, 22, 23, 29–32). For example, meat, fish, eggs, and dairy products have all been reported to increase plasma and/or urinary TMAO concentrations, although the strength of association varies (11, 22, 23, 29–32). Moreover, although vegetarians generally have lower plasma TMAO concentrations, the strictness of a vegetarian diet (i.e., vegan compared with lacto-ovo-vegetarian) does not affect TMAO concentrations (32). Interestingly, increases in TMAO concentrations in response to a l-carnitine challenge have been shown to vary according to whether individuals were omnivores or vegetarians (21). In addition, sources of fish (e.g., freshwater compared with saltwater) and cooking methods (e.g., deep frying compared with stir frying) correlate differently with urinary TMAO concentrations (15). Collectively, these observations highlight that TMAO concentrations can be altered by the diet, which may be important in cases where TMAO concentrations are considered cardiotoxic (66).
Consistent with previous reports, we found that the frequency of consumption of animal protein sources in our Australian cohort was associated with TMAO and l-carnitine concentrations in both generations. In addition, reported animal protein intake frequency was associated with DMG and choline concentrations in the parent subgroup exclusively. By contrast, the reported habitual intake of dairy products was not associated with any of the precursors, in contrast to observations from a German dietary study (29). Finally, betaine did not show evidence of association with the diet in either generation, consistent with previous reports (30). Differences in dietary associations between studies may be confounded by country-specific dietary habits, health status of the population studied, gut microbial composition and diversity, diet misreporting, timing of blood collection, as well as general lifestyle and dietary patterns. Therefore, diet–metabolite associations must be contextualized for each population/individual and extrapolations must be made cautiously.
TMAO originating from different dietary sources (diet, microbiome, etc.) has the same structure and therefore cannot be resolved analytically (29). This knowledge gap is an obstacle to understanding whether TMAO contributes to cardiovascular disease risk. However, preformed TMAO (found in fish) is more readily metabolized than TMAO that is synthesized from its precursors, thus fish-based TMAO is more readily available, and urinary TMAO itself has been identified as a good biomarker of fish intake (23, 67). Given the reported beneficial health effects of fish consumption (68, 69), time-dependent analyses of TMAO concentrations in both urine and plasma linked to robust dietary and metabolic data may be important to stratify individuals with high TMAO concentrations/high TMAO-production capacity by differential metabolic/dietary status, as has been previously performed (21, 46).
Limitations of this study
There are a number of limitations to our study. Despite the large sample size (n = 2490 individuals), our study was cross-sectional and semi-fasted plasma was only collected from individuals at 1 time point, and with different fasting times which would have affected our metabolite measures as well as their associations with reported dietary intakes. In addition, dietary intakes were self-reported, which is subject to bias (70, 71), and did not include detailed information on portion size or energy content. Moreover, the ratio of adult males to females in our study was unbalanced (1:10), which limited our power to identify some sex-specific differences, or to examine the effect of age in males. Furthermore, our cohort is relatively wealthier than the Australian population (>78% of our population scored in the middle to least disadvantaged Socio-Economic Indexes for Areas score quintiles compared with ∼62% in the general Australian population) (72, 73). Finally, the collection and analysis of our participants’ stool samples would have strengthened our conclusions given that the gut microbiome is important in TMAO formation (23), but was not feasible as part of the biomedical assessment.
Future directions
Identifying the drivers of TMAO concentrations (e.g., fish, red meat, diseases, medication, gut dysbiosis, stress, exercise) and the metabolic profiles (e.g., inflammatory markers, lipid and glycemic profiles) of individuals with high TMAO concentrations/increased TMAO-production capacity will improve the stratification of these individuals and their responses to interventions that target metabolic health outcomes. Therefore, future studies should be designed to combine measurements of fasting TMAO concentrations (in plasma and urine) with 1) TMAO concentrations in response to dietary/precursor (e.g., l-carnitine) challenges at different time points; and 2) measures of context-specific regulation of the genetic, transcriptional, gut microbial, and metabolic machinery involved in the metabolism of TMAO (21, 46). Given the emerging putative link between TMAO concentrations and cardiovascular and metabolic outcomes, we plan to analyze some of these associations.
Conclusion
Plasma TMAO concentrations are subject to familial, age, and sex-specific influences. Therefore, given the well-documented differences in cardiovascular disease risk in association with sex, age, and environmental influences, the relation between TMAO concentrations and cardiovascular preclinical phenotypes warrants further investigation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—MW, RS, and DPB: designed the CheckPoint study; MW and JMOS: designed, and obtained the funding for, the substudy of micronutrients; SA: conducted the laboratory work, performed all statistical analyses, and wrote the paper; KL and SAC: provided data support; BJ: provided statistical support; EBT: assisted with laboratory analyses; KL, SAC, BJ, EBT, JAK, MW, RS, DPB, and JMOS: discussed and edited the manuscript; MW, RS, DPB, and JMOS: supervised SA; and all authors: read and approved the final manuscript.
Notes
Supported by Ministry of Business, Innovation and Employment catalyst grant (The New Zealand-Australia Life Course Collaboration on Genes, Environment, Nutrition and Obesity) UOAX1611 (to JMOS, MW, RS, and DPB). The Checkpoint study was supported by National Health and Medical Research Council (NHMRC) of Australia project grants 1041352 and 1109355, The Royal Children's Hospital Foundation grant 2014-241, Murdoch Children's Research Institute, The University of Melbourne, National Heart Foundation of Australia grant 100660, Financial Markets Foundation for Children grants 2014-055 and 2016-310, and the Victorian Deaf Education Institute. Research at the Murdoch Children's Research Institute is supported by the Victorian Government's Operational Infrastructure Support Program. SA is the recipient of a New Zealand International Doctoral Research Scholarship 2017. MW was supported by NHMRC Research Fellowships 1046518 and 1160906 and Cure Kids New Zealand. DPB was supported by NHMRC Research Fellowship 1064629, Investigator Grant GTN1175744, and National Heart Foundation of Australia: Honorary Future Leader Fellowship 100369.
Author disclosures: The authors report no conflicts of interest.
The funding bodies did not play any role in the study. This article uses unit record data from Growing Up in Australia, the Longitudinal Study of Australian Children. The study is conducted in partnership between the Department of Social Services (DSS), the Australian Institute of Family Studies (AIFS), and the Australian Bureau of Statistics (ABS). The findings and views reported in this article are those of the authors and should not be attributed to the DSS, the AIFS, or the ABS.
Supplemental Figures 1 and 2 and Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Data described in the article will be made available upon request after application and approval by our teams.
Abbreviations used: CheckPoint, Child Health CheckPoint; DMG, dimethylglycine; FMO3, flavin monooxygenase 3; HFBA, heptafluorobutyric acid; MS-MS, tandem mass spectrometry; QC, quality control; TMA, trimethylamine; TMAO, trimethylamine N-oxide; UHPLC, ultra-high-performance liquid chromatography.
Contributor Information
Stephanie Andraos, Liggins Institute, The University of Auckland, Auckland, New Zealand.
Katherine Lange, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia.
Susan A Clifford, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia.
Beatrix Jones, Department of Statistics, Faculty of Science, The University of Auckland, Auckland, New Zealand.
Eric B Thorstensen, Liggins Institute, The University of Auckland, Auckland, New Zealand.
Jessica A Kerr, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia.
Melissa Wake, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia.
Richard Saffery, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia.
David P Burgner, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia; Department of Paediatrics, Monash University, Clayton, Victoria, Australia.
Justin M O'Sullivan, Email: justin.osullivan@auckland.ac.nz, Liggins Institute, The University of Auckland, Auckland, New Zealand.
References
- 1. Fennema D, Phillips IR, Shephard EA. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab Dispos. 2016;44:1839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Z, Tang WHW, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, McEntyre CJ, Elmslie JL, Atkinson W, Molyneux SL et al.. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS One. 2014;9:e114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li XS, Obeid S, Wang Z, Hazen BJ, Li L, Wu Y, Hurd AG, Gu X, Pratt A, Levison BS et al.. Trimethyllysine, a trimethylamine N-oxide precursor, provides near- and long-term prognostic value in patients presenting with acute coronary syndromes. Eur Heart J. 2019;40:2700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qi J, You T, Li J, Pan T, Xiang L, Han Y, Zhu L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta‐analysis of prospective studies. J Am Heart Assoc. 2017;6:e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, de Alteriis G, Tenore GC, Colao A, Savastano S. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients. 2018;10:1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243:638–44. [DOI] [PubMed] [Google Scholar]
- 10. Kaysen GA, Johansen KL, Chertow GM, Dalrymple LS, Kornak J, Grimes B, Dwyer T, Chassy AW, Fiehn O. Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr. 2015;25:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meyer KA, Benton TZ, Bennett BJ, Jacobs DR, Lloyd-Jones DM, Gross MD, Carr JJ, Gordon-Larsen P, Zeisel SH. Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J Am Heart Assoc. 2016;5:e003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L et al.. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao X, Tian Y, Randell E, Zhou H, Sun G. Unfavorable associations between serum trimethylamine N-oxide and l-carnitine levels with components of metabolic syndrome in the Newfoundland population. Front Endocrinol (Lausanne). 2019;10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mente A, Chalcraft K, Ak H, Davis AD, Lonn E, Miller R, Potter MA, Yusuf S, Anand SS, McQueen MJ. The relationship between trimethylamine-N-oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can J Cardiol. 2015;31:1189–94. [DOI] [PubMed] [Google Scholar]
- 15. Yu D, Shu X-O, Rivera ES, Zhang X, Cai Q, Calcutt MW, Xiang Y-B, Li H, Gao Y-T, Wang TJ et al.. Urinary levels of trimethylamine-N-oxide and incident coronary heart disease: a prospective investigation among urban Chinese adults. J Am Heart Assoc. 2019;8:e010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong Z, Liang Z, Guo M, Hu S, Shen Z, Hai X. The association between plasma levels of trimethylamine N-oxide and the risk of coronary heart disease in Chinese patients with or without type 2 diabetes mellitus. Dis Markers. 2018:1578320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reiner MF, Müller D, Gobbato S, Stalder O, Limacher A, Bonetti NR, Pasterk L, Méan M, Rodondi N, Aujesky D et al.. Gut microbiota-dependent trimethylamine-N-oxide (TMAO) shows a U-shaped association with mortality but not with recurrent venous thromboembolism. Thromb Res. 2019;174:40–7. [DOI] [PubMed] [Google Scholar]
- 18. Tveitevåg Svingen GF, Ueland PM, Pedersen EKR, Schartum-Hansen H, Seifert R, Ebbing M, Løland KH, Tell GS, Nygård O. Plasma dimethylglycine and risk of incident acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2013;33:2041–8. [DOI] [PubMed] [Google Scholar]
- 19. Svingen GF, Schartum-Hansen H, Ueland PM, Pedersen ER, Seifert R, Ebbing M, Bønaa KH, Mellgren G, Nilsen DW, Nordrehaug JE et al.. Elevated plasma dimethylglycine is a risk marker of mortality in patients with coronary heart disease. Eur J Prev Cardiol. 2015;22:743–52. [DOI] [PubMed] [Google Scholar]
- 20. Konstantinova SV, Tell GS, Vollset SE, Nygård O, Bleie Ø, Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138:914–20. [DOI] [PubMed] [Google Scholar]
- 21. Wu W-K, Chen C-C, Liu P-Y, Panyod S, Liao B-Y, Chen P-C, Kao H-L, Kuo H-C, Kuo C-H, Chiu THT et al.. Identification of TMAO-producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut. 2019;68:1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW et al.. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, Sutter JL, Caudill MA. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61:1600324. [DOI] [PubMed] [Google Scholar]
- 24. Cho CE, Caudill MA. Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire?. Trends Endocrinol Metab. 2017;28:121–30. [DOI] [PubMed] [Google Scholar]
- 25. Malinowska AM, Szwengiel A, Chmurzynska A. Dietary, anthropometric, and biochemical factors influencing plasma choline, carnitine, trimethylamine, and trimethylamine-N-oxide concentrations. Int J Food Sci Nutr. 2017;68:488–95. [DOI] [PubMed] [Google Scholar]
- 26. Zhu W, Wang Z, Tang WHW, Hazen SL. Gut microbe-generated trimethylamine N-oxide from dietary choline is prothrombotic in subjects. Circulation. 2017;135:1671–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang AQ, Mitchell S, Smith R. Fish odour syndrome: verification of carrier detection test. J Inherit Metab Dis. 1995;18:669–74. [DOI] [PubMed] [Google Scholar]
- 28. Pelletier CC, Croyal M, Ene L, Aguesse A, Billon-Crossouard S, Krempf M, Lemoine S, Guebre-Egziabher F, Juillard L, Soulage CO. Elevation of trimethylamine-N-oxide in chronic kidney disease: contribution of decreased glomerular filtration rate. Toxins (Basel). 2019;11:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krüger R, Merz B, Rist MJ, Ferrario PG, Bub A, Kulling SE, Watzl B. Associations of current diet with plasma and urine TMAO in the KarMeN study: direct and indirect contributions. Mol Nutr Food Res. 2017;61:1700363. [DOI] [PubMed] [Google Scholar]
- 30. Zuo H, Svingen GFT, Tell GS, Ueland PM, Vollset SE, Pedersen ER, Ulvik A, Meyer K, Nordrehaug JE, Nilsen DWT et al.. Plasma concentrations and dietary intakes of choline and betaine in association with atrial fibrillation risk: results from 3 prospective cohorts with different health profiles. J Am Heart Assoc. 2018;7:e008190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allenspach M, Müller D, Linseisen J, Rohrmann S, von Eckardstein A. Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J Nutr. 2015;146:283–9. [DOI] [PubMed] [Google Scholar]
- 32. Obeid R, Awwad HM, Keller M, Geisel J. Trimethylamine-N-oxide and its biological variations in vegetarians. Eur J Nutr. 2017;56(8):2599–609. [DOI] [PubMed] [Google Scholar]
- 33. Dagenais GR, Leong DP, Rangarajan S, Lanas F, Lopez-Jaramillo P, Gupta R, Diaz R, Avezum A, Oliveira GBF, Wielgosz A et al.. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2019;395(10226):785–94. [DOI] [PubMed] [Google Scholar]
- 34. Skilton MR, Celermajer DS, Cosmi E, Crispi F, Gidding SS, Raitakari OT, Urbina EM. Natural history of atherosclerosis and abdominal aortic intima-media thickness: rationale, evidence, and best practice for detection of atherosclerosis in the young. J Clin Med. 2019;8:1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leal‐Witt MJ, Llobet M, Samino S, Castellano P, Cuadras D, Jimenez‐Chillaron JC, Yanes O, Ramon‐Krauel M, Lerin C. Lifestyle intervention decreases urine trimethylamine N‐oxide levels in prepubertal children with obesity. Obesity. 2018;26:1603–10. [DOI] [PubMed] [Google Scholar]
- 36. Hsu C-N, Lu P-C, Lo M-H, Lin I-C, Chang-Chien G-P, Lin S, Tain Y-L. Gut microbiota-dependent trimethylamine N-oxide pathway associated with cardiovascular risk in children with early-stage chronic kidney disease. Int J Mol Sci. 2018;19:3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edwards B. Growing Up in Australia: the Longitudinal Study of Australian Children. [Internet] In: Family Matters Issue 95. Melbourne: Australian Institute of Family Studies; 2014; [cited 2019 Dec 6] pp. 5–14. Available from: https://aifs.gov.au/publications/family-matters/issue-95/growing-australia-longitudinal-study-australian-children. [Google Scholar]
- 38. Sanson A, Johnstone R; The LSAC Research Consortium & FaCS LSAC Project Team. Growing Up in Australia takes its first steps. Family Matters. 2004;(67):46–53. [Google Scholar]
- 39. Clifford SA, Davies S, Wake M; Child Health CheckPoint Team. Child Health CheckPoint: cohort summary and methodology of a physical health and biospecimen module for the Longitudinal Study of Australian Children. BMJ Open. 2019;9(Suppl 3):3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andraos S, Goy M, Albert BB, Kussmann M, Thorstensen EB, O'Sullivan JM. Robotic automation of a UHPLC/MS-MS method profiling one-carbon metabolites, amino acids, and precursors in plasma. Anal Biochem. 2020;592:113558. [DOI] [PubMed] [Google Scholar]
- 41. Flood V, Webb K, Rangan A. Recommendations for short questions to assess food consumption in children for the NSW Health Surveys. Sydney: NSW Centre for Public Health Nutrition; 2005. [Google Scholar]
- 42. Saloheimo T, González SA, Erkkola M, Milauskas DM, Meisel JD, Champagne CM, Tudor-Locke C, Sarmiento O, Katzmarzyk PT, Fogelholm M. The reliability and validity of a short food frequency questionnaire among 9–11-year olds: a multinational study on three middle-income and high-income countries. Int J Obes Suppl. 2015;5:S22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. R Core Team, 2020. R: A language and environment for statistical computing. [Internet] Vienna (Austria): R Foundation for Statistical Computing; [cited 2019 Aug 8] Available from: https://www.r-project.org/. [Google Scholar]
- 44. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 45. Chambers JM, Freeny A, Heiberger RM. Analysis of variance; designed experiments. In: Chambers JM, Hastie TJ, Statistical models in s. New York; London: Chapman & Hall/CRC; 1993. pp. 145–93. [Google Scholar]
- 46. Manor O, Zubair N, Conomos MP, Xu X, Rohwer JE, Krafft CE, Lovejoy JC, Magis AT. A multi-omic association study of trimethylamine N-oxide. Cell Rep. 2018;24:935–46. [DOI] [PubMed] [Google Scholar]
- 47. Koukouritaki SB, Simpson P, Yeung CK, Rettie AE, Hines RN. Human hepatic flavin-containing monooxygenases 1 (FMO1) and 3 (FMO3) developmental expression. Pediatr Res. 2002;51:236–43. [DOI] [PubMed] [Google Scholar]
- 48. Bouchemal N, Ouss L, Brassier A, Barbier V, Gobin S, Hubert L, de Lonlay P, Le Moyec L. Diagnosis and phenotypic assessment of trimethylaminuria, and its treatment with riboflavin: 1H NMR spectroscopy and genetic testing. Orphanet J Rare Dis. 2019;14:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hartiala J, Bennett BJ, Tang WHW, Wang Z, Stewart AFR, Roberts R, McPherson R, Lusis AJ, Hazen SL, Allayee H. Comparative genome-wide association studies in mice and humans for trimethylamine N-oxide, a proatherogenic metabolite of choline and l-carnitine. Arterioscler Thromb Vasc Biol. 2014;34:1307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aslibekyan S, Irvin MR, Hidalgo BA, Perry RT, Jeyarajah EJ, Garcia E, Shalaurova I, Hopkins PN, Province MA, Tiwari HK et al.. Genome- and CD4+ T-cell methylome-wide association study of circulating trimethylamine-N-oxide in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN). J Nutr Intermed Metab. 2017;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ellul S, Wake M, Clifford SA, Lange K, Würtz P, Juonala M, Dwyer T, Carlin JB, Burgner DP, Saffery R. Metabolomics: population epidemiology and concordance in Australian children aged 11–12 years and their parents. BMJ Open. 2019;9:106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Winther SA, Øllgaard JC, Tofte N, Tarnow L, Wang Z, Ahluwalia TS, Jorsal A, Theilade S, Parving H-H, Hansen TW et al.. Utility of plasma concentration of trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes Care. 2019;42:1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang A, Mitchell S, Smith R. Dietary precursors of trimethylamine in man: a pilot study. Food Chem Toxicol. 1999;37:515–20. [DOI] [PubMed] [Google Scholar]
- 54. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M et al.. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rist MJ, Roth A, Frommherz L, Weinert CH, Krüger R, Merz B, Bunzel D, Mack C, Egert B, Bub A et al.. Metabolite patterns predicting sex and age in participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) study. PLoS One. 2017;12:e0183228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Randrianarisoa E, Lehn-Stefan A, Wang X, Hoene M, Peter A, Heinzmann SS, Zhao X, Königsrainer I, Königsrainer A, Balletshofer B et al.. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep. 2016;6:26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coecke S, Debast G, Phillips I, Vercruysse A, Shephard E, Rogiers V. Hormonal regulation of microsomal flavin-containing monooxygenase activity by sex steroids and growth hormone in co-cultured adult male rat hepatocytes. Biochem Pharmacol. 1998;56:1047–51. [DOI] [PubMed] [Google Scholar]
- 58. Mitchell SC, Zhang AQ. Methylamine in human urine. Clin Chim Acta. 2001;312:107–14. [DOI] [PubMed] [Google Scholar]
- 59. Shimizu M, Cashman JR, Yamazaki H. Transient trimethylaminuria related to menstruation. BMC Med Genet. 2007;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mitchell SC, Smith RL. A physiological role for flavin-containing monooxygenase (FMO3) in humans?. Xenobiotica. 2010;40:301–5. [DOI] [PubMed] [Google Scholar]
- 61. Ayesh R, Mitchell SC, Zhang A, Smith RL. The fish odour syndrome: biochemical, familial, and clinical aspects. BMJ. 1993;307:655–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R et al.. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Draper CF, Duisters K, Weger B, Chakrabarti A, Harms AC, Brennan L, Hankemeier T, Goulet L, Konz T, Martin FP et al.. Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci Rep. 2018;8:14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sui Z, Raubenheimer D, Cunningham J, Rangan A. Changes in meat/poultry/fish consumption in Australia: from 1995 to 2011–2012. Nutrients. 2016;8:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mitchell SM, Milan AM, Mitchell CJ, Gillies NA, D'Souza RF, Zeng N, Ramzan F, Sharma P, Knowles SO, Roy NC et al.. Protein intake at twice the RDA in older men increases circulatory concentrations of the microbiome metabolite trimethylamine-N-oxide (TMAO). Nutrients. 2019;11:2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N‐oxide promotes vascular inflammation through signaling of mitogen‐activated protein kinase and nuclear factor‐κB. J Am Heart Assoc. 2016;5:e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, Slimani N, Zamora-Ros R, Rundle M, Frost G et al.. A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr. 2017;105:600–8. [DOI] [PubMed] [Google Scholar]
- 68. Bagge CN, Strandhave C, Skov CM, Svensson M, Schmidt EB, Christensen JH. Marine n-3 polyunsaturated fatty acids affect the blood pressure control in patients with newly diagnosed hypertension – a 1-year follow-up study. Nutr Res. 2017;38:71–8. [DOI] [PubMed] [Google Scholar]
- 69. Jayedi A, Shab-Bidar S, Eimeri S, Djafarian K. Fish consumption and risk of all-cause and cardiovascular mortality: a dose-response meta-analysis of prospective observational studies. Public Health Nutr. 2018;21:1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pryer JA, Vrijheid M, Nichols R, Kiggins M, Elliott P. Who are the “low energy reporters” in the dietary and nutritional survey of British adults?. Int J Epidemiol. 1997;26:146–54. [DOI] [PubMed] [Google Scholar]
- 71. Brennan L. Moving toward objective biomarkers of dietary intake. J Nutr. 2018;148:821–2. [DOI] [PubMed] [Google Scholar]
- 72. Australian Bureau of Statistics (ABS). Population. [Internet][cited 2019 Dec 6]. Belconnen (ACT): ABS; 2019. Available from: https://www.abs.gov.au/population. [Google Scholar]
- 73. Australian Bureau of Statistics (ABS). Census of population and housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2016. [Internet] [accessed 2020 Jun 24] Belconnen (ACT): ABS; 2018. Available from: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2071.0~2016~Main%20Features~Socio-Economic%20Advantage%20and%20Disadvantage~123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.