Abstract
Background
Left ventricular systolic dysfunction (LVSD) occurs frequently after acute ST-segment elevation myocardial infarction (STEMI). The predisposing factors and underlying mechanism of post-infarct LVSD are not fully understood. The present study mainly investigated the correlation between glycaemic gap, a novel index of stress-induced hyperglycaemia (SIH), and post-infarct LVSD.
Methods
A total of 274 first STEMI patients were enrolled in this cross-sectional study. Transthoracic echocardiography was performed within 48 h after admission and at 6 months after discharge to obtain left ventricular ejection fraction (LVEF). The change in LVEF was calculated as LVEF at 6 months after discharge minus baseline LVEF. Additionally, post-infarct LVSD was defined as LVEF ≤ 50%. Most importantly, glycaemic gap was calculated as admission blood glucose (ABG) minus the estimated average glucose over the previous 3 months.
Results
In patients without diabetes mellitus (DM), multivariate linear regression analysis revealed that both glycaemic gap (Beta = − 1.214, 95% CI − 1.886 to − 0.541, p < 0.001) and ABG (Beta = − 1.124, 95% CI − 1.795 to − 0.453, p = 0.001) were associated with change in LVEF. In DM patients, only glycaemic gap was still associated with change in LVEF, although this association was not observed in univariate linear regression analysis. Regarding the association between SIH and post-infarct LVSD, multivariate logistic regression analysis revealed that both glycaemic gap (OR = 1.490, 95% CI 1.043 to 2.129, p = 0.028) and ABG (OR = 1.600, 95% CI 1.148 to 2.229, p = 0.005) were associated with an increased risk of having post-infarct LVSD in non-DM patients. However, after multivariate adjustment in DM patients, only glycaemic gap (OR = 1.399, 95% CI 1.021 to 1.919, p = 0.037) remained associated with an increased risk of having post-infarct LVSD. Furthermore, the predictive value of glycaemic gap for post-infarct LVSD was not inferior to ABG in non-DM patients (p = 0.499), and only glycaemic gap, instead of ABG, could significantly predict post-infarct LVSD in DM patients (AUC = 0.688, 95% CI 0.591 to 0.774, p = 0.002).
Conclusions
Glycaemic gap was strongly associated with a change in LVEF and an increased risk of having post-infarct LVSD in patients following STEMI. In STEMI patients with DM, glycaemic gap could provide more valuable information than ABG in identifying patients at high risk of developing post-infarct LVSD.
Keywords: Left ventricular systolic dysfunction, Change in LVEF, Stress-induced hyperglycaemia, Glycaemic gap, Admission blood glucose, ST-segment elevation myocardial infarction
Background
Despite rapid development in acute treatment and second prevention in recent years, post-infarct left ventricular systolic dysfunction (LVSD) occurs frequently [1, 2]. Post-infarct LVSD, which is defined as reduced left ventricular ejection fraction (LVEF), is strongly correlated with adverse cardiovascular outcomes such as heart failure (HF), re-infarction, and increased cardiovascular mortality [3–6].
The pathophysiology of post-infarct LVSD is complex and poor understood [5]. Much evidence has revealed that processes such as myocardial injury associated with infarct size, left ventricular remodelling, myocardial stress, oxidative stress, and local and systemic inflammatory responses are all involved in the pathophysiology of post-infarct LVSD [5, 7–9]. Recent studies further indicated that stress-induced hyperglycaemia (SIH) and concomitant metabolic perturbations also play an extremely important role in the initiation and progression of post-infarct LVSD [10–12].
Critical illness such as acute myocardial infarction (AMI), sepsis, and major surgeries could lead to transient hyperglycaemia, termed SIH, through multiple potential mechanisms in patients with or without diabetes mellitus (DM) [13]. Admission blood glucose (ABG) has been mainly regarded as an index of SIH in previous studies [13]. The ABG level is impacted by both acute physiological stress and chronic baseline glycaemic levels, so it does not reflect the extent of SIH accurately in an acutely ill state [14, 15]. Furthermore, reports by Esposito et al. and Monnier et al. suggested that acute glucose fluctuations induced by SIH could be more harmful than sustained hyperglycaemia [16, 17]. To assess the effect of SIH more accurately, glycaemic gap and stress hyperglycaemia ratio (SHR), which eliminates the interference of chronic baseline glycaemic levels, were proposed as a novel index of SIH [18–20]. Considerable evidence have confirmed both glycaemic gap and SHR were strongly correlated with adverse cardiovascular outcome in AMI patients [15, 21, 22], but we knew little about the association between these indexes and post-infarct LVSD. Therefore, we mainly investigated the correlation between glycaemic gap and post-infarct LVSD in the present study.
Methods
Study population
In this cross-sectional study, we consecutively recruited ST-segment elevation myocardial infarction (STEMI) patients admitted to Beijing Anzhen Hospital, Capital Medical University from January 2018 to January 2019. Inclusion criteria were as follows: first STEMI diagnosed according to the European Society of Cardiology and American College of Cardiology Committee criteria [23, 24], emergent treatment of only the infarct-related artery by primary percutaneous coronary intervention (PCI) within 12 h, and other diseased vessels managed by elective PCI within 1 month if required. Exclusion criteria were defined as follows: age < 18 years or age > 80 years, previous history of myocardial infarction or coronary revascularization, thrombolysis prior to primary PCI, without stent implantation, serious valvular heart disease and primary cardiomyopathies, estimated glomerular filtration rate < 30 mL/min per 1.73 m2, active inflammatory or neoplastic process on admission, chronic requirement of steroid or immunosuppressive therapy. Additionally patients with hemopathy including hemolytic anemia, sickle cell anemia, thalassemia, and megaloblastic anemia were also excluded from our present study.
The study protocol was developed in compliance with the Declaration of Helsinki, and the study was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. Before the beginning of the study, we obtained written informed consent from each patient.
Therapy and management
All enrolled patients received optimal treatment according to current guidelines for the management of acute STEMI patients [23, 24]. All the patients were prescribed a loading dose of aspirin 300 mg, clopidogrel 600 mg or ticagrelor 180 mg, and intravenously unfractionated heparin (60–70 IU/kg, maximum 5000 IU) immediately after diagnosis of STEMI. In the cardiac catheterization laboratory, all patients received a second dose of unfractionated heparin according to weight (70–100 IU/kg). The experienced interventional cardiologist blinded to the study protocol performed the primary PCI in compliance with current guidelines [23, 24]. After primary PCI, dual antiplatelet therapy in the form of aspirin plus ticagrelor or clopidogrel was recommended for 1 year if there were no contraindications. In addition, concomitant medications such as beta-blockers, statins, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs) were all recommended unless contraindicated. Most importantly, lifestyle interventions, including smoking cessation, weight control, optimal blood pressure and blood glucose control, were also recommended.
Data collection
When patients reached the emergency department, peripheral venous blood samples were collected immediately to measure ABG using a standardized biochemical assay. High-performance liquid chromatography analysers were used to examine glycated haemoglobin (HbA1c) levels. Then, we obtained the estimated average glucose (eAG) level over the previous 3 months through the following equation: eAG = 28.7 * HbA1c − 46.7 [14, 15, 18], and glycaemic gap was calculated as ABG minus eAG. SHR, another index of relative hyperglycaemia, was defined as ABG/eAG.
For biomarker analyses, cardiac troponin I (cTnI) and hypersensitive C-reactive protein (hs-CRP) were monitored dynamically to identify peak values. The other biological parameters, such as uric acid, homocysteine, triglyceride, low-density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c), were all measured in a fasting state.
Demographic and clinical information, including age, sex, height, weight, smoking, DM, hypertension, and medication use at discharge, were all obtained through the standard questionnaire. In the questionnaire, DM was defined as having a prior history of DM (treated with diet or anti-diabetic medications) or having newly diagnosed DM with fasting plasma glucose ≥ 7.0 mmol/L, HbA1c ≥ 6.5% or 2-h plasma value ≥ 11.1 mmol/L during an oral glucose tolerance test [25]. Coronary angiogram data such as number of diseased vessels (stenosis ≥ 50% of diameter of coronary artery), thrombolysis in myocardial infarction (TIMI) flow grade pre- and post-PCI, and length of stents were all recorded by two independent cardiologists. Additionally, we defined slow flow/no-reflow as TIMI flow ≤ 2 post-PCI.
Echocardiography
All patients included underwent examination of transthoracic echocardiography within 48 h after admission and 6 months after discharge to obtain LVEF with the modified Simpson rule. Transthoracic echocardiography was performed following recommendations from the American Society of Echocardiography [26]. Most importantly, transthoracic echocardiography was performed by the same two independent echocardiographers who have worked more than 5 years and were blinded to the study protocol with a GE Vivid 7 ultrasound machine (GE Healthcare, Piscataway, NJ, USA). Change in LVEF was defined as LVEF at 6 months post-STEMI minus baseline LVEF. Post-infarct LVSD was defined as LVEF ≤ 50%.
Statistics
For continuous variables, a normal distribution was assessed using the Kolmogorov–Smirnov test. Then, continuous data were expressed as the mean ± standard deviation or median (interquartile ranges) and compared by Student’s t-test or the Mann–Whitney U test. Categorical variables were summarized as numbers (percentages) and analysed by the Chi-square test. To interrogate the association between change in LVEF and other variables, multivariate linear regression analysis was implemented (age, sex, total ischaemic time, number of diseased vessels, and variables with p < 0.1 in the univariate linear regression were included in the model). Additionally, the correlation between post-infarct LVSD and other variables was determined by multivariate logistic regression analysis (the model included age, sex, and variables with p < 0.1 in the univariate logistic regression). Furthermore, we assessed the predictive value of glycaemic gap and ABG through receiver operating characteristic (ROC) curve analysis. The predictive values of glycaemic gap and ABG were evaluated by the area under curve (AUC), and differences in the AUC were assessed by the DeLong test. Statistical analyses were performed using SPSS 20.0 (SPSS., Chicago, IL, USA) and MedCalc V.11.4 (MedCalc, Inc., Ostend, Belgium). p < 0.05 was regarded as statistically significant.
Results
Basic characteristics of the studied population
A total of 274 STEMI patients were enrolled for the final analyses in the present study after excluding 5 patients who died within 6 months after primary PCI and 3 patients who did not attend the 6-month visit. The patients enrolled were divided into 2 subgroups (non-DM group and DM group). Then, the non-DM (0.995 mmol/L) and DM (2.427 mmol/L) groups were further divided according to the median glycaemic gap level.
Table 1 shows that the mean age of the patients recruited was 57.78 years, and the prevalence of DM, current smoking, and hypertension was 39.1%, 65.7%, and 54.0%, respectively. Furthermore, as revealed in Additional file 1: Table S1, 48.5% of the patients had culprit vessel lesions in the left anterior descending artery (LAD), and 60.2% of the patients had multivessel disease. Additionally, as demonstrated in Additional file 1: Table S1, 94.5% of patients achieved TIMI flow grade 3 after intervention, which means that almost all patients recruited received timely and successful primary PCI.
Table 1.
Baseline characteristics
| Clinical information | Total (274) | Non-DM, n = 167 | p-value | DM, n = 107 | p-value | ||
|---|---|---|---|---|---|---|---|
| Group 1 (84) | Group 2 (83) | Group 3 (54) | Group 4 (53) | ||||
| Age (years) | 57.78 ± 11.42 | 55.18 ± 12.49 | 58.19 ± 9.95 | 0.087 | 58.54 ± 10.60 | 60.49 ± 12.08 | 0.376 |
| Male | 236 (86.1%) | 77 (91.7%) | 70 (84.3%) | 0.160 | 44 (81.5%) | 45 (84.9%) | 0.797 |
| BMI (kg/m2) | 25.29 ± 2.62 | 25.34 ± 2.20 | 25.22 ± 2.84 | 0.754 | 25.51 ± 2.58 | 25.10 ± 2.94 | 0.441 |
| Smoker | 180 (65.7%) | 59 (70.2%) | 53 (63.9%) | 0.413 | 33 (61.1%) | 35 (66.0%) | 0.689 |
| DM | 107 (39.1%) | – | – | – | 54 (100.0%) | 53 (100.0%) | 1.000 |
| Hypertension | 148 (54.0%) | 48 (57.1%) | 40 (48.2%) | 0.280 | 29 (53.7%) | 31 (58.5%) | 0.698 |
| Killip classification | 0.245 | 0.309 | |||||
| Class I | 255 (93.1%) | 75 (89.3%) | 80 (96.4%) | 52 (96.3%) | 48 (90.6%) | ||
| Class II | 13 (4.7%) | 6 (7.1%) | 2 (2.4%) | 2 (3.7%) | 3 (5.7%) | ||
| Class III | 2 (0.7%) | 1 (1.2%) | 1 (1.2%) | – | – | ||
| Class IV | 4 (1.5%) | 2 (2.4%) | 0 | 0 | 2 (3.8%) | ||
| Laboratory examination | |||||||
| Uric acid (μmol/L) | 359.70 ± 86.43 | 377.26 ± 91.46 | 356.86 ± 79.79 | 0.126 | 338.67 ± 95.03 | 357.74 ± 75.17 | 0.252 |
| Homocysteine (μmol/L) | 12.50 (9.38, 17.43) | 14.25 (10.00, 23.35) | 12.90 (9.30, 18.10) | 0.133 | 11.05 (9.18, 15.25) | 12.30 (9.15, 16.60) | 0.413 |
| eGFR (mmol/L) | 99.56 (91.58, 107.78) | 99.44 ± 15.01 | 98.19 ± 13.19 | 0.571 | 99.70 ± 14.06 | 94.18 ± 17.80 | 0.077 |
| Tg (mmol/L) | 1.45 (1.01, 2.05) | 1.52 (1.06, 2.03) | 1.29 (0.78, 1.70) | 0.016 | 1.76 ± 0.93 | 2.48 ± 2.08 | 0.022 |
| LDL-C (mmol/L) | 3.14 ± 0.98 | 3.26 ± 1.05 | 3.19 ± 0.93 | 0.555 | 2.95 ± 0.83 | 3.07 ± 1.08 | 0.517 |
| HDL-C (mmol/L) | 1.05 (0.88, 1.20) | 1.07 ± 0.25 | 1.13 ± 0.28 | 0.156 | 0.98 ± 0.21 | 1.06 ± 0.31 | 0.094 |
| Peak cTnI (ng/mL) | 30.80 (12.40, 68.52) | 22.35 (10.20, 48.77) | 40.97 (14.26, 75.00) | 0.019 | 33.38 ± 30.59 | 47.47 ± 31.88 | 0.022 |
| Peak hs-CRP (mg/L) | 4.75 (2.27, 10.09) | 4.72 (2.50, 7.58) | 4.93 (1.37, 11.05) | 0.804 | 4.53 (2.25, 7.98) | 5.29 (2.86, 15.36) | 0.323 |
| ABG (mmol/L) | 8.35 (7.02, 10.84) | 6.59 ± 0.64 | 8.66 ± 1.47 | < 0.001 | 10.03 ± 2.49 | 13.88 ± 2.95 | <0.001 |
| HBA1C (%) | 6.00 (5.58, 6.90) | 5.71 ± 0.35 | 5.62 ± 0.40 | 0.139 | 7.64 ± 1.56 | 7.48 ± 1.31 | 0.556 |
| Pre-hospital medications | |||||||
| Insulin | 11 (4.0%) | – | – | 4 (7.4%) | 7 (13.2%) | 0.359 | |
| DPP-4 inhibitors | 22 (8.9%) | – | – | 9 (16.7%) | 13 (24.5%) | 0.347 | |
| Other hypoglycemic agents | 62 (22.7%) | – | – | 34 (63.0%) | 28 (52.8%) | 0.331 | |
| Medications at discharge | |||||||
| Aspirin | 274 (100.0%) | 84 (100.0%) | 83 (100.0%) | 1.000 | 54 (100.0%) | 53 (100.0%) | 1.000 |
| Clopidogrel/ticagrelor | 274 (100.0%) | 84 (100.0%) | 83 (100.0%) | 1.000 | 54 (100.0%) | 53 (100.0%) | 1.000 |
| Statin | 270 (98.5%) | 84 (100.0%) | 80 (96.4%) | 0.121 | 54 (100.0%) | 52 (98.1%) | 0.495 |
| Beta-blockers | 215 (78.5%) | 68 (81.0%) | 61 (73.5%) | 0.273 | 45 (83.3%) | 41 (77.4%) | 0.474 |
| ACEI/ARB | 175 (63.9%) | 52 (61.9%) | 48 (57.8%) | 0.637 | 38 (70.4%) | 37 (69.8%) | 1.000 |
Non-DM and DM patients were further divided into subgroups according to median value of glycemic gap (Group 1: glycemic gap ≤ 0.995 mmol/L; Group 2: glycemic gap > 0.995 mmol/L; Group 3: glycemic gap ≤ 2.427 mmol/L; Group 4: glycemic gap > 2.427 mmol/L)
DM diabetes mellitus, BMI body mass index, eGFR estimated glomerular filtration rate, Tg triglycerides, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, cTnI cardiac troponin I, hs-CRP hypersensitive C-reactive protein, ABG admission blood glucose, FBG fasting blood glucose, HBA1C glycated hemoglobin, DPP-4 inhibitors dipeptidyl peptidase-4 inhibitors, ACEI/ARB angiotensin-converting enzyme inhibitor/angiotensin receptor blocker
After subgroup analysis, we discovered that STEMI patients with high glycaemic gaps in the non-DM (40.97 (14.26, 75.00) vs. 22.35 (10.20, 48.77), p = 0.019) and DM (47.47 ± 31.88 vs. 33.38 ± 30.59, p = 0.022) groups both tended to have higher peak cTnI levels. High glycaemic gaps in non-DM (8.66 ± 1.47 vs. 6.59 ± 0.64, p < 0.001) and DM (13.88 ± 2.95 vs. 10.03 ± 2.49, p < 0.001) patients were also associated with higher ABG levels. Additionally, high glycaemic gap in DM patients was also associated with higher triglyceride (2.48 ± 2.08 vs. 1.76 ± 0.93, p = 0.022) levels, but not in non-DM patients.
Glycaemic gap associated with change in LVEF
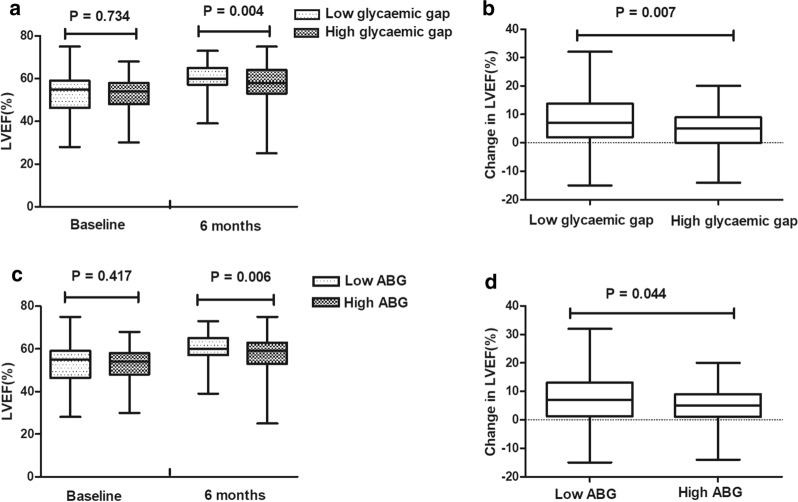
In non-DM patients, compared with the low glycaemic gap group, the high glycaemic gap group was associated with lower LVEF at 6 months post-STEMI (56.96 ± 9.12 vs. 60.55 ± 6.38, p = 0.004, Fig. 1a), and the change in LVEF (4.29 ± 6.82 vs. 7.50 ± 8.33, p = 0.007, Fig. 1b) was also significantly smaller in the high glycaemic gap group patients, despite the baseline LVEF (52.67 ± 7.71 vs. 53.11 ± 8.65, p = 0.734) being similar. Additionally, as presented in Fig. 1c, d, both the LVEF at the 6-month follow-up (57.06 ± 9.11 vs. 60.45 ± 6.44, p = 0.006) and the change in LVEF (4.69 ± 6.87 vs. 7.11 ± 8.42, p = 0.044) were significantly lower in the high ABG group than in the low ABG group in non-DM patients. Most importantly, as shown in Table 2, glycaemic gap (Beta = − 1.214, 95% CI − 1.886 to − 0.541, p < 0.001) and ABG (Beta = − 1.124, 95% CI − 1.795 to − 0.453, p = 0.001) remained associated with the change in LVEF in non-DM patients after adjusting for age, sex, BMI, peak hs-CRP, baseline LVEF, total ischaemic time, and number of diseased vessels.
Fig. 1.
LVEF (a) and change in LVEF (b) in non-DM patients in the high glycaemic gap group and low glycaemic gap group at 6-month follow-up. LVEF (c) and change in LVEF (d) in non-DM patients in the high ABG group and low ABG group at 6-month follow-up. LVEF left ventricular ejection fraction, DM diabetes mellitus, ABG admission blood glucose. Change in LVEF was defined as LVEF at 6 months post-STEMI minus baseline LVEF
Table 2.
Multivariate linear regression for the correlation between change in LVEF and other variables in non-DM
| Non-DM patients | Univariate linear regression | Multivariate linear regression (Mode A, R2 = 0.379) | Multivariate linear regression (Mode B, R2 = 0.373) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Beta | 95% CI | p-value | Beta | 95% CI | p-value | Beta | 95% CI | p-value |
| Age | − 0.029 | − 0.134 to 0.076 | 0.582 | 0.077 | − 0.015 to 0.169 | 0.099 | 0.098 | 0.004 to 0.193 | 0.042 |
| Sex | − 3.811 | − 7.428 to − 0.193 | 0.039 | − 4.107 | − 7.250 to − 0.964 | 0.011 | − 4.337 | − 7.490 to − 1.183 | 0.007 |
| BMI | 0.463 | − 0.003 to 0.928 | 0.051 | 0.286 | − 0.109 to 0.680 | 0.155 | 0.318 | − 0.078 to 0.714 | 0.115 |
| Peak hs-CRP | − 0.152 | − 0.304 to 0.001 | 0.051 | − 0.185 | − 0.312 to − 0.058 | 0.005 | − 0.190 | − 0.318 to − 0.063 | 0.004 |
| Baseline LVEF | − 0.469 | − 0.596 to − 0.342 | < 0.001 | − 0.492 | − 0.614 to − 0.370 | < 0.001 | − 0.498 | − 0.621 to − 0.375 | < 0.001 |
| Total ischemic time | − 0.004 | − 0.012 to 0.004 | 0.318 | − 0.005 | − 0.011 to 0.001 | 0.114 | − 0.005 | − 0.011 to 0.002 | 0.148 |
| No. of diseased vessels | − 0.516 | − 1.958 to 0.927 | 0.481 | − 0.171 | − 1.364 to 1.022 | 0.778 | − 0.174 | − 1.374 to 1.026 | 0.775 |
| ABG | − 0.866 | − 1.634 to − 0.099 | 0.027 | − 1.124 | − 1.795 to − 0.453 | 0.001 | |||
| Glycemic gap | − 1.116 | − 1.901 to − 0.331 | 0.006 | − 1.214 | − 1.886 to − 0.541 | < 0.001 | |||
Change in LVEF was defined as LVEF at 6-month follow-up minus baseline LVEF
DM diabetes mellitus, BMI body mass index, hs-CRP hypersensitive C-reactive protein, LVEF left ventricular ejection fraction, Total ischemic time the period from symptom onset to reopening of infarction-associated artery, ABG admission blood glucose
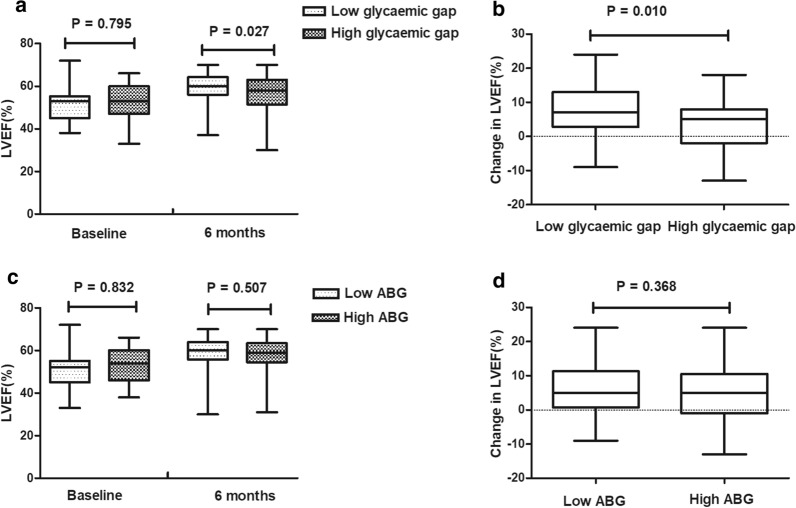
As demonstrated in Fig. 2a, b, a high glycaemic gap was still associated with lower LVEF (56.13 ± 9.38 vs. 59.61 ± 6.28, p = 0.027) and change in LVEF (3.43 ± 7.80 vs. 7.31 ± 7.51, p = 0.010) at the 6-month follow-up in DM patients. However, as presented in Fig. 2c, d, we observed no difference in LVEF and change in LVEF between the high ABG group and the low ABG group in DM patients at the 6-month follow-up. In DM patients, univariate linear regression revealed that both glycaemic gap (Beta = − 0.366, 95% CI − 0.942 to 0.210, p = 0.210) and ABG (Beta = − 0.281, 95% CI − 0.734 to 0.172, p = 0.221) were not correlated with change in LVEF.
Fig. 2.
LVEF (a) and change in LVEF (b) in DM patients in the high glycaemic gap group and low glycaemic gap group at 6-month follow-up. LVEF (c) and change in LVEF (d) in DM patients in the high ABG group and low ABG group at 6-month follow-up. LVEF left ventricular ejection fraction, DM diabetes mellitus, ABG admission blood glucose. Change in LVEF was defined as LVEF at 6 months post-STEMI minus baseline LVEF
Glycaemic gap was strongly correlated with post-infarct LVSD
In non-DM patients, the proportion of post-infarct LVSD in high glycaemic gap patients was significantly higher than that in low glycaemic gap patients at the 6-month follow-up (18.1% vs. 6.0%, p = 0.018, Fig. 3a), although the proportion of post-infarct LVSD was similar between subgroups at baseline (34.9% vs. 34.5%, p > 0.99, Fig. 3a). Additionally, as revealed in Fig. 3b, post-infarct LVSD at the 6-month follow-up also occurred more often in the high ABG group patients (18.1% vs. 6.0%, p = 0.018). Furthermore, as revealed in Table 3, multivariate logistic regression revealed that an augmented glycaemic gap (OR = 1.490, 95% CI 1.043 to 2.129, p = 0.028) and ABG (OR = 1.600, 95% CI 1.148 to 2.229, p = 0.005) were associated with an increased risk of having post-infarct LVSD after adjusting for age, sex, peak cTnI, HDL-c, and peak hs-CRP in non-DM patients.
Fig. 3.
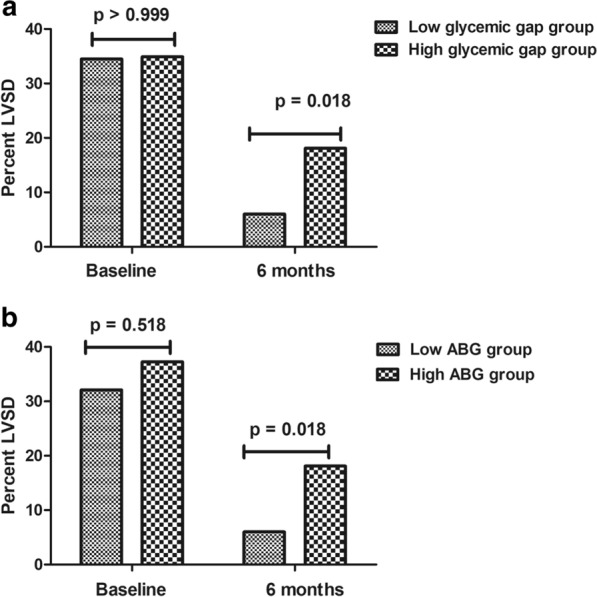
The proportion of post-infarct LVSD in non-DM patients in the high glycaemic gap group and low glycaemic gap group at baseline and 6-month follow-up (a). The proportion of post-infarct LVSD in non-DM patients in the high ABG group and low ABG group at baseline and 6-month follow-up (b). LVSD left ventricular systolic dysfunction, DM diabetes mellitus, ABG admission blood glucose. Post-infarct LVSD was defined as LVEF ≤ 50% after infarction
Table 3.
Multivariate logistic regression for the correlation between post-infarct LVSD and other variables in non-DM and DM patients
| Non-DM patients | Univariate logistic regression | Multivariate logistic regression (Mode A) | Multivariate logistic regression (Mode B) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p-value | OR | 95% | p-value | OR | 95% | p-value |
| Age | 0.986 | 0.947 to 1.027 | 0.494 | 0.950 | 0.897 to 1.005 | 0.073 | 0.939 | 0.884 to 0.996 | 0.038 |
| Sex | 0.341 | 0.109 to 1.071 | 0.065 | 0.216 | 0.046 to 1.017 | 0.053 | 0.195 | 0.041 to 0.934 | 0.041 |
| Peak cTnI | 1.025 | 1.008 to 1.042 | 0.003 | 1.015 | 0.997 to 1.033 | 0.106 | 1.015 | 0.997 to 1.034 | 0.111 |
| HDL-C | 5.707 | 1.167 to 27.919 | 0.032 | 3.972 | 0.600 to 26.284 | 0.153 | 4.626 | 0.679 to 31.509 | 0.118 |
| Peak hs-CRP | 1.074 | 1.020 to 1.131 | 0.006 | 1.070 | 1.010 to 1.135 | 0.022 | 1.075 | 1.013 to 1.141 | 0.017 |
| ABG | 1.499 | 1.146 to 1.962 | 0.003 | 1.600 | 1.148 to 2.229 | 0.005 | |||
| Glycemic gap | 1.494 | 1.120 to1.993 | 0.006 | 1.490 | 1.043 to 2.129 | 0.028 | |||
| DM patients | Univariate logistic regression | Multivariate logistic regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| Age | 1.028 | 0.977 to 1.081 | 0.287 | 1.013 | 0.945 to 1.085 | 0.722 | |||
| Sex | 3.038 | 0.894 to 10.326 | 0.075 | 0.317 | 0.057 to 1.780 | 0.192 | |||
| Peak cTnI | 1.048 | 1.023 to 1.073 | < 0.001 | 1.051 | 1.023 to 1.080 | < 0.001 | |||
| HDL-C | 6.183 | 0.975 to 39.221 | 0.053 | 0.588 | 0.073 to 4.736 | 0.617 | |||
| Peak hs-CRP | 1.025 | 0.964 to 1.090 | 0.434 | ||||||
| ABG | 1.106 | 0.947 to 1.292 | 0.203 | ||||||
| Glycemic gap | 1.267 | 1.027 to 1.565 | 0.027 | 1.399 | 1.021 to 1.919 | 0.037 | |||
LVSD was defined as LVEF ≤ 50%
LVSD left ventricular systolic dysfunction, DM diabetes mellitus, cTnI cardiac troponin I, HDL-C high-density lipoprotein cholesterol, hs-CRP hypersensitive C-reactive protein, ABG admission blood glucose
As shown in Fig. 4a, although the proportion of post-infarct LVSD at baseline was similar between subgroups in DM patients, high glycaemic gap patients were still associated with a higher proportion of post-infarct LVSD at the 6-month follow-up compared with low glycaemic gap patients (22.6% vs. 5.6%, p = 0.013). In line with this finding, as indicated in Table 3, univariate logistic regression revealed that an elevated glycaemic gap was strongly associated with an increased risk of having post-infarct LVSD at 6 months post-STEMI (OR = 1.267, 95% CI 1.027 to 1.565, p = 0.027). After adjusting for age, sex, peak cTnI, and HDL-c through multivariate logistic regression, the correlation persisted in DM patients (OR = 1.399, 95% CI 1.021 to 1.919, p = 0.037). However, as indicated in Fig. 4b, there were no significant differences between the high ABG and low ABG groups in the prevalence of post-infarct LVSD at baseline and 6 months post-STEMI. Additionally, univariate logistic regression also revealed that ABG also was not correlated with post-infarct LVSD at the 6-month follow-up (OR = 1.106, 95% CI 0.947 to 1.292, p = 0.203).
Fig. 4.
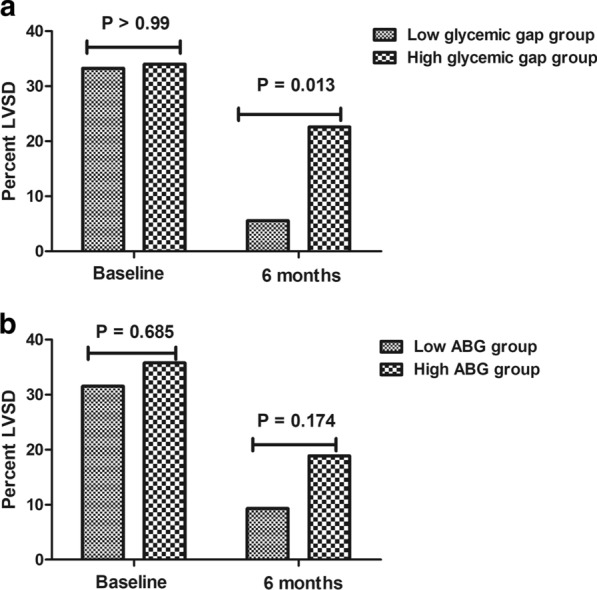
The proportion of post-infarct LVSD in DM patients in the high glycaemic gap group and low glycaemic gap group at baseline and 6-month follow-up (a). The proportion of post-infarct LVSD in DM patients in the high ABG group and low ABG group at baseline and 6-month follow-up (b). LVSD left ventricular systolic dysfunction, DM diabetes mellitus, ABG admission blood glucose. Post-infarct LVSD was defined as LVEF ≤ 50% after infarction
Predictive value of glycaemic gap compared to ABG for post-infarct LVSD at the 6-month follow-up
After ROC analysis (Table 4), we demonstrated that both glycaemic gap (AUC = 0.697, 95% CI 0.622 to 0.766, p = 0.001) and ABG (AUC = 0.713, 95% CI 0.638 to 0.781, p = 0.002) could provide a moderate predictive value for post-infarct LVSD at the 6-month follow-up, and the predictive value of glycaemic gap was not inferior to ABG (p = 0.499) in non-DM patients. However, as shown in Table 4, only glycaemic gap had a moderate predictive value for post-infarct LVSD at 6 months after STEMI (AUC = 0.688, 95% CI 0.591 to 0.774, p = 0.002) in DM patients.
Table 4.
Predictive value of glycemic gap versus ABG for post-infarct LVSD at 6-month follow-up
| AUC | 95% CI | p for AUC | P for δAUC | Cut-off value | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| Non-DM patients | |||||||||
| ABG | 0.713 | 0.638 to 0.781 | 0.002 | – | 8.27 | 70.00% | 78.23% | 30.4% | 95.0% |
| Glycemic gap | 0.697 | 0.622 to 0.766 | 0.001 | 0.499 | 1.858 | 55.00% | 78.91% | 26.2% | 92.8% |
| DM patients | |||||||||
| ABG | 0.621 | 0.522 to 0.713 | 0.08 | ||||||
| Glycemic gap | 0.688 | 0.591 to 0.774 | 0.002 | 2.197 | 86.67% | 54.35% | 23.6% | 96.2% | |
ABG admission blood glucose, LVSD left ventricular systolic dysfunction, DM diabetes mellitus, AUC area under curve by receiver-operating characteristic curve analysis, δ AUC difference of AUC, PPV positive predictive values, NPV negative predictive values
Discussion
Our present study demonstrated 3 significant findings. First, glycaemic gap, as a novel index of SIH, was associated with changes in LVEF and the increased risk of having post-infarct LVSD in STEMI patients with or without DM. Second, the correlation between change in LVEF, post-infarct LVSD, and ABG only persisted in non-DM patients. Third, in regard to identifying patients at high risk of having post-infarct LVSD, glycaemic gap could provide a superior discriminatory ability compared with ABG in DM patients.
Considerable evidences suggested glycaemic excursion is not only associated with risk of cardiovascular disease but also associated with major adverse cardiovascular and cerebrovascular events [27, 28]. Glycaemic gap, as a novel marker of glycaemic excursion that quantifies the magnitude of a relative glycaemic rise from chronic glycaemia in an acutely ill state, was proposed to better assess the effect of SIH [20]. Previous studies have reported glycaemic gap is associated with severity of disease and unfavorable prognosis in patients with critical illness [29]. Our present study further confirmed that peak cTnI was significantly higher in patients with a high glycaemic gap level regardless of diabetic status, which suggests SIH measured by glycaemic gap is associated with infarct size assessed by peak cTnI. According to previous studies, the potential mechanisms of the relationship between SIH and myocardial injury included secretion of excessive cortisol and catecholamine, inflammation and oxidative stress, endothelial dysfunction, relative insulin deficiency, and prothrombotic state [13, 16, 17, 30].
Recent clinical trials have confirmed ABG levels are associated with poor prognosis in STEMI patients without DM, but the association is relative weak in DM patients [31, 32]. However, the association between ABG and myocardial function after STEMI in patients with DM or without DM is not well investigated at present, and direct evidence is limited. Capes et al. reported that ABG was correlated with an increased risk of congestive heart failure or cardiogenic shock in non-DM patients, but the correlation was not observed in DM patients [31]. Teraguchi et al. further demonstrated that myocardial salvage index (MSI) assessed cardiac magnetic resonance (CMR) in patients with SIH (ABG ≥ 10 mmol/L) was lower than that in patients without SIH among non-DM patients; however, no significant difference was observed in MSI between patients with or without SIH in DM patients [33]. In line with these studies, our present study revealed that ABG was associated with changes in LVEF and the risk of having post-infarct LVSD in non-DM patients, but the association was not observed in DM patients. There may be several explanations for the discrepant findings. First, not taking baseline glucose levels into consideration in DM patients may be the main reason. There is a wide range in the level of chronic glycaemic control among DM patients, from satisfactory to poor; therefore, it is necessary to consider baseline glucose levels when investigating the correlation between SIH and the risk of post-infarct LVSD. Second, many DM patients achieve good glycaemic control with optimal glucose-lowering treatment, while others do not.
Instead of ABG, both glycaemic gap and SHR were associated with major adverse cardiac events (MACEs) and could provide moderate predictive value for the occurrence of MACEs in AMI patients with DM [15, 22, 34]. Liao et al. further demonstrated glycaemic gap rather than ABG was linked to all-cause mortality in acute heart failure (AHF) patients with DM [35]. Moreover, they also discovered glycaemic gap could provide higher predictive value than ABG for all-cause mortality, cardiovascular mortality, and acute respiratory failure [35]. Extending prior studies, our present study demonstrated for the first time that glycaemic gap, not ABG, was associated with the change in LVEF and risk of having post-infarct LVSD in DM patients. Comparing with ABG, glycaemic gap could provide a superior predictive ability for post-infarct LVSD in DM patients. Therefore, glycaemic gap could be used to assess prognosis and identify patients at high risk for developing post-infarct LVSD in the management of AMI patients, particularly in patients with DM. However, we should note that the accuracy of glycaemic gap is still moderate, and its performance should be further explored by prospective longitudinal studies before putting it into practice.
Additionally, Lee et al. reported that relative hyperglycaemia during glucose-lowering treatment is more strongly associated with adverse outcomes than absolute glycaemia in patients following AMI [36]. The results suggested that using relative, not absolute, glycaemic thresholds for intervention and therapeutic glycaemic targets could improve adverse outcomes following AMI [36]. Therefore, it is necessary for future randomized controlled trials to investigate whether a glucose-lowering treatment target on glycaemic gap or SHR could reduce the risk of developing post-infarct LVSD in STEMI patients.
We acknowledge that the present study has several limitations. First, this was a single-centre study with a limited sample size. Therefore, experimental and multi-center clinical studies with large sample size are needed to further verify our findings in the future. Second, this was an observational study, which failed to assess the causal link between the relative hyperglycaemia glycaemic gap and post-infarct LVSD. Third, we evaluated changes in LVEF and post-infarct LVSD only through transthoracic echocardiography, which may limit the value of our research. To improve accuracy and provide more valuable information, future studies could employ CMR or single-photon emission computed tomography to assess associated parameters including LVEF, infarct size, microvascular obstruction, and MSI. Finally, we only recruited STEMI patients who underwent primary PCI successfully in the present study. Thus, our findings remain to be verified in general patients with acute coronary syndrome.
Conclusions
Our present study demonstrated for the first time that glycaemic gap, as a novel index of SIH, was associated with the change in LVEF and the risk of having post-infarct LVSD at the 6-month follow-up in STEMI patients after primary PCI. Compared to ABG, glycaemic gap could provide a superior predictive value for post-infarct LVSD in DM patients and an equivalent predictive value in non-DM patients. Prospective studies are needed to further investigate whether glycaemic control using glycaemic gap could reduce the risk of having post-infarct LVSD, especially in DM patients.
Supplementary information
Additional file 1: Table S1. Procedural characteristics of patients enrolled.
Acknowledgements
None.
Abbreviations
- LVSD
Left ventricular systolic dysfunction
- LVEF
Left ventricular ejection fraction
- HF
Heart failure
- SIH
Stress-induced hyperglycaemia
- AMI
Acute myocardial infarction
- DM
Diabetes mellitus
- ABG
Admission blood glucose
- SHR
Stress hyperglycaemia ratio
- STEMI
ST-segment elevation myocardial infarction
- PCI
Percutaneous coronary intervention
- ACEIs/ARBs
Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers
- HbA1c
Glycated haemoglobin
- eAG
Estimated average glucose
- cTnI
Cardiac troponin I
- hs-CRP
Hypersensitive C-reactive protein
- LDL-c
Low-density lipoprotein cholesterol
- HDL-c
High-density lipoprotein cholesterol
- TIMI
Thrombolysis in myocardial infarction
- ROC
Receiver operating characteristic
- AUC
Area under curve
- LAD
Left anterior descending artery
- MSI
Myocardial salvage index
- CMR
Cardiac magnetic resonance
- MACEs
Major adverse cardiac events
- AHF
Acute heart failure
Authors’ contributions
YZ and KSL interpreted the data and wrote the manuscript. ZNJ and HGZ designed the study protocol and supervised the project. ZNJ, HGZ, SM and RFJ performed primary PCI. XL, MLC, and KYZ enrolled and managed the participants. All authors read and approved the final manuscript.
Funding
No.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study protocol was developed in compliance with the Declaration of Helsinki, and the study was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. Before the beginning of the study, we obtained written informed consent from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-020-01075-8.
References
- 1.Puymirat E, Simon T, Steg PG, Schiele F, Gueret P, Blanchard D, Khalife K, Goldstein P, Cattan S, Vaur L, et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA. 2012;308(10):998–1006. doi: 10.1001/2012.jama.11348. [DOI] [PubMed] [Google Scholar]
- 2.Weir RAP, McMurray JJV, Velazquez EJ. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol. 2006;97(10-supp-S1):13–25. doi: 10.1016/j.amjcard.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Kümler T, Gislason GH, Køber L, Torp-Pedersen C. Persistence of the prognostic importance of left ventricular systolic function and heart failure after myocardial infarction: 17-year follow-up of the TRACE register. Eur J Heart Fail. 2010;12(8):805–811. doi: 10.1093/eurjhf/hfq071. [DOI] [PubMed] [Google Scholar]
- 4.Im MS, Kim H, Kim S, Lim W, Seo J, Chung W, Zo J, Kim M, Park K, Koo B. Different prognostic factors according to left ventricular systolic function in patients with acute myocardial infarction. Int J Cardiol. 2016;221:90–96. doi: 10.1016/j.ijcard.2016.06.100. [DOI] [PubMed] [Google Scholar]
- 5.Swiatkiewicz I, Taub PR. The usefulness of C-reactive protein for the prediction of post-infarct left ventricular systolic dysfunction and heart failure. Kardiol Pol. 2018;76(5):821–829. doi: 10.5603/KP.a2018.0091. [DOI] [PubMed] [Google Scholar]
- 6.Swiatkiewicz I, Kozinski M, Magielski P, Gierach J, Fabiszak T, Kubica A, Sukiennik A, Navarese EP, Odrowaz-Sypniewska G, Kubica J. Usefulness of C-reactive protein as a marker of early post-infarct left ventricular systolic dysfunction. Inflamm Res. 2012;61(7):725–734. doi: 10.1007/s00011-012-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Bijl P, Abou R, Goedemans L, Gersh BJ, Holmes DR, Ajmone Marsan N, Delgado V, Bax JJ. Left ventricular post-infarct remodeling. JACC Heart Failure. 2020;8(2):131–140. doi: 10.1016/j.jchf.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Parviz Y, Vijayan S, Lavi S. A review of strategies for infarct size reduction during acute myocardial infarction. Cardiovasc Revasc Med. 2017;18(5):374–383. doi: 10.1016/j.carrev.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Świątkiewicz I, Magielski P, Kubica J, Zadourian A, DeMaria AN, Taub PR. Enhanced inflammation is a marker for risk of post-infarct ventricular dysfunction and heart failure. Int J Mol Sci. 2020;21(3):807. doi: 10.3390/ijms21030807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauters C, Ennezat PV, Tricot O, Lauwerier B, Lallemant R, Saadouni H, Quandalle P, Jaboureck O, Lamblin N, Le Tourneau T. Stress hyperglycaemia is an independent predictor of left ventricular remodelling after first anterior myocardial infarction in non-diabetic patients. Eur Heart J. 2006;28(5):546–552. doi: 10.1093/eurheartj/ehl546. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, Umemura T, Nakamura S, Yoshida M. Impact of acute hyperglycemia on left ventricular function after reperfusion therapy in patients with a first anterior wall acute myocardial infarction. Am Heart J. 2003;146(4):674–678. doi: 10.1016/S0002-8703(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 12.Yang CD, Shen Y, Lu L, Ding FH, Yang ZK, Zhang RY, Shen WF, Jin W, Wang XQ. Insulin resistance and dysglycemia are associated with left ventricular remodeling after myocardial infarction in non-diabetic patients. Cardiovasc Diabetol. 2019;18(1):100. doi: 10.1186/s12933-019-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandalia H, Gokani A. Stress hyperglycaemia. Lancet. 2009;324(8406):811–812. doi: 10.1016/s0140-6736(84)90734-7. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Kim T, Yoon K, Chung WS, Ahn Y, Jeong M, Seung K, Lee S, Chang K. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol. 2017;241:57–63. doi: 10.1016/j.ijcard.2017.02.065. [DOI] [PubMed] [Google Scholar]
- 15.Gao S, Liu Q, Ding X, Chen H, Zhao X, Li H. Predictive value of the acute-to-chronic glycemic ratio for in-hospital outcomes in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Angiology. 2020;71(1):38–47. doi: 10.1177/0003319719875632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monnier L, Mas E, Ginet C, Michel FO, Villon L, Cristol J, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 17.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 18.Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, Burt MG, Doogue MP. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocr Metab. 2015;100(12):4490–4497. doi: 10.1210/jc.2015-2660. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Liu Z, Miao J, Zheng W, Yang Q, Ye X, Zhuang X, Peng F. High stress hyperglycemia ratio predicts poor outcome after mechanical thrombectomy for ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28(6):1668–1673. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Chen P, Tsai S, Wang J, Tzeng Y, Wang Y, Chu C, Chu S, Liao W. An elevated glycemic gap predicts adverse outcomes in diabetic patients with necrotizing fasciitis. PLoS ONE. 2019;14(10):e223126. doi: 10.1371/journal.pone.0223126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima T, Hikoso S, Nakatani D, Suna S, Dohi T, Mizuno H, Okada K, Kitamura T, Kida H, Oeun B, et al. Impact of hyperglycemia on long-term outcome in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2020;125(6):851–859. doi: 10.1016/j.amjcard.2019.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Liao W, Lin C, Lee C, Wu Y, Chang W, Hsu C, Wang J, Tsai S. An elevated glycemic gap is associated with adverse outcomes in diabetic patients with acute myocardial infarction. Sci Rep. 2016;6(1):1. doi: 10.1038/srep27770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39(2):119–177. [Google Scholar]
- 24.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, De Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association Standards of Medical Care in Diabetes-20182. Classification and Diagnosis of Diabetes. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32(1):1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi H, Iwahashi N, Kirigaya J, Kataoka S, Minamimoto Y, Gohbara M, Abe T, Okada K, Matsuzawa Y, Konishi M, et al. Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome. Cardiovasc Diabetol. 2018;17:116. doi: 10.1186/s12933-018-0761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee G, Kim SM, Choi S, Kim K, Jeong S, Son JS, Yun J, Park SM. The effect of change in fasting glucose on the risk of myocardial infarction, stroke, and all-cause mortality: a nationwide cohort study. Cardiovasc Diabetol. 2018;17:51. doi: 10.1186/s12933-018-0694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donagaon S, Dharmalingam M. Association between glycemic gap and adverse outcomes in critically ill patients with diabetes. Indian J Endocrinol Metab. 2018;22(2):208. doi: 10.4103/ijem.IJEM_580_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worthley MI, Holmes AS, Willoughby SR, Kucia AM, Heresztyn T, Stewart S, Chirkov YY, Zeitz CJ, Horowitz JD. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol. 2007;49(3):304–310. doi: 10.1016/j.jacc.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 31.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 32.Ishihara M, Kojima S, Sakamoto T, Kimura K, Kosuge M, Asada Y, Tei C, Miyazaki S, Sonoda M, Tsuchihashi K, et al. Comparison of blood glucose values on admission for acute myocardial infarction in patients with versus without diabetes mellitus. Am J Cardiol. 2009;104(6):769–774. doi: 10.1016/j.amjcard.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 33.Teraguchi I, Imanishi T, Ozaki Y, Tanimoto T, Kitabata H, Ino Y, Ishibashi K, Komukai K, Hirata K, Akasaka T. Impact of stress hyperglycemia on myocardial salvage following successfully recanalized primary acute myocardial infarction. Circ J. 2012;76(11):2690–2696. doi: 10.1253/circj.cj-12-0303. [DOI] [PubMed] [Google Scholar]
- 34.Marenzi G, Cosentino N, Milazzo V, Metrio MD, Bartorelli AL. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study. Diabetes Care. 2018;41(4):c171732. doi: 10.2337/dc17-1732. [DOI] [PubMed] [Google Scholar]
- 35.Liao W, Wang J, Lin C, Yang C, Hsu C, Chu S, Chu C, Tsai S. Elevated glycemic gap predicts acute respiratory failure and in-hospital mortality in acute heart failure patients with diabetes. Sci Rep. 2019;9(1):1–9. doi: 10.1038/s41598-019-42666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee TF, Burt MG, Heilbronn LK, Mangoni AA, Wong VW, McLean M, Cheung NW. Relative hyperglycemia is associated with complications following an acute myocardial infarction: a post hoc analysis of HI-5 data. Cardiovasc Diabetol. 2017;16(1):157. doi: 10.1186/s12933-017-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Procedural characteristics of patients enrolled.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.