Abstract
Renal dysfunctions are major predictors of co-morbidities and mortality in HIV infected individuals. Unconventional T cells have been shown to regulate kidney functions. However, there is dearth of information on the effect of HIV associated nephropathies on γδ and DN T cells. It is also not clear whether γδ T cell perturbations observed during the early stages of HIV infection occur before immune activation. In this study, we investigated the relationship between creatinine and urea on the number of unconventional T cells in HIV infected individuals at the early and chronic stages of infection. Persons in the chronic stage of infection were divided into treatment naïve and exposed groups. Treatment exposed individuals were further subdivided into groups with undetectable and detectable HIV-1RNA in their in their blood.
Creatinine and urea levels were significantly higher among persons in the early HIV infection compared to the other groups. Proportions of γδ T, γδ+CD8, γδ+CD16 cells were also significantly reduced in the early stage of HIV infection (P<0.01). Markers of immune activation, CD4+HLA-DR and CD8+HLA-DR, were also significantly reduced during early HIV infection (P<0.01). Taken together, our findings suggest that high levels of renal markers as well as reduced proportions of gamma delta T cells are associated with the early stages of HIV infection. This event likely occurs before systemic immune activation reaches peak levels. This study provides evidence for the need for early HIV infection diagnosis and treatment.
Keywords: γδ T cells, DNT cells, Renal dysfunctions, Interleukin 17, HIV-1 RNA viral load, Neutrophils
1. Introduction
HIV associated renal dysfunctions are major predictors of end stage renal diseases, AIDS defining events, cardiovascular diseases, metabolic diseases; all of which cause mortality in HIV infected individuals1,2. The incidence of HIV associated chronic kidney disease have increased in sub-Sahara Africa as well as in persons with African descent in North America3–6. Chronic kidney disease is a major cause of death among HIV infected individuals in sub-Sahara Africa and in persons with Africa ancestry of which the risk of death is increased six times6. Renal disease in HIV infection has been shown to be associated with systemic inflammation, immune activation and an impaired immune system1,7. Our previous study and others showed that when HIV associated nephropathies is diagnosed before acute/early HIV seroconversion is identified, it presents with uremia, high serum creatinine levels and proteinuria3,8.
Unconventional T cells, especially gamma delta (γδ) and double negative T cells (DNT), which are unique subclasses of innate immune T cells have been shown to perform regulatory functions in humans9,10. DNT cells make up 1–5% of T cells in blood and lymphoid tissues and express either αβ or γδ T cell receptors, although a major percentage expresses αβ T cell receptor9. DN T cells expressing the αβ T cell receptor are CD3+/CD4−/CD8−, and can be found in the peripheral blood, mucosa and gut associated lymphoid tissues11. DNT cells have been previously described to be elevated in HIV infected individuals in order to regulate immune activation and restore immune cells to normal proportions11. Although, there are functional overlaps between αβ and γδ T cell receptors (TCRs), the antigen recognition format and activation for each subsets of these cells are different10,12. Unlike αβ T cells, γδ T cells do not process and present antigens through major histocompatibility (MHC) molecules13. Unconventional T cells display beneficial roles during HIV infection as they exhibit cell lytic and cytokine secretion functions14.
γδ T cells, neutrophils and cytokines such as IL-17, IL-23, IFN-γ and TNF-α have been shown to regulate kidney functions13,15,16. Lower proportions of γδ T cells have been associated with kidney disorders namely diabetic kidney diseases10, renal carcinoma17 and end stage renal diseases7. Studies have also shown lower numbers and frequencies of γδ T cells in tuberculosis18 and Hepatitis B infected patients with renal impairments19,20. However, there is dearth of information on the impact of HIV associated nephropathies on γδ and DN T cells proportions and subsets during early HIV infection. It is also not clear whether γδ T cell perturbations observed during the early stages of HIV infection occur before immune activation. This study was therefore designed to investigate the proportions of γδ, γδ+CD8, γδ+Cd16 as well as DNT in HIV infected individuals at different stages of infection. The timing of immune activation in relation to γδ T cells defects in these individuals was also determined.
2. Material and Methods
2.1. Patients
The study participants gave written informed consent before their blood samples were collected. Seventeen individuals at the early stage of HIV-1 infection (EHIV) were enrolled, out of which 10 were recruited from persons who presented for malaria parasite diagnosis as previously described8. The others were recruited from prospective volunteer blood donors whose samples were reactive for early HIV-1 infection defined by HIV-1 DNA detection along with 4th generation HIV ELISA (detection of both antigen and antibody) positive, and negative with 3rd generation HIV ELISA (detection of HIV antibody only). This definition is based on the updated CDC algorithm for laboratory testing for the diagnosis of early and chronic HIV-1 infection21,22 Twenty-three chronically infected HIV-1 cART –naïve patients (CHIV) and 41 chronically HIV-1 infected patients on cART were randomly selected from the APIN CDC-funded PEPFAR Treatment Centre at the University College Hospital, Ibadan, Nigeria. Twenty three out of the 41 chronically HIV-1 infected patients on therapy had undetectable viral load (LVL) as defined by HIV-1 RNA copies per mL less than 20, while the remaining 18 patients in this group had detectable viral load (HVL) defined by HIV-1 RNA copies per ml greater than 150 after being on treatment for at least 2 years. HIV infected individuals in Nigeria as well as in most countries in Sub-Saharan Africa are identified and recruited for treatment at the chronic stages of infection8. The approximate time of HIV exposure for CHIV, HVL and LVL groups are not less than 2, 6 and 7 years respectively. HIV-1 DNA was also detected in all the samples collected from persons in EHIV group while only HIV-1 RNA was detected from other groups. A control population was established by random selection among HIV negative individuals invited to participate in the study (n = 27).
2.2. Study Population
The study participants were enrolled from July 2015 to December 2017 at the HIV diagnostic unit of the Department of Virology, College of Medicine, Ibadan, Nigeria. The analysis reported here is a sub study of a prospective cohort of early HIV-1 infected adults.
2.3. HIV diagnosis and clinical follow up
The study participants were over 18 years of age and residents of Ibadan, Oyo state, Nigeria. Patients’ were identified as early HIV infection if HIV-1 DNA from their blood samples was detected as positive along with a 4th generation HIV ELISA that detects both antigen and antibody and negative with a 3rd generation HIV ELISA that detects only HIV antibody. Those patients with positive HIV-1 DNA with both 4th and 3rd generation ELISA were classified as chronic HIV infections as previously described8. Information and procedures regarding HIV diagnosis and monitoring have been previously described8.
Determination of absolute CD4 cell counts, haematological and clinical chemistry parameters were performed on freshly collected blood samples using a Sysmex Partec ®Cyflow Counter II flow cytometer (Sysmex Partec GmbH, Gorlitz), BC-5800 ®Auto Hematology Analyzer (Mindray Bio Electronics, Shenzhen) and Roche cobas® c111 Blood Chemistry Analyzer (Roche Diagnostics, Indianapolis) respectively according to manufacturers’ instructions. Each sample was analyzed to determine absolute counts of CD4 T cells, neutrophils, monocytes, lymphocytes, basophils and eosinophils. Levels of creatinine and urea as markers of renal function were also determined. Peripheral blood mononuclear cells (PBMCs) were isolated from the cellular fraction of 5ml of blood by Ficoll density gradients and stored in liquid nitrogen until analyzed.
2.4. Serological assays
HIV-1 infection status of each patient was determined by testing aliquots of their plasma sample with a 4th generation Genscreen™ ULTRA HIV-Ag-Ab ELISA kit (Biorad, Hercules, California) followed by testing with a 3rd generation AiD™ anti-HIV 1+2 ELISA kit (Wantai, Beijing, China). All the assays were performed under strict biosafety conditions according to the manufacturer’s recommendation. Quantitation of serum Human Interleukin 17 (IL-17) and Human Tumour necrosis factor alpha (TNF-α) was determined by using a commercial ELISA kit (Elabscience, USA) according to the manufacturer’s instruction.
2.5. Viral load testing
Plasma HIV-1 viral load (copies/ml) was determined using the COBAS® Ampliprep/COBAS TaqMan96®HIV-1 Test, v2:0 (Roche Molecular Diagnostics, Branchburg, NJ, USA) according to manufacturer’s instruction or by an in house real-time PCR protocol
2.6. Immunophenotyping
Plasma and cellular fractions of each blood sample were separated by centrifugation, and peripheral blood mononuclear cells (PBMCs) were isolated from the cellular portion by gradient centrifugation and stored in liquid nitrogen at 5million PBMCs/ml in new bovine serum containing 10% DMSO. The PBMCs were thawed and washed in RPMI medium (Invitrogen, Carlsbad, CA, USA) containing 10% new born bovine serum, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 2mM L-glutamine and 10mM Hepes buffer on days of assay. Frozen PBMCs were thawed and left on bench for 2 h before the phenotypic analysis. The following panels of antibodies were used: anti-CD3 FITC, anti-CD4 PE and anti-CD19 PE from Sysmex Partec; anti-CD3 PerCP and anti- CD27 PE/Cy5 from Beckman Coulter; anti-ɣɖ PE, anti-CD16 PE/Cy5, anti-CD8 APC, anti-αβ FITC, anti-HLA-DR and DRAQ 5™ viability dye from Biolegend. Previously isolated and stored PBMCs were washed twice in phosphate buffered saline (PBS) with 2% serum and then 100μl of cocktail antibodies was added to 2 × 106 cells and incubated for 15mins. Thereafter the cells were stained with diluted DRAQ 5™ and allowed to incubate for maximum of 15 minutes before acquisition. At least 100,000 live lymphocytes were acquired on a four colour flow cytometer (Partec Cube 6, Sysmex Partec, Germany).
Absolute QC Count Check Beads green (Partec, GMBH, Munster, Germany) particles with standard settings were used for quality control. Phenotypes and fluorescence intensity measurements was consistent in all experiments. Live cells stained with single dye were used for compensation while fluorescence minus one panels (FMO) were part of the gating strategy. As shown in supplementary figure 1, Unconventional T cells such as gamma delta were gated as CD3+ ɣɖ, gdCD8 as CD3+ ɣɖ +CD8, gdCD16 as CD3+ ɣɖ +CD16; double negative T cells (DNT cells) as αβ-CD8/CD4. While activated CD4 and CD8 T cells were gated as CD3+CD4+HLA-DR and CD3+CD8+HLA-DR respectively, B cells, naïve and memory were gated as CD19+CD27- and CD19+CD27+ respectively. Forward angle and side scatter light gating were used to exclude cell debris from the analysis. The final analysis was performed using the Flowjo software (version 10; FlowJo, LLC, Ashland OR, USA).
2.7. Statistical analysis
Within and across groups’ analysis of quantitative variables were done using ANOVA test and Tukey’s multiple comparison test. Chi-square tests were used for non-parametric analysis. Pearson’s r correlation was used to find linear relationships between variables. P-values were considered significant at 95% confidence limit (p<0.05) unless otherwise stated. Data were analyzed and compared using Graph Pad Prism version 7.00 for Windows, Graph Pad Software, La Jolla California USA.
3. Results
3.1. Baseline characteristics of the study participants
Twenty seven HIV-1-negative (HCC), 17 Early HIV infected (EHIV), 23 chronically HIV infected ART naïve (CHIV) and 41 Chronic HIV infected cART exposed individuals were enrolled for this study (Table 1). Information on participants’ age, gender, absolute CD4 counts, plasma HIV-1 RNA levels, CD4/CD8 ratio, neutrophil counts as well as monocyte/lymphocyte ratio are presented in Table 1. CHIV had the lowest absolute CD4 counts (p<0.0001) while LVL had lower absolute neutrophil counts (P<0.01) and monocyte/lymphocyte ratio (P<0.01) compared to CHIV. There were no significant differences in parameters measured between EHIV and HCC (Table 2).
Table 1:
Baseline characteristics of study participants.
| Healthy Controls (HCC) | Early HIV (EHIV) | Chronic HIV ART naïve (CHIV) | |||
|---|---|---|---|---|---|
| Persons with low viral load (LVL) | Persons with high viral load (HVL) | ||||
| (n=27) | (n=17) | (n=23) | (n=23) | (n=18) | |
| Gender(F/M) | 9/18 | 2/15 | 13/10 | 15/8 | 6/12 |
| Mean Age (years) | 27(21–41) | 35(27–48) | 43(23–69) | 47(34–69) | 47(34–69) |
| Mean CD4(cells/l) | 819(494–1575) | 662(401–881) | 206(68–476) | 631(175–853) | 283(172–370) |
| Mean Plasma VL(cp/ml) | N/A | 5,640,000(5,000,000–6,350,000) | 207,000(53,300–1,470,000) | TND | 8,620(66.8–86,300) |
| CD4/CD8 ratio | 5.54(4.80–10.8) | 5.7(1.92–9.91) | 3.09(1.72–6.12) | 10.7(3.63–18.0) | 0.896(0.114–3.90) |
| Neutrophil (x109) | 1.585(1.350–2.185) | 2.450(1.923–3.268) | 1.910(1.700–3.380) | 1.660(1.365–2.510) | 2.340(1.970–2.775) |
| Monocyte/Lymphocyte ratio | 0.106(0.0739–0.142) | 0.159(0.102–0.182) | 0.209(0.133–0.309) | 0.129(0.0990–0.164) | 0.168(0.135–0.218) |
Values shown for gender, age, CD4+ T cells, HIV RNA copies/ml, CD4/CD8 ratio, neutrophils, monocyte/lymphocyte ratio are medians and interquartile ranges. Statistical tests used: ANOVA and Tukey’s multiple comparison tests was used for age, CD4+ T cells, HIV RNA copies/ml, CD4/CD8 ratio, neutrophils, monocyte/lymphocyte ratio. N/A—Not applicable. ns – Not significant.
Table 2.
P‐Value of baseline characteristics of study participants.
| EHIV vs CHIV | EHIV vs LVL | EHIV vs HVL | EHIV vs HCC | |
|---|---|---|---|---|
| Gender (F/M | ns | ns | ns | ns |
| Mean Age (years) | 0.0001 | 0.0001 | 0.0001 | ns |
| Mean CD4(cells/l) | 0.0001 | ns | 0.0001 | ns |
| Mean Plasma VL (cp/ml) | 0.0001 | N/A | 0.0001 | N/A |
| CD4/CD8 ratio | ns | ns | ns | ns |
| Neutrophils | ns | ns | ns | ns |
| Monocyte/Lymphocyte ratio | 0.01 | ns | ns | ns |
Abbreviations: N/A, not applicable; ns, not significant.
3.2. Early HIV infection and renal dysfunctions
The levels of serum urea and creatinine were significantly higher among early HIV-1 infected individuals compared to ART naïve persons at the chronic stage of infection and healthy controls (Fig 1A and B). Also, ART naive persons at the chronic stage of infection had significantly lower levels of serum creatinine and urea compared to those on treatment. The hypothesis inferred from the results was that pro inflammatory cytokines – IL-17 as well as TNF-alpha associated with immune regulation of kidney functions15,23 were impacted by high urea and creatinine levels associated with HIV infection. To investigate this, levels of these cytokines in a subset of the samples were determined. It was observed that the levels of IL-17 and TNF-alpha were significantly lower in early HIV infected groups compared to the other groups. However, TNF-alpha was higher in EHIV group compared to the health controls (Fig. 1C and D).As shown in Fig. 2 (B) and (C), the level of serum IL-17 significantly correlated inversely with absolute CD4 T cell counts and directly with HIV-1 RNA viral load. Similarly, the level of serum urea correlated directly with HIV-1 RNA viral load but inversely with absolute CD4 T cell counts (Fig. 2D and E).
Fig. 1. Early HIV infection is associated with renal dysfunctions.
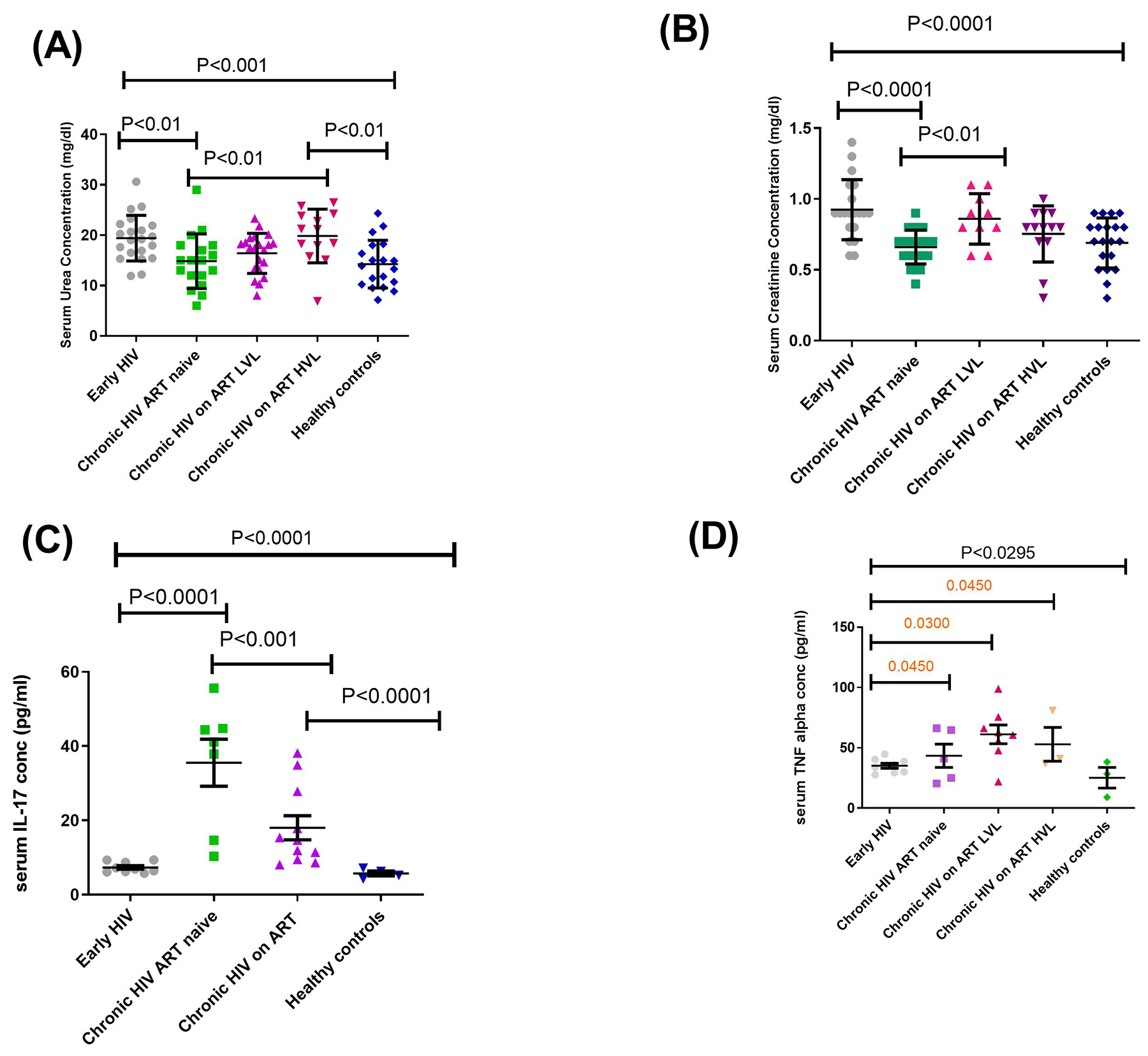
Levels of (A) serum urea concentration (mg/dl), (B) serum creatinine concentration (mg/dl), (C) serum IL-17 concentration (pg/ml), (D) serum TNF alpha (pg/ml). Each symbol represents an individual. Early HIV: persons at early stages of HIV infection (HIV Ab negative; HIV Ag positive, HIV DNA positive), Chronic HIV ART naïve: persons at chronic stages of HIV infection not on ART (HIV Ab positive; HIV Ag positive; HIV DNA positive), Chronic HIV on ART LVL: persons on ART for over 6months with undetectable viral load, Chronic HIV on ART HVL: persons on ART for over 6months with detectable viral load. Horizontal lines and errors bars represent standard error of the mean (SEM), 25th and 75th percentiles. Statistical test: ANOVA and Tukey’s multiple comparison tests.
Fig. 2. In the HIV-infected cohort, levels of serum IL-17 and urea are directly and inversely correlated with HIV-1 log RNA viral load copies and CD4 T cells proportions.
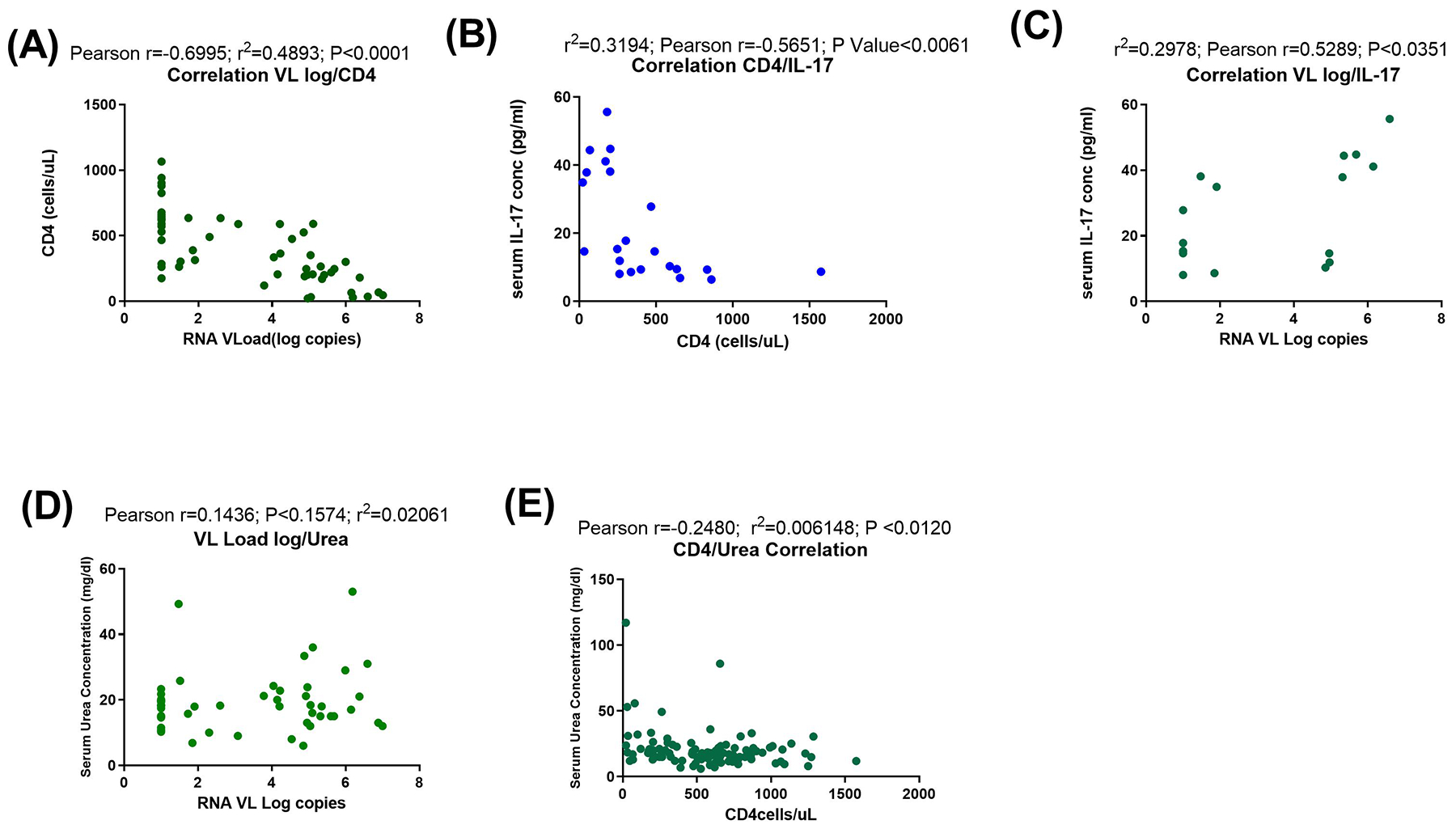
Correlation analyses between (A) HIV-1 log RNA viral load copies and CD4 T cells, (B) CD4 T cells and serum IL-17 concentration (pg/ml), (C) HIV-1 log RNA viral load copies and serum IL-17 (pg/ml), (D) HIV-1 log RNA viral load copies and serum urea level concentration (mg/dl) and (E) CD4 T cells and serum urea level concentration (mg/dl). Blue and green symbols represent study participants. Statistical test: Pearson correlation.
3.3. Proportions of γδ T cells and subsets, DNT cells as well as neutrophils are reduced during early HIV infection.
γδ T cells especially those expressing CD16 and CD8 have been shown to exhibit cell lytic as well as cytokine secretion functions13,24–26. It was observed in this study that γδ T cells and subsets were significantly reduced during EHIV compared to CHIV. DNT cells proportions were also reduced compared to CHIV although the difference was not significant. CHIV group had significantly higher levels of γδ, γδ+CD8 and γδ+CD16 proportions compared to the treatment groups. This was also similar to what was observed for DNT cells except that the differences did not reach significant levels among the groups for this cell type (Fig. 3).
Fig. 3. Proportions of gamma delta T cells (ɣɖT cells) and subsets, double-negative T cells (DNT cells) as well as neutrophils are reduced during early HIV infection.
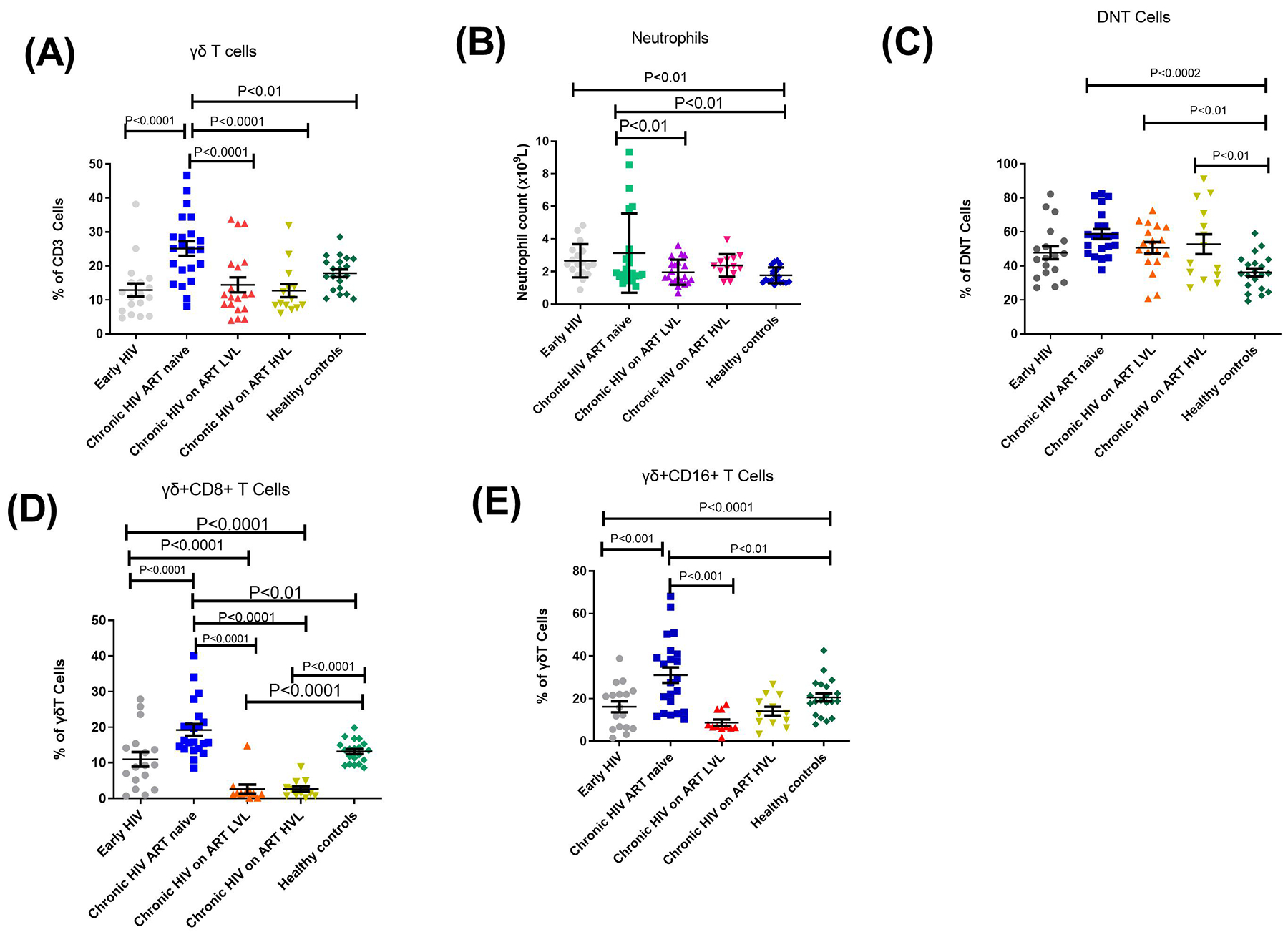
Proportions of (A) gamma delta T cells (as % of CD3 cells, (B) Neutrophil counts (x109), (C) double negative T cells (DNT cells), (D) gamma delta + CD8 T cells, (E) gamma delta + CD16 T cells as % of gamma delta T cells . Each symbol represents an individual. Early HIV: persons at early stages of HIV infection (HIV Ab negative; HIV Ag positive, HIV DNA positive), Chronic HIV ART naïve: persons at chronic stages of HIV infection not on ART (HIV Ab positive; HIV Ag positive; HIV DNA positive), Chronic HIV on ART LVL: persons on ART for over 6months with undetectable viral load, Chronic HIV on ART HVL: persons on ART for over 6months with detectable viral load. Horizontal lines and errors bars represent standard error of the mean (SEM), 25th and 75th percentiles. Statistical test: ANOVA and Tukey’s multiple comparison tests.
Studies have previously described the activating roles of neutrophils towards γδ and DNT cells27. As shown in Fig. 3, absolute neutrophil counts were lower in early HIV infected individuals compared to CHIV group, although this difference was not significant. Neutrophil counts among EHIV group were significantly higher than healthy controls. CHIV group had significantly higher neutrophil counts compared to the two treatment groups: LVL and HVL. γδ T cells significantly correlated directly with neutrophil counts levels and inversely with creatinine and urea levels in cART naïve persons, although the relationship between γδ T cells and urea was not significant (see Fig. 4).
Fig. 4. ɣɖ T cells and subsets correlate with neutrophil counts and serum IL-17 concentration.
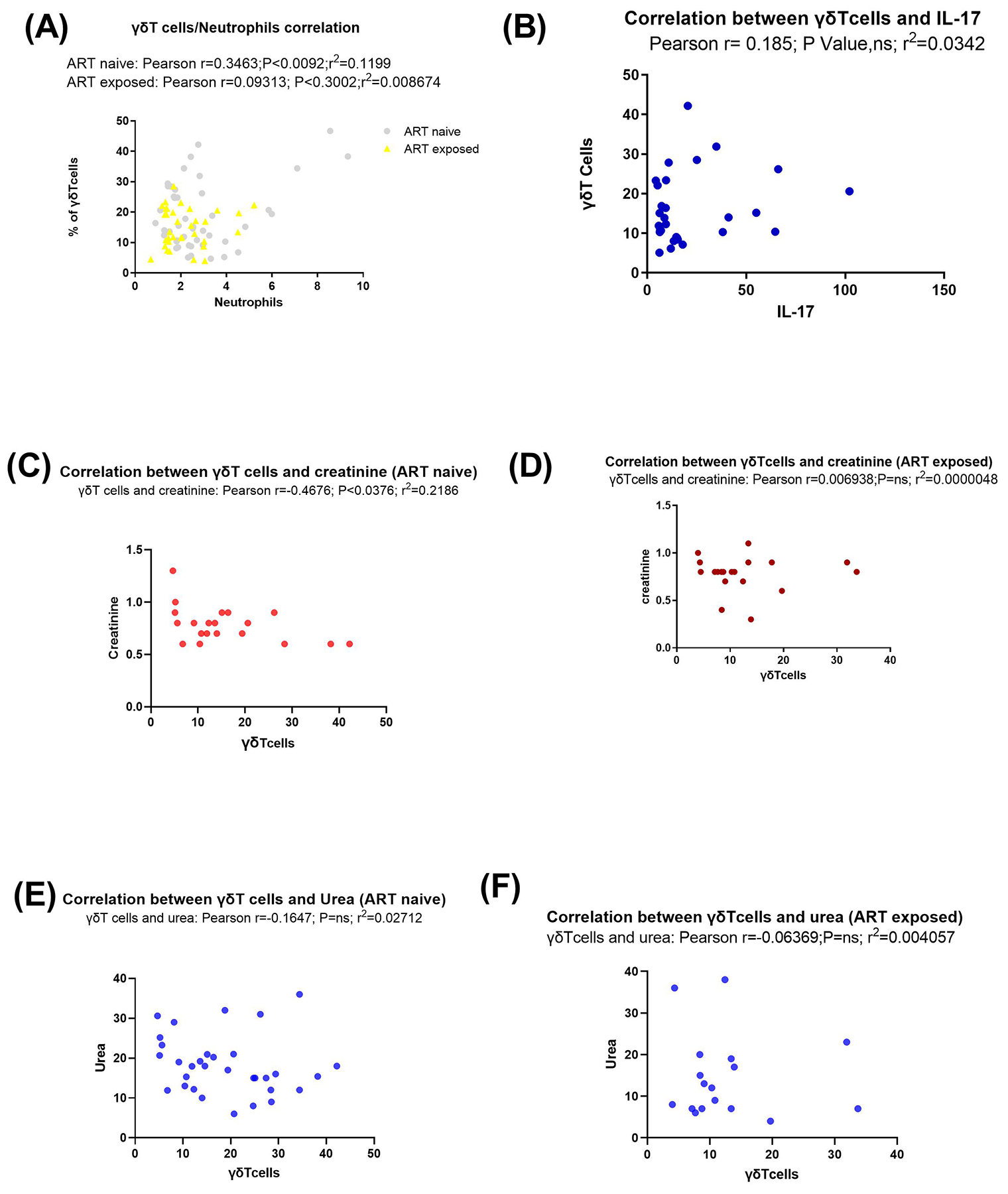
Correlation analyses between (A) gamma delta T cells and neutrophil counts (x109), grey symbols represent ART naïve individuals while yellow symbols represent ART exposed individuals (B) gamma delta T cells and serum IL-17 concentration (pg/ml), (C) gamma delta T cells and creatinine among ART naïve individuals, (D) gamma delta T cells and creatinine among ART exposed, (E) gamma delta T cells and urea among ART naïve individuals and (F) gamma delta T cells and urea among ART exposed individuals. Blue symbols represent urea levels while red symbols represent creatinine levels among study participants. Statistical test: Pearson correlation.
3.4. Immune activation is associated with chronic HIV-1 infection
HLA-DR markers on T cells have been previously described as a marker of primary immune activation28. CD8+HLA-DR proportions were higher among EHIV group compared to the groups on treatment. However, CD8+HLA-DR proportions were reduced in the EHIV group compared to the CHIV group although the difference was not significant. This was different to what was observed for the proportions of CD8T cells which was higher in EHIV compared to CHIV. HVL group had a significantly higher CD8 proportion compared to EHIV (Fig. 5). We hypothesized that the early HIV infection stage investigated in this study preceded the period in which immune activation has reached the peak. To verify this assumption, we analyzed the proportions of CD8, CD4+HLA-DR and CD8+HLA-DR in a subset of EHIV groups (n=5) after six months of infection.
Fig. 5. Immune activation is associated with chronic HIV-1 infection.
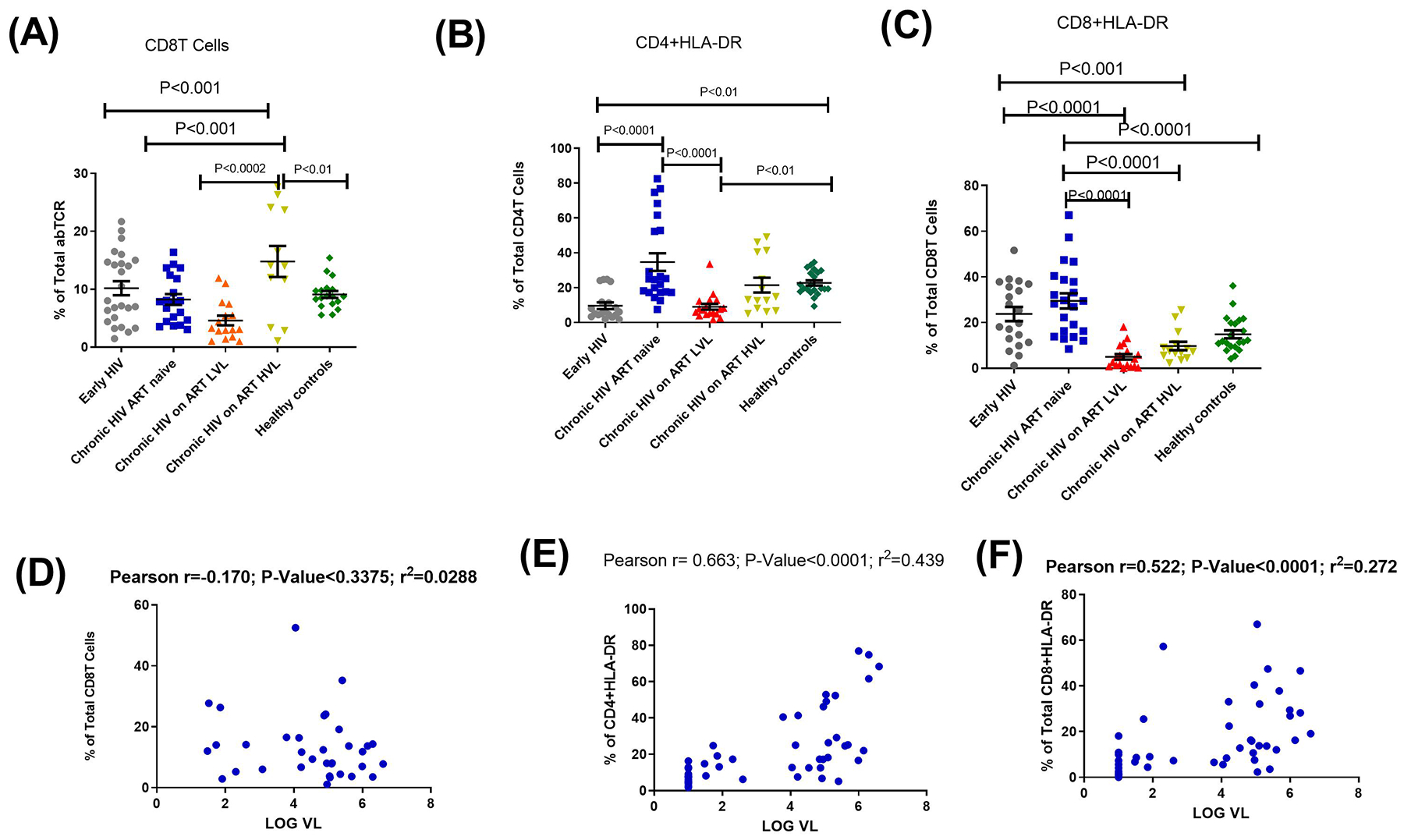
Proportions of (A) CD8 T cells as percentage of abTCR, (B) CD4+HLA-DR T cells as percentage of total CD4 (C) CD8 + HLA-DR T cells as percentages of total CD8 T cells. Each symbol represents an individual. Early HIV: persons at early stages of HIV infection (HIV Ab negative; HIV Ag positive, HIV Gag DNA positive), Chronic HIV ART naïve: persons at chronic stages of HIV infection not on ART (HIV Ab positive; HIV Ag positive; HIV Gag DNA positive), Chronic HIV on ART LVL: persons on ART for over 6months with undetectable viral load, Chronic HIV on ART HVL: persons on ART for over 6months with detectable viral load. Horizontal lines and errors bars represent standard error of the mean (SEM), 25th and 75th percentiles. Statistical test: ANOVA and Tukey’s multiple comparison tests. Correlation analyses between (D) CD8 T cells (E) CD4+HLA-DR T cells (F) CD8 + HLA-DR T cells and Log HIV-1 RNA viral load copies. Blue symbols represent study participants. Statistical test: Pearson correlation.
As shown in Fig.6, we observed that levels of CD8 T cells did not significantly improve among these individuals while CD4+HLA-DR and CD8+HLA-DR proportions increased within the six months period. This difference reached significant level for CD4+HLA-DR. Correlation analysis of DNT and γδ T cells with markers of immune activation showed that cART impacts on immune-regulatory functions of these innate immune cells (see supplementary figure 2). Without cART, DNT and γδ T cells significantly correlated directly with CD4+HLA-DR proportions but reverse was the case among persons under treatment.
Fig. 6. Immune activation is ongoing after six months of HIV infection among early infected individuals.
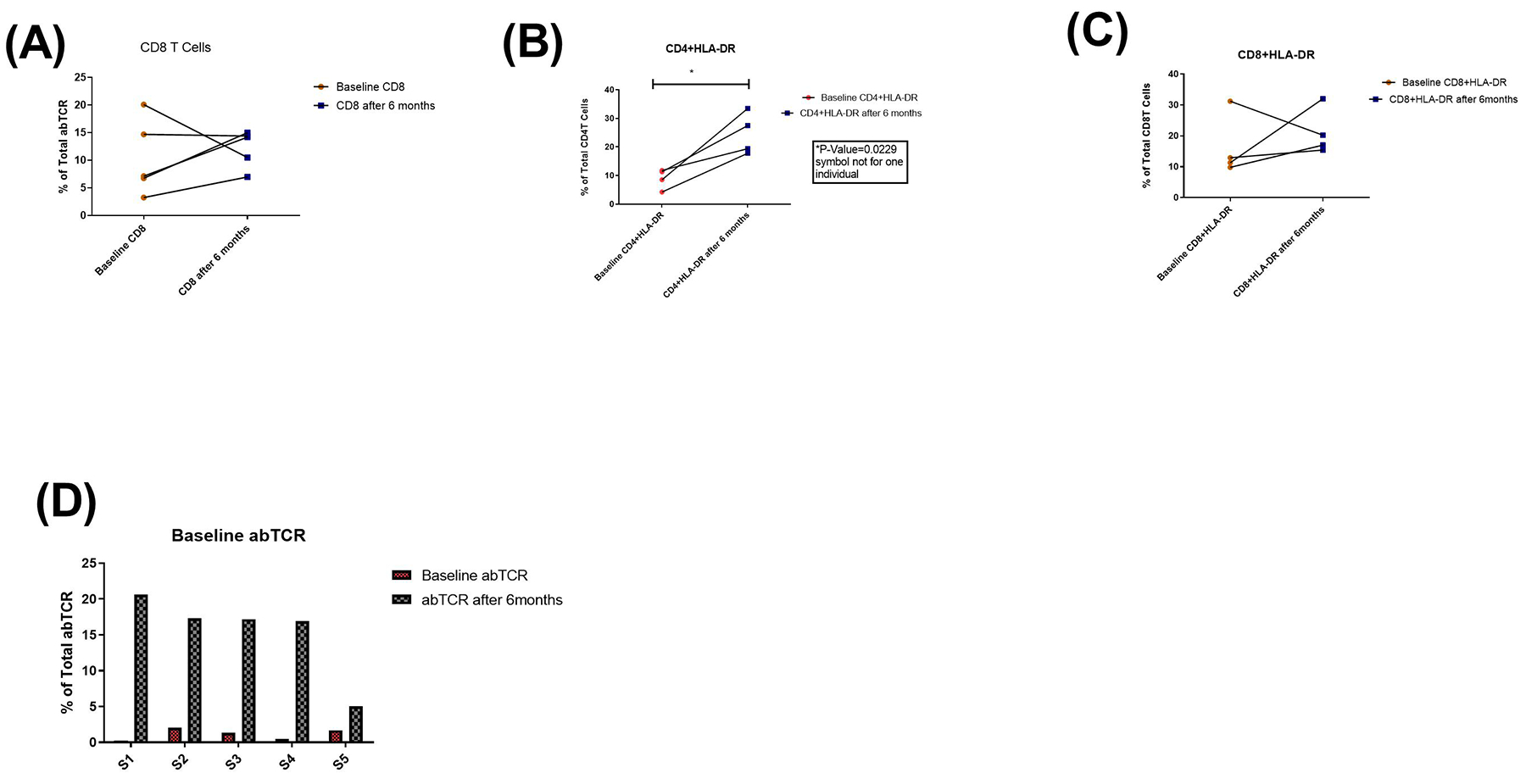
Five Individuals longitudinal follow-up in untreated patients with early HIV infection. Immune cell activation was assessed in untreated patients by measuring the frequency of (A) CD8 T cells (B) CD4+HLA-DR (C) CD8+HLA-DR and (D) alpha beta T cell receptors at baseline and six months after.
Recently, it was shown that γδ T cells influence B cells development as well as affect the production of auto and natural antibodies29,30. Although EHIV had the lowest proportion of total B cells, this difference was not significant. They, however, had significantly higher levels of naïve (P<0.01) and memory B cells (P<0.0001) compared to CHIV group. However, mean proportions of naïve and memory B cells of healthy controls were slightly higher than EHIV group, although the differences were not significant (Fig. 7 A–C). Individuals on ART (HVL and LVL) had significantly higher proportions of naïve and memory B cells compared to the other groups studied (Fig. 7A–C). Correlation analysis also revealed that γδ T cells significantly correlated directly with total and memory B cells (Fig. 7D and E). This data therefore suggest that inhibition of γδ T cells during early HIV infection due to renal dysfunctions may have impact on their downstream functions especially those related to innate immunity like antibody-dependent cell-mediated cytotoxicity24 as well as generation of natural antibodies29.
Fig. 7. ɣɖ T cells correlate directly with B and memory B cells.
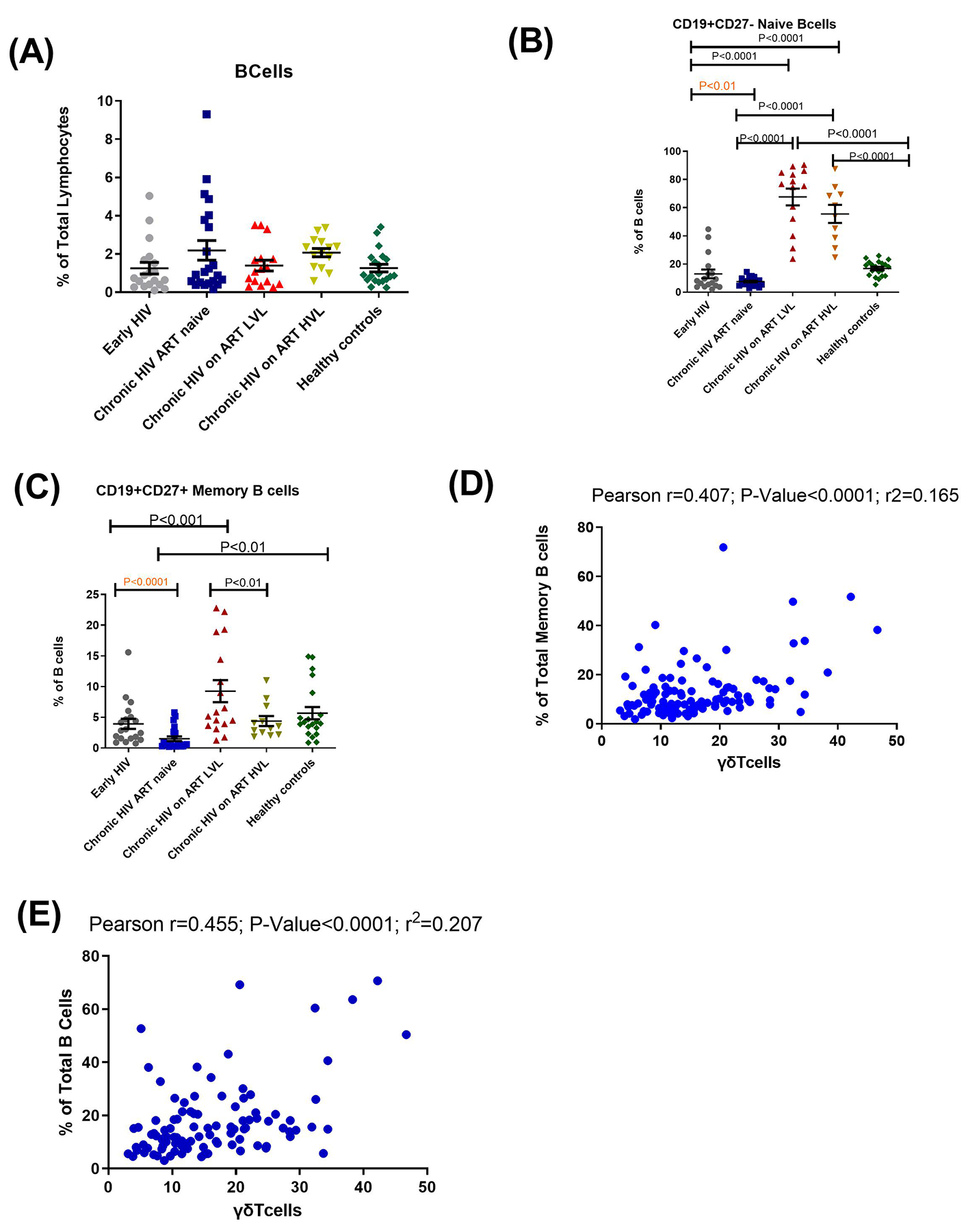
Proportions of (A) B cells, (B) naïve B cells and (C) memory B cells. Each symbol represents an individual. Early HIV: persons at early stages of HIV infection (HIV Ab negative; HIV Ag positive, HIV Gag DNA positive), Chronic HIV ART naïve: persons at chronic stages of HIV infection not on ART (HIV Ab positive; HIV Ag positive; HIV Gag DNA positive), Chronic HIV on ART LVL: persons on ART for over 6months with undetectable viral load, Chronic HIV on ART HVL: persons on ART for over 6months with detectable viral load. Horizontal lines and errors bars represent standard error of the mean (SEM), 25th and 75th percentiles. Statistical test: ANOVA and Tukey’s multiple comparison tests. Correlation analyses between (D) proportions of memory B cells and (E) Total B cells and gamma delta T cells. Blue symbols represent study participants. Statistical test: Pearson correlation.
4. Discussion
Results of this study suggest that there is reduction of γδ T cells and their subsets during early HIV infection. This reduction is associated with high levels of creatinine and urea despite the fact that this group of infected people were not on ART. Subsets of these cells that are involved in cytotoxic functions seem to be particularly affected. It is possible that systemic immune activation peaked after a reasonable compartment of γδ and DNT cells have been depleted which may occur during seeding of HIV virions into renal reservoirs as previously described31,32. In most sub Saharan African countries, treatment initiation for HIV infected individuals’ starts in the chronic stage of infection despite the recently introduced “test and treat” policy33, reason being that most HIV infected individuals are detected at this period8,33. Early HIV immunotherapy may be useful in preventing the depletion of γδ and DNT T cells proportions very early in infection before systemic immune activation. This will in turn control renal dysfunctions and further aid immune reconstitution of CD4 bearing T cells after prolonged cART11.
Several reports have shown HIV associated nephropathies during early HIV infection, especially before treatment initiation34–36. However, there is conflicting information as to whether the prevalence of this condition among early HIV infected individuals is high or low. Findings from this study have shown that renal dysfunction may be more prevalent among early HIV infected treatment naïve Africans than previously recognized. This may be due to an increased risk of “HIV associated nephropathy susceptible APOL1 variants” among Africans37,38. High replicative and transmitting capacities of non-subtype B HIV virions in infecting renal tubules and lymphocytes may also be a factor for this disorder34,39.
A subset of γδ T cells have been shown to serve as reservoir for HIV infection40. Finding in this study that IL-17 levels were higher among CHIV persons compared to EHIV is similar to reports from previous studies41,42. IL-17 production has previously been largely attributed to γδ, DNT and neutrophils. This IL-17/neutrophil/ γδ T cell production axis has been shown to be critical to HIV-1 disease progression14,41,42. Data from this study showed that high HIV-1 RNA viral load as well as urea levels may impact negatively on the IL-17/neutrophil/ γδ T cells axis thereby limiting their activation during the early stages of infection. Other infections with similar effect on IL-17/neutrophil/ γδ T cell axis have been previously described18,41.
The evidence provided from this study showed that the proportions of neutrophils and γδ T cell subsets were significantly reduced during early HIV infection. This is also similar to previous reports that investigated these parameters in HIV infected individuals11,12,14,41. However, the results of this study suggest that this inhibition may be related to the low immune activation state impacted by high creatinine and urea levels, more so that these immune cells significantly correlated positively with markers of immune activation in the absence of antiretroviral therapy.
Chronic immune activation, which is a hallmark of HIV infection43, has been shown recently to be significantly over expressed at the first month of infection after which it undergoes a prompt decrease before another rapid increase towards seroconversion12,44. The initial over expression may coincide with the period of high viral replication and seeding into various cellular reservoirs45. Depleted γδ and DNT cells have been shown not to fully recover even after prolonged cART46–48. This depletion, early in the infection may be a likely predisposing factor for end stage renal disease and other co-morbidities commonly observed among HIV infected Africans and African-Americans despite prolonged cART use44,49. Higher levels of γδ T and its subsets found during chronic HIV infection has been attributed to immune activation of IL-17 producing γδ T cells fractions14.
γδ T cells have been shown to regulate humoral responses including auto antibodies, modulate size and production of pre-immune peripheral B cells as well as control levels of circulating immunoglobulins16,29,30,50. It has been previously shown that memory B cells defects during HIV infection may be due the impact of the virus on T cells that provide B cells help in germinal centers43,51–53. As observed in previous studies, treated HVL and LVL groups had significantly higher proportions of the naïve and memory B cells compared to other groups54,55. Also, as observed in our study, treated LVL groups had higher proportions of naïve and memory B cells compared to HVL. This supports previous studies which suggest that high viremia impacts B cell reconstitution after ART exposure43,54,56. We also observed that memory B cells defined by (CD19+CD27) were increased during early HIV compared to the chronic stage before commencement of ART. Previous studies have associated this finding to the observation that HIV infected persons have preserved memory B cell functions after ART exposure56,57. It seems that reconstitution of memory B cells can be attributed to control of HIV through the use of ART.
Accordingly, this study has shown that the lack of γδ T cells immune reconstitution to healthy levels despite cART may play a major role in B cells defects observed during HIV disease progression. This abnormally may have started from the early stages of infection. A limitation of this study was our inability to separate the expansion of Vδ1+ subsets from the concomitant depletion of Vδ2+ during HIV infection. These subsets have been shown to respond differently at different stages of HIV infection14. It would be interesting to determine which of these subsets are particularly impacted during early HIV infection especially among Africans.
In conclusion, our data has provided insights into the reduction of γδ T cells during early stages of HIV infection. This event is associated with high levels of urea and creatinine and occurs before systemic immune activation reaches set points. The kidney may possibly serve as a reservoir of HIV as this underlines the direct impact of the virus on this organ. Our findings suggest that depletion of these T cells early in HIV infection has grave implications on disease progression even after prolonged ART use. Early HIV infection diagnosis and treatment is hereby recommended.
Supplementary Material
S1 Fig. Representative flow-cytometry plots showing the gating strategy for identifying various subsets of ɣɖ T cells.
S2 Fig. Representative flow-cytometry plots showing the gating strategy for identifying double negative T (DNT) cells.
S3 Fig. Representative flow-cytometry plots showing the gating strategy for identifying CD4 and CD8 T cells.
S4 Fig. Representative flow-cytometry plots showing the gating strategy for identifying CD4+HLA-DR and CD8+HLA-DR.
S5 Fig. Correlations of double negative (DN) T cells, ɣɖ T cells, CD4+HLA-DR and CD8+HLA-DR among ART exposed as well as unexposed individuals. Healthy control (n=27), Early HIV (n=17), Chronic HIV (n=23), Chronic HIV on ART with low viral load (n=23) and Chronic HIV on ART with high viral load (n=18) individuals were recruited for this study Fresh blood of patients were collected; peripheral blood mononuclear cells were isolated and stored in liquid nitrogen. Flow cytometry was performed as described in methods section. Correlations of DNT cells, CD4+HLA-DR and CD8+HLA-DR among (A) ART unexposed; (B) ART exposed as well as correlations of ɣɖ T cells, CD4+HLA-DR and CD8+HLA-DR among (C) ART unexposed and (D) ART exposed were compared among the groups. Pearson correlation test was used for statistical analysis.
Acknowledgments
We thank all individuals who participated in this study. We are also grateful to Dr. Daniel Muema (AHRI, KwaZulu-Natal, South Africa) and Dr. Adedayo Faneye (Department of Virology, UI, Nigeria) for the discussions. Special appreciation to Dr. Eunice Nduati (KEMRI-Welcome Trust, Kilifi, Kenya) for providing some of the antibodies used for flow cytometry. We thank Mrs. Fadimu and Mrs. Adeyefa (both of Blood bank, University College Hospital, Ibadan, Nigeria) for linking us up with volunteer blood donors. Also, we are grateful to Mr. Samuel Olawole and Miss. Bisola Aleru for assistance during laboratory analyses. License for the Flowjo software was donated by Treestar Corp (Flowjo Africa Scheme).
a. Funding
This study was supported by Medical Education Partnership Initiative Nigeria (MEPIN) mentored research award through National Institute of Health (NIH) USA grant funded by Fogarty International Centre, the office of AIDS Research and National Human Genome Research Institute of NIH, the Health Resources and Services Administration (HRSA) and the Office of the U.S. Global AIDS Coordinator under award number R24TW008878 to BO. BO is also a recipient of the HIV Research Trust, UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- γδ
gamma-delta
- DN
Double negative
- CD
Cluster of differentiation
- ELISA
Enzyme linked immunosorbent assay
- PBMCs
Peripheral blood mononuclear cells
Footnotes
Ethics Statement
This study was carried out in accordance with the recommendations of the University of Ibadan/ University College Hospital (UI/ UCH) Biomedical Research and Ethics Committee (UI/EC/15/0076) and the Oyo State Ministry of Health Committee on Human Research (AD13/479/951). The protocol was approved by these committees. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The results were delinked from all patient identifiers.
Conflict of Interest Statement
None declared
7. References
- 1.Gupta SK, Kitch D, Tierney C, Melbourne K, Ha B, Mccomsey GA, et al. Markers of renal disease and function are associated with systemic inflammation in HIV infection *. HIV Med. 2015;16:591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waheed S, Atta MG. Predictors of HIV-associated nephropathy. Informahealthcare. 2014;(5):555–63. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg AZ, Naicker S, Winkler CA, Kopp JB. HIV-associated nephropathies : epidemiology , pathology , mechanisms and treatment. Nat Publ Gr [Internet]. 2015;11(3):150–60. Available from: 10.1038/nrneph.2015.9 [DOI] [PubMed] [Google Scholar]
- 4.Mikulak J, Singhal PC. HIV-1 and Kidney Cells : Better Understanding of Viral Interaction. Nephron Exp Nephrol. 2010;11021:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Laroche M, Desbuissons G, Rouvier P, Barin F, Deray G, Caumes E, et al. APOL1 variants may induce HIV-associated nephropathy during HIV primary infection. J Antimicrob Chemother. 2017;72(5):1539–41. [DOI] [PubMed] [Google Scholar]
- 6.Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa : a systematic review and meta-analysis. Lancet Glob Heal [Internet]. 2014;2(3):e174–81. Available from: 10.1016/S2214-109X(14)70002-6 [DOI] [PubMed] [Google Scholar]
- 7.Betjes MGH. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Publ Gr [Internet]. 2013;1–11. Available from: 10.1038/nrneph.2013.44 [DOI] [PubMed] [Google Scholar]
- 8.Olusola BA, Olaleye DO, Odaibo GN. Early HIV infection among persons referred for malaria parasite testing in Nigeria. Arch Virol. 2017;163(2):439–45. [DOI] [PubMed] [Google Scholar]
- 9.Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, et al. Isolation and characterization of human antigen-specific TCRalpha beta CD4-CD8-double-negative regulatory T cells. Blood. 2017;105(7):2828–36. [DOI] [PubMed] [Google Scholar]
- 10.Pauza CD, Poonia B, Li H, Cairo C, Chaudhry S. γδ T cells in HIV disease : past , present , and future. Front Immunol. 2015;5(January):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiaofan L, Bin S, Huan X, Xin Z, Zhiying L, Yunxia J, et al. Low Double-Negative CD3+CD4−CD8− T Cells Are Associated with Incomplete Restoration of CD4+ T Cells and Higher Immune Activation in HIV-1 Immunological Non-Responders. Front Immunol. 2016;7(December):13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatnagar N, Girard P-M, Lopez-Gonzalez M, Didier C, Collias L, Corinne J, et al. Potential Role of V δ 2 + γδ T Cells in Regulation of Immune Activation in Primary HIV Infection. Front Immunol. 2017;8(September):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyan S, Kabelitz D. When neutrophils meet T cells : Beginnings of a tumultuous relationship with underappreciated potential. Eur J immunol. 2014;44:627–33. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Li W, Li N, Jiao Y, Chen D, Cui L, et al. Gamma delta T Cells Are Involved in Acute HIV Infection and Associated with AIDS Progression. PLoS One. 2014;9(9):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, et al. IL-17 produced by neutrophils regulates IFN- γ – mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120(1):331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters C, Kabelitz D, Wesch D. Regulatory functions of γδ T cells. Cell Mol Life Sci [Internet]. 2018. June 8 [cited 2018 Nov 6];75(12):2125–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29520421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawand M, Julie D-M, Marie-Caroline D-N. Key Features of Gamma-Delta T-Cell Subsets in Human Diseases and Their immunotherapeutic implications. Front Immunol. 2017;8(June):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juno JA, Waruk JLM, Harris A, Mesa C, Lopez C, Bueti J, et al. Gamma delta T-cell function is inhibited in end stage renal disease and impacted by latent tuberculosis infection. Kidney Int [Internet]. 2017;92(4):1003–14. Available from: 10.1016/j.kint.2017.03.036 [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Niu C, Cui J. Gamma-delta (γδ) T cells: friend or foe in cancer development? J Transl Med [Internet]. 2018. [cited 2018 Sep 7];16:3 Available from: 10.1186/s12967-017-1378-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, Zhang D, Zhen W, Shi Q, Liu Y, Ling N, et al. Characteristics of Circulating T Cell Receptor gdT Cells from Individuals Chronically Infected with Hepatitis B Virus (HBV): An Association between Vd2 Subtype and Chronic HBV Infection. JID [Internet]. 2008. [cited 2018 Sep 9];198(1):1643–50. Available from: https://academic.oup.com/jid/article-abstract/198/11/1643/961244 [DOI] [PubMed] [Google Scholar]
- 21.Pilcher CD, Fiscus S a, Nguyen TQ, Foust E, Wolf L, Williams D, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005;352(18):1873–83. [DOI] [PubMed] [Google Scholar]
- 22.Nasrullah M, Wesolowski LG, Ethridge SF, Cranston K, Pentella M, Myers RA, et al. Acute infections, cost and time to reporting of HIV test results in three U.S. State Public Health Laboratories. J Infect [Internet]. 2016; Available from: 10.1016/j.jinf.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papotto PH, Ribot JC, Silva-santos B. IL-17 + γδ T cells as kick-starters of inflammation. Nat Rev Immunol. 2017;18(6):604–11. [DOI] [PubMed] [Google Scholar]
- 24.Braakman E, van de Winkel JGJ, van Krimpen BA, Jansze M, Bolhuis RLH. CD16 on human γδ T lymphocytes: Expression, function, and specificity for mouse IgG isotypes. Cell Immunol. 1992;143(1):97–107. [DOI] [PubMed] [Google Scholar]
- 25.He X, Liang H, Hong K, Li H, Peng H, Zhao Y, et al. The Potential Role of CD16 + Vγ2Vδ2 T Cell-Mediated Antibody-Dependent Cell-Mediated Cytotoxicity in Control of HIV Type 1 Disease. AIDS Res Hum Retroviruses [Internet]. 2013;29(12):1562–70. Available from: http://www.liebertpub.com/doi/10.1089/aid.2013.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neves PCC, Rudersdorf RA, Galler R, Bonaldo MC, Santana MGV de, Mudd PA, et al. CD8+ gamma-delta TCR+ and CD4+ T cells produce IFN-γ at 5–7 days after yellow fever vaccination in Indian rhesus macaques, before the induction of classical antigen-specific T cell responses. Vaccine. 2010;28(51):8183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hensley-mcbain T, Klatt NR. The Dual Role of Neutrophils in HIV Infection. Curr HIV/AIDS Rep. 2018;15(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arruvito L, Payaslián F, Baz P, Billordo A, Pandolfi J, Arribalzaga E, et al. Identification and Clinical Relevance of Naturally Occurring Human CD8 + HLA-DR + Regulatory T Cells. J Immunol. 2014;193:4469–76. [DOI] [PubMed] [Google Scholar]
- 29.Born WK, Huang Y, Reinhardt RL, Huang H, Sun D, O’Brien RL. γδ T Cells and B Cells [Internet]. 1st ed. Vol. 134, Advances in Immunology. Elsevier Inc.; 2017. 1–45 p. Available from: 10.1016/bs.ai.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 30.Rezende RM, Lanser AJ, Rubino S, Kuhn C, Skillin N, Moreira TG, et al. γδ T cells control humoral immune response by inducing T follicular helper cell differentiation. Nat Commun. 2018;9(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, et al. Renal Epithelium Is a Previously Unrecognized Site of HIV-1 Infection. J Am Soc Nephrol. 2000;11:2079–87. [DOI] [PubMed] [Google Scholar]
- 32.Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, Cara A, et al. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med. 2002;8(5):522–6. [DOI] [PubMed] [Google Scholar]
- 33.Adetunji AA, Kuti MA, Audu RA, Muyibi SA, Imhansoloeva M, Mosuro OA, et al. Discordant rapid HIV tests: lessons from a low-resource community. HIV Med [Internet]. 2018. [cited 2018 Sep 14];19(1):72–6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5807078/pdf/nihms939773.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winston Jonathan A, Bruggeman Leslie A, Ross Micheal D, Jacobson Jeffrey, Ross Leora, D’Agati Vivette D, et al. NEPHROPATHY AND ESTABLISHMENT OF A RENAL RESERVOIR OF HIV TYPE 1 DURING PRIMARY INFECTION. N Engl J Med [Internet]. 2001. [cited 2018 Sep 14];344(26). Available from: www.nejm.org [DOI] [PubMed] [Google Scholar]
- 35.Ananworanich J, Datta AA, Fletcher JL, Townamchai N, Chomchey N, Kroon E, et al. Acute tubular nephropathy in a patient with acute HIV infection: review of the literature. AIDS Res Ther [Internet]. 2014. [cited 2018 Sep 14];11(344):1–6. Available from: http://www.aidsrestherapy.com/content/11/1/34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin ML, Palella F, Shah S, Lerma E, Butter J, Kanwar YS. HIV-associated nephropathy occurring before HIV antibody seroconversion. Am J Kidney Dis [Internet]. 2001. May [cited 2018 Sep 14];37(5):e39.1–e39.5. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0272638605900036 [DOI] [PubMed] [Google Scholar]
- 37.Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-peek T, et al. APOL1 Risk Variants Are Strongly Associated with HIV-Associated Nephropathy in Black South Africans. J Am Soc Nephrol. 2015;26:2882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Laroche Marine Desbuissons Geoffroy, Phillippe Rouvier, Francis Barin, Gilbert Deray, Eric Caumes, et al. APOL1 variants may induce HIV- associated nephropathy during HIV primary infection. J Antimicrob Chemother [Internet]. 2017. [cited 2018 Sep 14];10:1–3. Available from: https://watermark.silverchair.com/dkw563.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAhcwggITBgkqhkiG9w0BBwagggIEMIICAAIBADCCAfkGCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMAIab9VYiFmnnHgzXAgEQgIIBynHwlNE4agrFa1H0vFAZFqh2-62GQ_Ln_BJmpSOEuzygE6Wf [DOI] [PubMed] [Google Scholar]
- 39.Claiborne DT, Prince JL, Scully E, Macharia G, Micci L, Lawson B, et al. Replicative fitness of transmitted HIV-1 drives acute immune activation, proviral load in memory CD4+ T cells, and disease progression. PNAS [Internet]. 2015. [cited 2018 Sep 14];112(12):1480–9. Available from: www.pnas.org/cgi/doi/10.1073/pnas.1421607112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soriano-sarabia N, Archin NM, Bateson R, Dahl NP, Crooks M, Kuruc JD, et al. Peripheral V γ 9V δ 2 T Cells Are a Novel Reservoir of Latent HIV Infection. PLoS Pathog. 2015;11(10):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z, Lu X, Hu Z, Luo Z, Jiang W, Wu H, et al. Syphilis Infection Differentially Regulates the Phenotype and Function of γδ T Cells in HIV-1-Infected Patients Depends on the HIV-1 Disease Stage. Front Immunol. 2017;8(August):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, et al. Differentiation , phenotype , and function of interleukin-17 – producing human V γ 9V δ 2 T cells. Blood. 2018;118(1):129–39. [DOI] [PubMed] [Google Scholar]
- 43.Muema DM, Macharia GN, Olusola BA, Hassan AS, Fegan GW, Berkley JA, et al. Proportions of circulating follicular helper T cells are reduced and correlate with memory B cells in HIV-infected children. PLoS One. 2017;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucia P, Victor U, Jorge C, Erica P, Laura F-S, Chenjerai J, et al. Dynamics of cD4 and cD8 T-cell subsets and inflammatory Biomarkers during early and chronic hiV infection in Mozambican adults. Front Immunol. 2018;8(January):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature [Internet]. 2014. August 20 [cited 2018 Sep 15];512(7512):74–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25042999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kosub DA, Lehrman G, Milush JM, Zhou D, Chacko E, Leone A, et al. Gamma/Delta T-cell functional responses differ after pathogenic human immunodeficiency virus and nonpathogenic simian immunodeficiency virus infections. J Virol [Internet]. 2008. February [cited 2018 Sep 15];82(3):1155–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18045946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martini F, Urso R, Gioia C, De Felici A, Narciso P, Amendola A, et al. gammadelta T-cell anergy in human immunodeficiency virus-infected persons with opportunistic infections and recovery after highly active antiretroviral therapy. Immunology [Internet]. 2000. August [cited 2018 Sep 15];100(4):481–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10929075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casetti R, De Simone G, Sacchi A, Rinaldi A, Viola D, Agrati C, et al. V γ 9V δ 2 T-Cell Polyfunctionality Is Differently Modulated in HAART-Treated HIV Patients according to CD4 T-Cell Count. PLoS One. 2015;10(7):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chevalier MF, Didier C, Girard P, Manea ME, Rinaldo CR, Keating SM, et al. CD4 T-Cell Responses in Primary HIV Infection: Interrelationship with Immune Activation and Virus Burden. Front Immunol. 2016;7(September):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Y, Getahun A, Heiser RA, Detanico TO, Aviszus K, Kirchenbaum GA, et al. Gammadelta T cells shape pre-immune peripheral B cell populations. J Immunol. 2016;196(1):217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pallikkuth S, Parmigiani A, Silva SY, George VK, Fischl M, Pahwa R, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood [Internet]. 2012. August 2 [cited 2018 Sep 15];120(5):985–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22692510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human Circulating PD-1+CXCR3−CXCR5+ Memory Tfh Cells Are Highly Functional and Correlate with Broadly Neutralizing HIV Antibody Responses. Immunity [Internet]. 2013. October 17 [cited 2018 Sep 15];39(4):758–69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24035365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen K, Altfeld M, Alter G, Stamatatos L. Early Preservation of CXCR5+ PD-1+ Helper T Cells and B Cell Activation Predict the Breadth of Neutralizing Antibody Responses in Chronic HIV-1 Infection. J Virol [Internet]. 2014. November 15 [cited 2018 Sep 15];88(22):13310–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25210168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muema DM, Macharia GN, Hassan AS, Mwaringa SM, Fegan GW, Berkley JA, et al. Control of Viremia Enables Acquisition of Resting Memory B Cells with Age and Normalization of Activated B Cell Phenotypes in HIV-Infected Children. J Immunol. 2015. August 1;195(3):1082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, et al. B cells in early and chronic HIV infection: Evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. 2010. December 16;116(25):5571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moir S, Fauci AS. B cells in HIV infection and disease. Vol. 9, Nature Reviews Immunology. 2009. p. 235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, et al. B cells in early and chronic HIV infection : evidence for preservation of immune function associated with early initiation of antiretroviral therapy. 2018;116(25):5571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Fig. Representative flow-cytometry plots showing the gating strategy for identifying various subsets of ɣɖ T cells.
S2 Fig. Representative flow-cytometry plots showing the gating strategy for identifying double negative T (DNT) cells.
S3 Fig. Representative flow-cytometry plots showing the gating strategy for identifying CD4 and CD8 T cells.
S4 Fig. Representative flow-cytometry plots showing the gating strategy for identifying CD4+HLA-DR and CD8+HLA-DR.
S5 Fig. Correlations of double negative (DN) T cells, ɣɖ T cells, CD4+HLA-DR and CD8+HLA-DR among ART exposed as well as unexposed individuals. Healthy control (n=27), Early HIV (n=17), Chronic HIV (n=23), Chronic HIV on ART with low viral load (n=23) and Chronic HIV on ART with high viral load (n=18) individuals were recruited for this study Fresh blood of patients were collected; peripheral blood mononuclear cells were isolated and stored in liquid nitrogen. Flow cytometry was performed as described in methods section. Correlations of DNT cells, CD4+HLA-DR and CD8+HLA-DR among (A) ART unexposed; (B) ART exposed as well as correlations of ɣɖ T cells, CD4+HLA-DR and CD8+HLA-DR among (C) ART unexposed and (D) ART exposed were compared among the groups. Pearson correlation test was used for statistical analysis.


