Abstract
The pathogenesis of choroid plexus papillomas, intraventricular papillary neoplasms most often occurring sporadically in children and young adults, remains poorly understood. To identify pathways operative in the development of choroid plexus papillomas, gene expression profiles obtained from laser-microdissected human choroid plexus papilloma cells (n = 7) were compared with that of normal choroid plexus epithelial cells laser microdissected from autopsy tissue (n = 8). On DNA microarray data analysis, 53 probe sets were differentially expressed in choroid plexus papilloma tumor cells (>7-fold). Up-regulation of TWIST1, WIF1, TRPM3, BCLAF1, and AJAP1, as well as down-regulation of IL6ST was confirmed using quantitative reverse transcription-PCR. Knockdown of Twist1 gene expression in the rat choroid plexus epithelial cell line Z310 significantly reduced proliferation as assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and cell invasion in a Matrigel assay, whereas cell migration was not affected. Screening for expressional changes of cancer-related genes upon Twist1 knockdown revealed up-regulation of Cdknla, Cflar, and Serpinb2 and down-regulation of Figf. To conclude, using gene expression profiling, several genes differentially expressed in human choroid plexus papillomas could be identified. Among those, TWIST1 is highly expressed in choroid plexus papillomas and promotes proliferation and invasion.
Introduction
Choroid plexus papillomas are rare intraventricular papillary neoplasms derived from the choroid plexus epithelium that preferentially occur in children and young adults (1, 2). Confined to areas where choroid plexus is normally found, i.e., the lateral, third, and fourth cerebral ventricles, choroid plexus papillomas often impede cerebrospinal fluid flow and manifest clinically with hydrocephalus. On histopathologic examination, choroid plexus papillomas are benign tumors (grade I WHO) whose architecture closely resembles that of the nonneoplastic choroid plexus. Although the prognosis of choroid plexus papillomas is generally favorable if gross total resection can be achieved, recurrence and malignant progression may occur (3, 4).
The pathogenesis of sporadic choroid plexus papillomas, which represent the majority of cases, remains uncertain. Although choroid plexus tumors have been described in association with germline mutations of TP53 in Li-Fraumeni Syndrome (5) and SMARCB1 in the rhabdoid predisposition syndrome (6), mutations of TP53 accompanied by accumulation of p53-protein are very rare in sporadic choroid plexus papillomas (7–9) and inactivating mutations of SMARCB1 resulting in loss of INI1 protein expression are absent (10). Gene expression profiling has been successfully used to identify genes involved in the pathogenesis of a variety of pediatric brain tumors (11, 12) but not yet been used in choroid plexus tumors.
We thus aimed to identify pathways involved in the pathogenesis of choroid plexus papillomas by comparing gene expression profiles of cells laser microdissected from human choroid plexus papillomas and nonneoplastic choroid plexus epithelium.
Materials and Methods
Materials.
For laser microdissection and subsequent gene expression profiling, snap-frozen surgical samples from seven sporadic choroid plexus papillomas were available. Median age of the four males and three females was 27 y (range, 0–45 y). Three tumors were located in the lateral ventricles and four in the vicinity of the fourth ventricle. In all cases, the diagnosis of choroid plexus papilloma (grade I WHO) was confirmed neuropathologically according to current WHO criteria (2,13). The diagnostic work-up also included INI1 protein expression status, which was found to be positive in all tumors.
Laser microdissection.
Upon assessment of total RNA quality and integrity of the tissue samples by 2100-Bioanalyzer runs using the RNA-6000-Nano-LabChip (Agilent), cryosections (10 μm) were prepared on a cryostat and mounted on foil slides (P.A.L.M. Microlaser Technologies GmbH). Sections were Cresyl violet stained and air dried. Laser microdissection was performed using a Zeiss system equipped with an UVA-laser. A total of 10,000 cells were microdissected from each sample and collected in microcentrifuge tubes containing Buffer RLT (RNeasy Micro kit; Qiagen). Total RNA from these samples was then extracted using the RNeasy Micro kit (Qiagen). All steps were carried out under RNase-free conditions.
RNA amplification.
RNA was amplified using the GeneChip Two-Cycle cDNA Synthesis kit (Affymetrix) and subsequently in vitro transcribed to complementary RNA using the GeneChip IVT Labeling kit (Affymetrix). After confirmation of cRNA integrity, fragmented cRNA was hybridized for 16 h under constant rotation at 45 °C to U133A2 gene chips (Affymetrix) representing 18,400 transcripts and variants of ~ 14,500 human genes. After washing and staining (Fluidics Station 400; Affymetrix), fluorescence was scanned on a GeneChip Scanner 3000 (Affymetrix). Data files (GEO accession GSE14098) were evaluated using the Expressionist Pro software package (GeneData). After global chip quality control and fluorescence gradient correction using Expressionist Pro Refiner, differentially expressed probe sets were identified using Expressionist Pro Analyst (Genedata). To screen for differentially expressed candidate genes, normalized data were filtered for probe sets displaying expression levels at least 7-fold higher or lower in choroid plexus tumor cells compared with microarray data sets from eight samples representing laser-microdissected normal choroid plexus epithelial cells from human autopsy tissues that had been generated under comparable conditions (14).
Quantitative RT-PCR.
Upon RNA isolation from human tissue samples using the RNAeasy kit (Qiagen) and reverse transcription of total RNA using SuperScript II reverse transcriptase (Invitrogen), cDNA was amplified on a GeneAmp 5700 Sequence Detection System (Applied Biosystems) using commercially available primers and probes specific for the respective human transcripts (Applied Biosystems) in triplicates. Data were normalized for β-actin expression using the comparative threshold cycle method. RNA from rat cell culture samples was isolated using the RNeasy Plus kit (Qiagen) following manufactures instructions. Total RNA (1 μg) was transcribed into cDNA with the High Capacity cDNA reverse Transcription kit (Applied Biosystems) in a reaction volume of 20 μL. After cDNA synthesis, an aliquot (1 μL) of the reaction mixture was used for quantitative RT-PCR measurement in duplicates using the TaqMan GeneExpression assay TWIST1 (Rn00585470_s1). Data were normalized for glyceraldehyde-3-phosphate dehydrogenase expression (Rn99999916_s1) using the comparative threshold cycle method.
Cell culture.
Immortalized rat choroid plexus epithelial cells Z310 (15) were grown in culture medium [DMEM supplemented with 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, gentamicin (40 μg/mL), and 1% epidermal growth factor (10 ng/mL)] at 37°C in 5% CO2. All cell culture reagents were purchased from PAA.
Immunohistochemistry and immunocytochemistry.
Immunohistochemistry on paraffin-embedded formalin-fixed samples from 8 choroid plexus papillomas that had been treated within the Société Internationale d’ Oncologie Pédiatrique choroid plexus tumor study (CPT-SIOP-2000; ref. 16) as well as a tissue array representing duplicate probes (0.6 mm Ø) from 40 normal choroid plexus samples obtained at autopsy was carried out using a rabbit antiserum directed against TWIST-1 (T6451; Sigma-Aldrich; 1:400) and the avidin-biotin method on an automated staining system (TechMate; DAKO). TWIST-1 staining was evaluated semiquantitatively by scoring the percentage of stained cells [0 (absent), 1 (<10%), 2 (10–50%), 3 (51–80%), 4 (81–100%)] as well staining intensity [0 (absent), 1 (weak), 2 (distinct), 3 (strong)] by 2 independent neuropathologists (MH and AJ). Both scores were then multiplied to give a maximal staining score of 12. Staining for choroid plexus markers transthyretin and Kir7.1 (antibody kindly provided by Dr. S. Hirose, Department of Biological Sciences, Tokyo Institute of Technology, Japan) as well as INI1 protein was performed as described previously (14).
In Z310 cells, TWIST-1 protein expression was examined by immunofluorescence using a rabbit antiserum directed against TWIST-1 (sc-15393; Santa Cruz Biotechnology; 1:10) and an anti-rabbit-IgG TRITC-conjugated secondary antibody (T6778; Sigma-Aldrich; 1:400). Furthermore, Z310 cytospin preparations were stained for choroid plexus markers transthyretin and Kir7.1 as well as INI1 protein.
Western blot.
Lysis, SDS-PAGE and blotting were done as described previously (17) using a rabbit anti-p53 antibody (#9282, Cell Signaling; 1:1000).
RNAi.
Two different siRNA oligonucleotides for TWIST1 inhibition (Rn_Twist1_1 HP siRNA, SI02900128 and Rn_Twist1_3 HP siRNA, SI02900142), siRNA oligonucleotide for Tp53 inhibition (Rn_Tp53_4_HP siRNA,SI02047304) and scrambled siRNA for negative control were purchased from Qiagen. A total of 0.8 × 105 Z310 cells were seeded the day before siRNA transfection in 24-well plates in 500μL culture medium. Cells were transfected by adding 3 μL HiPerfect Transfection Reagent (Qiagen) and 1.5 μL siRNA dissolved in culture medium without FCS (5 nmol/L) per well according to manufacturer’s recommendations. For double-knockdown experiments, 1.5 μL siRNA1 plus 1.5 μL siRNA2 and 3 μL HiPerfect Transfection Reagent were dissolved in culture medium without FCS (5 nmol/L each). Cells were maintained in full culture medium for 24, 48, and 72 h before analysis, and the extent of RNA knockdown under these conditions was determined by quantitative RT-PCR.
Proliferation.
Cellular growth was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, Z310 cells were seeded at a density of 1 × 104 per well in a 96-well plate 24 h after siRNA transfection. For each time point, an exponential dilution series of cells was used with 1 × 105 cells as starting dilution. After 3 h, the medium was replaced by 200 μL MTT solution (0.5 mg/mL) and incubated for 3 h at 37°C and 5% CO2. MTT solution was discarded and 200 μL of isopropanol was added to dissolve the formazan crystals. Measurement was done at 570 nm using an ELISA reader upon 0, 24, 48, and 72 h (corresponding to 24, 48, 72, and 96 h after transfection). Experiments were independently performed thrice, and 10 to 24 wells were evaluated for each experimental condition.
Migration.
Cell migration was analyzed using a wound-healing assay (18). Briefly, Z310 cells were seeded at a density of 0.8 × 105 per well in a 24-well plate. Twenty-four hours after siRNA transfection, cell monolayers were scratched using the back side of a standard 100 μL pipette tip. After being washed thrice with PBS, scratches including the flanking front lines of cells, were microphotographed (400-fold magnification) and then incubated under standard conditions. Migration into the scratched area was documented at 24 and 48 h after wounding. Scratch closure by migrating cells was compared between Twist1-knockdown cells and control transfected cells relative to the total area of the scratches and evaluated using the analysis FIVE Software (Soft Imaging System). Experiments were independently performed thrice; and four to eight scratches were evaluated for each experimental condition.
Invasion.
Transwell plates (BD BioCoat growth factor reduced Matrigel Invasion Chamber; Becton Dickinson) were used to measure tumor invasion upon Twist1 knockdown. Cells were grown under standard conditions and Twist1 knockdown was performed as described above. Twenty-four hours after transfection, cells were trypsinized, washed with PBS, resuspended in 0.1% bovine serum albumin (BSA)-DMEM, and 500 μL (5 × 104 cells) were added to the Matrigel-coated upper chamber. The lower chamber contained 0.1% BSA-DMEM. Plates were incubated at 37°C, 5% CO2 for 24 h. After incubation, the noninvading cells and the Matrigel were removed from the upper surface of the membrane using a cotton swab. Inserts were removed, washed twice with PBS, and then cells were fixed using 3.7% formaldehyde. Cells were stained with Hoechst for 3 min and the membrane was transferred to a microscope slide. Membranes were microphotographed (400-fold magnification) and the number of stained cells was quantified using Analysis FIVE software (Olympus). Experiments were independently performed twice in quintuplicates.
Gene expression of cancer related genes.
The effect of Twist1 knockdown on the expression of a panel of 84 cancer related genes was determined using the Rat Cancer PathwayFinder PCR Array (Bioscience Corporation). Briefly, upon RNA isolation of Z310 cells transfected with siRNA (Rn_Twist1_1 HP siRNA, SI02900128) or scrambled control for 24 h, 2 μg of total RNA were transcribed into cDNA using the High Capacity cDNA reverse Transcription kit (Applied Biosystems) and subjected to quantitative RT-PCR according to the manufacturer’s instructions. Resulting gene expression profiles from two independent experiments were evaluated.
Statistics.
Comparison of quantitative PCR and immunohistochemistry data were done by Mann-Whitney U test. Functional studies that involved multiple comparisons were evaluated by Kruskal-Wallis test followed by Mann-Whitney U test. P value of <0.05 was considered significant. Statistical analyses were performed using SPSS version 15.0 (SPSS, Inc.).
Results
TWIST1 and other genes are differentially expressed in laser-microdissected human choroid plexus papilloma cells.
As shown in Fig. 1A, gene expression profiling yielded a number of 53 probe sets to be differentially expressed in laser-microdissected choroid plexus papilloma cells compared with nonneoplastic choroid plexus epithelial cells (>7-fold; Supplementary Table S1). Differential expression of six of these genes known to play a role in tumor biology was confirmed using RT-PCR showing significant up-regulation of TWIST1 coding for the transcription factor TWIST-1, Wnt inhibitory factor 1 (WIF1), transmembrane protein Shrew-1 (AJAP1), BCL2-associated transcription factor 1 (BCLAF1, a transcriptional repressor that interacts with members of the BCL2 family of proteins), and transient receptor potential channel (TRPM3) as well as down-regulation of interleukin-6 signal transducer (IL6ST) in choroid plexus papilloma cells (Fig. 1B). Because differential expression of TWIST1 was most pronounced, this gene was chosen for further analysis. As shown in Fig. 1C, TWIST-1 protein expression scores were significantly higher in choroid plexus papillomas (10 ± 3; mean ± SD) compared with nonneoplastic choroid plexus samples (3 ± 2; P < 0.001).
Figure 1.

Identification and validation of differentially expressed genes in choroid plexus papilloma cells. Expression levels (arbitrary units) of 53 genes found to be differentially expressed (>7-fold) in laser-microdissected human choroid plexus papilloma cells compared with normal choroid plexus (A), validation of expression of selected genes using quantitative RT-PCR (B; fold-change compared with nonneoplastic choroid plexus; columns, mean; bars, SE; n = 4–6; *, P < 0.05), and confirmation of differential expression of TWIST-1 on protein level in choroid plexus papillomas compared with nonneoplastic choroid plexus (C). Insets, representative stainings of choroid plexus papilloma and normal choroid plexus.
TWIST-1 is expressed in immortalized rat choroid plexus epithelial cells and involved in proliferation but not migration.
As shown in Fig. 2A, distinct cytoplasmic staining of TWIST-1 was present in the SV40-immortalized rat choroid plexus epithelial cell line Z310, which also showed immunoreactivity for choroid plexus tumor markers transthyretin (strong cytoplasmic staining in the majority of cells) and Kir7.1 (Hasselblatt et al., 2006; distinct membranous staining in 20% of cells) as well as retained nuclear INI1 staining. As shown in Fig. 2B, expression of Twist1 mRNA was effectively inhibited upon transfection with two specific siRNAs for up to 72 hours compared with control (scrambled siRNA; P < 0.01; n = 4). Twist1 knockdown resulted in a significant reduction of proliferative activity in Z310 cells: upon transfection with both Twist1 siRNAs, the increase in proliferative activity was most markedly reduced at the 24-hour time point (201% ± 8% and 185% ± 7%, respectively, versus 279% ± 15%; mean ± SD; n = 3; P < 0.01); the reduction remaining significant compared with control for up to 72 hours (Fig. 2C). As shown in Fig. 2D, silencing of Tp53 for 24 hours resulted in reduced expression of p53 protein compared with control scrambled siRNA and increased proliferative activity. Upon double knock-down of Tp53 and Twist1 (Tp53 X Twist1_1), the antiproliferative effect of Twist1 knockdown was abolished.
Figure 2.
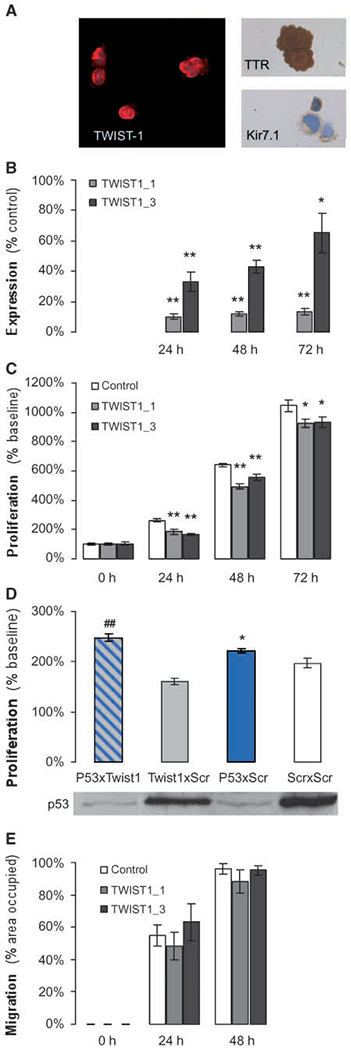
Expression of TWIST-1 in immortalized rat choroid plexus epithelial cells and effect on proliferation and migration. Expression of TWIST-1 protein along with choroid plexus markers transthyretin and Kir7.1 in the choroid plexus epithelial cell line Z310 (A). Upon transfection with the two specific siRNAs TWIST1_1 and TWIST1_3, TWIST-1 mRNA expression is inhibited for up to 72 h compared with control scrambled siRNA (B; columns, mean; bars, SE; n = 4; **, P < 0.01;*, P < 0.05) and proliferative activity as measured by MTT assay is significantly reduced (C; columns, mean; bars, SE; n = 3; **, P < 0.01; *, P < 0.05). Silencing of Tp53 (Tp53 XScr) for 24 h results in reduced expression of p53 protein compared with control scrambled siRNA (Scr X Scr) and increased proliferative activity (D; columns, mean results from one representative of two independent experiments, each performed in octuplicate; bars, SE; *, P < 0.05). Upon simultaneous silencing of Tp53 and Twist1 (Tp53 X Twist1_1), the antiproliferative effect of Twist1 knockdown is abolished (##, P < 0.01). Cell migration is not significantly affected upon silencing of Twist1 expression with the two specific siRNAs TWIST1_1 and TWIST1_3, (E; columns, mean; bars, SE; n = 3).
Twist1 knockdown does not affect cell migration.
We next examined the effect of Twist1 knockdown on migration in Z310 cells. As shown in Fig. 2E, however, no significant difference in the percentage of scratch-area occupied was observed upon transfection with both TWIST1 siRNAs compared with scrambled control (24 hours: 48.3% ± 12.3% and 63.1% ± 16.0% versus 55.1% ± 9.3%; 48 hours: 88.2% ± 10.3% and 95.3% ± 4.1% versus 95.9% ± 4.6%; mean ± SD, n = 3, not significant).
Twist1 knockdown results in decreased cell invasion and differential expression of cancer-related genes.
As shown in Fig. 3A, transfection with Twist1 specific siRNA TWIST1_1 resulted in decreased infiltrative capacity of Z310 cells in a Matrigel assay (33.6% ± 6.4% of control; mean ± SD, results from 1 representative of 2 independent experiments, each performed in quintuplicate; P < 0.01). Being interested in which downstream genes are involved in the functional effects observed upon Twist1 knockdown, we screened a panel of 84 cancer-related genes for expressional changes. As shown in Fig. 3B, transfection with Twist1 specific siRNA TWIST1_1 resulted in differential expression (>1.5-fold in 2 independent experiments) of 5 genes. In addition to confirmational down-regulation of Twist1 (—3.5-fold), down-regulation of encoding for vascular endothelial growth factor D (Figf) as well as up-regulation of cyclin-dependent kinase inhibitor-1A (Cdkn1a), FLICE inhibitory protein (Cflar), and plasminogen activator inhibitor type 2 (Serpinb2) was observed.
Figure 3.
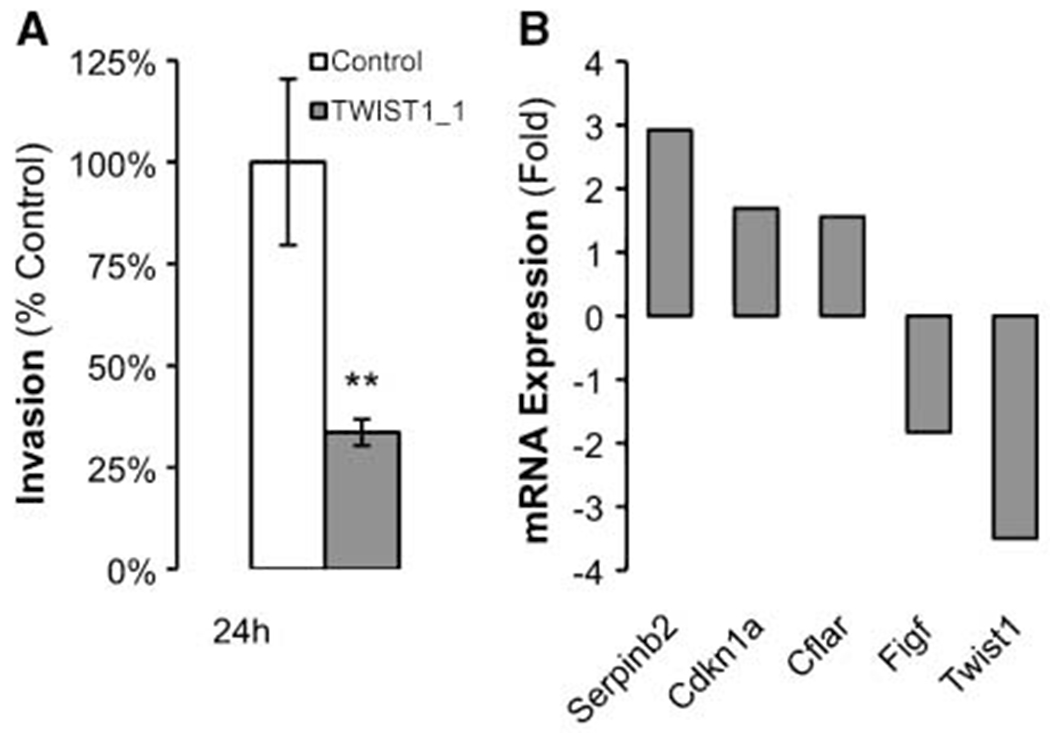
Effect of Twist1 knockdown on cell invasion and differential expression of cancer-related genes. Gene silencing with siRNA TWIST1_1 affects cell invasion compared with control in a Matrigel assay (A; columns, mean representative experiment performed in quadruplicate; bars, SE; **, P < 0.01) and results in differential expression (>1.5-fold) of 5 of 84 cancer-related genes examined by quantitative RT-PCR (B, mean from two independent experiments).
Discussion
Using gene expression profiling, a number of genes differentially expressed in normal versus neoplastic cells of choroid plexus could be identified. The finding that the transcription factor TWIST-1 is among the genes highly expressed in human choroid plexus papilloma cells suggests a role of TWIST-1 in the development of choroid plexus papillomas. TWIST-1 is tightly linked to the p53 signaling pathway and inhibits the tumor suppressor activity of p53 and ADP ribosylation factor (19). Indeed, the observed effects of Twist1 silencing on proliferation were abolished upon Tp53 knockdown, pointing toward a dependency on functional p53 signaling.
One limitation of all functional studies on choroid plexus tumor biology remains the absence of well-established human cancer cell lines. Because Z310 cells possess choroid plexus epithelial cell characteristics, shows strong proliferative activity, and has been generated by transfection with SV40 (15), implicated in the pathogenesis of choroid plexus tumors in rodents (20), despite all limitations this immortalized rat choroid plexus epithelial cell line represent the best model available to date.
In malignant gliomas, TWIST1 has been shown to be highly expressed and to be correlated with tumor grade (21). In contrast to previously reported functional data in SF767 glioma cells showing enhanced cell invasion, but not cell proliferation upon overexpression of TWIST1 (21), knockdown of Twist1 expression in immortalized choroid plexus epithelial cells impaired both proliferation and invasion. This discrepancy might be related to the different methodologic approach but could also point toward a different biological role of TWIST-1 in glial and choroid plexus epithelial cell lines.
The role of TWIST-1 as a promoter of invasive growth in metastasizing malignant tumors and an inducer of epithelial-mesenchymal transition in epithelial cells is well-established (22, 23). Because choroid plexus papillomas in vivo do usually not exert infiltrative growth, the observed effect of TWIST-1 on tumor cell infiltration is unexpected. It is tempting to speculate that TWIST-1 might aid the capability of choroid plexus tumor cells to invade the basal membrane of the choroid plexus epithelium, which represents an early and crucial step in tumor formation and progression.
Interestingly, Twist1 knockdown not only resulted in up-regulation of cyclin-dependent kinase inhibitor-1A, known to antagonize the effect of SV40 large T antigen on cell cycle progression (24), but was also associated with up-regulation of the SerpinB2 gene encoding for plasminogen activator inhibitor type 2, recently shown to decrease infiltrative tumor growth and metastasis (25). Our finding of down-regulated Figf in response to Twist1 gene silencing, however, is well in line with previous observations of induced angiogenesis upon TWIST-1 up-regulation in hepatocellular carcinoma (26).
In addition to TWIST1, differential expression of several other genes in choroid plexus papillomas could be validated by RT-PCR. Up-regulation of inhibitory factors WIF1 and BCLAF1 might possibly reflect a compensatory response to activation of Wnt and Bcl-2 signaling, respectively. TRPM3 (melastatin-3) is a cation channel of the transient receptor potential superfamily, which has been shown to be expressed in the nonneoplastic mouse choroid plexus epithelium (27). Although the role of transient receptor potential proteins in the development and progression of cancer is evolving (28), up-regulation of TRPM3 expression has not yet been described in tumor cell biology. Expression of AJAP1 encoding the transmembrane protein shrew-1, known to interact with E-cadherin-catenin complexes and to enhance the invasiveness of tumor cells (29), has not been described in choroid plexus papillomas. Further studies on the function of these and other genes will hopefully contribute to a better understanding of the complex molecular pathogenesis of choroid plexus papillomas.
To conclude, using gene expression profiling, several genes differentially expressed in tumor cells from human choroid plexus papillomas could be identified. Among those, TWIST-1 is highly expressed in choroid plexus tumors and involved in cell proliferation and invasion.
Supplementary Material
Acknowledgments
Grant support: Deutsche Krebshilfe (grant 108263).
We thank Drs. Ohgaki (IARC, Lyon, France), Reifenberger (Department of Neuropathology, Heinrich-Heine-University, Düosseldorf, Germany), Bergmann (Institute of Neuropathology, Klinikum Bremen-Mitte, Bremen, Germany), and Beschorner (Institute of Brain Research, University of Tüobingen, Germany) for generously sharing fresh-frozen choroid plexus papilloma samples.
Footnotes
Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Rickert CH, Paulus W. Tumors of the choroid plexus. Microsc Res Tech 2001;52:104–11. [DOI] [PubMed] [Google Scholar]
- 2.Paulus W, Brandner S. Choroid plexus tumours In: Louis DN, Ohgaki H, Wiestler O, Cavenee WK, editors. World Health Organization classification of tumors Pathology and genetics of tumours of the nervous system. 4th ed Lyon: IARC Press; 2007. p. 82–5. [Google Scholar]
- 3.Jeibmann A, Hasselblatt M, Gerss J, et al. Prognostic implications of atypical histologic features in choroid plexus papilloma. J Neuropathol Exp Neurol 2006;65: 1069–73. [DOI] [PubMed] [Google Scholar]
- 4.Jeibmann A, Wrede B, Peters O, Wolff JE, Paulus W, Hasselblatt M. Malignant progression in choroid plexus papillomas. J Neurosurg 2007;107:199–202. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford J, Chu CE, Duddy PM, et al. Investigations on a clinically and functionally unusual and novel germline p53 mutation. Br J Cancer 2002;86: 1592–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sevenet N, Sheridan E, Amram D, Schneider P, Handgretinger R, Delattre O. Constitutional mutations of the hSNF5/INI1 gene predispose to a variety of cancers. Am J Hum Genet 1999;65:1342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohgaki H, Eibl RH, Schwab M, et al. Mutations of the p53 tumor suppressor gene in neoplasms of the human nervous system. Mol Carcinog 1993;8:74–80. [DOI] [PubMed] [Google Scholar]
- 8.Jay V, Ho M, Chan F, Malkin D. P53 expression in choroid plexus neoplasms: an immunohistochemical study. Arch Pathol Lab Med 1996;120:1061–5. [PubMed] [Google Scholar]
- 9.Carlotti CG Jr, Salhia B, Weitzman S, et al. Evaluation of proliferative index and cell cycle protein expression in choroid plexus tumors in children. Acta Neuropathol 2002;103:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Mueller W, Eum JH, Lass U, et al. No evidence of hSNF5/INI1 point mutations in choroid plexus papilloma. Neuropathol Appl Neurobiol 2004;30:304–7. [DOI] [PubMed] [Google Scholar]
- 11.de Bont JM, Kros JM, Passier MM, et al. Differential expression and prognostic significance of SOX genes in pediatric medulloblastoma and ependymoma identified by microarray analysis. Neuro-oncol 2008;10:648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modena P, Lualdi E, Facchinetti F, et al. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol 2006;24:5223–33. [DOI] [PubMed] [Google Scholar]
- 13.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasselblatt M, Bohm C, Tatenhorst L, et al. Identification of novel diagnostic markers for choroid plexus tumors: a microarray-based approach. Am J Surg Pathol 2006;30:66–74. [DOI] [PubMed] [Google Scholar]
- 15.Zheng W, Zhao Q. Establishment and characterization of an immortalized Z310 choroidal epithelial cell line from murine choroid plexus. Brain Res 2002;958: 371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrede B, Peters O, Hasselblatt M, et al. An interim report of the International CPT-SIOP-2000. March 2007. Pediatr Blood Cancer 2007;49:426. [Google Scholar]
- 17.Mertsch S, Schmitz N, Jeibmann A, Geng JG, Paulus W, Senner V. Slit2 involvement in glioma cell migration is mediated by Robo1 receptor. J Neurooncol 2008;87:1–7. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez LG, Wu X, Guan JL. Wound-healing assay In: Guan J-L, editor. Cell Migration. Totowa (NJ): Humana Press; 2005. p. 23–9. [DOI] [PubMed] [Google Scholar]
- 19.Puisieux A, Valsesia-Wittmann S, Ansieau S. A twist for survival and cancer progression. Br J Cancer 2006;94:13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmiter RD, Chen HY, Messing A, Brinster RL. SV40 enhancer and large-T antigen are instrumental in development of choroid plexus tumours in transgenic mice. Nature 1985;316:457–60. [DOI] [PubMed] [Google Scholar]
- 21.Elias MC, Tozer KR, Silber JR, et al. TWIST is expressed in human gliomas and promotes invasion. Neoplasia 2005;7:824–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004;117:927–39. [DOI] [PubMed] [Google Scholar]
- 23.Lee TK, Poon RT, Yuen AP, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res 2006;12:5369–76. [DOI] [PubMed] [Google Scholar]
- 24.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993;75: 805–16. [DOI] [PubMed] [Google Scholar]
- 25.Croucher DR, Saunders DN, Lobov S, Ranson M. Revisiting the biological roles of PAI2 (SERPINB2) in cancer. Nat Rev Cancer 2008;8:535–45. [DOI] [PubMed] [Google Scholar]
- 26.Niu RF, Zhang L, Xi GM, et al. Up-regulation of Twist induces angiogenesis and correlates with metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res 2007; 26:385–94. [PubMed] [Google Scholar]
- 27.Oberwinkler J, Lis A, Giehl KM, Flockerzi V, Philipp SE. Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J Biol Chem 2005;280: 22540–8. [DOI] [PubMed] [Google Scholar]
- 28.Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta 2007;1772:937–46. [DOI] [PubMed] [Google Scholar]
- 29.Schreiner A, Ruonala M, Jakob V, et al. Junction protein shrew-1 influences cell invasion and interacts with invasion-promoting protein CD147. Mol Biol Cell 2007;18:1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


