Abstract
Purpose
The purpose of this study is to investigate a sol–gel transition property and content release profiles for thermosensitive poly-(D,L-lactide-co-glycolide)-block-poly-(ethylene glycol)-block-poly-(D,L-lactide-co-glycolide) (PLGA-b-PEG-b-PLGA) hydrogels carrying paclitaxel, rapamycin, and LS301, and to present a proof-of-concept that PLGA-b-PEG-b-PLGA hydrogels carrying paclitaxel, rapamycin, and LS301, called TheranoGel, exhibit excellent theranostic activity in peritoneal ES-2-luc ovarian cancer xenograft mice.
Methods
Thermosensitive PLGA-b-PEG-b-PLGA hydrogels carrying paclitaxel, rapamycin, and LS301, individually or in combination, were prepared via a lyophilization method, characterized with content release kinetics, and assessed with theranostic activity in ES-2-luc xenograft mice.
Results
A thermosensitive PLGA-b-PEG-b-PLGA sol–gel system was able to entrain 3 poorly water-soluble payloads, paclitaxel, rapamycin, and LS301 (TheranoGel). TheranoGel made a sol-to-gel transition at 37°C and slowly released 3 drugs at a simultaneous release rate in response to the physical dissociation of hydrogels in vitro. TheranoGel enabled loco-regional delivery of multi-drugs by forming a gel-depot in the peritoneal cavity of ES-2-luc xenograft mice. An intraperitoneal (IP) administration of TheranoGel resulted in excellent therapeutic and diagnostic activities, leading to the improved peritoneal surgery in ES-2-luc xenograft mice.
Conclusions
TheranoGel prepared via a facile lyophiliation method enabled successful IP delivery of multi-drugs and exhibited excellent theranostic activity in vivo.
Keywords: hydrogels, intraperitoneal, ovarian cancer, theranostics, thermosensitive
INTRODUCTION
Ovarian cancer is one of the most lethal female gynecological diseases (1, 2). Ovarian cancer cells tend to spread in the abdomino-pelvic cavity (known as the peritoneal cavity) as the primary disease site and further disseminate to the non-peritoneal organs, such as spleen, liver, lung, and brain. Due to the unique anatomical feature of the abdomen and distribution pattern of ovarian cancer cells, intraperitoneal (IP) chemotherapy has been extensively investigated for treating peritoneal ovarian cancer patients (1). Several clinical trials adopting IP chemotherapy provided evidence that IP chemotherapy offered a pharmacokinetic advantage in the peritoneal cavity as compared to systemic exposure. For example, peritoneal cavity levels of IP paclitaxel and IP cisplatin were found to be >1000 times and 10–20 times, respectively, greater than their levels in the systemic compartment (3, 4). Therefore, IP delivery of drugs and/or perhaps optical imaging agents deserves to gain attentions as a promising strategy to treat and/or image peritoneal ovarian cancer by providing an opportunity to expose ovarian cancer cells present within the peritoneal cavity to the substantially higher concentrations of cytotoxic drugs and/or optical imaging agents. A thermoresponsive PLGA-b-PEG-b-PLGA sol–gel carrying paclitaxel, known as OncoGel™, has been extensively tested in preclinical and clinical studies, which enabled localized delivery of paclitaxel (a microtubule stabilizer), a potent yet poorly water-soluble anticancer agent (5). OncoGel™ made a solto-gel transition at 37°C promoting formation of a PLGA-b-PEG-b-PLGA gel-depot carrying paclitaxel at the intratumoral injection site in patients with esophageal, brain and breast cancers. This gel-depot slowly released paclitaxel over 50 days in its parent form from OncoGel™ in vitro, and this gel-depot injected subcutaneously in rats was found to be completely resorbed from the injection site within 4–6 weeks in vivo. Most importantly, OncoGel™ demonstrated therapeutic efficacy with acceptable safety profiles in pre-clinical and clinical studies. Given these unique physicochemical properties of a thermo-responsive PLGA-b-PEG-b-PLGA sol–gel system, we previously employed PLGA-b-PEG-b-PLGA sol-gels to deliver multi-drugs to the peritoneal cavity of ovarian cancer-bearing mice (6). In our previous study, PLGA-b-PEG-b-PLGA sol-gels were able to incorporate 3 poorly water-soluble cytotoxic drugs, paclitaxel, 17-allylamino-17-demethoxygeldanamycin (17-AAG, a heat-shock protein inhibitor), and rapamycin (an mTOR inhibitor), termed Triogel, seeking localized delivery of a synergistic 3-drug combination for the treatment of ovarian cancer (6). Notably, PLGA-b-PEG-b-PLGA hydrogels had a capacity for 3 poorly water-soluble drugs at ca. mg/mL level and released all 3 drugs simultaneously at a gradual release rate. In an in vivo study, Triogel successfully delivered multi-drugs to the peritoneal cavity of ES-2-luc ovarian cancer-bearing mice and was able to control local proliferation of ovarian cancer cells and ascites, resulting in extended longevity of the treated animals.
Although IP chemotherapy appears to enhance therapeutic effects in peritoneal ovarian cancer patients, in order to ensure desired therapeutic outcome and prolonged survival of patients, surgical dissection of microscopic residual tumor tissues is inevitable. In this study, we attempted to extend the use of a thermo-responsive PLGA-b-PEG-b-PLGA sol–gel system to surgical gynecology by highlighting a proof-of-concept that a thermo-responsive PLGA-b-PEG-b-PLGA sol–gel system can be utilized as a promising nanocarrier for IP delivery of cytotoxic drugs (paclitaxel and rapamycin) and a near-infrared (NIR) optical imaging agent (LS301, cysteineglycine-arginine-aspartic acid-serine-proline-cysteine-lysine peptide conjugated to cypate), permitting IP chemotherapy followed by intraoperative image-guided surgery in a peritoneal ovarian cancer mouse model; As reported elsewhere, inhibition of mTOR using rapamycin has been shown to increase and prolong sensitivity of various cancer cells to paclitaxel (7–9). Achilefu et al. demonstrated that an intravenous (IV) injection of LS301 clearly delineated tumor margins and small nodules invisible to the naked eyes in 4T1 breast cancer bearing mice (10). In this study, a theranostic activity of a single IP injection of PLGA-b-PEG-b-PLGA sol-gels carrying paclitaxel, rapamycin, and LS301 (called TheranoGel) was tested in an ES-2-luc xenograft model of human peritoneal ovarian cancer, which was superior than a theranostic activity of a single IP or IV administration of PEG-b-PLA polymeric micelles carrying paclitaxel, rapamycin, and LS301 (called TheranoMic).
MATERIALS AND METHODS
Preparations of TheranoGel and TheranoMic
TheranoGel (PLGA-b-PEG-b-PLGA (1500-b-1000-b-1500) (Polyscitech, West Lafayette, IN) hydrogels carrying paclitaxel (LC Laboratories, Woburn, MA), rapamycin (LC Laboratories, Woburn, MA), and LS301 (kindly provided by Dr. Samuel Achilefu) and TheranoMic (PEG-b-PLA (4000-b-2000) (Polymer Source, Dorval, Canada) micelles carrying paclitaxel, rapamycin, and LS301) were prepared by the lyophilization method as previously described (6). PLGA-b-PEG-b-PLGA triblock copolymers (250 mg) dissolved in 1 mL of cold water (5°C) were mixed with 6 mg of paclitaxel, 6 mg of rapamycin, and 44 μg of LS301 in 1 mL of tert-butanol at 60°C and lyophilized for 24 h. The lyophilized cake was then rehydrated with 1 mL of cold DI water at 4°C and gentrly stirred for >6 h in the cold room at 300 rpm. The rehydrated solution was passed through a 0.2 μm regenerated cellulose filter to remove unincorporated drugs.
Likewise, TheranoMic was prepared by the lyophilization method: 60 mg of PEG-b-PLA diblock copolymers, 6 mg of paclitaxel, 6 mg of rapamycin, and 44 μg of LS301 were dissolved in 1 mL of tert-butanol at 60°C, and 1 mL of water at 60°C was added to the polymer/drug mixture prior to the lyophilizaiton process. The lyophilized cake was then rehydrated with DI water and gently swirled for 10 min. The rehydrated solution was passed through a 0.2 μm regenerated cellulose filter to remove unincorporated drugs.
TheranoGel and TheranoMic were diluted with cold and room temperature acetonitrile in solution, respectively, for the quantification of paclitaxel and rapamycin by reverse phase (RP)-HPLC. Diluted samples (10 μL) were injected into Zobrax SP-C8 Rapid Resolution catridge (4.6 × 75 mm, 3.5 μm, Agilent, Santa Clara, CA). The flow rate was 1.0 mL/min. The separation of paclitaxel and rapamycin was carried out in an isocratic mode with 55% acetonitrile and 45% water (containing 0.1% phasphoric acid and 1% of methanol), and paclitaxel and rapamycin were monitored at 2.8 and 8.6 min, respectively. The content level of LS301 was determined by UV-visible measurements at λmax of 745 nm on a Cary 100 Bio UV-visible spectrophotometer (Varian, Palo Alto, California).
Physical Characterizations of TheranoGel and TheranoMic
Z-average diameters of TheranoGel and TheranoMic were determined by dynamic light scattering (DLS) measurements using Zetasizer Nano-ZS (Malvern Instruments, United Kingdom) at 10, 25, or 37°C with a detection angle of 173° and a He-Ne ion laser (4 mW, λmax = 633 nm) for the incident beam. Autocorrelation functions were created based on the cumulant analysis using the Stokes-Einstein equation. Prior to the measurements, TheranoGel and TheranoMic were diluted with distilled water to give a polymer concentration ≥0.6 mg/mL, which represents the polymer level above the critical micelle concentration (CMC) and does not significantly change viscosity of diluted samples of TheranoGel at 37°C.
Release of drugs from TheranoGel and TheranoMic was assessed by a dialysis method. Aqueous solutions (100 μL) of TheranoMic and TheranoGel were put into dialysis cassettes (n = 3/each time point) (MWCO 20,000, Thermo Fischer Scientific Inc., Rockford, IL), and dialysis cassettes were placed in 2 L of distilled water at 37°C with stirring. At 0, 0.5, 1, 2, 3, 6, 9, 24, and 48 h, 3 cassettes were removed to collect TheranoGel gels and TheranoMic solutions. Collected samples were diluted with acetonitrile for the quantification of residual drug contents in hydrogels and micellar solutions. Drug release was profiled on a first-order association using GraphPad Prism version 6 (La Jolla, CA).
IP Human Ovarian Cancer Xenograft and Treatments
All animal studies were approved by the UW-Madison’s Institutional Animal Care and Use Committee and conducted in accordance with the institutional and NIH guidelines. Female 6–8 week-old athymic nude mice were purchased from Harlan Laboratories (Madison, WI). General anesthesia was induced with 1.5% isoflurane/oxygen and maintained with 1% isoflurane/oxygen level during the course of the studies. All aninals were euthanized at the time of reaching a moribund condition by medical grade carbon dioxide with a flow rate of 10–30% of the euthanasia chamber volume/min. ES-2-luc cells (106 cells/200 μL each animal) were intraperitoneally injected into the peritoneal cavity of anesthetized mice to mimic patient conditions with intraperitoneal ovarian cancer; intraperitoneally injected ES-2-luc cells formed ascites and solid tumors which invade the peritoneal wall and the diaphragm and aggregate in the mesentery and the omentum (11, 12). Drug treatments were initiated 96 h post cancer cell inoculation. ES-2-luc ovarian cancer-bearing mice (n = 4) were treated with a single injection of (i) IP empty PLGA-b-PEG-b-PLGA hydrogels, (ii) IP TheranoGel carrying paclitaxel, rapamycin, and LS301 at 60, 60 mg/kg, and 440 μg/kg, respectively, (iii) IP TheranoMic carrying paclitaxel, rapamycin, and LS301 at 60, 60 mg/kg, and 440 μg/kg, respectively, or (iv) IV TheranoMic carrying paclitaxel, rapamycin, and LS301 at 60, 60 mg/kg, and 440 μg/kg, respectively. Body weights, general appearance and behavior, and mortality of animals were monitored during the animal studies. Due to the poor aqueous solubility of paclitaxel and rapamycin, administrations of free drugs without drug delivery carriers (polymeric micelles or hydrogels) were not included.
Bioluminescence and Fluorescence Imagings
Xenogen IVIS 200 Series equipped with a 150 W quartz halogen lamp and a 1 mW power scanning laser was used to obtain bioluminescence and fluorescence images in vitro, in vivo, and ex vivo. Bioluminescence images were acquired by a charged couple device (CCD) camera with the following conditions: exposure time = 1 s, medium binning, f/stop = 1, field of view = 22.4 cm, subject height = 1.5 cm, and resolution = 60 μm/pixel. In vitro, D-luciferin at 10 μg was added to each well 5 min prior to obtaining bioluminescence images. In vivo, D-luciferin was intraperitoneally injected at 113 mg/kg 5 min prior to the whole-body bioluminescence imaging. The dynamics of ES-2-luc proliferations and distributions were shown as color-coded images (inverted rainbow) using Live Imaging software for qualitative and quantitative analyses. Intensities of bioluminescence signal expressed by ES-2-luc cancer cells, ascites, and tissues were quantified by averaging the total counts of the region of interest (ROI) in bioluminescence images. Whole-body bioluminescence images were recorded at 0, 24, 48, and 72 h post treatments.
Fluorescence images were also acquired by the CCD camera with the following conditions: exposure time = 1 s, medium binning, f/stop = 2, field of view = 22.4 cm, and subject height = 1.5 cm. A filter used to detect LS301 was set at 745 nm excitation and 800 nm emission. The dynamics of LS301 distributions were shown as color-coded images (red hot) using Live Imaging software for qualitative analysis. Fluorescence intensity of LS301 was expressed in an average radiant efficiency ([p/sec/cm2/sr]/[μM/cm2]). Whole-body fluorescence images were recorded at 10 min, 6, 24, 48, and 72 h post treatments.
Intraoperative Fluorescence Imaging Acquisitions
Fluobeam800 is a portable hand-held 2-dimensional NIR fluorescence imaging system equipped with a black-and white CCD camera (exposure time = 1 msec to 1 s) and an integrated NIR light source (100 mW) optimized with excitation at 780 nm and emission at >800 nm. Intraoperative fluorescence images of sacrificed animals were acquired as raw images in the identical settings: focal distance = 22 cm, field of view: 10 × 7 cm2, and resolution: 110 μm/pixel. Fluorescence images were displayed on the screen as either black-and-white static images with the maximum image frame rate of 9 images/sec or real-time videos with the maximum image frame rate of 25 images/sec.
Surgical Procedure
Three days after a single IV or IP injection of each formulation, ES-2-luc-bearing mice received an IP injection of D-Luciferin at 113 mg/kg 5 min prior to the whole-body bioluminescence imaging. After obtaining the whole-body bioluminescence and fluorescence imagings, the animals were sacrified immediately by medical grade carbon dioxide with a flow rate of 10–30% of the euthanasia chamber volume/min. Surgical procedure was performed on sacrified animals within 30 min after an IP injection of D-Luciferin, and all animals underwent a mid-line incision to extensively explore peritoneal regions of the animals. ES-2-luc-bearing mice treated with (i) an IP empty PLGA-b-PEG-b-PLGA hydrogels were surgically dissected to remove tumor tissues under the conventional white-light surgerical procedure. ES-2-lucbearing mice treated with formulations (ii)-(iv) underwent intraoperative fluorescence image-guided surgery. ES-2-luc ovarian cancer xenograft mice which received formulations (ii)-(iv) were sacrificed on day 3 and surgically explored upon the abdominal mid-line incision. Animals were placed under the Fluobeam800 to obtain intraoperative NIR fluorescence images. Surgical resection of fluorescence-positive (FLU+) tumor-like tissues were preformed guided by intraoperative images displayed on the computer screen. When the surgery was deemed satisfactory, dissected tumor-like tissues and carcass were scanned using Xenogen IVIS200 Series to obtain ex vivo bioluminescence and fluorescence images. Surgical outcome was evaluated by calculating % total counts of bioluminescence-positive (LUC+) tumor tissues left in the carcass among total counts of LUC+ tumor tissues removed plus LUC+ tumor tissues left in the carcass.
Statistical Analysis
For statistical analysis, one-way ANOVA at 5% significance level combined with Tukey’s multiple comparison tests by GraphPad Prison ver 6 was used.
RESULTS
Preparation of TheranoGel and TheranoMic
TheranoGel (PLGA-b-PEG-b-PLGA hydrogels) and TheranoMic (PEG-b-PLA micelles) carrying paclitaxel, rapamycin, and LS301 at ca. 6, 6 mg/mL, and 44 μg/mL, respectively, were successfully prepared by the lyophilization method (Fig. 1a). Lyophilization yielded a lyophilized cake of TheranoGel, and upon the addition of cold water at 5°C with gentle stirring at 300 rpm, the lyophilized cake was completely reconstituted into a solution within 6 h. At room temperature, TheranoGel was partially congealed, and at 37°C, TheranoGel was completely transformed into a compressed gel, demonstrating a successful sol-to-gel transition at body temperature.
Fig. 1.
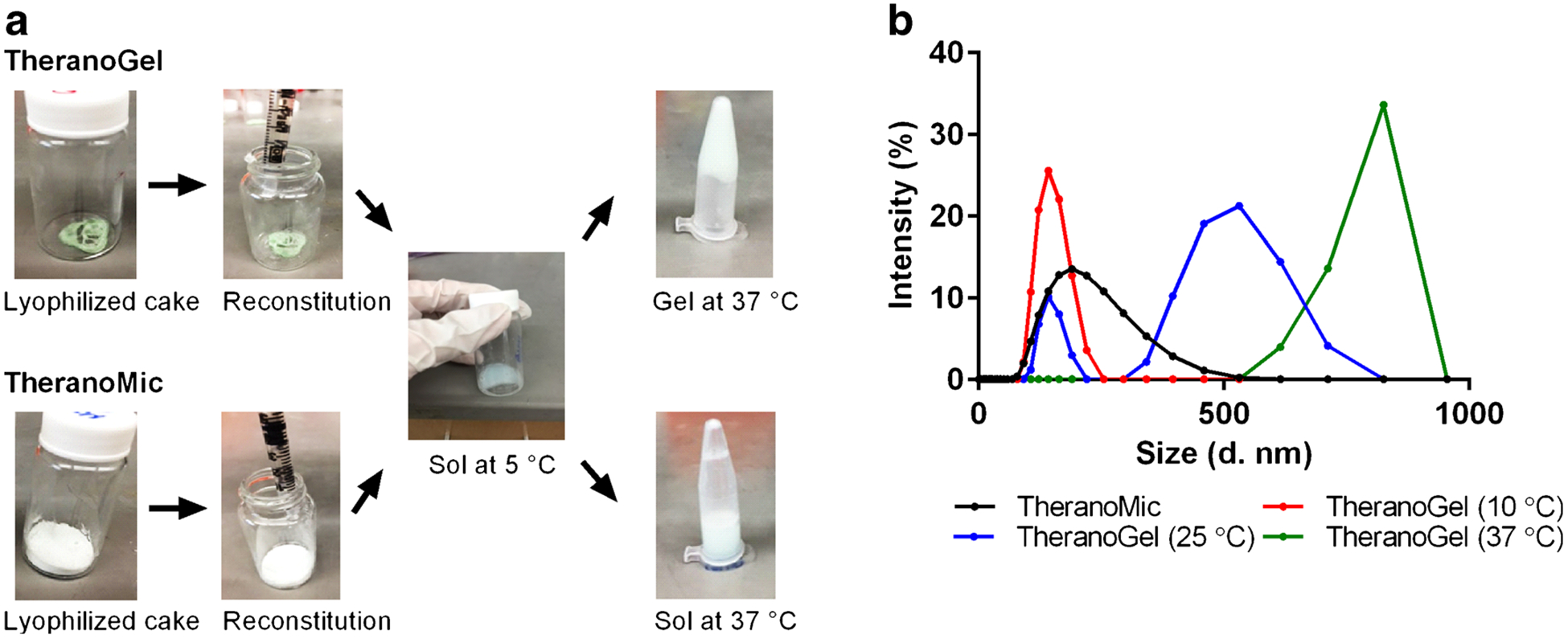
Step-wise preparations of TheranoGel and TheranoMic (a) and assessment of particle size distributions of TheranoGel and TheranoMic at 10, 25, and 37°C (b).
A lyophilized cake of TheranoMic was completely rehydrated upon the addition of warm water within 10 min. Regardless of the temperature changes, reconstituted TheranoMic existed in a solution state at all time.
Figure 1b briefly presents the particle size distribution of TheranoGel and TheranoMic at 10, 25, and 37°C. The z-average diameter of TheranoGel was ca. 140 nm (PDI 0.3) at 10°C. At 25°C, there were two populations with z-average diameter at ca. 140 nm and ca. 450 nm (PDI 0.5). At 37°C, the z-average diameter of TheranoGel was ca. 800 nm (PDI ≥ 0.6). Regardless of temperature changes, the z-average diameter of TheranoMic was ca. 190 nm (PDI ≤ 0.2).
In vitro release profile of drugs from TheranoGel at 37°C demonstrated that all 3 drugs were slowly and simultaneously released, reaching ca. 26% content release within 48 h whereas TheranoMic released 3 drugs at a rapid rate, reaching 68–70% content release within 48 h (Fig. 2). Applying in vitro drug release data to the one phase association, it is estimated that the half-lives of drug release are ca. 101 (R2 = 0.9714), 102 (R2 = 0.9690), and 97 h (R2 = 0.9642) for paclitaxel, rapamycin, and LS30, respectively, from TheranoGel and ca. 18 (R2 = 0.9814), 20 (R2 = 0.9765), and 20 h (R2 = 0.9621) for paclitaxel, rapamycin, and LS301, respectively, from TheranoMic. Complete physical erosion of TheranoGel occurred in 7 days.
Fig. 2.

Release profiles of paclitaxel, rapamycin, and LS301 from TheranoGel (a) and TheranoMic (b) at 37°C(****p < 0.0001; TheranoGel versus TheranoMic at 48 h).
Evaluation of TheranoGel in the ES-2-luc Xenograft Model of Intraperitoneal Ovarian Cancer
TheranoGel and TheranoMic carrying paclitaxel, rapamycin, and LS301 at 60, 60 mg/kg, and 440 μg/kg, respectively, were evaluated in an ES-2-luc xenograft model of human peritoneal ovarian cancer (Fig. 3). First, therapeutic efficacy of each formulation was monitored longitudinally by scanning whole-body bioluminescence images for 3 days (Fig. 3a), and bioluminescence intensity of ES-2-luc cells in the peritoneal cavity was calculated as % bioluminescence intensity (%BLI) of the bioluminescence signal in the animals at each day relative to the bioluminescence signal observed in the animals at day 0 (Fig. 3b). In the control, after a single IP injection of empty hydrogels, animals developed tumor burden significantly from 100 to 3480 ± 445% in %BLI within 3 days. A single IP injection of TheranoGel appeared to be effective in treating ES-2-luc ovarian cancer showing a remarkable decrease of tumor burden from 100% down to 7 ± 1% in %BLI within 3 days. A single IV or IP injection of TheranoMic did not show tumor burden resection, demonstrating increased %BLI from 100 to 110 ± 21% for IP TheranoMic and from 100 to 471 ± 236% for IV TheranoMic. There was no notable toxicity observed in ES-2-luc-bearing mice upon injections of all formulations.
Fig. 3.
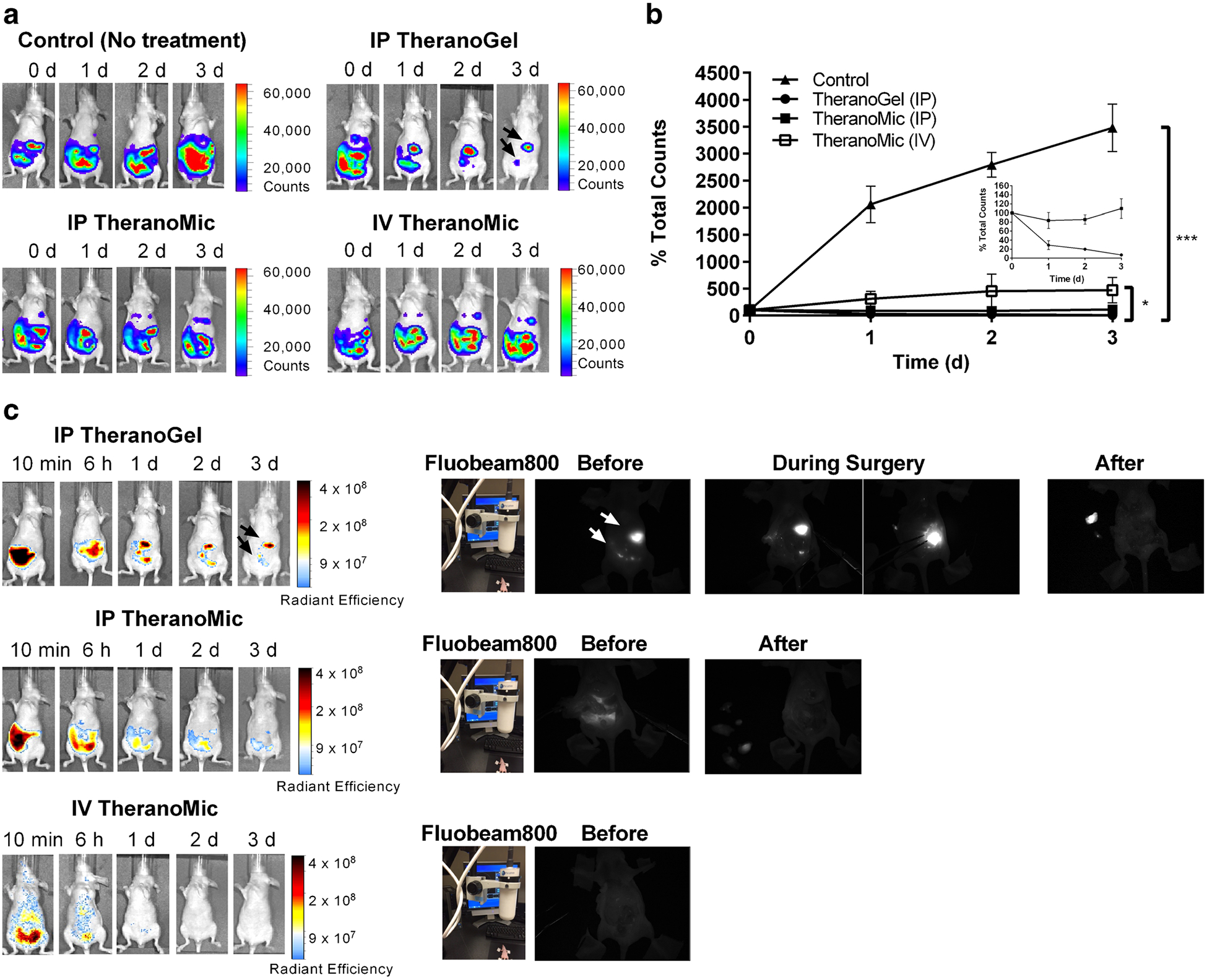
Assessment of therapeutic activities of a single injection of IP empty hydrogels, IP TheranoGel, IP TheranoMic, and IV TheranoMic in whole-body bioluminescence images (a) and relative bioluminescence intensity of ROIs in a time-dependent manner (b). Assessment of diagnostic activities of a single injection of IP TheranoGel, IP TheranoMic, and IV TheranoMic in fluorescence images post-treatment, before, during, and after the surgery (c) (*p < 0.05; TheranoMic (IV) versus TheranoGel (IP) at day 3, ***p < 0.001; control versus TheranoGel (IP) at day 3).
Diagnostic activity of each formulation was monitored longitudinally by scanning whole-body fluorescence images for 3 days (Fig. 3c and SI). A single IP injection of TheranoGel not only debulked tumor tissues but also successfully illuminated residual tumor tissues; Objects delineated in fluorescence images at days 1, 2, and 3, exactly matched with objects delineated in bioluminescence images at days 1, 2, and 3. A single IV or IP injection of TheranoMic provided non-specific and the weaker fluorescence signal in the peritoneal cavity at day 3, presumably due to the rapid dilution of drugs upon the faster content release from micellar solutions. The practical feasibility of each formulation for the intraoperative image guidance was assessed in ES-2-luc-bearing mice using Fluobeam800. According to the black-and-white images provided by Fluobeam800, FLU+ tumor-like tissues were brightly illuminated (white-in-color) before, during, and after the surgery in ES-2-luc-bearing mice treated with IP TheranoGel; One large and a couple of small FLU+ tissues were strongly illuminated in the carcass of animals before and during the surgical procedure. After the surgery, FLU+ tumor-like tissues removed from the carcass and placed on the left side of the carcass were also clearly visible. Meanwhile, the carcass remained dark in the Fluobeam images after the surgery. ES-2-luc cancer cells and tissues were much more weakly visualized upon an injection of IP TheranoMic under the IVIS and Fluobeam800. There were no distinguishable FLU+ tissues identified on days 1–3 post treatment of IV TheranoMic.
Removed tissues were evaluated to confirm if those were genuinely cancerous tissues by obtaining bioluminescence images of the removed tumor-like tissues and carcass (Fig. 4). For the control (no treatment, the conventional surgery), % BLU+ of the carcass over the total BLU+ of the carcass and removed tumor-like tissues were 48 ± 16%, demonstrating that ca. 48% of tumor tissues were left undissected. Guided by intraoperative optical fluorescence images, the animals treated with an IP TheranoGel carried 5 ± 2% of residual tumor tissues in the peritoneum after the surgery, indicating that ca. 95% of tumor tissues were successfully removed. % BLU+ of the carcass of animal treated with an IP TheranoMic or IV TheranoMic were 20 ± 11 and 58 ± 18%.
Fig. 4.
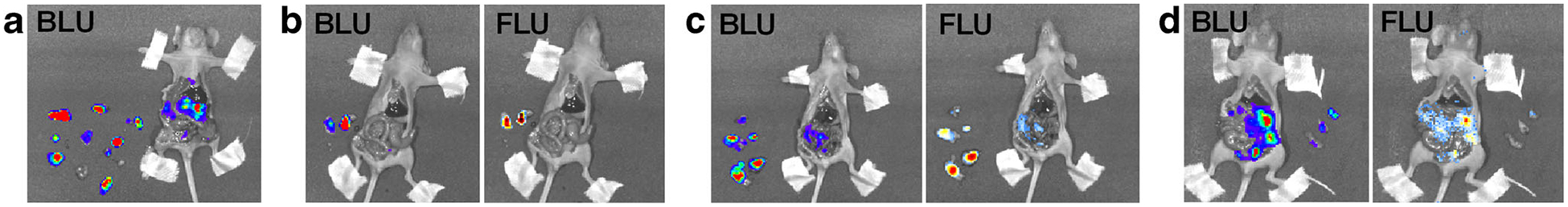
Bioluminescence (BLU) and fluorescence (FLU) images of ES-2-luc-bearing mice and dissected tumor-like tissues after the surgery on day 3 post treatment of a single injection of IP empty hydrogels (control) (a), IP TheranoGel (b), IP TheranoMic (c), and IV TheranoMic (d).
DISCUSSION
The fundamental goal of IP drug delivery is to increase the exposure of the peritoneal cancer cells to the delivered drugs while decreasing systemic toxicity in patients with ovarian cancer or gastrointestinal malignancies (3). Under this rationale, PLGA-b-PEG-b-PLGA sol-gels can be exploited to fulfill the major requirements for IP delivery of drugs: (i) multi-drug solubilization, (ii) facile preparation, (iii) convenient administration, (iv) biocompatibility, and (v) sustained release of drugs.
(i and ii) PLGA-b-PEG-b-PLGA triblock copolymers showed a unique ability to act as a carrier of hydrophobic drugs, including paclitaxel, rapamycin, and LS301 at 6, 6 mg/mL, and 44 μg/mL, respectively (called TheranoGel). Paclitaxel, rapamycin, and LS301 were simply incorporated in PLGA-b-PEG-b-PLGA sol-gels by the freeze-drying method, resulting in a solid that could be reconstituted with cold sterile water for injection. Unlike other hydrogels, preparation of TheranoGel carrying multi-drugs by freeze-drying did not require UV irradiation or additives such as plasticizers (13).
(iii and iv) TheranoGel made a complete sol-to-gel transition at 37°C within 2–5 min and quickly turned back to solution at 5°C within 5 min. The sol-to-gel transition of PLGA-b-PEG-b-PLGA is known to be governed by an interplay of hydrophobic interaction of PLGA blocks and hydrogen bond ing of PEG with water, which vary with temperature (6, 14, 15). Below ambient temperature, PLGA-b-PEG-b-PLGA exists as unimers in water with a low capacity for drug solubilization. Above the critical micelle temperature, unimers assemble into micelles that have a unique flower-like supramolecular structure that has loops of PEG protruding into water to form a shell region and a core of PLGA that can host a poorly water-soluble anticancer agent, resulting in drug solubilization. Above the critical gelation temperature, bridging of PLGA-b-PEG-b-PLGA polymers among PLGA-b-PEG-b-PLGA micelles, loss of hydrogen bonding between PEG and water and enhanced hydrophobic interaction occurs, leading to aggregation and a sol-to-gel transition, defined as a loss of flowability. Notably, PLGA-b-PEG-b-PLGA gels retain a capacity for drug solubilization. At higher temperatures, hydrophobic interaction of PLGA increases for PLGA-b-PEG-b-PLGA gels, resulting in phase separation. This sol-to-gel transition has been proven by assessing the distribution of particle sizes of TheranoGel at 10, 25, and 37°C. At 10°C, TheranoGel carried particles roughly at 140 nm presumably due to the formation of flower petal-like loops of triblock co-polymers. At 25°C, TheranoGel carried particles roughly at 140 and 450 nm presumably due to the coexistence of loops and flower-like micelles. At 37°C, it is predicted to yield an aggregated micellar network by showing an average particle size at ca. 800 nm. Meanwhile, TheranoMic (PEG-b-PLA micelles carrying paclitaxel, rapamycin, and LS301) existed in a solution state with an average particle size of ca. 190 nm regardless of temperature changes because PEG-b-PLA micelles at the concentration of 50 mg/mL do not involve in temperature-responsive sol–gel transitions. This sol-to-gel transition of TheranoGel at body temperature can promote instant formation of a gel-depot at the injection site in patients, allowing loco-regional delivery of drugs. It is envisioned that TheranoGel can be easily and safely implemented into clinical practice; Rehydration of lyophilized TheranoGel in cold sterile water followed by withdrawal of TheranoGel at a sol-state using cold injection tools (syringe and needle) will prevent clogging of needles. Furthermore, PLGA-b-PEG-b-PLGA hydrogels have been shown their proven safety profiles and biocompatibility/biodegradability in patients. It has been previously reported that without notable side toxicity, a couple of pieces of PLGA-b-PEG-b-PLGA hydrogel depots were found in the peritoneal cavity of nude mice at day 6 after an IP administration (6).
(v) TheranoGel (gel) exhibited slow in vitro release profiles of paclitaxel, rapamycin, and LS301, reaching <30% content release within 48 h whereas TheranoMic (sol) released 3 drugs more rapidly, reaching >60% content release within 48 h. Two dissimilar release patterns of drugs for TheranoGel and TheranoMic supported the assertion that hydrophobic drugs were released based on the different release mechanisms; release of hydrophobic drugs for PLGA-b-PEG-b-PLGA hydrogels is driven by initial diffusion followed by a prolonged polymer degradation whereas release of hydrophobic drugs incorporated in PEG-b-PLA micelles is primarily diffusion controlled (16). It is expected that slower release profiles for TheranoGel can permit longer residence time of drugs in the peritoneal cavity. Having considered anatomical/physiological characteristics of the peritoneal cavity, peritoneal ovarian cancer and pharmacokinetic advantages of IP delivery of drugs, gradual release of drugs directly into the peritoneal cavity could result in a profound increase in the exposure of the cavity to the released drugs for an extended periods of time while reducing systemic and local toxicity in patients. On the other hand, uncontrolled exposure of peritoneal cavity to the free drugs at substantially greater concentrations (10–1000 folds) may induce dose-limiting local side effect. Considering challenges of IP administration regarding side effects such as abdominal pain, nausea, and vomiting, being able to reduce injection frequency using TheranoGel can be one of the major advantages in a practical aspect whereas IP administration of TheranoMic or free drugs/agents may require multi-dosing regimens to meet the desired drug residence in the peritoneal cavity of patients for extended periods of time.
The in vivo activity of IP TheranoGel carrying paclitaxel, rapamycin, and LS301 was examined in ES-2-luc xenograft mice of human peritoneal ovarian cancer. TheranoMic carrying paclitaxel, rapamycin, and LS301 was IV or IP injected in ES-2-luc xenograft mice to incorporate different factors (injection routes and drug release rates) potentially affecting theranostic results. It is noted that dissolution of free drugs in injectable solutions followed by injections of free drugs in vivo was not possible because of the low intrinsic solubility of free drugs. A single IP injection of TheranoGel exerted an excellent anticancer activity by decreasing tumor burden from 100% down to 7 ± 1% within 3 days. Animals administered with a single IP injection of TheranoMic maintained their tumor burden from 100 to 110 ± 21% in 3 days. A single IV injection of TheranoMic was not effective in arresting tumor proliferation in 3 days, resulting in a notable increase of tumor burden from 100 to 471 ± 236%. As reported elsewhere, IP delivery of drugs has shown to be effective in increasing regional drug concentrations and controlling formation of malignant ascites (3, 6). The in vivo results in this study lend to the strong support to the rationale of IP drug delivery; IP delivery of drugs appears to be more effective in controlling ascites formation and tumor progression than IV delivery of drugs in peritoneal ovarian cancer (IP TheranoMic versus IV TheranoMic). Slower release of drugs for TheranoGel appears to heighten the therapeutic activity of delivered drugs presumably by gradual exposure of the peritoneal cavity to drugs followed by slow exit of drugs from the peritoneal cavity (IP TheranoGel versus IP TheranoMic).
Most importantly, IP delivery of LS301 (an optical fluorescence imaging agent) via PLGA-b-PEG-b-PLGA sol-gels provided remarkably accurate optical images of tumor tissues in ES-2-luc xenograft mice whereas IV delivery of LS301 via PEG-b-PLA micelles lacked accuracy in visualizing tumor tissues. LS301 is a molecular probe consisting of cysteine glycine-arginine-aspartic acid-serine-proline-cystein-lysine peptide sequence and an NIR fluorescence imaging agent, cypate (10). LS301 has been shown to preferentially bind to tumor margins in vivo (10). Previously, it has been reported that IV delivery of LS301 via 20% DMSO/80% saline solution clearly visualized tumor tissues in a 4T1-luc orthotropic mammary tumor model (10). Tumor tissues were further dissected under the intraoperative image-guidance using wireless surgical goggles. Surprisingly, residual tumor tissues and small nodules that were not visible to the naked eyes could be detected in the intraoperative optical fluorescence images. Achilefu et al. stated in the article that LS301 rapidly distributed in the systemic circulation immediately after IV injection of LS301 and, the highest tumor-to-background contrast ratio in fluorescence was obtained within 1 h post injection. In our in vivo study using an ES-2-luc peritoneal ovarian cancer model, IP TheranoGel could result on a superior diagnostic activity than IV TheranoMic mainly because of the nature of peritoneal ovarian cancer; IP delivery of LS301 increased a loco-regional concentration of LS301 and as a result, improved the diagnostic activity in peritoneal ovarian cancer over IV delivery of LS301. Furthermore, intraperitoneally delivered LS301 via a gel-depot for extended periods of time may continuously expose the peritoneal cavity to LS301 molecules, presenting the most accurate and brightest visualization of tumor tissues. Another reason systemically delivered LS301 were not effective in illuminating tumor tissues in our in vivo study was due to the delayed acquisition of in vivo fluorescence images for the intraoperative image-guided surgery (at day 3 post injection). Visualization of tumor tissues emitted by LS301 molecules accumulated in tumor tissues could be more optimal if scanned within a few h after an injection of IV ThernoMic.
The superior diagnostic activity of IP TheranoGel was extended to the successful peritoneal surgery guided by the realtime feedback of optical fluorescence images of LS301 accumulated in tumor tissues and cells. Tumor tissues were brightly visible in ES-2-luc xenograft mice before and during the peritoneal surgery. It is evident that intraoperative optical fluorescence images permitted identification of submillimeter sized tumors including large tumor tissues as large as ca. 6 mm in diameter (Supplementary video SII) and small tumor tissues as small as ca. 1 mm in diameter (Supplementary vide SIII). Considering the assumption that the tumor debulking could be optimized by removing all tumor tissues >1 cm in size, the real-time feedback of fluorescence images could improve tumor detection and provide efficient surgical tumor resection (1). After the surgery, only 5% tumor tissues were left undissected in animals treated with an IP TheranoGel. IP and IV TheranoMic were not as effective as IP TheranoGel in visualizing tumor tissues before, during, and after surgery, leaving substantial amount of tumor tissues behind in surgical objects.
CONCLUSION
In summary, thermosensitive PLGA-b-PEG-b-PLGA entrained paclitaxel, rapamycin, and LS301 (called TheranoGel) in solution at 10°C, made a sol-to-gel transition at 37°C, and simultaneously released all three components with an extended release profile. A single IP injection of TheranoGel resulted in efficient tumor debulking and accurate delineation of tumor tissues in intraoperative NIR fluorescence images and ultimately, improved the surgical out come in ES-2-luc ovarian cancer-bearing mice. This proof of-concept study highlighted advances of IP delivery of multi drugs in treating and visualizing peritoneal ovarian cancer while providing pharmacological flexibility of a gel-depot based drug delivery system, and its safety profile permitting a rapid transition of promising preclinical findings into clinical testings.
A future study will assess biodistribution of payloads in tumor tissues and evaluate theranostic activity of TheranoGel in peritoneal ovarian cancer-bearing rats to provide insights by actually simulating the series of treatment plan, primary surgery; IP injection of TheranoGel; closure of abdomen; intraoperative optical NIR image-guided second-look surgery; and monitoring longitudinal treatment outcome in advanced ovarian cancer. Moreover, we envision that hydrogel vehicle itself will serve as a physical barrier between surgical wound and internal organs and prevent postsurgical peritoneal adhesion and adhesion-associated side effects such as small-bowel obstruction, chronic abdominal and pelvic pain, and female infertility (17, 18).
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by St. Louis College of Pharmacy Faculty Research Incentive Fund.
ABBREVIATIONS
- 17-AAG
17-allylamino-17-demethoxygeldanamycin
- BLI
Bioluminescence intensity
- BLU
Bioluminescence
- FLU
Fluorescence
- IP
Intraperitoneal
- IV
Intravenous
- LS301
Cysteine-glycine-arginine-aspartic acid-serine-proline-cystein-lysine-cypate
- Luc
Luciferase
- mTOR
Mammalian target of rapamycin
- NIR
Near infrared
- PEG-b-PLA
Poly-(ethylene glycol)-block-(D,L-lactide)
- PLGA-b-PEG-b-PLGA
(D,L-lactide-co-glycolide)-block-poly-(ethylene glycol)-block-poly-(D,L-lactide-co-glycolide)
- TheranoGel
PLGA-b-PEG-b-PLGA hydrogels carrying paclitaxel, rapamycin, and LS301
- TheranoMic
PEG-b-PLA micelles carrying paclitaxel, rapamycin, and LS301
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11095-016-1968-3) contains supplementarymaterial, which is available to authorized users.
REFERENCES
- 1.Fagotti A, Gallotta V, Romano F, Fanfani F, Rossitto C, Naldini A, et al. Peritoneal carcinosis of ovarian origin. World J Gastrointest Oncol. 2010;2(2):102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ushijima K Treatment for recurrent ovarian cancer-at first relapse. J Oncol. 2010;2010:497429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markman M Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4(5):277–83. [DOI] [PubMed] [Google Scholar]
- 4.Markman M, Walker JL. Intraperitoneal chemotherapy of ovarian cancer: a review, with a focus on practical aspects of treatment. J Clin Oncol. 2006;24(6):988–94. [DOI] [PubMed] [Google Scholar]
- 5.Elstad NL, Fowers KD. OncoGel (ReGel/paclitaxel)–clinical applications for a novel paclitaxel delivery system. Adv Drug Deliv Rev. 2009;61(10):785–94. [DOI] [PubMed] [Google Scholar]
- 6.Cho H, Kwon GS. Thermosensitive poly-(d, l-lactide-co-glycolide)block-poly(ethylene glycol)-block-poly-(d, l-lactide-co-glycolide) hydrogels for multi-drug delivery. J Drug Target. 2014;22(7):669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellard SL, Clemons M, Gelmon KA, Norris B, Kennecke H, Chia S, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol. 2009;27(27): 4536–41. [DOI] [PubMed] [Google Scholar]
- 8.Mita MM, Mita AC, Chu QS, Rowinsky EK, Fetterly GJ, Goldston M, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26(3):361–7. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell A, Faivre S, Buris HA 3rd, Rea D, Papadimitrakopoulou V, Shand N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26(10):1588–95. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Bauer AQ, Akers WJ, Sudlow G, Liang K, Shen D, et al. Hands-free, wireless goggles for near-infrared fluorescence and real-time image-guided surgery. Surgery. 2011;149(5):689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho H, Lai TC, Kwon GS. Poly(ethylene glycol)-block-poly(epsilon-caprolactone) micelles for combination drug delivery: evaluation of paclitaxel, cyclopamine and gossypol in intraperitoneal xenograft models of ovarian cancer. J Control Release. 2013;166(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw TJ, Senterman MK, Dawson K, Crane CA, Vanderhyden BC. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol Ther. 2004;10(6): 1032–42. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed EM. Hydrogel: Preparation, characterization, and applications: a review. J Adv Res. 2015;6(2):105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DSSMS, Kim SW, Lee H, Park I, Chang T. Novel thermore-versible gelation of biodegradable PLGA-block-PEO-block-PLGA triblock copolymers in aqueous solution. Macromol Rapid Commun. 2001;22(8):587–92. [Google Scholar]
- 15.Qiao M, Chen D, Ma X, Liu Y. Injectable biodegradable temperature-responsive PLGA-PEG-PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int J Pharm. 2005;294(1–2):103–12. [DOI] [PubMed] [Google Scholar]
- 16.Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 17.Kamel RM. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):111–8. [DOI] [PubMed] [Google Scholar]
- 18.Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. recent advances in prevention and management. Dig Surg. 2001;18(4):260–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


