Abstract
Background:
Chronic venous ulcers sometimes fail to heal in spite of adequate treatment. Nonhealing is possibly because of chronic activation of β-2 adrenergic receptor (B2-AR) in the keratinocytes by endogenously generated catecholamines, which inhibits keratinocyte migration. Blocking of B2-AR using β-blockers has been reported to promote wound healing through keratinocyte migration, angiogenesis, and fibroblast migration. Topical timolol, a B2-AR antagonist, is used to promote wound healing.
Aims and Objectives:
The aim of this study was to compare the efficacy of topical timolol versus saline in chronic venous leg ulcers and to compare the mean reduction in ulcer area at the end of 4 weeks.
Materials and Methods:
Twenty patients were randomized into two groups. Patients in Group 1 were treated with one drop of 0.5% topical timolol every alternate days for 4 weeks. Ulcer margins were measured and ulcer area calculated every week for 4 weeks. Similar dressing and measurement were carried out for patients with saline for 4 weeks. Healing rate was assessed by the percentage of reduction of ulcer area of both groups at week 4.
Results:
The mean reduction in the ulcer size in timolol group was 86.80%, and in saline group 43.82% at the end of 4 weeks.
Conclusion:
Topical timolol is an easy, noninvasive therapy that can be recommended for chronic ulcer.
Keywords: Healing, timolol, venous leg ulcer
INTRODUCTION
Venous stasis ulcers account for 80%–90% of all leg ulcers. The current treatment for venous ulcers is compression and wound care with dressings. All of which have not showed any significant improvement in healing. Keratinocyte migration is a key component in wound healing and it is required for skin reepithelization and complete wound repair. β-2 adrenergic receptor (B2-AR) is expressed primarily on human keratinocytes.[1] Activation of this receptor inhibits keratinocyte migration. Blocking of B2-AR pathway using B2-AR antagonists (β-blockers) has been reported to promote wound healing through different mechanisms, including keratinocyte migration, promotion of angiogenesis, increased dermal fibroblast migration, and epidermal differentiation.[2] Timolol, a commercially available B2-AR antagonist, used as an intraocular glaucoma medication has been shown to promote wound healing when instilled in chronic leg ulcers. The purpose of this study was to assess the efficacy of topical timolol in wound healing.
MATERIALS AND METHODS
Twenty patients diagnosed with chronic venous ulcer of lower extremities, presenting to the OPD over the study period and satisfying the inclusion and exclusion criteria, were included in the study after obtaining informed consent.
The inclusion criteria of the study included patients who were over 18 years old, venous ulcers lasting for at least 4 weeks with no improvement with good wound care, dressings, and compression stockings.
The exclusion criteria of the study included infected ulcers, patients on systemic β-blockers, patients with asthma and COPD, and patients with venous leg ulcers on systemic treatment. Each patient after randomization was allotted into treatment or control group.
Group 1 (treatment group). Ten patients with chronic venous leg ulcer receiving topical timolol
Group 2 (control group). Ten patients with chronic venous ulcer receiving saline dressing.
All patients were advised to continue compression stockings.
Group 1. One milliliter of timolol contains 5mg of the drug. Each milliliter contains 20 drops. Hence, each drop contains 0.25mg of the drug. To each patient, one drop was instilled every 2 cm² of wound edge and then covered with sterile dressing. This was repeated every alternate days.
Group 2. One drop of saline was instilled every 2 cm² of wound and then covered with sterile dressing every alternate days.
Ulcer margin was measured by measuring the greatest length and the greatest width, perpendicular to the greatest length every week for a maximum period of 4 weeks. Digital photographs were taken during each visit.
The primary end point is to evaluate the ulcer reduction rate at the end of 4 weeks. The improvement was also evaluated with photography and the ulcer reduction rate in both groups was calculated at the end of 1 month after beginning the treatment. The healing rate between the study and control group was compared statistically using Satistical Package for Social Sciences Software Version 24.0. Armonk, NY: IBM Corp.
RESULTS
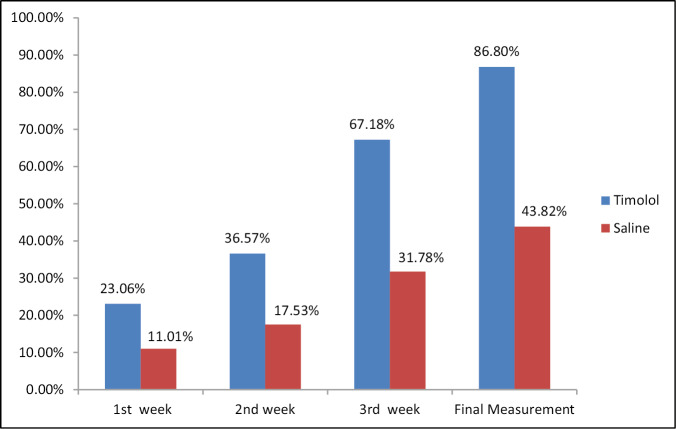
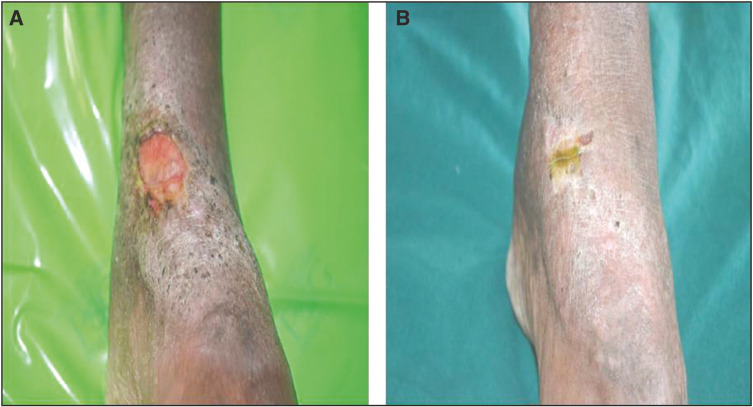
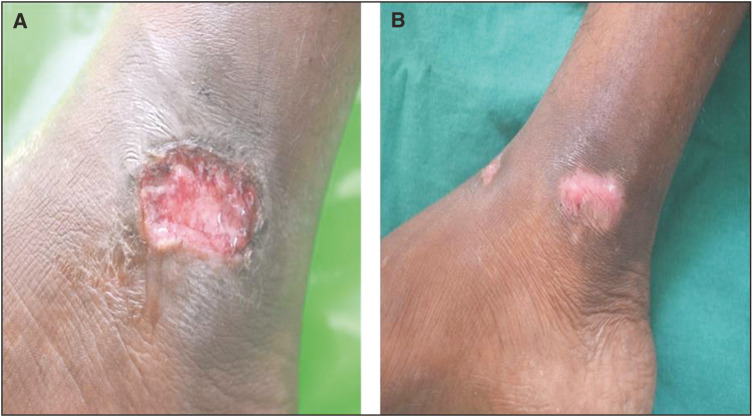
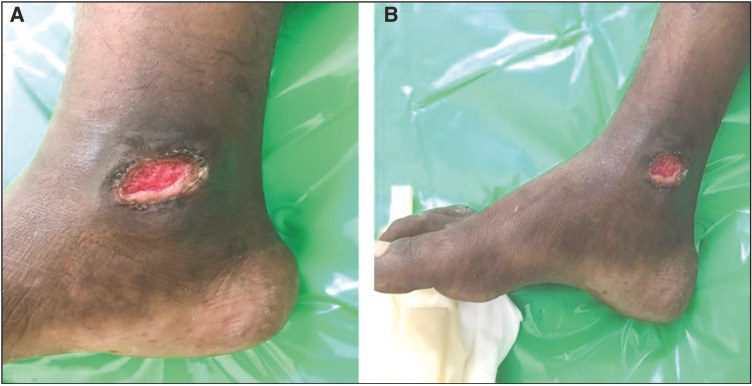
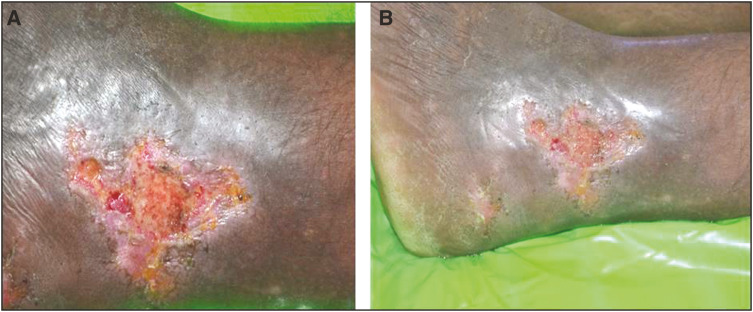
A total of 20 patients meeting the inclusion and exclusion criteria were enrolled in the study. All the patients were suffering from chronic venous ulcers. The result was assessed on the basis of ulcer area reduction. The mean ulcer area reduction after 1 week in the timolol group was 23.06%, after 2 weeks it was 36.57%, at 3 weeks it was 67.18%, and after 4 weeks it was 86.80% [Table 1]. However in the saline group, it was 11.01%, 17.53%, 31.78%, and 43.82%, respectively [Table 2]. The overall mean reduction in the ulcer area was 86.80% in the patients with timolol dressing as compared to 43.82% in the patients treated with saline dressing [Figure 5]. There was a complete closure of the ulcer in five patients (50%) in the timolol group [Figures 1 and 2]. There was no case of complete closure of the ulcer in the saline group [Figures 3 and 4].
Table 1.
Percentage of improvement in patients treated with timolol dressing
| S. no. of patients | Initial measurement in cm2 | Measurement after 1 week | Measurement after 2 weeks | Measurement after 3 weeks | Final measurement | Percentage of improvement |
|---|---|---|---|---|---|---|
| 1 | 10 | 6.6 | 4 | 1.2 | 0 | 100 |
| 2 | 6.21 | 4.12 | 2.56 | 1 | 0 | 100 |
| 3 | 2.52 | 2.17 | 1.54 | 1.2 | 0.7 | 72.2 |
| 4 | 9 | 7.3 | 6.4 | 4.2 | 1.2 | 86.66 |
| 5 | 3.5 | 3.1 | 2.76 | 2 | 1.12 | 68 |
| 6 | 24.36 | 18.75 | 14.06 | 7.36 | 5.8 | 76.19 |
| 7 | 9.24 | 7.3 | 7.2 | 2.04 | 0 | 100 |
| 8 | 19.25 | 17.27 | 16.83 | 14.1 | 6.16 | 65.71 |
| 9 | 31.5 | 22 | 19.25 | 2.7 | 0 | 100 |
| 10 | 4.16 | 2.58 | 2.4 | 0.6 | 0 | 100 |
| Mean percentage of improvement | 0% | 23.06% | 36.57% | 67.184% | 86.8% | 86.8% |
Table 2.
Percentage of improvement in patients treated with saline dressing
| S. no. of patients | Initial measurement in cm2 | Measurement after 1 week | Measurement after 2 weeks | Measurement after 3 weeks | Final measurement | Percentage of improvement |
|---|---|---|---|---|---|---|
| 1 | 1.4 | 1.2 | 1.0 | 0.8 | 0.55 | 60.71 |
| 2 | 3.8 | 3.1 | 2.7 | 2.1 | 1.72 | 54.73 |
| 3 | 31.35 | 30 | 28.5 | 23 | 20 | 36.2 |
| 4 | 1.6 | 1.5 | 1.4 | 1.25 | 1.06 | 33.75 |
| 5 | 6.21 | 5.94 | 5.8 | 5.5 | 4.40 | 29.14 |
| 6 | 13.6 | 13.1 | 12.8 | 12.5 | 11.9 | 12.5 |
| 7 | 5.4 | 4.7 | 4.5 | 3.8 | 3.12 | 42.2 |
| 8 | 9.5 | 8.1 | 7.1 | 5.25 | 3.9 | 58.94 |
| 9 | 3.7 | 3.1 | 3.0 | 2.1 | 1.63 | 55.94 |
| 10 | 7.4 | 6.3 | 5.7 | 4.1 | 3.4 | 54.05 |
| Mean percentage of improvement | 0% | 11.01% | 17.53% | 31.78% | 43.82% | 43.82% |
Figure 5.
Bar diagram showing the comparison of the mean improvement in timolol group and saline group after every week and also after the treatment for 4 weeks
Figure 1.
(A) Before application of timolol dressing. (B) After the application of timolol dressing at the end of 4 week
Figure 2.
(A) Before application of timolol dressing. (B) After the application of timolol dressing at the end of 4 weeks
Figure 3.
(A) Before application of saline dressing. (B) After application of saline dressing at the end of 4 weeks
Figure 4.
(A) Before application of saline dressing. (B) After application of saline dressing at the end of 4 weeks
Although there was a reduction in the ulcer size in the timolol group as compared to the saline group at the end of 4weeks, it was not statistically significant at P = 0.104 [Table 3].
Table 3.
Group statistics showing standard deviation (SD) and P value
| Groups | N | Mean | SD | P value | |
|---|---|---|---|---|---|
| Initial measurement | Timolol | 10 | 11.9 | 9.7 | 0.519 |
| Saline | 10 | 8.4 | 8.8 | ||
| Measurement after first week | Timolol | 10 | 9.1 | 7.3 | 0.806 |
| Saline | 10 | 7.7 | 8.5 | ||
| Measurement after second week | Timolol | 10 | 7.7 | 6.5 | 0.963 |
| Saline | 10 | 7.2 | 8.2 | ||
| Measurement after third week | Timolol | 10 | 3.6 | 4.1 | 0.340 |
| Saline | 10 | 6.0 | 6.8 | ||
| Final measurement | Timolol | 10 | 1.4 | 2.4 | 0.104 |
| Saline | 10 | 5.1 | 6.1 |
There were no local or systemic side effects noted.
DISCUSSION
Chronic ulcers are a common problem, particularly in older people resulting in repeated admissions. The treatment of chronic ulcer presents a therapeutic challenge and includes good wound care, dressings, compression therapy, and lifestyle modifications. Dressings often used for chronic ulcers include hydrocolloid, hydrogel foams, pastes, silver impregnated dressings, and simple nonadherent dressings. A meta-analysis of 42 randomized controlled trials with more than 1000 patients showed no significant difference among dressing types.[3] Hydrocolloid dressings that are expensive are not shown to have a healing benefit over simple nonadherent dressings. There is no clear evidence to support the use of one dressing over the other and so the choice of dressing depends on the cost and ease of application.[3]
B2-AR receptors are present on cells in multiple organ systems, including the skin. Within skin, receptors are present on keratinocytes, fibroblasts, and melanocytes. B2-AR is the dominant subtype expressed on keratinocyte surfaces.[1] The influence of this receptor has been studied by observing the effects of agonist and antagonist at the receptor. In addition, the epidermis contains the full range of enzymes necessary to synthesize catecholamines such as norepinephrine and epinephrine to bind to various adrenergic receptors and delay wound healing.[4] B2-AR antagonists have been reported to promote wound healing, and one potential mechanism of action via keratinocyte migration. Keratinocyte migration occurs by the facilitation of chemotaxis, the polarization of cells, and activation of extracellular signal-related kinases essential in the signaling of promigratory pathways. The B2-AR activation inhibits keratinocyte migration by activating the serine/threonine phosphatase 2A, which downregulates phosphorylation of extracellular signal-related kinases necessary for migration.[5] Therefore, B2-AR antagonists prevent the phosphorylation of phosphatase 2A and downstream extracellular signal-related kinase promotion, inducing a promigratory pathway in keratinocytes. The B2-AR antagonists improve the ability of keratinocytes to respond to such migratory signals, whereas the B2-AR agonists decrease keratinocytes ability to respond.[6] Angiogenesis and dermal fibroblast proliferation are also regulated by B2-AR. The B2-AR antagonists have been found to promote angiogenesis in chick chorioallantoic membrane assays and in vivo murine wound models. Dermal fibroblast migration is also increased when exposed to B2-AR antagonists, and epidermal differentiation is improved with B2-AR antagonists.[7,8] Catecholamines, such as epinephrine and norepinephrine, are endogenously generated agonists, and these are also generated by the wound epithelium. Therefore, it is possible that one pathogenic mechanism contributing to nonhealing skin wounds is the chronic activation of B2-AR by endogenously generated catecholamines.[9] Blocking of B2-AR pathway using pharmacological antagonists promotes keratinocyte migration and re-epithelization in vitro, in human skin, in an ex vivo skin wound model, and in vivo in animal preclinical studies.[10,11]
People with burns have been treated with systemic B2-AR antagonist proponalol to diminish the effect of excessive catecholamine response after burn injury. Mohammadi et al.[12] carried out a randomized double-blind trial in burn patients. They included 79 burn patients, 37 in the study group and 42 in the control group. The participants in the study group who received oral propranolol had better wound healing.
A case report by Tang et al.[13] showed that topical timolol application produced complete wound healing in a refractory wound on the mid back.
A cohort study by Chakkittakandiyil et al.[14] showed the efficacy, tolerability, and safety of timolol maleate 0.5% solution in treating infantile hemangiomas. Thus, Manahan et al.[15] used topical timolol as a safe and inexpensive drug to treat chronic ulcer. Braun et al.[16] used topical timolol in five patients with recalcitrant wounds; 0.5% topical timolol was instilled in chronic, recalcitrant wounds of these five patients, and improvement was noted in all five, including three patients who had complete healing. The mean reduction in wound size after 7 weeks was 78.2%. Lev-Tov et al.[17] successfully treated chronic venous leg ulcer with topical timolol.
A study by Thomas et al.[18] showed that the patients treated with topical timolol had a significantly higher ulcer healing rate of 61.79% in the study group and 29.62% in the control group at the end of 12 weeks. Analysis showed that there were significant differences in percentage change in ulcer of the study and control groups. Thus, treatment using topical application of timolol resulted in faster healing rate compared with conventional treatment.
Our study showed that patients in timolol group (86.80%) had significant reduction in the size of the ulcer as compared to the saline group (43.82%) after 4 weeks of treatment. There was no side effects and the treatment was well tolerated.
Our research attempts to study the efficacy of topical timolol on healing of chronic venous leg ulcer. Topical timolol is cost-effective, easily available as a 0.5% solution, and is therefore a treatment option in chronic ulcers. This is the first randomized controlled study comparing timolol and saline on healing rate of chronic venous ulcers. The limitation of our study is the sample size. However, more studies are needed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sivamani RK, Lam ST, Isseroff RR. Beta adrenergic receptors in keratinocytes. Dermatol Clin. 2007;25:643–53, x. doi: 10.1016/j.det.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pullar CE, Chen J, Isseroff RR. PP2A activation by beta2-adrenergic receptor agonists: novel regulatory mechanism of keratinocyte migration. J Biol Chem. 2003;278:22555–62. doi: 10.1074/jbc.M300205200. [DOI] [PubMed] [Google Scholar]
- 3.Palfreyman S, Nelson EA, Michaels JA. Dressings for venous leg ulcers: systematic review and meta-analysis. BMJ. 2007;335:244. doi: 10.1136/bmj.39248.634977.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126:1948–65. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- 5.Pullar CE, Chen J, Isseroff RR. PP2A activation by beta2-adrenergic receptor agonists: novel regulatory mechanism of keratinocyte migration. J Biol Chem. 2003;278:22555–62. doi: 10.1074/jbc.M300205200. [DOI] [PubMed] [Google Scholar]
- 6.Pullar CE, Zhao M, Song B, Pu J, Reid B, Ghoghawala S. Beta-adrenergic receptor agonists delay while antagonists accelerate epithelial wound healing: evidence of an endogenous adrenergic network within the corneal epithelium. J Cell Physiol. 2007;211:261–72. doi: 10.1002/jcp.20934. [DOI] [PubMed] [Google Scholar]
- 7.Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117:397–405. doi: 10.1242/jcs.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pullar CE, Isseroff RR. The beta 2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J Cell Sci. 2006;119:592–602. doi: 10.1242/jcs.02772. [DOI] [PubMed] [Google Scholar]
- 9.Sivamani RK, Shi B, Griffiths E, Vu SM, Lev-Tov HA, Dahle S, et al. Acute wounding alters the beta2-adrenergic signaling and catecholamine synthetic pathways in keratinocytes. J Invest Dermatol. 2014;134:2258–66. doi: 10.1038/jid.2014.137. [DOI] [PubMed] [Google Scholar]
- 10.Pullar CE, Rizzo A, Isseroff RR. Beta-adrenergic receptor antagonists accelerate skin wound healing: evidence for a catecholamine synthesis network in the epidermis. J Biol Chem. 2006;281:21225–35. doi: 10.1074/jbc.M601007200. [DOI] [PubMed] [Google Scholar]
- 11.Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009; 6:e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammadi AA, Bakhshaeekia A, Alibeigi P, Hasheminasab MJ, Tolide-ei HR, Tavakkolian AR, et al. Efficacy of propranolol in wound healing for hospitalized burn patients. J Burn Care Res. 2009;30:1013–7. doi: 10.1097/BCR.0b013e3181b48600. [DOI] [PubMed] [Google Scholar]
- 13.Tang JC, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135–8. doi: 10.1111/j.1524-4725.2011.02200.x. [DOI] [PubMed] [Google Scholar]
- 14.Chakkittakandiyil A, Phillips R, Frieden IJ, Siegfried E, Lara-Corrales I, Lam J, et al. Timolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas: a retrospective, multicenter, cohort study. Pediatr Dermatol. 2012;29:28–31. doi: 10.1111/j.1525-1470.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- 15.Manahan MN, Peters P, Scuderi S, Surjana D, Beardmore GL. Topical timolol for a chronic ulcer–a case with its own control. Med J Aust. 2014;200:49–50. doi: 10.5694/mja13.10823. [DOI] [PubMed] [Google Scholar]
- 16.Braun LR, Lamel SA, Richmond NA, Kirsner RS. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400–2. doi: 10.1001/jamadermatol.2013.7135. [DOI] [PubMed] [Google Scholar]
- 17.Lev-Tov H, Dahle S, Moss J, Isseroff RR. Successful treatment of a chronic venous leg ulcer using a topical beta-blocker. J Am Acad Dermatol. 2013;69:e204–5. doi: 10.1016/j.jaad.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Thomas B, Kurien JS, Jose T, Ulahannan SE, Varghese SA. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg Venous Lymphat Disord. 2017;5:844–50. doi: 10.1016/j.jvsv.2017.04.019. [DOI] [PubMed] [Google Scholar]