Abstract
Background
The aim of this study was to assess the relationship between serum lipoprotein (a) (Lp[a]) concentration and the requirement for peripheral artery disease (PAD) operations or incidence of major adverse cardiovascular events.
Methods and Results
A total of 1472 people with PAD presenting with intermittent claudication (n=355), abdominal aortic aneurysm (n=989) or critical limb ischemia (n=128) were prospectively recruited from 4 outpatient clinics in Australia. Lp(a) was measured in serum samples collected at recruitment using an immunoassay. Participants were followed for a median (interquartile range) of 2.4 (0.1–6.1) years to record requirement for any PAD operation, defined to include any open or endovascular PAD intervention (lower limb peripheral revascularization, abdominal aortic aneurysm repair, other aneurysm repair, or carotid artery revascularization). Myocardial infarctions, strokes, and deaths were also recorded. The association of Lp(a) with events was assessed using Cox proportional hazard analysis adjusting for traditional risk factors. Participants with Lp(a) ≥30 mg/dL had a greater requirement for any PAD operation (hazard ratio, 1.20, 95% CI, 1.02–1.41) and lower limb peripheral revascularization alone (hazard ratio 1.33, 95% CI, 1.06–1.66) but no increased risk of major adverse cardiovascular events or all‐cause mortality. Lp(a) ≥50 mg/dL and a 40 mg/dL increase in Lp(a) were also associated with an increased risk of lower limb peripheral revascularization alone but not with other outcomes.
Conclusions
In participants with PAD referred for hospital management those with high Lp(a) had greater requirement for lower limb peripheral revascularization but Lp(a) was not consistently associated with other clinical events.
Keywords: Peripheral artery disease, surgical treatment, lipoprotein (a)
Subject Categories: Peripheral Vascular Disease
Clinical Perspective
What Is New?
High serum lipoprotein (a) was associated with a small increased requirement for lower limb revascularization but not consistently associated with other clinical events in people referred for management of peripheral artery disease.
What Are the Clinical Implications?
More research is required to test whether lipoprotein (a) lowering therapies are beneficial for people with established arterial disease, such as those with peripheral artery disease.
Nonstandard Abbreviations and Acronyms.
Lp(a) lipoprotein (a)
PAD peripheral artery disease
MACE major adverse cardiovascular events
AAA abdominal aortic aneurysm
HR hazard ratio
LDL‐C low‐density lipoprotein‐cholesterol
eGFR estimated glomerular filtration rate
CHD coronary heart disease
IQR interquartile range
Diseases of the abdominal aorta and its branches (peripheral artery disease; PAD), are a collection of chronic occlusive and aneurysmal diseases, commonly presenting as intermittent claudication, abdominal aortic aneurysm (AAA) and critical limb ischemia.1, 2, 3, 4 People with these PAD presentations have an incidence of major cardiovascular events (MACE; myocardial infarction, stroke or cardiovascular death) and requirement for arterial interventions, such as lower limb peripheral revascularization or AAA repair, of ≈20% and 30%, respectively, during short‐term follow‐up.5, 6, 7, 8, 9, 10
Apolipoprotein B‐containing lipoproteins, such as low‐density lipoprotein‐cholesterol (LDL‐C), are strongly implicated in cardiovascular disease.11 There is now a range of effective drugs for lowering LDL‐C, such as statins and PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors, which have been shown to substantially reduce MACE.8, 12 Lipoprotein (a) (Lp [a]) is an LDL‐like particle with a highly glycosylated lipoprotein, called apolipoprotein (a), that has potent atherothrombotic and inflammatory properties.13 Circulating concentrations of Lp (a) are highly heritable and transmitted in families as a co‐dominant trait.13 Population and case‐control studies suggest that a high circulating concentration of Lp (a) is an important risk factor for the development of PAD.14, 15, 16, 17, 18, 19, 20 The association of elevated circulating concentrations of Lp (a) with clinical events in people with established PAD has not been well studied. A smaller number of studies have investigated the association of elevation of Lp (a) with MACE21 and peripheral events22, 23 in people with established PAD. These studies have had a number of limitations, such as small sample sizes,22, 23 studying populations of people presenting with PAD mixed with those presenting with other cardiovascular diseases,21 not including the full spectrum of PAD presentations,21, 22, 23 and focusing on imaging outcomes such as patency22 rather than clinical end points.
Lp (a) is implicated in promoting atherosclerosis progression, thrombosis, and inflammation, which are all pathological processes thought to be critical in stimulating PAD progression and the need for surgical intervention.24 There is, therefore, a need to clarify the association of elevation in Lp (a) with requirement for peripheral artery operations and the incidence of MACE in a heterogeneous population of people with established PAD. The aims of the current study were to examine the associations of elevation in serum Lp (a) with requirement for peripheral artery operations and the incidence of MACE in a heterogeneous population of people with established PAD.
Methods
Requests for access to data, analytic methods, and study materials should be made to the corresponding author.
Study Design and Participants
This investigation was designed as part of an ongoing prospective cohort study that commenced in 2002 and aimed to identify risk factors associated with the outcomes of people with PAD.25, 26 Participants were recruited from outpatient vascular services in Australia, including The Townsville University Hospital, the Mater Hospital Townsville, Gosford Vascular Services and The Royal Brisbane and Women's Hospital. The current study included participants with intermittent claudication, critical limb ischemia or an AAA diagnosed by a vascular specialist.27, 28 Intermittent claudication was diagnosed in people with a history of leg pain on walking and absence of lower limb pulses, ankle‐brachial index ≤0.9 or imaging evidence of a lower limb artery stenosis of ≥50% or occlusion.25, 26 AAA was diagnosed if the orthogonal maximum outer to outer infra‐renal aortic wall diameter was ≥30 mm measured from ultrasound or computed tomographic angiography.25, 26 Critical limb ischemia was diagnosed in people with symptoms of rest pain, gangrene or arterial ulceration and evidence of limb ischemia.25, 26 Written informed consent was obtained from all participants upon entry into the study. The study was performed in accordance with the Declaration of Helsinki and ethical approval was granted from institutional ethics committees (HREC/13/QTHS/125 and HREC/14/QTHS/203).
Blood Analyses
At recruitment, participants had a fasting blood test, to measure total cholesterol, triglyceride, LDL‐C, high‐density lipoprotein‐cholesterol, and C‐reactive protein, as previously described.28, 29 Serum was isolated at recruitment and stored at −80°C until later batch analysis of Lp (a). Lp (a) was measured by an automated latex enhanced immunoassay (Quantia Lp (a) assay, Abbott Laboratories). Briefly, the Quantia Lp (a) assay is a turbidimetric immunoassay for the estimation of Lp (a) in human serum in an Architect autoanalyzer C16000 (Abbott Diagnostics) and is based on the principle of an agglutination reaction.30, 31 The interassay coefficient of variation for samples was <7%. Lp (a) measurement using this method has been significantly correlated with an liquid chromatography–mass spectrometry method (n=50, r=0.972, P<0.001). The definition of elevated Lp (a) is controversial with some guidelines considering ≥30 mg/dL abnormal32 but others proposing ≥50 mg/dL.33 In the current study ≥30 mg/dL was selected for the primary analysis since this threshold has been previously associated with increased clinical events in prior studies of other high‐risk populations22, 34 and also within meta‐analyses.33 Sensitivity analyses were performed using other thresholds, including ≥50 mg/dl and a 40 mg/dL (approximate standard deviation in the population) increase. Serum creatinine was measured as previously described26 and estimated glomerular filtration rate calculated using the Chronic Kidney Disease Epidemiology Collaboration formula.26
Definitions of Risk Factors and Medications Recorded
Smoking history was classified as current, former, or never smokers.25, 35 Hypertension and diabetes mellitus were defined by a past history of diagnosis or treatment for these conditions.25, 35 Coronary heart disease (CHD) was defined as a history of myocardial infarction, angina, or previous treatment for CHD.35 All prescribed medications including antiplatelet agents, statins, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, beta‐blockers, and metformin were recorded at the time of recruitment.
Definition and Assessment of Cardiovascular and Peripheral Vascular Outcomes
Participants were followed up as part of normal care. Outpatient follow‐up was performed according to local clinical practice. Participants were offered at least annual follow‐up. Outcome data were recorded during clinical reviews on prospectively defined case report forms. Hospital charts and electronic records were also reviewed by a vascular specialist. Outcome data were also obtained from linked hospital admission records as previously described.25, 26, 36, 37 Linked data were obtained from the Queensland Hospital admitted patient data collection which is regularly audited to minimize inaccuracies.38 PAD operations recorded included lower limb (open and endovascular) peripheral revascularization, carotid artery revascularization, open and endovascular AAA repair, and other aneurysm repair.6, 10, 36 The primary outcome was requirement for any PAD operation, defined as including any of the peripheral artery interventions listed above. Secondary outcomes were MACE, lower limb (open or endovascular) peripheral revascularization, and AAA (open or endovascular) repair. The tertiary outcome was all‐cause mortality. MACE was defined as the first occurrence of a major cardiovascular event including myocardial infarction, stroke, or cardiovascular death. Decisions about requirement for operative interventions were at the discretion of the treating consultant surgeon but were in line with current international guidelines,39, 40 including lifestyle limiting intermittent claudication failing to respond to conservative therapy, symptomatic or large asymptomatic AAA, and critical limb ischemia.10, 41
Sample Size
We aimed to have adequate power to test our hypothesis that Lp (a) ≥30 mg/dL was associated with a greater requirement for any PAD operation. Our prior studies suggest that PAD operations are common in people with established PAD occurring in between 30% and 40% during short‐term follow‐up.6, 10, 36 Monte‐Carlo simulations suggest that a multivariable regression model is powered sufficiently when 10 outcome events per degree of freedom of the predictor variables are observed.42 We estimated that the 2‐year requirement for any PAD operation would be ≈30% and planned to adjust for 10 variables, some with multiple degrees of freedom, including age, sex, smoking (current, former or never smokers), presentation (intermittent claudication, AAA, or critical limb ischemia), diabetes mellitus, hypertension, CHD, statin prescription, LDL‐C, and estimated glomerular filtration rate in the regression models. Based on these estimates we felt that a sample size of >1000 participants would be well powered to test the main hypothesis.
Data Analysis
Data were analyzed using the SPSS v 25 (IBM, Armonk, NY) software package. Continuous data that were not normally distributed, as confirmed using the Shapiro–Wilk test, and were presented as median and interquartile range. Between‐group comparisons were conducted using the Mann–Whitney U and Kruskal–Wallis tests. Categorical variables were compared using Pearson Chi squared test. Kaplan–Meier analysis was used to calculate the observed incidence of PAD operations and MACE and log‐rank test performed to statistically compare incidence rates. For these analyses P<0.05 were considered significant. Cox proportional hazard analyses assessed the association of serum Lp (a) ≥30 mg/dL with events adjusted for other risk factors. Analyses were adjusted for age, sex, smoking (current, former, or never smokers), presenting problem (intermittent claudication, AAA, or critical limb ischemia), diabetes mellitus, hypertension, CHD, statin prescription, LDL‐C, and estimated glomerular filtration rate. Sensitivity analyses examined the association of events with Lp (a) ≥50 mg/dL and Lp (a) as a continuous variable using a 40 mg/dL increase (approximate SD in the cohort). For Cox regression analyses, Lp (a) was considered associated with the event concerned when the 95% CI of the hazard ratio did not cross one. The effect of age on the association of Lp (a) ≥30 mg/dL with events was also examined. For these analyses, participants were divided into those aged <72 and ≥72 years (approximate median age of the cohort). The full Cox regression models are available from the corresponding author.
Results
Participants and Outcomes
Between March 2002 and March 2018, 1472 people with intermittent claudication (n=355), AAA (n=989) and critical limb ischemia (n=128) were recruited. Participants’ age and sex, history of smoking and diabetes mellitus, and prescription of aspirin and metformin varied significantly according to the presenting problem (Table 1). Serum concentrations of total cholesterol, LDL‐C, and C‐reactive protein, and estimated glomerular filtration rate also varied significantly between participants with different presenting problems (Table 1). Participants were followed for a median (interquartile range) of 2.4 (0.1–6.1) years. During that time participants required a total of 1084 PAD operations, including 672 lower limb peripheral revascularizations, 353 AAA repairs, 31 carotid artery revascularizations, and 28 repairs of other aneurysms. The participants also had a total of 230 myocardial infarctions and 75 strokes. There were a total of 368 deaths of which 242 were attributed to cardiovascular disease.
Table 1.
Comparison of Risk Factors in Participants With Different Presenting Problems
| Risk Factor | Presenting Peripheral Artery Disease Problem | P Value | ||
|---|---|---|---|---|
| Intermittent Claudication (n=355) | AAA (n=989) | Critical Limb Ischemia (n=128) | ||
| Age, y | 66.4 (59.7–73.3) | 74.0 (68.3–79.0) | 68.7 (59.6–76.2) | <0.001* |
| Men | 265 (74.6%) | 808 (81.7%) | 81 (63.3%) | <0.001* |
| Smoking | <0.001* | |||
| Current | 133 (37.5%) | 286 (28.9%) | 35 (27.3%) | |
| Previous | 182 (51.3%) | 574 (58.0%) | 60 (46.9%) | |
| Never | 40 (11.3%) | 129 (13.0%) | 33 (25.8%) | |
| Hypertension | 264 (74.4%) | 765 (77.4%) | 95 (74.2%) | 0.439 |
| Diabetes mellitus | 120 (33.8%) | 199 (20.1%) | 63 (49.2%) | <0.001* |
| Coronary heart disease | 170 (47.9%) | 507 (51.3%) | 66 (51.6%) | 0.533 |
| Aspirin | 258 (72.7%) | 589 (59.6%) | 84 (65.6%) | <0.001* |
| Other anti‐platelets | 67 (18.9%) | 195 (19.7%) | 19 (14.8%) | 0.415 |
| Statin | 257 (72.4%) | 665 (67.2%) | 83 (64.8%) | 0.138 |
| Fibrates | 13 (3.7%) | 29 (2.9%) | 7 (5.5%) | 0.297 |
| Calcium channel blocker | 105 (29.6%) | 263 (26.6%) | 39 (30.5%) | 0.423 |
| Beta‐blockers | 112 (31.5%) | 374 (37.8%) | 42 (32.8%) | 0.081 |
| Angiotensin converting enzyme inhibitor | 141 (39.7%) | 407 (41.2%) | 61 (47.7%) | 0.286 |
| Angiotensin receptor blocker | 86 (24.2%) | 223 (22.5%) | 32 (25.0%) | 0.713 |
| Metformin | 82 (23.1%) | 95 (9.6%) | 40 (31.3%) | <0.001* |
| Estimated glomerular filtration rate (mL/min per 1.73 m2)† | 83.0 (64.8–94.0) | 68.0 (52.0–83.0) | 75.5 (58.3–92.0) | <0.001* |
| Total cholesterol‡ | 4.40 (3.60–5.30) | 4.30 (3.60–5.10) | 4.00 (3.40–4.75) | 0.001* |
| Triglyceride | 1.60 (1.10–2.30) | 1.40 (1.10–2.00) | 1.35 (1.00–2.00) | 0.086 |
| Low‐density lipoprotein† | 2.30 (1.70–3.10) | 2.30 (1.80–3.00) | 1.95 (1.60–2.60) | <0.001* |
| High‐density lipoprotein‡ | 1.20 (1.00–1.48) | 1.14 (0.95–1.40) | 1.20 (0.95–1.50) | 0.126 |
| C‐reactive protein (mg/L)§ | 3.0 (1.0–5.0) | 3.0 (1.9–6.0) | 5.0 (2.2–10.0) | <0.001* |
| Lipoprotein (a) (mg/dL) | 18.7 (7.4–52.9) | 18.3 (9.4–55.2) | 15.5 (5.8–40.8) | 0.067 |
| Lipoprotein (a) levels | 0.261 | |||
| <30 mg/dL | 221 (62.3%) | 632 (63.9%) | 90 (70.3%) | |
| ≥30 mg/dL | 134 (37.7%) | 357 (36.1%) | 38 (29.7%) | |
Shown are numbers (%) or medians and interquartile ranges. Lipids were reported in mmol/L. AAA indicates abdominal aortic aneurysm.
*P<0.01. Missing in †8, ‡1, and §36.
Association of High Serum Lp (a) With Risk Factors
At recruitment, 943 (64.1%) and 529 (35.9%) participants had Lp (a) < and ≥30 mg/dL, respectively. Three hundred and eighty‐nine (26.4%) and 154 (10.5%) participants had Lp (a) ≥50 and ≥100 mg/dL, respectively. As previously reported in other cohorts,43, 44 participants with Lp (a) ≥30 mg/dL were significantly more likely to be prescribed statins than those with lower Lp (a) levels (Table 2). Participants with Lp (a) ≥30 mg/dL also had significantly higher serum total cholesterol and LDL‐C than those with lower Lp (a) levels (Table 2).
Table 2.
Comparison of Risk Factors in Participants With Different Serum Lipoprotein (a) Concentrations
| Risk Factor | Serum Lipoprotein Concentration (mg/dL) | P Value | |
|---|---|---|---|
| <30 (n=943) | ≥30 (n=529) | ||
| Age | 72.0 (66.2–77.4) | 71.3 (65.0–78.1) | 0.451 |
| Men | 737 (78.2%) | 417 (78.8%) | 0.763 |
| Smoking | 0.163 | ||
| Current | 275 (29.2%) | 179 (33.8%) | |
| Previous | 533 (56.5%) | 283 (53.5%) | |
| Never | 135 (14.3%) | 67 (12.7%) | |
| Hypertension | 708 (75.1%) | 416 (78.6%) | 0.123 |
| Diabetes mellitus | 234 (24.8%) | 148 (28.0%) | 0.184 |
| Coronary heart disease | 464 (49.2%) | 279 (52.7%) | 0.193 |
| Aspirin | 583 (61.8%) | 348 (65.8%) | 0.130 |
| Other anti‐platelets | 172 (18.2%) | 109 (20.6%) | 0.268 |
| Statin | 621 (65.9%) | 384 (72.6%) | 0.008* |
| Fibrates | |||
| Calcium channel blocker | 248 (26.3%) | 159 (30.1%) | 0.122 |
| Beta‐blockers | 339 (35.9%) | 189 (35.7%) | 0.932 |
| Angiotensin‐converting enzyme inhibitor | 386 (40.9%) | 223 (42.2%) | 0.648 |
| Angiotensin receptor blocker | 225 (23.9%) | 116 (21.9%) | 0.399 |
| Metformin | 145 (15.4%) | 72 (13.6%) | 0.359 |
| Estimated glomerular filtration rate (mL/min per 1.73 m2)† | 71.0 (56.0–86.0) | 73.0 (54.0–88.0) | 0.277 |
| Total cholesterol‡ | 4.25 (3.50–5.00) | 4.40 (3.70–5.10) | 0.009* |
| Triglyceride | 1.50 (1.10–2.10) | 1.40 (1.00–2.10) | 0.157 |
| Low‐density lipoprotein† | 2.20 (1.70–3.00) | 2.40 (1.90–3.10) | 0.004* |
| High‐density lipoprotein‡ | 1.14 (0.95–1.40) | 1.20 (1.00–1.46) | 0.088 |
| C‐reactive protein (mg/L)§ | 3.0 (1.9–6.0) | 3.00 (1.9–6.2) | 0.938 |
Shown are numbers (%) or medians and interquartile ranges. Lipids were reported in mmol/L.
*P<0.01. Missing in †8, ‡1, and §36.
Association of High Serum Lp (a) With First Occurrence of Clinical Events
According to Kaplan Meier analyses, the requirements for the following procedures over 3 years were: any PAD operation 51.2% and 56.3% (P=0.041 by log rank test; Figure 1), lower limb peripheral revascularization 25.3% and 30.4% (P=0.029 by log rank test; Figure 2), and AAA repair 27.1% and 28.7% (P=0.282 by log rank test; Figure 3) in participants with Lp (a) < and ≥30 mg/dL, respectively. The incidences of MACE were 17.7% and 19.7% (P=0.250 by log‐rank test; Figure 4) and all‐cause mortality were 15.3% and 13.0% (P=0.885 by log‐rank test; Figure 5) in participants with Lp (a) < and ≥30 mg/dL, respectively. After adjustment for other risk factors, Lp (a) ≥30 mg/dL was associated with greater incidence of any PAD operation and lower limb peripheral revascularization alone but not AAA repair (Table 3 and Table S1). The incidence of MACE and all‐cause mortality were not associated with Lp (a) ≥30 mg/dL (Table 3).
Figure 1.
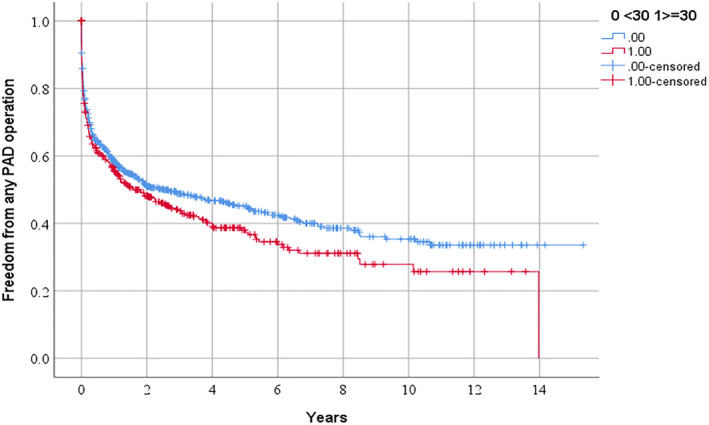
Freedom from requirement for any peripheral artery disease operation in people referred for management of peripheral artery disease in relation to serum lipoprotein (a) ≥30 mg/dL.
PAD indicates peripheral artery disease.
Figure 2.
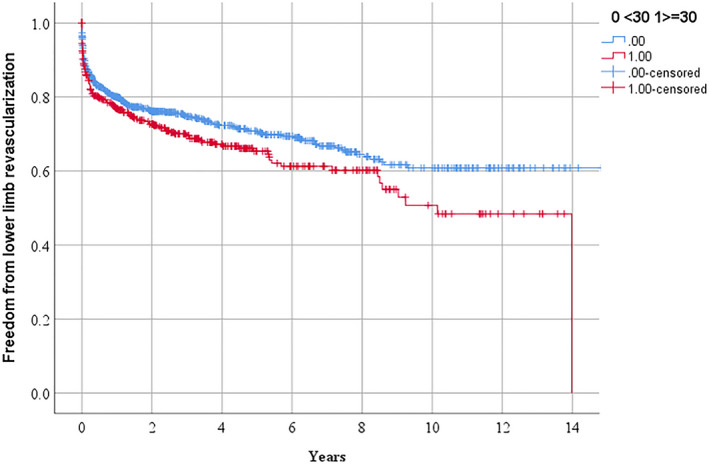
Freedom from requirement for lower limb peripheral revascularization in people referred for management of peripheral artery disease in relationship to serum lipoprotein (a) ≥30 mg/dL.
Figure 3.
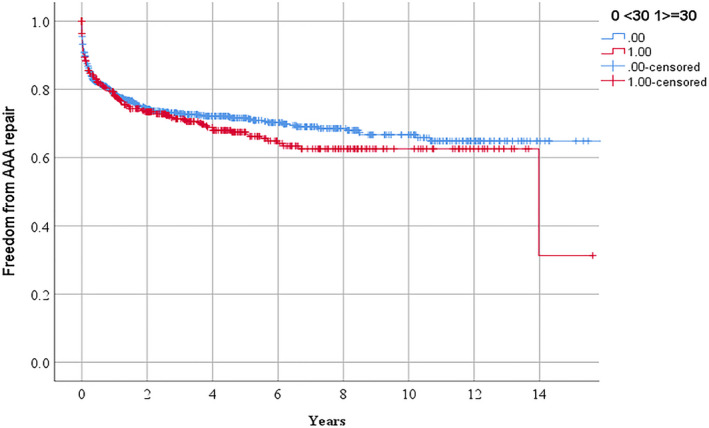
Freedom from requirement for abdominal aortic aneurysm repair in people referred for management of peripheral artery disease in relationship to serum lipoprotein (a) ≥30 mg/d.
AAA indicates abdominal aortic aneurysm.
Figure 4.
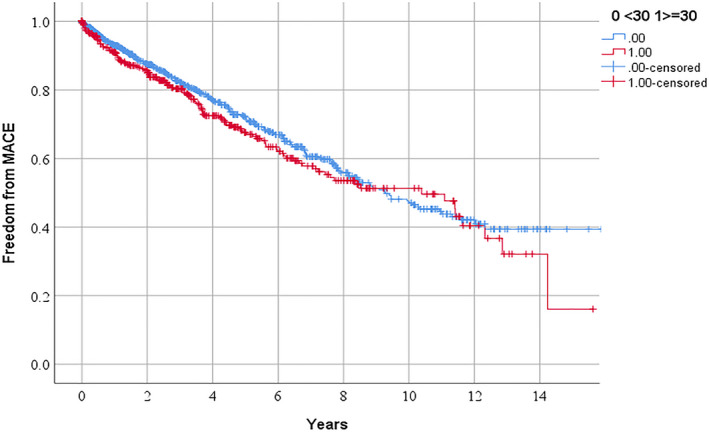
Freedom from major adverse cardiovascular events in people referred for management of peripheral artery disease in relationship to serum lipoprotein (a) ≥30 mg/dL.
MACE indicates major cardiovascular event (ie, myocardial infarction, stroke, or cardiovascular death).
Figure 5.
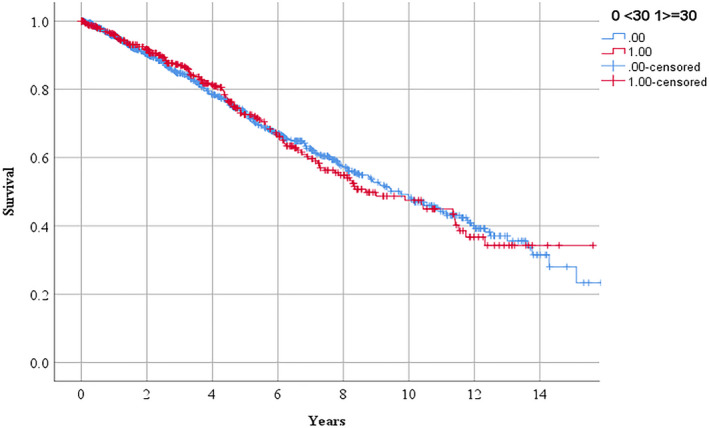
Survival in people referred for management of peripheral artery disease in relationship to serum lipoprotein (a) ≥30 mg/dL.
Table 3.
Association of Lipoprotein (a) ≥30 or ≥50 mg/mL With Clinical Events in People With PAD
| Event Type or Model | Serum Lipoprotein Concentration (mg/mL) | |||
|---|---|---|---|---|
| ≥30 mg/mL | ≥50 mg/mL | |||
| Hazard model | Unadjusted | Adjusted | Unadjusted | Adjusted |
| Any PAD operation | 1.18 (1.01–1.38) | 1.20 (1.02–1.41) | 1.08 (0.91–1.29) | 1.10 (0.92–1.31) |
| Lower limb peripheral revascularization | 1.27 (1.02–1.58) | 1.33 (1.06–1.66) | 1.17 (0.92–1.47) | 1.29 (1.00–1.65) |
| AAA repair | 1.13 (0.91–1.41) | 1.14 (0.92–1.43) | 1.15 (0.91–1.45) | 1.06 (0.83–1.34) |
| MACE | 1.14 (0.91–1.41) | 1.16 (0.93–1.45) | 1.06 (0.84–1.35) | 1.12 (0.88–1.43) |
| All‐cause mortality | 1.02 (0.82–1.26) | 1.08 (0.87–1.35) | 1.04 (0.82–1.32) | 1.15 (0.90–1.47) |
Shown are hazard ratios and 95% CIs for models that were unadjusted or adjusted for age, sex, smoking history, diabetes mellitus, hypertension, coronary heart disease, presenting problem, statin prescription, low‐density lipoprotein‐cholesterol, and estimated glomerular filtration rate. Adjusted analyses did not include 16 participants because of missing low‐density lipoprotein‐cholesterol or estimated glomerular filtration rate results. AAA indicates abdominal aortic aneurysm; MACE, major cardiovascular events; and PAD, peripheral arterial disease.
Association of High Serum Lp (a) With Total Occurrence of Each Clinical Event
Of the 640 participants who had any PAD operation, 422 had 1 operation and 218 had multiple operations (122 participants had 2, 41 had 3, 28 had 4, 11 had 5, 4 had 6, 5 had 7, 3 had 8, 1 had 9, 1 had 10, 1 had 11, and 1 had 15). The total numbers of clinical events in participants with Lp (a) <30 and ≥30 mg/dL are shown in Table 4.
Table 4.
Total Numbers of Peripheral Artery Disease Operations and Cardiovascular Events in Participants With Different Serum Lipoprotein (a) Concentrations
| Event or Follow‐Up | Lipoprotein (mg/dL) | |
|---|---|---|
| <30 (n=943) | ≥30 (n=529) | |
| Follow‐up, y | 2.3 (0.1–6.2) | 2.6 (0.1–5.6) |
| All‐cause mortality | 242 (25.7%) | 126 (23.8%) |
| Cardiovascular death | 156 (16.5%) | 86 (16.3%) |
| Total myocardial infarction events | 137 | 93 |
| Number of participants who the myocardial infarction events occurred in | 86 (9.1%) | 54 (10.2%) |
| Total stroke events | 47 | 28 |
| Number of participants who the stroke events occurred in | 39 (4.1%) | 24 (4.5%) |
| Total of any peripheral artery disease operations | 64 | 436 |
| Number of participants who the peripheral artery disease operations occurred in | 393 (41.7%) | 247 (46.7%) |
| Total of lower limb revascularizations | 392 | 280 |
| Number of participants who the lower limb revascularizations occurred in | 203 (21.5%) | 137 (25.9%) |
| Total abdominal aortic aneurysm repairs | 219 | 134 |
| Number of participants who abdominal aortic aneurysm repairs occurred in | 206 (21.8%) | 130 (24.6%) |
Shown are median (interquartile range) or numbers (%).
Sensitivity Analyses
After adjustment for other risk factors, Lp (a) ≥50 mg/dL or a 40 mg/dL increase in Lp (a) were associated with a greater requirement for lower limb peripheral revascularization but not with other events (Tables 3 and 5). In participants aged <72 years, Lp (a) ≥30 mg/dL was associated with a greater requirement for any PAD operation but not with other events (Table 6). In participants aged ≥72 years, Lp (a) ≥30 mg/dL was associated with a greater requirement for lower limb peripheral revascularization and all‐cause mortality but not with other events (Table 6).
Table 5.
Association of a 40 mg/dL Higher Lipoprotein (a) with Clinical Events in People With PAD
| Event | Cox Proportional Hazard Model | |
|---|---|---|
| Unadjusted | Adjusted | |
| Any PAD operation | 1.04 (0.98–1.11) | 1.05 (0.98–1.12) |
| Lower limb peripheral revascularization | 1.09 (1.00–1.18) | 1.11 (1.02–1.21) |
| AAA repair | 1.06 (0.97–1.15) | 1.04 (0.95–1.15) |
| MACE | 1.00 (0.91–1.09) | 1.02 (0.93–1.12) |
| All‐cause mortality | 0.97 (0.88–1.06) | 1.00 (0.90–1.10) |
Shown are hazard ratios and 95% CIs for models that are unadjusted or adjusted for age, sex, smoking history, diabetes mellitus, hypertension, coronary heart disease, presenting problem, statin prescription, low‐density lipoprotein‐cholesterol and estimated glomerular filtration rate. Adjusted analyses did not include 16 participants because of missing low‐density lipoprotein‐cholesterol, or estimated glomerular filtration rate results. AAA indicates abdominal aortic aneurysm; MACE, major cardiovascular events; and PAD, peripheral arterial disease.
Table 6.
Association of Lipoprotein (a) ≥30 mg/mL With Clinical Events in People With PAD Aged <72 or ≥72 Years
| Event | Age (y) | |
|---|---|---|
| <72 | ≥72 | |
| Any PAD operation | 1.27 (1.03–1.58) | 1.18 (0.91–1.53) |
| Lower limb peripheral revascularization | 1.19 (0.89–1.58) | 1.71 (1.15–2.55) |
| AAA repair | 1.37 (0.99–1.89) | 1.09 (0.79–1.50) |
| MACE | 1.12 (0.80–1.57) | 1.23 (0.90–1.67) |
| All‐cause mortality | 0.73 (0.50–1.06) | 1.35 (1.01–1.80) |
Shown are hazard ratios and 95% CIs for models that were adjusted for age, sex, smoking history, diabetes mellitus, hypertension, coronary heart disease, presenting problem, statin prescription, low‐density lipoprotein‐cholesterol and estimated glomerular filtration rate. Adjusted analyses did not include 16 participants because of missing low‐density lipoprotein‐cholesterol, or estimated glomerular filtration rate results. AAA indicates abdominal aortic aneurysm; MACE, major cardiovascular events; and PAD, peripheral artery disease.
Discussion
The main finding of this study was that participants with high serum Lp (a) were more likely to require lower limb peripheral revascularization. This finding was consistent in analyses using different definitions of high Lp (a) but not present in a sensitivity analysis limited to younger participants. High Lp (a) was not consistently associated with other clinical events including the incidence of MACE and mortality.
The rate of major cardiovascular events has been reported to be higher in people with PAD than those presenting with other cardiovascular diseases.8 The cohort investigated in this study had a high rate of clinically important events. During a median follow‐up of ≈2.5 years, about 40% of participants had at least 1 PAD operation and one quarter died. These event rates are higher than a number of recent reports from randomized trials although these typically include highly selected people.7, 8 The participants included in the current study had a high frequency of cardiovascular risk factors, such as old age, critical limb ischemia, current smoking, diabetes mellitus, and CHD, which likely explains the high incidence of clinical events found. As has been previously reported in other studies, the prescription of best medical management was not optimal in the current cohort.45 About 30% of participants were not prescribed statins for example. This also likely contributed to the high incidence of events found. More effective methods are needed for implementation of best medical management in people with PAD.
Lp (a) has been suggested as a target for reducing clinical events using novel treatments to lower its circulating concentration.46, 47, 48 In the current study, high serum Lp (a) was consistently associated with a greater requirement for lower limb peripheral revascularization. Participants with Lp (a) ≥30 mg/mL, however, only had a 1.2‐fold increased rate of lower limb peripheral revascularization. Also, the risk of lower limb peripheral revascularization was only mildly elevated in people with Lp (a) ≥50 mg/mL (hazard ratio 1.10). Furthermore, high Lp (a) was not associated with other events, such as the incidence of MACE. These findings differ from those reported from other cohorts.21, 49 A study of a mixed cohort of 1503 people with coronary, cerebrovascular, or PAD reported that those with Lp (a) ≥50 mg/mL had hazard ratios of 19.5 (95% CI, 10.5–36.1) and 54.5 (95% CI, 25.4–116.7) for myocardial infarction and ischemic stroke, respectively.21 The disparate findings of the current study could relate to the characteristics of the participants involved, such as their high‐risk profile, high incidence of clinical events, and the inclusion of people with unique presentations, such as critical limb ischemia. Further studies are needed in similar PAD populations to clarify the importance of high Lp (a) as a risk factor for clinical events. Overall, however, the findings of the current study do not make a strong case for novel therapies to lower Lp (a) in people with PAD.
The current study has a number of strengths and weaknesses. A large heterogeneous population of people with PAD was included meaning findings are most relevant to similar populations. Information about a large range of vascular interventions was collected allowing detailed analysis of predictors of PAD operations. Analyses were adjusted for a large number of potential confounding factors. Sensitivity analyses were also performed. This study also has a number of limitations. It was an observational study and while high Lp (a) was not associated with a number of clinical events, such as MACE, this does not rule out a benefit of Lp (a) lowering. The findings of randomized trials are frequently disparate from those in observational studies. The current study involved a large number of analyses. While these analyses largely tested the same hypothesis (ie, that high serum Lp (a) was associated with an increased risk of clinical events), it needs to be recognized that such multiple testing introduces a risk of false discovery. Furthermore, despite including 1472 participants it is possible the study was underpowered to detect some of the associations that were tested particularly within the subgroups of younger and older participants. The distribution of Lp (a) concentrations did not include sufficiently large number of people with concentrations >100 mg/dL to fully assess the associations of circulating Lp (a) concentrations. Finally, recruitment for the current study occurred over a prolonged period from 2002 to 2018. This has a number of implications. Firstly, medical management has varied substantial during this period, which may have implications for generalizing the results. Secondly, some of the samples were stored for a long period before analysis. Samples were stored at −80°C and thus we believe this had no impact on the findings, however this remains possible.
In conclusion, this study emphasizes the high incidence of clinical events in people with PAD. High serum Lp (a) was associated with a greater requirement for lower limb peripheral revascularization but not consistently associated with other important clinical events.
Sources of Funding
Funding from the National Health and Medical Research Council (1063476 and 1022752), James Cook University, The Townsville Hospital and Health Services Study, Education and Research Trust Fund, and Queensland Government supported this work.
Disclosures
Professor Golledge holds a Practitioner Fellowships from the National Health and Medical Research Council (1117061) and a Senior Clinical Research Fellowship from the Queensland Government, Australia. The remaining authors have no disclosures to report.
Supporting information
Table S1
J Am Heart Assoc.2020;9:e015355 DOI: 10.1161/JAHA.119.015355
For Sources of Funding and Disclosures, see page 10.
References
- 1. Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156–170. [DOI] [PubMed] [Google Scholar]
- 2. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 3. Sampson UK, Norman PE, Fowkes FG, Aboyans V, Song Y, Harrell FE Jr, Forouzanfar MH, Naghavi M, Denenberg JO, McDermott MM, et al. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob Heart. 2014;9:159–170. [DOI] [PubMed] [Google Scholar]
- 4. Golledge J. Lower-limb arterial disease. Lancet. 1997;350:1459–1465. [DOI] [PubMed] [Google Scholar]
- 5. Thomas Manapurathe D, Moxon JV, Krishna SM, Rowbotham S, Quigley F, Jenkins J, Bourke M, Bourke B, Jones RE, Golledge J. Cohort study examining the association between blood pressure and cardiovascular events in patients with peripheral artery disease. J Am Heart Assoc. 2019;8:e010748 DOI: 10.1161/JAHA.118.010748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris DR, Skalina TA, Singh TP, Moxon JV, Golledge J. Association of computed tomographic leg muscle characteristics with lower limb and cardiovascular events in patients with peripheral artery disease. J Am Heart Assoc. 2018;7:e009943 DOI: 10.1161/JAHA.118.009943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonaca MP, Bhatt DL, Storey RF, Steg PG, Cohen M, Kuder J, Goodrich E, Nicolau JC, Parkhomenko A, López-Sendón J, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67:2719–2728. [DOI] [PubMed] [Google Scholar]
- 8. Bonaca MP, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, Tokgozoglu L, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the Fourier trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk). Circulation. 2018;2018:338–350. [DOI] [PubMed] [Google Scholar]
- 9. Anand SS, Eikelboom JW, Bosch J, Dyal L, Aboyans V, Abola MT, Branch KRH, Keltai K, Bhatt DL, Verhamme P, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71:2306–2315. [DOI] [PubMed] [Google Scholar]
- 10. Golledge J, Moxon JV, Rowbotham S, Pinchbeck J, Yip L, Velu R, Quigley F, Jenkins J, Morris DR. Risk of major amputation in patients with intermittent claudication undergoing early revascularization. Br J Surg. 2018;105:699–708. [DOI] [PubMed] [Google Scholar]
- 11. Volta A, Hovingh GK, Grefhorst A. Genetics of familial hypercholesterolemia: a tool for development of novel lipid lowering pharmaceuticals? Curr Opin Lipidol. 2018;29:80–86. [DOI] [PubMed] [Google Scholar]
- 12. Golledge J, Ward NC, Watts GF. Lipid management in people with peripheral artery disease. Curr Opin Lipidol. 2019;30:470–476. [DOI] [PubMed] [Google Scholar]
- 13. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. [DOI] [PubMed] [Google Scholar]
- 14. Klarin D, Lynch J, Aragam K, Chaffin M, Assimes TL, Huang J, Lee KM, Shao Q, Huffman JE, Natarajan P, et al. Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nat Med. 2019;25:1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tunstall-Pedoe H, Peters SAE, Woodward M, Struthers AD, Belch JJF. Twenty-year predictors of peripheral arterial disease compared with coronary heart disease in the Scottish Heart Health Extended Cohort (SHHEC). J Am Heart Assoc. 2017;6:e005967 DOI: 10.1161/JAHA.117.005967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laschkolnig A, Kollerits B, Lamina C, Meisinger C, Rantner B, Stadler M, Peters A, Koenig W, Stöckl A, Dähnhardt D, Böger CA, et al. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc Res. 2014;103:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gurdasani D, Sjouke B, Tsimikas S, Hovingh GK, Luben RN, Wainwright NW, Pomilla C, Wareham NJ, Khaw KT, Boekholdt SM, et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2012;32:3058–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones GT, van Rij AM, Cole J, Williams MJ, Bateman EH, Marcovina SM, Deng M, McCormick SP. Plasma lipoprotein(a) indicates risk for 4 distinct forms of vascular disease. Clin Chem. 2007;53:679–685. [DOI] [PubMed] [Google Scholar]
- 19. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28:354–362. [DOI] [PubMed] [Google Scholar]
- 20. Kotani K, Sahebkar A, Serban MC, Ursoniu S, Mikhailidis DP, Mariscalco G, Jones SR, Martin S, Blaha MJ, Toth PP, et al. Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Lipoprotein(a) Levels in Patients With Abdominal Aortic Aneurysm. Angiology. 2017;68:99–108. [DOI] [PubMed] [Google Scholar]
- 21. Sanchez Munoz-Torrero JF, Rico-Martin S, Alvarez LR, Aguilar E, Alcala JN, Monreal M; Investigators FRENA . Lipoprotein (a) levels and outcomes in stable outpatients with symptomatic artery disease. Atherosclerosis. 2018;276:10–14. [DOI] [PubMed] [Google Scholar]
- 22. Giovanetti F, Gargiulo M, Laghi L, D'Addato S, Maioli F, Muccini N, Borghi C, Stella A. Lipoprotein(a) and other serum lipid subfractions influencing primary patency after infrainguinal percutaneous transluminal angioplasty. J Endovasc Ther. 2009;16:389–396. [DOI] [PubMed] [Google Scholar]
- 23. Hishikari K, Hikita H, Nakamura S, Nakagama S, Mizusawa M, Yamamoto T, Doi J, Utsugi Y, Sudo Y, Kimura S, et al. Usefulness of lipoprotein(a) for predicting clinical outcomes after endovascular therapy for aortoiliac atherosclerotic lesions. J Endovasc Ther. 2017;24:793-9. [DOI] [PubMed] [Google Scholar]
- 24. Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57:1953–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Golledge J, Cronin O, Iyer V, Bradshaw B, Moxon JV, Cunningham MA. Body mass index is inversely associated with mortality in patients with peripheral vascular disease. Atherosclerosis. 2013;229:549–555. [DOI] [PubMed] [Google Scholar]
- 26. Golledge J, Ewels C, Muller R, Walker PJ. Association of chronic kidney disease categories defined with different formulae with major adverse events in patients with peripheral vascular disease. Atherosclerosis. 2014;232:289–297. [DOI] [PubMed] [Google Scholar]
- 27. Parr AM, Buttner PP, Shahzad AM, Golledge JM. Relation of infra-renal abdominal aortic calcific deposits and cardiovascular events in patients with peripheral artery disease. Am J Cardiol. 2010;105:895–899. [DOI] [PubMed] [Google Scholar]
- 28. Golledge J, Jayalath R, Oliver L, Parr A, Schurgers L, Clancy P. Relationship between CT anthropometric measurements, adipokines and abdominal aortic calcification. Atherosclerosis. 2007;197:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moxon JV, Lazzaroni SM, Boult M, Velu R, Fitridge RA, Golledge J. Circulating biomarkers are not associated with endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2018;67:770–777. [DOI] [PubMed] [Google Scholar]
- 30. Simo JM, Camps J, Gomez F, Ferre N, Joven J. Evaluation of a fully-automated particle-enhanced turbidimetric immunoassay for the measurement of plasma lipoprotein(a). population-based reference values in an area with low incidence of cardiovascular disease. Clin Biochem. 2003;36:129–134. [DOI] [PubMed] [Google Scholar]
- 31. Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stefanutti C JU, Watts GF, Harada-Shiba M, Cossu M, Schettler VJ, De Silvestro G, Soran H, Van Lennep JR, Pisciotta L, Klör HU, et al; MIGHTY MEDIC Multinational Society . Toward an international consensus-Integrating lipoprotein apheresis and new lipid-lowering drugs. J Clin Lipidol. 2017;11:858–871. [DOI] [PubMed] [Google Scholar]
- 33. Wilson DP, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, Orringer CE. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13:374–392. [DOI] [PubMed] [Google Scholar]
- 34. Ohashi H, Ohno M, Watanabe S, Sakata S. Lipoprotein(a) as a risk factor for coronary artery disease in hemodialysis patients. Kidney Int Suppl. 1999;71:S242–S244. [DOI] [PubMed] [Google Scholar]
- 35. Golledge J, Leicht A, Crowther RG, Clancy P, Spinks WL, Quigley F. Association of obesity and metabolic syndrome with the severity and outcome of intermittent claudication. J Vasc Surg. 2007;45:40–46. [DOI] [PubMed] [Google Scholar]
- 36. Morris DR, Singh TP, Moxon JV, Smith A, Stewart F, Jones RE, Golledge J. Assessment and validation of a novel angiographic scoring system for peripheral artery disease. Br J Surg. 2017;104:544–554. [DOI] [PubMed] [Google Scholar]
- 37. Moxon JV, Jones RE, Wong G, Weir JM, Mellett NA, Kingwell BA, Meikle PJ, Golledge J. Baseline serum phosphatidylcholine plasmalogen concentrations are inversely associated with incident myocardial infarction in patients with mixed peripheral artery disease presentations. Atherosclerosis. 2017;263:301–308. [DOI] [PubMed] [Google Scholar]
- 38. Queensland Health . Queensland hospital admitted data collection manual 2015-2016. Published by the State of Queensland. Available at: https://www.health.qld.gov.au/hsu/collections/qhapdc. Accessed February 5, 2020.
- 39. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, Dick F, van Herwaarden J, Karkos C, Koelemay M, et al. Editor's choice ‐ European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto‐iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93. [DOI] [PubMed] [Google Scholar]
- 40. Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, et al. Editor's choice—2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55:305–368. [DOI] [PubMed] [Google Scholar]
- 41. Golledge J, Pinchbeck J, Rowbotham S, Jenkins J, Bourke M, Bourke B, Norman PE, Jones R, Moxon JV. Editor's choice—metformin prescription is associated with a reduction in the combined incidence of surgical repair and rupture related mortality in patients with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:94–101. [DOI] [PubMed] [Google Scholar]
- 42. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. [DOI] [PubMed] [Google Scholar]
- 43. Tsimikas S, Gordts P, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41:192–193. [DOI] [PubMed] [Google Scholar]
- 44. Yahya R, Berk K, Verhoeven A, Bos S, van der Zee L, Touw J, Erhart G, Kronenberg F, Timman R, Sijbrands E, et al. Statin treatment increases lipoprotein(a) levels in subjects with low molecular weight apolipoprotein(a) phenotype. Atherosclerosis. 2019;289:201–205. [DOI] [PubMed] [Google Scholar]
- 45. Nastasi DR, Moxon JV, Trollope A, Golledge J. Prescription of pharmacotherapy and the incidence of stroke in patients with symptoms of peripheral artery disease. Stroke. 2018;49:2953–2960. [DOI] [PubMed] [Google Scholar]
- 46. Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double‐blind, placebo‐controlled, dose‐ranging trials. Lancet. 2016;388:2239–2253. [DOI] [PubMed] [Google Scholar]
- 47. Khan TZHL, Arai AE, Rhodes S, Pottle A, Wage R, Banya W, Gatehouse PD, Giri S, Collins P, Pennell DJ, et al. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross‐over trial. Eur Heart J. 2017;38:1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poller WC, Berger A, Dreger H, Morgera S, Enke‐Melzer K. Lipoprotein apheresis in patients with peripheral artery disease and lipoprotein(a)‐hyperlipoproteinemia: 2‐year follow‐up of a prospective single center study. Atheroscler Suppl. 2017;30:174–179. [DOI] [PubMed] [Google Scholar]
- 49. Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, Willeit P, Young R, Surendran P, Karthikeyan S, et al; European Prospective Investigation Into Cancer and Nutrition–Cardiovascular Disease (EPIC‐CVD) Consortium . Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)‐lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 2018;3:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


