Abstract
Background
People with a Fontan circulation experience a range of physical, psychosocial and neurodevelopmental challenges alongside, or caused by, their cardiac condition, with significant consequences for health‐related quality of life (HRQOL). We meta‐analyzed HRQOL outcomes reported by people with a Fontan circulation or their proxies and evaluated predictors of poorer HRQOL.
Methods and Results
Six electronic databases were searched for peer‐reviewed, English‐language articles published before March 2019. Standardized mean differences (SMD) were calculated using fixed and random‐effects models. Fifty articles reporting on 29 unique studies capturing HRQOL outcomes for 2793 people with a Fontan circulation and 1437 parent‐proxies were analyzed. HRQOL was lower in individuals with a Fontan circulation compared with healthy referents or normative samples (SMD, −0.92; 95% CI, −1.36 to −0.48; P<0.001). Lower scores were reported across all HRQOL domains, with the largest differences found for physical (SMD, −0.90; 95% CI, −1.13 to −0.67; P<0.001) and school/work functioning (SMD, −0.71; 95% CI, −0.90 to −0.52; P<0.001). Meta‐regression analyses found no significant predictors of self‐reported physical functioning, but older age at Fontan operation was associated with poorer emotional functioning (β=−0.124; P=0.004), and diagnosis of hypoplastic left heart was associated with poorer social functioning (β=−0.007; P=0.048). Sensitivity analyses showed use of the PedsQL Core Module was associated with lower HRQOL scores compared with the Short‐Form Health Survey‐36.
Conclusions
HRQOL outcomes for people with a Fontan circulation are lower than the general population. Optimal care acknowledges the lifelong impact of the Fontan circulation on HRQOL and offers targeted strategies to improve outcomes for this growing population.
Keywords: chronic illness, congenital heart disease, Fontan circulation, health‐related quality of life, mental health, psychological stress
Subject Categories: Meta Analysis, Congenital Heart Disease, Quality and Outcomes, Pediatrics
Nonstandard Abbreviations and Acronyms
- BNP
brain natriuretic peptide
- CHD
congenital heart disease
- CHQ
Child Health Questionnaire
- HLHS
hypoplastic left heart syndrome
- HRQOL
health‐related quality of life
- PedsQL
Pediatric Quality of Life Core Module
- PR
parent‐report
- SF‐36
Short‐Form Health Survey‐36
- SR
self‐report
- VO2
oxygen uptake
Clinical Perspective
What Is New?
This review and meta‐analysis of health‐related quality of life (HRQOL) in people with a Fontan circulation, synthesizes the findings of 50 articles reporting on 2,793 patients and 1,437 parent‐proxies.
People of all ages with a Fontan circulation report lower total HRQOL compared with referents, and poorer outcomes across all HRQOL domains, with a particularly large effect for physical functioning.
Parents report lower HRQOL for their child with a Fontan circulation compared to parental reports for children from the general community.
Meta‐regression analyses revealed emotional and social functioning are more likely than physical functioning to be moderated by demographic and medical factors.
What Are the Clinical Implications?
The Fontan circulation has a lifelong impact on HRQOL and wellbeing, and targeted strategies to improve long‐term outcomes for this growing population are needed.
The Fontan procedure is the final in a series of surgeries performed to palliate single‐ventricle congenital heart disease (CHD), a class of highly complex CHD in which it is impossible to create a 2‐ventricle circulation. Over 80% of children with single‐ventricle CHD who progress to a Fontan circulation survive into adulthood, translating into a rapidly growing population of people living with a high burden of disease.1
Patients who have undergone the Fontan operation experience a range of comorbidities related to their cardiac condition and associated medical interventions. The impact of resulting stressors on the developing child can be profound, and individuals with a Fontan circulation report physical, psychological, neurodevelopmental, and social challenges across their lifespan.2, 3 After surgery, patients and their families anticipate progressive functional limitations, serious cardiac and noncardiac morbidities, and the possibility of Fontan circulatory failure of sufficient severity to require cardiac transplantation or cause premature death. These lifelong challenges and risks can influence patients’ overall well‐being and health‐related quality of life (HRQOL).
HRQOL is a multidimensional concept including domains related to physical, psychological, social, and occupational functioning.4, 5 Despite recognition that people with a Fontan circulation are at risk of poor HRQOL,6 there is no consensus on the individual and environmental factors that influence this outcome. It is unclear, for example, whether CHD complexity itself is a risk factor for poorer HRQOL. While 3 reviews found greater CHD complexity was associated with lower HRQOL in children and adults,7, 8, 9 2 reviews reported no association.10, 11 Clinical factors, such as daily medication use, longer hospitalizations, and greater number of medical interventions, are associated with worse HRQOL among people with complex CHD.8, 11 Greater psychological stress, fewer social supports, and lower family socioeconomic status are also correlated with lower HRQOL12, 13, 14, 15; however, no meta‐analyses have examined the relative impact of these factors in people with a Fontan circulation.
With the Fontan population currently estimated at 70 000 individuals worldwide and predicted to double over the next 20 years,16 there is an imperative to better understand HRQOL.17 This review aimed to: (1) meta‐analyze HRQOL outcomes reported by children, adolescents, and adults with a Fontan circulation and/or their proxies in comparison to the general population; (2) identify individual and environmental factors that predict HRQOL in people with a Fontan circulation and determine moderating effects; (3) examine associations between healthcare use (eg, frequency of visits to cardiac services), health service costs and HRQOL; and (4) critically appraise the quality of existing literature to set priorities for future clinical practice and research advancement.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses Statement18 for the purposes of identifying articles, extracting data, and synthesizing evidence. The protocol was registered with PROSPERO (CRD42015016610).
Data Sources and Search Strategy
Six electronic databases (Medline, CINHAL, Cochrane, Embase, PsycINFO, and Scopus) were searched for peer‐reviewed studies, and autoalerts were created using the same unique search algorithm for each database, with studies published through March 7, 2019 incorporated into the review. Search terms defining the target population were combined with key HRQOL terms (Table S1).19 Reference lists of included studies were manually scanned to identify additional articles. Prolific author searching was used to identify additional articles.
Eligibility Criteria
Eligible studies met all following criteria: (1) reported on a sample of individuals with a Fontan circulation; (2) used a validated, quantitative self‐ or proxy‐reported HRQOL measure; and (3) were published in an English‐language, peer‐reviewed format. Studies that defined participants only by univentricular diagnosis were included if >80% of the sample had a Fontan circulation. All study designs and comparison group types were considered eligible.
Study Selection and Data Extraction
Initially, one researcher (K.M.) screened all titles for duplicates and ineligible articles. Remaining abstracts and resulting full texts were then independently screened by 2 researchers (K.M., N.K.). In cases where eligibility was unclear, a third researcher (G.S.) was consulted or the corresponding author contacted (Figure 1). One researcher (K.M.) extracted data from each article, and a second checked for accuracy (N.K.). Disagreements were resolved through discussion and consensus. Among articles comparing people with a Fontan circulation with healthy controls or normative data, averages (means, medians) and distribution of self‐ and parent‐reported HRQOL scores were extracted for meta‐analysis. For articles that did not report the SD of scores,20, 21, 22, 23 the Cochrane RevMan calculator24 was used to estimate SD. Plot digitizer software (http://plotdigitizer.sourceforge.net/) was used to extract scores reported in graphs.23, 25 Where ≥2 articles reported on the same sample, data from the most recently published article were meta‐analyzed.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses diagram illustrating the systematic search process.
HRQOL indicates health‐related quality of life.
Risk of Bias Analysis
Risk of bias was independently assessed for each study by 2 reviewers (K.M., D.C.). Assessments were performed using the 14‐item criteria proposed by Kmet et al,26 and item scores from 0 to 2 were assigned. A total mean summary score was then calculated, with higher scores indicating greater methodological rigor and lower risk of bias (>0.8=strong, 0.71–0.79=good, 0.50–0.70=adequate, and <0.50=limited).
Data Synthesis and Meta‐Analysis
Based on an a priori assumption of outcome measurement heterogeneity, a narrative synthesis was used to report evidence from all 50 captured articles. Statistical analyses were performed using the Comprehensive Meta‐Analysis Program, Version 3 (CMA 3.0; Englewood, NJ).27 Standardized mean difference (SMD) scores were the primary summary measure, allowing for comparison of effect sizes across HRQOL measures.28 All meta‐analyses were initially conducted using a random‐effects model, as described by DerSimonian and Laird,29 as variation in the true effect size between studies caused by clinical (eg, CHD complexity, comorbidities) or methodological (eg, study design, risk of bias) heterogeneity was evident following data extraction.30 Fixed‐effects models have also demonstrated validity in the presence of heterogeneity;31 thus, the primary meta‐analyses were repeated using a fixed‐effects model to enhance the practical inference of our results. Random‐ and fixed‐effects analyses were performed for overall self‐ and parent‐reported HRQOL. For each reporting method (self or proxy), separate analyses were then performed for each HRQOL domain, including physical and psychosocial summary domains and emotional, social, and school/work functioning. For articles in which the number of control participants was not reported, a conservative approach assuming the number equal to that of the patient group was used. Statistical heterogeneity between studies was assessed using Cochran's Q.32 The I2 statistic27 was used as an estimate of the percentage of total between‐study variance, with I2 ≥50% indicating substantial heterogeneity.33 A series of random‐effects univariate meta‐regression analyses were conducted to determine the effect of continuous moderator variables on the SMD, only if the number of studies was ≥4. Demographic, clinical, and social psychological variables were specified a priori, but only 4 variables (hypoplastic left heart syndrome [HLHS] diagnosis, mean age at Fontan procedure, mean age at HRQOL assessment, and sex) met criteria for regression analysis. Regression coefficients (β) were used to indicate the estimated increase in the effect size per unit increase in the moderator variable. The proportion of between‐study variation explained by each moderator variable was calculated as R 2. Interactions among moderator variables were not tested because of insufficient power. Post hoc sensitivity analyses were performed to explore the potential effect of HRQOL measure. Only the PedsQL Core Module and Short‐Form Health Survey‐36 provided sufficient data for these analyses. For all statistical analyses, significance was set at P<0.05. Additional predictors of HRQOL not assessed via meta‐analysis were captured using narrative synthesis.
Publication bias was investigated by visual inspection of the funnel plot for asymmetry on all outcome measures. Egger's weighted regression method34 and the Begg‐Mazumdar rank correlation method35 were used to assess potential publication bias. If bias was detected (P<0.05), Duval and Tweedie's Trim and Fill procedure36 with random‐effects modeling was used to estimate the impact of bias on meta‐analytic results.
Results
Fifty articles examining HRQOL outcomes of individuals with a Fontan circulation were identified, after screening of 3907 titles or abstracts and review of 126 full texts (Figure 1). Captured articles reported on the outcomes of 29 unique samples, including 2793 patients and 1437 parent‐proxies. Risk of bias was low, with a mean quality rating of 0.92 across the 50 articles (Table 1), and no studies were excluded because of bias. Studies sampled individuals from the United States (n=31),2, 3, 6, 22, 23, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 Europe (n=14),20, 21, 25, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 and Australia (n=2),74, 75 whereas 3 samples were recruited from multiple countries.76, 77, 78 Most articles (76%) were published between 2010 and 2019.
Table 1.
Characteristics of Included Studies
| Article | Country | Risk of Bias Score* | No. of Participants | Mean or Median Age at HRQOL Assessment, y | Sex | Diagnosis | Mean or Median Age at Fontan, y | Mean or Median Time Since Fontan, y | SR or PR | HRQOL Measure(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Anderson et al3 2008† | United States | 0.86 | 546 | 11.9 | 327 male, 219 female |
TA: 119 HLHS: 112 DILV: 80 Heterotaxia: 42 DORV: 41 PA with intact ventricular septum: 33 MA: 31 Abnormal tricuspid valve: 22 Atrioventricular canal defect: 22 Other: 38 |
3.4 | NR | PR | CHQ |
| Atz et al37 2007† | United States | 0.81 | 546 | 12.2 | 327 male, 219 female | NR | 5.1 | 7.2 | SR, PR | CHQ |
| Atz et al39 2011† | United States | 0.77 | 536 | 11.9 | 318 male, 218 female |
DILV: F=47, NF=31 MA: F=21, NF=9 TA: F=75, NF=46 Unbalanced atrioventricular canal: F=14, NF=9 Heterotaxia: F=28, NF=12 HLHS: F=83, NF=26 Other: F=93, NF=42 |
F: 3.5 NF: 3.3 | NR | PR | CHQ |
| Atz et al38 2013† | United States | 0.95 | 546 | 11.9 | 327 male, 219 female |
TA: 119 HLHS: 112 DILV: 80 Heterotaxia: 42 DORV: 41 PA with intact ventricular septum: 33 MA: 31 Abnormal tricuspid valve: 22 Atrioventricular canal defect: 22 Other: 38 |
SCC: 3.5 No SCC: 3.2 | 8.1 | SR, PR | CHQ |
| Atz et al41 2015† | United States | 0.86 | 427 | 18.4 | 249 male, 178 female | NR | NR | 15.2 | SR, PR | CHQ, PedsQL, SF‐36 |
| Atz et al40 2017† | United States | 0.95 | 373 | 21.2 | 222 male, 151 female | NR | 3.3 | 17.8 | SR, PR | CHQ, PedsQL, SF‐36 |
| Banka et al42 2011† | United States | 0.85 | 539 | 11.9 | NR | NR | Coil: 3.9, No coil: 3.2 | 8.6 | PR | CHQ |
| Blaufox et al43 2008† | United States | 0.81 | 521 | 11.9 | 315 male, 206 female | NR | 3.4 | NR | PR | CHQ |
| Callegari et al78 2019 | Germany and Italy | 1.00 | 232 | 25.6 | 92 male, 140 female | NR | NR | NR | SR | SF‐36 |
| Cohen et al44 2010† | United States | 1.00 | 544 | 11.9 | NR | NR | NR | 8.4 | PR | CHQ |
| Czosek et al76 2015 | United States and United Kingdom | 1.00 | 318 | 11.9 | 182 male, 136 female | NR | NR | NR | SR, PR | PedsQL |
| d'Udekem et al74 2009 | Australia | 0.77 | 36 | 21.6 | 19 male, 17 female |
DILV: 14 TA: 8 DORV: 3 Atrioventricular canal: 3 PA with intact ventricular septum: 2 Straddling atrioventricular valve: 1 Other: 5 |
5 | 16.6 | SR | SF‐36 |
| Dulfer et al20 2014 | The Netherlands | 0.85 | 44 | NR | NR | NR | 3 | NR | SR, PR | SF‐36, TACQOL |
| Dulfer et al63 2016 | The Netherlands | 0.90 | 79 | NR | 47 male, 32 female | NR | NR | NR | SR, PR | TACQOL |
| Friedland‐Little et al45 2017 | United States | 0.81 | 31 | ECMO: 7.8 No ECMO: 6.8 | 24 male, 7 female |
HLHS: 27 DILV: 1 TA with d‐MGA: 1 Unbalanced atrioventricular septal defects: 1 DORV with hypoplastic LV: 1 |
NR | NR | SR, PR | PedsQL |
| Goldberg et al46 2014 | United States | 0.90 | 232 | 3.0 | 145 male, 87 female | HLHS: 207 | NR | NR | PR | PedsQL |
| Goldstein et al22 2011 | United States | 0.90 | 51 | 15.0 | 31 male, 20 female |
HLHS: 25 DILV: 8 TA: 6 AVSD with hypoplastic LV: 3 TGA with hypoplastic RV: 3 PA with intact ventricular septum: 3 Other: 3 |
6.2 | NR | SR, PR | PedsQL |
| Gratz et al66 2009 | Germany | 0.86 | 31 | 22.1 | 16 male, 15 female | NR | 2.9 | NR | SR | SF‐36 |
| Hedlund et al71 2016† | Sweden | 0.72 | 30 | 14.2 | 16 male, 14 female | NR | 2.7 | 11.4 | SR, PR | PedsQL |
| Hedlund et al70 2017† | Sweden | 0.77 | 30 | 14.2 | 16 male, 14 female | NR | NR | NR | SR, PR | PedsQL |
| Heye et al77 2019 | Germany and Switzerland | 0.95 | 46 | 3.1 | 31 male, 15 female |
HLHS: 26 PA with VSD: 2 PA with intact ventricular septum: 1 TA: 3 DILV with TGA: 1 l‐TGA with hypoplastic LV: 1 Imbalanced AVSD: 6 Borderline LV: 4 DORV with TGA: 2 |
2.7 | 0.83 | PR | PedsQL |
| Hock et al67 2018 | Germany | 0.95 | 78 | 12.0 | 57 male, 21 female |
HLHS: 27 TA: 13 DILV: 12 d‐MGA: 10 Atrioventricular anomaly: 5 HRHS: 4 Other: 7 |
2.3 | NR | SR | KINDL‐R |
| Idorn et al21 2013 | Denmark | 0.81 | 158 | NR | 93 male, 65 female |
TA: 43 DILV: 39 AVSD: 15 HLHS: 12 PA with intact ventricular septum: 12 Other: 37 |
NR | NR | SR, PR | PedsQL, SF‐36 |
| Jacobsen et al47 2016† | United States | 0.86 | 14 | 10.4 | 8 male, 6 female |
TA: 4 HLHS: 4 DORV:1 Unbalanced AVSD: 2 d‐TGA with MA: 1 HRHS: 1 Coarctation with MA, LV hypoplasia: 1 |
NR | 7.3 | SR, PR | PedsQL |
| Jacobsen et al48 2018† | United States | 0.86 | 11 | 10.5 | 5 male, 6 female | NR | NR | 7.4 | SR, PR | PedsQL |
| Karamlou et al49 2013 | United States | 0.90 | 25 | NR | NR | NR | NR | NR | SR | CHQ, PedsQL |
| Kukreja et al50 2015 | United States | 0.86 | 49 | 26 | 25 male, 24 female | NR | NR | NR | SR | SF‐36 |
| Lambert et al51 2009† | United States | 0.81 | 328 | 13.9 | 195 male, 133 female | NR | 3.6 | NR | SR, PR | CHQ |
| Manlhiot et al23 2009 | United States | 0.90 | 68 | 13.0 | 24 male, 44 female |
DILV: 20 TA: 15 HLHS: 4 Other: 29 |
4 | NR | SR, PR | CHQ, PedsQL |
| McCrindle et al2 2006† | United States | 0.95 | 537 | 11.9 | 323 male, 214 female | NR | NR | NR | PR | CHQ |
| McCrindle et al52 2007† | United States | 0.95 | 147 | 11.6 | 91 male, 56 female | NR | 3.5 | 8.1 | SR, PR | CHQ |
| McCrindle et al55 2010† | United States | 0.90 | 511 | 11.9 | 311 male, 200 female | NR | 3.4 | NR | PR | CHQ |
| McCrindle et al54 2014† | United States | 0.90 | 325 | 13.9 | 193 male, 132 female |
TA: 80 HLHS: 58 DILV: 53 Heterotaxia: 22 MA: 18 AVSD: 12 Other: 76 |
NR | NR | SR | CHQ |
| McCrindle et al53 2014† | United States | 0.90 | 245 | 16.2 | 144 male, 101 female | NR | 2.9 | NR | PR | CHQ |
| Mellion et al56 2014‡ | United States | 0.95 | 208 | Children: 9.6, Adolescents: 14.8 | 123 male, 85 female |
HLHS: 57 DILV: 28 DORV: 3 MA: 17 TA: 39 Unbalanced atrioventricular canal: 19 RV‐aorta with PA: 18 Superinferior ventricle: 4 Conotruncal anomalies: 135 TGA: 99 DORV: 16 Truncus arteriosus: 20 |
NR | NR | SR, PR | PedsQL |
| Menon et al57 2018 | United States | 0.95 | 299 | NR | 174 male, 125 female | HLHS: 98 | NR | NR | SR, PR | PedsQL |
| Müller et al69 2009 | Germany | 0.81 | 57 | NR | 39 male, 18 female | NR | NR | NR | SR | CHQ, SF‐36 |
| Müller et al68 2012 | Germany | 0.81 | 57 | NR | 33 male, 24 female | NR | NR | NR | SR | SF‐36 |
| Overgaard et al25 2011 | Denmark | 0.90 | 62 | NR | 34 male, 28 female |
DILV: 20 TA: 17 MA: 6 DORV: 6 AVSD: 4 PA with intact ventricular septum: 3 HLHS: 2 Other: 4 |
NR | NR | SR | SF‐36 |
| Pike et al59 2012 | United States | 0.95 | 54 | 26.0 | 26 male, 28 female |
TA: 19 DILV: 13 Hypoplastic RV: 8 HLHS: 4 DORV: 3 Ebstein abnormality: 1 AVSD: 2 Other: 4 |
NR | NR | SR | SF‐36 |
| Prakash et al58 2010† | United States | 0.95 | 123 | 12.1 | 69 male, 54 female |
TA: 36 DILV: 22 HLHS: 14 d‐TGA, PA: 9 Heterotaxia, DORV, SV: 9 PA with intact ventricular septum: 7 l‐TGA, DORV, PA: 6 Unbalanced atrioventricular canal: 3 Other: 17 |
NR | NR | PR | CHQ |
| Smas‐Suska et al72 2018 | Poland | 0.81 | 40 | 26.0 | 24 male, 16 female |
Right ventricular hypoplasia: 25 Pulmonary stenosis: 9 Left ventricular hypoplasia: 3 DORV: 2 Complete atrioventricular canal: 1 |
4.8 | 20.5 | SR | SF‐36 |
| Stephenson et al60 2010† | United States | 0.95 | 520 | 11.9 | 315 male, 205 female |
Single LV, DILV, and TA: IART=18, No IART=174 Single RV, DIRV, MA, HLHS: IART=5, No IART=135 SV, Unbalanced atrioventricular canal defect: IART=1, No IART=19 Other: IART=10, No IART=116 SV, Heterotaxia: IART=4, No IART=34 |
3.4 | 8.4 | PR | CHQ |
| Sutherland et al75 2018 | Australia | 0.90 | 17 | 15 | 10 male, 7 female |
DILV: 3 TA:5 HLHS: 3 TGA: 2 DORV: 1 PA with intact ventricular septum: 1 AVSD: 2 |
NR | NR | SR, PR | PedsQL |
| Uzark et al6 2016† | United States | 0.95 | 408 | 18.5 | 237 male, 171 female | NR | 3.8 | 15.2 | SR | CHQ, PedsQL, SF‐36 |
| van den Bosch et al64 2004 | The Netherlands | 0.68 | 36 | NR | 18 male, 18 female |
TA: 21 DILV: 9 Other: 6 |
12 | NR | SR | SF‐36 |
| Williams et al61 2009† | United States | 0.95 | 476 | NR | 280 male, 187 female |
PA: 29 HLHS: 100 Atrioventricular valve, heterotaxia, unbalanced AVSD: 52 TA: 114 Anomalous venous return: 8 |
3.4 | 8.8 | PR | CHQ |
| Williams et al62 2013† | United States | 0.72 | 546 | 11.9 | NR |
SV, DILV: No pacemaker=62, pacemaker=20 SV, DIRV: No pacemaker=9, pacemaker=1 SV, MA: No pacemaker=29, pacemaker=1 SV, TA: No pacemaker=105, pacemaker=15 Unbalanced AVCD: No pacemaker=19, pacemaker=2 Heterotaxia: No pacemaker=33, pacemaker=7 HLHS: No pacemaker=100, pacemaker=10 Other: No pacemaker=118, pacemaker=15 |
NR | NR | PR | CHQ |
| Wolff et al65 2018 | The Netherlands | 0.90 | 21 | 27.0 | 7 male, 14 female |
TA: 9 DILV: 6 AVSD: 4 PA with intact ventricular septum: 2 |
6 | NR | SR | SF‐36 |
| Yildiz et al73 2011 | Turkey | 0.81 | 20 | 19.0 | 10 male, 10 female |
TA with pulmonary stenosis: 10 PA with intact ventricular septum: 5 DILV: 3 DORV: 1 PA with VSD: 1 |
NR | NR | SR | PedsQL |
AVCD indicates atrioventricular canal defect; AVSD, atrioventricular septal defect; CHQ, Child Health Questionnaire; DILV, double‐inlet left ventricle; DIRV, double‐inlet right ventricle; d‐MGA, d‐malposed great arteries; DORV, double‐outlet right ventricle; d‐TGA, dextrotransposition of the great arteries; ECMO, extracorporeal membrane oxygenation; F, fenestration; HLHS, hypoplastic left heart syndrome; HRHS, hypoplastic right heart syndrome; HRQOL, health‐related quality of life; IART, intra‐atrial reentrant tachycardia; KINDL‐R, The Revised Children's Quality of Life Questionnaire; l‐TGA, levotransposition of the great arteries; LV, left ventricle; MA, mitral atresia; NF, no fenestration; NR, not reported; PA, pulmonary atresia; PedsQL, PedsQL Core Module; PR, parent‐report; RV, right ventricle; SCC, superior cavopulmonary connection; SF‐36, Short‐Form Health Survey‐36; SR, self‐report; SV, single ventricle; TA, tricuspid atresia; TACQOL, TNO AZL Children's Quality of Life; TGA, transposition of the great arteries; and VSD, ventricular septal defect.
Higher scores indicate greater methodological rigor and lower risk of bias.
Articles reporting on the same or overlapping study cohorts.
Articles reporting on multiple congenital heart disease diagnoses per participant.
Mean patient age at HRQOL assessment ranged from 3.0 to 27.0 years; 16 samples included people of all ages, 10 sampled children and adolescents, and 3 sampled adults only. Most samples (86%) included a larger proportion of male than female participants. Average time since Fontan completion ranged from 0.83 to 20.5 years, and mean age at Fontan operation ranged from 2.3 to 12.0 years. Nineteen studies relied on both parent‐ and self‐reported HRQOL, 18 used self‐report, and 13 captured only parent proxy–reported HRQOL. Across all studies, 8 HRQOL measures were used; most common were the PedsQL Core Module79 (PedsQL n=17; 14 samples; 1604 patients; 1181 parent‐proxies), Short‐Form Health Survey‐3680 (SF‐36; n=16; 14 samples; 806 patients), and Child Health Questionnaire81 (n=22; 4 samples; 665 patients; 546 parent‐proxies). Twenty‐two articles provided sufficient data for meta‐analysis. Of these, 13 studies compared HRQOL outcomes of people with a Fontan circulation with those of an optimal healthy control sample, and 9 studies compared HRQOL scores with values derived from a normative sample (3 from a “healthy” sample, 6 from a “general community” sample). Given the diversity in comparator samples, the terms “referents” or “comparators” will be used herein to define control or comparison groups.
Health‐Related Quality of Life
Self‐reported HRQOL
Overall, self‐reported HRQOL was significantly lower in individuals with a Fontan circulation compared with referents (SMD −0.92; 95% CI, −1.36 to −0.48; P<0.001; k=8; Table 2, Figure 2). Fontan patients reported lower scores across all HRQOL domains, with a large effect for physical functioning (SMD −0.90; 95% CI, −1.13 to −0.67; P<0.001; k=26) and moderate effects for school/work (SMD −0.71; 95% CI, −0.90 to −0.52; P<0.001; k=10) and for psychosocial (SMD −0.63; 95% CI, −0.87 to −0.39; P<0.001; k=14) and social functioning (SMD −0.56; 95% CI, −0.73 to −0.39; P<0.001; k=21) compared with referents. Emotional functioning was also poorer compared with referents; however, the effect size was small (SMD −0.35; 95% CI, −0.54 to −0.15; P=0.001; k=23). Fixed‐effects analyses also found overall self‐reported HRQOL was lower in individuals with a Fontan circulation compared with referents (SMD −0.89; 95% CI, −0.99 to −0.80; P<0.0001; k=8). Fixed‐effect analyses followed the same pattern across all self‐reported HRQOL domains, ranging from −0.84 to −0.26 SDs below the mean for referents (all P<0.001; Table S2).
Table 2.
Meta‐Analysis Results Comparing Mean Self‐ and Parent‐Reported HRQOL Scores for People With a Fontan Circulation With Healthy Referents
| Variable | No. of Comparisons | No. of Participants | Test Statistics | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fontan Patients | Healthy Referents | SMD | 95% CI | P Value | I2 | Q | P Value | |||
| Self‐reported outcomes | ||||||||||
| Total HRQOL | 8 | 768 | 7697 | −0.92 | −1.36 | −0.48 | <0.0001* | 93.89 | 114.62 | <0.0001* |
| Physical functioning | 26 | 1694 | 13 043 | −0.90 | −1.13 | −0.67 | <0.0001* | 90.30 | 257.60 | <0.0001* |
| Psychosocial functioning | 14 | 1009 | 5963 | −0.63 | −0.87 | −0.39 | <0.0001* | 84.03 | 81.41 | <0.0001* |
| Emotional functioning | 23 | 1603 | 10 590 | −0.35 | −0.54 | −0.15 | 0.0001* | 85.87 | 148.60 | <0.0001* |
| Social functioning | 21 | 1246 | 10 321 | −0.56 | −0.73 | −0.39 | <0.0001* | 75.54 | 77.68 | <0.0001* |
| School/work functioning | 10 | 882 | 7986 | −0.71 | −0.90 | −0.52 | <0.0001* | 68.45 | 28.53 | 0.001* |
| Parent‐reported outcomes | ||||||||||
| Total HRQOL | 7 | 538 | 11 110 | −1.05 | −1.41 | −0.69 | <0.0001* | 87.82 | 49.25 | <0.0001* |
| Physical functioning | 8 | 802 | 11 482 | −0.99 | −1.22 | −0.76 | <0.0001* | 79.08 | 33.45 | <0.0001* |
| Psychosocial functioning | 8 | 802 | 11 502 | −0.83 | −1.18 | −0.48 | <0.0001* | 91.66 | 83.88 | <0.0001* |
| Emotional functioning | 6 | 508 | 11 060 | −0.69 | −1.01 | −0.39 | <0.0001* | 83.36 | 30.05 | <0.0001* |
| Social functioning | 6 | 508 | 11 051 | −0.74 | −1.12 | −0.36 | <0.0001* | 89.30 | 46.74 | <0.0001* |
| School/work functioning | 4 | 239 | 9226 | −0.68 | −0.96 | −0.40 | <0.0001* | 64.75 | 8.51 | <0.0001* |
HRQOL indicates health‐related quality of life; and SMD, standardized mean difference.
Statistically significant at P<0.05.
Figure 2. Forest plots of random‐effects analysis of self‐reported health‐related quality of life (HRQOL), presented separately for total HRQOL (A), physical functioning (B), and psychosocial functioning (C).
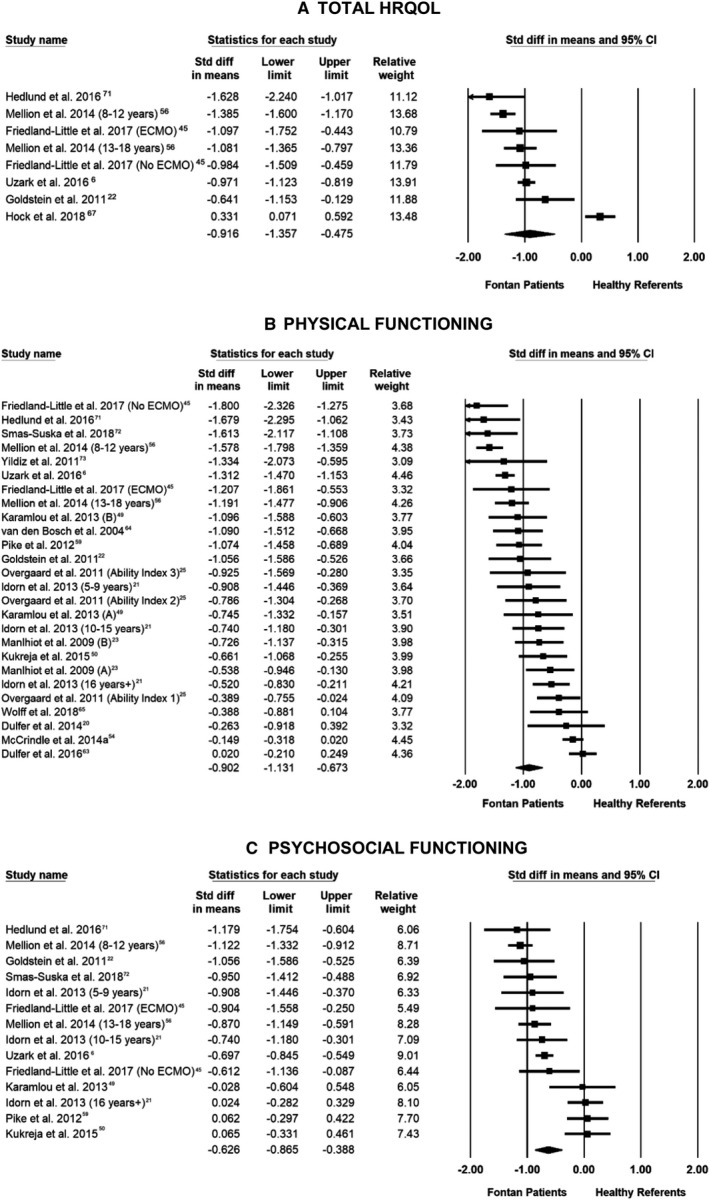
Random‐effect meta‐analysis of between‐group effect sizes. Box sizes are proportional to the weight of each study in the analysis, and the lines represent their 95% CIs. The thickest part of the diamond represents the pooled standardized mean difference with a width proportional to the 95% CI. ECMO indicates extracorporeal membrane oxygenation.
Proxy‐reported HRQOL
Parents reported lower overall HRQOL for their child with a Fontan circulation compared with parents of referents (SMD −1.05; 95% CI, −1.41 to −0.69; P<0.001; k=7; Table 2, Figure S1). All HRQOL domains were lower for individuals with a Fontan circulation compared with referents, ranging from −0.68 to −0.99 SDs below the mean for controls (all P<0.001; Table 2). Findings from fixed‐effects analyses did not differ markedly from those of the primary (random‐effects model) results; parents reported lower scores for their child across all HRQOL domains, including total HRQOL (SMD −0.99; 95% CI, −1.10 to −0.87; P<0.0001; k=7) and physical (SMD −0.93; 95% CI, −1.02 to −0.84; P<0.0001; k=8), psychosocial (SMD −0.66; 95% CI, −0.75 to −0.57; P<0.0001; k=8), emotional (SMD −0.65; 95% CI, −0.76 to −0.54; P<0.0001; k=6), social (SMD −0.72; 95% CI, −0.84 to −0.61; P<0.0001; k=6), and school/work (SMD −0.78; 95% CI, −0.93 to −0.64; P<0.0001; k=4) functioning (Table S2).
Moderators of HRQOL
HLHS diagnosis
Using meta‐regression, we found studies with a higher proportion of HLHS patients tended to report lower self‐reported social functioning (β=−0.007; 95% CI, 0.015 to −0.0001; P=0.048). Conversely, HLHS diagnosis was associated with a smaller difference in parent‐reported scores compared with referents for all domains, including total HRQOL (β=0.012; 95% CI, 0.009–0.016; P<0.001) and physical (β=0.010; 95% CI, 0.006–0.013; P<0.001), emotional (β=0.009; 95% CI, 0.005–0.013; P<0.001), social (β=0.012; 95% CI, 0.008–0.016; P<0.001), and school/work (β=0.009; 95% CI, 0.002–0.015; P=0.004) functioning (Table 3).
Table 3.
Moderators of Self‐ and Parent‐Reported HRQOL, Presented Separated for Total HRQOL and Functional Domain Scores, Based on Meta‐Regression Results
| Moderators | HRQOL Domain | No. of Comparisons | No. of Participants | Meta‐Regression Statistics | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fontan Patients | Healthy Referents | Slope | 95% CI | P Value | R 2 | ||||
| HLHS, % with diagnosis | Self‐reported | ||||||||
| Total HRQOL | 6 | 298 | 6666 | 0.004 | −0.006 | 0.014 | 0.440 | 0.00 | |
| Physical functioning | 15 | 1046 | 7346 | −0.008 | −0.019 | 0.001 | 0.108 | 0.17 | |
| Psychosocial functioning | 12 | 558 | 7041 | −0.006 | −0.016 | 0.003 | 0.203 | 0.18 | |
| Emotional functioning | 12 | 949 | 7285 | −0.002 | −0.012 | 0.007 | 0.660 | 0.02 | |
| Social functioning | 12 | 627 | 7052 | −0.007 | −0.015 | −0.0001 | 0.048* | 0.33 | |
| School/work functioning | 7 | 378 | 6699 | −0.001 | −0.006 | 0.005 | 0.868 | 0.00 | |
| Parent reported | |||||||||
| Total HRQOL | 7 | 538 | 11 110 | 0.012 | 0.009 | 0.016 | <0.0001* | 1.00 | |
| Physical functioning | 7 | 538 | 11 091 | 0.010 | 0.006 | 0.013 | <0.0001* | 1.00 | |
| Psychosocial functioning | 7 | 538 | 11 111 | 0.013 | 0.009 | 0.016 | <0.0001* | 1.00 | |
| Emotional functioning | 6 | 508 | 11 060 | 0.009 | 0.005 | 0.013 | <0.0001* | 0.95 | |
| Social functioning | 6 | 508 | 11 051 | 0.012 | 0.008 | 0.016 | <0.0001* | 1.00 | |
| School/work functioning | 4 | 239 | 9226 | 0.009 | 0.002 | 0.015 | 0.004* | 1.00 | |
| Age at Fontan operation | Self‐reported | ||||||||
| Total HRQOL | 2 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | |
| Physical functioning | 10 | 1183 | 5953 | −0.015 | −0.145 | 0.115 | 0.818 | 0.00 | |
| Psychosocial functioning | 6 | 590 | 545 | 0.101 | −0.122 | 0.357 | 0.374 | 0.07 | |
| Emotional functioning | 10 | 1092 | 3498 | −0.124 | −0.21 | −0.038 | 0.004* | 0.48* | |
| Social functioning | 8 | 735 | 3230 | 0.039 | −0.05 | 0.128 | 0.393 | 0.00 | |
| School/work functioning | 3 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | |
| Parent reported | |||||||||
| Total HRQOL | 2 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | |
| Physical functioning | 2 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | |
| Psychosocial functioning | 2 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | |
| Emotional functioning | 1 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | |
| Social functioning | 1 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | |
| School/work functioning | 0 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | |
| Age at HRQOL assessment | Self‐reported | ||||||||
| Total HRQOL | 8 | 768 | 7697 | 0.004 | −0.158 | 0.166 | 0.961 | 0.00 | |
| Physical functioning | 21 | 1602 | 11 129 | 0.007 | −0.039 | 0.053 | 0.758 | 0.00 | |
| Psychosocial functioning | 14 | 1009 | 7450 | 0.043 | 0.013 | 0.073 | 0.004* | 0.51 | |
| Emotional functioning | 17 | 1511 | 8676 | 0.021 | −0.010 | 0.053 | 0.185 | 0.00 | |
| Social functioning | 15 | 1154 | 8407 | 0.034 | 0.011 | 0.058 | 0.004* | 0.33 | |
| School/work functioning | 10 | 882 | 7986 | −0.003 | −0.061 | 0.055 | 0.913 | 0.00 | |
| Parent reported | |||||||||
| Total HRQOL | 7 | 538 | 11 110 | −0.077 | −0.119 | −0.035 | 0.0003* | 0.85 | |
| Physical functioning | 8 | 802 | 11 482 | −0.039 | −0.090 | 0.012 | 0.135 | 0.00 | |
| Psychosocial functioning | 8 | 802 | 11 482 | −0.045 | −0.141 | 0.050 | 0.353 | 0.00 | |
| Emotional functioning | 6 | 508 | 11 060 | −0.065 | −0.089 | −0.040 | <0.0001* | 1.00 | |
| Social functioning | 6 | 508 | 11 051 | −0.071 | −0.130 | −0.011 | 0.018* | 0.64 | |
| School/work functioning | 4 | 239 | 9226 | −0.071 | −0.193 | 0.050 | 0.250 | 0.00 | |
| Sex, % male | Self‐reported | ||||||||
| Total HRQOL | 8 | 768 | 7697 | 0.018 | −0.016 | 0.053 | 0.296 | 0.29 | |
| Physical functioning | 20 | 1594 | 10 443 | −0.013 | −0.035 | 0.008 | 0.236 | 0.00 | |
| Psychosocial functioning | 13 | 995 | 7383 | −0.009 | −0.034 | 0.014 | 0.433 | 0.00 | |
| Emotional functioning | 18 | 1533 | 10 418 | −0.003 | −0.022 | 0.015 | 0.718 | 0.00 | |
| Social functioning | 16 | 1176 | 10 149 | −0.011 | −0.025 | 0.002 | 0.093 | 0.17 | |
| School/work functioning | 10 | 882 | 7986 | −0.001 | −0.018 | 0.014 | 0.827 | 0.00 | |
| Parent reported | |||||||||
| Total HRQOL | 7 | 538 | 11 110 | 0.027 | −0.002 | 0.056 | 0.075 | 0.24 | |
| Physical functioning | 7 | 538 | 11 091 | 0.021 | −0.004 | 0.047 | 0.111 | 0.20 | |
| Psychosocial functioning | 7 | 538 | 11 111 | 0.029 | −0.001 | 0.058 | 0.056 | 0.27 | |
| Emotional functioning | 6 | 508 | 11 060 | 0.021 | −0.006 | 0.049 | 0.128 | 0.19 | |
| Social functioning | 6 | 508 | 11 051 | 0.017 | −0.016 | 0.051 | 0.317 | 0.09 | |
| School/work functioning | 4 | 239 | 9226 | 0.026 | 0.008 | 0.042 | 0.003* | 1.00 | |
HLHS indicates hypoplastic left heart syndrome; and HRQOL, health‐related quality of life.
Statistically significant at P<0.05.
Age at Fontan operation
Older patient age at Fontan operation was associated with worse self‐reported emotional functioning (β=−0.124; 95% CI, −0.210 to −0.038; P=0.004) compared with referents.
Patient age at HRQOL assessment
Older age at HRQOL assessment was associated with better self‐reported psychosocial (β=0.043; 95% CI, 0.013–0.073; P=0.004) and social (β=0.034; 95% CI, 0.011–0.058; P=0.004) functioning (Table 3). In terms of parent‐reported outcomes, older patient age at assessment was associated with poorer total HRQOL (β=−0.077; 95% CI, −0.119 to −0.035; P=0.0003) and emotional (β=−0.065; 95% CI, −0.089 to −0.040; P<0.0001) and social (β=−0.071; 95% CI, −0.130 to −0.011; P=0.018) functioning.
Sex
Relative to studies with a higher proportion of female patients, studies with a higher proportion of male patients yielded a smaller difference in parent‐reported school functioning compared with referents (β=0.026; 95% CI, 0.008–0.042; P=0.003).
Publication Bias
For self‐reported social functioning, visual inspection of the funnel plot and Egger's test indicated publication bias. Trim and Fill estimation still yielded a significant effect size (SMD −0.79; 95% CI, −0.98 to −0.06), and fail‐safe N82 indicated it would take inclusion of 969 studies reporting null results for the findings to lose statistical significance. Visual inspection of the funnel plot suggested all other self‐ and parent‐reported HRQOL domains were symmetric. Results of Egger's test and Begg‐Mazumdar rank correlation test supported this assumption (P>0.05).
Sensitivity Analyses to Examine Potential Measurement Effects
Across studies, mean patient age at PedsQL assessment ranged from 3.0 to 18.5 years. When meta‐analyses were restricted to only PedsQL scores, self‐reported HRQOL remained lower among individuals with a Fontan circulation compared with healthy referents across all PedsQL domains, including total HRQOL (SMD −1.11; 95% CI, −1.32 to −0.90; P<0.0001; k=7) and physical (SMD −1.18; 95% CI, −1.39 to −0.97; P<0.0001; k=12), psychosocial (SMD −0.83; 95% CI, −1.01 to −0.65; P<0.0001; k=10), emotional (SMD −0.58; 95% CI, −0.79 to −0.37; P<0.0001; k=10), social (SMD −0.89; 95% CI, −1.01 to −0.77; P<0.0001; k=10), and school/work (SMD −0.77; 95% CI, −0.91 to −0.63; P<0.0001; k=9) functioning (Table S3). Compared with results of the primary analyses, when analyses included only PedsQL scores, effect sizes across self‐reported HRQOL domains increased. Parent‐proxy PedsQL scores followed the same pattern; parent‐reported physical (SMD −1.02; 95% CI, −1.32 to −0.73; P<0.0001; k=7) and psychosocial (SMD −0.92; 95% CI, −1.29 to −0.56; P<0.0001; k=7) functioning was poorer compared with healthy referents, and these findings did not differ markedly from those of the primary analyses. Meta‐regression analyses performed using only PedsQL scores found HLHS diagnosis was associated with a smaller difference in parent‐reported physical (β=0.010; 95% CI, 0.007–0.014; P<0.0001) and psychosocial (β=0.013; 95% CI, 0.010–0.017; P<0.0001) functioning (Table S4). Older patient age at HRQOL assessment was associated with poorer parent‐reported physical (β=−0.060; 95% CI, −0.098 to −0.022; P=0.021) and psychosocial functioning (β=−0.081; 95% CI, −0.123 to −0.040; P<0.0001) compared with healthy referents.
Across studies, mean patient age at SF‐36 assessment ranged from 20.7 to 27 years. When meta‐analyses included only SF‐36 scores, people with a Fontan circulation reported lower physical (SMD −0.77; 95% CI, −1.01 to −0.53; P<0.0001; k=10) and social (SMD −0.21; 95% CI, −0.42 to −0.01; P=0.044; k=10) functioning compared with healthy referents (Table S3). Mental health component (SMD −0.18; 95% CI, −0.60 to 0.24; P=0.405; k=4) and domain scores (SMD −0.23; 95% CI, −0.57 to 0.12; P=0.197; k=11) did not differ between Fontan patients and healthy referents. Regression analyses performed using only SF‐36 scores found older age at Fontan operation was associated with lower self‐reported mental health scores (β=−0.225; 95% CI, −0.314 to −0.136; P<0.0001). Studies with a higher proportion of female patients yielded a smaller difference in self‐reported physical functioning compared with referents (β=−0.041; 95% CI, −0.075 to −0.007; P=0.018; Table S5).
Factors Associated With HRQOL Identified via Narrative Synthesis
Demographic, clinical, social, and psychological factors associated with poorer self‐ and parent‐reported HRQOL outcomes are summarized in Table 4.
Table 4.
Demographic, Clinical, and Psychological Factors Associated With Poorer Self‐ or Parent‐Reported HRQOL Outcomes
| Variable | No. of Studies Examining Factor | HRQOL Domains | |||||
|---|---|---|---|---|---|---|---|
| Total HRQOL | Physical Functioning | Psychosocial Functioning | Emotional Functioning | Social Functioning | School/Work Functioning | ||
| Demographic factors | |||||||
| Lower household income | 5 | ↓ SR,57 ∅ PR77 | ∅ SR,6 ↓ PR,2 ∅ PR53, 77 | ↓ SR,6 ↓ PR2 | ∅ SR,6 ∅ PR77 | ∅ SR,6 ∅ PR77 | ↓ SR6 |
| Lower patient education | 1 | ∅ SR72 | ∅ SR72 | ||||
| Higher maternal education | 2 | ∅ SR6 | ∅ SR,6 ↑ PR53 | ∅ SR6 | ∅ SR6 | ∅ SR6 | ↑ SR6 |
| Parent unemployment | 1 | ↓ PR2 | ∅ PR2 | ||||
| Patient unemployment | 1 | ∅ SR72 | ∅ SR72 | ||||
| Parent married | 1 | ∅ PR77 | ∅ PR77 | ∅ PR77 | ∅ PR77 | ||
| Patient married | 1 | ∅ SR72 | ∅ SR72 | ||||
| Having a sibling | 1 | ↓ SR23 | ∅ SR23 | ∅ SR23 | ∅ SR23 | ↓ SR23 | |
| Clinical factors, perioperative | |||||||
| Dominant right ventricle | 7 | ∅ SR67 | ∅ SR,41, 63, 72 ↓ PR,41 ∅ PR3, 53, 63 | ∅ SR3, 72 | ∅ SR,63, 74 ∅PR63 | ∅ SR,63 ∅ PR63 | |
| SCC before Fontan | 3 | ∅ PR3, 38, 53 | ↓ PR3, 38 | ||||
| Intracardiac LT Fontan (type) | 7 | ∅ SR,6, 23, 40, 63, 72 ∅ PR53, 63 | ∅ SR72 | ∅ SR,23, 63, 74 ↓ PR63 | ↓ SR,23 ∅ SR63 | ↓ SR23 | |
| No fenestration at Fontan | 4 | ∅ SR,72 ↓ PR,2 ∅ PR39, 53 | ∅ SR,72 ∅ PR39 | ||||
| Greater weight at Fontan | 1 | ↓ PR2 | ∅ PR2 | ||||
| Prenatal diagnosis | 1 | ∅ PR77 | ∅ PR77 | ∅ PR77 | ∅ PR77 | ||
| Preterm birth | 1 | ∅ PR77 | ∅ PR77 | ∅ PR77 | ∅ PR77 | ||
| Shunt type at Norwood | 1 | ∅ PR46 | ∅ PR46 | ∅ PR46 | ∅ PR46 | ∅ PR46 | ∅ PR46 |
| Heterotaxy syndrome | 1 | ∅ SR,37 ∅ PR37 | ∅ SR,37 ∅ PR37 | ||||
| ECMO before Fontan | 1 | ∅ SR,45 ∅ PR45 | ∅ SR,45 ∅ PR45 | ∅ SR,45 ∅ PR45 | ∅ SR,45 ∅ PR45 | ∅ SR,45 ∅ PR45 | ∅ SR,45 ∅ PR45 |
| Coil embolization of APCs | 1 | ∅ PR42 | |||||
| Clinical factors, postoperative | |||||||
| Greater time since surgery | 3 | ∅ PR77 | ↓ SR,23 ∅ SR,72 ∅ PR77 | ∅ SR72 | ↓ SR,23 ∅ PR77 | ∅ SR,23 ∅ PR77 | ↓ SR23 |
| Greater No. of procedures after Fontan | 1 | ↓ SR,63 ↓ PR63 | ∅ SR,63∅ PR63 | ∅ SR,63∅ PR63 | |||
| Greater No. of medications | 2 | ↓ PR77 | ↓ PR2, 77 | ↓ PR77 | |||
| Use of β blocker | 1 | ↓ PR43 | ∅ PR43 | ||||
| Use of class III antiarrhythmic agent | 1 | ↓ PR43 | ∅ PR43 | ||||
| Arrythmia | 4 | ↓ PR2, 43, 53, 60 | ↓ PR,2, 60 ∅ PR43 | ||||
| Protein‐losing enteropathy | 1 | ↓ PR53 | |||||
| Pacemaker | 2 | ↓ PR43, 62 | ∅ PR43, 62 | ||||
| Atrioventricular valve regurgitation | 3 | ∅ SR,72 ↓ PR55 | ∅ SR72 | ∅ SR74 | |||
| Elevated brain natriuretic peptide | 6 | ↓ SR40, 54 ∅ SR,72 ↓ PR,55 ∅ PR53 | ∅ SR,72 ∅ PR55 | ∅ SR74 | |||
| Elevated serum albumin | 1 | ↑ SR72 | ∅ SR72 | ||||
| Lower alanine aminotransferase | 1 | ↑ SR72 | ∅ SR72 | ||||
| Lower resting heart rate | 1 | ↑ PR43 | ∅ PR43 | ||||
| Higher peak heart rate | 2 | ↑ SR,72 ↑ PR43 | ∅ SR,72 ∅ PR43 | ||||
| Higher chronotropic index | 2 | ↑ SR,40 ∅ PR55 | |||||
| Higher resting O2 saturation | 4 | ↑ SR,40 ∅ SR,72 ↑ PR55 | ∅ SR72 | ∅ SR74 | |||
| Higher % predicted VO2 at peak exercise | 6 | ↑ SR,40 ∅ SR,21, 72 ↑ PR52, 55 | ∅ SR,21, 72 ∅ PR52, 55 | ∅ SR21, 74 | ∅ SR21 | ∅ SR21 | |
| Higher % predicted VO2 at anaerobic threshold | 3 | ↑ PR52, 55 | ∅ PR52, 55 | ∅ SR74 | |||
| Higher % predicted maximum work rate | 3 | ↑ SR,40 ↑ PR52, 55 | ↑ PR,55 ∅ PR52, 55 | ||||
| Higher % predicted maximum oxygen pulse | 4 | ∅ SR,40 ↑ PR55 ↓ PR53 | ↑ PR55 | ||||
| Higher ejection fraction | 4 | ↓ SR,23 ∅ SR,63, 72∅ PR53, 63 | ∅ SR72 | ↓ SR,23 ∅ SR63, 74 ∅ PR63 | ↓ SR,23 ∅ SR,63 ∅ PR63 | ↓ SR23 | |
| Lower ventricular end‐systolic volume | 2 | ↑ PR,55 ∅ PR53 | ∅ PR55 | ||||
| Lower ventricular end‐diastolic volume | 3 | ↓SR,63 ↑ PR,55 ∅ PR53, 63 | ∅ PR55 | ∅ SR,63 ↓ PR63 | ↓ SR,63 ∅ PR63 | ||
| Higher VE/VCO2 | 2 | ∅ SR,63, 72 ∅ PR63 | ∅ SR72 | ∅ SR,63 ∅ PR63 | ∅ SR,63 ∅ PR63 | ||
| Reduced FEV1 | 2 | ↓ SR72, 78 | ∅ SR72 | ||||
| Better peripheral vascular function | 1 | ∅ SR22 | ∅ SR22 | ↑ SR22 | |||
| Presence of sinus node dysfunction | 1 | ∅ SR,63 ∅ PR63 | ∅ SR,63↓ PR63 | ∅ SR,63 ∅ PR63 | |||
| Better secondary ventricle function | 1 | ∅ PR58 | |||||
| Increased physical activity | 2 | ∅ SR,69 ∅ PR52 | ∅ PR52 | ↑ SR69 | ∅ SR69 | ||
| Abnormal body mass index | 2 | ↓ SR57 | ∅ PR44 | ∅ PR44 | |||
| Delayed puberty | 1 | ∅ SR57 | |||||
| Shorter stature | 1 | ↓ PR44 | ↓ PR44 | ||||
| Psychological factors | |||||||
| Behavioral or learning problems | 2 | ↓ PR,2 ∅ PR53 | ↓ PR2 | ||||
| Greater psychological distress | 1 | ∅ PR,2∅ PR53 | ↓ PR2 | ||||
All associations at the P<0.05 level. ↓ indicates poorer HRQOL score; ↑, better HRQOL score; ∅, no association; APC, aortopulmonary collateral; ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in 1 second; HRQOL, health‐related quality of life; LT, lateral tunnel; PR, parent reported; SCC, superior cavopulmonary connection; SR, self‐reported; VE/VCO2, minute ventilation and carbon dioxide production; and VO2, oxygen uptake.
Demographic factors
Five studies examined HRQOL and household income. Three cross‐sectional studies found lower household income to be associated with lower parent‐reported physical functioning,2 and lower self‐ and parent‐reported psychosocial functioning2, 6 and overall HRQOL.57 Two studies found no significant association with any HRQOL domain.53, 77 Patients with siblings reported poorer physical and school functioning; however, this factor was examined in only one study.23 Two studies found higher maternal education was associated with better self‐reported school/work functioning6 and parent‐reported physical functioning.53 One study examined patient educational attainment, employment, and marital status; no association with any HRQOL domain was found.72 Associations between parent marital status and HRQOL have also not been found.77
Clinical factors
Of the 7 studies investigating HRQOL and ventricular morphological characteristics, only 1 found children with dominant right ventricle had lower parent‐reported physical functioning,41 whereas 6 found no association.3, 53, 63, 67, 72, 74 Three studies examined HRQOL and timing of superior cavopulmonary connection. Two cross‐sectional studies found parent‐reported psychosocial functioning is lower for children who undergo a superior cavopulmonary connection before their Fontan procedure.3, 38 No association between prior superior cavopulmonary connection and parent‐reported physical functioning was found.38, 53 Seven studies investigated HRQOL and Fontan type; 6 found no association with physical6, 23, 40, 63, 72 and emotional23, 63, 74 functioning when assessed cross‐sectionally, 1 found no association over time,53 and 1 found children with an intracardiac lateral tunnel Fontan reported poorer social and school/work functioning compared with siblings.23 Fenestration at time of Fontan was examined in 4 studies; 1 reported children without a fenestration had lower parent‐reported physical functioning,2 and 3 found no association.39, 53, 72 Associations between HRQOL and prenatal diagnosis,77 preterm birth,77 type of shunt at Norwood procedure,46 coil embolization of the aortopulmonary collateral vessels,42 heterotaxy syndrome,37 and use of extracorporeal membrane oxygenation after Norwood operation45 have not been found.
Of 3 studies, 1 reported longer time since Fontan surgery was associated with lower physical, emotional, and social functioning in adolescents,23 but this association was not found in children77 or adults.72 Parent‐reported physical functioning was lower for patients who had more procedures after Fontan (only 1 study),63 and those taking a greater number of medications (2 of 2 studies),2, 77 particularly β blockers43 and class III antiarrhythmic drugs.43 The same was found for patients with arrhythmias (4 of 4 studies)2, 43, 53, 60 or protein‐losing enteropathy at follow‐up (only 1 study),53 and children with a pacemaker (2 of 2 studies).43, 62 Parents of children who presented with moderate to severe atrioventricular valve regurgitation reported lower physical functioning scores, although this association was evident only for children who had their Fontan at age ≤2 years (1 of 3 studies).55 Two studies found no association between the degree of atrioventricular valve regurgitation and self‐reported HRQOL.72, 74 Across the 6 studies assessing BNP (brain natriuretic peptide) levels, 3 found higher BNP was weakly associated with lower self‐ and parent‐reported physical functioning40, 54, 55 and 3 found no association with any HRQOL domain.53, 72, 74 Higher serum albumin and lower alanine aminotransferase levels were associated with better self‐reported physical functioning in adults;72 however, these factors were examined by only one study.
Objective measures of better cardiopulmonary function, such as lower resting heart rate (1 study),43 higher peak heart rate (2 of 2 studies),43, 72 higher peak work rate (3 of 3 studies),40, 52, 55 and higher resting O2 saturation (2 of 4 studies),40, 55 were associated with better physical functioning. Three of 6 studies found higher predicted oxygen uptake (VO2) at peak exercise was associated with better physical functioning,40, 52, 55 whereas 3 reported no association with physical21, 72 or emotional functioning.74 Higher predicted VO2 at anerobic threshold was associated with better parent‐reported physical functioning across 2 of 3 studies.52, 55 Of the 3 studies that investigated echocardiographic variables (eg, end‐diastolic and end‐systolic volume, stroke volume, ejection fraction, and ventricular mass) using multivariate modeling, 2 found a weak relationship40, 54 and 1 reported no association with HRQOL.55 Similarly, 1 of 2 studies found lower ventricular end‐systolic volumes corresponded with better parent‐reported physical functioning,55 and 1 found no association.53 Across the 3 studies assessing ventricular end‐diastolic volume, 1 found lower scores were associated with better parent‐reported physical functioning,55 whereas 2 found no association.53, 63 Two (of two) studies found reduced forced expiratory volume in 1 second (FEV1) was associated with lower physical functioning.72, 78 Physical activity levels and HRQOL was investigated by 2 studies. One study reported greater total daily activity was weakly associated with better self‐reported psychosocial functioning,69 although no associations with parent‐reported HRQOL outcomes were found.52 Across 2 (of 2) studies, self‐reported physical functioning was predictive of clinical outcomes; poorer self‐reported physical functioning was associated with higher risk of death or heart transplantation over follow‐up.40, 41 Atz et al41 found patients with an elevated brain natriuretic peptide and low physical functioning score were 6 times more likely to die or undergo transplant.
People with better peripheral vascular functioning reported better psychosocial functioning, but only one study examined this, and the correlation was modest.22 Short stature was examined in one study; short height was associated with lower parent‐reported physical and psychosocial functioning.44 Of 2 studies investigating HRQOL and body mass index at follow‐up, 1 found abnormal (higher or lower) body mass index was associated with lower self‐reported HRQOL57 One study found no difference in parent‐reported HRQOL outcomes between patients with abnormal body mass index.44
Psychological factors
Two studies examined HRQOL and psychological factors. Presence of behavioral, attentional, or learning problems or greater anxiety or depression was associated with lower parent‐reported psychosocial functioning in children and adolescents.2 Learning problems were also associated with lower parent‐reported physical functioning2 when assessed cross‐sectionally,2 but not longitudinally.53
Healthcare use and costs
No study examined HRQOL and healthcare use or health service costs.
Discussion
This review, the first to use meta‐analytic methods to investigate HRQOL in this population, synthesizes the findings of 50 articles reporting on outcomes of 2793 people with a Fontan circulation and 1437 parent‐proxies. We found people of all ages with a Fontan circulation report lower total HRQOL compared with referents, and poorer outcomes across all HRQOL domains, with a particularly large effect for physical functioning. Parents also report lower HRQOL for their child with a Fontan circulation compared with parental reports for healthy children or children from the general community. While greater CHD severity is known to be associated with lower self‐ and parent‐reported HRQOL,7, 8, 9 our work demonstrates the high physical and psychological burden experienced by people with a Fontan circulation.
Meta‐analytic findings were generally robust; however, restricting analyses to only SF‐36 scores rendered differences between Fontan patients and healthy referents on psychosocial and emotional functioning non‐significant. Sensitivity analyses found studies measuring HRQOL using the PedsQL reported larger effect sizes compared with studies using the SF‐36. Mean age at PedsQL assessment ranged from 3.0 to 18.5 years, whereas mean age at SF‐36 assessment ranged from 20.7 to 27 years. Previous reviews have found adults with CHD report similar outcomes to healthy controls for psychosocial10, 11 and emotional83 functioning. Moreover, Kahr et al84 found SF‐36 scores were not significantly different between adult CHD patients and healthy controls across all HRQOL domains. Effect size variation across HRQOL measures may be attributable to sample age differences; however, the potential effect of HRQOL measure on outcomes cannot be ruled out.
Meta‐regression analyses revealed emotional and social functioning are more likely than physical functioning to be moderated by demographic and medical factors, such as diagnosis of hypoplastic left heart, age at Fontan operation, and age at HRQOL assessment. Neurodevelopmental impairments in children with CHD have also been shown to strongly predict psychosocial health in adolescence.85 The high prevalence of neuropsychological deficits in children with a Fontan circulation86, 87, 88 may predispose patients to poorer psychosocial outcomes in adulthood. According to the “disability paradox,” peoples’ perceptions of their physical health are embedded in their illness, such that individuals with a chronic illness may have no reference point for “normal” physical functioning as experienced by the general community.89 This may explain why individual factors are less likely to predict physical functioning in people with single‐ventricle CHD, who are born with their illness. Post hoc analyses indicate the associations between social functioning and HLHS and age at HRQOL assessment may not be robust to differences in HRQOL measure. Prospective studies examining predictive factors across people of all ages with a Fontan circulation using a common HRQOL measure are required to determine the strength of this result.
While patients with HLHS, on average, reported lower social functioning compared with others with a Fontan circulation, HLHS diagnosis was associated with better parent‐reported HRQOL across all functional domains. It is possible parents may adjust (or lower) expectations of their child in the context of early counseling on the uncertainty of long‐term outcomes. Mahle et al90 found despite a higher incidence of neurocognitive deficits, most parents of children with HLHS perceived their child's health as “excellent” and described their child's school performance and exercise ability as “average or above.” With male patients representing a greater proportion of HLHS patients than female patients, our findings may be influenced by sex effects, though we did not find meta‐analytic evidence of this.
Compared with younger patients, those older at time of Fontan operation reported poorer emotional functioning. While evidence suggests older age at Fontan does not increase the incidence of physical complications, such as arrhythmias and PLE,91 little is known about the psychological consequences of delaying Fontan surgery. Among other CHD groups, older age at surgery has been associated with higher anxiety and depression at follow‐up.92 From a developmental perspective, older children may have greater capacity to comprehend environmental stress,93 potentially leading to greater acute distress and poorer long‐term psychological outcomes. This finding is potentially confounded by older age at HRQOL assessment. Over half our sample (60%) were adults at the time of HRQOL assessment and likely underwent the Fontan procedure at an older age compared with contemporary practice; thus, our findings may reflect a bias toward adult outcomes.
Parents of older children at HRQOL assessment reported lower overall child HRQOL, as well as lower emotional and social functioning. This difference was not reflected in self‐reported scores, with older patients reporting better psychosocial functioning than younger patients; however, post hoc analyses indicate this finding may be sensitive to HRQOL measure. Across the reviewed studies, the highest mean age reported was 27 years. It is possible with an aging Fontan population, we will see an increase in challenges that influence psychosocial outcomes, such as greater physical morbidity, difficulties associated with childbearing, and fears of premature death. It is also difficult to determine the true discrepancy between self‐ and parent‐reported outcomes, as the average patient age was higher among studies relying on self‐report compared with parent‐report. Of the 20 studies that included both parent‐report and self‐report, only 3 tested for differences and 2 of these found parent‐reported outcomes were significantly lower than the child's own assessment.
Limitations of Captured Studies and the Current Review
While increasing attention is focused on HRQOL as a clinical indicator of health outcomes in the Fontan population, there remains considerable methodologic and measurement variation across studies. Conceptual challenges exist in CHD HRQOL research, including lack of common theoretical frameworks and operational definitions,94 making it difficult to compare findings between studies and across time. Only 8 studies examined HRQOL using a longitudinal design, limiting causal inference. Changes in medical practices over time (eg, younger age at Fontan procedure in contemporary practice compared with previous surgical eras), and improving survival rates for HLHS patients, may introduce bias. Chance of collinearity between variables is also high,95 making it difficult to determine the precise effect of risk factors on adverse HRQOL outcomes. In addition, the relatively young age of our cohort may limit the generalizability of our findings to older Fontan patients, who likely experience poorer HRQOL as morbidities arise with age. Factors known to be associated with HRQOL, such as socioeconomic deprivation, neurodevelopmental impairment, greater number of surgeries, and longer length of hospitalization, were not able to be meta‐analyzed because of insufficient data, leaving the potential moderating effect of these variables undetermined. Similarly, while high psychological distress and limited social support are known to exert stronger influence on HRQOL within CHD cohorts than clinical factors,11, 15, 96, 97 we found only 2 studies examining this association, indicating an important knowledge gap. Furthermore, none of the reviewed studies examined HRQOL and healthcare use or fiscal costs; thus, the extent to which HRQOL is influenced by healthcare practices and vice versa remains unclear. Meaningful data could not be extracted from some studies because of incomplete reporting of raw data and lack of control conditions. Evidence of publication bias was found for self‐reported social functioning; however, the result remained significant after the Trim and Fill procedure. While people with a Fontan circulation reported lower social functioning compared with healthy referents, the magnitude of this difference should be interpreted cautiously.
Our meta‐analyses relied on aggregated study‐level data, rather than individual‐level data. Using individual‐level data may provide greater detail and increase the chance of detecting predictor effects on HRQOL. Exclusion of non–English‐language studies may also limit the generalizability of results.
Priorities for Clinical Practice and Research Advancement
Optimal care acknowledges the lifelong impact of the Fontan circulation on HRQOL and well‐being. Targeted strategies are required to ensure long‐term outcomes of this growing cohort continue to improve.17 Population‐based registries, such as the Australian and New Zealand Fontan Registry98 and the U.S. National Pediatric Cardiology Quality Improvement Collaborative (NPC‐QIC),99 provide encouraging examples of large, rigorous, multisite collaborations designed to overcome the limitations of single‐center initiatives. Additional recommendations include the following: (1) use of conceptually driven HRQOL frameworks to inform research questions, methods, and the development and trial of screening and intervention protocols; (2) consistency in measurement and reporting of outcomes and predictive, mediating, and moderating factors to ensure results can be pooled; and (3) longitudinal assessment of HRQOL to capture developmentally sensitive patient‐ and parent‐reported outcomes, as well as potential changes in outcomes over time and with clinical advances. Culturally and developmentally tailored perioperative psychoeducation and psychological care is recommended to bolster psychological resilience, shared decision making, and coping skills among patients and their families.100
Conclusions
The Fontan procedure has led to a pathway of survival for people born with single‐ventricle CHD, yet as this cohort ages, the burden of accompanying physical, neurodevelopmental, and psychological morbidities are becoming increasingly evident. This meta‐analysis confirms people with a Fontan circulation and their proxies report poorer HRQOL when compared with the general community. While the quality of available evidence is high, our knowledge of the role of moderating factors and psychosocial variables is limited. Considerable work is needed to strengthen our knowledge of the determinants of HRQOL and institute targeted preventive approaches to improve outcomes for this population.
Sources of Funding
K. H. Marshall is the recipient of a University of New South Wales Scientia PhD Scholarship. Dr Kasparian is the recipient of a National Heart Foundation of Australia Future Leader Fellowship (101229) and a 2018 to 2019 Harkness Fellowship in Health Care Policy and Practice from The Commonwealth Fund. This work was supported by an National Health and Medical Research Council (NHMRC) of Australia Project Grant (APP1081001).
Disclosures
Dr D'Udekem is a Clinician Practitioner Fellow of the National Health and Medical Research Council (1082186) and has received consulting fees from Merck Sharp & Dohme and Actelion. Dr Opotowsky has received consulting fees from Actelion and Novartis. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5
Figure S1
J Am Heart Assoc. 2020;9:e014172 DOI: 10.1161/JAHA.119.014172
References
- 1. Iyengar AJ, Shann F, Cochrane AD, Brizard CP, d'Udekem Y. The Fontan procedure in Australia: a population‐based study. J Thorac Cardiovasc Surg. 2007;134:1353–1354. [DOI] [PubMed] [Google Scholar]
- 2. McCrindle BW, Williams RV, Mitchell PD, Hsu DT, Paridon SM, Atz AM, Li JS, Newburger JW. Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation. 2006;113:1123–1129. [DOI] [PubMed] [Google Scholar]
- 3. Anderson PAW, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health‐related quality of life. J Nurs Scholarsh. 2005;37:336–342. [DOI] [PubMed] [Google Scholar]
- 5. United States Department of Health and Human Services . Healthy People 2020: Foundation Health Measure Report. 2010. Available at: https://www.healthypeople.gov/sites/default/files/HRQoLWBFullReport.pdf. Accessed July 28, 2019.
- 6. Uzark K, Zak V, Shrader P, McCrindle B, Radojewski E, Varni J, Daniels K, Handisides J, Hill K, Lambert L, et al. Assessment of quality of life in young patients with single ventricle after the Fontan operation. J Pediatr. 2016;170:166–172.e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ladak LA, Hasan BS, Gullick J, Gallagher R. Health‐related quality of life in congenital heart disease surgery in children and young adults: a systematic review and meta‐analysis. Arch Dis Child. 2019;104:340–347. [DOI] [PubMed] [Google Scholar]
- 8. Latal B, Helfricht S, Fischer JE, Bauersfeld U, Landolt MA. Psychological adjustment and quality of life in children and adolescents following open‐heart surgery for congenital heart disease: a systematic review. BMC Pediatr. 2009;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drakouli M, Petsios K, Giannakopoulou M, Patiraki E, Voutoufianaki I, Matziou V. Determinants of quality of life in children and adolescents with CHD: a systematic review. Cardiol Young. 2015;25:1027–1036. [DOI] [PubMed] [Google Scholar]
- 10. Schrøder M, Boisen KA, Reimers J, Teilmann G, Brok J. Quality of life in adolescents and young adults with CHD is not reduced: a systematic review and meta‐analysis. Cardiol Young. 2016;26:415–425. [DOI] [PubMed] [Google Scholar]
- 11. Fteropoulli T, Stygall J, Cullen S, Deanfield J, Newman SP. Quality of life of adult congenital heart disease patients: a systematic review of the literature. Cardiol Young. 2013;23:473–485. [DOI] [PubMed] [Google Scholar]
- 12. Ferguson MK, Kovacs AH. Quality of life in children and young adults with cardiac conditions. Curr Opin Cardiol. 2013;28:115–121. [DOI] [PubMed] [Google Scholar]
- 13. Bertoletti J, Marx GC, Hattge Júnior SP, Pellanda LC. Quality of life and congenital heart disease in childhood and adolescence. Arq Bras Cardiol. 2014;102:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia Guerra G, Robertson CM, Alton GY, Joffe AR, Dinu IA, Nicholas D, Ross DB, Rebeyka IM; Western Canadian Complex Pediatric Therapies Follow‐up Group . Quality of life 4 years after complex heart surgery in infancy. J Thorac Cardiovasc Surg. 2013;145:482–488.e482. [DOI] [PubMed] [Google Scholar]
- 15. Denniss DL, Sholler GF, Costa DSJ, Winlaw DS, Kasparian NA. Need for routine screening of health‐related quality of life in families of young children with complex congenital heart disease. J Pediatr. 2019;205:21–28.e22. [DOI] [PubMed] [Google Scholar]
- 16. Schilling C, Dalziel K, Nunn R, Du Plessis K, Shi WY, Celermajer D, Winlaw D, Weintraub RG, Grigg LE, Radford DJ, et al. The Fontan epidemic: population projections from the Australia and New Zealand Fontan Registry. Int J Cardiol. 2016;219:14–19. [DOI] [PubMed] [Google Scholar]
- 17. Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, Hsia T‐Y, Hsu DT, Kovacs AH, McCrindle BW, et al. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation. 2019;140:e234–e284. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA‐P Group . Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moons P, Budts W, De Geest S. Critique on the conceptualisation of quality of life: a review and evaluation of different conceptual approaches. Int J Nurs Stud. 2006;43:891–901. [DOI] [PubMed] [Google Scholar]
- 20. Dulfer K, Duppen N, Kuipers I, Schokking M, Domburg R, Verhulst F, Helbing W, Utens E. Aerobic exercise influences quality of life of children and youngsters with congenital heart disease: a randomized controlled trial. J Adolesc Health. 2014;55:65–72. [DOI] [PubMed] [Google Scholar]
- 21. Idorn L, Jensen AS, Juul K, Overgaard D, Nielsen NP, Sorensen K, Reimers JI, Sondergaard L. Quality of life and cognitive function in Fontan patients, a population‐based study. Int J Cardiol. 2013;168:3230–3235. [DOI] [PubMed] [Google Scholar]
- 22. Goldstein BH, Golbus JR, Sandelin AM, Warnke N, Gooding L, King KK, Donohue JE, Gurney JG, Goldberg CS, Rocchini AP, et al. Usefulness of peripheral vascular function to predict functional health status in patients with Fontan circulation. Am J Cardiol. 2011;108:428–434. [DOI] [PubMed] [Google Scholar]
- 23. Manlhiot C, Knezevich S, Radojewski E, Cullen‐Dean G, Williams WG, McCrindle BW. Functional health status of adolescents after the Fontan procedure—comparison with their siblings. Can J Cardiol. 2009;25:e294–e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cochrane Training . RevMan Calculator. 2014. Available at: http://training.cochrane.org/resource/revman-calculator. Accessed March 31, 2019.
- 25. Overgaard D, Schrader AM, Lisby KH, King C, Christensen RF, Jensen HF, Idorn L, Sondergaard L, Moons P. Patient‐reported outcomes in adult survivors with single‐ventricle physiology. Cardiology. 2011;120:36–42. [DOI] [PubMed] [Google Scholar]
- 26. Kmet LM, Lee RC, Cook LS. Standard Quality Assessment Criteria for Evaluating Primary Research Papers From a Variety of Fields. Edmonton, Canada: Alberta Heritage Foundation for Medical Research; 2004. [Google Scholar]
- 27. Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta‐Analysis Version 3. Englewood, NJ: Biostat; 2013. [Google Scholar]
- 28. Grissom RJ, Kim JJ. Effect Sizes for Research: A Broad Practical Approach. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2005. [Google Scholar]
- 29. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 30. Rao G, Lopez‐Jimenez F, Boyd J, D'Amico F, Durant NH, Hlatky MA, Howard G, Kirley K, Masi C, Powell‐Wiley TM, et al. Methodological standards for meta‐analyses and qualitative systematic reviews of cardiac prevention and treatment studies: a scientific statement from the American Heart Association. Circulation. 2017;136:e172–e194. [DOI] [PubMed] [Google Scholar]
- 31. Rice K, Higgins JPT, Lumley T. A re‐evaluation of fixed effect(s) meta‐analysis. J R Stat Soc Ser A Stat Soc. 2018;181:205–227. [Google Scholar]
- 32. Borenstein M, Hedges L, Higgins JP, Rothstein H. Introduction to Meta‐Analysis. Chicester, UK: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 33. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 36. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 37. Atz AM, Cohen MS, Sleeper LA, McCrindle BW, Lu M, Prakash A, Breitbart RE, Williams RV, Sang CJ, Wernovsky G. Functional state of patients with heterotaxy syndrome following the Fontan operation. Cardiol Young. 2007;17:44–53. [DOI] [PubMed] [Google Scholar]
- 38. Atz AM, Travison TG, McCrindle BW, Mahony L, Glatz AC, Kaza AK, Breitbart RE, Colan SD, Kaltman JR, Margossian R, et al. Cardiac performance and quality of life in patients who have undergone the Fontan procedure with and without prior superior cavopulmonary connection. Cardiol Young. 2013;23:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Atz AM, Travison TG, McCrindle BW, Mahony L, Quartermain M, Williams RV, Breitbart RE, Lu M, Radojewski E, Margossian R, et al. Late status of Fontan patients with persistent surgical fenestration. J Am Coll Cardiol. 2011;57:2437–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atz AM, Zak V, Mahony L, Uzark K, D'Agincourt N, Goldberg DJ, Williams RV, Breitbart RE, Colan SD, Burns KM, et al. Longitudinal outcomes of patients with single ventricle after the Fontan procedure. J Am Coll Cardiol. 2017;69:2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Atz AM, Zak V, Mahony L, Uzark K, Shrader P, Gallagher D, Paridon SM, Williams RV, Breitbart RE, Colan SD, et al. Survival data and predictors of functional outcome an average of 15 years after the Fontan procedure: the Pediatric Heart Network Fontan Cohort. Congenit Heart Dis. 2015;10:E30–E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Banka P, Sleeper L, Atz A, Cowley C, Gallagher D, Gillespie M, Graham E, Margossian R, McCrindle B, Sang C, et al. Practice variability and outcomes of coil embolization of aortopulmonary collaterals before Fontan completion: a report from the Pediatric Heart Network Fontan Cross‐Sectional Study. Am Heart J. 2011;162:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blaufox AD, Sleeper LA, Bradley DJ, Breitbart RE, Hordof A, Kanter RJ, Stephenson EA, Stylianou M, Vetter VL, Saul JP. Functional status, heart rate, and rhythm abnormalities in 521 Fontan patients 6 to 18 years of age. J Thorac Cardiovasc Surg. 2008;136:100–107.e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen M, Zak V, Atz A, Printz B, Pinto N, Lambert L, Pemberton V, Li J, Margossian R, Dunbar‐Masterson C, et al. Anthropometric measures after Fontan procedure: implications for suboptimal functional outcome. Am Heart J. 2010;160:1092–1098, 1098.e1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Friedland‐Little J, Uzark K, Yu S, Lowery R, Aiyagari R, Hirsch‐Romano J. Functional status and quality of life in survivors of extracorporeal membrane oxygenation after the Norwood operation. Ann Thorac Surg. 2017;103:1950–1955. [DOI] [PubMed] [Google Scholar]
- 46. Goldberg C, Lu M, Sleeper L, Mahle W, Gaynor J, Williams I, Mussatto K, Ohye R, Graham E, Frank D, et al. Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr. 2014;165:490–496.e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jacobsen RM, Ginde S, Mussatto K, Neubauer J, Earing M, Danduran M. Can a home‐based cardiac physical activity program improve the physical function quality of life in children with Fontan circulation? Congenit Heart Dis. 2016;11:175–182. [DOI] [PubMed] [Google Scholar]
- 48. Jacobsen R, Danduran M, Mussatto K, Hill GD, Ginde S. Can a home‐based cardiac physical activity program improve and sustain quality of life and exercise capacity in children with Fontan circulation? Prog Pediatr Cardiol. 2018;50:12–16. [Google Scholar]
- 49. Karamlou T, Poynter J, Walters H, Rhodes J, Bondarenko I, Pasquali S. Long‐term functional health status and exercise test variables for patients with pulmonary atresia with intact ventricular septum: a Congenital Heart Surgeons Society study. J Thorac Cardiovasc Surg. 2013;145:1018–1025; discussion 1025‐1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kukreja M, Bryant AS, Cleveland DC, Dabal R, Hingorani N, Kirklin JK. Health‐related quality of life in adult survivors after the Fontan operation. Semin Thorac Cardiovasc Surg. 2015;27:299–306. [DOI] [PubMed] [Google Scholar]
- 51. Lambert LM, Minich L, Newburger JW, Lu M, Pemberton VL, McGrath EA, Atz AM, Xu M, Radojewski E, Servedio D, et al. Parent‐ versus child‐reported functional health status after the Fontan procedure. Pediatrics. 2009;124:e942–e949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McCrindle BW, Williams RV, Mital S, Clark BJ, Russell JL, Klein G, Eisenmann JC. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child. 2007;92:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McCrindle BW, Zak V, Breitbart RE, Mahony L, Shrader P, Lai WW, Burns KM, Colan SD, Williams RV, Goldberg D, et al. The relationship of patient medical and laboratory characteristics to changes in functional health status in children and adolescents after the Fontan procedure. Pediatr Cardiol. 2014;35:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCrindle BW, Zak V, Pemberton VL, Lambert LM, Vetter VL, Lai WW, Uzark K, Margossian R, Atz AM, Cook A, et al. Functional health status in children and adolescents after Fontan: comparison of generic and disease‐specific assessments. Cardiol Young. 2014;24:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McCrindle BW, Zak V, Sleeper LA, Paridon SM, Colan SD, Geva T, Mahony L, Li JS, Breitbart RE, Margossian R, et al. Laboratory measures of exercise capacity and ventricular characteristics and function are weakly associated with functional health status after Fontan procedure. Circulation. 2010;121:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mellion K, Uzark K, Cassedy A, Drotar D, Wernovsky G, Newburger JW, Mahony L, Mussatto K, Cohen M, Limbers C, et al. Health‐related quality of life outcomes in children and adolescents with congenital heart disease. J Pediatr. 2014;164:781–788.e781. [DOI] [PubMed] [Google Scholar]
- 57. Menon SC, Al‐Dulaimi R, McCrindle BW, Goldberg DJ, Sachdeva R, Goldstein BH, Seery T, Uzark KC, Chelliah A, Butts R, et al. Delayed puberty and abnormal anthropometry and its associations with quality of life in young Fontan survivors: a multicenter cross‐sectional study. Congenit Heart Dis. 2018;13:463–469. [DOI] [PubMed] [Google Scholar]
- 58. Prakash A, Travison TG, Fogel MA, Hurwitz LM, Powell AJ, Printz BF, Puchalski MD, Shirali GS, Yoo SJ, Geva T. Relation of size of secondary ventricles to exercise performance in children after Fontan operation. Am J Cardiol. 2010;106:1652–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pike NA, Evangelista LS, Doering LV, Eastwood J‐A, Lewis AB, Child JS. Quality of life, health status, and depression: comparison between adolescents and adults after the Fontan procedure with healthy counterparts. J Cardiovasc Nurs. 2012;27:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stephenson EA, Lu M, Berul CI, Etheridge SP, Idriss SF, Margossian R, Reed JH, Prakash A, Sleeper LA, Vetter VL, et al. Arrhythmias in a contemporary Fontan cohort: prevalence and clinical associations in a multicenter cross‐sectional study. J Am Coll Cardiol. 2010;56:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Williams IA, Sleeper LA, Colan SD, Lu M, Stephenson EA, Newburger JW, Gersony WM, Cohen MS, Cnota JF, Atz AM, et al. Functional state following the Fontan procedure. Cardiol Young. 2009;19:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Williams RV, Travison T, Kaltman JR, Cecchin F, Colan SD, Idriss SF, Lu M, Margossian R, Reed JH, Silver ES, et al. Comparison of Fontan survivors with and without pacemakers: a report from the Pediatric Heart Network Fontan Cross‐Sectional Study. Congenit Heart Dis. 2013;8:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dulfer K, Bossers SS, Utens EM, Duppen N, Kuipers IM, Kapusta L, van Iperen G, Schokking M, ten Harkel AD, Takken T, et al. Does functional health status predict health‐related quality of life in children after Fontan operation? Cardiol Young. 2016;26:459–468. [DOI] [PubMed] [Google Scholar]
- 64. van den Bosch AE, Roos‐Hesselink JW, Van Domburg R, Bogers AJ, Simoons ML, Meijboom FJ. Long‐term outcome and quality of life in adult patients after the Fontan operation. Am J Cardiol. 2004;93:1141–1145. [DOI] [PubMed] [Google Scholar]
- 65. Wolff D, van de Wiel HBM, de Muinck Keizer ME, van Melle JP, Pieper PG, Berger RMF, Ebels T, Weijmar Schultz WCM. Quality of life and sexual well‐being in patients with a Fontan circulation: an explorative pilot study with a mixed method design. Congenit Heart Dis. 2018;13:319–326. [DOI] [PubMed] [Google Scholar]
- 66. Gratz A, Hess J, Hager A. Self‐estimated physical functioning poorly predicts actual exercise capacity in adolescents and adults with congenital heart disease. Eur Heart J. 2009;30:497–504. [DOI] [PubMed] [Google Scholar]
- 67. Hock J, Reiner B, Neidenbach RC, Oberhoffer R, Hager A, Ewert P, Muller J. Functional outcome in contemporary children with total cavopulmonary connection—health‐related physical fitness, exercise capacity and health‐related quality of life. Int J Cardiol. 2018;255:50–54. [DOI] [PubMed] [Google Scholar]
- 68. Muller J, Hess J, Hager A. Minor symptoms of depression in patients with congenital heart disease have a larger impact on quality of life than limited exercise capacity. Int J Cardiol. 2012;154:265–269. [DOI] [PubMed] [Google Scholar]
- 69. Muller J, Christov F, Schreiber C, Hess J, Hager A. Exercise capacity, quality of life, and daily activity in the long‐term follow‐up of patients with univentricular heart and total cavopulmonary connection. Eur Heart J. 2009;30:2915–2920. [DOI] [PubMed] [Google Scholar]
- 70. Hedlund ER, Lundell B, Soderstrom L, Sjoberg G. Can endurance training improve physical capacity and quality of life in young Fontan patients? Cardiol Young. 2018;28:438–446. [DOI] [PubMed] [Google Scholar]
- 71. Hedlund ER, Lundell B, Villard L, Sjöberg G. Reduced physical exercise and health‐related quality of life after Fontan palliation. Acta Paediatr. 2016;105:1322–1328. [DOI] [PubMed] [Google Scholar]
- 72. Smas‐Suska M, Dluzniewska N, Werynski P, Pajak J, Plazak W, Olszowska M, Podolec P, Tomkiewicz‐Pajak L. What determines the quality of life of adult patients after Fontan procedure? Cardiol J. 2018;25:72–80. [DOI] [PubMed] [Google Scholar]
- 73. Yildiz CE, Zahmacioglu O, Koca B, Oktay V, Gokalp S, Eroglu AG, Cetin G, Oztunc F. Self perception and quality of life of adolescents who had undergone open‐heart surgery due to cyanotic congenital heart disease in their infancy. Turk Pediatri Ars. 2011;46:220–227. [Google Scholar]
- 74. d'Udekem Y, Cheung MM, Setyapranata S, Iyengar AJ, Kelly P, Buckland N, Grigg LE, Weintraub RG, Vance A, Brizard CP, et al. How good is a good Fontan? Quality of life and exercise capacity of Fontans without arrhythmias. Ann Thorac Surg. 2009;88:1961–1969. [DOI] [PubMed] [Google Scholar]
- 75. Sutherland N, Jones B, Westcamp Aguero S, Melchiori T, du Plessis K, Konstantinov IE, Cheung MMH, d'Udekem Y. Home‐ and hospital‐based exercise training programme after Fontan surgery. Cardiol Young. 2018;28:1299–1305. [DOI] [PubMed] [Google Scholar]
- 76. Czosek RJ, Cassedy AE, Wray J, Wernovsky G, Newburger JW, Mussatto KA, Mahony L, Tanel RE, Cohen MI, Franklin RC, et al. Quality of life in pediatric patients affected by electrophysiologic disease. Heart Rhythm. 2015;12:899–908. [DOI] [PubMed] [Google Scholar]
- 77. Heye KN, Knirsch W, Scheer I, Beck I, Wetterling K, Hahn A, Hofmann K, Latal B, Reich B, Landolt MA. Health‐related quality of life in pre‐school age children with single‐ventricle CHD. Cardiol Young. 2019;29:162–168. [DOI] [PubMed] [Google Scholar]
- 78. Callegari A, Neidenbach R, Milanesi O, Castaldi B, Christmann M, Ono M, Muller J, Ewert P, Hager A. A restrictive ventilatory pattern is common in patients with univentricular heart after Fontan palliation and associated with a reduced exercise capacity and quality of life. Congenit Heart Dis. 2019;14:147–155. [DOI] [PubMed] [Google Scholar]
- 79. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. [DOI] [PubMed] [Google Scholar]
- 80. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36), I: conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 81. Landgraf JM, Abetz L, Ware JE. The Child Health Questionnaire (CHQ) User's Manual. Boston, MA: HealthAct; 1999. [Google Scholar]
- 82. Orwin RG. A fail‐safe N for effect size in meta‐analysis. J Educ Stat. 1983;8:157–159. [Google Scholar]
- 83. Jackson JL, Misiti B, Bridge JA, Daniels CJ, Vannatta K. Emotional functioning of adolescents and adults with congenital heart disease: a meta‐analysis. Congenit Heart Dis. 2015;10:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kahr PC, Radke RM, Orwat S, Baumgartner H, Diller GP. Analysis of associations between congenital heart defect complexity and health‐related quality of life using a meta‐analytic strategy. Int J Cardiol. 2015;199:197–203. [DOI] [PubMed] [Google Scholar]
- 85. Robson VK, Stopp C, Wypij D, Dunbar‐Masterson C, Bellinger DC, DeMaso DR, Rappaport LA, Newburger JW. Longitudinal associations between neurodevelopment and psychosocial health status in patients with repaired D‐transposition of the great arteries. J Pediatr. 2019;204:38–45.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Goldberg CS, Schwartz EM, Brunberg JA, Mosca RS, Bove EL, Schork MA, Stetz SP, Cheatham JP, Kulik TJ. Neurodevelopmental outcome of patients after the Fontan operation: a comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr. 2000;137:646–652. [DOI] [PubMed] [Google Scholar]
- 87. Bellinger DC, Watson CG, Rivkin MJ, Robertson RL, Roberts AE, Stopp C, Dunbar‐Masterson C, Bernson D, DeMaso DR, Wypij D, et al. Neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the Fontan procedure. J Am Heart Assoc. 2015;4:e002302 DOI: 10.1161/JAHA.115.002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Uzark K, Lincoln A, Lamberti JJ, Mainwaring RD, Spicer RL, Moore JW. Neurodevelopmental outcomes in children with Fontan repair of functional single ventricle. Pediatrics. 1998;101:630–633. [DOI] [PubMed] [Google Scholar]
- 89. Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med. 1999;48:977–988. [DOI] [PubMed] [Google Scholar]
- 90. Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school‐aged and adolescent children with hypoplastic left heart syndrome. Pediatrics. 2000;105:1082–1089. [DOI] [PubMed] [Google Scholar]
- 91. Pace Napoleone C, Oppido G, Angeli E, Giardini A, Resciniti E, Gargiulo G. Results of the modified Fontan procedure are not related to age at operation. Eur J Cardiothorac Surg. 2010;37:645–650. [DOI] [PubMed] [Google Scholar]
- 92. Utens EM, Verhulst FC, Duivenvoorden HJ, Meijboom FJ, Erdman RA, Hess J. Prediction of behavioural and emotional problems in children and adolescents with operated congenital heart disease. Eur Heart J. 1998;19:801–807. [DOI] [PubMed] [Google Scholar]
- 93. Rudolph KD, Dennig MD, Weisz JR. Determinants and consequences of children's coping in the medical setting: conceptualization, review, and critique. Psychol Bull. 1995;118:328–357. [DOI] [PubMed] [Google Scholar]
- 94. Moons P, Van Deyk K, Budts W, De Geest S. Caliber of quality‐of‐life assessments in congenital heart disease: a plea for more conceptual and methodological rigor. Arch Pediatr Adolesc Med. 2004;158:1062–1069. [DOI] [PubMed] [Google Scholar]
- 95. Yoo W, Mayberry R, Bae S, Singh K, Peter He Q, Lillard JW Jr. A study of effects of multicollinearity in the multivariable analysis. Int J Appl Sci Technol. 2014;4:9–19. [PMC free article] [PubMed] [Google Scholar]
- 96. DeMaso DR, Calderon J, Taylor GA, Holland JE, Stopp C, White MT, Bellinger DC, Rivkin MJ, Wypij D, Newburger JW. Psychiatric disorders in adolescents with single ventricle congenital heart disease. Pediatrics. 2017;139:e20162241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Casey FA, Stewart M, McCusker CG, Morrison ML, Molloy B, Doherty N, Craig BG, Sands AJ, Rooney N, Mulholland HC. Examination of the physical and psychosocial determinants of health behaviour in 4‐5‐year‐old children with congenital cardiac disease. Cardiol Young. 2010;20:532–537. [DOI] [PubMed] [Google Scholar]
- 98. Iyengar AJ, Winlaw DS, Galati JC, Gentles TL, Weintraub RG, Justo RN, Wheaton GR, Bullock A, Celermajer DS, d'Udekem Y. The Australia and New Zealand Fontan Registry: description and initial results from the first population‐based Fontan registry. Intern Med J. 2014;44:148–155. [DOI] [PubMed] [Google Scholar]
- 99. Martin GR, Anderson JB, Vincent RN. Impact registry and national pediatric cardiology quality improvement collaborative: contributions to quality in congenital heart disease. World J Pediatr Congenit Heart Surg. 2019;10:72–80. [DOI] [PubMed] [Google Scholar]
- 100. LeRoy S, Elixson EM, O'Brien P, Tong E, Turpin S, Uzark K. Recommendations for preparing children and adolescents for invasive cardiac procedures: a statement from the American Heart Association Pediatric Nursing Subcommittee of the Council on Cardiovascular Nursing in Collaboration with the Council on Cardiovascular Diseases of the Young. Circulation. 2003;108:2550–2564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figure S1


