Abstract
Background
Laboratory studies demonstrate glucose‐insulin‐potassium (GIK) as a potent cardioprotective intervention, but clinical trials have yielded mixed results, likely because of varying formulas and timing of GIK treatment and different clinical settings. This study sought to evaluate the effects of modified GIK regimen given perioperatively with an insulin‐glucose ratio of 1:3 in patients undergoing cardiopulmonary bypass surgery.
Methods and Results
In this prospective, randomized, double‐blinded trial with 930 patients referred for cardiac surgery with cardiopulmonary bypass, GIK (200 g/L glucose, 66.7 U/L insulin, and 80 mmol/L KCl) or placebo treatment was administered intravenously at 1 mL/kg per hour 10 minutes before anesthesia and continuously for 12.5 hours. The primary outcome was the incidence of in‐hospital major adverse cardiac events including all‐cause death, low cardiac output syndrome, acute myocardial infarction, cardiac arrest with successful resuscitation, congestive heart failure, and arrhythmia. GIK therapy reduced the incidence of major adverse cardiac events and enhanced cardiac function recovery without increasing perioperative blood glucose compared with the control group. Mechanistically, this treatment resulted in increased glucose uptake and less lactate excretion calculated by the differences between arterial and coronary sinus, and increased phosphorylation of insulin receptor substrate‐1 and protein kinase B in the hearts of GIK‐treated patients. Systemic blood lactate was also reduced in GIK‐treated patients during cardiopulmonary bypass surgery.
Conclusions
A modified GIK regimen administered perioperatively reduces the incidence of in‐hospital major adverse cardiac events in patients undergoing cardiopulmonary bypass surgery. These benefits are likely a result of enhanced systemic tissue perfusion and improved myocardial metabolism via activation of insulin signaling by GIK.
Clinical Trial Registration
URL: clinicaltrials.gov. Identifier: NCT01516138.
Keywords: cardiopulmonary bypass, insulin, cardiac surgery, tissue perfusion
Subject Categories: Cardiovascular Surgery
Clinical Perspective
What Is New?
-
•
In this randomized, double-blind, and placebo-controlled trial, a modified glucose-insulin-potassium regimen with an insulin-glucose ratio of 1:3 exerted significant cardioprotection and reduced the incidence of in-hospital major adverse cardiac events in patients undergoing cardiopulmonary bypass surgery, without further increasing blood glucose levels compared with control.
-
•
These data also provided the first piece of direct evidence of alleviated postoperative hyperlactemia and preserved renal function by the modified glucose-insulin-potassium.
What Are the Clinical Implications?
-
•
Our findings support the cardioprotection of the modified glucose-insulin-potassium (insulin-glucose ratio 1:3) and suggest that glucose-insulin-potassium could be part of the perioperative medication in cardiopulmonary bypass surgery, especially in developing countries, attributable to its low cost and wide applicability.
Mounting experimental evidence has documented significant cardioprotection of glucose‐insulin‐potassium (GIK) in ischemic hearts. 1 , 2 , 3 , 4 However, clinical trials have not consistently shown the beneficial effects of this therapy, partly because of different formulas and administration timing of GIK in these studies. 5 , 6 , 7 , 8 , 9 The ratio of insulin to glucose in previous GIK formulas was usually between 1:6 and 1:4, which often led to significant elevation of blood glucose in GIK‐treated patients. 5 , 6 , 7 , 8 , 9 Although the optimal level of glycemic control in patients undergoing cardiac surgery as well as other critically ill patients remains controversial, 10 , 11 , 12 , 13 perioperative hyperglycemia is generally associated with adverse outcome in patients undergoing cardiopulmonary bypass (CPB). 12 , 13 , 14 Furthermore, our previous study demonstrated that hyperglycemia reduces the beneficial effects of GIK. 15 Therefore, we hypothesized that adjusting the insulin‐glucose ratio of GIK may avoid further increasing blood glucose and thus maximize the protection of GIK for patients undergoing CPB.
The optimum timing of GIK infusion is another critical issue. Strong experimental evidence pointed out that GIK should be given early to be able to reach the ischemic myocardium before reperfusion. 16 , 17 The application of this concept has been confirmed by several large clinical trials in patients with acute myocardial infarction or acute coronary syndromes (ACS). 5 , 8 , 18 GIK given early (≈ 90 minutes) after myocardial ischemia reduces the incidence of cardiac arrest and in‐hospital mortality, 5 while late application (after reperfusion) of GIK fails to exert beneficial effects. 8 , 18 Although this treatment regimen greatly limits its application as an effective treatment for acute myocardial infarction, preventive GIK before myocardial ischemia is certainly achievable during cardiac surgical procedure, which might slow the onset of irreversible injury and result in the best recovery of myocardial function via increased myocardial glycogen content and production of glycolytic ATP during ischemia. 13 , 19 Therefore, this double‐blinded, randomized, controlled clinical trial was designed to test the effect of GIK with an insulin‐glucose ratio of 1:3, starting 10 minutes before anesthesia and lasting for 12.5 hours, on the incidence of major adverse cardiac events (MACE) in 930 patients undergoing cardiac surgery with CPB.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request and approval for the principal investigator.
Patient Population and Study Design
The study was approved by the Ethics Committee of Xijing Hospital, Xian, China, registered with ClinicalTrials.gov (identifier: NCT01516138), and carried out in accordance with the Declaration of Helsinki. Written, informed consent was obtained from all patients or their legal surrogates before study inclusion. A total of 2909 patients referred for cardiac surgery with CPB at Xijing Hospital between February 2012 and March 2013 were screened for eligibility. Inclusion criteria included age between 18 and 70 years, elective cardiac surgery with first‐time CPB, and left ventricular ejection fraction ≥30%. Exclusion criteria were previous cardiac surgery, emergent surgery, cardiac surgery without the use of CPB, severe heart failure as defined by left ventricular ejection fraction <30%, enrollment in another clinical trial, pregnant or lactating woman or positive pregnancy test, diabetes mellitus, severe renal insufficiency or respiratory insufficiency, serious preoperative illness (eg, sepsis, active systemic infection, and active malignancy requiring treatment), history of drug abuse, bleeding diathesis or coagulopathy, and subject who refuses blood transfusion.
Study Intervention
Subjects were randomly assigned to the GIK versus control arm. Modified GIK included 20% glucose (200 g/L), 66.7 U/L regular insulin (Novo Nordisk A/S, Bagsvaerd, Denmark), and 80 mmol/L KCl. The ratio of insulin to glucose is 1:3 in this formula (while in most traditional GIK it is 1:5 or 1:6). Intravenous administration started 10 minutes before anesthesia at a rate of 1 mL/kg per hour for 12.5 hours. Subjects in the control arm received acetated Ringer's solution (6.12 g/L CH3COONa, 5.85 g/L NaCl, 0.3 g/L KCl, and 0.33 g/L CaCl2) at the same rate for 12.5 hours. Blood glucose and potassium were closely monitored during infusion by sampling 30 minutes before onset of CPB, every 20 minutes while on CPB, and then every hour after CPB up to 12.5 hours with protocol‐based management of blood glucose and potassium. All the subjects received the standard of care plus GIK or control treatment.
Randomization
A computer‐generated block randomization sequence was used. Investigators were required to confirm patient eligibility. Randomization was performed in the block size of 6 without taking into account any of the patient's characteristics (such as age or sex). Information was kept in a sealed envelope and opened by a trial investigator at the time of patient enrollment. Intervention and control drug infusion packets were identical in appearance and contained no treatment‐identifying information. The interventions (GIK vs. balanced salt solution) were blinded to surgeons, patients, and assessors.
Anesthesia, Cardiopulmonary Bypass, and Surgery
All patients received a standard anesthetic protocol comprising sufentanil 0.5 to 1 μg/kg, midazolam 2 mg, etomidate 0.1 to 0.2 mg/kg, and rocuronium 0.6 to 0.8 mg/kg, and all patients were fully heparinized (3 mg/kg) to achieve an activated clotting time >400 seconds. Standard CPB was established by experienced cardiac surgeons with the use of a hollow‐fiber oxygenator (Medtronic, Minneapolis, MN) and a roller pump (Stockert‐sc, Germany) with ascending aortic and bicaval cannulations. During CPB, moderate hemodilution (hematocrit 20–25%) with mild systemic hypothermia (30°–32°C) was used. Cold blood antegrade perfusion was used for cardioplegia in patients. The crystalloid high‐potassium solution contained (in mmol/L): 140 Na+, 75 K+,16 Mg2+, 1.2 Ca2+, 104 Cl–, 20 HCO3 –, 10 glucose, and 0.7 lidocaine (pH 7.6–7.7). After aortic cross clamping, the cardioplegic arrest was obtained with the 4:1 blood/crystalloid high‐potassium solution antegrade infusion at 4°C, with a flow rate of 300–330 mL/min for 4 minutes. For patients without aortic valve regurgitation, cardioplegia was infused through a double‐lumen needle in the ascending aorta for antegrade administration. For those patients with aortic regurgitation, the aorta was opened promptly after the cross clamp was applied, the left and right coronary artery ostia were cannulated, and the cold blood cardioplegia was delivered though a Y bifurcated catheter. This procedure was reproduced every 25 to 30 minutes to maintain cardioplegia. All operations were performed by experienced cardiac surgeons using standard techniques; type of surgery and procedural strategies were applied at the surgeon's discretion. All patients were admitted postoperatively into the cardiac intensive care unit (ICU) for further treatment.
Outcome Measures
The primary outcome measurement was composite in‐hospital MACE that included all‐cause death, low cardiac output syndrome, acute myocardial infarction, cardiac arrest with successful resuscitation, congestive heart failure and arrhythmia (ie, postoperative atrial fibrillation), supraventricular tachycardia, nonsustained ventricular tachycardia, and ventricular fibrillation. Low cardiac output syndrome was defined as cardiac index <2.2 L/min per m2 or the need of intra‐aortic balloon pumping or infusion of vasoactive agents for ≥30 minutes to maintain systolic blood pressure at ≥90 mm Hg. Acute myocardial infarction, assessed by an independent blinded cardiologist, was defined as new Q waves in an ECG or creatine kinase–myocardial bands (CK‐MB) at ≥5 times the upper normal limit with classic angina symptoms. Congestive heart failure was defined as physician documentation or report of clinical symptoms of heart failure, described as unusual dyspnea on light exertion, recurrent dyspnea occurring in the supine position, fluid retention; or jugular venous distention, pulmonary edema on physical examination, or pulmonary edema on chest roentgenogram. Postoperative atrial fibrillation was defined as new‐onset atrial fibrillation (AF) requiring treatments; that is, AF persisted for >60 minutes or recurrent episodes of AF 48 hours after surgery. Patients with a history of AF were excluded. Secondary outcomes included in‐hospital renal complications, redo operation, stroke, infection, prolonged ventilation, and stay in the ICU and hospital, as well as mortality during 3‐year follow‐up. Renal complications were defined by an increase in the serum creatinine level by a factor of ≥2 from baseline, a urine output of no more than 0.5 mL/kg per hour for 12 hours, or the use of acute hemodialysis. Infection was culture‐proven septicemia or pneumonia. Prolonged ventilation was defined as ventilatory support for >24 hours. Left ventricular ejection fraction at 24 hours after surgery and before patient discharge and CK‐MB during the 48 hours after surgery were also prespecified outcome measures.
Measurements
Transthoracic 2‐dimensional Doppler echocardiography (M‐mode) was carried out on admission, 24 hours after the surgery, and immediately before discharge. The measurements reflect the averages over 3 to 5 cycles based on the American Society of Echocardiography Guidelines. Left ventricular ejection fraction was assessed using the biplane Simpson method.
Arterial blood samples were collected before the surgery (baseline); 5 minutes after commencing CPB; 5 minutes after aortic cross clamp (ACC); 5 minutes after ACC removal; and 1, 6, 12, 24, and 48 hours after completion of the surgery. Blood glucose and lactate concentration were determined by using a standard blood gas and electrolyte analyzer (GEM Premier 3000, Instrumentation Laboratory, Bedford, MA). CK‐MB, cardiac troponin I, blood urea nitrogen and creatinine at preoperation and 24 and 48 hours after surgery were measured by using an Access AccuTnI assay system (Beckman‐Coulter, Fullerton, CA). The total released amounts of CK‐MB, cardiac troponin I, blood urea nitrogen, and creatinine were represented as 0‐ to 48‐hour area under the curve after surgery.
To evaluate myocardial extraction/excretion of glucose, oxygen, and lactate, blood samples were collected simultaneously from the radial artery and the coronary sinus immediately before ACC and at 5 minutes after ACC removal. Coronary sinus samples were obtained from a coronary sinus cardioplegia cannula, and the number of patients who had these measurements was 16 in GIK and 20 in control. Myocardial extraction/excretion was calculated as (arterial concentration − coronary sinus concentration) / arterial concentration × 100%. Oxygen content in blood samples was calculated using the formula: oxygen content = 1.39 × Hb × O2 Sat. 20 For lactate, a positive value indicates lactate uptake, whereas a negative value indicates lactate excretion. 21
Western Blotting and Transmission Electron Microscopy
Left ventricular biopsies were obtained from patients (10 in GIK and 10 in control) undergoing aortic valvular replacement or double valvular replacement with concomitant septal myectomy caused by left ventricular outflow tract obstruction, having resection of fibrous‐muscular attachments between papillary muscle and ventricular septum as an indispensable procedure for surgery. All these patients had signed to agree to surgical specimen disposal before surgery. Biopsies were obtained 15 minutes after ACC removal and immediately snap‐frozen in liquid nitrogen for western blotting with antibodies against Akt (protein kinase B), phospho‐Akt (Ser473) (Cell Signaling Technology, Danvers, MA), insulin receptor substrate‐1 (IRS‐1), phospho‐IRS‐1 (Tyr612, Millipore), or glyceraldehyde 3‐phosphate dehydrogenase (Abcam, Cambridge, UK). Some cardiac biopsies were fixed in Karnovsky's reagent (0.1 mol/L sodium cacodylate with 2% paraformaldehyde and 2.0% glutaraldehyde, pH 7.4), postfixed in 2% osmium tetroxide, dehydrated in ethanol in propylene oxide, and embedded in Poly/Bed812 (Polysciences, Warrington, PA). Semithin sections (2 μm) were stained with toluidine blue (Sigma‐Aldrich, St Louis, MO), and thin sections (60 nm) were obtained with NOVA ultratome (LKB Vertriebs GmbH, Vienna, Austria), stained with lead citrate and examined with a Tecnai G2 transmission electron microscope (FEI, Hillsboro, OR). Patients with cardiac biopsies were excluded from the biomarker evaluations.
Statistical Analysis
Power analysis indicated that at α level of 0.05 (2‐tailed), an evaluable sample of 815 subjects would provide 85% power to detect a relative 10% reduction in the primary end point for GIK versus control from 40% to 30% 6 , 9 . At least 896 subjects would be required for randomization to account for an anticipated attrition and patients withdrawing consent.
All analyses were conducted using the intent‐to‐treat cohort, composed of all randomized participants. Continuous variables were presented as mean plus/minus SD and analyzed with Student's t‐test if normally distributed. Continuous variables not following normal distribution were presented as medians and interquartile range, and analyzed with Mann‐Whitney U‐test. Categorical variables were analyzed with chi‐square or Fisher's exact test. The primary outcome measure was expressed as odds ratio with 95% CI. Three‐year mortality was analyzed by the Kaplan–Meier method and compared by the log‐rank test. Blood glucose and lactate levels over time were analyzed with the use of mixed models, which included surgeons and study subjects as random effects. Multiple testing was adjusted with Bonferroni's method. All analyses were performed with SPSS 12.0 (SPSS Inc, Chicago, IL). Statistical significance was set at P<0.05 (2‐tailed).
Results
Study Population and Baseline Characteristics
As shown in Figure 1, a total of 930 patients were eligible for randomization (n=465 per arm). Demographics were comparable between the 2 arms (Table 1). The 2 groups did not significantly differ with respect to smoking, hypertension, previous myocardial infarction, and preoperative medications including β‐blockers, digitalis glycosides, and statins. The mean European System for Cardiac Operative Risk Evaluation score was 4.19±1.77 in the GIK arm and 4.24±1.77 in the control arm (P=0.699).
Figure 1.
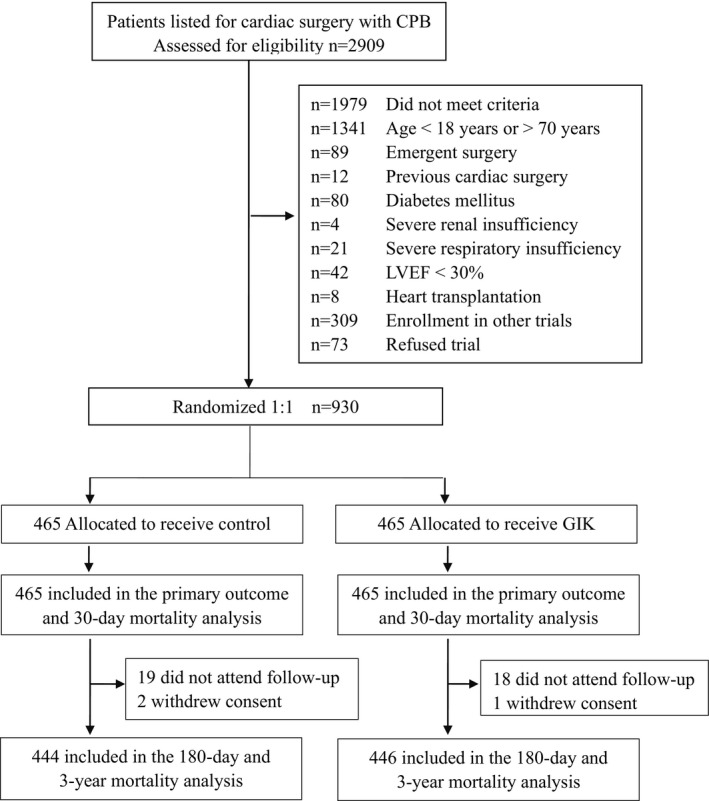
Number of patients assessed, enrolled, and randomized in the trial. CPB indicates cardiopulmonary bypass; GIK, glucose‐insulin‐potassium; LVEF, left ventricular ejection fraction.
Table 1.
Preoperative and Intraoperative Data
| Variables | Control (n=465) | GIK (n=465) | P Value |
|---|---|---|---|
| Age, mean±SD, y | 42.5±13.5 | 42.3 ±13.6 | 0.565 |
| BMI, mean±SD, kg/m2 | 23.8±4.6 | 23.4 ±4.2 | 0.446 |
| Male sex | 206 (44.3) | 199 (42.8) | 0.643 |
| Current smoker | 179 (38.5) | 164 (35.3) | 0.308 |
| Hypertension | 36 (7.7) | 45 (9.9) | 0.295 |
| Previous MI | 9 (1.9) | 11 (2.4) | 0.651 |
| COPD | 28 (6.0) | 22 (4.7) | 0.383 |
| Preoperative β‐blockers | 58 (12.5) | 57 (12.3) | 0.921 |
| Preoperative digitalis glycosides | 133 (28.6) | 145 (41.6) | 0.390 |
| Preoperative statins | 27 (5.8) | 25 (0.54) | 0.775 |
| Logistic EuroSCORE, mean±SD | 4.24±1.77 | 4.19±1.77 | 0.699 |
| NYHA class | |||
| II | 333 (71.6) | 320 (68.8) | 0.351 |
| III/IV | 132 (28.4) | 145 (31.2) | 0.351 |
| LVEF, mean±SD, % | 54.9±8.2 | 55.0±7.4 | 0.840 |
| LVEF category | |||
| Moderate (30–50%) | 124 (26.7) | 116 (24.5) | 0.549 |
| Good (>50%) | 341 (73.3) | 349 (75.1) | 0.549 |
| Type of surgery | |||
| Congenital | 174 (37.4) | 174 (37.4) | >0.999 |
| Valve | 240 (51.6) | 243 (52.3) | 0.844 |
| CABG only | 34 (7.3) | 33 (7.1) | 0.899 |
| Cardiac myxoma | 17 (3.7) | 15 (3.2) | 0.719 |
| Operation time, mean±SD, min | 191.4±80.5 | 189.6±74.0 | 0.721 |
| ACC time, mean±SD, min | 51.5±31.2 | 50.6±30.4 | 0.676 |
| CPB time, mean±SD, min | 106.5±48.0 | 103.3±46.7 | 0.313 |
EuroSCORE is the European System for Cardiac Operative Risk evaluation. Values are numbers (percentages) or mean± SD. ACC indicates aortic cross clamp; BMI indicates body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; GIK, glucose‐insulin‐potassium; LVEF, left ventricular ejective fraction; NYHA, New York Heart Association; previous MI, myocardial infarction within 90 days.
The majority of patients undergoing cardiac surgery in this study had valvular heart diseases (52.3% in GIK, 51.6% in control). Congenital heart diseases took the second place (37.4% in GIK, 37.4% in control). The percentage of subjects receiving coronary artery bypass grafting surgery was comparably low (7.1% in GIK, 7.3% in control). Detailed surgery classification is described in Table 2. In summary, there was balance in the type of cardiac surgery and in the composition of the background diseases between the GIK and control arms. Furthermore, the 2 arms did not differ in operation time, cross‐clamping time, and CPB time.
Table 2.
Surgery Classification of the Patients
| Surgery | Control (n=465), n (%) | GIK (n=465), n (%) | P Value |
|---|---|---|---|
| Valve surgery* | 240 (51.6) | 243 (52.3) | 0.844 |
| AVR with or without CABG | 56 (12.0) | 58 (12.5) | 0.842 |
| BENTALL | 10 (2.2) | 9 (1.9) | 0.817 |
| DVR | 60 (12.9) | 69 (14.8) | 0.393 |
| AVR +TVP | 2 (0.4) | 0 (0.0) | 0.250 |
| DVR +TVP | 2 (0.4) | 2 (0.4) | >0.999 |
| MVR | 93 (20.0) | 85 (18.3) | 0.505 |
| MVR+TVP | 14 (3.0) | 15 (3.2) | 0.850 |
| TVR | 3 (0.6) | 5 (1.1) | 0.732 |
| Congenital | 174 (37.4) | 174 (37.4) | >0.999 |
| Noncyanotic heart disease | 156 (33.5) | 159 (34.2) | 0.835 |
| Atrial septal repair† | 56 (12.0) | 52 (11.2) | 0.682 |
| Ventricular septal repair‡ | 100 (21.5) | 107 (23.0) | 0.581 |
| Cyanotic heart disease | 18 (3.9) | 15 (3.2) | 0.595 |
| Tetralogy of Fallot repair | 8 (1.7) | 7 (1.5) | 0.795 |
| TAPVC/PAPVC repair | 10 (2.2) | 8 (1.7) | 0.634 |
| CABG | 34 (7.3) | 33 (7.1) | 0.899 |
| Resection of cardiac myxoma | 17 (3.7) | 15 (3.2) | 0.719 |
AVR indicates aortic valvular replacement; BENTALL, aortic valvular replacement with root of the ascending aorta replacement; CABG, coronary artery bypass grafting; DVR, double valvular replacement; GIK, glucose‐insulin‐potassium; MVR, mitral valvular replacement/repair; TAPVC/PAPVC, total/partial anomalous pulmonary venous connection; TVP, tricuspid valve repair; TVR, tricuspid valve replacement.
Valve surgery indicates valvular replacement or repair due to congenital, rheumatic, or infective valvular diseases.
Atrial septal repair indicates atrial septal repair with or without patent ductus arteriosus and partial endocardial cushion defect.
Ventricular septal repair includes ventricular septal repair with or without atrial septal repair, patent ductus arteriosus, pulmonary stenosis, and rupture aneurysm of aortic sinus.
Outcomes
The data set for primary measure included all 930 subjects. As shown in Table 3, the rate of in‐hospital MACE was significantly lower in the GIK arm. GIK therapy significantly reduced the incidence of low cardiac output syndrome and congestive heart failure. GIK also decreased the incidence of new‐onset AF and nonsustained supraventricular tachycardia but not ventricular fibrillation. Incidence of cardiac arrest with successful resuscitation and acute myocardial infarction did not differ between the 2 arms.
Table 3.
Primary Outcome of the Patients
| Variables | Control (n=465) | GIK (n=465) | OR (95% CI) | P Value |
|---|---|---|---|---|
| Major adverse cardiac events | 177 (38.1) | 120 (25.8) | 0.57 (0.43–0.75) | <0.001 |
| In‐hospital mortality | 12 (2.6) | 8 (1.7) | 0.66 (0.27–1.63) | 0.366 |
| Low cardiac output syndrome | 97 (20.9) | 73 (15.7) | 0.71 (0.51–0.99) | 0.042 |
| Acute myocardial infarction | 1 (0.2) | 1 (0.2) | 1.00 (0.06–16.04) | >0.999 |
| Cardiac arrest with successful resuscitation | 4 (0.9) | 3 (0.6) | 0.75 (0.17–3.36) | 0.704 |
| Congestive heart failure | 26 (5.6) | 10 (2.2) | 0.37 (0.18–0.78) | 0.007 |
| Arrhythmia | ||||
| POAF | 56 (12.0) | 29 (6.2) | 0.47 (0.30–0.78) | 0.002 |
| SVT | 29 (6.2) | 14 (3.0) | 0.47 (0.24–0.90) | 0.019 |
| NSVT | 18 (3.9) | 7 (1.5) | 0.39 (0.16–0.94) | 0.026 |
| VF | 9 (1.9) | 6 (1.3) | 0.66 (0.23–1.88) | 0.435 |
Values are numbers (percentages). Composite adverse cardiac events included all‐cause mortality in hospital, low cardiac output syndrome, acute myocardial infarction, cardiac arrest with successful resuscitation, congestive heart failure, and arrhythmia. Low cardiac outcome syndrome was defined as cardiac index <2.2 L/(min.m2) or need of intra‐aortic balloon pumping or infusion of epinephrine, milrinone, or dobutamine to maintain systolic blood pressure ≥90 mm Hg. Acute myocardial infarction, assessed by an independent blinded cardiologist, was defined as new Q waves in ECG or CK‐MB at ≥5 times the upper normal limit with classic angina symptoms. Congestive heart failure was defined as physician documentation or report of the clinical symptoms of heart failure, described as unusual dyspnea on light exertion, recurrent dyspnea occurring in the supine position, fluid retention or jugular venous distention, pulmonary edema on physical examination, or pulmonary edema on chest roentgenogram. Arrhythmia included POAF, SVT, NSVT, and VF. POAF was defined as new‐onset atrial fibrillation requiring treatments; that is, atrial fibrillation persisted for >60 minutes or recurrent episodes of atrial fibrillation 48 hours after surgery. GIK indicates glucose‐insulin‐potassium; NSVT, nonsustained ventricular tachycardia; OR, odds ratio; POAF, postoperative atrial fibrillation; SVT, supraventricular tachycardia; VF, ventricular fibrillation.
Twenty of the 930 patients died in hospital (1.7% in GIK versus 2.6% in control; odds ratio, 0.66; 95% CI, 0.27–1.63; P=0.366). Nine patients died from multiple‐organ failure (5 in GIK, 4 in control). Eleven patients died from acute cardiovascular collapse (4 in GIK, 7 in control). No further deaths were observed during 30 days of follow‐up. During 3‐year follow‐up, 37 patients could not be contacted (19 in control, 18 in GIK), 3 patients withdrew consent (2 in control, 1 in GIK), and 12 patients died (7 in control, 5 in GIK). No significant difference in mortality was observed at 3‐year follow‐up (2.8% in GIK versus 4.1% in control; P=0.278). Long‐term follow‐up is ongoing to determine whether a modified GIK regimen improves patient prognosis.
Secondary outcomes are shown in Table 4. The incidence of renal complications in patients undergoing cardiac surgery with CPB was significantly lower in the GIK arm. Hospital stay did not differ between the 2 arms, but stay in the ICU was significantly shorter in the GIK arm. The number of patients requiring intensive care for >3 days was also markedly lower in the GIK arm. Time on mechanical ventilation support did not differ between the 2 arms; however, the number of patients on ventilation support for >24 hours was significantly lower in the GIK arm. There was no statistically significant difference in the proportion of patients who required redo operation or in the rate of postoperative stroke and sepsis between the 2 arms.
Table 4.
Secondary Outcomes of the Patients
| Variables | Control (n=465) | GIK (n=465) | P Value |
|---|---|---|---|
| Renal complications | 41 (8.8) | 23 (4.9) | 0.020 |
| Redo operation | 18 (3.9) | 20 (4.3) | 0.740 |
| Stroke | 6 (1.3) | 3 (0.6) | 0.503 |
| Infection | 18 (3.9) | 15 (3.2) | 0.595 |
| Length of stay in ICU, d | 3 (2–4) | 3 (2–3) | 0.005 |
| Length of stay in ICU >3 d | 119 (25.6) | 88 (18.9) | 0.015 |
| Mechanical ventilation, h | 21 (12–24) | 20 (15–24) | 0.023 |
| Prolonged ventilation >24 h | 115 (24.7) | 81 (17.4) | 0.006 |
Values are numbers (percentages) or medians and interquartile range. Renal complications was defined by an increase in the serum creatinine level by a factor of ≥2 from baseline, a urine output of no more than 0.5 mL/kg per hour for 12 hours, or the use of acute hemodialysis. GIK indicates glucose‐insulin‐potassium; ICU, intensive care unit.
Blood Glucose and Lactate Levels
Blood glucose gradually increased after CPB and reached a peak at 6 hours after surgery in both arms (Figure 2A). Neither the entire temporal profile of blood glucose nor the peak glucose level differed between the 2 arms. Hypoglycemia (defined as a blood glucose level of ≤40 mg/dL) occurred in 3 patients in the GIK arm and 1 patient in the control arm. Similarly, blood lactate rose slowly during CPB and shot up at 6 hours after surgery in both arms (Figure 2B). However, GIK therapy significantly reduced lactate levels at 1 hour (3.5±2.0 versus 4.0±2.1 mmol/L in control; P<0.001), 6 hours (5.0±3.1 versus 6.5±4.0 mmol/L in control; P<0.001), and 12 hours (4.6±2.8 versus 5.6±3.2 mmol/L in control; P<0.001) after surgery.
Figure 2.
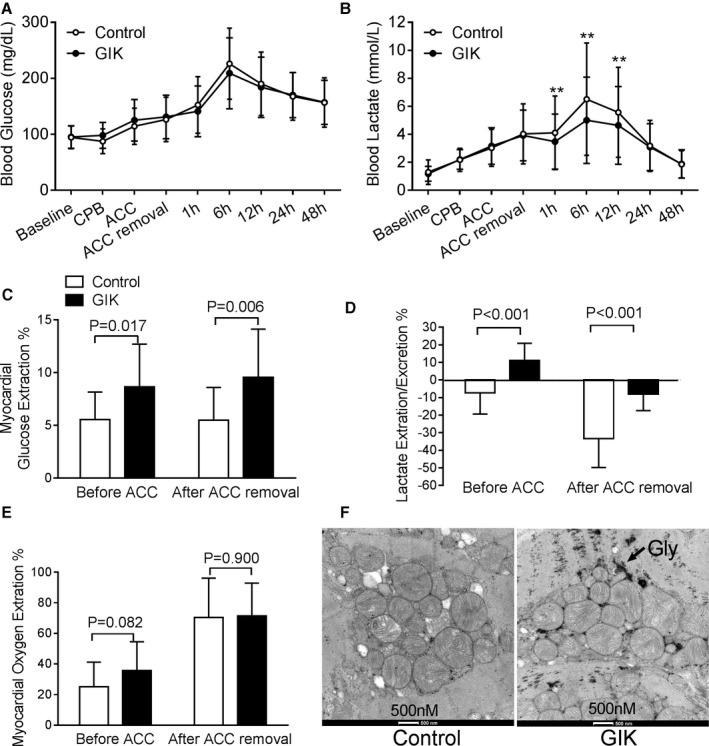
Regulation of glucose metabolism by GIK. Blood glucose (A) and lactate levels (B) between the GIK and control arms before the surgery (baseline), 5 minutes after commencing CPB, 5 minutes afterACC), 5 minutes after ACC removal and 1, 6, 12, 24, and 48 hours after surgery. Values are mean±SD for all the patients in both arms. **P<0.01 vs control. Percentage of myocardial extraction/excretion of glucose (C), lactate (D), and oxygen (E) immediately before ACC and 5 minutes after ACC removal. Positive value indicates myocardial extraction, whereas negative indicates myocardial excretion. Values are mean±SD for 16 patients in the GIK arm and 20 patients in the control arm. (F) Glycogen abundance in cardiac biopsies performed 15 minutes after ACC removal by transmission electron micrograph. ACC indicates aortic cross clamp; CPB, cardiopulmonary bypass; GIK, glucose‐insulin‐potassium.
Postoperative LVEF
As shown in Table 1, the preoperative LVEF was comparable between the 2 arms. The number of patients who had a >50% LVEF was similar between the 2 arms. A significant decline of LVEF was observed at 24 hours after operation. As shown in Table 5, compared with the control arm, the number of subjects with a >50% LVEF significantly increased in the GIK arm 24 hours after operation and before discharge. In addition, the numbers of subjects with LVEF between 30% and 50% went down in the GIK arm. The number of patients with a <30% postoperative LVEF was similar between the 2 arms. Patients who died in hospital were considered to have an LVEF below 30%.
Table 5.
Postoperative LVEF of the Patients
| Variables | Control n=465 | GIK n=465 | P Value |
|---|---|---|---|
| Postoperative 24 h | |||
| Poor (<30%) | 5 (1.1) | 5 (1.1) | >0.999 |
| Moderate (30%–50%) | 300 (64.5) | 269 (57.8) | 0.037 |
| Good (>50%) | 160 (34.4) | 191 (41.1) | 0.036 |
| Before discharge | |||
| Poor (<30%) | 9 (1.9) | 7 (1.5) | 0.608 |
| Moderate (30%–50%) | 135 (29.0) | 99 (21.3) | 0.007 |
| Good (>50%) | 321 (69.0) | 359 (77.2) | 0.005 |
Values are numbers (percentages). GIK indicates glucose‐insulin‐potassium; LVEF, left ventricular ejection fraction.
Cardiac and Renal Biomarkers
Preoperative CK‐MB and cardiac troponin I levels were comparable between the GIK and control arms (Table 6). Patients with cardiac biopsies were excluded from the biomarker evaluations (10 in GIK and 10 in control). The median area under the curve from 0 to 48 hours after surgery for CK‐MB was significantly lower in (1596 [1167–2133] U/L versus 1680 [1260–2304] U/L in control; P=0.014). Similar results were found for the 0‐ to 48‐hour area under the curve for cardiac troponin I measurements (166 [88–288] ng/mL in GIK versus 190 [114–335] ng/mL in control; P=0.01). Preoperative blood urea nitrogen and creatinine levels were comparable between the 2 arms. However, the area under the curve from 0 to 48 hours after surgery for both creatinine (5064 [4404–5904] versus 5323 [4500–6192] μmol/L in control; P=0.017) and blood urea nitrogen (396 [324–492] U/L versus 420 [347–516] mmol/L in control; P=0.003) decreased significantly in GIK‐treated patients.
Table 6.
Cardiac and Renal Biomarkers
| Variables | Control | GIK | P Value |
|---|---|---|---|
| CK‐MB, median (IQR), U/L | n=455 | n=455 | |
| Preoperative | 11.2 (8.4–14.4) | 11.0 (8.0–14.0) | 0.766 |
| Postoperative 0–48 h AUC | 1680 (1260–2304) | 1572 (1164–2112) | 0.002 |
| cTnI, median (IQR), ng/mL | n=455 | n=455 | |
| Preoperative | 0.01 (0.01–0.02) | 0.02 (0.02–0.02) | 0.121 |
| Postoperative 0–48 h AUC | 193 (114–335) | 167 (91–288) | 0.012 |
| Cr, median (IQR), μmol/L | n=465 | n=465 | |
| Preoperative | 88.5 (80–98) | 89 (81–98) | 0.646 |
| Postoperative 0–48 h AUC | 5323 (4500–6192) | 5064 (4404–5904) | 0.017 |
| BUN, median (IQR), mmol/L | n=465 | n=465 | |
| Preoperative | 5 (4–6) | 5 (4–6) | 0.140 |
| Postoperative 0–48 h AUC | 420 (347–516) | 396 (324–492) | 0.003 |
AUC indicates area under the curve; BUN, blood urea nitrogen; CK‐MB, creatine kinase–myocardial bands; Cr, creatinine; cTnI, cardiac troponin I; GIK, glucose‐insulin‐potassium; IQR, interquartile range.
Regulation by GIK of Myocardial Glucose Metabolism
Myocardial glucose metabolism was evaluated by measurement of arterial and coronary sinus concentrations of glucose, oxygen, and lactate immediately before ACC and 5 minutes after ACC removal in 36 patients (20 GIK and 16 control subjects). As shown in Figure 2C, GIK increased myocardial glucose uptake before ACC as well as after the release of ACC. These increases resulted in more abundant glycogen store in the hearts of GIK‐treated patients than those in the controls by electron microscopic analysis (Figure 2F). In addition, myocardial oxygen extraction did not differ between the 2 arms at either time point (P=0.082 and P=0.900, respectively; Figure 2E). After commencing CPB but before ACC, lactate was excreted by the heart in the control arm but extracted by the heart in the GIK arm (Figure 2D). At 5 minutes after reperfusion, lactate excretion was significantly lower in the GIK arm.
IRS‐1 and Akt phosphorylation were studied in cardiac biopsies from 20 patients (10 GIK and 10 control subjects). As shown in Figure 3, there was a 1.8‐fold increase in the ratio of phospho‐IRS‐1 to IRS and a 2.3‐fold increase in the ratio of phospho‐Akt to total Akt in GIK‐treated patients, suggesting that activation of insulin signaling may contribute to regulation of myocardial glucose metabolism by GIK therapy described above.
Figure 3.
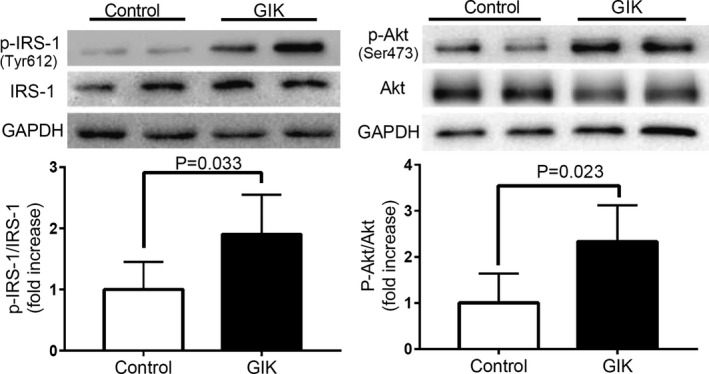
Activation of insulin signaling in the hearts of GIK‐treated patients. Representative western blots and quantification of IRS‐1, Tyr phosphorylated IRS‐1, Akt, Ser473 phosphorylated Akt in cardiac biopsies. Each bar denotes mean±SD intensities quantified by densitometric analysis of the immunoblots. n=10 per group. Akt indicates protein kinase 1; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; GIK, glucose‐insulin‐potassium; IRS‐1, insulin receptor substrate 1; p‐Akt, phospho‐Akt; p‐IRS‐1, phospho‐IRS‐1.
Discussion
We have made 3 major observations in the present study. First, this is the first trial to test the effectiveness of a modified GIK regimen in which insulin‐glucose ratio is adjusted to 1:3 and GIK is administered perioperatively. Second, this is by far the largest GIK surgical trial, with 930 patients undergoing CPB surgery included. Third, we found that GIK significantly reduced the incidence of in‐hospital MACE in patients with CPB. Mechanistically, this treatment improved myocardial metabolic state as manifested by increased glucose uptake, more glycogen stores, and less lactate excretion in GIK‐treated patients. Our data also present the first piece of direct evidence of alleviated postoperative hyperlactemia and preserved renal function by GIK. This modified GIK therapy also decreased the incidences of prolonged ICU stay and extended ventilator support in CPB patients.
Since the first application of GIK in ischemic heart disease in the 1960s, GIK therapy has gone through altering periods of varying attention. 1 , 22 , 23 Previous clinical trials about GIK focused largely on the management of acute coronary syndrome, 5 , 7 while only few studies were specific to the setting of cardiac surgery with small population and most studies were conducted in the setting of coronary artery bypass grafting surgery. 6 , 22 , 23 , 24 In contrast, the majority of patients involved in this study underwent valve surgery or congenital repair surgery because of high prevalence of these 2 heart diseases in the northwest of China. Our results showed that GIK improved cardiac hemodynamic performance, as manifested by lower incidence of low cardiac outcome syndrome, reduced need for inotropic support, and improved LVEF after surgery, which was in agreement with previous reports about GIK 6 . However, compared with previous GIK formulas with low insulin‐glucose ratios, 5 , 6 , 8 GIK with an insulin‐glucose ratio of 1:3 did not further elevate blood glucose, indicating a better glycemic control of this modified regimen in patients with cardiac surgery. Although blood glucose gradually increased during CPB and exceeded 200 mg/dL at 6 hours after operation in both GIK and control arms, our data still demonstrate the benefits of GIK infusion in CPB surgery, suggesting the direct cardioprotective effects of insulin in addition to its preventive effects against hyperglycemia. 13 , 15
During ischemia and reperfusion, the heart breaks down endogenous glycogen stores and/or increases its extraction of circulating glucose to maintain glycolytic flux, which is critical for preserving cellular viability. 1 In this trial, GIK therapy was started about 10 minutes before general anesthesia, which resulted in higher glucose uptake in the heart even before ischemia and consequently increased myocardial glycogen store. Sustained GIK infusion during ischemia continued to enhance myocardial glycolytic flux. All these actions increase production of glycolytic ATP, prevent total cellular ATP levels from falling below a critical threshold, and thus slow the onset of irreversible myocardial injury. Furthermore, stimulation of glucose metabolism by GIK improves oxygen use efficiency during ischemia because the oxidation of glucose as substrate is more efficient than fatty acids. 1 , 25 Our findings that preserved contractile function without increase of myocardial oxygen extraction in the GIK arm suggest that GIK could improve myocardial performance without affecting oxygen consumption.
Myocardial lactate release has been advocated as a negative end point in clinical trials evaluating perioperative myocardial protection. Rao et al 26 observed that aortic cross‐clamping induces anaerobic myocardial metabolism with a net release of lactate. Persistent lactate release during reperfusion suggests a delayed recovery of normal aerobic metabolism and may lead to depressed myocardial function. In this trial, GIK therapy reduced myocardial lactate release at early reperfusion, suggesting an improved anaerobic metabolism during ischemia or a more rapid recovery of aerobic metabolism during reperfusion. This may contribute to myocardial protection and functional recovery observed in GIK‐treated patients. However, we only used the concentration differences between arterial and coronary sinus blood to evaluate myocardial metabolism. The actual substrate and oxygen uptake or release is also determined by blood flow, so great caution should be exercised in the interpretation of these data.
Blood lactate is a sensitive marker of the magnitude of anaerobic metabolism and tissue oxygen deficit during cardiac surgery with CPB. 27 , 28 Tissue hypoperfusion with lactic acidosis during CPB may occur despite normal blood gas concentrations. 29 In high‐risk patients undergoing general surgery, there is a positive correlation between the estimated intraoperative oxygen deficit and postoperative lactate concentration. 30 Hyperlactemia during CPB has been proposed to be a potential marker for postoperative morbidity and mortality. 27 Change in lactate over time can be a reliable marker of a response to therapeutic interventions and subsequent outcomes. 30 We observed a significant reduction of systemic blood lactate in GIK‐treated patients, which suggests improved tissue perfusion during CPB surgery and potentially contributes to better outcomes such as decreased postoperative complications and shorter stay in the ICU.
Study Limitations
First, the current study was conducted in a single center of China and predominantly selected a Han Chinese population of patients with valvular heart diseases and congenital heart diseases. The prevalence of these 2 diseases is high, and the number of cardiac surgical procedures is growing in China, 31 while the percentage of coronary artery bypass grafting surgery is comparably low because most patients with coronary artery diseases prefer percutaneous coronary intervention, as it is less invasive and less costly. Second, considering the “younger and healthier” patients enrolled in this study (mean age, 42 years; mean body mass index, 23 kg/m2) as compared with the typical cardiac surgery patient profiles reported in the Western literature, and the absence of diabetes mellitus in this trial, our results should be interpreted with caution. Further studies are needed to evaluate the safety and effectiveness of a modified GIK regimen in elderly patients and those with diabetes mellitus.
Conclusions
Our modified GIK regimen (insulin‐glucose ratio 1:3) administered perioperatively reduces the incidence of MACE in patients undergoing CPB surgery. These benefits are likely the result of enhanced systemic tissue perfusion and improved myocardial metabolism via activation of insulin signaling by GIK.
Sources of Funding
This work was supported by the National High Technology Research and Development Program of China (2014AA020542), National Natural Science Foundation of China (31771265, 31371150, 81870273), National Key Basic Research Program of China (2013CB531204), and Shaanxi Science and Technology Youth Fellowship and Outstanding Youth Fellowship of Fourth Military Medical University (2019XC089).
Disclosures
None.
(J Am Heart Assoc. 2020;9:e012376 DOI: 10.1161/JAHA.119.012376.)
Contributor Information
Jia Li, Email: jiali816@fmmu.edu.cn.
Feng Gao, Email: fgao@fmmu.edu.cn.
References
- 1. Grossman AN, Opie LH, Beshansky JR, Ingwall JS, Rackley CE, Selker HP. Glucose‐insulin‐potassium revived: current status in acute coronary syndromes and the energy‐depleted heart. Circulation. 2013;127:1040–1048. [DOI] [PubMed] [Google Scholar]
- 2. Yu Q, Gao F, Ma XL. Insulin says no to cardiovascular disease. Cardiovasc Res. 2011;89:516–524. [DOI] [PubMed] [Google Scholar]
- 3. Li J, Zhang H, Wu F, Nan Y, Ma H, Guo W, Wang H, Ren J, Das UN, Gao F. Insulin inhibits tumor necrosis factor‐alpha induction in myocardial ischemia/reperfusion: role of Akt and endothelial nitric oxide synthase phosphorylation. Crit Care Med. 2008;36:1551–1558. [DOI] [PubMed] [Google Scholar]
- 4. Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA, Ma XL. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia‐reperfusion: the roles of pi3‐kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105:1497–1502. [DOI] [PubMed] [Google Scholar]
- 5. Selker HP, Beshansky JR, Sheehan PR, Massaro JM, Griffith JL, D'Agostino RB, Ruthazer R, Atkins JM, Sayah AJ, Levy MK, Richards ME, Aufderheide TP, Braude DA, Pirrallo RG, Doyle DD, Frascone RJ, Kosiak DJ, Leaming JM, Van Gelder CM, Walter GP, Wayne MA, Woolard RH, Opie LH, Rackley CE, Apstein CS, Udelson JE. Out‐of‐hospital administration of intravenous glucose‐insulin‐potassium in patients with suspected acute coronary syndromes: the immediate randomized controlled trial. JAMA. 2012;307:1925–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howell NJ, Ashrafian H, Drury NE, Ranasinghe AM, Contractor H, Isackson H, Calvert M, Williams LK, Freemantle N, Quinn DW, Green D, Frenneaux M, Bonser RS, Mascaro JG, Graham TR, Rooney SJ, Wilson IC, Pagano D. Glucose‐insulin‐potassium reduces the incidence of low cardiac output episodes after aortic valve replacement for aortic stenosis in patients with left ventricular hypertrophy: results from the hypertrophy, insulin, glucose, and electrolytes (hinge) trial. Circulation. 2011;123:170–177. [DOI] [PubMed] [Google Scholar]
- 7. Mamas MA, Neyses L, Fath-Ordoubadi F. A meta‐analysis of glucose‐insulin‐potassium therapy for treatment of acute myocardial infarction. Exp Clin Cardiol. 2010;15:e20–e24. [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta SR, Yusuf S, Diaz R, Zhu J, Pais P, Xavier D, Paolasso E, Ahmed R, Xie C, Kazmi K, Tai J, Orlandini A, Pogue J, Liu L. CREATE‐ECLA Trial Group Investigators. Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute ST‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial. JAMA. 2005;293:437–446. [DOI] [PubMed] [Google Scholar]
- 9. Bothe W, Olschewski M, Beyersdorf F, Doenst T. Glucose‐insulin‐potassium in cardiac surgery: a meta‐analysis. Ann Thorac Surg. 2004;78:1650–1657. [DOI] [PubMed] [Google Scholar]
- 10. Gunst J, Doig GS. The optimal blood glucose target in critically ill patients: more questions than answers. Intensive Care Med. 2017;43:110–112. [DOI] [PubMed] [Google Scholar]
- 11. Yatabe T, Inoue S, Sakaguchi M, Egi M. The optimal target for acute glycemic control in critically ill patients: a network meta‐analysis. Intensive Care Med. 2017;43:16–28. [DOI] [PubMed] [Google Scholar]
- 12. Thiessen S, Vanhorebeek I, Van den Berghe G. Glycemic control and outcome related to cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol. 2015;29:177–187. [DOI] [PubMed] [Google Scholar]
- 13. Ng KW, Allen ML, Desai A, Macrae D, Pathan N. Cardioprotective effects of insulin: how intensive insulin therapy may benefit cardiac surgery patients. Circulation. 2012;125:721–728. [DOI] [PubMed] [Google Scholar]
- 14. Doenst T, Wijeysundera D, Karkouti K, Zechner C, Maganti M, Rao V, Borger MA. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1144. [DOI] [PubMed] [Google Scholar]
- 15. Yu Q, Zhou N, Nan Y, Zhang L, Li Y, Hao X, Xiong L, Lau WB, Ma XL, Wang H, Gao F. Effective glycaemic control critically determines insulin cardioprotection against ischaemia/reperfusion injury in anaesthetized dogs. Cardiovasc Res. 2014;103:238–247. [DOI] [PubMed] [Google Scholar]
- 16. van der Horst IC. Acute coronary syndromes: early metabolic modulation–a solution for MI? Nat Rev Cardiol. 2012;9:377–378. [DOI] [PubMed] [Google Scholar]
- 17. Apstein CS, Opie LH. A challenge to the metabolic approach to myocardial ischaemia. Eur Heart J. 2005;26:956–959. [DOI] [PubMed] [Google Scholar]
- 18. van der Horst IC, Zijlstra F, van ‘t Hof AW, Doggen CJ, de Boer MJ, Suryapranata H, Hoorntje JC, Dambrink JH, Gans RO, Bilo HJ, Zwolle Infarct Study Group . Glucose‐insulin‐potassium infusion inpatients treated with primary angioplasty for acute myocardial infarction: the glucose‐insulin‐potassium study: a randomized trial. J Am Coll Cardiol. 2003;42:784–791. [DOI] [PubMed] [Google Scholar]
- 19. Hausenloy DJ, Boston-Griffiths E, Yellon DM. Cardioprotection during cardiac surgery. Cardiovasc Res. 2012;94:253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lilleberg J, Nieminen MS, Akkila J, Heikkila L, Kuitunen A, Lehtonen L, Verkkala K, Mattila S, Salmenpera M. Effects of a new calcium sensitizer, levosimendan, on haemodynamics, coronary blood flow and myocardial substrate utilization early after coronary artery bypass grafting. Eur Heart J. 1998;19:660–668. [DOI] [PubMed] [Google Scholar]
- 21. Albacker TB, Carvalho G, Schricker T, Lachapelle K. Myocardial protection during elective coronary artery bypass grafting using high‐dose insulin therapy. Ann Thorac Surg. 2007;84:1920–1927; discussion 1920–1927. [DOI] [PubMed] [Google Scholar]
- 22. Fan Y, Zhang AM, Xiao YB, Weng YG, Hetzer R. Glucose‐insulin‐potassium therapy in adult patients undergoing cardiac surgery: a meta‐analysis. Eur J Cardiothorac Surg. 2011;40:192–199. [DOI] [PubMed] [Google Scholar]
- 23. Schipke JD, Friebe R, Gams E. Forty years of glucose‐insulin‐potassium (GIK) in cardiac surgery: a review of randomized, controlled trials. Eur J Cardiothorac Surg. 2006;29:479–485. [DOI] [PubMed] [Google Scholar]
- 24. Ellenberger C, Sologashvili T, Kreienbuhl L, Cikirikcioglu M, Diaper J, Licker M. Myocardial protection by glucose‐insulin‐potassium in moderate‐ to high‐risk patients undergoing elective on‐pump cardiac surgery: a randomized controlled trial. Anesth Analg. 2018;126:1133–1141. [DOI] [PubMed] [Google Scholar]
- 25. Zhang HX, Zang YM, Huo JH, Liang SJ, Zhang HF, Wang YM, Fan Q, Guo WY, Wang HC, Gao F. Physiologically tolerable insulin reduces myocardial injury and improves cardiac functional recovery in myocardial ischemic/reperfused dogs. J Cardiovasc Pharmacol. 2006;48:306–313. [DOI] [PubMed] [Google Scholar]
- 26. Rao V, Ivanov J, Weisel RD, Cohen G, Borger MA, Mickle DA. Lactate release during reperfusion predicts low cardiac output syndrome after coronary bypass surgery. Ann Thorac Surg. 2001;71:1925–1930. [DOI] [PubMed] [Google Scholar]
- 27. Ranucci M, De Toffol B, Isgro G, Romitti F, Conti D, Vicentini M. Hyperlactatemia during cardiopulmonary bypass: determinants and impact on postoperative outcome. Crit Care. 2006;10:R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perz S, Uhlig T, Kohl M, Bredle DL, Reinhart K, Bauer M, Kortgen A. Low and “supranormal” central venous oxygen saturation and markers of tissue hypoxia in cardiac surgery patients: a prospective observational study. Intensive Care Med. 2011;37:52–59. [DOI] [PubMed] [Google Scholar]
- 29. Fiaccadori E, Vezzani A, Coffrini E, Guariglia A, Ronda N, Tortorella G, Vitali P, Pincolini S, Beghi C, Fesani F. Cell metabolism in patients undergoing major valvular heart surgery: relationship with intra and postoperative hemodynamics, oxygen transport, and oxygen utilization patterns. Crit Care Med. 1989;17:1286–1292. [DOI] [PubMed] [Google Scholar]
- 30. Waxman K, Nolan LS, Shoemaker WC. Sequential perioperative lactate determination. Physiological and clinical implications. Crit Care Med. 1982;10:96–99. [DOI] [PubMed] [Google Scholar]
- 31. Lei C, Berra L, Rezoagli E, Yu B, Dong H, Yu S, Hou L, Chen M, Chen W, Wang H, Zheng Q, Shen J, Jin Z, Chen T, Zhao R, Christie E, Sabbisetti VS, Nordio F, Bonventre JV, Xiong L, Zapol WM. Nitric oxide decreases acute kidney injury and stage 3 chronic kidney disease after cardiac surgery. Am J Respir Crit Care Med. 2018;198:1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]


