Abstract
Background
Trials and registries associated female sex and high age with unfavorable outcomes in abdominal aortic aneurysm treatment. Many studies showed an inverse correlation between annual hospital volume and in‐hospital mortality. The volume‐outcome relationship has not been investigated separately for women and men or across the age range. The aim was to analyze whether sex and age are effect modifiers or confounders of the volume‐outcome association.
Methods and Results
In a nationwide setting, all in‐hospital cases from 2005 to 2014 with a diagnosis of intact abdominal aortic aneurysm and procedure codes for endovascular or open aortic repair were included. Primary outcome was in‐hospital mortality. Using a multilevel multivariable regression model, hospital volume was modeled as a continuous variable. Separate analyses were performed for women and men and for predefined age groups. A total of 94 966 cases were included (12% women; median age, 72 years). Mortality was 4.9% in women and 3.0% in men (3.2% overall). Mortality increased with age. Although there was no significant volume‐outcome association in women (P=0.57), there was in men (P=0.02). The strongest volume‐outcome association was found in younger men. The younger female subpopulation was found to show a trend for an inverse volume‐outcome relationship, whereas an opposite association was found for the women aged >79 years.
Conclusions
Women have a higher mortality risk after elective abdominal aortic aneurysm treatment. Sex and age are modifiers of the volume‐outcome relationship. Unlike in male patients, in women there is no consistent effect of hospital volume on outcome.
Keywords: endovascular aortic repair, hospital performance, hospital volume, in‐hospital mortality, secondary data analysis, sex differences, sex specific
Subject Categories: Risk Factors, Women, Health Services, Quality and Outcomes, Mortality/Survival
Clinical Perspective
What Is New?
To our best knowledge, this is the first study to analyze the interaction between sex, age, and hospital caseload in abdominal aortic aneurysm surgery.
Increasing annual hospital caseload seems to lower the risk for in‐hospital mortality in male patients of all age groups.
The effect in female patients is more complex, and hospital volume does not have a consistent effect on outcome.
What Are the Clinical Implications?
In‐hospital mortality is likely to be reduced in male patients, if they are treated in high‐volume hospitals.
In women, who innately bear a higher risk of in‐hospital death, the anticipated effect of centralization on mortality seems not to be as strong as in men.
Nonstandard Abbreviations and Acronyms.
AAA abdominal aortic aneurysm
DRG diagnosis related groups
EVAR endovascular aortic repair
iAAA intact AAA
ICD‐10‐GM international classification of diseases, tenth revision, German modification
OAR open aortic repair
OR odds ratio
rAAA ruptured AAA
Similar to the situation with other cardiovascular diseases, women are reported to have unfavorable outcomes after treatment of abdominal aortic aneurysms (AAAs) by endovascular repair (EVAR) or open aortic repair (OAR).1 Treatment strategies for women therefore require optimization.
When analyzing men and women together, associations between annual hospital volume and outcome, particularly death, after elective AAA surgery have been described across several countries and healthcare systems.2, 3, 4, 5, 6, 7 This led to inclusion of minimal threshold recommendations in the treatment guidelines from the Society of Vascular Surgery8 and the European Society of Vascular and Endovascular Surgery.9 The ideal threshold is still elusive, and varies depending on study design and healthcare system. In addition, it remains unclear whether only high‐risk subgroups of patients are affected by worse outcomes in low‐volume hospitals, as has been shown for percutaneous cardiac interventions.10 Objectification and clarification are necessary, as centralization of AAA care is increasingly discussed for improvement of outcomes.
In a recent analysis of nationwide German data on outcomes after AAA surgery, it was shown that the lowest risk for in‐hospital death was achieved in centers treating >75 cases per year (OAR and EVAR combined).11 A second analysis revealed female sex and increasing age to be important risk factors for mortality.12 To date, the volume‐outcome relationship has not been investigated separately for women and men or young and elderly patients. Therefore, the objective of the present study was to analyze whether sex and age are effect modifiers or confounders of the association between hospital volume and mortality.
Methods
Data Source
A retrospective data analysis was performed using the 2005 to 2014 hospital episode microdata that are statutorily gathered by the German Federal Statistical Office.13 As reporting of inpatient cases is regulated by law (§21 Hospital Remuneration Act), this database enables a complete nationwide survey (except military and psychiatry services). Using controlled remote data processing, it can be used for secondary data analyses for scientific purposes. Individual patient data or institutional identifiers for hospitals were not available to the authors. The method of controlled remote data processing was established and published in earlier studies.11, 12, 14, 15, 16, 17
Because of the sensitive nature of the data collected for this study, application to the German Federal Statistical Office is necessary for primary data access. Qualified researchers trained in human subject confidentiality protocols may apply at https://www.forschungsdatenzentrum.de/de/bedingungen and use data for secondary data analyses according to defined conditions.
The authors followed the guideline for Good Practice of Secondary Data Analysis18 as well as the STROSA2 (Standardisierte BerichtsRoutine für Sekundärdaten Analysen [Standardised Reporting Routine for Secondary Data Analysis]) guideline,19 which is a modification of RECORD (Reporting of studies Conducted using Observational Routinely‐collected Data)20 focusing on the peculiarities of the German healthcare system. The local ethics committee of the Medical Faculty, Technical University of Munich, approved the study (reference 21/16 S). Informed consent was not required.
Case Selection and Population
As in‐hospital episodes are recorded without an individual patient identifier, this study describes cases rather than individual patients. Between January 1, 2005, and December 31, 2014, all cases with a principal or secondary diagnosis of intact AAA (iAAA; International Classification of Diseases, Tenth Revision, German Modification [ICD‐10‐GM] I71.4; includes all AAA without signs of rupture) and specific treatment (defined as specific procedure codes, “operationen‐ und prozedurenschlüssel”, for infrarenal EVAR or OAR; Table S1) were included. To exclude primary hybrid procedures and secondary conversions (EVAR ‐> OAR), cases with codes for both OAR and EVAR were excluded, as the exact strategy cannot be identified using administrative data. For detailed ICD‐10 and operationen‐ und prozedurenschlüssel codes, see Table S1; a flowchart of case selection is presented in Figure 1.
Figure 1.
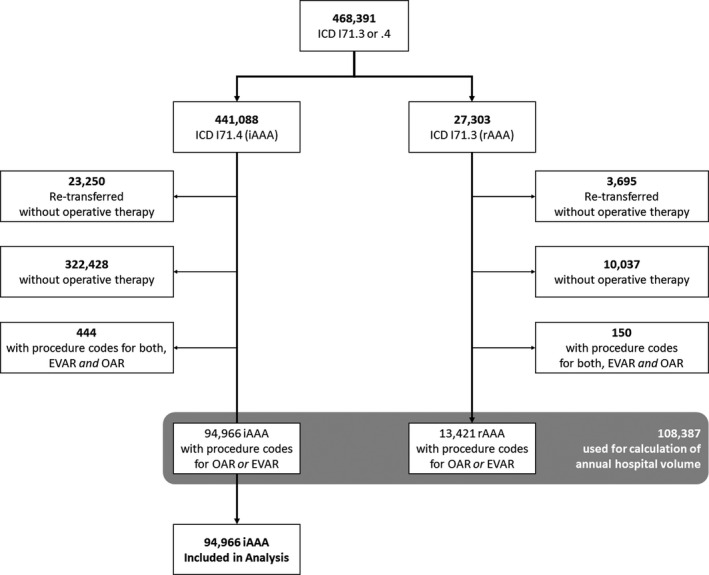
Case flowchart.
EVAR indicates endovascular aortic repair; OAR, open aortic repair.
Hospital Volume
Annual hospital caseload was considered as a surrogate indicator of the theoretical (hidden) construct of “center experience to treat AAA.” For each year, the individual hospital volume of AAA repairs (ICD‐10‐GM I71.3 for ruptured AAA and I71.4 for iAAA) was calculated. Hospitals were identified by institutional code and site code, which were harmonized over the years by the German Federals Statistical Office. Hospital volume was statistically modeled as a continuous variable.
Patient Characteristics and Treatment
In accordance with a recent study on volume and outcome after AAA repair in the same population,11 baseline patient (sex, age, and type of admission) and treatment characteristics (type of surgery [ie, OAR or EVAR] and length of hospital stay) are described. Comorbidities were identified by use of specific codes for primary or secondary diagnoses (Table S1).21 The following comorbidities were included: chronic ischemic heart disease, peripheral arterial disease, diabetes mellitus, renal disease, chronic pulmonary disease, and malignant disease. The modified Elixhauser score was calculated as a measure of comorbidity burden and for multivariable adjustment.22 Age groups (<65, 65–69, 70–74, 75–79, and >79 years) were identical to earlier publications in which they were arbitrarily set,12, 23 to achieve comparability.
Outcomes
The primary outcome was in‐hospital mortality after iAAA repair. Any death during hospital stay has to be reported by law. Therefore, data can be regarded as accurate.11
Statistical Analysis
Patient characteristics in men and women were compared using the Bonferroni‐corrected t test. The Cochran‐Armitage test for trend was used to analyze trends in mortality of the different age groups.
A multilevel logistic regression model allowing for clustering effects of cases treated in the same hospital was fit to the data. Year of treatment was entered as random effect into the model. Besides the variables of interest (age, sex, and volume), Elixhauser comorbidity score and type of treatment (EVAR or OAR) were used as predictor variables. Using a series of Wald tests and interpreting plots of the crude in‐hospital mortality stratified by sex and age, the volume effect was modeled as second‐degree polynomial. Moreover, the fitted model should be able to adjust a volume‐outcome effect with respect to age and sex; thus, pairwise interactions among the variables of interest were included. Finally, this resulted in 13 parameters to be estimated from data with the underlying mathematical formula:
CIs (Figures 2, 3 through 4) were calculated using the empirical covariance matrix and a 95% threshold. In Figure 4, each line behaves like the first derivative of the corresponding line of Figure 3. CIs are displayed in the figures for the above‐mentioned age groups, represented by the median age of the group. To create Figures 5 and 6, the youngest and oldest age group needed to be limited. As the German Federal Statistical Office does not provide minimum and maximum values, the 2.5% and 97.5% group‐specific quantiles were used (50 and 90 years, respectively).
Figure 2.
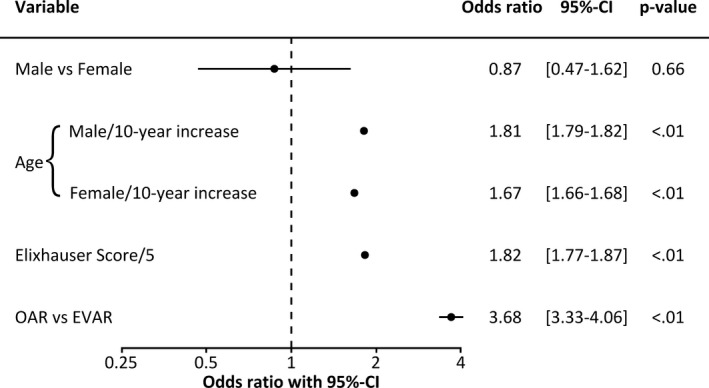
Association between in‐hospital mortality (primary outcome) and sex, age, comorbidity, and type of treatment: Forest plot of the underlying model. Sex was evaluated at median volume (14 cases) and median male age (72 years); age was evaluated at median volume (14 cases).
To account for clustering of patients within hospitals, and to account for effect of calendar year, the year of treatment and hospital site code were entered as random effects.
Figure 3.
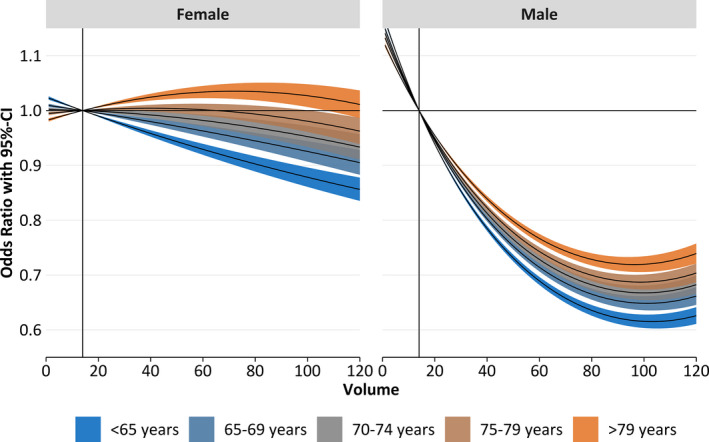
Odds ratios and 95% CIs of the volume‐outcome relationship by sex (women [left] and men [right]) and age (indicated by color). The median annual volume (14 cases; indicated by vertical line) was set as reference. Odds ratios were compared with the median annual hospital volume (14 cases) and corresponding 95% CI evaluated at the median age within each age group.
Figure 4.
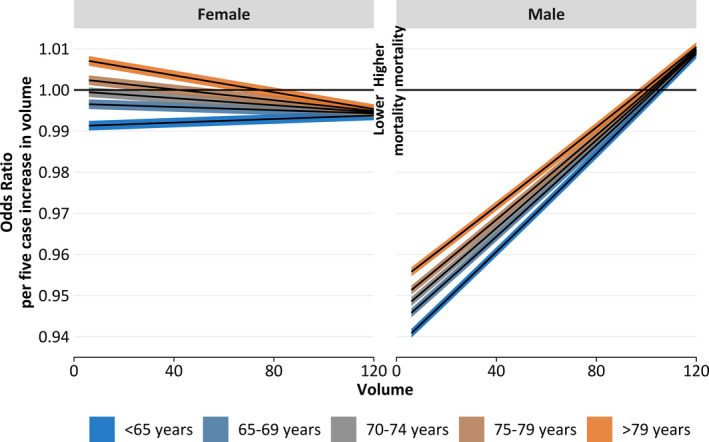
Odds ratio of volume compared with an increased volume (5 additional cases a year) with 95% CIs by sex (women [left] and men [right]) and age (indicated by color) for all cases (open aortic repair and endovascular aortic repair [EVAR]). Each line behaves like the first derivative of the corresponding line of Figure 3. At each volume point, the regression coefficients were treated as normally distributed random variables. Then, all variables except the volume were set constant and the linear predictor of the 2 time points was calculated and subtracted. Then, the corresponding estimator of the SE was calculated. Finally, the linear predictor difference with 95% CI was retransformed to the odds ratio scale.
Figure 5.
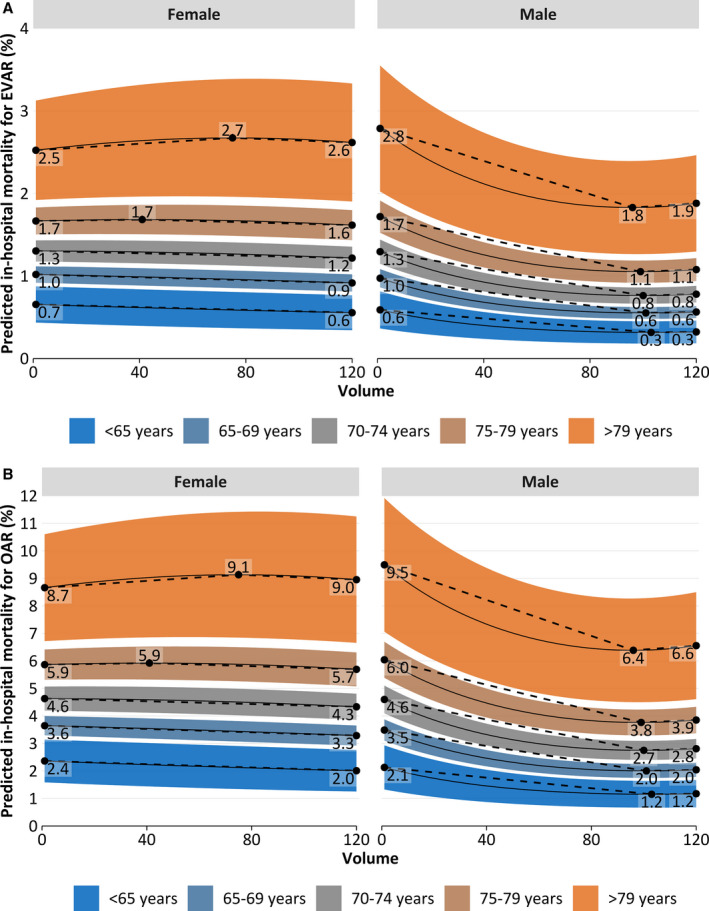
Predicted in‐hospital mortality depending on annual hospital volume by sex (women [left] and men [right]) and age (indicated by color) for endovascular aortic repair (A) and open aortic repair (OAR) (B), amended by highlighted extrema (black dots).
Figure 6.
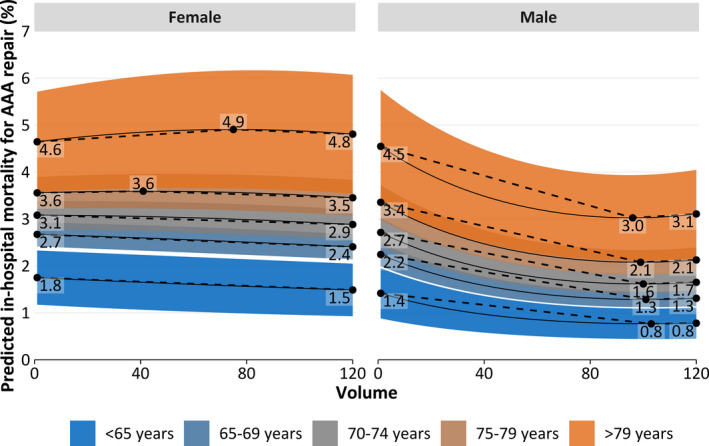
Predicted in‐hospital mortality depending on annual hospital volume by sex (women [left] and men [right]) and age (indicated by color) for all cases (open aortic repair and endovascular aortic repair [EVAR]), amended by highlighted extrema (black dots). Averaged on Figure 5A and 5B by EVAR proportion within each stratum. AAA indicates abdominal aortic aneurysm.
For controlled remote data processing and statistical analysis, SAS software (version 9.2; Microsoft Windows; 2015 SAS Institute Inc, Cary, NC) was used. Findings have been visualized by R (version 3‐5‐1)24 and the ggplot2‐package.25
Results
Study Cohort and Mortality
The cohort consisted of 94 966 cases with iAAA receiving treatment in a median of 501 hospitals per annum (total >700 hospitals). Of the total cases, 11 363 (12%) were women. Median age of female patients was 74 years (lower‐upper quartile, quartile 25%–75%, 69–80 years), whereas the median age of male patients was 72 years (quartile 25%–75%, 66–77 years). Overall, the patients had a high comorbidity burden, indicated by a median Elixhauser score of 5 (males, 5 [quartile 25%–75%, 0–9]; females, 5 [quartile 25%–75%, 1–10]). On comparing male and female patients, differences were found on the frequency of chronic ischemic heart disease (men, 36%; women, 26%; P<0.001) and peripheral arterial disease (men, 30%; women, 36%; P<0.001). Female patients received endovascular therapy less frequently (men, 57%; women, 51%; P<0.001) and hospitalization was longer (median length of hospital stay in men, 11 days [quartile 25%–75%, 8–16 days]; median length of hospital stay in women, 13 days [quartile 25%–75%, 9–18 days]; P<0.001). The rate of EVAR procedures is significantly associated with age in both female (P<0.001, Cochran‐Armitage test for trend) and male patients (P<0.001). Patient characteristics and treatment details are given in Tables 1 and 2.
Table 1.
Characteristics of All Cases (N=94 966) Receiving Treatment of iAAAs in This Study
| Characteristic | Men | Women | P Value |
|---|---|---|---|
| No. of cases | 83 603 | 11 363 | ··· |
| Age, median (quartile0.25–quartile0.75), y | 72 (66–77) | 74 (69–80) | <0.01 |
| Elixhauser score, median (quartile0.25–quartile0.75) | 5 (0–9) | 5 (1–10) | <0.01 |
| Comorbidities, n (%) | |||
| Chronic ischemic heart disease | 30 100 (36) | 2980 (26) | <0.01 |
| Peripheral arterial disease | 25 173 (30) | 4056 (36) | <0.01 |
| Chronic pulmonary disease | 15 460 (19) | 2204 (19) | 0.21 |
| Diabetes mellitus | 14 512 (17) | 1711 (15) | <0.01 |
| Renal disease | 16 750 (20) | 2316 (20) | 1.00 |
| Any malignancy | 2941 (3.5) | 253 (2.2) | <0.01 |
| Endovascular therapy, n (%) | 47 727 (57) | 5749 (51) | <0.01 |
| Length of stay, median (quartile0.25–quartile0.75), d | 11 (8–16) | 13 (9–18) | <0.01 |
P value calculated with Bonferroni‐corrected t test for continuous variables and Bonferroni‐corrected χ2 test for nominal variables. iAAA indicates intact abdominal aortic aneurysm.
Table 2.
Characteristics of All Cases (N=94 966) Receiving Treatment of iAAAs in This Study, by Age Group
| Characteristic | Age Group, y | ||||
|---|---|---|---|---|---|
| <65 | 65–69 | 70–74 | 75–79 | >79 | |
| No. of cases | |||||
| Men | 16 889 | 15 734 | 20 901 | 17 173 | 12 906 |
| Women | 1486 | 1674 | 2625 | 2645 | 2933 |
| Elixhauser score, median (quartile0.25–quartile0.75) | |||||
| Men | 2 (0–7) | 3 (0–8) | 5 (0–9) | 5 (0–10) | 5 (2–12) |
| Women | 4 (0–7) | 5 (0–9) | 5 (0–10) | 5 (1–10) | 6 (2–12) |
| Comorbidities, n (%) | |||||
| Chronic ischemic heart disease | |||||
| Men | 5030 (30) | 5539 (35) | 7730 (37) | 6746 (39) | 5055 (39) |
| Women | 303 (20) | 419 (25) | 676 (26) | 760 (29) | 822 (28) |
| Peripheral arterial disease | |||||
| Men | 5219 (31) | 4822 (31) | 6309 (30) | 5206 (30) | 3617 (28) |
| Women | 630 (42) | 702 (42) | 958 (37) | 917 (35) | 849 (29) |
| Chronic pulmonary disease | |||||
| Men | 2607 (15) | 2919 (19) | 4092 (20) | 3431 (20) | 2411 (19) |
| Women | 298 (20) | 401 (24) | 565 (22) | 513 (19) | 427 (15) |
| Diabetes mellitus | |||||
| Men | 2698 (16) | 2871 (18) | 3872 (19) | 3108 (18) | 1963 (15) |
| Women | 177 (12) | 240 (14) | 437 (17) | 438 (17) | 419 (14) |
| Renal disease | |||||
| Men | 1868 (11) | 2477 (16) | 4165 (20) | 4354 (25) | 3886 (30) |
| Women | 160 (11) | 284 (17) | 486 (19) | 612 (23) | 774 (26) |
| Any malignancy | |||||
| Men | 372 (2.2) | 509 (3.2) | 804 (3.8) | 737 (4.3) | 519 (4.0) |
| Women | 31 (2.1) | 42 (2.5) | 55 (2.1) | 55 (2.1) | 70 (2.4) |
| Endovascular therapy, n (%) | |||||
| Men | 7811 (46) | 7785 (49) | 11 933 (57) | 10 678 (62) | 9520 (74) |
| Women | 533 (36) | 621 (37) | 1222 (47) | 1454 (55) | 1919 (65) |
| Length of stay, median (quartile0.25–quartile0.75), d | |||||
| Men | 11 (8–14) | 11 (8–15) | 11 (8–16) | 11 (8–17) | 11 (8–17) |
| Women | 12 (9–17) | 13 (9–18) | 13 (9–18) | 13 (9–19) | 14 (9–20) |
iAAA indicates intact abdominal aortic aneurysm.
Overall mortality was 3.2%: However, mortality was >1.5 times higher in women (4.9%) than in men (3.0%; P<0.001). In cases treated with OAR, mortality was 5.2% (men, 5.0%; women, 7.0%; P<0.001), compared with 1.6% after EVAR (men, 1.5%; women, 2.9%; P<0.001). Mortality increased with patient age for both EVAR and OAR (P<0.001; Table 3).
Table 3.
In‐Hospital Mortality After Open and Endovascular iAAA Repair
| Variable | All Cases | <65 y | 65–69 y | 70–74 y | 75–79 y | >79 y | P Value |
|---|---|---|---|---|---|---|---|
| Overall (P<0.01) | |||||||
| No. of cases | |||||||
| Men | 83 603 | 16 889 | 15 734 | 20 901 | 17 173 | 12 906 | ··· |
| Women | 11 363 | 1486 | 1674 | 2625 | 2645 | 2933 | ··· |
| Death until discharge, n (%) | |||||||
| Men | 2482 (3.0) | 229 (1.4) | 314 (2.0) | 551 (2.6) | 732 (4.3) | 656 (5.1) | <0.01 |
| Women | 557 (4.9) | 30 (2.0) | 65 (3.9) | 119 (4.5) | 143 (5.4) | 200 (6.8) | <0.01 |
| OAR (P<0.01) | |||||||
| No. of cases | |||||||
| Men | 35 876 | 9078 | 7949 | 8968 | 6495 | 3386 | ··· |
| Women | 5614 | 953 | 1053 | 1403 | 1191 | 1014 | ··· |
| Death until discharge, n (%) | |||||||
| Men | 1776 (5.0) | 195 (2.1) | 251 (3.2) | 415 (4.6) | 505 (7.8) | 410 (12.1) | <0.01 |
| Women | 393 (7.0) | 21 (2.2) | 52 (4.9) | 98 (7.0) | 94 (7.9) | 128 (12.6) | <0.01 |
| EVAR (P<0.01) | |||||||
| No. of cases | |||||||
| Men | 47 727 | 7811 | 7785 | 11 933 | 10 678 | 9520 | ··· |
| Women | 5749 | 533 | 621 | 1222 | 1454 | 1919 | ··· |
| Death until discharge, n (%) | |||||||
| Men | 706 (1.5) | 34 (0.4) | 63 (0.8) | 136 (1.1) | 227 (2.1) | 246 (2.6) | <0.01 |
| Women | 164 (2.9) | 9 (1.7) | 13 (2.1) | 21 (1.7) | 49 (3.4) | 72 (3.8) | <0.01 |
P values of the Bonferroni‐corrected χ2 test comparing men vs women are given next to treatment modality. P value of Cochran‐Armitage test for trend is given in the right column. EVAR indicates endovascular aortic repair; iAAA, intact abdominal aortic aneurysm; and OAR, open aortic repair.
The median caseload of hospitals and their distribution within volume quartiles are presented in Tables S2 and S3.
Volume‐Outcome Relationship and Interaction With Sex and Age
The results of the fitted multilevel logistic regression model are illustrated in Figures 2, 3, 4, 5 through 6.
Figure 2 shows the odds ratios (ORs) of the statistical model underlying the further analysis. As sex interacted with both age and volume, the ORs are observed by inserting the median male age (72 years) and/or the median volume (14 cases), which resulted in an OR of 0.87 [95% CI 0.47–1.62]. Increasing age is significantly associated with mortality in both male (OR, 1.81 [1.79–1.82] per 10‐year increase; P<0.01) and female patients (OR, 1.67 [1.66–1.68] per 10‐year increase; P<0.01). Increased Elixhauser comorbidity score (OR, 1.82 [1.77–1.87] per 5‐point increase; P<0.01) and an OAR procedure (OR, 3.68 [3.33–4.96]; P<0.01) are significant risk factors for in‐hospital mortality.
The accuracy of the statistical model (predicted mortality) was cross‐checked with actual outcomes (observed mortality). The results are presented in Tables S4 and S5.
Figure 3 shows the ORs and 95% CIs for the volume‐outcome relationship (reference, 14 cases/year) differentiated by age group and sex. Overall, the predicted effect of hospital volume is only statistically evident for male patients. This is indicated by the maximum benefit of male patients (OR, 0.60–0.75; P=0.02), which is more pronounced than the maximum benefit of women (OR, 0.85–1.00; P=0.57). Furthermore, the volume‐outcome effect is more distinct in younger patients, as indicated by staggered OR curves in both sexes, starting with the youngest subgroups at the bottom (lowest OR; Figure 3). This means that the strongest volume‐outcome association was found in the younger male subpopulation, as indicated by the slopes in Figure 4. These figures also show an inverse volume‐outcome relationship in the younger female subpopulation, whereas an opposite association was found for the women aged >79 years.
The volume intervals related to beneficial (meaning that higher volumes are related to lower mortality) or possible adverse effects on outcome are displayed in Table 4. Positive volume effects were found for all age groups, but the range of volume differs in each group. In female patients aged <70 years, a positive effect was found all over the viewed volume range of 1 to 120 cases per year, and if the caseload exceeded 40, 68, or 94 in the 70 to 74, 75 to 79, and ≥80‐year age groups, respectively. For male patients, a positive effect of increasing annual hospital volume was shown for all age groups up to an annual volume between 89 and 97 cases (depending on age group). Conversely, if the annual volume exceeded a certain value (98–103, depending on age group), a negative effect was seen. In Figure 4, each line behaves like the first derivative of the corresponding line of Figure 3 (ie, the bigger the magnitude in Figure 3, the steeper the graph in Figure 4; negative/positive regions in Figure 4 are associated with decreasing/increasing regions in Figure 3).
Table 4.
Volume Range (1–120) Allocated Into Areas of Effect Quality (Beneficial, No, and Adverse) for Each Sex and Age Group Individually
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| OR<1 | OR=1 | OR>1 | OR<1 | OR=1 | OR>1 | ||
| (Positive) | (No) | (Negative) | (Positive) | (No) | (Negative) | ||
| Age group, y | 50–64 | 1–120 | ··· | ··· | 1–97 | 98–103 | >103 |
| 65–69 | 1–120 | ··· | ··· | 1–96 | 96–101 | >102 | |
| 70–74 | >40 | 1–40 | ··· | 1–94 | 95–100 | >100 | |
| 75–79 | >68 | 7–68 | 1–6 | 1–93 | 94–98 | >98 | |
| 80–90 | >94 | 44–94 | 1–43 | 1–89 | 90–97 | >97 | |
Cutting points were calculated on the basis of Figure 3, particularly the cut points of the 95% CIs and the OR=1.0 line. OR indicates odds ratio.
Predicted mortality specific for EVAR and OAR is plotted in Figure 5. Predicted overall in‐hospital mortality is plotted in Figure 6 (weighted for EVAR proportion).
Discussion
Overall, an inverse relationship between the annual hospital volume of iAAA repairs and in‐hospital mortality was confirmed. No significant volume‐outcome effect was evident among female patients. Interestingly, the effect was more pronounced in young male patients, who are usually deemed to have a lower perioperative risk. Female sex and age were identified as independent risk factors for in‐hospital mortality. Sex and age were modifiers of the volume‐outcome relationship. The age effect was not confounded by sex. Furthermore, increasing age, comorbidity, and OAR were associated with a higher risk for in‐hospital mortality.
Interaction Between Volume, Sex, Age, and Mortality
To the best of the authors’ knowledge, this is the first study to examine effect modification of the volume‐outcome relationship by patient sex and age. The volume‐outcome effect is widely known2, 3, 4, 5, 6, 7 and has been demonstrated in the same nationwide German setting before.11 In a further analysis of risk factors for AAA treatment in Germany, female sex and age were associated with in‐hospital mortality.12 Taking these results together, the hypothesis of the present study was that the volume‐outcome effect would be more pronounced in higher‐risk patients (older age and women). This was supported by analogous findings in percutaneous coronary artery interventions, where a volume‐outcome effect could only be shown for high‐risk procedures.10 Looking at the results of the present study, this hypothesis cannot be confirmed for iAAA treatment. In general, the volume‐outcome effect was not evident for women. Looking at Figure 1 in detail, the model predicts no advantage of treating older women in hospitals with a higher annual caseload but rather a nearly linear decrease of the OR among younger women. In contrast, for male patients, a positive effect of increasing annual hospital volume was shown for all age groups up to an annual volume of ≈100 cases. In hospitals treating >100 cases per year, mortality increased again. This increase of mortality in high‐volume hospitals is known from other studies, and several possible reasons have been discussed. The most likely causes are selection bias and confounding by unobserved/undocumented factors (eg, cases with complex aortic pathological conditions, teaching status of hospitals, and individual surgeon volume in high‐volume hospitals).11, 26 Although it was not the aim of the present study to define an overall case threshold for AAA repair, it seems that the recent guideline recommendations of the Society of Vascular Surgery (10 OAR/10 EVAR cases per year)8 and European Society of Vascular and Endovascular Surgery (30 AAA cases per year)9 may be set too low, and almost all patients might potentially benefit from higher thresholds.
Possible reasons for the worse in‐hospital outcomes in women compared with men have been discussed in detail recently.12, 27, 28 Apart from more complicated AAA morphological characteristics (calcification, shorter neck, and smaller access vessels), the greater age and possible underdiagnosed cardiovascular and pulmonary disease in women are reasonable explanations. Thus, women may benefit from more extensive preoperative workup (eg, noninvasive cardiac evaluation and lung function tests).
A recent analysis showed that young male patients face the lowest risk in AAA repair.12 In the present analysis, this patient group showed the most pronounced relative effect of annual hospital volume on postoperative mortality. A conclusion on why particularly patients who are deemed to be at a lower risk are more affected by annual hospital volume and why no benefit can be shown for female patients cannot be drawn from the present analysis. A possible explanation might be “failure to rescue,” which describes the inability of a hospital or team to save a patient′s life if complications occur. In other settings, such as esophageal and pancreatic surgery,29, 30 but also for cardiac and AAA surgery,31 failure to rescue is used to explain the volume‐outcome effect on mortality. Complication rates in these studies were not dependent on volume. Mortality and consequently failure to rescue rates were lower in high‐volume hospitals. Complication rates and their dependency on volume after AAA surgery in Germany have been analyzed in the same setting as the present study.11 Although an inverse association of hospital volume with complication rates was seen for bleeding complications (indicated by amount of blood transfusion) and peripheral arterial embolism, other severe complication rates (acute myocardial infarction, acute stroke, and mesenteric ischemia) were not associated with annual AAA volume.11 Transferring these findings to the present study, this might imply that the patients deemed to be at low risk for surgery might benefit the most from the ability to rescue in high‐volume hospitals. Further studies are needed to confirm this possible explanation.
Strengths and Limitations
The main strength of this secondary data analysis of German routine data is its nationwide coverage and accuracy on the “hard” outcome of in‐hospital death. Nevertheless, the findings have to be interpreted in the context of several limitations that have also been discussed in detail in former publications.14, 32
This observational study can only prove statistical associations, rather than causal relationships. Furthermore, the administrative nature of the data set does not allow adjustment of the statistical model for important factors, such as anatomical factors (especially aneurysm extent and diameter), urgency of treatment, and patient frailty. Adjusting the data for Elixhauser score, which was developed to provide a predictive score for in‐hospital mortality in administrative data sets, was regarded as the best tool to adjust analyses for comorbidity burden.22
The record of a case ends at discharge; therefore, only in‐hospital outcomes can be reported. Furthermore, median length of hospital stay was 2 days longer in women compared with men (13 versus 11 days). However, for the main research question (sex differences in the volume‐outcome effect after iAAA surgery), the risk of bias attributable to different length of hospital stay was considered low.
Potential miscoding could have biased case selection and grouping. Although upcoding of secondary diagnoses is considered unlikely (because of frequent monitoring of the medical service by health insurance companies), secondary diagnoses might not be coded if they are not relevant for reimbursement. Nonetheless, it is important to mention that data collection and validation for this data set are regulated by German Social Law, and German hospitals are statutorily committed to submit their data. Last, further follow‐up after hospital discharge is not possible using this data set; therefore, a possible effect of hospital volume on long‐term survival after AAA repair cannot be evaluated.
Conclusions
Operations on iAAA remain high‐risk procedures, even in the endovascular era. Overall, an inverse relationship between annual hospital volume of iAAA repairs and in‐hospital mortality was confirmed. Interestingly, the effect was most pronounced in young male patients who are usually deemed to have a lower perioperative risk. No significant volume‐outcome effect was evident when all female patients were taken together. In younger female patients, a positive, but barely relevant, effect of hospital volume on mortality could still be shown. Sex and age were modifiers of the volume‐outcome relationship. The age effect was not confounded by sex. As the vast majority of patients are men, these findings do not contradict demands for centralization of AAA, irrespective of the type of AAA procedure. However, for female patients, centralization may not lead to a relevant reduction of their perioperative risk. Further studies are needed to identify the underlying causal factors and improve AAA treatment for women.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1‐S5
References 21 and 22
Acknowledgments
We would like to thank Mrs Melanie Scheller from the Research Data Center of the German Federal Statistical Office for her competent and pleasant assistance during planning and performing the data analysis. In addition, we would like to thank Mrs Jutta Spindler and Mrs Sabine Nemitz (German Federal Statistical Office) for supporting our research.
(J Am Heart Assoc. 2020;9:e014534 DOI: 10.1161/JAHA.119.014534.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Stoberock K, Kolbel T, Atlihan G, Debus ES, Tsilimparis N, Larena‐Avellaneda A, Behrendt CA, Wipper S. Gender differences in abdominal aortic aneurysm therapy—a systematic review. Vasa. 2018;47:267–271. [DOI] [PubMed] [Google Scholar]
- 2. Hafez H. National Vascular Database analysis: the relationship between AAA repair volume and outcome. Br J Surg. 2012;99:4.22441847 [Google Scholar]
- 3. Holt PJ, Karthikesalingam A, Hofman D, Poloniecki JD, Hinchliffe RJ, Loftus IM, Thompson MM. Provider volume and long‐term outcome after elective abdominal aortic aneurysm repair. Br J Surg. 2012;99:666–672. [DOI] [PubMed] [Google Scholar]
- 4. Holt PJ, Poloniecki JD, Khalid U, Hinchliffe RJ, Loftus IM, Thompson MM. Effect of endovascular aneurysm repair on the volume‐outcome relationship in aneurysm repair. Circ Cardiovasc Qual Outcomes. 2009;2:624–632. [DOI] [PubMed] [Google Scholar]
- 5. Holt PJ, Poloniecki JD, Loftus IM, Michaels JA, Thompson MM. Epidemiological study of the relationship between volume and outcome after abdominal aortic aneurysm surgery in the UK from 2000 to 2005. Br J Surg. 2007;94:441–448. [DOI] [PubMed] [Google Scholar]
- 6. Landon BE, O'Malley AJ, Giles K, Cotterill P, Schermerhorn ML. Volume‐outcome relationships and abdominal aortic aneurysm repair. Circulation. 2010;122:1290–1297. [DOI] [PubMed] [Google Scholar]
- 7. Phillips P, Poku E, Essat M, Woods HB, Goka EA, Kaltenthaler EC, Walters S, Shackley P, Michaels J. Procedure volume and the association with short‐term mortality following abdominal aortic aneurysm repair in European populations: a systematic review. Eur J Vasc Endovasc Surg. 2017;53:77–88. [DOI] [PubMed] [Google Scholar]
- 8. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e2. [DOI] [PubMed] [Google Scholar]
- 9. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, Dick F, van Herwaarden J, Karkos C, Koelemay M, et al. Editor's choice—European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto‐iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93. [DOI] [PubMed] [Google Scholar]
- 10. Zahn R, Gottwik M, Hochadel M, Senges J, Zeymer U, Vogt A, Meinertz T, Dietz R, Hauptmann KE, Grube E, et al. Volume‐outcome relation for contemporary percutaneous coronary interventions (PCI) in daily clinical practice: is it limited to high‐risk patients? Results from the Registry of Percutaneous Coronary Interventions of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Heart. 2008;94:329–335. [DOI] [PubMed] [Google Scholar]
- 11. Trenner M, Kuehnl A, Salvermoser M, Reutersberg B, Geisbuesch S, Schmid V, Eckstein H‐H. Editor's choice: high annual hospital volume is associated with decreased in hospital mortality and complication rates following treatment of abdominal aortic aneurysms: secondary data analysis of the nationwide German DRG statistics from 2005 to 2013. Eur J Vasc Endovasc Surg. 2018;55:185–194. [DOI] [PubMed] [Google Scholar]
- 12. Trenner M, Kuehnl A, Reutersberg B, Salvermoser M, Eckstein HH. Nationwide analysis of risk factors for in‐hospital mortality in patients undergoing abdominal aortic aneurysm repair. Br J Surg. 2018;105:379–387. [DOI] [PubMed] [Google Scholar]
- 13. FDZ der Statistischen Ämter des Bundes und der Länder: Fallpauschalenbezogene Krankenhausstatistik (research data centers of the federal statistical office: DRG statistics). Own calculations 2005‐2016.
- 14. Kuehnl A, Erk A, Trenner M, Salvermoser M, Schmid V, Eckstein HH. Incidence, treatment and mortality in patients with abdominal aortic aneurysms—a secondary analysis of diagnosis‐related groups (DRG) data from 2005‐2014. Dtsch Arztebl Int. 2017;114:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuehnl A, Salvermoser M, Erk A, Trenner M, Schmid V, Eckstein HH. Spatial analysis of hospital incidence and in hospital mortality of abdominal aortic aneurysms in Germany: secondary data analysis of nationwide hospital episode (DRG) data. Eur J Vasc Endovasc Surg. 2018;55:852–859. [DOI] [PubMed] [Google Scholar]
- 16. Nimptsch U, Krautz C, Weber GF, Mansky T, Grutzmann R. Nationwide in‐hospital mortality following pancreatic surgery in Germany is higher than anticipated. Ann Surg. 2016;264:1082–1090. [DOI] [PubMed] [Google Scholar]
- 17. Reutersberg B, Salvermoser M, Trenner M, Geisbusch S, Zimmermann A, Eckstein HH, Kuehnl A. Hospital incidence and in‐hospital mortality of surgically and interventionally treated aortic dissections: secondary data analysis of the nationwide German diagnosis‐related group statistics from 2006 to 2014. J Am Heart Assoc. 2019;8:e011402 DOI: 10.1161/JAHA.118.011402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swart E, Gothe H, Geyer S, Jaunzeme J, Maier B, Grobe TG, Ihle P. Good Practice of Secondary Data Analysis (GPS): guidelines and recommendations [in German]. Gesundheitswesen. 2015;77:120–126. [DOI] [PubMed] [Google Scholar]
- 19. Swart E, Bitzer EM, Gothe H, Harling M, Hoffmann F, Horenkamp‐Sonntag D, Maier B, March S, Petzold T, Röhrig R, et al. STandardisierte BerichtsROutine für Sekundärdaten Analysen (STROSA)—ein konsentierter Berichtsstandard für Deutschland, Version 2. Das Gesundheitswesen. 2016;78:145–160. [DOI] [PubMed] [Google Scholar]
- 20. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sorensen HT, von Elm E, Langan SM. The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 22. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. [DOI] [PubMed] [Google Scholar]
- 23. Schermerhorn ML, O'Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–474. [DOI] [PubMed] [Google Scholar]
- 24. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria; 2018. [Google Scholar]
- 25. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer‐Verlag; 2016. [Google Scholar]
- 26. Kuehnl A, Tsantilas P, Knappich C, Schmid S, Konig T, Breitkreuz T, Zimmermann A, Mansmann U, Eckstein HH. Significant association of annual hospital volume with the risk of inhospital stroke or death following carotid endarterectomy but likely not after carotid stenting: secondary data analysis of the statutory German carotid quality assurance database. Circ Cardiovasc Interv. 2016;9:e004171. [DOI] [PubMed] [Google Scholar]
- 27. Hultgren R, Vishnevskaya L, Wahlgren CM. Women with abdominal aortic aneurysms have more extensive aortic neck pathology. Ann Vasc Surg. 2013;27:547–552. [DOI] [PubMed] [Google Scholar]
- 28. Sweet MP, Fillinger MF, Morrison TM, Abel D. The influence of gender and aortic aneurysm size on eligibility for endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2011;54:931–937. [DOI] [PubMed] [Google Scholar]
- 29. Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high‐risk surgery. Med Care. 2011;49:1076–1081. [DOI] [PubMed] [Google Scholar]
- 30. Nimptsch U, Haist T, Krautz C, Grutzmann R, Mansky T, Lorenz D. Hospital volume, in‐hospital mortality, and failure to rescue in esophageal surgery. Dtsch Arztebl Int. 2018;115:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gonzalez AA, Dimick JB, Birkmeyer JD, Ghaferi AA. Understanding the volume‐outcome effect in cardiovascular surgery: the role of failure to rescue. JAMA Surg. 2014;149:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trenner M, Eckstein HH, Kallmayer MA, Reutersberg B, Kühnl A. Secondary analysis of statutorily collected routine data. Gefässchirurgie. 2019;24:220–227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S5
References 21 and 22


