Abstract
Background
Risk stratification of Chagas disease patients in the limited‐resource setting would be helpful in crafting management strategies. We developed a score to predict 2‐year mortality in patients with Chagas cardiomyopathy from remote endemic areas.
Methods and Results
This study enrolled 1551 patients with Chagas cardiomyopathy from Minas Gerais State, Brazil, from the SaMi‐Trop cohort (The São Paulo‐Minas Gerais Tropical Medicine Research Center). Clinical evaluation, ECG, and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) were performed. A Cox proportional hazards model was used to develop a prediction model based on the key predictors. The end point was all‐cause mortality. The patients were classified into 3 risk categories at baseline (low, <2%; intermediate, ≥2% to 10%; high, ≥10%). External validation was performed by applying the score to an independent population with Chagas disease. After 2 years of follow‐up, 110 patients died, with an overall mortality rate of 3.505 deaths per 100 person‐years. Based on the nomogram, the independent predictors of mortality were assigned points: age (10 points per decade), New York Heart Association functional class higher than I (15 points), heart rate ≥80 beats/min (20 points), QRS duration ≥150 ms (15 points), and abnormal NT‐proBNP adjusted by age (55 points). The observed mortality rates in the low‐, intermediate‐, and high‐risk groups were 0%, 3.6%, and 32.7%, respectively, in the derivation cohort and 3.2%, 8.7%, and 19.1%, respectively, in the validation cohort. The discrimination of the score was good in the development cohort (C statistic: 0.82), and validation cohort (C statistic: 0.71).
Conclusions
In a large population of patients with Chagas cardiomyopathy, a combination of risk factors accurately predicted early mortality. This helpful simple score could be used in remote areas with limited technological resources.
Keywords: Chagas cardiomyopathy, Chagas disease, mortality, risk prediction, risk score
Subject Categories: Cardiomyopathy, Inflammatory Heart Disease
Clinical Perspective
What Is New?
A simple score accurately predicts 2‐year mortality in patients with Chagas cardiomyopathy.
The score included variables that could be readily assessed at public health primary care units.
The score provides a robust tool for risk stratification among Chagas community‐based patients.
What Are the Clinical Implications?
The score provides insights on how patient care and healthcare resources can be optimized.
Risk stratification of Chagas disease patients in the limited‐resource setting would be helpful for patient management.
Identification of high‐risk patients is useful to guide appropriate therapy.
Introduction
Chagas disease is a major public health burden in Latina America and has become a potentially serious emerging problem in nonendemic countries.1, 2 Millions of people are chronically infected, with the largest numbers living in Brazil and Argentina.3, 4 Although most infected people remain asymptomatic, it is estimated that a third of individuals infected with Trypanosoma cruzi will progress to Chagas cardiomyopathy decades after the initial infection.5
Chagas cardiomyopathy has a wide spectrum of clinical presentations, with ECG abnormality early in the disease course, but can progress over time to dilated cardiomyopathy with heart failure, ventricular arrhythmias, conduction disturbances, thromboembolic events, and sudden cardiac death.6 Chagas cardiomyopathy is a major cause of cardiovascular‐related deaths in endemic areas,7, 8 with ≈12 000 attributed deaths per year worldwide.1, 9 The disease also causes substantial disability, with a large proportional contribution of years of life lost to its total disability‐adjusted life‐years.10
Over the past several decades, large‐scale rural‐to‐urban migration has spread the disease to cities and to nonendemic countries.2 However, Chagas disease has remained largely confined to poor rural areas of Latin America and is recognized by the World Health Organization as a neglected tropical disease that primarily affects low‐income populations in endemic areas.1 A substantial number of infected individuals still live in remote poor areas and are at risk for disease progression and death.11 In rural endemic areas, access to diagnosis and treatment is limited, which may result in high mortality from Chagas disease.12 Consequently, a score that could provide risk stratification for patients with Chagas disease would be useful for generalist physicians working at the primary health services.
The 2018 American Heart Association scientific statement on Chagas cardiomyopathy recommends performing risk stratification in all patients with chronic Chagas disease.6 However, the use of the Rassi score for mortality risk stratification requires 24‐hour Holter monitoring, stress testing, and echocardiography, which may not be available in rural areas in endemic countries.13 A simple risk stratification score using available tests that can be applied in endemic populations would be helpful for patient management.
The SaMi‐Trop (The São Paulo‐Minas Gerais Tropical Medicine Research Center) is a large cohort of well‐characterized patients with Chagas cardiomyopathy within a limited region of Minas Gerais State, Brazil,14 where the participants were systematically followed with standardized assessment of clinical outcomes. We used this robust cohort to develop a risk‐assessment algorithm that integrates all relevant variables to be applied in primary healthcare settings. The purpose of this study was to develop a simple point‐based risk score to predict 2‐year mortality in patients with Chagas cardiomyopathy from remote endemic areas.
Methods
The data, analytic methods, and study materials will be made available by the corresponding author on reasonable request from other researchers for purposes of reproducing the results or replicating the procedure.
Derivation Cohort
The baseline characteristics of the cohort have been described in detail previously.14 Briefly, patients were selected using the database encompassing the population of 21 municipalities in the north of Minas Gerais (Figure 1). This study is part of the National Institutes of Health–sponsored SaMi‐Trop prospective cohort study,14 one of the largest cohort study of Chagas disease conducted worldwide. The SaMi‐Trop cohort included patients with chronic Chagas cardiomyopathy from among those who used the Telehealth Network, a program that uses telecommunication and virtual technology designed to support primary health care in Minas Gerais State, Brazil.15
Figure 1.
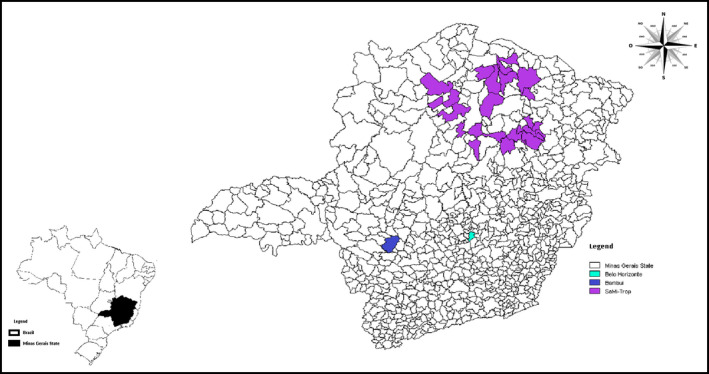
Map of Minas Gerais State showing the 21 municipalities enrolled for the derivation cohort (SaMi‐Trop) and Bambuí city, where the population was selected for the validation cohort.
Initially, eligible participants were selected based on self‐reporting of Chagas disease and the presence of ECG abnormalities that characterize the cardiac form of the disease. In brief, 12‐lead ECG abnormalities included Q‐wave abnormalities, primary ST‐ and T‐wave changes, complete intraventricular block, atrioventricular conduction changes or pacemaker use, atrial fibrillation or flutter, or other major arrhythmias.16, 17
Subsequently, all participants were tested for T cruzi infection using 2 serologic assays, a T cruzi lysate‐based enzyme immunoassay and a recombinant enzyme immunoassay (Wiener Lab). The final cohort included only patients with 2 positive serologic tests against T cruzi.
The baseline visit was performed between 2013 and 2014 at public health primary care units by previously trained staff. Patients were interviewed using a standardized questionnaire with sociodemographic information, health‐related behaviors, comorbidities, heart disease symptoms, especially functional capacity assessed by the New York Heart Association (NYHA) classification, and blood collection. The NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) test was also obtained. NT‐proBNP (Roche Diagnostics) was categorized according to age‐specific cut points for heart failure.18
A resting 12‐lead ECG was recorded at baseline using a PC‐based ECG machine (TEB). The ECG recordings were sent electronically via the Telehealth system and read by a trained cardiologist. The report was subsequently returned to the patient's physician.19 ECGs were classified using the Minnesota Code criteria with variables derived from the median complex of the Glasgow University software measurement matrix.19 ECG measurements and codes were reviewed by a second cardiologist who was blinded to clinical findings and serologic statuses. Patients who presented with normal ECG at baseline and were classified as having an indeterminate form were not selected for the study. Initially, 1959 patients with confirmed Chagas disease from 21 municipalities were eligible for the study. After ECG review, 375 patients (19.1%) who had normal ECG were excluded, and a total of 1584 patients were enrolled.
Among the 1584 study participants, 33 patients (2%) were excluded at the end of the follow‐up for unknown date of death (n=13), missing values of NT‐proBNP (n=4), or NYHA functional class (n=15), and one patient without the date of last contact was excluded. Therefore, 1551 patients who had complete data and who fulfilled the inclusion criteria were included in this study (Figure 2).
Figure 2.
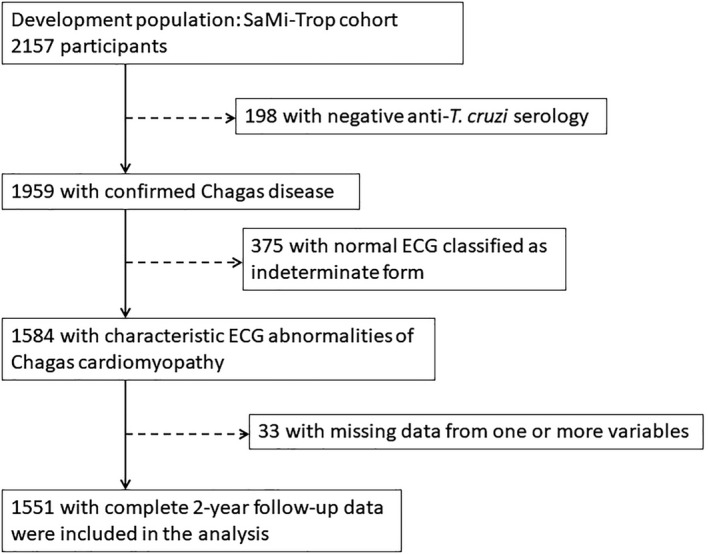
Flowchart of the study population.
After 2 years of follow‐up, all patients were contacted for a second visit at public health primary care units (2015–2016). The study was approved by the Brazilian National Institutional Review Board (CONEP), No. 179.685/2012. In this investigation, all human subjects were adults who gave written informed consent.
End Point Definition
The end point was all‐cause mortality at 2‐year follow‐up. Deaths were mostly identified using death certificates obtained from the Health Department of Minas Gerais or the local municipality authorities, which provided information about locale, cause, and date of death.
The death certificate is a nationally required document for all registered deaths; however, national coverage is not 100%. In fact, it was estimated in a recent study as 82.7% for the period of 2010–2016.20 Some of our cohort patients did not attend their scheduled visit at 2 years of follow‐up and had no identified death certificate, but their vital statuses were known via contact with the families or community health workers. For a few of those participants, details of cause and date of death were missing.
Validation Cohort
A second set of patients who also lived in an endemic area for Chagas disease, located in the midwest of Minas Gerais State, was selected as the validation cohort (Figure 1). The cohort study, which consists of all residents aged ≥60 years from Bambuí town, one of the oldest known endemic area for Chagas disease, has been described previously.21 Baseline data collection was performed from February to May 1997, including standardized interviews, overall blood tests, and ECGs.16 The same variables assessed in the derivation cohort were also available in the validation cohort. The only difference was the use of BNP to replace NT‐proBNP measured in the derivation cohort. A cutoff value ≥100 pg/mL was categorized as abnormal BNP.22 Of the 1462 participants, 557 had 3 positive serologic tests for Chagas disease and were included. Death certificate verification was performed during annual follow‐up visits. For validation purposes, we analyzed mortality at 2 years of follow‐up. The Bambuí Cohort Study of Aging was approved by the ethics board of the Fundação Oswaldo Cruz, Brazil.
Statistical Analysis
Baseline characteristics were reported by survivorship status at the end of 2 years of follow‐up. We presented frequency and percentages for categorical variables and mean±SD or median and interquartile range for continuous variables. For continuous variables, Student unpaired t test or Mann–Whitney U test were used to compare survivors and nonsurvivors for categorical variables; the χ2 test was used to assess differences.
Cox proportional hazards regression was performed to identify independent predictors of all‐cause mortality. In an initial step, we used a model in which prespecified variables associated with mortality in Chagas disease were included: age, sex, NYHA functional class, benznidazole treatment, and NT‐proBNP.13, 22, 23 In a second step, ECG variables that were significantly associated with death in the univariable analysis were entered the final model, including heart rate, QRS duration, QT interval, and low QRS voltage. The presence of atrial fibrillation; use of a pacemaker; and left bundle‐branch block, which occurred in <5% of the population, were not included in the final model. Interaction terms between the several explanatory variables were also considered during modeling.
Unadjusted associations between continuous predictors and mortality were examined using restricted cubic spline functions, including heart rate and QRS duration. The estimated spline function indicated that the association between these variables and mortality was not linear; therefore, we categorized them to enter dichotomously into the final prediction model. NT‐proBNP was entered in the score model as a dichotomous variable corrected by age. The score was obtained with a simple sum of points attributed to each predictor of the final model.
Subsequently, with the aim of providing a practical tool for risk stratification, the nomogram was developed to calculate the predictive risk. A risk score to predict 2‐year mortality was obtained using a scale from 0 to 100 points. The patients were classified into 3 categories of risk: low risk (<2%), intermediate risk (≥2% to 10%), and high risk (≥10%) of death in 2 years. The risk categories were defined based on a previous study assessing the risk score for mortality in Chagas disease.13 The mortality rates at 10 years in low, intermediate, and high risk were 10%, 44%, and 84%, respectively, which correspond to 2‐year mortality in low, intermediate, and high risk of 2%, 8.8%, and 16.8%, respectively. The total number of points in the nomogram corresponding to 2‐year risk of death in the 3 categories can be obtained. The corresponding score cutoffs were determined based on the total number of points in the nomogram corresponding to 2‐year risk of death in the 3 categories by tracing a vertical line from total points to 2‐year risk of death. Consequently, low risk (<2%) corresponds to roughly 50 points, intermediate risk (≥2% to 10%) is between 50 and 100 points, and high risk (≥10%) is >100 points.
The score risk model was internally validated by means of a bootstrap resample method to assess model optimism24; calibration was based on the Hosmer and Lemeshow test and discrimination on the receiver operating characteristic curve and C statistic. In addition, 1000 times cross‐validation was run, splitting into training (70%) and test (30%) samples, and discrimination and calibration were evaluated in the test group. External validation was performed by applying the final model to an independent population of patients with Chagas disease (the Bambuí cohort) to assess its discrimination and calibration in predicting 2‐year mortality.
Statistical analysis was performed using SPSS for Windows v22.0 (IBM Corp) and R v3.4.0 (R Foundation for Statistical Computing).
Results
Patient Characteristics
The mean age was 59.4±12.7 years (range: 18–96), 1024 participants were women (66%), and 836 (54%) participants were in NYHA functional class I at the time of recruitment into the study. The baseline characteristics of the overall population, comparing those who survived with those who died, are summarized in Table 1. Previous treatment with benznidazole was reported by 368 patients (24%). Right bundle‐branch block plus left anterior fascicular block was present in 215 patients (14%), and atrial fibrillation in 80 patients (5%) at enrollment.
Table 1.
Characteristics of the Study Population at Baseline, Stratified According to All‐Cause Mortality
| Overall Population (N=1551) | Survivors (n=1441) | Deceased (n=110) | P Value | |
|---|---|---|---|---|
| Age, y | 59.4±12.7 | 58.9±12.6 | 66.2±13.3 | <0.001 |
| Male sex | 527 (34) | 479 (33) | 48 (44) | 0.026 |
| NYHA functional class II or higher | 715 (46) | 643 (45) | 72 (66) | <0.001 |
| Syncopea | 357 (23) | 330 (23) | 27 (25) | 0.193 |
| Diabetes mellitus | 156 (10) | 148 (10) | 8 (7) | 0.314 |
| Arterial hypertension | 535 (34) | 502 (35) | 33 (30) | 0.304 |
| Chronic kidney disease | 115 (7) | 100 (7) | 15 (14) | 0.010 |
| NT‐proBNP, pg/mL) | 167 (68/472) | 149 (63/397) | 1133 (413/3213) | <0.001 |
| Abnormal NT‐proBNPb | 214 (14) | 158 (11) | 56 (51) | <0.001 |
| Medications | ||||
| Benznidazole treatmentc | 368 (26) | 357 (25) | 11 (10) | 0.001 |
| Loop diuretics | 327 (21) | 274 (19) | 53 (48) | <0.001 |
| ACEIs | 475 (31) | 447 (31) | 28 (26) | 0.258 |
| Angiotensin receptor blockers | 452 (29) | 403 (28) | 49 (45) | <0.001 |
| Spironolactone | 284 (18) | 245 (17) | 39 (36) | <0.001 |
| β‐Blockers (carvedilol) | 339 (22) | 288 (20) | 51 (46) | <0.001 |
| Digoxin | 132 (9) | 115 (8) | 17 (16) | 0.006 |
| Amiodarone | 386 (25) | 338 (23) | 48 (44) | 0.001 |
| ECG findings | ||||
| Heart rate, beats/min | 66±14.1 | 66±13.5 | 70±17.9 | 0.028 |
| Atrial fibrillation | 80 (5) | 66 (5) | 14 (13) | <0.001 |
| QTc interval, ms | 446.7±30.3 | 446.0±30.2 | 458.8±29.8 | <0.001 |
| QRS duration, ms | 120.4±29.0 | 119.3±28.3 | 136.3±34.7 | <0.001 |
| Pacemaker | 64 (4) | 48 (3) | 16 (15) | <0.001 |
| Isolated RBBB | 379 (24) | 358 (25) | 21 (19) | 0.167 |
| RBBB plus LAFB | 215 (14) | 196 (14) | 19 (17) | 0.283 |
| LBBB | 74 (5) | 60 (4) | 14 (13) | <0.001 |
| Ventricular ectopic beats | 52 (3) | 45 (3) | 7 (6) | 0.071 |
| Low QRS voltage | 109 (7) | 95 (6.6) | 14 (13) | 0.015 |
Data are expressed as mean±SD, median (interquartile range), or absolute numbers (percentage). ACEI indicates angiotensin‐converting enzyme inhibitor; LAFB, left anterior fascicular block; LBBB, left bundle‐branch block; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; RBBB, right bundle‐branch block.
Reported by the patients.
Considering age‐related cutoff values.
Previous treatment with benznidazole was not informed by 124 patients (8%).
During a median follow‐up of 24 months (range: 0.83 to 39.96 months [3.33 years]), 110 patients died, with a mortality incidence rate of 3.505 deaths per 100 person‐years. Survivors were significantly younger, with a predominance of women; had better NYHA functional class; received further treatment with benznidazole; and the levels of NT‐proBNP were lower compared with those who died. The presence of comorbidities did not vary among the participants, except that chronic kidney disease was present in a higher percentage of those who died than those who survived.
Compared with the survivors, the participants who died presented more ECG abnormalities. Atrial fibrillation, larger QTc interval, longer QRS duration, and presence of a pacemaker were found more frequently in deceased patients than in survivors.
By multivariable analysis, 5 variables were maintained in the final model: age, NYHA functional class, heart rate, QRS duration, and NT‐proBNP (Table 2). Figure 3 shows Kaplan–Meier survival curves by NYHA functional class II or higher, abnormal NT‐proBNP, QRS duration ≥150 ms, heart rate ≥80 beats/min, age groups, and according to risk category.
Table 2.
Multivariable Cox Proportional Hazards Regression Model for Predicting 2‐Year Mortality
| Variable | Β Coefficient | HR (95% CI) | P Value |
|---|---|---|---|
| Age (decade)a | 0.358 | 1.431 (1.229–1.665) | <0.001 |
| NYHA class II or higher | 0.487 | 1.628 (1.088–2.437) | 0.018 |
| Heart rate ≥80 beats/min | 0.592 | 1.808 (1.169–2.796) | 0.008 |
| QRS duration ≥150 ms | 0.486 | 1.626 (1.070–2.470) | 0.023 |
| Abnormal NT‐proBNPb | 1.772 | 5.885 (3.931–8.812) | <0.001 |
HR indicates hazard ratio; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
HR for each 10‐year increase in age.
NT‐proBNP was considered abnormal by age range as follows: <50 years=NT‐proBNP >450 pg/mL; 50–75 years=NT‐proBNP >900 pg/mL; >75 years=NT‐proBNP=1800 pg/mL.
Figure 3.
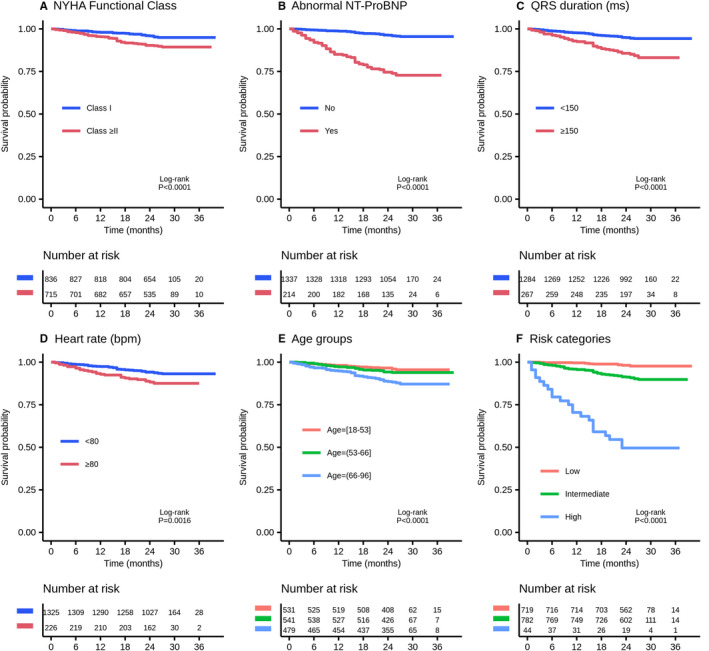
Kaplan–Meier survival curves by New York Heart Association (NYHA) functional class ≥II (A), abnormal NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) (B), QRS duration ≥150 ms (C), heart rate ≥80 beats/min (D), age groups (E), and risk category (F).
The final model validated in the test sample showed a C statistic of 0.816 (95% CI, 0.772–0.859) and is well calibrated (Hosmer–Lemeshow test, P=0.452). Illustration of observed versus predicted mortality by decile of predicted risk in the development set is shown in Figure 4A. No evidence showed overfitting, and the optimism result was 97.4%. Cross‐validation with replication showed optimal discrimination of the model, with an average C statistic of 0.806 (95% CI, 0.736–0.869) and calibration with an average of Hosmer–Lemeshow P value of 0.306.
Figure 4.
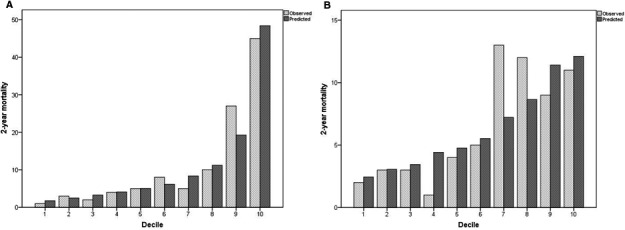
Calibration plots by decile for the 2‐year mortality risk prediction model in the derivation cohort (A) and in the validation cohort (B).
The nomogram produced based on the regression coefficient for each independent predictor of mortality can be seen in Figure 5. The scores for each variable, which were summed to give total points, are shown in Table 3. According to the nomogram, abnormal NT‐proBNP adjusted by age was the strongest predictor of death. A low‐risk score was set as a predicted probability of dying in 2 years <2% (<50 points); intermediate risk was ≥2% to 10% (50–100 points), and the high‐risk category had predicted risk of >10% (>100 points). As an example, using the point scale in the nomogram (Figure 6), a 65‐year‐old woman (61 points) presented with NYHA functional class I (0 point) with abnormal NT‐proBNP (55 points), heart rate of 85 beats/min (18 points), and QRS duration of 160 ms (15 points). The total sum of points is 149, which classifies her into the high‐risk group with predicted probability of 2‐year mortality of 38%. The observed 2‐year mortality rates in the low‐, intermediate‐, and high‐risk groups were 0%, 3.6%, and 32.7%, respectively.
Figure 5.
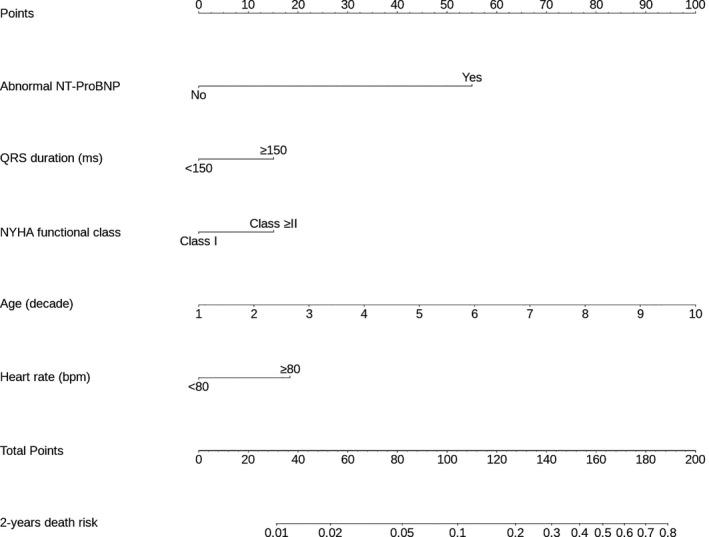
Nomogram for the final model. Each variable corresponds to a point (top). These points are then summed to translate into a 2‐year risk of death (bottom). A low‐risk score was 2% (<60 points), intermediate risk was ≥2% to 10% (60–100 points), and high risk was >10% (>100 points). NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Table 3.
Simple Classification Based on Points Score
| Variable | Points |
|---|---|
| Abnormal NT‐proBNP | 55 |
| QRS duration ≥150 ms | 15 |
| NYHA functional class higher than I | 15 |
| Age (per 10 y) | 10 |
| Heart rate (≥80 beats/min) | 20 |
NT‐proBNP indicates N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Figure 6.
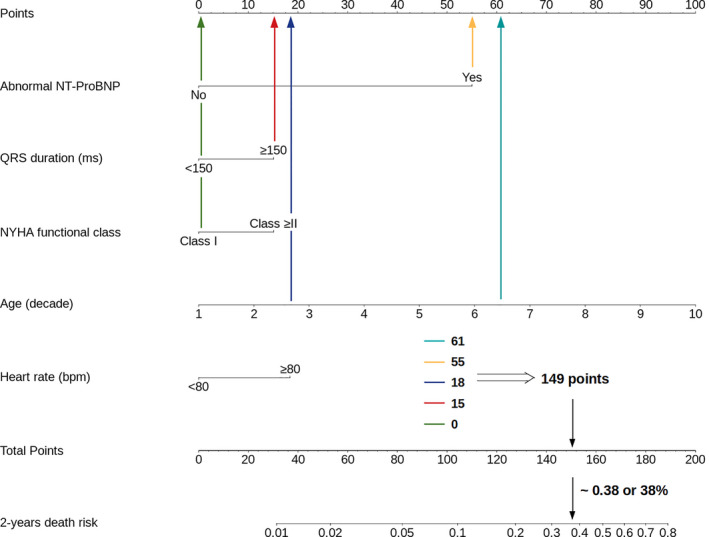
Case: 65‐year‐old woman (61 points) with Chagas cardiomyopathy presented in NYHA functional class I (0 points) with abnormal NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide; 55 points), heart rate of 85 beats/min (18 points), and QRS duration of 160 ms (15 points). The total sum of points is 149, which classifies her as high risk with a predicted probability of 2‐year mortality of 38%.NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Validation Cohort
The median age of the 557 patients in the validation cohort was 68 years (range: 60–92 years), and 376 (68%) were women. Most patients were in NYHA functional class I (78%) at enrollment. The characteristics of the validation cohort compared with those of the derivation cohort are shown in Table 4. These patients were older and had higher prevalence of hypertension but fewer ECG abnormalities compared with the derivation cohort. Abnormal natriuretic peptide was also more frequently detected in this population, whereas other prognostic variables in Chagas disease including male sex and atrial fibrillation were similar between the cohorts.
Table 4.
Overall Baseline Characteristics of the Derivation Cohort Compared With the Validation Cohort
| Variables | Derivation Cohort (n=1551) | Validation Cohort (n=557) | P Value |
|---|---|---|---|
| Age, y | 59.4±12.7 | 69.4±7.1 | <0.001 |
| Male sex | 527 (34) | 181 (33) | 0.525 |
| NYHA functional class II or higher | 715 (46) | 124 (22) | <0.001 |
| Diabetes mellitus | 156 (10) | 57 (10) | 0.877 |
| Arterial hypertension | 535 (35) | 285 (51) | <0.001 |
| Chronic kidney disease | 115 (7) | 33 (6) | 0.242 |
| Abnormal natriuretic peptidea | 214 (14) | 321 (58) | <0.001 |
| Heart rate, beats/min | 66.3±13.9 | 70.2±13.5 | <0.001 |
| QRS duration, ms | 120.4±28.9 | 102.0±25.4 | <0.001 |
| QTc interval, ms | 435.5±43.6 | 400.4±43.8 | <0.001 |
| Atrial fibrillation | 80 (5) | 32 (6) | 0.596 |
| Pacemaker | 64 (4) | 6 (1) | 0.001 |
| RBBB plus LAFB | 215 (14) | 51 (9) | 0.004 |
| LBBB | 74 (5) | 18 (3) | 0.127 |
LAFB indicates left anterior fascicular block; LBBB, left bundle‐branch block; NYHA, New York Heart Association; RBBB, right bundle‐branch block.
BNP levels in the validation cohort: median of 119.5 (63/206); range: 15–1882 pg/mL.
The score was then applied to the external validation cohort. In the validation cohort, the observed 2‐year mortality rates in the low‐, intermediate‐, and high‐risk groups were 3.2%, 8.7%, and 19.1%, respectively. The discrimination of the model was good (C statistic=0.71; 95% CI, 0.64–0.77), and the model was well calibrated (the risk score predicted 63 deaths and 63 occurred). A Hosmer–Lemeshow P=0.165 confirmed no significant difference between observed and predicted mortality (Figure 4B).
Discussion
A simple score for predicting 2‐year mortality risk in Chagas cardiomyopathy was developed and validated to be used in remote areas with limited technological resources. The score included only 5 variables that can be readily assessed at public health primary care units and provides a robust tool for risk stratification in community‐based patients with Chagas cardiomyopathy. Internal validation by bootstrapping excluded significant overfitting during the development of the score. For ease of use, the final model was represented by a nomogram. The score model had high discriminatory ability for all‐cause mortality with adequate calibration in the derivation and the validation cohorts. The score can be easily implemented in clinical practice at limited‐resources settings and may contribute to improvements in risk stratification of patients with Chagas cardiomyopathy from endemic regions.
Chagas cardiomyopathy continues to be an important cause of premature death, affecting 20% to 40% of people infected with T cruzi during their lifetime.25 However, Chagas disease is an underreported cause of death.7 According to a previous study, Chagas disease was mentioned in only 58% of death certificates of seropositive patients in Brazil, which suggests the official mortality rates are probably underestimated.7 In this context, it is possible that access to health care is limited in remote areas, where there is a low ability of practitioners serving communities to identify patients at high risk of death, in whom optimization of heart failure treatment is indicated.
The severity of heart disease is the main determinant of mortality in Chagas cardiomyopathy.26, 27 However, previous studies addressing the predictors of mortality in Chagas disease have several limitations.28, 29, 30, 31, 32, 33, 34, 35 In particular, wide variation in prognosis for individual patients highlights the need to identify key factors implicated in disease progression and death. More accurate mortality risk prediction in Chagas cardiomyopathy would be helpful for clinical‐decision guidance, particularly in remote areas, where the lack of access to appropriate treatment continues to be a challenge.6
Risk‐Stratification Scores in Chagas Disease
Three risk scores have been proposed to predict mortality in patients with Chagas disease.13, 36, 37 The score model developed by Rassi et al13 identified 6 independent prognostic factors, including male sex, symptoms assessed by NYHA functional class, and severity of cardiac involvement based on chest X‐ray, ECG, 24‐hour Holter, and echocardiography findings. A risk score derived by the combination of points attributed to each of these features accurately classified patients into a low‐, medium‐, or high‐risk group, with 10‐year mortality rates of 10%, 44%, and 84%, respectively. The score was validated using an external population, and the respective 10‐year mortality rates were 9%, 37%, and 85%. In addition, the score by Rassi et al applied to the population with mild to moderate Chagas cardiomyopathy from the BENEFIT trial was able to predict risk of composite cardiac events, including death, resuscitated cardiac arrest, sustained ventricular tachycardia, insertion of a pacemaker or implantable cardioverter‐defibrillator, cardiac transplantation, new heart failure, stroke, or systemic embolism.38 Event rates in low‐, intermediate‐, and high‐risk patients were 3%, 6%, and 9% per year, respectively. This score is the standard currently used to predict death in Chagas disease.
Subsequently, Ribeiro et al36 showed that 3 risk factors including depressed left ventricular ejection fraction, ventricular tachycardia assessed at either 24‐hour Holter monitoring or stress testing, and prolonged filtered QRS complex accurately predicted death in ambulatory patients with Chagas disease. The score showed optimal discrimination to identify patients at low, moderate, and high risk of death, comparable to the score by Rassi et al13. The study was limited by the small number of patients enrolled; the majority were asymptomatic with mild heart disease, and no internal or external validation was performed.
Recently, de Souza et al37 developed a risk score for sudden death, based on 4 risk factors encompassing premature ventricular complexes, severe left ventricular dysfunction, syncope, and QT dispersion by ECG. The score demonstrated good performance to predict the risk of sudden death, which is the most common cause of death overall in patients with Chagas disease. This study included an urban cohort from a reference center for Chagas disease, which might not be applicable to the general Chagas disease patient population. Indeed, it has not been validated using an independent external population.
These previously reported risk scores for prediction of mortality in Chagas cardiomyopathy have several limitations. Specifically, the scores rely on the availability of many diagnostic tests, including 24‐hour Holter monitoring, stress testing, echocardiography, or special examinations such as signal averaged ECG and QT dispersion. These models have been too complex to be integrated into daily practice in rural endemic areas, with a limited role in risk stratification in the primary care setting.
The present risk score offers specific advantages over previous risk scores. First, a large contemporary cohort of patients with Chagas cardiomyopathy living in rural endemic areas was used to determine the most important prognostic factors and to derive the mortality prediction score model.14 Our methodology focused on identifying a minimal number of variables for risk assessment that can be obtained in a setting with limited resources, which could affect the clinical management of patients. The variables were obtained at public health primary care units by previously trained staff using a standardized questionnaire, a laboratory test, and a standard ECG evaluation. Consequently, the proposed score can be applied in endemic areas and may provide insights into how patient care and healthcare resources can be optimized. Appropriate risk stratification of patients in this limited‐resource setting would minimize unnecessary referrals to distant tertiary centers. Moreover, identification of high‐risk patients is useful for prioritizing interventions, including devices or cardiac transplantation, and for close follow‐up with optimization of medical treatment. Although many infected individuals moved from rural zones to cities and to other countries, most patients still live in small towns and rural areas. Martins‐Melo et al12 studied the spatial distribution of the mortality rate in Brazil and highlighted that 24 municipalities concentrated 3% of deaths from Chagas disease, and most were small towns with <40 000 inhabitants.
Given the lack of a health service structure in remote areas, the ECGs were analyzed in a central reading unit supported by Telehealth Network of Minas Gerais. Indeed, given wide availability and low cost, ECG is the single most important tool for Chagas disease risk stratification.16, 17, 39 In this score, we included 2 easy‐to‐obtain ECG variables derived from simple measurements: QRS duration and heart rate. A wide QRS, which encompasses intraventricular conduction delay of either right or left bundle‐branch block,16, 32, 34 was a predictor of mortality but only for values ≥150 ms, which is consistent with the cutoff QRS duration used for recognizing those patients who benefit more from cardiac resynchronization in general cardiology. Heart rate was also an independent predictor of mortality in our population. In particular, the risk of mortality increased with increasing heart rate in a linear relationship, but significantly increased risk was observed at 80 beats/min. In agreement with our results, a recent meta‐analysis of prospective cohort studies in the general population showed that patients with a resting heart rate >80 beats/min had a relative risk of 1.45 (95% CI, 1.34–1.57) for all‐cause mortality.40
Second, the score was based on key factors associated with increased risk of mortality. The natriuretic peptides levels, either BNP or NT‐proBNP, increase in parallel with the degree of left ventricular dysfunction and hemodynamic stress.41 Furthermore, the value of natriuretic peptides as reliable prognostic markers in various cardiac conditions is well documented.42 In the setting of Chagas disease, the elevation of natriuretic peptide concentration was associated with left ventricular dysfunction.43, 44 A previous study including 1398 participants from an endemic area showed that elevated levels of BNP predicted total and stroke mortality in elderly T cruzi–infected patients.45 The advantage of using NT‐proBNP is the ease of obtaining the blood sample; processing can be performed up to 24 hours after collection, allowing transportation from remote areas. The present highlights the powerful prognostic value of NT‐proBNP, especially in remote areas where echocardiography is not available. Interestingly, a previous study showed that natriuretic peptide concentrations provide incremental prognostic information in heart failure patients regardless of left ventricular ejection fraction assessed by echocardiography.46
Third, the score was validated in an external cohort of patients from endemic areas. However, the derivation and validation cohorts differed in terms of the predictors incorporated in the risk score, which may explain the smaller rate of death among high‐risk patients in the validation cohort. The score model does not estimate noncardiovascular mortality that may be prevalent in the validation population with elderly patients. Furthermore, the patient characteristics included in the validation cohort indicate less severity of Chagas heart disease. The majority were asymptomatic, without ECG changes related to Chagas disease, with only minor ECG abnormalities in 12% of this population. In addition, we used BNP in the validation cohort to replace NT‐proBNP measured in the derivation cohort. Although a systematic review comparing BNP with NTproBNP in the setting of heart failure showed no significant difference between the natriuretic peptides,47 higher‐than‐normal levels of BNP are often found in elderly patients and were more prevalent in the validation cohort without overt heart failure. Therefore, despite important differences between the derivation and validation sets, the score showed good discrimination for predicting death in the external population of patients with Chagas disease with similar living conditions.
Our study has some limitations. The first limitation is related to the population used for external validation, which may be not the most appropriate. Patients with different characteristics, especially older patients, may have contributed for the differences in mortality rates among high‐risk patients in the validation cohort compared with the derivation cohort. However, to perform external validation, the derivation and validation cohorts rarely have identical risk stratifications. It would be relevant to point out that internal validation using bootstrapping methods was performed, which provided results that corroborated those from the external validation. Future external validation may be needed to confirm our findings.
A second limitation refers to the unavailability of BNP or NT‐proBNP in the limited‐resource areas. Indeed, the Brazilian Universal Health System only recently incorporated natriuretic peptides in the row of complementary exams available in primary health care, and it is possible that this examination is not easily available in the rural areas of other Latin American countries. However, natriuretic peptide testing has been increasingly used in clinical practice, is associated with improved patient outcomes and important cost‐savings,48 and should be available in all healthcare settings.49 Third, although previous treatment with benznidazole has been shown to be a powerful protective factor in this sample,23 we decided not to include this variable in our model because the effectiveness of the specific treatment has been a controversial point, with negative results in more advanced cases and large regional heterogeneity in the response to treatment.50 Finally, our outcome is death from all cases, which, in our original sample, was mostly related to Chagas disease. It would be useful to have different prognostic scores for deaths from different mechanisms, such as sudden cardiac death and progressive heart failure, to guide the use of specific therapies like implantable cardiodefibrillators or cardiac transplantation. In addition, because the score was developed in patients infected with T cruzi II, which is the prevalent type in Brazil, the definitive validation of this score will require applying it in other regions affected by other subtypes of T cruzi.
In conclusion, a risk score to predict death in 2 years was developed and validated, including older age, abnormal NT‐proBNP by age cutoff values, long QRS duration, and higher heart rate. This score could be used by physicians who work in primary care areas, predicting individual risks and directing appropriate therapy.
Sources of Funding
The SaMi‐Trop cohort study is supported by the National Institutes of Health (P50 AI098461‐02 and U19AI098461‐06), the Brazilian National Research Council, CNPq (467043/2014‐0 and 310679/2016‐8), and the Minas Gerais State Research Agency, FAPEMIG (REDE 018‐14 and PPM 00428‐17). Di Lorenzo Oliveira is a postdoctoral student from the Institute of Tropical Medicine/University of São Paulo.
Disclosures
None.
Acknowledgments
The authors thank all SaMi‐Trop patients, the Bambui study population, and the health team in each municipality for their valuable contribution to this study. We thank John Carpenter (Ribeirão Preto, SP, Brazil) for the English‐language revision.
(J Am Heart Assoc. 2020;9:e014176 DOI: 10.1161/JAHA.119.014176.)
References
- 1. Mondiale O. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;6:33–43. [PubMed] [Google Scholar]
- 2. Bonney KM. Chagas disease in the 21st century: a public health success or an emerging threat? Parasite. 2014;21:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Requena‐Mendez A, Aldasoro E, de Lazzari E, Sicuri E, Brown M, Moore DA, Gascon J, Munoz J. Prevalence of Chagas disease in Latin‐American migrants living in Europe: a systematic review and meta‐analysis. PLoS Negl Trop Dis. 2015;9:e0003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gascon J, Bern C, Pinazo M‐J. Chagas disease in Spain, the United States and other non‐endemic countries. Acta Trop. 2010;115:22–27. [DOI] [PubMed] [Google Scholar]
- 5. Sabino EC, Ribeiro AL, Salemi VM, Di Lorenzo Oliveira C, Antunes AP, Menezes MM, Ianni BM, Nastari L, Fernandes F, Patavino GM, Sachdev V, Capuani L, de Almeida‐Neto C, Carrick DM, Wright D, Kavounis K, Goncalez TT, Carneiro‐Proietti AB, Custer B, Busch MP, Murphy EL. Ten‐year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi‐seropositive former blood donors. Circulation. 2013;127:1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverria LE, Dutra WO, Gascon J, Morillo CA, Oliveira‐Filho J. Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American Heart Association. Circulation. 2018;138:e169–e209. [DOI] [PubMed] [Google Scholar]
- 7. Capuani L, Bierrenbach AL, Pereira Alencar A, Mendrone A Jr, Ferreira JE, Custer B, P Ribeiro AL, Cerdeira Sabino E. Mortality among blood donors seropositive and seronegative for Chagas disease (1996–2000) in São Paulo, Brazil: a death certificate linkage study. PLoS Negl Trop Dis. 2017;11:e0005542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cucunubá ZM, Okuwoga O, Basáñez M‐G, Nouvellet P. Increased mortality attributed to Chagas disease: a systematic review and meta‐analysis. Parasit Vectors. 2016;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis. 2013;13:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hotez PJ, Alvarado M, Basanez MG, Bolliger I, Bourne R, Boussinesq M, Brooker SJ, Brown AS, Buckle G, Budke CM, Carabin H, Coffeng LE, Fevre EM, Furst T, Halasa YA, Jasrasaria R, Johns NE, Keiser J, King CH, Lozano R, Murdoch ME, O'Hanlon S, Pion SD, Pullan RL, Ramaiah KD, Roberts T, Shepard DS, Smith JL, Stolk WA, Undurraga EA, Utzinger J, Wang M, Murray CJ, Naghavi M. The Global Burden of Disease Study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8:e2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Viotti R, Vigliano CA, Alvarez MG, Lococo BE, Petti MA, Bertocchi GL, Armenti AH. The impact of socioeconomic conditions on chronic Chagas disease progression. Rev Esp Cardiol. 2009;62:1224–1232. [DOI] [PubMed] [Google Scholar]
- 12. Martins‐Melo FR, Ramos AN Jr, Alencar CH, Lange W, Heukelbach J. Mortality of Chagas’ disease in Brazil: spatial patterns and definition of high‐risk areas. Trop Med Int Health. 2012;17:1066–1075. [DOI] [PubMed] [Google Scholar]
- 13. Rassi A Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, Rassi GG, Hasslocher‐Moreno A, Sousa AS, Scanavacca MI. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med. 2006;355:799–808. [DOI] [PubMed] [Google Scholar]
- 14. Cardoso CS, Sabino EC, Oliveira CD, de Oliveira LC, Ferreira AM, Cunha‐Neto E, Bierrenbach AL, Ferreira JE, Haikal DS, Reingold AL, Ribeiro AL. Longitudinal study of patients with chronic Chagas cardiomyopathy in Brazil (SaMi‐Trop project): a cohort profile. BMJ Open. 2016;6:e011181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alkmim MB, Figueira RM, Marcolino MS, Cardoso CS, Pena de Abreu M, Cunha LR, da Cunha DF, Antunes AP, Resende AG, Resende ES, Ribeiro AL. Improving patient access to specialized health care: the Telehealth Network of Minas Gerais, Brazil. Bull World Health Organ. 2012;90:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ribeiro AL, Marcolino MS, Prineas RJ, Lima‐Costa MF. Electrocardiographic abnormalities in elderly Chagas disease patients: 10‐year follow‐up of the Bambui Cohort Study of Aging. J Am Heart Assoc. 2014;3:e000632 DOI: 10.1161/JAHA.113.000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rojas LZ, Glisic M, Pletsch‐Borba L, Echeverria LE, Bramer WM, Bano A, Stringa N, Zaciragic A, Kraja B, Asllanaj E, Chowdhury R, Morillo CA, Rueda‐Ochoa OL, Franco OH, Muka T. Electrocardiographic abnormalities in Chagas disease in the general population: a systematic review and meta‐analysis. PLoS Negl Trop Dis. 2018;12:e0006567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG, Cohen‐Solal A, Dahlstrom U, DeMaria A, Di Somma S, Filippatos GS, Fonarow GC, Jourdain P, Komajda M, Liu PP, McDonagh T, McDonald K, Mebazaa A, Nieminen MS, Peacock WF, Tubaro M, Valle R, Vanderhyden M, Yancy CW, Zannad F, Braunwald E. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. [DOI] [PubMed] [Google Scholar]
- 19. Marcolino MS, Palhares DM, Ferreira LR, Ribeiro AL. Electrocardiogram and Chagas disease: a large population database of primary care patients. Glob Heart. 2015;10:167–172. [DOI] [PubMed] [Google Scholar]
- 20. Collaborators GBDCoD . Global, regional, and national age‐sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lima‐Costa MF, Firmo JO, Uchoa E. Cohort profile: the Bambui (Brazil) Cohort Study of Ageing. Int J Epidemiol. 2011;40:862–867. [DOI] [PubMed] [Google Scholar]
- 22. Lima‐Costa MF, Cesar CC, Peixoto SV, Ribeiro ALP. Plasma β‐type natriuretic peptide as a predictor of mortality in community‐dwelling older adults with Chagas disease: 10‐year follow‐up of the Bambuí Cohort Study of Aging. Am J Epidemiol. 2010;172:190–196. [DOI] [PubMed] [Google Scholar]
- 23. Cardoso CS, Ribeiro ALP, Oliveira CDL, Oliveira LC, Ferreira AM, Bierrenbach AL, Silva JLP, Colosimo EA, Ferreira JE, Lee T‐H. Beneficial effects of benznidazole in Chagas disease: NIH SaMi‐Trop cohort study. PLoS Negl Trop Dis. 2018;12:e0006814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 25. Nunes MC, Dones W, Morillo CA, Encina JJ, Ribeiro AL; Council on Chagas Disease of the Interamerican Society of C . Chagas disease: an overview of clinical and epidemiological aspects. J Am Coll Cardiol. 2013;62:767–776. [DOI] [PubMed] [Google Scholar]
- 26. Nunes MC, Carmo AA, Rocha MO, Ribeiro AL. Mortality prediction in Chagas heart disease. Expert Rev Cardiovasc Ther. 2012;10:1173–1184. [DOI] [PubMed] [Google Scholar]
- 27. Rassi A Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115:1101–1108. [DOI] [PubMed] [Google Scholar]
- 28. Espinosa R, Carrasco HA, Belandria F, Fuenmayor AM, Molina C, González R, Martínez O. Life expectancy analysis in patients with Chagas’ disease: prognosis after one decade (1973–1983). Int J Cardiol. 1985;8:45–56. [DOI] [PubMed] [Google Scholar]
- 29. Hagar JM, Rahimtoola SH. Chagas’ heart disease in the United States. N Engl J Med. 1991;325:763–768. [DOI] [PubMed] [Google Scholar]
- 30. Espinosa RA, Pericchi LR, Carrasco HA, Escalante A, Martinez O, Gonzalez R. Prognostic indicators of chronic chagasic cardiopathy. Int J Cardiol. 1991;30:195–202. [DOI] [PubMed] [Google Scholar]
- 31. Mady C, Cardoso RH, Barretto AC, da Luz PL, Bellotti G, Pileggi F. Survival and predictors of survival in patients with congestive heart failure due to Chagas’ cardiomyopathy. Circulation. 1994;90:3098–3102. [DOI] [PubMed] [Google Scholar]
- 32. Carrasco HA, Parada H, Guerrero L, Duque M, Duran D, Molina C. Prognostic implications of clinical, electrocardiographic and hemodynamic findings in chronic Chagas’ disease. Int J Cardiol. 1994;43:27–38. [DOI] [PubMed] [Google Scholar]
- 33. Bestetti RB, Dalbo CM, Arruda CA, Correia Filho D, Freitas OC. Predictors of sudden cardiac death for patients with Chagas’ disease: a hospital‐derived cohort study. Cardiology. 1996;87:481–487. [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez‐Salas LA, Klein E, Acquatella H, Catalioti F, Davalos VV, Gomez‐Mancebo JR, Gonzalez H, Bosch F, Puigbo JJ. Echocardiographic and clinical predictors of mortality in chronic Chagas’ disease. Echocardiography. 1998;15:271–278. [DOI] [PubMed] [Google Scholar]
- 35. Viotti RJ, Vigliano C, Laucella S, Lococo B, Petti M, Bertocchi G, Ruiz Vera B, Armenti H. Value of echocardiography for diagnosis and prognosis of chronic Chagas disease cardiomyopathy without heart failure. Heart. 2004;90:655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ribeiro AL, Cavalvanti PS, Lombardi F, Nunes Mdo C, Barros MV, Rocha MO. Prognostic value of signal‐averaged electrocardiogram in Chagas disease. J Cardiovasc Electrophysiol. 2008;19:502–509. [DOI] [PubMed] [Google Scholar]
- 37. de Souza AC, Salles G, Hasslocher‐Moreno AM, de Sousa AS, Alvarenga Americano do Brasil PE, Saraiva RM, Xavier SS. Development of a risk score to predict sudden death in patients with Chaga's heart disease. Int J Cardiol. 2015;187:700–704. [DOI] [PubMed] [Google Scholar]
- 38. Morillo CA, Marin‐Neto JA, Avezum A, Sosa‐Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med. 2015;373:1295–1306. [DOI] [PubMed] [Google Scholar]
- 39. Maguire JH, Hoff R, Sherlock I, Guimaraes AC, Sleigh AC, Ramos NB, Mott KE, Weller TH. Cardiac morbidity and mortality due to Chagas’ disease: prospective electrocardiographic study of a Brazilian community. Circulation. 1987;75:1140–1145. [DOI] [PubMed] [Google Scholar]
- 40. Zhang D, Shen X, Qi X. Resting heart rate and all‐cause and cardiovascular mortality in the general population: a meta‐analysis. CMAJ. 2016;188:E53–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Di Angelantonio E, Chowdhury R, Sarwar N, Ray KK, Gobin R, Saleheen D, Thompson A, Gudnason V, Sattar N, Danesh J. B‐type natriuretic peptides and cardiovascular risk: systematic review and meta‐analysis of 40 prospective studies. Circulation. 2009;120:2177–2187. [DOI] [PubMed] [Google Scholar]
- 43. Ribeiro AL, dos Reis AM, Barros MV, de Sousa MR, Rocha AL, Perez AA, Pereira JB, Machado FS, Rocha MO. Brain natriuretic peptide and left ventricular dysfunction in Chagas’ disease. Lancet. 2002;360:461–462. [DOI] [PubMed] [Google Scholar]
- 44. Talvani A, Rocha MO, Cogan J, Maewal P, de Lemos J, Ribeiro AL, Teixeira MM. Brain natriuretic peptide and left ventricular dysfunction in chagasic cardiomyopathy. Mem Inst Oswaldo Cruz. 2004;99:645–649. [DOI] [PubMed] [Google Scholar]
- 45. Lima‐Costa MF, Matos DL, Ribeiro AL. Chagas disease predicts 10‐year stroke mortality in community‐dwelling elderly: the Bambui Cohort Study of Aging. Stroke. 2010;41:2477–2482. [DOI] [PubMed] [Google Scholar]
- 46. van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B‐type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. [DOI] [PubMed] [Google Scholar]
- 47. Clerico A, Fontana M, Zyw L, Passino C, Emdin M. Comparison of the diagnostic accuracy of brain natriuretic peptide (BNP) and the N‐terminal part of the propeptide of BNP immunoassays in chronic and acute heart failure: a systematic review. Clin Chem. 2007;53:813–822. [DOI] [PubMed] [Google Scholar]
- 48. Athanasakis K, Arista I, Balasopoulos T, Boubouchairopoulou N, Kyriopoulos J. How peptide technology has improved costs and outcomes in patients with heart failure. Expert Rev Pharmacoecon Outcomes Res. 2016;16:371–382. [DOI] [PubMed] [Google Scholar]
- 49. Figal DAP, Casademont J, Lobos JM, Piñera P, Bayes‐Genis A, González‐Juanatey JR. Natriuretic peptides: consensus call for use. Rev Esp Cardiol. 2016;69:817–819. [DOI] [PubMed] [Google Scholar]
- 50. Pérez‐Molina JA, Pérez‐Ayala A, Moreno S, Fernandez‐Gonzalez MC, Zamora J, Lopez‐Velez R. Use of benznidazole to treat chronic Chagas’ disease: a systematic review with a meta‐analysis. J Antimicrob Chemother. 2009;64:1139–1147. [DOI] [PubMed] [Google Scholar]


