Abstract
Background
Ultrasound (US) guidance provides the unique opportunity to control the puncture zone of the artery during transfemoral transcatheter aortic valve replacement and may decrease major vascular complications (VC) and life‐threatening or major bleeding complications. This study aimed to evaluate the clinical impact of US guidance using a propensity score–matched comparison.
Methods and Results
US guidance was implemented as the default approach for all transfemoral transcatheter aortic valve replacement cases in our institution in June 2013. We defined 3 groups of consecutive patients according to the method of puncture (fluoroscopic/US guidance) and the use of a transcatheter heart valve. Patients in the US‐guided second‐generation group (Sapien XT [Edwards Lifesciences, Irvine, CA], Corevalve [Medtronic, Dublin, Ireland]) were successfully 1:1 matched with patients in the fluoroscope‐guided second‐generation group (n=95) with propensity score matching. In a second analysis we described the consecutive patients of the US‐guided third‐generation group (Evolut‐R [Medtronic], Sapien 3 [Edwards Lifesciences], n=308). All vascular and bleeding complications were reduced in the US‐guided second‐generation group compared with the fluoroscope‐guided second‐generation group: VC (16.8% versus 6.3%; P=0.023); life‐threatening or major bleeding (22.1% versus 6%; P=0.004); and VC related to vascular access (12.6% versus 4.2%; P=0.052). In the US‐guided third‐generation group the rates of major VC and life‐threatening or major bleeding were 3.2% (95% CI, 1.6% to 5.9%) and 3.6% (95% CI, 1.8% to 6.3%). In the overall population (n=546), life‐threatening or major bleeding was associated with a 1.7‐fold increased mortality risk (P=0.02).
Conclusions
We demonstrated that US guidance effectively reduced VC and bleeding complications for transfemoral transcatheter aortic valve replacement and should be considered the standard puncture method.
Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02628509.
Keywords: bleeding, fluoroscopy, transcatheter aortic valve replacement, ultrasound, vascular complications
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Ultrasound, Catheter-Based Coronary and Valvular Interventions
Clinical Perspective
What Is New?
Data on the impact of ultrasound guidance for percutaneous femoral artery puncture in transcatheter aortic valve replacement are scarce.
This study is the first to use propensity score matching to show the potential strong clinical benefit of ultrasound guidance for the large‐bore vascular access required in percutaneous transfemoral transcatheter aortic valve replacement.
What Are the Clinical Implications?
These data support the concept of implementing ultrasound guidance as the standard puncture method for transcatheter aortic valve replacement or other percutaneous procedures requiring large‐bore access.
Nonstandard Abbreviations and Acronyms.
AF atrial fibrillation
ASD absolute standardized difference
AVA aortic valve area
BMI body mass index
CAD coronary artery disease
CFA common femoral artery
COPD chronic obstructive pulmonary disease
HR hazard ratio
IL inguinal ligament
IQR interquartile range
LT/MB life‐threatening or major bleeding
LVEF left ventricular ejection fraction
NYHA New York Heart Association
OR odds ratio
PAD peripheral artery disease
PFA profound femoral artery
RBC red blood cells
SE‐valve self‐expandable valve
SFA superficial femoral artery
SFAR sheath‐to–femoral artery ratio
STS‐PROM Society of Thoracic Surgeons Predicted Risk of Mortality
TAVR transcatheter aortic valve replacement
TF transfemoral
THV transcatheter heart valve
TIA transient ischemic attack
US ultrasound
VARC Valve Academic Consortium
VC vascular complications
Introduction
Percutaneous transfemoral transcatheter aortic valve replacement (TF‐TAVR) has changed the treatment of severe aortic valve stenosis and is a safe, efficient, and reproducible procedure. Numerous factors such as a smaller delivery system, broader use of percutaneous closure system, better patient selection, and increased operator experience have contributed to a reduction in vascular complications (VC). However, even the latest‐generation transcatheter heart valve (THV) requires large‐bore access, and major VC or life‐threatening (LT) bleeding represents the most frequent adverse outcome.1 Several studies demonstrated an increased risk of mortality associated with major VC2 and bleeding complications or transfusions.3 Thus, precise cannulation of the common femoral artery (CFA) is extremely important to secure delivery sheath insertion, ensure the appropriate deployment of vascular closure devices, reduce access‐site complications, and improve clinical outcomes. Fluoroscopic guidance remains the most frequently used method for CFA cannulation in TAVR as initially described, taught, and recommended in the early TAVR era.4 However, ultrasound (US) guidance might offer a substantial benefit to cannulating arterial access because it confers the unique opportunity to obtain real‐time valuable anatomic information about localization of the femoral bifurcation or the presence of anterior wall calcification. This enables control of the needle's path and angle during puncture in the ideal central and calcium‐free horizontal segment of the CFA. Randomized clinical trials have already demonstrated the superiority of US guidance for the cannulation of central venous access to VC.5 Before the present work only 1 small observational study reported a benefit of US versus fluoroscopic guidance on a composite end point combining transfusion and vascular and bleeding complications without the use of a propensity score–matching methodology.6 The scarcity of data in structural procedures requiring large‐bore arterial access could explain the lack of adoption of US guidance.7
The present study aimed to evaluate the impact of the implementation of real‐time US guidance for TF‐TAVR on individual vascular and bleeding complications using a propensity score‐matching comparison.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
TAVR was first offered at our institution in 2008. US guidance for vascular access was implemented as the default approach in June 2013 for all percutaneous TF‐TAVR and used by all operators after a short training course.
Thus, we defined the 3 periods and groups of consecutive patients according to the percutaneous puncture method (fluoroscopic or US guidance) and the THV generation (second or third generation) (Figure 1).
US‐guided second‐generation group. TF‐TAVR with second‐generation THV (Sapien XT [Edwards Lifesciences], Corevalve [Medtronic]) and performed under US guidance. This group refers to the period from June 2013 to November 2014 (n=119).
Fluoroscopy‐guided second‐generation group. The last (n=119) TF‐TAVR with second‐generation THV and under fluoroscopic guidance (January 2012 to June 2013). Patients treated before January 2012 were not included to mitigate the effects of the learning‐curve phase.
US‐guided third‐generation group. TF‐TAVR with third‐generation THV (Sapien 3 [Edwards Lifesciences], Evolut‐R [Medtronic]) from November 2014 to December 2018 (n=308).
Figure 1.
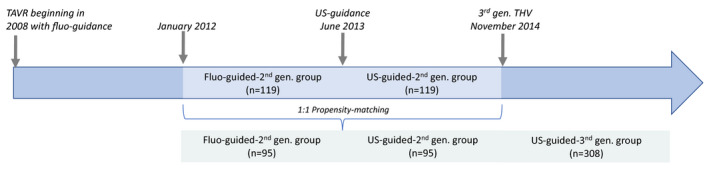
Study design.
Fluo indicates fluoroscope; gen., generation; TAVR, transcatheter aortic valve replacement; THV, transcatheter heart valve; and US, ultrasound.
In the main analysis we compared the outcomes of patients from the US‐guided second‐generation group and from the fluoroscope‐guided second‐generation group using propensity score–matching based on parameters previously described as being associated with TAVR outcomes including vascular or bleeding complications (Data S1).
In the secondary analysis, we described consecutive patients of the US‐guided third‐generation group.
Data Collection and Management
All adverse events were assessed according to VARC‐2 (Valve Academic Consortium‐2) classification.8 Procedural 30‐day outcomes and yearly follow‐up were documented in all patients. No external funding was obtained to support the study. The WITAVI (Von Willebrand Factor As a Biological Sensor of Blood Flow in Percutaneous Cardiac Procedure) registry protocol was approved by local ethics committee (NCT02628509). All patients were included in the registry after providing written informed consent.
Procedural Management
At our institution all patients received an aspirin loading dose of 250 mg before the procedure with ongoing aspirin therapy after the procedure. Clopidogrel was not administered unless the patient was already receiving clopidogrel treatment. No clopidogrel loading was done. None of the patients received ticagrelor or prasugrel. Oral anticoagulation was systematically discontinued before the procedure and restarted after TAVR. The patients underwent percutaneous TF‐TAVR procedures as previously reported.9 Vascular preclosing technique with Perclose Proglide device (Abbott, Lake Forest, IL) was systematic for all the TF‐TAVR in our institution. The sheath‐to–femoral artery ratio was calculated from the femoral artery diameters obtained from multidetector computed tomography and the prespecified inner sheath dimensions.
Vascular Access in the US‐Guided Second‐ and Third‐Generation Groups
The CFA was punctured under US guidance. The US probe was placed in a transparent sterile sheath. An ultrasound survey in basic 2‐dimensional mode was performed to assess vessel anatomy and determine the exact location of the femoral artery bifurcation. The ideal cannulation site was identified as the middle of the anterior wall of a calcium‐free horizontal segment of the CFA with a diameter superior to the sheath. The vessels were punctured on the longitudinal (long‐axis) and/or transverse (short‐axis) view according to the operator's preference without a needle guide. The same operator held the probe with 1 hand while directing the needle in the other hand (1‐person technique). The needle's path was visualized as a hyperechoic point (short‐axis) or a hyperechoic shaft (long‐axis) until the vessel was entered. US guidance was used for main and secondary arterial access and venous access. Veins were typically identified as more easily compressible and pulseless vessels (Figure 2).
Figure 2.
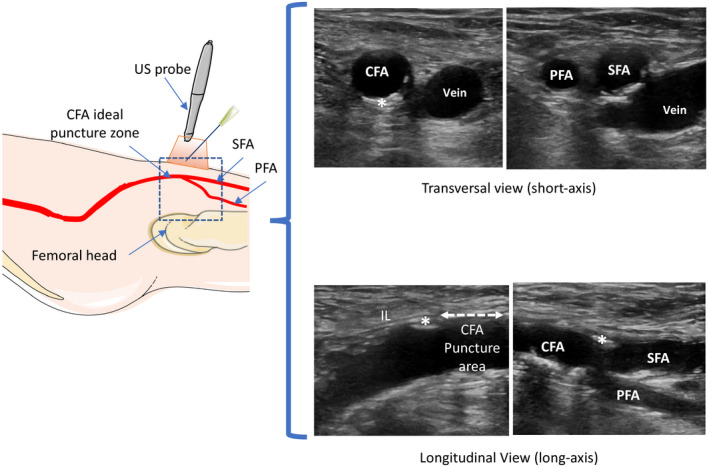
Schematic representation of ultrasound (US) survey required to localize the femoral bifurcation between the superficial femoral artery (SFA) and the profound femoral artery (PFA) to determine the ideal puncture zone: below the inguinal ligament (IL) (parallel strands of echogenic fibers), in the middle of the noncalcified anterior wall, and in the horizontal segment of the common femoral artery (CFA).
Imaging in longitudinal (long‐axis) and/or transverse (short‐axis) views. *Calcification.
Vascular Access in the Fluoroscope‐Guided Second‐Generation Group
The CFA was punctured under fluoroscopic guidance. First, the secondary femoral access was cannulated after pulse palpation and identification of external anatomical landmarks and/or fluoroscopic guidance with the aim of hitting the artery above the middle of the femoral head. A pigtail catheter was positioned to the contralateral iliac artery using the crossover technique to opacify the femoral bifurcation of the main access. The appropriate calcium‐free puncture zone was chosen, and the needle trajectory was performed under fluoroscopic guidance as previously described.10
Clinical Outcome
Clinical follow‐up was obtained in all patients at a median 19 months (interquartile range, 13–29).
All procedural and 30‐day end points (VC and bleeding) were adjudicated according to the VARC‐2 definitions by 2 independent cardiologists not involved in the procedure.8
To specifically address the complications associated with the cannulation technique, we further restricted the major VC to access‐site major VC (major VC excluding aortic dissection, annulus rupture, aortic rupture, and left ventricle perforation). Cutaneous bleeding that required prolonged manual compression was not considered bleeding if no hematoma was observed.
Clinical follow‐up data during the first 30 days after TAVR and vital status at 2 years were obtained for all patients.
Statistical Analyses
Full details are available in Data S1. We assessed the effect of US guidance on vascular and bleeding complications and procedural outcomes after considering the potential confounding factors by using a prespecified propensity‐score method.11, 12 In the primary analysis a propensity score was used to assemble well‐balanced groups (propensity score–matched cohorts); in the sensitivity analysis, propensity scoring was used to weight each subject using the inverse probability of treatment weighting (stabilized inverse propensity score as weight) and generate an inverse probability of treatment weighting cohort. The propensity score was estimated using a nonparsimonious multivariate logistic regression model with the treatment group as the dependent variable; all of the characteristics are listed in Table as covariates. Patients from the US‐guided second‐generation group were matched 1:1 to patients in the fluoroscope‐guided second‐generation group according to the propensity score using the greedy nearest neighbor–matching algorithm with a caliper width of 0.2 SD of the logit of the propensity score.12, 13
Table 1.
Baseline and Procedural Characteristics Before and After Propensity‐Score Matching
| Before Propensity‐Score Matching | After Propensity‐Score Matching | |||||
|---|---|---|---|---|---|---|
| Fluoroscope‐Guided Second‐Generation Group(n=119) | US‐Guided Second‐Generation Group(n=119) | ASD (%) | Fluoroscope‐Guided Second‐Generation Group(n=95) | US‐Guided Second‐Generation Group(n=95) | ASD (%) | |
| Clinical characteristics | ||||||
| Age, y | 81.9 (5.3) | 81.7 (7.6) | 4.3 | 81.7 (5.4) | 82.1 (6.9) | 1.7 |
| Female sex | 65 (54.6) | 74 (62.2) | 15.4 | 54 (56.8) | 59 (62.1) | 10.2 |
| BMI, kg/m2 | 27.0 (5.0) | 26.8 (5.0) | 4.3 | 27.0 (4.9) | 27.1 (4.8) | 8.5 |
| NYHA class III or IV | 77 (64.7) | 67 (56.3) | 17.2 | 59 (62.1) | 60 (63.2) | 7.1 |
| STS‐PROM (%) |
5.3 (4.4–7.4) 6.2 (3.1) |
5.3 (4.3–6.8) 6.2 (4) |
5.6 | 5.3 (4.4–7.5) | 5.4 (4.4–7.0) | 4 |
| Anticoagulant | 30 (25.2) | 32 (26.9) | 3.8 | 24 (25.3) | 29 (30.5) | 13.7 |
| Comorbidities | ||||||
| Diabetes mellitus | 34 (28.6) | 39 (32.8) | 9.1 | 28 (29.5) | 31 (32.6) | 5.8 |
| Hypertension | 94 (79.0) | 92 (77.3) | 4.1 | 74 (77.9) | 77 (81.1) | 7.8 |
| CAD | 68 (57.1) | 61 (51.3) | 11.8 | 51 (53.7) | 50 (52.6) | 3.1 |
| Prior stroke/TIA | 12 (10.1) | 17 (14.3) | 12.9 | 9 (9.5) | 15 (15.8) | 19.7 |
| COPD | 45 (37.8) | 38 (31.9) | 12.4 | 36 (37.9) | 32 (33.7) | 10.1 |
| PAD | 40 (33.6) | 36 (30.3) | 7.2 | 30 (31.6) | 33 (34.7) | 7.2 |
| Prior AF | 39 (32.8) | 46 (38.7) | 12.3 | 24 (25.3) | 26 (27.4) | 4.7 |
| Creatinine >2 mg/dL | 3 (2.5) | 6 (5.0) | 13.2 | 3 (3.2) | 4 (4.2) | |
| Echography parameters | ||||||
| LVEF (%) | 60.0 (50.0–65.0) | 60.0 (50.0–60.0) | 14.3 | 60.0 (50.0–65.0) | 60.0 (50.0–60.0) | 8.6 |
| AVA, cm2 | 0.7 (0.2) | 0.7 (0.2) | 16.9 | 0.7 (0.2) | 0.7 (0.2) | 20.2 |
| Mean aortic gradient, mm Hg | 47.3 (15.7) | 45.6 (14.8) | 11.2 | 46.7 (15.4) | 46.3 (14.4) | 4.1 |
| Procedural characteristics | ||||||
| Ongoing clopidogrel | 43 (36.1) | 34 (28.6) | 16.2 | 31 (32.6) | 30 (31.6) | 1.9 |
| Main access sheath size | 18.0 (18.0–18.0) | 18.0 (18.0–18.0) | 26.1 | 18.0 (18.0–18.0) | 18.0 (18.0–18.0) | 24.0 |
| Secondary access sheath size | 7.0 (6.0–7.0) | 6.0 (6.0–7.0) | 36.8 | 7.0 (6.0–7.0) | 6.0 (6.0–7.0) | 29.1 |
| Perclose vascular closure system | 100 | 100 | 0 | 100 | 100 | 0 |
| SE‐valve | 26 (21.8) | 23 (19.3) | 6.2 | 18 (18.9) | 23 (24.2) | 12.8 |
| THV size | ||||||
| 23 mm | 21 (17.6) | 43 (36.1) | 17 (17.9) | 32 (33.7) | ||
| 26 mm | 82 (68.9) | 63 (52.9) | 68 (71.6) | 51 (53.7) | ||
| 29 mm | 11 (9.2) | 13 (10.9) | 6 (6.3) | 12 (12.6) | ||
| 31 mm | 5 (4.2) | 0 (0.0) | 4 (4.2) | 0 (0.0) | ||
| SFAR >1.05 | 19 (16.0) | 18 (15.1) | 2.3 | 13 (13.7) | 13 (13.7) | 1.9 |
AF indicates atrial fibrillation; ASD, absolute standardized difference; AVA, aortic valve area; BMI, body mass index (weight in kilograms divided by the square of the height in meters); CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PAD, peripheral artery disease; SE‐valve, self‐expandable valve (Corevalve, Medtronic); SFAR, sheath‐to–femoral artery ratio; STS‐PROM, Society of Thoracic Surgeons Predicted Risk of Mortality score; THV, transcatheter heart valve; and TIA, transient ischemic attack.
In the propensity score–matched cohort, intergroup comparisons were made using a generalized linear mixed model (binomial distribution, logit function) for binary outcomes or a linear mixed model for continuous outcomes by including matched blocks as random effects to account for the matched design. In the inverse probability of treatment weighting cohort, comparisons were made using logistic (binary outcomes) or a linear regression model using the stabilized inverse propensity score as the weight.
We also compared survival during follow‐up between the US‐guided second generation group and the fluoroscope‐guided second‐generation group in the matched cohort by using Cox regression models with a robust sandwich variance estimator to account for the matched design. Using the fluoroscope‐guided second‐generation group as the reference group, hazard ratio (HRs) and their 95% CIs were derived from these Cox regression models as treatment effect size measures. We assessed the proportional hazard assumption using Schoenfeld residuals plots.14 Survival during the first year between life‐threatening or bleeding complications was compared using the same methods.
Finally, the procedural outcomes and vascular and bleeding complications were described with 95% CIs in patients in the US‐guided third‐generation group. Statistical testing was conducted at the 2‐tailed α‐level of 0.05. Data were analyzed using SAS software (SAS Institute, Cary, NC) v 9.4.
Results
Impact of US Guidance on Vascular and Bleeding Complications
Among the 119 patients in the US‐guided second‐generation group, 95 were successfully propensity‐score matched in a 1:1 ratio with patients of fluoroscope‐guided second‐generation group, resulting in similar baseline clinical characteristics, echocardiographic parameters, and procedural parameters between the groups.
Patient characteristics by puncture method before and after propensity‐score matching and after handling missing values by multiple imputation are presented in Table. The distributions of propensity scores by treatments before matching are reported in Figure S1. In the propensity score–matched population, major VC occurred less frequently in the US‐guided second‐generation group than in the fluoroscope‐guided second‐generation group (6.3% versus 16.8%; odds ratio, 0.31; 95% CI, 0.12–0.85; P=0.02; Figure 3). This was also the case when the analysis was limited to major VC related to vascular access (4.2% versus 12.6%; odds ratio, 0.31; 95% CI, 0.1–1.0; P=0.05). The rate of LT/MB complications was also significantly reduced in the US‐guided second‐generation group (6.3% versus 22.1%; odds ratio, 0.24; 95% CI, 0.09–0.63; P<0.01). The details of the VC are presented in Figure 4. A reduction in mean fluoroscopic time and a concomitant significant reduction of the radiation dose assessed by the mean air kerma was also observed in the US‐guided second‐generation group (847±602 versus 569±365; odds ratio, −277; 95% CI, −435 to −120). No difference was seen in the volume of contrast injected or the rate of acute kidney injury (Figure 3).
Figure 3.
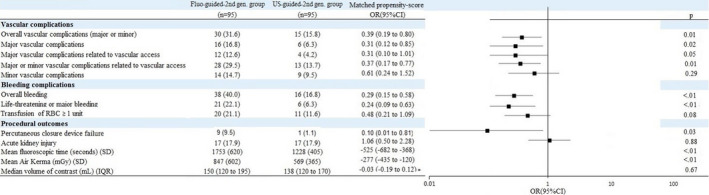
Vascular, bleeding, and periprocedural complications by fluoroscope‐guided or US‐guided vascular access.
Propensity score–matching analysis. IQR indicates interquartile range; OR, odds ratio; RBC, red blood cells; and US, ultrasound.
Figure 4.
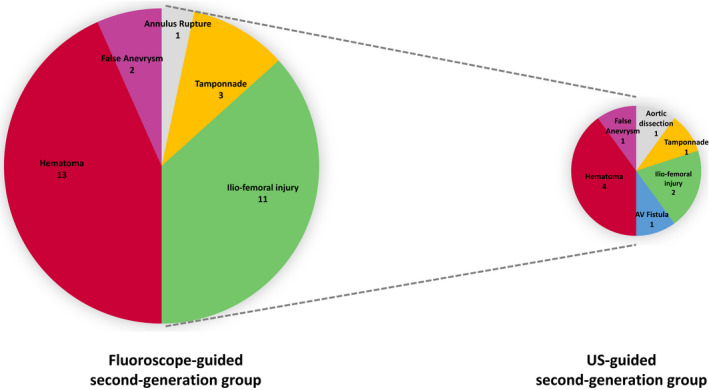
Details of major and minor vascular complications according to fluoroscope‐guided or US‐guided vascular access: Propensity score matching analysis (relative areas of each pies are proportional to the total number of complications). AV indicates arteriovenous; and US, ultrasound.
Similar treatment effect sizes were observed in the inverse probability of treatment weighting cohort, with differences in major VC, major VC related to vascular access, minor VC, overall bleeding, LT/MB, and transfusion of ≥1 unit of red blood cells that reached significance (Figure S2).
After propensity‐score matching, the 1‐year survival rate was 72% in the US‐guided second‐generation group and 70% in the fluoroscope‐guided second‐generation group. There was no intergroup difference in survival (HR, 0.68; 95% CI, 0.42–1.10; P=0.13). The procedural outcomes before propensity‐score matching in the unadjusted analyses are presented in Table S1. The 1‐year survival rate was 80% in the fluoroscope‐guided second‐generation group and 86% in the US‐guided second‐generation group. We observed a trend toward higher survival in the US‐guided second‐generation group (HR, 0.67; 95% CI, 0.44–1.00; P=0.05).
In all patients who underwent THV implantation in the second‐generation group, we observed a trend toward a higher 1‐year mortality associated with LT/MB complications (HR, 2.09; 95% CI, 0.98–4.42; P=0.05).
Vascular and Bleeding Complications With US Guidance in Third‐Generation THV
The main characteristics of the patients of the third‐generation group (n=308) are summarized in Table S2. Patients were a median 84 (interquartile range, 79.9–87.0) years of age and had a mean Society of Thoracic Surgeons score of 5.8%. Patients were predominantly female (56.8%); 43% were New York Heart Association class III/IV before TAVR. The Sapien 3 (Edwards Lifesciences) was implanted in 80.2% of the patients versus the Evolut‐R (Medtronic) in the other 18.8%.
The survival rate at 1 year was 86±2.3%. The US‐guided puncture achieved a rate of major VC of 3.2% (95% CI, 1.6% to 5.9%) and of LT/MB of 3.6% (95% CI, 1.8% to 6.3%) (Table S3).
Vascular/Bleeding Complications and Long‐Term Mortality in Overall Population
In the overall population (patients with THV of second and third generations, n=546), survival during the first year was strongly impaired by LT/MB complications (HR, 2.70; 95% CI, 1.43–5.09, P<0.01). The VC rates according to the type of guidance used and the generation of THV are presented in Figure 5.
Figure 5.
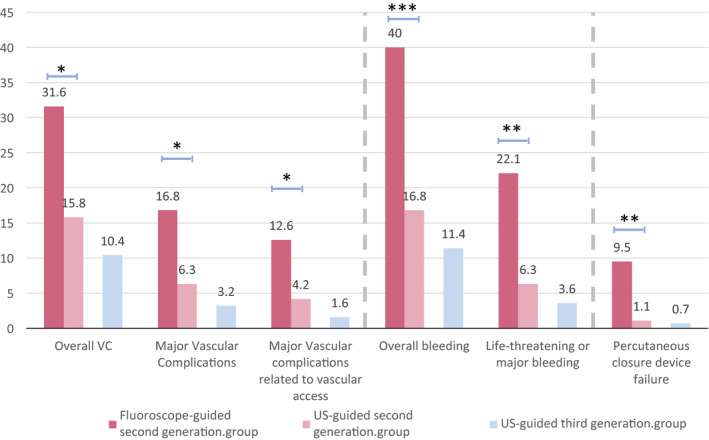
Vascular complications rate in the fluoroscope‐guided second‐generation (propensity score–matched), US‐guided second‐generation (propensity score–matched), and US‐guided third‐generation population.
*P<0.05; **P<0.01; ***P<0.001. US indicates ultrasound; and VC, vascular complications.
Discussion
The present study of a cohort of 546 patients is the largest to specifically evaluate the impact of US guidance on the incidence of individual VC and bleeding end points in TF‐TAVR.
The main result of the report is that US guidance is associated with a strong and significant reduction in the rate of major VC and major bleeding (Figure 3).
The TF approach currently represents more than 85% of TAVR procedures.15 Operator experience and the progressive reduction in the profiles of newer delivery systems have made TF‐TAVR safer, but major VC and LT/MB bleeding rates remain estimated at 4.6% (95% CI, 3.6% to 5.6%) and 12.1% (95% CI, 3.4% to 20.8%),1 representing 1 of the most frequent complications of TAVR procedures,16 impacting mortality at 30 days and 1 year.2, 17
Thus, obtaining adequate and safe vascular access is key to TAVR procedure success. Vascular access has been historically widely performed and taught using a combination of anatomical landmarks and fluoroscopic guidance via opacification of the CFA from the contralateral access (crossover technique).4, 10, 18 The potential benefit of US guidance over conventional fluoroscopic guidance is frequently claimed, but its impact has never been evaluated via propensity comparison matching until now.
Impact of US Guidance on Outcomes
The US‐ and fluoroscope‐guided second‐generation groups had demographics and procedural characteristics similar to those of the PARTNER‐2 cohorts (Sapien XT [Edwards Lifesciences] in intermediate‐ to high‐risk patients, PARTNER‐2A19 and in surgery‐prohibited patients, PARTNER‐2B).20 However, the implementation of US guidance in 2013 in our center decreased the VC by more than half to only 6.3% in the US‐guided second‐generation group, which is lower than the 8.5% and 9.5% reported in the PARTNER‐2A and 2B trials, respectively. As recommended by the VARC‐2, we detailed specifically the VC related to vascular access (excluding the VC occurring at a distance from the puncture zone) and showed a 69% reduction of these events directly related to arterial puncture quality.
In the same magnitude, US guidance had a huge impact on bleeding events with a reduction by more than half of the total bleeding complications (from 40% to 16.8%), including LT/MB episodes (from 22.1% to 6.3%), which is lower than the 10.4% and 25.3% rates reported in the PARTNER‐2A19 and ‐2B20 cohorts, respectively. In the THV of the third‐generation group, the rates of major bleeding and VC requiring surgical or percutaneous interventions were 8.9% and 7.7%, respectively, in the latest issue of the French registry.15 In our cohort of patients at intermediate surgical risk (Society of Thoracic Surgeons score, 5.8±3.5), US guidance achieved a very low rate of 3.2% (95% CI, 1.6% to 5.9%) of major VAC and 3.6% of LT/MB (95% CI, 1.8% to 6.3%), which is comparable to the remarkable outcomes achieved recently in the PARTNER‐3 trial in a very selected and low‐risk (Society of Thoracic Surgeons score, 1.8±0.5) population (2.2% major VC and 3.6% LT/MB rates).21
US Guidance in Interventional Procedures
US guidance is recommended by the international guidelines of anesthesia and intensive care medicine to secure the insertion of central venous catheters.5 This high‐level recommendation is the fruit of several randomized trials that have demonstrated the superiority of US guidance over the anatomical landmarks technique for the cannulation of central venous access to reduce VC and the number of attempts to improve the overall success rate while decreasing the cost of hospitalization with a very short learning curve.22
In interventional cardiology, although several opinion leaders and society guidelines23 supported the practice of US guidance for interventional procedures, especially in cases with technical difficulties,24, 25, 26 the data supporting this practice are scarce.
The FAUST (Femoral Arterial Access With Ultrasound Trial) randomized trial compared 1004 patients with respect to US versus fluoroscopic guidance for CFA cannulation in procedures requiring a small size sheath (mean size, 5.9 French). This study demonstrated only a small benefit of US guidance with an improved success rate at the first attempt and a reduction in time required for access, accidental venipuncture rates, and minor VC only (driven by a reduction of hematoma).27 The subanalysis of the SAFE‐PCI (Study of Access Site for Enhancement of PCI for Women) demonstrated that the use of micropuncture and ultrasonic localization was associated with the best access outcomes, as low as radial access.28 Concerning TAVR procedures, 1 observational study reported a reduction in the composite end point of transfusion and vascular and bleeding complications associated with US guidance.6 The lack of data demonstrating a strong impact of US guidance on individual end points could explain the suboptimal adoption of US guidance in routine practice. No data exist on structural procedure practices, but a recent international web survey reported that the use of US guidance combined with palpation and fluoroscopy to stick the CFA for coronary angiogram was low at 36% in North America.29 In 2010, another survey showed that only 13% of clinicians routinely used US for femoral access despite 88% answering that US was readily available in the cardiac catheterization laboratory.7
Assets of US Guidance for Structural Procedures
Requiring large‐bore access, TAVR procedures are very dependent on vascular puncture quality to avoid VC. US guidance allows more granular and comprehensive imaging of the anatomy and the structures of the vessel to identify the ideal intended landing zone for the needle.
The 4 main advantages of US guidance are these: (1) precise localization of the femoral bifurcation; (2) identification of the calcified zones; (3) controlling of the entry of the needle in the middle of the anterior wall of the artery; and (4) decreasing the dose of radiation administered to operators and patients. Using this technique, the operator is able to identify the femoral bifurcation, avoid calcified segments, and control the trajectory of the needle to ensure true entry in the middle of the anterior wall of the CFA.
These features are of major clinical relevance because they prevent unexpected arterial damage and transfixing puncture, improve the efficacy of percutaneous closure devices, and avoid major vascular and bleeding complications.
Limitations
One of the primary limitations of this study is its retrospective nature and nonrandomized design of the intergroup comparison (US‐guided versus fluoroscope‐guided). To overcome these issues, a careful propensity score–matched comparison was used to control for the other potential differences among 15 key variables between the 2 groups. Such methodology has previously been shown to be highly predictive of the results of randomized studies.30 The comparison of 2 populations treated during time periods that are not contemporary could also be considered a limitation in that time cannot be included in the parameters of the propensity scoring. Operators were more experienced in the second than in the first study period. Such potential bias was minimized by excluding from our study those patients treated during the period with the steepest learning curve, during the initial TAVI experience at our center from 2008 to 2012. To further limit this bias, we also included patients treated during 2 immediately consecutive and relatively short periods (immediately before and after the implementation of US guidance). Finally, the lack of power and small study size of the main comparison (n=190) likely explains the absence of a benefit of US guidance on patient survival despite an effect on LT/MB complications. Interestingly, in the overall and larger population of 546 patients, we confirmed the previously reported noxious impact of LT/MB complications on mortality.
Conclusions
The indications for TAVR are expanding to younger and lower‐risk patients, but reducing the vascular and bleeding burdens during structural interventions should be a constant for operators. Here we demonstrated that elective real‐time US‐guided cannulation of the femoral artery is an efficient way to reduce the risk of vascular and bleeding complications and improve procedural and clinical outcomes. These data support the concept of implementing US guidance as the standard puncture method for TAVR or other percutaneous procedures requiring large‐bore access.
Sources of Funding
F. Vincent received a research grant from the Federation Française de Cardiologie. This work was supported by Lille‐II University.
Disclosures
Dr Modine is a consultant for Boston Scientific, Medtronic, Edwards Lifesciences, Cephea, Microport, GE, and Abbott and has served as a proctor and received speaker fees from Medtronic. Dr Delhaye has received speaker and proctoring fees from Medtronic. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S3
Figures S1–S2
References 11–14, 31 and 32
Acknowledgments
The authors thank Alexandre Ung, Anaïs Gaul, Geneviève Pin, and Ludivine Masquelin (Lille University Hospital) for their contribution to the study as well as the members of the catheterization laboratory and cardiac operating room teams of the Department of Cardiology represented by their chief nurses (Toria El Walid, Fabienne Delesalle, and Pauline Dollet) for their commitment to the project.
J Am Heart Assoc. 2020;9:e014916 DOI: 10.1161/JAHA.119.014916
References
- 1. Barbanti M, Buccheri S, Rodés‐Cabau J, Gulino S, Généreux P, Pilato G, Dvir D, Picci A, Costa G, Tamburino C, et al. Transcatheter aortic valve replacement with new‐generation devices: a systematic review and meta‐analysis. Int J Cardiol. 2017;245:83–89. [DOI] [PubMed] [Google Scholar]
- 2. Généreux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, Davidson CJ, Eisenhauer AC, Makkar RR, Bergman GW, et al. Vascular complications after transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;60:1043–1052. [DOI] [PubMed] [Google Scholar]
- 3. Tchetche D, Van der Boon RMA, Dumonteil N, Chieffo A, Van Mieghem NM, Farah B, Buchanan GL, Saady R, Marcheix B, Serruys PW, et al. Adverse impact of bleeding and transfusion on the outcome post‐transcatheter aortic valve implantation: insights from the Pooled‐RotterdAm‐Milano‐Toulouse In Collaboration Plus (PRAGMATIC Plus) initiative. Am Heart J. 2012;164:402–409. [DOI] [PubMed] [Google Scholar]
- 4. Piazza N, Cribier A, De Palma R. The PCR‐EAPCI Textbook: Percutaneous Interventional Cardiovascular Medicine. Transcatheter aortic valve implantation. Available at: https://www.pcronline.com/eurointervention/textbook/pcr-textbook/table-of-contents/. Accessed November 24, 2017. [Google Scholar]
- 5. Frankel HL, Kirkpatrick AW, Elbarbary M, Blaivas M, Desai H, Evans D, Summerfield DT, Slonim A, Breitkreutz R, Price S, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—part I: general ultrasonography. Crit Care Med. 2015;43:2479–2502. [DOI] [PubMed] [Google Scholar]
- 6. Elbaz‐Greener G, Zivkovic N, Arbel Y, Radhakrishnan S, Fremes SE, Wijeysundera HC. Use of two‐dimensional ultrasonographically guided access to reduce access‐related complications for transcatheter aortic valve replacement. Can J Cardiol. 2017;33:918–924. [DOI] [PubMed] [Google Scholar]
- 7. Soverow J, Oyama J, Lee MS. Adoption of routine ultrasound guidance for femoral arterial access for cardiac catheterization. J Invasive Cardiol. 2016;28:311–314. [PubMed] [Google Scholar]
- 8. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G‐A, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 9. Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, Sauren B, Mohr F‐W, Walther T, Zickmann B, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high‐risk patients using the second‐ and current third‐generation self‐expanding CoreValve prosthesis: device success and 30‐day clinical outcome. J Am Coll Cardiol. 2007;50:69–76. [DOI] [PubMed] [Google Scholar]
- 10. Himbert D, Roy D, Brecker S, Brochet E, Depoix J‐P, Radu C, Laborde J‐C, Vahanian A. Tools & techniques: transcatheter aortic valve implantation: transfemoral approach. EuroIntervention. 2011;6:784–785. [DOI] [PubMed] [Google Scholar]
- 11. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 15. Auffret V, Lefevre T, Van Belle E, Eltchaninoff H, Iung B, Koning R, Motreff P, Leprince P, Verhoye JP, Manigold T, et al. Temporal trends in transcatheter aortic valve replacement in France: FRANCE 2 to FRANCE TAVI. J Am Coll Cardiol. 2017;70:42–55. [DOI] [PubMed] [Google Scholar]
- 16. Grube E, Sinning J‐M. The “Big Five” complications after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:370–372. [DOI] [PubMed] [Google Scholar]
- 17. Généreux P, Cohen DJ, Mack M, Rodes‐Cabau J, Yadav M, Xu K, Parvataneni R, Hahn R, Kodali SK, Webb JG, et al. Incidence, predictors, and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol. 2014;64:2605–2615. [DOI] [PubMed] [Google Scholar]
- 18. Toggweiler S, Leipsic J, Binder RK, Freeman M, Barbanti M, Heijmen RH, Wood DA, Webb JG. Management of vascular access in transcatheter aortic valve replacement: part 1: basic anatomy, imaging, sheaths, wires, and access routes. JACC Cardiovasc Interv. 2013;6:643–653. [DOI] [PubMed] [Google Scholar]
- 19. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 20. Webb JG, Doshi D, Mack MJ, Makkar R, Smith CR, Pichard AD, Kodali S, Kapadia S, Miller DC, Babaliaros V, et al. A randomized evaluation of the SAPIEN XT transcatheter heart valve system in patients with aortic stenosis who are not candidates for surgery. JACC Cardiovasc Interv. 2015;8:1797–1806. [DOI] [PubMed] [Google Scholar]
- 21. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 22. Saugel B, Scheeren TWL, Teboul J‐L. Ultrasound‐guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care. 2017;21:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naidu SS, Aronow HD, Box LC, Duffy PL, Kolansky DM, Kupfer JM, Latif F, Mulukutla SR, Rao SV, Swaminathan RV, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2016;88:407–423. [DOI] [PubMed] [Google Scholar]
- 24. Sandoval Y, Burke MN, Lobo AS, Lips DL, Seto AH, Chavez I, Sorajja P, Abu‐Fadel MS, Wang Y, Poulouse A, et al. Contemporary arterial access in the cardiac catheterization laboratory. JACC Cardiovasc Interv. 2017;10:2233–2241. [DOI] [PubMed] [Google Scholar]
- 25. Bertrand O, De Palma R, Meerkin D. Vascular access The PCR‐EAPCI Textbook. Available at: https://www.pcronline.com/eurointervention/textbook/pcr-textbook/table-of-contents/. [Google Scholar]
- 26. Rao SV, Stone GW. Arterial access and arteriotomy site closure devices. Nat Rev Cardiol. 2016;13:641–650. [DOI] [PubMed] [Google Scholar]
- 27. Seto AH, Abu‐Fadel MS, Sparling JM, Zacharias SJ, Daly TS, Harrison AT, Suh WM, Vera JA, Aston CE, Winters RJ, et al. Real‐time ultrasound guidance facilitates femoral arterial access and reduces vascular complications. JACC Cardiovasc Interv. 2010;3:751–758. [DOI] [PubMed] [Google Scholar]
- 28. Koshy LM, Aberle LH, Krucoff MW, Hess CN, Mazzaferri E, Jolly SS, Jacobs A, Gibson CM, Mehran R, Gilchrist IC, et al. Comparison of radial access, guided femoral access, and non‐guided femoral access among women undergoing percutaneous coronary intervention. J Invasive Cardiol. 2018;30:18–22. [PubMed] [Google Scholar]
- 29. Damluji AA, Nelson DW, Valgimigli M, Windecker S, Byrne RA, Cohen F, Patel T, Brilakis ES, Banerjee S, Mayol J, et al. Transfemoral approach for coronary angiography and intervention: a collaboration of International Cardiovascular Societies. JACC Cardiovasc Interv. 2017;10:2269–2279. [DOI] [PubMed] [Google Scholar]
- 30. Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, Lim S, Malaisrie SC, Kapadia S, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate‐risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. [DOI] [PubMed] [Google Scholar]
- 31. Mattei A. Estimating and using propensity score in presence of missing background data: an application to assess the impact of childbearing on wellbeing. Stat Methods Appl. 2009;18:257–273. [Google Scholar]
- 32. Toutenburg H Rubin DB. Multiple imputation for nonresponse in surveys. Stat Pap. 1990;31:180. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figures S1–S2
References 11–14, 31 and 32


