Abstract
Background
Direct‐acting oral anticoagulant (DOAC) dosing guidelines for atrial fibrillation recommend dose alteration based on age, renal function, body weight, and drug‐drug interactions. There is paucity of data describing the frequency and factors associated with prescription of potentially inappropriate doses.
Methods and Results
In the ongoing SAGE‐AF (Systematic Assessment of Geriatric Elements in Atrial Fibrillation) study, we performed geriatric assessments (frailty, cognitive impairment, sensory impairments, social isolation, and depression) for participants with atrial fibrillation (age ≥65 years, CHA2DS2VASc ≥2, no anticoagulant contraindications). We developed an algorithm to analyze DOAC dose appropriateness accounting for drug‐drug interactions, age, renal function, and body weight. We also examined whether geriatric impairments were related to inappropriate dosing. Of 1064 patients prescribed anticoagulants, 460 received a DOAC. Participants were aged 74±7 years, 49% were women, and 82% were white. A quarter (23%; n=105) of participants received inappropriate DOAC dose, of whom 82 (78%) were underdosed and 23 (22%) were overdosed. Among participants receiving an inappropriate dose, 12 (11%) were identified using the drug‐drug interactions criteria and would have otherwise been misclassified. In multivariable regression analyses, older age, higher CHA2DS2VASc score, and history of renal failure were associated with inappropriate DOAC dosing (P<0.05). Geriatric conditions were not associated with inappropriate dosing.
Conclusions
In this cohort, over 20% of older patients with atrial fibrillation treated with DOACs were prescribed an inappropriate dose, with most being underdosed. Drug‐drug interactions were common. Factors that influence prescription of guideline‐nonadherent doses may be perception of higher bleeding risk or presence of renal failure in addition to lack of familiarity with dosing guidelines.
Keywords: anticoagulant, atrial fibrillation, geriatrics, off‐label dosing
Subject Categories: Arrhythmias, Atrial Fibrillation, Quality and Outcomes, Anticoagulants
Clinical Perspective
What Is New?
In a diverse and contemporary cohort of older adults with atrial fibrillation, over 20% of patients were treated with an inappropriate direct‐acting oral anticoagulant dose, with most being underdosed. Drug‐drug interactions were common.
Prescription of inappropriate doses may be influenced by clinician perception of higher bleeding risk or presence of renal failure in addition to lack of familiarity with dosing guidelines.
What Are the Clinical Implications?
Clinicians should systematically assess for drug‐drug interactions, in addition to age, body weight, and renal function, for prescription of accurate direct‐acting oral anticoagulant dose.
Introduction
Direct‐acting oral anticoagulant (DOAC) medications including dabigatran, apixaban, rivaroxaban, and edoxaban are approved by the Food and Drug Administration (FDA) for the prevention of thromboembolic events in patients with nonvalvular atrial fibrillation (AF). DOACs have a class I recommendation in the current AF guidelines and are increasingly being used in clinical practice.1 Data from large randomized clinical trials have shown each of these individual agents to be noninferior to warfarin for prevention of systemic thromboembolism with a similar or better safety profile.2, 3, 4, 5 Unlike warfarin, they do not require intensive laboratory monitoring, making them easier to prescribe and use. However, their FDA approval was granted at specific doses with dose adjustments for age, renal function, body weight, or concomitant drug therapies.6, 7, 8, 9
Recent studies have shown that 10% to 15% patients receiving DOAC prescriptions were treated with potentially inappropriate doses.10, 11 Inappropriate dosing has potential clinical consequences, including thromboembolism, bleeding, and death. However, the reasons for choosing an off‐label dosing regimen in such patients are not clear based on previous data.11, 12, 13 Older patients with AF are especially susceptible to the adverse effects associated with inappropriate drug dosing attributable to decreased drug metabolism, increased prevalence of hepatic and renal dysfunction, and higher likelihood of drug‐drug interactions as a result of frequent polypharmacy. Previously published studies have either ignored or incompletely evaluated the prevalence and effect of drug‐drug interactions on DOAC dosing,10, 11, 12, 13 limiting their precision. In these studies, older age, female sex, and higher stroke and bleeding risk scores have been associated with off‐label prescribing.13 However, whether impairments common in the geriatric population and related to adverse drug‐related outcomes, such as cognition and frailty, affect DOAC dosing in these patients have not been examined.
Using data from a prospective cohort of elderly patients 65 years and older with robust phenotyping of geriatric impairments, we sought to describe the frequency of inappropriate dosing of DOACs while systematically accounting for drug‐drug interactions, renal function, and other factors related to dosing of DOAC medications. Second, we aimed to examine the clinical characteristics, including geriatric conditions, that might be associated with inappropriate dosing. We hypothesized that frailty would relate to higher odds of off‐label dosing, with frail patients with AF being more likely to be underdosed.
Methods
The data that support the findings of this study are available from SAGE‐AF (Systematic Assessment of Geriatric Elements in Atrial Fibrillation) study principal investigators upon reasonable request (david.mcmanus@umassmemorial.org and j.saczynski@northeastern.edu).
SAGE‐AF Cohort
The SAGE‐AF study is an ongoing large, multicenter prospective cohort study examining the relationship between the components of a comprehensive geriatric assessment and outcomes in AF among patients older than 65 years. Details of the SAGE‐AF study have been previously published.14 Briefly, SAGE‐AF enrolled patients with ambulatory AF aged 65 years and older with a CHA2DS2VASc score of at least 2 from 5 recruitment sites in Massachusetts and Georgia. Patients were excluded if they had an absolute contraindication to oral anticoagulation or if they had an additional indication for anticoagulation apart from AF, such as mechanical heart valves or venous thromboembolism. Participants who consented to the study underwent standard history and physical examinations in the context of their routine care on the same day as the SAGE‐AF interview. All participants provided written informed consent. Individual institutional review boards of the University of Massachusetts Medical School (#H‐00009079) and other participating enrollment sites approved the study.
Trained personnel abstracted clinical, demographic, laboratory, and treatment characteristics of these participants from the electronic health records. This included participants’ age, sex, race, education, income, insurance type, comorbidities (such as diabetes mellitus, hypertension, heart failure, anemia, chronic kidney disease, and prior stroke), treatment variables (ie, complete medication list), and laboratory values including serum creatinine and hemoglobin (within the past year). Using the relevant data, CHA2DS2‐VASc and HAS‐BLED risk scores were calculated based on previously validated methods.15 History of major bleeding, as defined by the International Society on Thrombosis and Hemostasis criteria,16 was also ascertained.
Geriatric Assessment
There were 6 components of the geriatric assessment including frailty, cognitive function, social support, depressive symptoms, vision, and hearing. Frailty was assessed using the Cardiovascular Health Survey (CHS) frailty scale,17 a biological model of frailty based on 5 components: unintentional weight loss, exhaustion, low physical activity, slow gait speed, and weakness as measured by grip strength. Scores on the CHS frailty index range from 0 to 5 (higher scores=more frailty). Participants with a score ≥3 were categorized as being frail. Cognition was assessed using the Montreal Cognitive Assessment (MoCA)18 with scores ranging from 0 to 30, with a score of <23 used to indicate cognitive impairment.19 A 5‐item modified version of the Social Support Scale and the 6‐item Social Network scale was used to assess social support.20 The Patient Health Questionnaire (PHQ‐9) was used to assess depressive symptoms.21 Patients self‐reported vision and hearing status based on standardized questionnaires evaluating symptoms of reduced vision such as difficulty in reading the print on newspapers or doing work or hobbies, difficulty in hearing during daily activities, or use of hearing aids.22
Determining Appropriateness of DOAC Dose
We used the guidelines from FDA‐approved package inserts6, 7, 8, 9 and the 2018 European Heart Rhythm Association (EHRA) practical guide on the use of non–vitamin K antagonist anticoagulants23 for each drug with respect to absolute contraindications (ie, creatinine clearance <15 mL/min and not on dialysis, presence of a mechanical heart valve, documented allergy to respective DOAC, active pathological bleeding), age, renal function, and weight to create an algorithm to evaluate the appropriateness of DOAC dosing. Creatinine clearance was calculated for each patient from the serum creatinine within 24 months of enrollment based on the Cockcroft‐Gault equation using ideal body weight.24
We created a comprehensive list of potentially interacting drugs based on EHRA recommendations23 and the FDA‐reviewed package inserts6, 7, 8, 9 for each DOAC. Briefly, all participants who had an absolute contraindication were deemed to be overdosed (Figure 1). If there were no absolute contraindications, the algorithm evaluated dose appropriateness based on age, renal function and body weight (for apixaban), renal function alone (for rivaroxaban and edoxaban), or renal function plus dronedarone use (for dabigatran). If the participant was correctly dosed by these parameters, we checked for drug‐drug interactions. Participants in whom the drug was contraindicated based on drug‐drug interactions were classified as either overdosed or underdosed based on whether the drug interaction caused an increase or decrease in serum levels of the DOAC. For example, ketoconazole causes a rise in serum levels of all DOACs; hence, concomitant use of ketoconazole and a DOAC was classified as an overdose. On the other hand, for participants taking concomitant diltiazem, reduced strength dose was deemed to be the “appropriate dose” if they also had a secondary indication (age, renal function, or body weight) for dose reduction. For the sake of uniformity, all yellow recommendations by the EHRA guidelines (consider dose reduction or different DOAC if ≥2 “yellow” factors are present) were considered as a requirement for dose reduction. Where there were discrepancies between the EHRA recommendations and the DOAC package insert, we used the EHRA recommendations as the gold standard for determining dose appropriateness. The exception to this rule was the EHRA recommendation to consider dose reduction for concomitant use of antiplatelet agents and HAS‐BLED score >3, which were not included in the algorithm.
Figure 1.
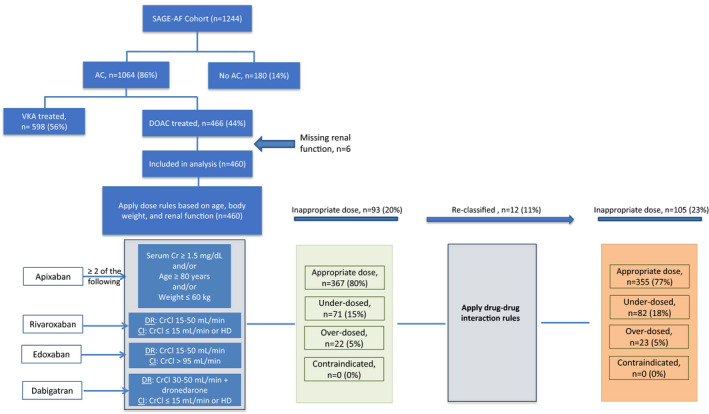
Breakdown of study sample of older adults with atrial fibrillation (SAGE‐AF [Systematic Assessment of Geriatric Elements in Atrial Fibrillation] cohort) treated with direct‐acting oral anticoagulant (DOAC) medications as appropriate dose or inappropriate dose, ie, above recommended dose (overdosed), below recommended dose (underdosed), or contraindicated. CrCl indicates creatinine clearance; DR, dose reduction; VKA, vitamin K antagonist.
Those SAGE‐AF participants taking a DOAC who met all of the above criteria were deemed to be on the guideline‐consistent DOAC dose for nonvalvular AF. Participants were categorized as guideline‐consistent, overdosed, or underdosed. These recommendations were drafted by the 2 pharmacists blinded to SAGE‐AF data (P.C. and W.T.) and were agreed upon by all authors. Two reviewers (S.R.S. and C.W.) blinded to the DOAC dosing algorithm independently reviewed all algorithm outputs (guideline‐consistent, underdosing, or overdosing) to validate its findings. Manual chart review findings were consistent with the output of the algorithm described above in all cases.
Statistical Analysis
We compared participants on the guideline‐directed dose of DOACs versus off‐label dose (either overdosed or underdosed) using chi‐square test for categorical variables and ANOVA for continuous variables. Because of the overall small number of participants who were overdosed, we decided to perform our subsequent analyses by grouping the underdosed and overdosed into a single “off‐label dose” category. We also performed logistic regression analysis to estimate associations between geriatric elements (cognitive function, frailty, social isolation, vision impairment, hearing impairment, depression) and inappropriate dosing of DOACs. For these analyses, we adjusted for characteristics that were significant (P<0.05) in our univariate analyses in Table 1.
Table 1.
Demographic and Clinical Characteristics of Older Adults With AF Treated With DOACs in Relation to Appropriateness of DOAC Dosing: SAGE‐AF
| Recommended Dose of DOAC (n=355) | Off‐Label Dose of DOAC (n=105) | P Value | |
|---|---|---|---|
| Age, mean (SD), y | 73.8 (6.3) | 76.6 (6.5) | <0.001* |
| Women | 167 (47.0) | 57 (54.3) | 0.19 |
| Non‐Hispanic white | 291 (82.0) | 86 (81.9) | 0.99 |
| Married or living as married | 212 (59.7) | 53 (50.5) | 0.10 |
| College graduate or above | 162 (45.6) | 42 (40.0) | 0.37 |
| Insurance status | |||
| Commercial | 45 (12.7) | 10 (9.5) | ··· |
| Medicare | 260 (73.2) | 85 (81.0) | 0.12 |
| Other | 49 (13.8) | 10 (9.5) | 0.48 |
| AF type | |||
| Paroxysmal | 227 (63.9) | 68 (64.8) | 0.35 |
| Persistent/long‐standing, persistent | 93 (26.2) | 23 (21.9) | 0.10 |
| Permanent | 11 (3.1) | 7 (6.7) | |
| Frailty category | |||
| Nonfrail | 133 (37.5) | 39 (37.1) | ··· |
| Prefrail | 174 (49.0) | 44 (41.9) | 0.11 |
| Frail | 48 (13.5) | 22 (21) | 0.07 |
| Cognitive impairment | 136 (38.3) | 49 (46.7) | 0.13 |
| Social isolation | 53 (14.9) | 16 (15.2) | 0.94 |
| Visual impairment | 129 (36.3) | 36 (34.3) | 0.70 |
| Hearing impairment | 117 (33.0) | 40 (38.1) | 0.33 |
| Depression | 99 (27.9) | 32 (30.5) | 0.61 |
| Anxiety | 89 (25.1) | 27 (25.7) | 0.89 |
| CHA2DS2‐VASc score, mean (SD) | 4.2 (1.6) | 4.7 (1.9) | 0.02* |
| HAS‐BLED score, mean (SD) | 2.8 (1.0) | 2.9 (1.0) | 0.40 |
| Medical history | |||
| Heart failure | 115 (32.4) | 43 (41.0) | 0.11 |
| Peripheral vascular disease | 42 (11.8) | 14 (13.3) | 0.68 |
| Hypertension | 316 (89.0) | 94 (89.5) | 0.88 |
| Diabetes mellitus | 104 (29.3) | 36 (34.3) | 0.33 |
| Major bleeding | 66 (18.6) | 29 (27.6) | 0.04* |
| Gastrointestinal bleeding | 37 (10.4) | 16 (15.2) | 0.94 |
| Stroke | 29 (8.2) | 11 (10.5) | 0.46 |
| Anemia | 92 (25.9) | 27 (25.7) | 0.97 |
| Chronic lung disease | 99 (27.9) | 24 (22.9) | 0.31 |
| Renal failure | 74 (20.9) | 37 (35.2) | 0.003* |
| Fall in the past 6 mo | 68 (19.2) | 22 (21.0) | 0.68 |
| Provider type | |||
| Cardiologist | 113 (31.8) | 26 (24.8) | 0.44 |
| Electrophysiologist | 236 (66.5) | 77 (73.3) | 0.71 |
| Internist | 6 (1.7) | 2 (1.9) | |
| Site | |||
| Massachusetts | 220 (62.0) | 55 (52.4) | ··· |
| Georgia | 135 (38.0) | 50 (47.6) | 0.08 |
| Aspirin use | 82 (23.1) | 19 (18.1) | 0.28 |
| Other antiplatelet use | 23 (6.5) | 9 (8.6) | 0.46 |
Continuous variables are presented as mean±SD and categorical variables as number (percentage). AF indicates atrial fibrillation; DOAC, direct‐acting oral anticoagulant; SAGE‐AF, Systematic Assessment of Geriatric Elements in Atrial Fibrillation.
P<0.05.
Results
Study Sample and Prevalence of Potentially Inappropriate DOAC Dosing
The study sample is described in Figure 1. SAGE‐AF recruited a total of 1244 participants from 2016 to 2018, 1064 (86%) of whom were prescribed oral anticoagulants for stroke prevention. Of those prescribed anticoagulants, 466 (44%) were prescribed a DOAC. We excluded 6 participants with missing renal function. This yielded a total of 460 participants for the present analysis. Of these, 235 (51%) were treated with apixaban, 181 (39%) with rivaroxaban, 40 (9%) with dabigatran, and 4 (1%) with edoxaban (Figure 2). The DOAC appropriateness algorithm evaluated each participants’ DOAC dose in a stepwise manner (Figure 1). None of the participants had an absolute contraindication for DOAC therapy. Once the age, renal function, and body weight criteria were applied, 71 (15%) were found to be overdosed and 22 (5%) were underdosed. After this, the drug‐drug interactions were factored in and 12 additional participants were found to be overdosed and 1 additional participant was underdosed based on the EHRA criteria. Overall, 355 patients (77%) were receiving doses that were consistent with the package inserts, 82 (18%) were underdosed, and 23 (5%) were overdosed. There were no significant differences in inappropriate dose prescription among the different DOACs (P=0.308).
Figure 2.

Frequency of direct‐acting oral anticoagulant (DOAC) prescription by: (A) type of DOAC: apixaban, rivaroxaban, dabigatran, and edoxaban; (B) frequency of guideline‐consistent, underdosed, and overdosed prescription for the entire cohort; (C) frequency of potentially inappropriate dosing by each DOAC type, among older patients with atrial fibrillation treated with DOAC in the SAGE‐AF (Systematic Assessment of Geriatric Elements in Atrial Fibrillation) study (n=460).
Baseline Characteristics
We compared the demographic, clinical, and geriatric characteristics of participants who were prescribed guideline‐consistent dosing and those who were underdosed and overdosed (Table S1). A higher proportion of participants who were overdosed reported visual impairment (52% versus 29%, P=0.04). Baseline characteristics of the cohort by dosing group are presented in Table 1. Compared with participants taking guideline‐consistent doses of DOAC, those taking potentially inappropriate doses were older (76.6±6.5 years versus 73.8±6.3 years, P<0.001), had higher CHA2DS2‐VASc scores (4.7±1.9 versus 4.2±1.6, P=0.02), and were more likely to have a history of major bleeding (27.6% versus 18.6%, P=0.04) and renal failure (35.2% versus 20.9%, P=0.003). A higher proportion of participants receiving potentially inappropriate doses were frail compared with those receiving the guideline‐consistent dose (21% versus 14%). However, this difference did not achieve statistical significance in our sample (P=0.07). There were no statistically significant differences in rates of potentially inappropriate DOAC dosing by sex, marital status, education, AF type, or study site (Table 1).
DOAC Dosing by Drug and Renal Impairment
We performed additional analyses to clarify reasons for inappropriate dosing and drug interactions. The rates of guideline‐consistent dose prescription were similar in participants taking apixaban who met dose reduction criteria versus those who appropriately received full‐dose apixaban (83% versus 79%). Among participants prescribed reduced‐dose rivaroxaban, 54% were taking the guideline‐consistent dose compared with 76% taking full‐dose rivaroxaban (Table S2).
Drug‐Drug Interactions
Overall, 115 (25%) of SAGE‐AF participants taking DOACs were coprescribed a medication with a potential DOAC‐drug interaction. A breakdown of the individual drugs that had potential DOAC‐drug interactions is shown in Table 2. Antifungal agents, such as ketoconazole, that are absolutely contraindicated with DOACs were used in 8 (2%) participants. Calcium channel blockers, ie, diltiazem and verapamil, were prescribed concomitantly with DOACs in 60 participants (13%). Amiodarone was prescribed in 26 cases (6%).
Table 2.
Frequency of Drug‐Drug Interactions by DOAC Prescribed in Older Patients With AF: SAGE‐AF
| Amiodarone | Diltiazem | Verapamil | Dronedarone | Erythromycin | Ketoconazole | Cyclosporine | Tacrolimus | Naproxen | |
|---|---|---|---|---|---|---|---|---|---|
|
Apixaban n=235 |
10 (4) | 28 (12) | 5 (2) | 4 (2) | 1 (<1) | 4 (2) | 0 | 2 (2) | 7 (3) |
| Rivaroxaban n=181 | 12 (7) | 21 (12) | 3 (2) | 1 (1) | 0 | 4 (2) | 0 | 0 | 4 (2) |
|
Dabigatran n=40 |
3 (8) | 3 (8) | 0 | 0 | 0 | 0 | 1 (3) | 0 | 1 (3) |
|
Edoxaban n=4 |
1 (25) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Total N=460 |
26 (6) | 52 (11) | 8 (2) | 5 (1) | 1 (<1) | 8 (2) | 1 (<1) | 2 (<1) | 12 (3) |
Continuous variables are presented as mean±SD and categorical variables as number (percentage). AF indicates atrial fibrillation; DOAC, direct‐acting oral anticoagulant; SAGE‐AF, Systematic Assessment of Geriatric Elements in Atrial Fibrillation.
Geriatric Elements and Potentially Inappropriate DOAC Dosing
Although we observed that a greater proportion of SAGE‐AF participants receiving a potentially inappropriate DOAC dose had visual impairment and were frail, in a logistic regression model adjusted for age, history of renal failure, history of major bleeding, and CHA2DS2‐VASc score, none of the geriatric elements—frailty, cognitive impairment, social isolation, visual impairment, hearing impairment, or elevated depressive symptoms—were associated with potentially inappropriate DOAC dosing (Table 3). However, advanced age (odds ratio, 1.1; 95% CI, 1–1.1), history of renal failure (odds ratio, 1.8; 95% CI, 1.1–3.0), and higher CHA2DS2‐VASc score (odds ratio, 1.1; 95% CI, 1.0–1.3) remained significantly associated with potentially inappropriate dosing in the adjusted model. We performed additional exploratory analyses looking at associations between the geriatric elements and underdosing only (Table S3), and results did not differ substantively from results in the overall sample.
Table 3.
Demographic, Clinical, and Geriatric Elements in Relation to Potentially Inappropriate Dosing of DOACs: SAGE‐AF
| Geriatric Elements | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Age | 1.1 (1.0, 1.1) | 1.1 (1.0, 1.1)* |
| History of renal failure | 2.1 (1.3, 3.3) | 1.8 (1.1, 3.0)* |
| History of major bleeding | 1.7 (1.0, 2.8) | 1.5 (0.9, 2.5) |
| CHA2DS2‐VASc score | 1.2 (1.0, 1.3) | 1.1 (1.0, 1.3)* |
| Frailty | 1.6 (0.8, 2.9) | 1.2 (0.6, 2.3) |
| Cognitive impairment | 1.4 (0.9, 2.2) | 1.1 (0.7, 1.8) |
| Social isolation | 1.0 (0.6, 1.9) | 1.0 (0.6, 1.9) |
| Visual impairment | 0.9 (0.6, 1.4) | 0.8 (0.5, 1.3) |
| Hearing impairment | 1.3 (0.8, 2.0) | 1.2 (0.7 1.9) |
| Elevated depressive symptoms | 1.1 (0.7, 1.8) | 1.1 (0.7, 1.7) |
DOAC indicates direct‐acting oral anticoagulant; OR, odds ratio; SAGE‐AF, Systematic Assessment of Geriatric Elements in Atrial Fibrillation.
P<0.05.
Discussion
To our knowledge, this is the first analysis examining potentially inappropriate DOAC dosing in an older cohort of participants with nonvalvular AF that includes an in‐depth phenotyping of both renal function and comprehensive analysis of polypharmacy and drug‐drug interactions. Our results suggest that in our cohort of older patients with AF, nearly a quarter were treated with potentially inappropriate DOAC doses and the majority were underdosed. Older age, history of renal failure, and higher stroke risk were associated with significantly increased odds of receiving a potentially inappropriate dose. Contraindications caused by drug‐drug interactions accounted for a significant proportion of the potentially inappropriate prescribing. Contrary to our hypothesis, our results suggest that age and stroke risk, but not the presence of geriatric conditions, relates to potentially inappropriate prescribing.
Previous findings report wide ranges of inappropriate dosing of DOACs. In ORBIT‐AF II (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II), 13% of participants were found to be taking off‐label doses.11 However, the authors of this study did not report drug‐drug interactions, which accounted for a significant proportion of the inappropriate dosing observed in our study. A recent prospective multicenter registry in Japan reported potentially inappropriate dosing in 26% of all participants taking a DOAC, without accounting for drug‐drug interactions, suggesting that our findings are valid but possibly underestimate the true rate of inappropriate dosing.25 Perhaps the lower body weight of Japanese participants with our US participants account for some of the differences between cohorts, since body weight factors into renal function calculation and constitutes one of apixaban's reduced dosing criteria. In contrast, a national registry from Europe that included 530 participants with AF observed a 32% prevalence rate of potentially inappropriate DOAC dosing.26 However, this study used a strict application of the EHRA guidelines. For example, the study mandated dose reduction for concomitant use of antiplatelet agents such as aspirin and higher bleeding risk, ie, HAS‐BLED score ≥3, which are not reflected in the package insert6, 7, 8, 9 or US guidelines.27 We took a more pragmatic approach to determining whether drug‐drug interactions existed and included only those interactions backed by pharmacokinetic or clinical data.
In our cohort, potentially inappropriate dosing was related to older age, poor renal function, and higher CHA2DS2‐VASc score. Reasons for potentially inappropriate dosing may include prescriber perception of higher bleeding risk in older patients or those with renal failure. Interestingly, participants with higher CHA2DS2‐VASc score, ie, higher thromboembolic risk, were more likely to be taking a potentially inappropriate DOAC dose, even after adjusting for renal failure and major bleeding. This may be driven by age. Since the majority of the potentially inappropriate prescribing we identified was underdosing, our results suggest that older patients and those at higher stroke risk may be at increased risk for receiving subtherapeutic doses of DOACs. This finding is of great clinical significance, particularly since DOAC dosing did not relate to objectively measured frailty, a validated marker associated with adverse reactions to cardiovascular medications.
We also looked at DOAC dose appropriateness by renal function and body weight as recommended in the package inserts and contemporary guidelines. Interestingly, there was no difference in the rates of inappropriate dosing between the full‐dose and dose‐reduced apixaban groups (21% versus 17%). Participants taking dose‐reduced rivaroxaban were more likely to be taking inappropriate doses than participants taking full‐dose rivaroxaban (46% versus 24%) (Table S4). Perhaps this is because the dose reduction criteria for apixaban (any 2 of 3 of the following: age ≥80 years, body weight ≤60 kg, and serum creatinine ≥1.5 mg/dL) are easier to apply correctly or because prescribers are using alternative calculators to determine DOAC dosing. However, because of the limited sample size in these analyses, these results should be interpreted with caution and replicated in larger samples.
Previous studies have investigated the association of frailty using a variety of frailty assessment scales with anticoagulation prescription. One meta‐analysis showed an association between frailty and nonprescription of anticoagulants.28 There have been no studies to our knowledge that investigated potentially inappropriate DOAC dosing in relation to frailty. In our cohort, components of a comprehensive geriatric assessment, including frailty, were not related to inappropriate dosing. These findings suggest that although prescribers consider age in OAC prescribing, they may not consider geriatric conditions in the context of DOAC dosing. Whether these geriatric factors affect outcomes of OAC in participants with AF still needs to be evaluated.
It is likely that the reasons behind prescription of potentially inappropriate doses are multifactorial. First, DOAC dosing recommendations can be complex and difficult to recall—for example, apixaban dose reductions are recommended based on serum creatinine, whereas dabigatran and rivaroxaban are based on creatinine clearance. Most commercial electronic health record systems report renal function as glomerular filtration rate based on the Modification of Diet in Renal Disease equation even though the pivotal trials with DOACs used creatinine clearance as a measure of renal function. There may be significant variations between the two.29 Previous studies have shown higher stroke risk with underdosing and elevated bleeding risk with inappropriate overdosing of DOACs; hence, adherence to guideline‐directed dosing is important nonetheless.11, 12, 13, 25 Second, drug‐drug interactions are numerous and can be cumbersome to apply, leading to variations in dosing. Perhaps, inclusion of drug interaction calculators in electronic health records would improve dose prescribing in such scenarios. Third, prescription of such doses may be intentional—clinicians may be looking at trends in renal function as opposed to a single point check, as we did, and thus may reduce doses based on higher perceived bleeding or fall risk or may choose to forgo recommended dose reduction in patients with a high thromboembolic risk profile. This is not something we could determine from our data but should be explored in future studies.
Study Strengths and Limitations
We leveraged data from a contemporary, comprehensive, diverse cohort of older patients with AF who had a high degree of comorbidity, and performed in‐depth phenotyping of drug dosing and interactions, clinical comorbidities, and frailty status. We developed an algorithm for DOAC dose appropriateness that can potentially be used with other data sets and electronic health record–based quality assessment tools to improve DOAC dosing and validated all cases with blinded chart review. However, there are certain limitations of this study. These data cannot elucidate reasons for potentially inappropriate dosing, or whether this inappropriate dosing was intentional and part of informed clinical decision‐making or represents an error. Since outcome data are still being collected, we cannot examine whether potentially inappropriate dosing is associated with increased risk of thromboembolic or bleeding outcomes as it has been in other cohorts. We will explore these associations as our follow‐up data become available. In addition, we collected participants’ renal function at the time of entry into the study. It is possible that prescribing physicians had access to multiple data points and based their dosing on trends in renal function. We checked for drug interactions for those drugs listed in the 2018 EHRA recommendations and package inserts. However, we acknowledge that the list of drugs metabolized by the P‐glycoprotein transporter and CYP3A4 enzyme is extensive and their individual effects on each of the 4 DOACs are not yet completely understood. We did not include antiplatelet agents in our drug‐drug interactions algorithm since concomitant use is justified according to some guidelines29 and there is no consensus on dose reduction for this interaction. The modest number of participants receiving certain DOACs and inappropriate dosing of these DOACs limited our statistical power to detect associations. Last, our algorithm needs to be independently validated in other cohorts to establish its utility for clinical use.
Conclusions
In this contemporary and well‐characterized cohort of older adults with AF receiving DOACs we observed that nearly one quarter were prescribed a potentially inappropriate dose, with most being underdosed. Systematic assessment of drug‐drug interactions is important for prescription of accurate DOAC dosing. Inappropriate dosing was more likely among older patients with higher CHA2DS2‐VASc scores and presence of renal dysfunction but was not associated with frailty or other geriatric conditions. Further research is needed to determine the association between dosing and clinical outcomes and strategies to prevent unintentional inappropriate dosing by prescribers.
Sources of Funding
This article was supported by grant R01HL126911 from the National Heart, Lung, and Blood Institute. Dr McManus’ time was also supported by grants R01HL137734, R01HL137794, R01HL13660, and R01HL141434 from the National Heart, Lung, and Blood Institute.
Disclosures
Dr McManus has received research grant support from Apple Computer, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Pfizer, Samsung, Philips Healthcare, and Biotronik; has received consultancy fees from Bristol‐Myers Squibb, Pfizer, Flexcon, Boston Biomedical Associates, and Rose Advisors; and had inventor equity in Mobile Sense Technologies, Inc. (CT) until 2019. The remaining authors have no disclosures to report.
Supporting information
Table S1. Demographic and Clinical Characteristics of Older Adults With AF Treated With DOACs in Relation to Appropriateness of DOAC Dosing: SAGE‐AF
Table S2. Frequency of Full‐Dose and Reduced‐Dose DOAC Prescription in Relation to Dose Appropriateness: SAGE‐AF
Table S3. Demographic, Clinical, and Geriatric Elements in Relation to Underdosing of DOACs: SAGE‐AF
Table S4. Age, Body Weight, and Renal Function in Relation to DOAC Dose Appropriateness: SAGE‐AF
(J Am Heart Assoc. 2020;9:e014108 DOI: 10.1161/JAHA.119.014108.)
References
- 1. Barnes GD, Lucas E, Alexander GC, Goldberg ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 3. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 4. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 5. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; for the ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 6. PRADAXA (dabigatran etexilatemesylate) [package insert]. Ridgefield, CT: Boehringer‐Ingelheim; 2011. [Google Scholar]
- 7. Xarelto (rivaroxaban) [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2011. [Google Scholar]
- 8. Eliquis (apixaban) [package insert]. Princeton, NJ: Bristol‐Myers Squibb; 2013. [Google Scholar]
- 9. Sayvasa (edoxaban) [package insert]. Parsippnay, NY: Daiichi Sankyo Inc; 2015. [Google Scholar]
- 10. Bell AD, Gross P, Heffernan M, Deschaintre Y, Roux JF, Purdham DM, Shuaib A. Appropriate use of antithrombotic medication in Canadian patients with nonvalvular atrial fibrillation. Am J Cardiol. 2016;117:1107–1111. [DOI] [PubMed] [Google Scholar]
- 11. Steinberg BA, Blanco RG, Ollis D, Kim S, Holmes DN, Kowey PR, Fonarow GC, Ansell J, Gersh B, Go AS, Hylek E, Mahaffey KW, Thomas L, Chang P, Peterson ED, Piccini JP; ORBIT‐AF Steering Committee Investigators . Outcomes registry for better informed treatment of atrial fibrillation II: rationale and design of the ORBIT‐AF II registry. Am Heart J. 2014;168:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non‐vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69:2779–2790. [DOI] [PubMed] [Google Scholar]
- 13. Steinberg BA, Shrader P, Pieper K, Thomas L, Allen LA, Ansell J, Chan PS, Ezekowitz MD, Fonarow GC, Freeman JV, Gersh BJ, Kowey PR, Mahaffey KW, Nacarrelli GV, Reiffel JA, Singer DE, Peterson ED, Piccini JP; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) II Investigators . Frequency and outcomes of reduced dose non‐vitamin K antagonist anticoagulants: results from ORBIT‐AF II (the outcomes registry for better informed treatment of atrial fibrillation II). J Am Heart Assoc. 2018;7:e007633 DOI: 10.1161/JAHA.117.007633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saczynski JS, Sanghai SR, Kiefe CI, Lessard D, Marino F, Waring ME, Parish D, Helm R, Sogade F, Goldberg R, Gurwitz J, Wang W, Mailhot T, Bamgbade B, Barton B, McManus DD. Geriatric elements and oral anticoagulant prescribing in older atrial fibrillation patients: SAGE AF. J Am Geriatr Soc. 2020;68:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lane DA, Lip GY. Use of the CHA2DS2‐VASc and HAS‐BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126:860–865. [DOI] [PubMed] [Google Scholar]
- 16. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 17. Fried LP, Tanen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 18. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 19. Saczynski JS, Inouye SK, Guess J, Jones RN, Fong TG, Nemeth E, Hodara A, Ngo L, Marcantonio ER. The Montreal Cognitive Assessment: creating a crosswalk with the Mini‐Mental State Examination. J Am Geriatr Soc. 2015;63:2370–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moser A, Stuck AE, Silliman RA, Ganz PA, Clough‐Gorr KM. The eight‐item modified Medical Outcomes Study Social Support Survey: psychometric evaluation showed excellent performance. J Clin Epidemiol. 2012;65:1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self‐report version of PRIME‐MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. [DOI] [PubMed] [Google Scholar]
- 22. Macphee GJ, Crowther JA, McAlpine CH. A simple screening test for hearing impairment in elderly patients. Age Ageing. 1988;17:347–351. [DOI] [PubMed] [Google Scholar]
- 23. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan‐Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbuchel H; ESC Scientific Document group . The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 24. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 25. Murata N, Okumura Y, Yokoyama K, Matumoto N, Tachibana E, Kuronuma K, Oiwa K, Matusmono M, Kojima T, Hanada S, Nomoto K, Arima K, Takahashi F, Kotani T, Ikeya Y, Fukushima S, Itoha S, Kondo K, Chiku M, Ohno Y, Onikura M, Hirayama A; SAKURA‐AF registry investigators . Clinical outcomes of off‐label dosing of direct oral anticoagulant therapy among Japanese patients with atrial fibrillation identified from the SAKURA AF registry. Circ J. 2019;83:727–735. [DOI] [PubMed] [Google Scholar]
- 26. Ruiz Ortiz M, Muniz J, Rana Miguez P, Roldan I, Marin F, Asuncion Esteve‐Pastor M, Cequier A, Martinez‐Selles M, Bertomeu V, Anguita M; FANTASIIA Study Investigators . Inappropriate doses of direct oral anticoagulants in real‐world clinical practice: prevalence and associated factors. A subanalysis of the FANTASIIA registry. Europace. 2018;20:1577–1583. [DOI] [PubMed] [Google Scholar]
- 27. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'Gara PT, Sabatine MS, Smith PK, Smith SC Jr. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease : a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction, 2014 ACC/AHA guideline for the management of patients with non–ST‐elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134:e123–e155. [DOI] [PubMed] [Google Scholar]
- 28. Oqab Z, Pournazari P, Sheldon RS. What is the impact of frailty on prescription of anticoagulation in elderly patients with atrial fibrillation? A systematic review and meta‐analysis. J Atr Fibrillation. 2018;10:1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic and Clinical Characteristics of Older Adults With AF Treated With DOACs in Relation to Appropriateness of DOAC Dosing: SAGE‐AF
Table S2. Frequency of Full‐Dose and Reduced‐Dose DOAC Prescription in Relation to Dose Appropriateness: SAGE‐AF
Table S3. Demographic, Clinical, and Geriatric Elements in Relation to Underdosing of DOACs: SAGE‐AF
Table S4. Age, Body Weight, and Renal Function in Relation to DOAC Dose Appropriateness: SAGE‐AF


