Abstract
Background
High blood pressure (BP) has long been recognized as a major health threat and, particularly, a major risk factor for stroke, cardiovascular disease, and end‐organ damage. However, the identification of a novel, alternative, integrative approach for the control of BP and cardiovascular protection is still needed.
Methods and Results
Sixty‐nine uncontrolled hypertension patients, aged 40 to 68 years, on antihypertensive medication were enrolled in 2 double‐blind studies. Forty‐five were randomized to placebo or a new nutraceutical combination named AkP05, and BP, endothelial function, and circulating nitric oxide were assessed before and at the end of 4 weeks of treatment. Twenty‐four patients were randomized to diuretic or AkP05 for 4 weeks and underwent a cardiopulmonary exercise test to evaluate the effects of AkP05 on functional capacity of the cardiovascular, pulmonary, and muscular systems. Vascular and molecular studies were undertaken on mice to characterize the action of the single compounds contained in the AkP05 nutraceutical combination. AkP05 supplementation reduced BP, improved endothelial function, and increased nitric oxide release; cardiopulmonary exercise test revealed that AkP05 increased maximum O2 uptake, stress tolerance, and maximal power output. In mice, AkP05 reduced BP and improved endothelial function, evoking increased nitric oxide release through the PKCα/Akt/endothelial nitric oxide synthase pathway and reducing reactive oxygen species production via NADPH‐oxidase inhibition. These effects were mediated by synergism of the single compounds of AkP05.
Conclusions
This is the first study reporting positive effects of a nutraceutical combination on the vasculature and exercise tolerance in treated hypertensive patients. Our findings suggest that AkP05 may be used as an adjunct for the improvement of cardiovascular protection and to better control BP in uncontrolled hypertension.
Keywords: exercise capacity, nitric oxide, vascular biology
Subject Categories: Vascular Biology, Hypertension
Clinical Perspective
What Is New?
This is the first demonstration that supplementation with a novel nutraceutical combination can improve functional capacity and cardiovascular protection in uncontrolled hypertensive patients on antihypertensive medication.
AkP05 has a direct effect on blood pressure, endothelial function, circulating nitric oxide levels, and exercise tolerance in humans.
The single components of AkP05 exert synergistic effects in maintenance of the redox axis for nitric oxide bioavailability, modulating oxidative stress status and enzyme activity.
What Are the Clinical Implications?
This novel nutraceutical combination is able to contribute to the reduction of blood pressure levels and to the improvement of endothelial function and exercise effort beyond classical pharmacological therapy.
The characterization of the beneficial cardiovascular properties of a combination of multiple naturally derived compounds, named AkP05, may lay the foundation for the development of new, natural adjunct therapies aimed at improving cardiovascular protection and contributing to the achievement of target blood pressure levels.
High blood pressure (BP) is a growing, worldwide epidemic with an enormous economic burden; as such, the care and prevention of arterial hypertension represent important challenges for the World Health Organization. Indeed, arterial hypertension is associated with higher cardiovascular morbidity and mortality at any age.1 A pivotal role in the pathogenesis of this condition has been attributed to endothelial dysfunction related to impairment of nitric oxide (NO) production2, 3 and to altered functional capacity of the cardiovascular, pulmonary, and muscular systems.4 Unfortunately, the pharmacological therapies available for the management of hypertensive patients often prove to be ineffective because patients are often reluctant to undergo multiple‐drug regimens, so adherence to therapy is reduced.4 Moreover, exercise capacity in hypertensive patients on antihypertensive medication has been reported to be reduced, further contributing to end‐organ damage.5 Thus, an integrative approach capable of improving cardiovascular protection is urgently needed.
We have recently reported that treatment with a combination of nutraceuticals (a mixture of Bacopa monnieri, extract of Ginkgo biloba leaves, extract of green tea leaves, and phosphatidylserine, named AkP05, Izzek; Damor Farmaceutici, Italy) is able to simultaneously improve cognitive function and arterial compliance, as assessed by the augmentation index, in a population of hypertensive patients in whom BP was already satisfactorily controlled.6
Since impairment of arterial compliance is the main determinant of age‐induced increase in BP, our aim was to test the action of the nutraceutical combination AkP05 on the modulation of BP in uncontrolled hypertension. In particular, we decided to evaluate the cardiovascular effects of supplementing current antihypertensive medication with this new nutraceutical combination, assessing BP homeostasis, endothelial function, NO serum level, and exercise tolerance (by a cardiopulmonary test) in hypertensive patients with poorly controlled BP. The molecular mechanisms recruited by AkP05 and its single components were dissected in experimental models.
Methods
Data supporting the findings of this study are available from the corresponding authors on reasonable request. For detailed methodologies, please, see Data S1. The proof‐of‐concept study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the University of Salerno, which designed and registered the study protocol (no 390‐01587470‐30081). Written informed consent was obtained from each subject.
Study Design
We enrolled 69 patients of both sexes, aged between 40 and 68 years (56±7.7), diagnosed with essential hypertension. Only hypertensive patients on pharmacologic therapy and with an unsatisfactory, but stable, BP control were screened for assessment of the exclusion criteria. During the observation period, there were no changes in the pharmacological therapy administered: from enrollment until the end of the study, all patients were given, in addition to their normal therapy, either placebo or AkP05 in 1 study and diuretic or AkP05 in the other, in order to rule out the possibility of interference of potential additional or different antihypertensive drugs on cardiovascular function.
Sample Size Calculation
Primary study/outcome
Based on results of previous observations,6 we hypothesized that 50% of the patients treated with AkP05 would reach optimal arterial BP values (mean systolic BP <125 mm Hg; mean diastolic BP <85 mm Hg) with respect to only 10% of patients assuming placebo. Based on this assumption and on a 2:1 randomization, 22 patients would be needed in the active treatment group versus 11 in the placebo group to have a statistical power of 80% (α=0.05, 2‐tailed). Considering a possible dropout of ≈20%, the total number of patients enrolled was 45 (30 patients in the active treatment group and 15 patients in the placebo group).
Secondary study/outcome
Since CPET represents a novel outcome of the study, a second group of uncontrolled hypertensive patients were enrolled for the assessment of cardiovascular, pulmonary, and muscular systems. Considering the nature of our proof‐of concept study, we performed a randomization 1:1 in which patients were underwent CPET assessment at time 0 (before assumption) and at 28 days after assumption of AkP05 or Diuretic.
Basing on the effect‐size that we considered clinically relevant (d=0.8) and a power of 80%, considering that we would like to perform a noninferiority study aimed at evaluate the differences between before and after assumption of the compound, with a 1‐tail t test, the estimation of the sample size suggests that 12 patients should be recruited for each experimental group (N=12 AkP05; N=12 Diuretic). This choice has been verified using G*power software.
Twenty‐Four Hour Arterial BP Measurement
Patients enrolled for secondary outcome underwent 24‐hour arterial BP measurement before and at the end of AkP05 or diuretic assumption for 4 weeks, with a walk200b arterial BP measurement monitoring device (Holter Recorder, Cardioline, Italy), which registers BP every 15 minutes during the day and every 30 minutes during the night. All subjects carried out normal daily activities.
Cardiopulmonary Exercise Test
All cardiopulmonary exercise tests (CPETs) were performed using a QUARK CPET breath‐by‐breath metabolic measurement system (Cosmed, Rome, Italy). A physician blind to treatment assignment did all tests. Detailed methodology has been described in Data S1.
Evaluation of Endothelial Function
Endothelial function was assessed via the reactive hyperemia index, using an EndoPAT 2000 device (Itamar Medical, Israel). Measurements were performed according to the manufacturer's instructions and were calculated using a computerized automated algorithm (software version 3.1.2) provided with the device. Briefly, subjects were in a supine position for a minimum of 15 minutes before measurements in a quiet, temperature‐controlled (21–24°C) room with dimmed lights. The subjects were asked to remain as still and silent as possible during the entire measurement period. Each recording consisted of 5 minutes of baseline measurement, 5 minutes of occlusion measurement, and 5 minutes of postocclusion measurement (hyperemic period). Occlusion of the brachial artery was performed on the nondominant upper arm. The occlusion pressure (200 mm Hg minimum, 300 mm Hg maximum) was at least 60 mm Hg above the systolic blood pressure (SBP).
Measurements of Serum Nitrite Levels
Nitrite is a central component of the NO cycle. The measurement of nitrite levels in serum was performed with a 280i Nitric Oxide Analyzer (Sievers, Italy). In detail, after withdrawal, blood was rapidly transferred to serum vacutainer tubes and centrifuged within 30 seconds for 5 minutes at 800g for serum separation. Only serum samples without visible hemolysis were used for measurements. Before analysis, a mixture of glacial acetic acid and 1.0 mL of 0.5 mol/L ascorbic acid was added to the purge bath to generate a calibration curve for the nitrite. This method is specific for nitrite, since the reaction mixture does not release NO from any other NOx metabolites. All samples were thawed in the dark just before analysis and kept on ice until injected. Every 4 injections, the reagents of the purge bath were changed. The concentration of nitrite in the samples was determined using a 280i Nitric Oxide Analyzer (Sievers Instruments).
Experimental Models
All experiments involving animals conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85‐23, revised 2011) and were approved by the IRCCS INM Neuromed review board. Because of the important influence of female hormones and the estrous cycle, in which hormones vary periodically (every 4 days, reducing significantly the reproducibility of the studies), all animal studies were performed on male mice. A detailed description of experimental procedures is provided in Data S1.
Vivo Vascular Reactivity Studies and Staining for Superoxide
Vascular reactivity studies were performed on second‐order branches of the mesenteric artery, as previously described.7 A detailed description of experimental procedures is provided in Data S1.
Cell Culture and NO Measurement
Commercially available human umbilical vein endothelial cells (HUVECs) or coronary artery smooth muscle cells were purchased from Lonza (Walkersville, MD) and grown in EGM‐2 and SmBM basal medium, respectively. The NO concentration in the supernatant was assayed as nitrite, the stable breakdown product of NO, with a chemiluminescence detector (Sievers 280i NO Analyzer), as previously described.8 A detailed description of methodology is provided in Data S1.
Fluorescence Microscopy
Free intracellular calcium concentration ([Ca2+]i) recordings were obtained by time‐resolved digital fluorescence microscopy on HUVECs or coronary artery smooth muscle cells loaded with the Ca2+ indicator Fura2‐AM (excitation, 340 and 380 nm; emission, 550 nm). Briefly, cells were incubated for 45 minutes at 37°C with 1 μmol/L Fura2‐AM, then placed in standard normal external solution (in mmol: NaCl, 140; KCl, 2.5; CaCl2, 2; MgCl2, 2; HEPES‐NaOH, 10; and glucose, 10; pH 7.3), and continuously superfused with a gravity‐driven perfusion system. Ca2+ transients were elicited by applying phosphatidylserine (PS) (0.4 mg/mL) for 2 minutes. The time courses of Ca2+ transients were quantified by measuring at each time point (frequency of acquisition 0.3 Hz) the ratio of fluorescence emission stimulating the dye at 340 and 380 nm (R) in the region of interest surrounding each cell present in the field. The rise in cytosolic Ca2+ concentration was expressed as the difference ΔR between the ratio values at peak and resting level.
In Situ NO Measurement
NO production in response to different stimuli, as reported in figure legends, was assessed and imaged using 4‐amino‐5‐methylamino‐2′,7‐difluorofluorescein diacetate (DAF‐FM) (Invitrogen, D‐23842), as previously described.7 Briefly, isolated mesenteric arteries were cleaned of fat and connective tissue and equilibrated for 30 minutes in Krebs solution at room temperature. DAF‐FM (5 μmol/L) was then added to the buffer for 30 minutes. Subsequently, vessels were washed twice with fresh Krebs solution and then stimulated as reported in figure legends. After stimulation, vessels were immediately snap‐frozen with OCT embedding compound in isopentane prechilled with liquid nitrogen. Frozen rings were then cut into 10‐μm‐thick sections using a CM1250 cryostat (Leica) and imaged by a fluorescence DS‐Ri1 microscope (Zeiss Axiophot 2) with excitation at 495 nm and emission at 515 nm. Subsequently, some sections were counterstained with hematoxylin–eosin and observed under a light microscope.
Protein Extraction and Immunoblot Analysis
Immunoblotting was performed as previously described.7 A detailed description of methodology is provided in Data S1.
NADPH Oxidase Activity
NADPH oxidase (NOX) activity in mouse mesenteric arteries was measured in control (untreated) vessels and in vessels treated with either angiotensin II (Ang II; 10−6 mol/L) or Ang II plus different compounds preincubated for 1 hour at a dose of 0.4 mg/mL. The chemiluminescence that occurred over the ensuing 5 minutes in response to addition of 100 μmol/L NADPH was recorded (LS6500 Multipurpose Scintillation Counter; Beckman Coulter, Fullerton, CA). In preliminary experiments, homogenates alone, without addition of NADPH, gave only minimal signals, and NADPH did not evoke lucigenin chemiluminescence in the absence of homogenate. A detailed description of methodology is provided in Data S1.
BP Measurements in Mice
SBP was noninvasively measured in conscious mice with a tail‐cuff method, using a BP‐2000 instrument (Visitech Systems) as previously described.9 Briefly, animals were placed in a holder on a temperature‐controlled platform (kept at 37°C), and recordings were performed in steady‐state conditions. BP values were averaged from at least 3 consecutive measurements.
The dose administered in vivo by gavage was calculated considering the human equivalent dose based on US Food and Drug Administration recommendations.10 In particular, considering that 1000 mg of whole AkP05 contains 300 mg Bacopa monnieri (BM), 50 mg Ginkgo biloba extract (GBE), 25 mg PS, and 40 mg green tea extract (GTE), we administered 150 mg/kg of AkP05 or 46 mg/kg of BM, 7.8 mg/kg of GBE, 4 mg/kg of PS, or 6.3 mg/kg of GTE.
Statistical Analysis
All statistical analyses were performed with Prism 8.01 (GraphPad). Student t test (2‐tailed) was used to calculate statistical significance of 2 independent groups. Data from all experiments are given as mean±SD, except for the vascular reactivity and tail‐cuff blood pressure experiments, for which data are given as mean±SEM. The z‐score test for 2 population proportions was used to determine the hypertensives who achieve target blood pressure in placebo and active treatment groups. Comparison between measurements before and after treatments have been performed using paired t test, whereas analysis of the differences between AkP05 or Diuretic group was performed using unpaired t test (2 tailed). Measured effect size was calculated by G*Power 3.1.9.4. When more than 2 independent groups were compared, we used 1‐way ANOVA followed by Tukey's post‐hoc test. To analyze the effects of treatments on dose‐dependent vasorelaxation, we performed a 2‐way repeated‐measures ANOVA with Tukey's post‐hoc test for multiple comparisons. A P<0.05 was considered statistically significant.
Results
AkP05 Reduces BP in Hypertensive Patients by Improving Endothelial Function and NO Release
Baseline characteristics and medication regimens of the first study population are reported in Tables S1 and S2: no statistically significant differences were found between the 2 groups. As expected, SBP and diastolic blood pressure in the placebo‐treated group remained unchanged throughout the observation period (Figure 1A and 1B). By contrast, AkP05 evoked a significant reduction in SBP, but no significant effect on diastolic blood pressure, by the end of the observation period (Figure 1C and 1D), a finding suggestive of the nutraceutical combination having an additive effect with antihypertensive drugs on the control of hypertension.
Figure 1.
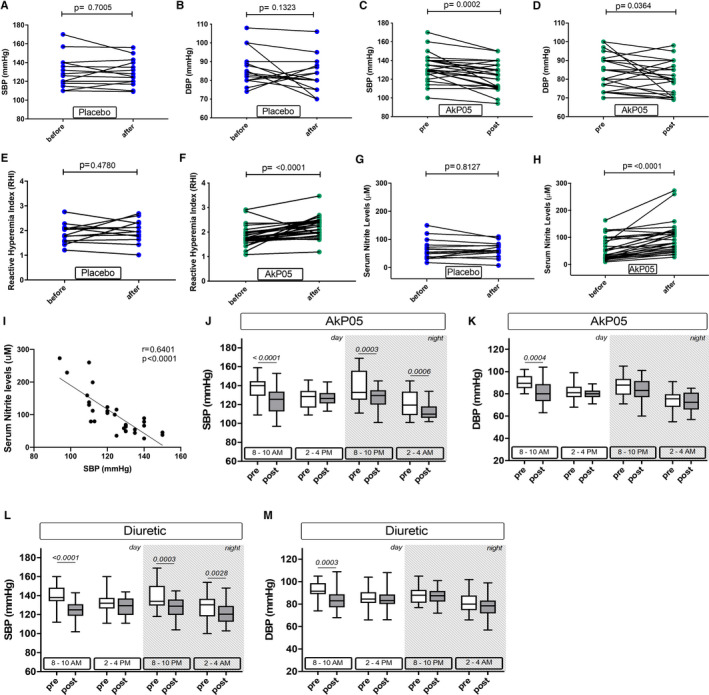
A through D, Systolic and diastolic blood pressure (SBP, DBP) in hypertensive patients before and after treatment with placebo (A,B), and before and after treatment with AkP05 (C and D). E and F, Reactive hyperemia index (RHI) in patients on placebo (E) or AkP05 (F). G and H, Serum concentration of nitrite in hypertensive patients before and after treatment with placebo (G) or AkP05 (H). Dot plots with mean (horizontal line). pre, baseline; post, end of 4 weeks of treatment. I, Correlation between serum nitrite levels and systolic blood pressure in AkP05‐treated hypertensive subjects (n=30). r=Pearson's correlation coefficient. J through M, 24‐hour blood pressure monitoring of hypertensive patients at 2 time points during the day (8–10 AM; 2–4 PM) and night (8–10 PM; 2–4 AM) before and after treatment with AkP05 (J and K) or a diuretic (L and M) for 4 weeks in addition to their usual antihypertensive drug regimen. Box and whisker plots, with the band indicating median, the box indicating first and third quartiles. DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
A useful noninvasive tool for the evaluation of vascular function, as well as for cardio‐ and cerebrovascular disease risk assessment, is reactive hyperemia index.11 As expected, the reactive hyperemia index did not change in the placebo group (Figure 1E), but treatment with AkP05 did improve it significantly (Figure 1F). We then measured nitrite levels—a well‐recognized biomarker of the state of NO metabolism—in the sera of patients: the level of circulating nitrite was significantly increased in the AkP05‐treated group, while remaining unchanged in the placebo group (Figure 1G and 1H). No difference in sodium excretion or serum electrolytes between the 2 groups was detected (data not shown). Of note, Pearson correlation revealed an association between the increase of nitrite and the reduction of SBP in the AkP05‐treated group (Figure 1I). This led us to hypothesize that AkP05 ameliorated endothelial function and decreased BP through a NO‐dependent‐mechanism. Moreover, since 53.3% of the AkP05 group reached the target BP of ≤125/85 mm Hg, versus only 6.66% of the placebo‐treated group (Table S3), this strongly supports the efficacy of AkP05 supplementation for better management of BP in hypertensive subjects. In addition, evaluation of the impact of medications on the achievement of target values revealed no differences between mono‐ or polytherapy when analyzing all patients together, nor between males and females (data not shown). This suggests that improvement of BP control is selectively related to AkP05 treatment in both sexes.
AkP05 Improves Exercise Tolerance and Reduces Cardiovascular Risk
Seeing that no changes were observed in the placebo‐treated group regarding BP, NO release, and endothelial function, subsequent clinical evaluations were not performed on these patients.
A second population of a total 24 patients who were not taking diuretics were then selected and randomized with a 1:1 ratio to take, in addition to their usual medication, either AkP05 or a diuretic (Table S4). These patients (n=12 treated additionally with AkP05, and n=12 age‐matched patients treated additionally with a diuretic) underwent 24‐hour arterial BP measurement monitoring and a CPET at baseline and after 4 weeks of treatment. At the end of the treatment period, SBP was significantly reduced in both groups in the morning (between 8 and 10 AM), (AkP05: from 137.3±13.6 to 124.2±14.3 mm Hg, P<0.0001; diuretic: from 140.3±11.68 to 124.8±10.55, P<0.0001) during the evening (between 8 and 10 PM) (AkP05: from 137.4±17.46 to 127.7±11.3 mm Hg, P=0.0003; diuretic: from 139.3±14.27 to 127.0±10.80 mm Hg, P=0.0003), and night (between 2 and 4 AM) (AkP05: from 121.8±14.1 to 112.0±8.1 mm Hg, P=0.0006; diuretic: from 128.8±14.34 to 120.8±11.5 mm Hg, P=0.0028); diastolic blood pressure was significantly reduced in both groups in the morning between 8 and 10 AM (AkP05: from 90.2±6.2 to 81.5±11.2 mm Hg, P=0.0004; diuretic: from 92.7±7.4 to 84.0±9.5 mm Hg, P<0.0003), but the decrease was not statistically significant at all other times (Figure 1J through 1M).
Of note, CPET revealed there was an increase in maximum VO2 during exercise and a significant reduction in maximum SBP only in the AkP05‐treated group. Moreover, exercise duration and maximum power generated during the test were indicative of the nutraceutical combination inducing significant improvements in both parameters (Table 1), a finding suggestive of it having an important impact on the patients’ functional capacity. The hypertensive patients treated with a diuretic also had reduced BP, but there were no changes in other CPET parameters. Moreover, the evaluation between the difference pre/post of AkP05 versus Diuretic group revealed a statistical significance and high effect size for SBPMAX, VO2/kg, MAX, Exercise duration, and maximal power (Table 2).
Table 1.
CPET Parameters Before and After Supplementation of Medication With AkP05 or a Diuretic
| Variable | AkP05 Group (n=12) | Measured Effect Size | Diuretic Group (n=12) | Measured Effect Size | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | P Value | Before | After | P Value | |||
| SBP rest, mm Hg | 136.1±10.5 | 124.6±11.7 | 0.0016 | −1.08 | 138.33±7.0 | 127.5±12.6 | >0.0001 | −1.91 |
| SBPMAX, mm Hg | 176.5±18.9 | 153.8±14.4 | 0.0031 | −0.97 | 175.6±16.8 | 182.9±11.4 | 0.1261 | 0.35 |
| DBP rest, mm Hg | 87.5±11.0 | 85.0±8.7 | 0.2030 | −0.25 | 88.3±14.9 | 87.3±10.9 | 0.4175 | −0.06 |
| DBPMAX, mm Hg | 110.7±12.1 | 103.1±14.5 | 0.0379 | −0.57 | 112.0±6.6 | 107. 8±11.3 | 0.1491 | −0.32 |
| METs, rest | 1.36±0.2 | 1.52±0.5 | 0.0947 | 0.40 | 1.3±0.4 | 1.5±0.5 | 0.1318 | 0.34 |
| METs, max | 8.4±1.8 | 8.7±1.6 | 0.0539 | 0.64 | 8.1±1.1 | 8.2±1.6 | 0.4534 | 0.03 |
| VO2/kg, rest (mL/min per kg) | 4.3±0.7 | 4.9±1.4 | 0.0930 | 0.41 | 4.2±1.0 | 4.3±1.0 | 0.3705 | 0.10 |
| VO2/kg, MAX (mL/min per kg) | 27.6±2.7 | 31.3±3.2 | >0.0001 | 1.80 | 28.0±3.3 | 27.7±3.3 | 0.1126 | −0.37 |
| Exercise duration (min) | 12.6±4.3 | 15.8±4.3 | 0.0050 | 0.90 | 12.3±1.5 | 12.1±2.0 | 0.3579 | −0.11 |
| Max. power (W) | 175.3±33.5 | 208.3±41.6 | 0.0219 | 1.46 | 176.9±28.1 | 168.1±32.8 | 0.0148 | −0.72 |
| FVC | 4.24±0.6 | 4.3±0.8 | 0.2044 | 0.25 | 4.4±0.8 | 4.4±0.8 | 0.4514 | 0.04 |
| FEV | 3.4±0.6 | 3.4±0.6 | 0.3966 | −0.08 | 3.4±0.3 | 3.3±0.3 | 0.0646 | −0.47 |
Data are mean±SD. Statistical analysis was performed using paired t test between before/after in the same patients’ group. Measured effect size was calculated by G*Power 3.1.9.4. CPET indicates cardiopulmonary exercise test; DBP rest, diastolic blood pressure measured at rest; DBPMAX, maximal diastolic blood pressure; FEV, forced expiratory volume in 1 second; FVC, forced vital capacity; Max. power (W), maximal power output; METs MAX, maximal 1 metabolic equivalent; METs rest, 1 metabolic equivalent measured at rest; SBP rest, systolic blood pressure measured at rest; SBPMAX, maximal systolic blood pressure; VO2/kg MAX, maximal respiratory oxygen uptake; VO2/kg rest, respiratory oxygen uptake measured at rest.
Table 2.
Unpaired t Test Results on Differences Between Pre/Post Supplementation With AkP05 or Diuretic
| Variable | Differences | Measured Effect Size | ||
|---|---|---|---|---|
| AkP05 (n=12) | Diuretic (n=12) | P Value | ||
| SBP rest, mm Hg | −11.3±10.5 | −10.8±5.7 | 0.8859 | 0.06 |
| SBPMAX, mm Hg | −22.7±23.3 | 7.1±20.5 | 0.0030 | 1.36 |
| VO2/kg, MAX, mL/min per kg | 3.74±2.1 | −0.29±0.8 | <0.0001 | 2.57 |
| Exercise duration, min | 3.18±3.5 | −0.15±1.4 | 0.0060 | 1.24 |
| Max. power, W | 33.08±22.7 | −8.75±12.1 | <0.0001 | 2.30 |
Data are presented as mean of differences±SD. Statistical analysis was performed using unpaired t test between mean of the differences before/after in AkP05 vs Diuretic group. Measured effect size for each variable was calculated using G*Power 3.1.9.4 with statistical test “Means: Difference between 2 independent means (2 groups), 2 tails.” Max. power (W) indicates maximal power output; SBPMAX, maximal systolic blood pressure; VO2/kg MAX, maximal respiratory oxygen uptake.
Thus, improvements in functional capacity of the cardiovascular, pulmonary, and muscular systems may be specifically attributable to the nutraceutical.
AkP05 Directly Evokes Vasorelaxation of Mouse Mesenteric Arteries
Based on the beneficial effects seen in the patients, and given the impossibility of characterizing the molecular mechanisms recruited by AKP05 in humans, we decide to investigate the vascular effects of the nutraceutical combination in experimental models.
We first investigated whether AkP05 modulated vascular function per se, using an ex vivo method in which mesenteric arteries were excised from mice and mounted on a myograph apparatus. Exposure to increasing doses of AkP05 evoked dose‐dependent vasorelaxation of arteries, an effect significantly reduced by pretreatment with the endothelial nitric oxide synthase (eNOS) inhibitor l‐nitro‐arginine methyl ester (l‐NAME) (Figure 2A). In agreement, molecular analysis of the vessels showed clear induction of eNOS phosphorylation on serine1177 upon exposure to AkP05 (Figure 2B). Moreover, staining of arteries with DAF‐FM demonstrated that NO was released from AkP05‐stimulated vessels (Figure 2C).
Figure 2.
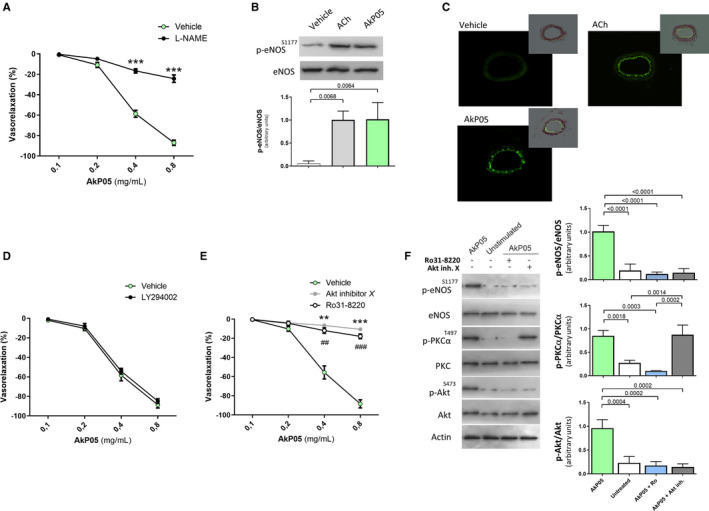
A, Dose–response curves of phenylephrine‐precontracted mesenteric arteries harvested from C57BL/6 mice to increasing doses of AkP05, in the presence and absence of the eNOS inhibitor l‐NAME (300 μmol/L). ***P<0.001 (n=5 for each group). B, Representative immunoblot (top) and densitometry (bottom) of p‐eNOS (S1177) and total eNOS in mesenteric arteries from C57BL/6 mice, exposed to vehicle, acetylcholine (ACh), or AkP05; (n=3 independent experiments). C, Representative micrographs of DAF‐FM fluorescent signal in mesenteric arteries treated with vehicle, ACh, or AkP05 (n=5 for each group). Inset: images of the fluorescent signal merged with images of sections counterstained with hematoxylin–eosin. Scale bar, 100 μm. D and E, Response of phenylephrine‐precontracted mesenteric arteries harvested from C57BL/6 mice to increasing doses of AkP05, in the presence of either the PI3K inhibitor LY294002 (D), Akt inhibitor X, or the PKC inhibitor Ro31‐8220 (E). **P<0.01; ## P<0.01; ***P<0.001; ### P<0.001 (n=5 for each group). F, Representative immunoblot blots (left) and densitometry (right) of p‐eNOS (S1177), total eNOS, p‐PKCα (T497), PKC antibody, p‐Akt (S473), total Akt, and β‐actin in mesenteric arteries from C57BL/6 mice exposed to ACh or AkP05 in the presence of Akt inhibitor X or Ro31‐8220; (n=3 for each group). eNOS indicates endothelial nitric oxide; l‐NAME, l‐nitro‐arginine methyl ester.
To investigate the mechanism through which AkP05 modulated NO metabolism, we performed a new set of ex vivo experiments using pharmacological inhibitors to block well‐known signaling pathways leading to eNOS activation.12 Inhibition of PI3K signaling with LY294002 did not modify AkP05‐evoked vasorelaxation (Figure 2D), excluding the involvement of this kinase in the nutraceutical's mechanism of action. By contrast, inhibition of Akt—one of the most important modulators of eNOS—abolished AkP05‐evoked vasorelaxation (Figure 2E).
Because classical PKC (protein kinase C) plays an important role in Akt activation,13 we then evaluated whether the PKC inhibitor Ro31‐8220 also removed AkP05's vasorelaxant ability. Indeed, PKC inhibition eliminated the nutraceutical's dose‐dependent vasorelaxation effect (Figure 2E), leading us to explore the sequence of proteins recruited in the pathway. Molecular analysis revealed that PKC inhibition completely blocked AkP05‐evoked Akt and eNOS activation, whereas upon Akt inhibition, eNOS continued to be inactive in the face of PKCα activation (Figure 2F). Thus, AkP05 recruits a PKCα/Akt/eNOS intracellular signaling cascade.
Differential Effects of Single Components on Vascular Function
After performing chemical characterization of the nutraceutical by UHPLC‐PDA‐MS/MS analyses (Data S2, Figure S1 and, Tables S5 and S6), we then set out to identify the component/s responsible for AkP05's vascular effects. We found that BM and PS were able to evoke dose‐dependent, eNOS‐inhibition‐sensitive vasorelaxation (Figure 3A and 3B), whereas neither GBE nor GTE modulated endothelial function (Figure 3C and 3D).
Figure 3.
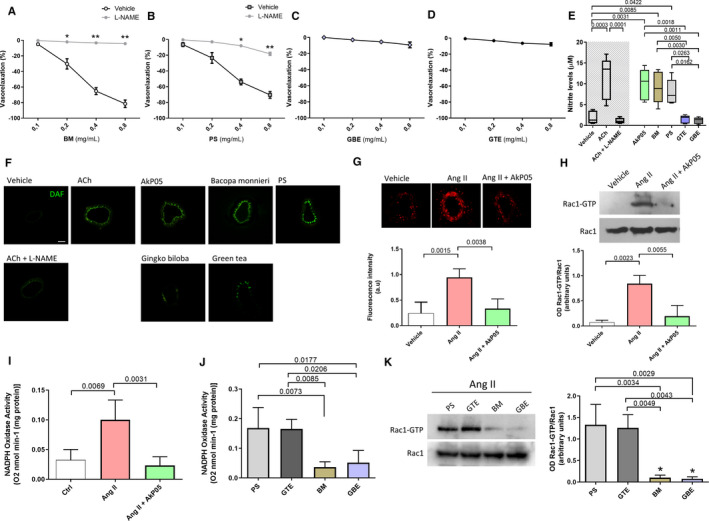
A through D, Dose–response curves of phenylephrine‐precontracted mesenteric arteries from C57BL/6 mice to increasing doses of single AkP05 components, in the presence and absence of the eNOS inhibitor l‐NAME (300 μmol/L). *P<0.05; **P<0.01 (n=4 for each group). BM, Bacopa monnieri; PS, phosphatidylserine; GBE, Ginkgo biloba extract; GTE, green tea extract. E, Nitrite levels measured in supernatants from HUVEC cultures treated with vehicle, 100 μmol/L acetylcholine (ACh), ACh plus l‐NAME, or the individual components contained in AkP05. Box and whisker representation; (N=5 for each group). F, Representative micrographs of DAF‐FM fluorescent signals from mesenteric arteries exposed to vehicle, ACh, ACh plus l‐NAME, AkP05, or single components (n=5 for each group). Scale bar, 100 μm. G, In situ detection of superoxide generation (top) and graph of total ROS production (bottom) in C57BL/6 mesenteric arteries exposed to vehicle, angiotensin II (Ang II), or Ang II after pretreatment with AkP05 (0.4 mg/mL for 1 hour), and qualitative measured with CM‐H2DCF/DA probe. Data are mean±SD; (4 independent experiments). H, Representative immunoblot (top) and densitometry (bottom) of phosphorylation levels of Rac1‐GTP and total Rac1 conducted on extracts of mouse mesenteric artery exposed to vehicle, Ang II, or Ang II plus AkP05; (n=3 independent experiments). I and J, The effect of AkP05 (I) and of its individual components (J) on nicotinamide adenine dinucleotide phosphate (NADPH)‐induced lucigenin chemiluminescence in C57BL/6 mice mesenteric arteries; (n=4). K, Representative immunoblot (left) and densitometry (right) conducted on extracts of mouse mesenteric artery exposed to Ang II plus BM, PS, GBE, or GTE. Semiquantitative analyses of phosphorylation levels of Rac1‐GTP and total Rac1 (n=3 independent experiments). eNOS indicates endothelial nitric oxide; l‐NAME, l‐nitro‐arginine methyl ester; HUVEC, human umbilical vein endothelial cell; ROS, reactive oxygen species.
These findings led us to quantitatively measure the stable form of NO (nitrite) released from HUVEC cultures exposed to single components. Only in the presence of AkP05, BM, or PS nitrite was released into the supernatant to a comparable extent as that observed with acetylcholine (ACh) (Figure 3E); GTE and GBE did not have any measurable effect on NO production in HUVECs, as also shown by DAF‐FM immunofluorescence signals (Figure 3F).
AkP05 Inhibits Oxidative Stress by Modulating NADPH Oxidase Activity
The level of NO can be modulated by increasing production through activation of eNOS as well as by improving its bioavailability, acting on oxidative stress modulation.14 In this regard, several nutraceutical compounds have potent antioxidant activities.15, 16 Thus, we tested the capacity of AkP05 to modulate reactive oxygen species released from vessels after exposure to Ang II, a potent inducer of reactive oxygen species.17
Pretreatment with AkP05 induced a marked reduction in Ang II‐evoked oxidative stress compared with vessels exposed to Ang II alone, as measured qualitatively by DHE (Figure 3G top) and quantitatively by CM‐H2DAF DA (Figure 3G bottom), a finding demonstrating that the nutraceutical combination has antioxidant properties.
Ang II induces reactive oxygen species production through the modulation of NOX, an enzyme that becomes active after the assembly of different subunits promoted by the small GTPase Rac1. Thus, in order to identify the molecular mechanism through which AkP05 reduces NOX activation, we performed a pull‐down assay on active Rac1. AkP05 significantly reduced Rac1 activation, as shown by reduced Rac1‐GTP compared with vessels exposed to Ang II alone (Figure 3H). To translate this result to the functional level, we measured NOX activity, finding a significant reduction of enzymatic activity after exposure to AkP05 (Figure 3I). The evaluation of single components on oxidative stress status revealed that BM and GBE were the only ones capable of reducing Ang II‐stimulated NOX activity in our time window (Figure 3J). The evaluation of Rac1 status in the presence of the different components revealed that BM and GBE were specifically able to reduce Rac1‐GTP formation, whereas GTE and PS were not (Figure 3K).
AkP05's Single Components Act Through Different Intracellular Mechanisms
Based on our observation of the direct NO‐dependent vascular action of the AkP05 components BM and PS, we tried to dissect the specific molecular mechanism recruited. Firstly, we tested the vasorelaxant properties of the components on ex vivo vessels under Akt inhibition. Of note, BM and PS both failed to modulate vascular function under this condition (Figure 4A and 4B), a finding suggesting that Akt represents a common node for the 2 compounds.
Figure 4.
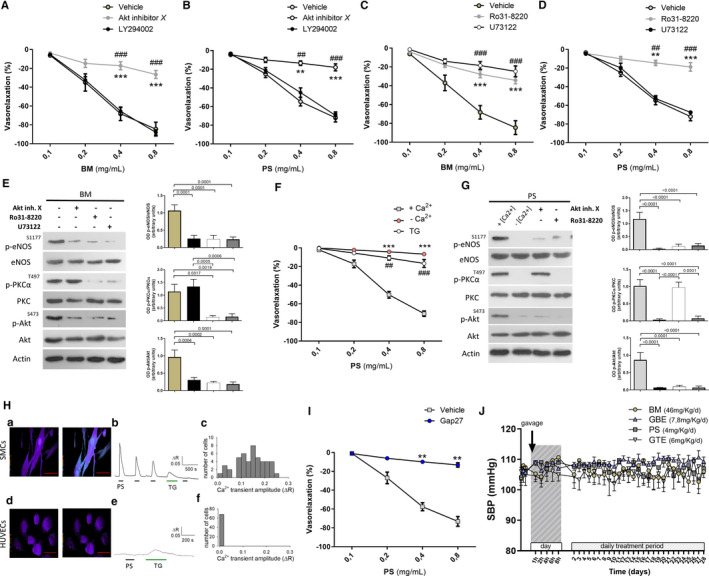
A through D, Dose–response curves of phenylephrine‐precontracted mesenteric arteries from C57BL/6 mice to increasing doses of Bacopa monnieri (A and C) or phosphatidylserine (B and D) under conditions of either Akt inhibition with inhibitor X or PI3K inhibition with LY294002 (A and B), or PKC inhibition with Ro31‐8220 or PLC inhibition with U73122 (C and D). **P<0.01, ***P<0.01; ## P<0.01, ### P<0.001 (n=4 for each group). BM, Bacopa monnieri; PS, phosphatidylserine. E, Representative immunoblots (left) and semiquantitative densitometric analysis (right) of protein extracts from mouse mesenteric arteries exposed to BM, for phosphorylation of eNOS on serine 1177, of PKCα on tyrosine 497, and of Akt on serine 473 (3 independent experiments). F, Dose–response curves of phenylephrine‐precontracted mesenteric arteries from C57BL/6 mice to increasing doses of PS in normal calcium medium (+ Ca2+), in the absence of calcium (− Ca2+), or in presence of thapsigargin (TG). ***P<0.01 vs + Ca2+; ## P<0.01, ### P<0.001 vs + Ca2+. (n=4). G, Representative immunoblots (left) and semiquantitative densitometric analysis (right) of protein extracts from mouse mesenteric arteries exposed to PS, for phosphorylation of eNOS on serine 1177, of PKCα on tyrosine 497, and of Akt on serine 473 (3 independent experiments). H, Top left, Digital images of the [Ca2+]i level before (left) and after (right) application of 0.4 mg/mL phosphatidylserine (PS) for 2 min to coronary artery smooth muscle cells (CASMCs) loaded with Fura2‐AM. [Ca2+]i is quantified as the ratio (R) between fluorescence emissions at 340 and 380 nm (acquisition at 510 nm), and represented as pseudocolors (red line=50 μm). Top middle, Typical time‐course of [Ca2+]i changes elicited in CASMCs by PS (horizontal black bar=2 min application) or thapsigargin (TG; 1 μmol/L) (horizontal green bar=5 min application); Top right, Histogram of PS‐induced Ca2+ transient amplitude distribution in CASMCs (47 cells from 7 fields); Bottom left, Same as in top but performed on human umbilical vein endothelial cells (HUVECs). Please note no change in [Ca2+]i following PS application; Bottom middle, Typical [Ca2+]i time‐courses in HUVECs during PS (horizontal black bar) or TG (horizontal green bar) application. Bottom right, Histogram of Ca2+ transient amplitude distribution in HUVECs, indicative of no response (68 cells from 10 fields). I, Dose–response curves of phenylephrine‐precontracted mesenteric arteries from C57BL/6 mice to increasing doses of phosphatidylserine (PS) in the presence of gap‐junction inhibition with GAP‐27. *P<0.05; **P<0.01 vs + Ca2+.# P<0.05; ## P<0.01 vs + Ca2+ (n=6). J, Systolic blood pressure (SBP) in wild‐type mice treated with vehicle or the different single components of AkP05 (n=3 for each group) at different time‐points from first gavage administration (day 1) and after 4 hours from continued daily administration for 4 weeks. eNOS indicates endothelial nitric oxide; PKC, protein kinase C; PLC, phospholipase C.
To find the upstream modulator of Akt, we tested the effects of BM and PS on vessels in the presence of a potent PI3K inhibitor.7 Under this condition, BM and PS continued to evoke dose‐dependent vasorelaxation (Figure 4A and 4B), a finding indicating that the kinase was not involved. In contrast, when we inhibited PKC—an important modulator of Akt—the vasorelaxant properties of both components were completely abolished (Figure 4C and 4D).
Among the mechanisms regulating PKC proteins, phospholipase C (PLC) plays a major role through the production of soluble intracellular second messengers.18 Upon pharmacological inhibition of PLC, the vascular action of BM was completely abolished (Figure 4C), an effect associated with a significant reduction of eNOS activation in vessels treated with inhibitors of PKC or PLC (Figure 4E). In contrast, inhibition of PLC did not alter response to PS (Figure 4D), a finding that led us to explore alternative intracellular signaling pathways involved in PKCα activation.
Intracellular calcium release is the leading mechanism regulating calcium‐dependent PKC. In the absence of Ca2+ or in the presence of thapsigargin—a noncompetitive inhibitor of sarco/endoplasmic reticulum Ca2+ efflux—PS did not evoke dose‐dependent vasorelaxation (Figure 4F), nor did it promote activation of PKCα or eNOS (Figure 4G). We then went on to evaluate Ca2+ mobilization by PS, measuring [Ca2+]i variations in HUVECs and coronary artery smooth muscle cells. PS evoked Ca2+ transients in all coronary artery smooth muscle cells tested (Figure 4H, top), but not in HUVECs (Figure 4H; bottom).
Smooth muscle cells and endothelial cells can be electrically and metabolically connected by gap junctions that allow the passage of currents and small signaling molecules, such as Ca2+, between cells.12, 19 Thus, to further investigate the effect of Ca2+ transients evoked by PS in smooth muscle cells, we performed vascular reactivity studies in the presence of GAP‐27, a mix of small peptides that block gap junctions by inhibiting connexin 43 and 37 (cx43, cx37). In the presence of GAP‐27, PS did not evoke any vascular effects, confirming a calcium‐dependent mechanism of action (Figure 4I).
Considering the important direct vascular action of the single components of AkP05, we investigated their modulation of BP levels in mice. Following the US Food and Drug Administration recommendations10 about the human equivalent dose conversion, we administered each component singly for 4 weeks via gavage, with the aim of identifying the cardiovascular active compound in the combination. Surprisingly, none of the individual compounds making up AkP05 was able to exert a hemodynamic effect on its own (Figure 4J).
Synergism of the Single Components Is Needed for AkP05's Hemodynamic Effect
Having seen that none of the individual components was able to modulate BP, we administered the complete nutraceutical combination orally at the human equivalent dose for 4 weeks in order to more reliably reflect the experimental condition applied to humans. Of note, SBP was significantly reduced after 4 hours from administration and continued to remain reduced for the whole observation period of 4 weeks (Figure 5A). This suggested that synergism between components is important for the hemodynamic action of AkP05. Moreover, vascular reactivity studies conducted on mesenteric arteries excised at the end of the treatment period revealed significantly increased Ach‐evoked endothelial vasorelaxation (Figure 5B), a finding consistent with the improvement of endothelial function observed in humans.
Figure 5.
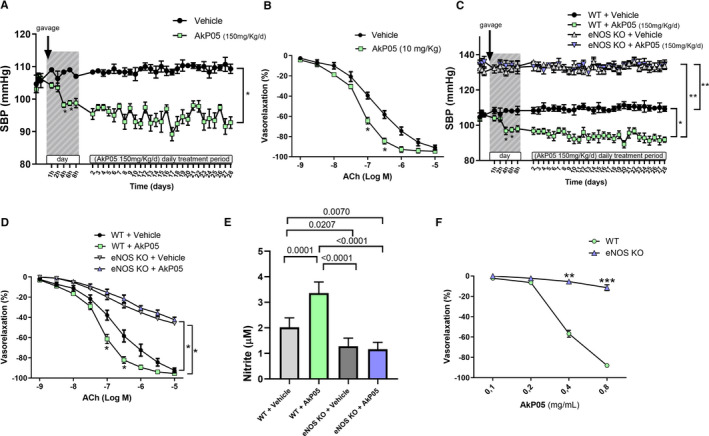
A, Time course of systolic blood pressure (SBP) measured noninvasively (A) in C57BL/6 mice receiving a daily oral dose of vehicle or AkP05 at different time‐points from first gavage administration (day 1) and after 4 hours from continued daily administration for 4 weeks. Arrow indicates start of administration via gavage. Data are mean±SEM (n=5 AkP05; n=3 Vehicle); *P<0.05 vs all. B, Dose–response curves to acetylcholine (ACh; 10−9 mol/L to 10−5 mol/L) of mesenteric arteries excised from mice at the end of treatment with of vehicle or AkP05; *P<0.05 (n=5 experiments). C, SBP in wild‐type (WT) and eNOS‐knockout (KO) mice during AkP05 or vehicle administration at different time‐points from first gavage administration (day 1) and after 4 hours from continued daily administration for 4 weeks. *P<0.05, **P<0.01; (n=5 for each group). D, Dose–response curves to acetylcholine (ACh; 10−9 mol/L to 10−5 mol/L) of mesenteric arteries obtained from wild‐type and eNOS deficient mice at the end of treatment with vehicle or AkP05; *P<0.05 (n=5 independent experiments). E, Nitrite concentration in serum collected at the end of 4‐week daily treatment in wild‐type and eNOS KO mice treated with vehicle or AkP05 (n=5). F, Dose–response curves of ex vivo mice mesenteric arteries from eNOS‐knockout mice are refractive to the vasorelaxant effect of AkP05. **P<0.01. ***P<0.001 (n=4 for each group).
The results obtained so far suggested that the vascular action of AkP05 is mediated by NO. Thus, to further corroborate our hypothesis, we administered the complete combination in eNOS‐knockout mice. As expected, administration of AkP05 did not evoke any hemodynamic effect in eNOS‐deficient mice (Figure 5C), strengthening the notion of an NO‐dependent action for AkP05 also in vivo. Of note, mesenteric arteries excised at the end of the treatment period from the eNOS‐deficient mice displayed altered vasorelaxation (Figure 5D). In addition, whereas serum nitrite was significantly increased in wild‐type mice treated with AkP05, such an increase was not seen in eNOS‐deficient mice (Figure 5E). Finally, ex vivo vascular reactivity studies on eNOS‐deficient vessels demonstrated an inability of the nutraceutical combination to evoke any direct dose‐dependent vasorelaxation in this setting (Figure 5F), a finding proving that eNOS represents the main target of AkP05's vascular action.
Discussion
Here, we demonstrate that the administration of a novel nutraceutical combination, called AkP05, to hypertensive patients on antihypertensive medication but with unsatisfactory BP control reduces BP, improves endothelial function, and increases serum NO concentration. These effects are associated with an increase of VO2 max uptake, stress tolerance, and maximal power output. To the best of our knowledge, this is the first study describing positive actions on the vasculature and exercise tolerance of a nutraceutical preparation in treated hypertensive patients with poor BP control.
Reduced endothelial function represents an early, subclinical stage of vascular alteration that precedes and accompanies the development of hypertension, contributing to increased cardiovascular complications.20, 21 Downregulated NO, caused by either reduced synthesis or by inactivation because of increased oxidative stress in the hypertensive setting, significantly contributes to endothelial dysfunction and to the rise of BP.22
Regarding exercise physiology, recent studies have reported that increasing NO production and bioavailability may enhance oxygen and nutrient delivery to tissues, improving exercise tolerance and recovery mechanisms. Based on this concept, we investigated the cardiovascular effects of AkP05 supplementation on these functional aspects, which represent clinically significant parameters defining the health status of hypertensive patients.23, 24 Over the past decades, CPET has garnered approval for the evaluation of cardiovascular status, exercise capacity, and gas exchange in hypertensive patients, demonstrating that the alteration of these parameters are predictive factors for hypertension progression, end‐organ damage, ischemic stroke, myocardial infarction, and total mortality. The good reliability and reproducibility of the test have made it a useful tool for the clinician. Higher maximal SBP and lower oxygen uptake during exercise have been considered powerful predictors of low survival rate in hypertensive patients. On this point, medicated hypertensive patients (regardless of whether disease is controlled or uncontrolled) have an exaggerated SBP increase upon maximal exercise testing,5, 6 a finding clearly suggesting an inability of common, current antihypertensive drugs to control BP throughout the patient's daily life.
Investigation into the possible beneficial roles of nutraceutical compounds on cardiovascular diseases is increasing, and a number of reports have described the effects produced by the single main compounds making up AkP05. One study assessed the possible cardiovascular action of Gingko biloba in humans, demonstrating that it had no effect on BP in elderly prehypertensive patients,25 whereas another reported that the administration of G. biloba for 3 months led to a significant reduction in SBP and diastolic blood pressure in prehypertensive adults aged 21 to 57 years.26 Thus, G. biloba may have different actions in different types of patients. A similar situation has emerged from the analysis of the literature on the effects of green tea in humans, extensively collected in a review by Khalesi et al,27 in which the actions of GTE consumption on BP modulation depended on the population studied and the duration of treatment. In contrast, only a single study has been performed on Bacopa monnieri (given at doses from 300 and 600 mg/d) in humans:28 it reported that continuous administration for 12 weeks did not exert any effect on BP. In our proof‐of‐concept study, we investigated the cardiovascular effect on uncontrolled hypertension of a combination of these natural compounds, which has already been shown to produce beneficial effects on the cognitive performance of hypertensive patients with controlled BP.6 We found that the use of AkP05 as an adjunct to therapy for hypertension may significantly aid in reducing maximal SBP response during exercise and in increasing maximal VO2 consumption, and so may contribute to cardiovascular risk reduction. Moreover, in agreement with the functional role of NO on exercise tolerance, AkP05‐treated patients had increased exercise duration and maximum power generated, a finding suggesting the effectiveness of the nutraceutical combination in inducing adaptations necessary for the improvement of the patients’ health status. Of note, in the parallel group of treated hypertensive patients in which AkP05 supplementation was substituted with the administration of a diuretic, there was a significant reduction of BP but no improvement in functional parameters, proving the specificity of AkP05‐evoked cardiovascular effects.
To test whether AkP05 exerts per se a direct action on vessels, we focused our attention on experimental models demonstrating both in ex vivo and in vivo studies that the effect of the new nutraceutical combination is mediated by a NO‐dependent mechanism since it failed to act in a condition of eNOS deficiency. Of note, daily oral administration for 4 weeks of AkP05 significantly reduced BP during the whole observation period, improved endothelial function, and increased the circulating level of nitrite, findings corroborating what we observed in hypertensive humans. In addition, its effects depend on the combination of the different compounds contained in AkP05, which alone are unable to exert any hemodynamic effect. To clarify this issue, we characterized the vascular action of the individual components on isolated vessels, finding that BM and PS—which are widely used to treat central nervous system disorders, and that promote mental health29 and cognitive performance30—evoked NO release through different molecular mechanisms converging on the PKCα/Akt signaling pathway necessary for phosphorylation of eNOS on its activation site. In particular, whereas BM recruits PLC, PS modulates intracellular calcium mobilization from smooth muscle to the endothelial layer.
A further notable finding emerging from our study on the single components is the antioxidant action exerted by BM and GBE, which blocks the main machinery generating vascular oxidative stress. Specifically, they inhibited activation of Rac‐1, a small GTP‐binding protein necessary for the activation of NADPH oxidase. In agreement, modulation of the PKCα/Akt/eNOS and Rac‐1/NADPH intracellular signaling pathways was crucial for AkP05's action on NO metabolism.
Strong correlation between the level of circulating NO and the modulation of exercise tolerance has been previously reported.31 Although it is generally accepted that regular physical activity stimulates NO release, which exerts beneficial effects on the vascular system and contributes to slowing down, suppressing, or even reversing cardiovascular diseases,32 it has been recently demonstrated in a small cohort of patients that assumption of nitrite supplementation for 3 weeks improves vasodilatory reserve and peak oxygen uptake in human subjects.33 In our present proof‐of‐concept study, significantly increased NO levels in the serum of hypertensive patients treated with the nutraceutical combination for 4 weeks led us to speculate that the improvements seen in exercise duration, maximal oxygen uptake, and maximal power output were because of an endogenous mechanism stimulating NO production.
The magnitude of the improvements produced by AkP05 supplementation suggests potential for the clinical management of hypertension. However, it is important to emphasize that although nutraceuticals can complement common antihypertensive therapy, they cannot replace it. Nonetheless, based on the efficacy revealed by our study, AkP05 might be considered as an adjunct for the treatment of hypertensive patients, improving both functional capacity and cardiovascular protection.
Study Limitations
Although we firmly believe that AkP05 has potent cardiovascular effects, it is important to emphasize that we have tested its properties only on a small population of treated hypertensive patients: it was difficult to enroll a large number of patients before assessing safety and efficacy of the combination. More studies are therefore needed to fully characterize and corroborate its efficacy in different types of cardiovascular risk patients. Second, we characterized the molecular mechanisms evoked by the single components of AkP05 without identifying the specific active compound/s (peptides or proteins) released following gastrointestinal digestion of the AkP05 tablet. Obviously, in order to improve the combination's effectiveness, more studies are needed to identify the active compounds released from each component. Our study also lacks pharmacokinetic characterization: this would have required serial blood sample collection to characterize the specific properties of AkP05, which would have been difficult to justify for this initial experimentation. Finally, this was a single‐center study, which may limit generalizability to other populations. Clearly, future studies are needed to eliminate these limitations.
Perspectives
Although more studies are needed to completely characterize the cardiovascular effects of AkP05, the blood pressure–lowering effect and increased effort tolerance evoked suggest that it could be used in cardiovascular disease prevention. Moreover, the identification of a novel nutraceutical combination able to improve NO production and bioavailability in the hypertensive condition may extend the clinician's armamentarium of approaches for better control of BP.
Disclosures
None.
Supporting information
Data S1. Supplemental Materials and Methods.
Data S2. Supplemental Results.
Table S1. Baseline Characteristics of Hypertensive Patients of First Study
Table S2. Pharmacological Therapies of Patients
Table S3. Statistical Analysis of Hypertensives That Achieve Target Blood Pressure in Placebo and Active Treated Groups
Table S4. Baseline Characteristics of Patients Undergoing Cardiopulmonary Exercise Test
Table S5. UHPLC‐PDA‐MS/MS Characterization of AkP05
Table S6. Quantification of the Most Abundant Peaks
Figure S1. AKP05 profile with UHPLC‐PDA‐MS/MS peak assignment.
(J Am Heart Assoc. 2020;9:e014923 DOI: 10.1161/JAHA.119.014923.)
Contributor Information
Bruno Trimarco, Email: trimarco@unina.it.
Carmine Vecchione, Email: cvecchione@unisa.it.
References
- 1. O'Donnell CJ, Ridker PM, Glynn RJ, Berger K, Ajani U, Manson JE, Hennekens CH. Hypertension and borderline isolated systolic hypertension increase risks of cardiovascular disease and mortality in male physicians. Circulation. 1997;95:1132–1137. [DOI] [PubMed] [Google Scholar]
- 2. Brandes RP. Endothelial dysfunction and hypertension. Hypertension. 2014;64:924–928. [DOI] [PubMed] [Google Scholar]
- 3. Hermann M, Flammer A, Luscher TF. Nitric oxide in hypertension. J Clin Hypertens (Greenwich). 2006;8:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chant B, Bakali M, Hinton T, Burchell AE, Nightingale AK, Paton JFR, Hart EC. Antihypertensive treatment fails to control blood pressure during exercise. Hypertension. 2018;72:102–109. [DOI] [PubMed] [Google Scholar]
- 5. Lim PO, MacFadyen RJ, Clarkson PB, MacDonald TM. Impaired exercise tolerance in hypertensive patients. Ann Intern Med. 1996;124:41–55. [DOI] [PubMed] [Google Scholar]
- 6. Giugliano G, Salemme A, De Longis S, Perrotta M, D'Angelosante V, Landolfi A, Izzo R, Trimarco V. Effects of a new nutraceutical combination on cognitive function in hypertensive patients. Immun Ageing. 2018;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carrizzo A, Conte GM, Sommella E, Damato A, Ambrosio M, Sala M, Scala MC, Aquino RP, De Lucia M, Madonna M, Sansone F, Ostacolo C, Capunzo M, Migliarino S, Sciarretta S, Frati G, Campiglia P, Vecchione C. Novel potent decameric peptide of spirulina platensis reduces blood pressure levels through a PI3K/AKT/eNOS‐Dependent Mechanism. Hypertension. 2019;73:449–457. [DOI] [PubMed] [Google Scholar]
- 8. Cudmore MJ, Hewett PW, Ahmad S, Wang KQ, Cai M, Al‐Ani B, Fujisawa T, Ma B, Sissaoui S, Ramma W, Miller MR, Newby DE, Gu Y, Barleon B, Weich H, Ahmed A. The role of heterodimerization between VEGFR‐1 and VEGFR‐2 in the regulation of endothelial cell homeostasis. Nat Commun. 2012;3:972. [DOI] [PubMed] [Google Scholar]
- 9. Carrizzo A, Ambrosio M, Damato A, Madonna M, Storto M, Capocci L, Campiglia P, Sommella E, Trimarco V, Rozza F, Izzo R, Puca AA, Vecchione C. Morus alba extract modulates blood pressure homeostasis through eNOS signaling. Mol Nutr Food Res. 2016;60:2304–2311. [DOI] [PubMed] [Google Scholar]
- 10. Reagan‐Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. [DOI] [PubMed] [Google Scholar]
- 11. Michelsen MM, Mygind ND, Pena A, Aziz A, Frestad D, Host N, Prescott E; Steering Committee of the i PS . Peripheral reactive hyperemia index and coronary microvascular function in women with no obstructive CAD: the iPOWER study. JACC Cardiovasc Imaging. 2016;9:411–417. [DOI] [PubMed] [Google Scholar]
- 12. Spinelli CC, Carrizzo A, Ferrario A, Villa F, Damato A, Ambrosio M, Madonna M, Frati G, Fucile S, Sciaccaluga M, Capunzo M, Cali G, Milanesi L, Maciag A, Puca AA, Vecchione C. LAV‐BPIFB4 isoform modulates eNOS signalling through Ca2+/PKC‐alpha‐dependent mechanism. Cardiovasc Res. 2017;113:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang L, Qiao G, Ying H, Zhang J, Yin F. TCR‐induced Akt serine 473 phosphorylation is regulated by protein kinase C‐alpha. Biochem Biophys Res Commun. 2010;400:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM, Antoniades C. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127:2209–2221. [DOI] [PubMed] [Google Scholar]
- 15. Carrizzo A, Puca A, Damato A, Marino M, Franco E, Pompeo F, Traficante A, Civitillo F, Santini L, Trimarco V, Vecchione C. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension. 2013;62:359–366. [DOI] [PubMed] [Google Scholar]
- 16. Forte M, Conti V, Damato A, Ambrosio M, Puca AA, Sciarretta S, Frati G, Vecchione C, Carrizzo A. Targeting nitric oxide with natural derived compounds as a therapeutic strategy in vascular diseases. Oxid Med Cell Longev. 2016;2016:7364138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griendling KK, Ushio‐Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Peptides. 2000;91:21–27. [DOI] [PubMed] [Google Scholar]
- 18. Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. [DOI] [PubMed] [Google Scholar]
- 19. Billaud M, Marthan R, Savineau JP, Guibert C. Vascular smooth muscle modulates endothelial control of vasoreactivity via reactive oxygen species production through myoendothelial communications. PLoS ONE. 2009;4:e6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. [DOI] [PubMed] [Google Scholar]
- 21. Carrizzo A, Lenzi P, Procaccini C, Damato A, Biagioni F, Ambrosio M, Amodio G, Remondelli P, Del Giudice C, Izzo R, Malovini A, Formisano L, Gigantino V, Madonna M, Puca AA, Trimarco B, Matarese G, Fornai F, Vecchione C. Pentraxin 3 induces vascular endothelial dysfunction through a P‐selectin/matrix metalloproteinase‐1 pathway. Circulation. 2015;131:1495–1505; discussion 1505. [DOI] [PubMed] [Google Scholar]
- 22. Lembo G, Iaccarino G, Vecchione C, Rendina V, Parrella L, Trimarco B. Insulin modulation of beta‐adrenergic vasodilator pathway in human forearm. Circulation. 1996;93:1403–1410. [DOI] [PubMed] [Google Scholar]
- 23. Dekleva M, Lazic JS, Pavlovic‐Kleut M, Mazic S, Stevanovic A, Soldatovic I, Markovic‐Nikolic N, Beleslin B. Cardiopulmonary exercise testing and its relation to oxidative stress in patients with hypertension. Hypertens Res. 2012;35:1145–1151. [DOI] [PubMed] [Google Scholar]
- 24. Wensel R, Opitz CF, Anker SD, Winkler J, Hoffken G, Kleber FX, Sharma R, Hummel M, Hetzer R, Ewert R. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation. 2002;106:319–324. [DOI] [PubMed] [Google Scholar]
- 25. Brinkley TE, Lovato JF, Arnold AM, Furberg CD, Kuller LH, Burke GL, Nahin RL, Lopez OL, Yasar S, Williamson JD; Ginkgo Evaluation of Memory Study Investigators . Effect of Ginkgo biloba on blood pressure and incidence of hypertension in elderly men and women. Am J Hypertens. 2010;23:528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalus JS, Piotrowski AA, Fortier CR, Liu X, Kluger J, White CM. Hemodynamic and electrocardiographic effects of short‐term Ginkgo biloba. Ann Pharmacother. 2003;37:345–349. [DOI] [PubMed] [Google Scholar]
- 27. Khalesi S, Sun J, Buys N, Jamshidi A, Nikbakht‐Nasrabadi E, Khosravi‐Boroujeni H. Green tea catechins and blood pressure: a systematic review and meta‐analysis of randomised controlled trials. Eur J Nutr. 2014;53:1299–1311. [DOI] [PubMed] [Google Scholar]
- 28. Peth‐Nui T, Wattanathorn J, Muchimapura S, Tong‐Un T, Piyavhatkul N, Rangseekajee P, Ingkaninan K, Vittaya‐Areekul S. Effects of 12‐week bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evid Based Complement Alternat Med. 2012;2012:606424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamkaew N, Scholfield CN, Ingkaninan K, Maneesai P, Parkington HC, Tare M, Chootip K. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J Ethnopharmacol. 2011;137:790–795. [DOI] [PubMed] [Google Scholar]
- 30. Downey LA, Kean J, Nemeh F, Lau A, Poll A, Gregory R, Murray M, Rourke J, Patak B, Pase MP, Zangara A, Lomas J, Scholey A, Stough C. An acute, double‐blind, placebo‐controlled crossover study of 320 mg and 640 mg doses of a special extract of Bacopa monnieri (CDRI 08) on sustained cognitive performance. Phytother Res. 2013;27:1407–1413. [DOI] [PubMed] [Google Scholar]
- 31. Tsukiyama Y, Ito T, Nagaoka K, Eguchi E, Ogino K. Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. J Clin Biochem Nutr. 2017;60:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nosarev AV, Smagliy LV, Anfinogenova Y, Popov SV, Kapilevich LV. Exercise and NO production: relevance and implications in the cardiopulmonary system. Front Cell Dev Biol. 2014;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zamani P, Tan V, Soto‐Calderon H, Beraun M, Brandimarto JA, Trieu L, Varakantam S, Doulias PT, Townsend RR, Chittams J, Margulies KB, Cappola TP, Poole DC, Ischiropoulos H, Chirinos JA. Pharmacokinetics and pharmacodynamics of inorganic nitrate in heart failure with preserved ejection fraction. Circ Res. 2017;120:1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Materials and Methods.
Data S2. Supplemental Results.
Table S1. Baseline Characteristics of Hypertensive Patients of First Study
Table S2. Pharmacological Therapies of Patients
Table S3. Statistical Analysis of Hypertensives That Achieve Target Blood Pressure in Placebo and Active Treated Groups
Table S4. Baseline Characteristics of Patients Undergoing Cardiopulmonary Exercise Test
Table S5. UHPLC‐PDA‐MS/MS Characterization of AkP05
Table S6. Quantification of the Most Abundant Peaks
Figure S1. AKP05 profile with UHPLC‐PDA‐MS/MS peak assignment.


