Abstract
Background
Supplementation with omega‐3 (n‐3) fatty acid or dietary fish may protect against atherosclerosis, but the potential mechanisms are unclear. Prior studies found modest triglyceride‐lowering effects and slight increases in LDL (low‐density lipoprotein) cholesterol. Limited evidence has examined n‐3 effects on more detailed lipoprotein biomarkers.
Methods and Results
We conducted a study of 26 034 healthy women who reported information on fish and n‐3 intake from a 131‐item food‐frequency questionnaire. We measured plasma lipids, apolipoproteins, and nuclear magnetic resonance spectroscopy lipoproteins and examined their associations with dietary intake of fish, total n‐3, and the n‐3 subtypes (eicosapentaenoic, docosahexaenoic, and α‐linolenic acids). Top‐ versus bottom‐quintile intake of fish and n‐3 were significantly associated with lower triglyceride and large VLDL (very‐low‐density lipoprotein) particles. Fish intake, but not total n‐3, was positively associated with total cholesterol, LDL cholesterol, apolipoprotein B, and larger LDL size, but only α‐linolenic acid was associated with lower LDL cholesterol. Total n‐3, docosahexaenoic acid, and α‐linolenic acid intake were also positively associated with larger HDL (high‐density lipoprotein) size and large HDL particles. High eicosapentaenoic acid intake was significantly associated with only a decreased level of VLDL particle concentration and VLDL triglyceride content. The n‐3 fatty acids had some similarities but also differed in their associations with prospective cardiovascular disease risk patterns.
Conclusions
Higher consumption of fish and n‐3 fatty acids were associated with multiple measures of lipoproteins that were mostly consistent with cardiovascular prevention, with differences noted for high intake of eicosapentaenoic acid versus docosahexaenoic acid and α‐linolenic acid that were apparent with more detailed lipoprotein phenotyping. These hypothesis‐generating findings warrant further study in clinical trials.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00000479.
Keywords: fish, n‐3, nuclear magnetic resonance lipoprotein subfractions
Subject Categories: Cardiovascular Disease, Diet and Nutrition, Women, Epidemiology
Clinical Perspective
What Is New?
It is unclear if differences exist in the lipid and lipoprotein profiles associated with dietary intake of fish, omega‐3 (n‐3) fatty acids, and each of the main n‐3 subtypes (eicosapentaenoic, docosahexaenoic, and α‐linolenic acids).
Although intake of fish and n‐3 fatty acids was significantly associated mostly with a lipoprotein profile consistent with cardiovascular benefit, there were notable differences for high intake of eicosapentaenoic acid (lower VLDL [very‐low‐density lipoprotein] particles and VLDL triglyceride) versus docosahexaenoic and α‐linolenic acids (larger LDL [low‐density lipoprotein] and HDL [high‐density lipoprotein] size) versus α‐linolenic acids (lower LDL cholesterol and apolipoprotein B100) that were apparent with detailed lipoprotein phenotyping using nuclear magnetic resonance spectroscopy.
What Are the Clinical Implications?
Among apparently healthy women, the n‐3 fatty acids differed in their lipoprotein profiles, with greater differences noted with detailed lipoprotein profiling compared with traditional lipids.
These findings may provide insight into potential mechanisms for cardiovascular disease benefit for n‐3 fatty acids.
Moderate consumption of fatty fish, a major source of omega‐3 (n‐3) fatty acids, is recommended by numerous dietary guidelines as a preventive strategy against atherosclerotic cardiovascular disease (CVD).1, 2, 3 Although some randomized trials and subsequent meta‐analyses have questioned the value of n‐3 fatty acid supplementation in CVD risk reduction,4 2 recent clinical trials—VITAL (Vitamin D and Omega‐3 Trial) and REDUCE IT (Reduction of Cardiovascular Events with Icosapent Ethyl‐Intervention)—found cardiovascular benefits with n‐3 supplementation.5, 6 Experimental studies have found pleiotropic effects of n‐3 fatty acids. In addition to anti‐arrhythmic effects,7 beneficial influences on a number of cardiometabolic risk factors have been demonstrated, including blood pressure,8 thrombosis, inflammation,9 vascular reactivity, and lipid levels.10, 11
Reported n‐3 fatty acid effects on lipids are mixed.12 When administered in supplemental doses, n‐3 fatty acids, particularly marine‐derived, produce a meaningful decrease in triglycerides,13 a modest increase in HDL (high‐density lipoprotein) cholesterol (HDL‐C),14 and an increase in LDL (low‐density lipoprotein) cholesterol (LDL‐C).15, 16 Typically, a decrease in triglycerides and an increase in HDL‐C would be expected to reduce CVD risk, but an increase in LDL‐C would typically be expected to increase CVD risk, producing an overall indeterminate effect. Habitual fish consumption or dietary n‐3 fatty acids, which contain lower amounts of n‐3 fatty acids compared with supplemental doses, have less substantial effects on traditional lipids.17
The association of habitual dietary n‐3 fatty acids with lipoprotein particle fractions is poorly characterized.18, 19 Studies examining the association of n‐3 fatty acids with lipoprotein particle subfractions have been limited to small nutritional studies of 4 g/day16, 20 or 5.9 g/day21 of supplemental doses of n‐3 fatty acids. It is increasingly appreciated that lipoprotein particles, and not their major lipid components, serve as both direct mediators of atherosclerosis and principal targets of lipid‐modifying therapies proven to reduce the risk of CVD.22, 23 Therefore, we investigated whether more detailed measures of LDL‐C, HDL‐C, and triglyceride‐rich remnant subfractions and other lipoprotein measures could provide insight into the CVD risk associations with dietary intake of fish, total n‐3, and the main n‐3 subtypes: eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and α‐linolenic acid (ALA). Using data from the WHS (Women's Health Study), a large cohort of female health professionals, we quantified the association between habitual fish intake and intake of n‐3 fatty acids and their subtypes with detailed lipoprotein subfraction profiles. We then examined these associations in relation to recently reported associations of these subfraction profiles with prospectively ascertained incident CVD events in the same population of participants24 to generate hypotheses regarding potential mechanisms of benefit for fish and n‐3 intake.
Methods
Study Population
Study participants were drawn from the WHS, a completed, randomized, double‐blind, placebo‐controlled trial of low‐dose aspirin and vitamin E in the primary prevention of CVD and cancer in US female healthcare professionals.25 The randomized intervention ended in 2004 with no significant reduction in the primary end points of the trial; therefore, the 2 intervention groups were combined for this analysis. All participants provided written informed consent, and the institutional review board of the Brigham and Women's Hospital (Boston, MA) approved the study protocol. At enrollment (1992–1996), study participants completed questionnaires on demographics, anthropometrics, medical history, and lifestyle behaviors. A blood sample at enrollment was requested, but not required, from the 39 876 women who were randomized; 28 345 women provided one. On an a priori basis, of the women who provided baseline blood samples, we excluded those who had prevalent diabetes mellitus (n=767), reported total energy intake of <600 or >3500 kcal/d (n=889), were missing >50% of the 131 items assessed on the food‐frequency questionnaire (FFQ; n=15), or were missing information on any lipid or lipoprotein variable (n=640). A total sample of 26 034 women were analyzed for the current study.
Dietary Assessment
A semiquantitative baseline FFQ, which was previously validated,8, 26, 27, 28, 29 captured information on 131 commonly consumed food items (including fish oil supplements). For each item, a portion size was specified, and each woman was asked how often, on average, during the past year she had consumed that amount. Nine responses were possible, ranging from “never or less than once a month” to “6 times a day.” A detailed description of the FFQ and the procedures used for calculating nutrient intake, as well as data on reproducibility and validity, were published previously.30 Nutrient scores were computed by multiplying the frequency of consumption of each unit of food from the FFQ by the nutrient content of that specific portion size of the food according to food composition tables from the US Department of Agriculture.31
Fish consumption was obtained through 4 items from the FFQ: participants were asked to report their average consumption of canned tuna fish (portion size: 85–113 g); other dark‐flesh fish such as mackerel, salmon, sardines, bluefish, and swordfish (portion size: 85–142 g); light‐flesh fish (portion size: 85–142 g); and shrimp, lobster, or scallops (or all 3) as a main dish. Possible responses included never, <1 time/month, 1 to 3 times/month, 1 time/week, 2 to 4 times/week, 1 time/day, 2 to 3 times/day, 4 to 5 times/day, and ≥6 times/day. We converted individual responses into servings per day by using the midpoint for each category. We summed the frequency of consumption of canned tuna; dark fish; other fish; and shrimp, lobster, and scallops as a main dish to obtain a fish variable and created quintiles of fish consumption.
Dietary n‐3 fatty acids were also derived from the FFQ. We calculated the marine‐derived intake of EPA and DHA by assigning grams per serving as follows: 1.51 g for dark‐flesh fish; 0.42 g for canned tuna fish; 0.48 g for light‐flesh fish; and 0.32 g for shrimp, lobster, or scallops. These n‐3 fatty acids values were derived by weighting the mean values of n‐3 fatty acids for the most commonly caught types of fish in US catches in 1984 (according to the US Department of Commerce), as described elsewhere.32 Intake of ALA fatty acids, obtained primarily from plant sources, and other n‐3 fatty acids was also estimated and used to calculate total n‐3 fatty acid intake. The total marine n‐3, EPA, DHA, and ALA fatty acid intakes were individually adjusted for energy intake using the residual method.33 For each adjusted n‐3 fatty acid intake, we created quintiles of the exposure variables.
Laboratory Measurements
EDTA blood samples were obtained at the time of enrollment into the WHS and stored in vapor‐phase liquid nitrogen (−170°C). In a laboratory (N. Rifai, Children's Hospital, Boston, MA) certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization Program, baseline samples were thawed and analyzed for standard lipids and apolipoproteins.24 Standard lipids were measured directly with reagents from Roche Diagnostics. Apolipoproteins B100 (apo B100) and A‐I were measured with immunoturbidometric assays (DiaSorin).
Samples for lipoprotein particle analysis by proton nuclear magnetic resonance (NMR) spectroscopy were shipped to LipoScience (now LabCorp), as reported previously (Lipoprofile® version 3).24 Particle concentrations of lipoproteins of different sizes were calculated from the measured amplitudes of their spectroscopically distinct lipid methyl group signals. Weighted‐average lipoprotein particle sizes (diameter, Ø nm) were derived from the sum of the diameter of each subclass multiplied by its relative mass percentage based on the amplitude of its methyl NMR signal.34 The particle diameter ranges are reported in Table S1.
Ascertainment of Other Clinical Factors
Demographic data were collected at baseline. Self‐reported baseline weight and height were used to compute the body mass index (weight in kilograms divided by height in meters squared). Self‐reported walking, stair climbing, and participation in 8 groups of recreational activities were obtained to estimate the energy expended on physical activity.35 In addition, information on prevalence of hypertension, hypercholesterolemia, parental history of diabetes mellitus, menopausal status, postmenopausal hormone therapy, smoking, and alcohol consumption was obtained at baseline.36
Statistical Analyses
Statistical analyses were conducted using SAS (v9.1; SAS Institute). All tests for significance were performed at α=0.05, 2‐tailed. Log transformations were used for triglycerides and for all NMR‐derived lipoprotein variables before calculating inferential statistics. The small LDL particle concentration variable presented a clearly bimodal distribution; therefore, we dichotomized and represented it with 2 different values to describe the mean scores, dividing the women into 2 groups according to their small LDL particle concentration (small A: <164 nmol/L; small B: ≥164 nmol/L).
We divided women according to quintiles of the exposure variables. We explored the median of the baseline demographic and anthropometric values across the quintiles of the exposure variables and used a Wilcoxon rank sum test (continuous variables) or a χ2 test (categorical variables) to compare the baseline values of the different groups.
To examine the relationship of intake of habitual fish, n‐3, and specific subtypes of n‐3 (EPA, DHA and ALA fatty acids) in relation to lipids and lipoprotein profiles, we computed the geometric mean (with 95% CI) for log‐transformed variables and the raw mean±SD for natural‐scale variables across quintiles of exposure variables. We then used multivariable linear regression models to calculate the least squares means (with 95% CI) and β coefficients (with SE) for all lipids and lipoprotein variables across quintiles of the nutritional exposure variables after adjusting for demographic, clinical, and dietary factors. We tested for trend across quintiles of the exposure variables using category medians modeled as a continuous variable. We used isocaloric multivariable nutrient‐density models with energy‐adjusted exposure variables in which the n‐3 fats replaced the percentage of energy from carbohydrates, as reported previously.37
For fish intake, multivariable models were adjusted for age and total energy (reported as model 1), and then additionally adjusted for lifestyle factors, including smoking, alcohol use, body mass index, exercise, menopausal status, use of hormone therapy, hypertension, antihypertensive treatment, hypercholesterolemia, treatment for high cholesterol, parental history of coronary heart disease, energy‐adjusted glycemic index, multivitamin use, aspirin use, red meat consumption, and fruit and vegetable consumption (reported as model 2).
For n‐3 intake, multivariable models were adjusted as described but were adjusted for energy‐adjusted saturated fats, energy‐adjusted monounsaturated fats, energy‐adjusted transfat, energy‐adjusted n‐6, and energy‐adjusted proteins. When EPA, DHA, and ALA were modeled, they were additionally adjusted.
To facilitate the visualization of differences between quintiles, we expressed the percentage of difference in the expected value of the outcome due to differences among quintile 2 (Q2) through Q5 compared with Q1 using equation (1) for the log‐transformed output variables and equation (2) for the natural‐scale outcome variables:
| (1) |
| (2) |
where i=2, 3, 4, and 5 corresponds to the β coefficients of Q2 through Q5 and μ corresponds to the quintile mean.
Finally, to understand the nature and extent of the effect of lipid and lipoprotein differences associated with fish and n‐3 intake on clinical CVD events, we used previously published results of the same cohort in which the contribution of the lipoprotein and lipid profiles to risk for prospectively ascertained CVD events was reported using Cox regression model hazard ratio (HR) values.24 By using the HR, we identified NMR‐derived variables that were associated with a lower risk for CVD, including larger HDL and LDL particle sizes, elevated levels of HDL‐C, and elevated large HDL particle concentration; conversely, variables that were associated with a higher risk for CVD, including increased levels of the small LDL particle concentration, increased levels of all subclasses of VLDL (very‐low‐density lipoprotein) particle concentrations, total cholesterol (TC), LDL‐C and triglyceride concentration.
To easily visualize the global impact on CVD events of all variables at the same time, we plotted the percentage of the difference in the expected value of outcome related to differences between the highest (Q5) and lowest (Q1) intake groups. The lipid and lipoprotein variables were sorted in descending order of their previously mentioned HRs on a colored red to green heat map according to their contribution toward CVD incident risk.
The data supporting the findings of this study are available to researchers on request from the WHS data usage review committee.
Results
Among the 26 034 apparently healthy women at baseline, habitual fish and energy‐adjusted fatty acid intake ranged from 0 to 5.1 servings/day for fish, 0.27 to 5.05 g/day for total n‐3, 0 to 1.72 g/day for DHA, 0.01 to 1.35 g/day for EPA, and 0.26 to 4.78 g/day for ALA. The baseline characteristics according to quintiles of total fish intake and n‐3 intake adjusted by total calorie intake are shown in Tables S2 and S3. Women consuming greater amounts of fish and n‐3 were older, had higher body mass index, and were more likely to be active and postmenopausal (and treated with hormone therapy) and to have hypertension but were less likely to be current smokers. Intake of fish and n‐3 was also positively associated with intake of fruits, nuts, vegetables, dietary magnesium, and cereal fiber and inversely associated with saturated and transfat intake.
Table 1 depicts the raw and multivariable adjusted means for LDL‐related variables (lipids, apolipoproteins, and NMR subfractions) with fish and total n‐3 intake. Greater fish intake quintile (Q5 versus Q1) was positively associated with higher mean levels for TC, LDL‐C, and apo B100 levels (P trend ranges from <0.0001 to 0.03), but these associations were not significant for n‐3 intake. When the more detailed NMR LDL measurements were analyzed, we found that only the large LDL particle concentration was higher for greater fish and n‐3 intake (up to 10±4%, P trend<0.01) but not the small LDL subfraction. We consistently found a positive association between fish (and n‐3 intake) with larger LDL mean particle size (P trend ranges from <0.001 to 0.03).
Table 1.
Raw Mean±SD and Adjusted Geometric Mean (95% CI) of LDL‐Related Variables According to Fish and n‐3 Intake
| Fish | n‐3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | P Trend | Q1 | Q3 | Q5 | P Trend | |
| n | 5839 | 5465 | 5617 | 5248 | 4991 | 5123 | ||
| Intake, median [min, max]a | 0.07 [0, 0.07] | 0.21 [0.2, 0.21] | 0.5 [0.43, 0.64] | 0.95 [0.86, 1.02] | 1.35 [1.31, 1.39] | 1.89 [1.77, 2.1] | ||
| LDL‐C, mg/dL | ||||||||
| Raw mean | 123±34 | 124±33 | 125±35 | 123±34 | 124±33 | 125±35 | ||
| Adjusted meanb | 123 (122–124) | 124 (123–124) | 125 (124–126) | 0.01 | 125 (124–126) | 124 (123–125) | 124 (123–125) | 0.61 |
| Adjusted meanc | 123 (122–124) | 124 (123–124) | 124 (123–125) | 0.03 | 124 (123–125) | 123 (122–124) | 123 (122–124) | 0.08 |
| TC, mg/dL | ||||||||
| Raw mean | 210±41 | 212±41 | 213±42 | 210±42 | 211±41 | 213±42 | ||
| Adjusted meanb | 210 (209–211) | 212 (211–213) | 213 (212–214) | <0.0001 | 212 (210–213) | 211 (210–212) | 212 (211–213) | 0.43 |
| Adjusted meanc | 210 (210–211) | 212 (211–212) | 212 (211–212) | 0.01 | 212 (211–213) | 211 (210–212) | 211 (210–212) | 0.36 |
| Apo B100, mg/dL | ||||||||
| Raw mean | 103±27 | 103±27 | 104±28 | 103±28 | 104±28 | 104±28 | ||
| Adjusted meanb | 103 (102–103) | 103 (102–104) | 104 (104–105) | <0.001 | 104 (103–105) | 104 (103–104) | 103 (103–104) | 0.73 |
| Adjusted meanc | 102 (102–103) | 103 (102–104) | 104 (103–104.2) | 0.005 | 103 (103–104) | 103 (103–104) | 103 (102–103.4) | 0.36 |
| LDL size (Ø nm) | ||||||||
| Geometric mean | 21.05 (21.03–21.06) | 21.08 (21.06–21.1) | 21.08 (21.06–21.09) | 21.06 (21.04–21.08) | 21.07 (21.05–21.09) | 21.07 (21.05–21.09) | ||
| Adjusted meanb | 21.04 (21.02–21.06) | 21.08 (21.06–21.09) | 21.09 (21.07–21.1) | <0.001 | 21.02 (21–21.04) | 21.08 (21.06–21.09) | 21.1 (21.08–21.12) | <0.0001 |
| Adjusted meanc | 21.06 (21.04–21.08) | 21.06 (21.05–21.08) | 21.09 (21.07–21.11) | 0.03 | 21.03 (21.01–21.05) | 21.08 (21.06–21.09) | 21.1 (21.08–21.12) | <0.001 |
| LDL particles | ||||||||
| Total, nm/L | ||||||||
| Geometric mean | 1188 (1178–1198) | 1188 (1179–1198) | 1208 (1198–1219) | 1188 (1177–1198) | 1196 (1185–1207) | 1209 (1198–1220) | ||
| Adjusted meanb | 1189 (1179–1199) | 1189 (1179–1199) | 1206 (1196–1216) | 0.005 | 1204 (1192–1216) | 1195 (1185–1206) | 1195 (1183–1207) | 0.49 |
| Adjusted meanc | 1182 (1173–1191) | 1192 (1183–1202) | 1195 (1186–1205) | 0.02 | 1196 (1185–1207) | 1190 (1180–1199) | 1186 (1174–1197) | 0.35 |
| IDL, nm/L | ||||||||
| Geometric mean | 146 (143–149) | 146 (143–149) | 146 (144–149) | 151 (149–154) | 144 (142–147) | 141 (139–144) | ||
| Adjusted meanb | 146 (143–148) | 146 (144–149) | 147 (144–149) | 0.78 | 147 (144–151) | 144 (142–147) | 145 (142–148) | 0.90 |
| Adjusted meanc | 147 (145–150) | 145 (143–148) | 145 (142–148) | 0.22 | 147 (143–150) | 144 (141–147) | 144 (141–148) | 0.75 |
| Large, nm/L | ||||||||
| Geometric mean | 466 (457–475) | 483 (473–492) | 492 (482–501) | 460 (450–469) | 483 (473–493) | 499 (489–509) | ||
| Adjusted meanb | 462 (453–471) | 483 (474–492) | 495 (486–505) | <0.0001 | 455 (445–466) | 485 (475–495) | 498 (486–510) | 0.001 |
| Adjusted meanc | 469 (460–479) | 479 (469–489) | 498 (488–508) | 0.006 | 458 (447–470) | 485 (475–496) | 496 (484–509) | 0.006 |
| Small A, nm/L | ||||||||
| Geometric mean | 62 (60.8–63.3) | 64.4 (63.2–65.6) | 64 (62.7–65.3) | 60.4 (59.1–61.7) | 63.9 (62.6–65.3) | 65.4 (64–66.7) | ||
| Adjusted meanb | 62 (60.8–63.2) | 64.3 (63.1–65.6) | 64.1 (62.9–65.3) | 0.03 | 62.2 (60.8–63.6) | 64 (62.7–65.3) | 63.5 (62.1–65) | 0.24 |
| Adjusted meanc | 62 (60.8–63.3) | 63.8 (62.6–65.1) | 63.8 (62.5–65.1) | 0.09 | 61.5 (60–63) | 64 (62.7–65.4) | 63.6 (62–65.2) | 0.13 |
| Small B, nm/L | ||||||||
| Geometric mean | 661 (650–671) | 654 (643–666) | 663 (653–674) | 658 (647–669) | 657 (646–668) | 669 (657–680) | ||
| Adjusted meanb | 661.8 (651.7–672) | 654 (643–666) | 661 (651–672) | 0.59 | 669 (657–682) | 657 (646–668) | 657 (644–670) | 0.34 |
| Adjusted meanc | 658 (647–668) | 658 (647–670) | 653 (642–663) | 0.92 | 668 (656–681) | 657 (646–668) | 650 (637–663) | 0.13 |
Data are shown as geometric mean (95% CI) for log‐transformed variables and raw mean±SD for natural‐scale variables across quintiles of exposure variable intake, except as noted. Apo indicates apolipoprotein; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; LDL‐C, low‐density lipoprotein cholesterol; n‐3, omega‐3; Q, quintile; TC, total cholesterol.
Fish intake shown as servings per day, n‐3 intake shown as grams per day.
Model 1: the adjusted means are estimated from linear regression models adjusted for age (continuous) and total energy (quintiles; plus energy‐adjusted saturated fats [quintiles], energy‐adjusted monounsaturated fats [quintiles], energy‐adjusted transfat [quintiles], energy‐adjusted total n‐6 [quintiles], and energy‐adjusted proteins [quintiles] for n‐3 intake models).
Model 2: adjusted as in model 1 but including smoking (current, past, never), alcohol use (rarely/never, 1–3 drinks/mo, 1–6 drinks/wk, and 1 drink/d), body mass index (continuous), exercise (quintiles of metabolic equivalent task hours per week), menopausal status (premenopausal, uncertain, postmenopausal), use of hormone therapy (current, past/never), hypertension (systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg), antihypertensive treatment (yes or no), hypercholesterolemia (total cholesterol of at least 240 mg/dL), treatment for high cholesterol (yes or no), parental history of coronary heart disease (yes or no), energy‐adjusted glycemic index (quintiles), multivitamin use (current, past, and never), aspirin use (current >1 time/wk), and red meat consumption and fruit and vegetable consumption (both quintiles).
Table 2 depicts the raw and the adjusted means for VLDL and remnant particle‐related variables with fish and n‐3 intake. Notably, the associations of the triglycerides and the NMR subfractions with intake of fish and total n‐3 were in the same direction—women consuming higher amounts of both exposure variables had up to 4% lower levels of total triglycerides (P trend=0.05 and <0.01, respectively) and NMR VLDL‐triglyceride content (P trend<0.0001) when Q1 and Q5 were compared. Women consuming greater amounts of fish (or n‐3) also had smaller VLDL size (P trend=0.07 and <0.01, respectively) and lower concentration of total VLDL particles (P trend=0.01 and <0.001, respectively). In particular, this group also had up to a 15% lower concentration of large VLDL particles (P trend<0.0001), lower medium VLDL particles (P trend≤0.01), and no significant differences in the small VLDL particles (P trend≥0.1) in the multivariable models.
Table 2.
Raw Mean±SD and Adjusted Geometric Mean (95% CI) of VLDL‐Related Variables According to Fish and n‐3 Intake
| Fish | n‐3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | P Trend | Q1 | Q3 | Q5 | P Trend | |
| n | 5839 | 5465 | 5617 | 5248 | 4991 | 5123 | ||
| Intake, median [min, max]a | 0.07 [0, 0.07] | 0.21 [0.2, 0.21] | 0.5 [0.43, 0.64] | 0.95 [0.86, 1.02] | 1.35 [1.31, 1.39] | 1.89 [1.77, 2.1] | ||
| TG, mg/dL | ||||||||
| Geometric mean | 123 (121–125) | 120 (119–122) | 122 (120–123) | 123 (121–125) | 123 (121–125) | 121 (119–123) | ||
| Adjusted mean | 123 (122–125) | 120 (119–122) | 121 (119–123) | 0.14 | 125 (123–127) | 123 (121–124) | 119 (117–121) | 0.007 |
| Adjusted mean | 122 (121–124) | 121 (120–123) | 119 (118–121) | 0.05 | 124 (122–126) | 122 (120–124) | 118 (116–120) | 0.001 |
| VLDL size (Ø nm) | ||||||||
| Geometric mean | 51.11 (50.93–51.29) | 50.96 (50.78–51.15) | 51.09 (50.91–51.28) | 51.51 (51.31–51.72) | 51 (50.81–51.19) | 50.65 (50.46–50.84) | ||
| Adjusted mean | 51.15 (50.97–51.34) | 50.97 (50.78–51.15) | 51.04 (50.85–51.22) | 0.84 | 51.28 (51.06–51.5) | 51.01 (50.82–51.21) | 50.85 (50.62–51.07) | 0.01 |
| Adjusted mean | 51.25 (51.06–51.43) | 51.01 (50.82–51.19) | 50.89 (50.7–51.08) | 0.07 | 51.27 (51.04–51.5) | 51.01 (50.81–51.21) | 50.75 (50.52–50.98) | 0.007 |
| VLDL particles | ||||||||
| Total, nm/L | ||||||||
| Geometric mean | 56.9 (56.3–57.6) | 55.6 (54.9–56.2) | 54.9 (54.2–55.6) | 55.4 (54.7–56) | 56.9 (56.2–57.6) | 56.3 (55.6–57.1) | ||
| Adjusted mean | 57.1 (56.4–57.8) | 55.5 (54.9–56.2) | 54.7 (54–55.4) | <0.0001 | 57.6 (56.8–58.5) | 56.7 (56–57.4) | 54.5 (53.7–55.3) | <0.0001 |
| Adjusted mean | 56.2 (55.5–56.9) | 55.7 (55.1–56.4) | 54.9 (54.2–55.6) | 0.01 | 57 (56.2–57.9) | 56.5 (55.8–57.3) | 54.6 (53.8–55.5) | <0.001 |
| Large, nm/L | ||||||||
| Geometric mean | 2.59 (2.53–2.66) | 2.4 (2.33–2.47) | 2.41 (2.34–2.48) | 2.71 (2.63–2.78) | 2.52 (2.44–2.59) | 2.29 (2.22–2.36) | ||
| Adjusted mean | 2.63 (2.56–2.7) | 2.4 (2.34–2.47) | 2.37 (2.3–2.43) | <0.0001 | 2.71 (2.62–2.8) | 2.51 (2.44–2.58) | 2.31 (2.23–2.38) | <0.0001 |
| Adjusted mean | 2.62 (2.55–2.7) | 2.41 (2.35–2.48) | 2.32 (2.26–2.39) | <0.0001 | 2.69 (2.6–2.78) | 2.48 (2.41–2.56) | 2.28 (2.2–2.36) | <0.0001 |
| Medium, nm/L | ||||||||
| Geometric mean | 13.3 (13.0–13.6) | 12.9 (12.6–13.2) | 12.8 (12.5–13.1) | 13.1 (12.8–13.4) | 13.3 (13.0–13.6) | 12.8 (12.5–13.1) | ||
| Adjusted mean | 13.4 (13.2–13.7) | 12.9 (12.6–13.2) | 12.7 (12.4–12.9) | <0.0001 | 13.6 (13.3–14) | 13.2 (12.9–13.5) | 12.4 (12.1–12.8) | <0.0001 |
| Adjusted mean | 13.2 (12.9–13.5) | 12.9 (12.6–13.2) | 12.6 (12.3–12.9) | 0.01 | 13.4 (13–13.8) | 13.1 (12.7–13.4) | 12.4 (12.1–12.8) | 0.003 |
| Small, nm/L | ||||||||
| Geometric mean | 36.4 (35.9–36.9) | 35.6 (35.1–36.2) | 35 (34.5–35.6) | 34.9 (34.4–35.5) | 36.4 (35.9–37) | 36.6 (36.0–37.2) | ||
| Adjusted mean | 36.4 (35.9–36.9) | 35.6 (35.1–36.1) | 35 (34.5–35.5) | <0.0001 | 36.5 (35.8–37.1) | 36.3 (35.8–36.9) | 35.3 (34.7–36) | 0.04 |
| Adjusted mean | 35.8 (35.3–36.3) | 35.8 (35.2–36.3) | 35.3 (34.8–35.9) | 0.13 | 36.2 (35.5–36.9) | 36.3 (35.7–36.9) | 35.5 (34.8–36.2) | 0.21 |
| VLDL‐TG, mg/dL | ||||||||
| Geometric mean | 76 (75.1–77) | 73.7 (72.7–74.7) | 73.2 (72.3–74.2) | 75.4 (74.4–76.5) | 75.6 (74.5–76.6) | 73.4 (72.4–74.4) | ||
| Adjusted mean | 76.4 (75.5–77.4) | 73.7 (72.7–74.7) | 72.8 (71.8–73.7) | <0.0001 | 77.5 (76.3–78.7) | 75.4 (74.4–76.4) | 71.9 (70.7–73) | <0.0001 |
| Adjusted mean | 75.6 (74.6–76.6) | 74 (73–74.9) | 72.5 (71.5–73.5) | <0.0001 | 76.6 (75.4–77.9) | 75.1 (74–76.1) | 71.7 (70.6–72.9) | <0.0001 |
Data are shown as geometric mean (95% CI) for log‐transformed variables and raw mean±SD for natural‐scale variables across quintiles of exposure variable intake, except as noted. Adjustments for models 1 and 2 are shown in Table 1. n‐3 indicates omega‐3; Q, quintile; TG, triglycerides; VLDL, very‐low‐density lipoprotein.
Fish intake shown as servings per day, n‐3 intake shown as grams per day.
Associations of fish and n‐3 intake with the HDL‐related variables are reported in Table 3. Greater fish and n‐3 intake was positively associated with a larger HDL size (P trend=0.05 and <0.001, respectively). The increased HDL size was consistent with a statistically significant redistribution between large and medium HDL subfractions for the same amount of HDL‐C.
Table 3.
Raw Mean±SD and Adjusted Geometric Mean (95% CI) of HDL‐Related Variables According to Fish and n‐3 Intake
| Fish | n‐3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | P Trend | Q1 | Q3 | Q5 | P Trend | |
| n | 5839 | 5465 | 5617 | 5248 | 4991 | 5123 | ||
| Intake, median [min, max]a | 0.07 [0, 0.07] | 0.21 [0.2, 0.21] | 0.5 [0.43, 0.64] | 0.95 [0.86, 1.02] | 1.35 [1.31, 1.39] | 1.89 [1.77, 2.1] | ||
| HDL‐C, mg/dL | ||||||||
| Raw mean | 53.3±14.7 | 54.7±15.5 | 54.2±15 | 53.1±14.4 | 54±14.8 | 54.8±15.3 | ||
| Adjusted mean | 53 (52.7–53.4) | 54.7 (54.3–55.1) | 54.5 (54.1–54.9) | <0.0001 | 53.2 (52.8–53.7) | 54.1 (53.6–54.5) | 54.5 (54.1–55) | 0.0078 |
| Adjusted mean | 53.8 (53.5–54.2) | 54.4 (54–54.8) | 54.3 (54–54.7) | 0.12 | 53.6 (53.2–54.1) | 54.1 (53.7–54.5) | 54.4 (53.9–54.9) | 0.13 |
| Apo A‐I, mg/dL | ||||||||
| Raw mean | 150±26 | 152±26 | 152±26 | 150±25 | 152±25 | 153±26 | ||
| Adjusted mean | 150 (149–150) | 152 (152–153) | 152 (152–153) | <0.0001 | 150 (149–151) | 152 (151–152) | 152 (151–153) | 0.001 |
| Adjusted mean | 151 (151–152) | 152 (151–152) | 152 (151–152) | 0.37 | 151 (150–152) | 152 (151–152) | 152 (151–153) | 0.15 |
| HDL size (Ø nm) | ||||||||
| Geometric mean | 9.18 (9.17–9.19) | 9.22 (9.21–9.23) | 9.2 (9.19–9.21) | 9.18 (9.17–9.2) | 9.2 (9.19–9.21) | 9.21 (9.19–9.22) | ||
| Adjusted mean | 9.17 (9.16–9.19) | 9.22 (9.21–9.23) | 9.21 (9.2–9.22) | <0.001 | 9.17 (9.15–9.18) | 9.2 (9.19–9.22) | 9.22 (9.2–9.23) | <0.0001 |
| Adjusted mean | 9.19 (9.18–9.21) | 9.21 (9.2–9.22) | 9.21 (9.2–9.23) | 0.05 | 9.17 (9.16–9.19) | 9.21 (9.19–9.22) | 9.23 (9.21–9.24) | <0.001 |
| HDL particles | ||||||||
| Total, μm/L | ||||||||
| Geometric mean | 36.7 (36.5–36.8) | 37.1 (36.9–37.3) | 36.9 (36.7–37.1) | 36.8 (36.6–37) | 37 (36.8–37.2) | 37 (36.8–37.2) | ||
| Adjusted mean | 36.6 (36.4–36.8) | 37.1 (36.9–37.3) | 37 (36.8–37.2) | 0.002 | 37 (36.8–37.2) | 37 (36.8–37.2) | 36.8 (36.6–37) | 0.17 |
| Adjusted mean | 36.9 (36.8–37.1) | 37 (36.8–37.2) | 36.8 (36.6–37) | 0.13 | 37.1 (36.9–37.3) | 37 (36.8–37.2) | 36.7 (36.5–36.9) | 0.002 |
| Large, μm/L | ||||||||
| Geometric mean | 5.36 (5.29–5.44) | 5.64 (5.56–5.73) | 5.5 (5.42–5.59) | 5.33 (5.25–5.42) | 5.49 (5.40–5.58) | 5.61 (5.53–5.7) | ||
| Adjusted mean | 5.32 (5.24–5.4) | 5.64 (5.56–5.73) | 5.56 (5.47–5.64) | <0.001 | 5.31 (5.22–5.41) | 5.51 (5.42–5.6) | 5.58 (5.48–5.69) | 0.002 |
| Adjusted mean | 5.46 (5.38–5.54) | 5.58 (5.51–5.67) | 5.56 (5.48–5.64) | 0.22 | 5.37 (5.28–5.47) | 5.54 (5.46–5.62) | 5.61 (5.51–5.71) | 0.01 |
| Medium, μm/L | ||||||||
| Geometric mean | 11.2 (11.1–11.4) | 11.4 (11.2–11.5) | 11.3 (11.2–11.5) | 11.6 (11.4–11.7) | 11.3 (11.1–11.4) | 11.1 (11.0–11.3) | ||
| Adjusted mean | 11.2 (11.1–11.4) | 11.4 (11.2–11.5) | 11.3 (11.2–11.5) | 0.26 | 11.5 (11.4–11.7) | 11.3 (11.1–11.4) | 11.1 (10.9–11.3) | 0.0035 |
| Adjusted mean | 11.4 (11.3–11.6) | 11.3 (11.2–11.5) | 11.2 (11–11.4) | 0.07 | 11.6 (11.4–11.8) | 11.3 (11.1–11.4) | 11.1 (10.9–11.3) | 0.0020 |
| Small, μm/L | ||||||||
| Geometric mean | 17.7 (17.6–17.8) | 17.6 (17.5–17.8) | 17.7 (17.5–17.8) | 17.4 (17.3–17.6) | 17.8 (17.6–18) | 17.9 (17.7–18) | ||
| Adjusted mean | 17.7 (17.6–17.9) | 17.6 (17.5–17.8) | 17.7 (17.5–17.8) | 0.54 | 17.7 (17.5–17.9) | 17.8 (17.6–17.9) | 17.6 (17.5–17.8) | 0.68 |
| Adjusted mean | 17.6 (17.5–17.8) | 17.6 (17.5–17.8) | 17.6 (17.4–17.7) | 0.52 | 17.7 (17.5–17.9) | 17.7 (17.6–17.9) | 17.5 (17.3–17.7) | 0.22 |
Geometric mean (95% CI) for log‐transformed variables and raw mean±SD for natural‐scale variables across quintiles of exposure variable intake, except as noted. Adjustments for models 1 and 2 are shown in Table 1. Apo indicates apolipoprotein; HDL, high‐density lipoprotein; HDL‐C, high‐density lipoprotein cholesterol; n‐3, omega‐3; Q, quintile.
Fish intake shown as servings per day, n‐3 intake shown as grams per day.
The associations of intake of the n‐3 subtypes (EPA, DHA, and ALA) in relation to the lipid and lipoprotein profiles are described in Tables S4 through S12. To summarize, intake of the different n‐3 subtypes had different patterns of association. After adjusting for potential confounders, the highest EPA intake was significantly associated with only a decreased level of VLDL particle concentration (P trend=0.03) and VLDL‐triglycerides (P trend=0.03; Tables S4, S7, and S10). In contrast, greater intake of DHA and ALA was associated with several significant differences in lipids and lipoproteins, such as LDL and HDL size and particle concentration. The most pronounced differences in LDL and HDL sizes were associated with DHA intake, which also had a higher concentration of large LDL particles (Table S5) and large HDL particles (Table S11). Although higher intake of all n‐3 subtypes showed an association with decreased levels of large VLDL particles, only the ALA intake was significant (Table S9) and associated with a significant decrease in LDL‐C.
The effect size of the energy‐adjusted exposure variables was determined by the modeling of the β coefficients and the P linear trend reflecting the same results extracted from the adjusted means analysis (see Table S13 for the correspondence between absolute and per‐1% and per‐5% differences). To easily visualize the association between the exposure variables and the lipid and lipoprotein profiles, we defined a model based on a variable computing the percentages of difference between Q2 through Q5 and Q1 of the exposure variables. Figure 1 depicts these results for LDL‐, VLDL‐, and HDL‐related variables according to fish and total n‐3 intake adjusted by anthropometric, clinical, and dietary factors. Generally, greater effects were seen for n‐3 intake than fish intake, although they were mostly consistent in direction. The adjusted percentages according to the different n‐3 subtype intakes are shown in Figure 2, with differences in particular noted for EPA compared with DHA or ALA (the latter 2 being more similar in lipoprotein associations).
Figure 1.
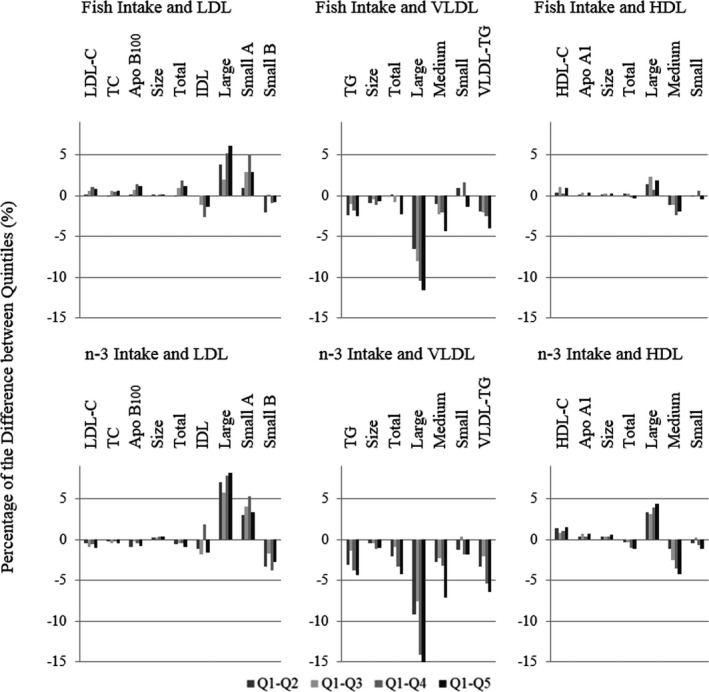
Percentage difference in outcome between quintiles (Qs; Q2–Q5) compared with Q1 of fish and n‐3 intake after adjusting for all demographic, clinical, and dietary factors. Individual distribution: fish: Q1, n=5839; Q2, n=5035; Q3, n=5465; Q4, n=4078; Q5, n=5617; n‐3: Q1, n=5248; Q2, n=5406; Q3, n=4991; Q4, n=5266; Q5, n=5123. Apo indicates apolipoprotein; HDL, high‐density lipoprotein; HDL‐C, high‐density lipoprotein cholesterol; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; LDL‐C, low‐density lipoprotein cholesterol; n‐3, omega‐3; TC, total cholesterol; TG, triglycerides; VLDL, very‐low‐density lipoprotein.
Figure 2.
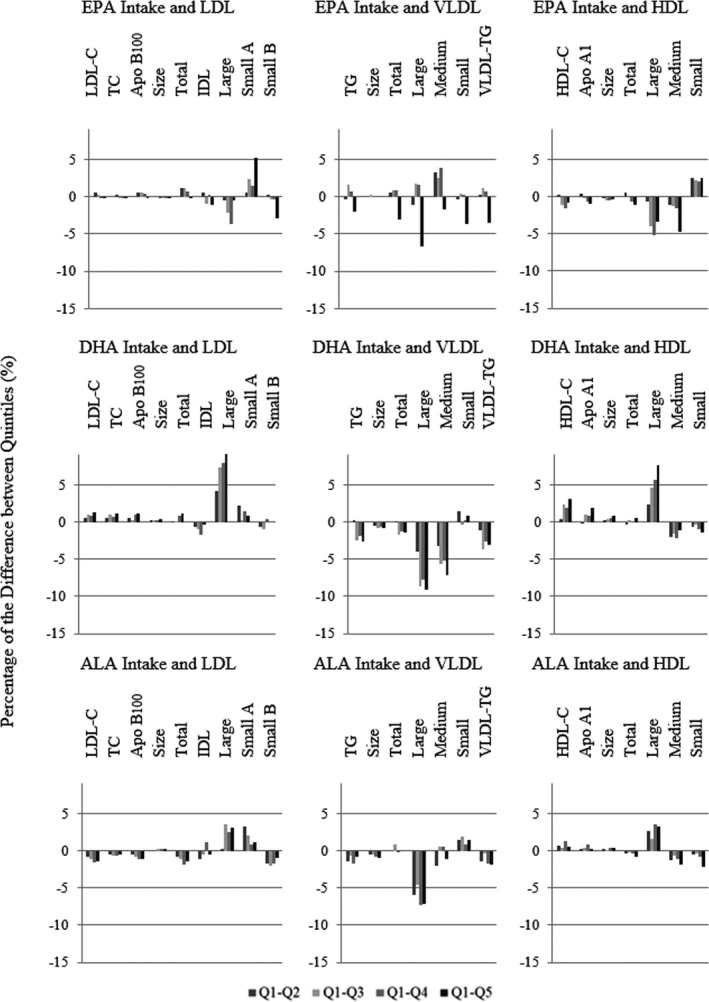
Adjusted percentage difference in lipids and lipoproteins between quintiles (Qs; Q2–Q5) compared with Q1 of n‐3 subtype intakes. ALA indicates α‐linolenic acid; Apo, apolipoprotein; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL, high‐density lipoprotein; HDL‐C, high‐density lipoprotein cholesterol; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; LDL‐C, low‐density lipoprotein cholesterol; n‐3, omega‐3; TC, total cholesterol; TG, triglycerides; VLDL, very‐low‐density lipoprotein.
Finally, we evaluated the associations between the different dietary variables in the lipid and lipoprotein profiles in relation to prospective CVD events in this population. Figure 3 is a heat map that shows the percentage of the difference in the expected value of outcome due to differences between the highest (Q5) and lowest (Q1) intake groups of all exposure variables in terms of risk of CVD events. Figure 4 summarizes the significant associations (P trend<0.05) between the exposure variables and lipid and lipoprotein subfractions.
Figure 3.
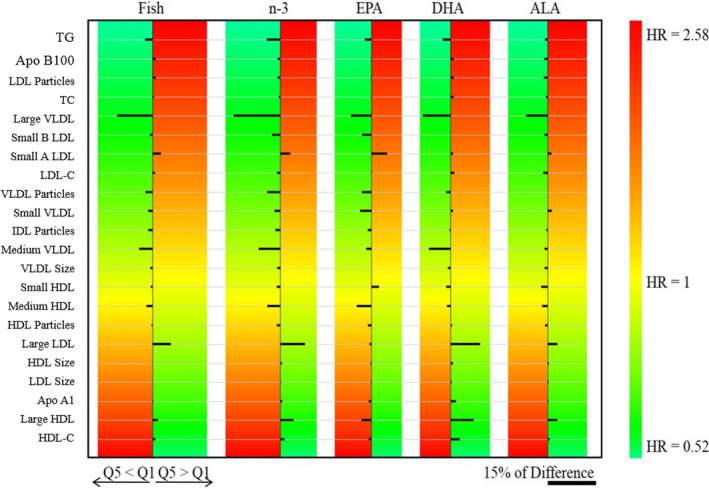
Heat map of percentage differences between adjusted means of greater and lower intake groups of the exposure variables (Q5–Q1) according to intake of fish, total n‐3, and n‐3 subtypes. Outcome variables are sorted according to previously reported hazard ratios (HRs) adjusted for nonlipid risk factors. Green is associated with lower risk of cardiovascular disease (CVD), and red is associated with higher risk of CVD. The right‐lower scale bar illustrates the magnitude (length) of a 15% difference between Q5 and Q1, in which smaller bars represent <15% difference and larger bars represent >15% difference. When the consumption of higher amounts of exposure variables decreases the outcome variable, Q5<Q1; when the consumption of higher amounts of exposure variables increases the outcome variable, Q5>Q1 (fish: Q1, n=5839; Q5, n=5617; n‐3: Q1, n=5248; Q5, n=5123; EPA: Q1, n=5370; Q5, n=4947; DHA: Q1, n=6351; Q5, n=4797; ALA: Q1, n=5286; Q5, n=5097). ALA indicates α‐linolenic acid; Apo, apolipoprotein; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL, high‐density lipoprotein; HDL‐C, high‐density lipoprotein cholesterol; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; LDL‐C, low‐density lipoprotein cholesterol; n‐3, omega‐3; Q, quintile; TC, total cholesterol; VLDL, very‐low‐density lipoprotein.
Figure 4.
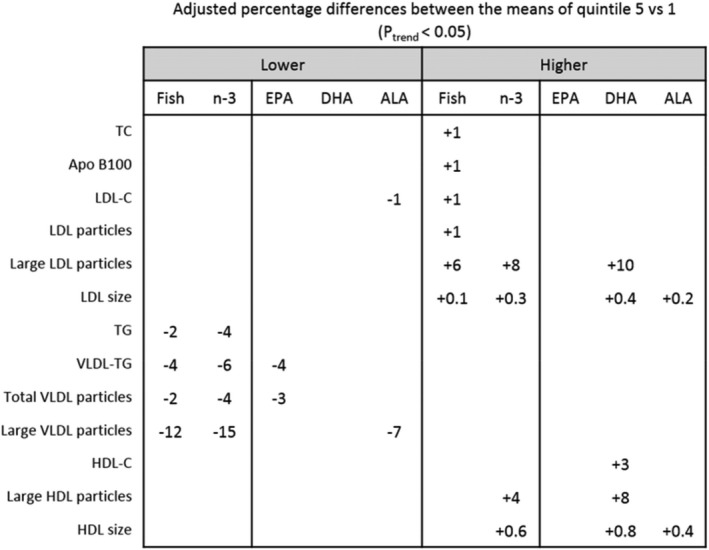
Percentage differences between the means of the greater and lower intake groups of the exposure variables (Q5–Q1) after adjusting for all demographic, clinical, and dietary factors that showed a significant association (P Trend<0.05) among fish, total n‐3, and the different n‐3 subtypes of fatty acid intake and lipid and lipoprotein subfractions. Blank spaces indicate no significant association (fish: Q1, n=5839; Q5, n=5617; n‐3: Q1, n=5248; Q5, n=5123; EPA: Q1, n=5370; Q5, n=4947; 6 DHA: Q1, n=6351; Q5, n=4797; ALA: Q1, n=5286; Q5, n=5097). ALA indicates α‐linolenic acid; Apo, apolipoprotein; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; TC, total cholesterol; LDL, low density lipoprotein; LDL‐C, low‐density lipoprotein cholesterol; TG, triglycerides; VLDL, very‐low‐density lipoprotein; HDL, high‐density lipoprotein; HDL‐C, high‐density lipoprotein cholesterol. For the adjusted means please see Table S14.
The n‐3 fatty acids had some similarities but also differed in their associations with lipid and lipoprotein CVD risk patterns. The higher intake of fish and total n‐3 was associated with a decrease of large VLDL particle concentration, but ALA was the only n‐3 that had a significant association with lower levels of LDL‐C. In contrast, higher DHA and ALA intake was associated with an increased size of LDL and HDL particles, factors known to be associated with a lower risk for CVD. EPA was associated with lower VLDL particles, a finding that would be expected to result in lower risk for CVD.
Discussion
In this large cohort of 26 034 female healthcare professionals, we found that habitual fish consumption and total dietary‐derived n‐3 fatty acids had generally similar and predominantly cardioprotective patterns of associations with lipid, apolipoprotein, and lipoprotein particle measurements. Notable exceptions include the observation that only higher consumption of fish (but not n‐3) was associated with higher levels of LDL‐C and apo B100; conversely, only dietary n‐3 fatty acids showed a significant increase in HDL size and in large HDL particles. The different intake of n‐3 subtypes had different patterns of association. DHA and ALA (but not EPA) were associated with larger HDL and LDL size, whereas only ALA was associated with lower LDL‐C. In contrast, high EPA intake was significantly associated with only a decreased level of VLDL particle concentration and VLDL‐triglycerides. Finally, associations became evident from analysis of detailed lipoprotein particle measurements and were not as readily detected with standard lipid measurements.
Both higher habitual fish and total n‐3 fatty acid intake were significantly associated with lower levels of triglycerides and a tendency toward increased levels of HDL‐C, but their associations with LDL‐C were discordant. Specifically, higher fish intake was associated with a significant but small increase in LDL‐C, whereas total dietary n‐3 fatty acid intake was associated with a trend toward reduced levels of LDL‐C that was mostly accounted for by the plant‐derived ALA. Both higher habitual fish and total n‐3 fatty acid intake were associated with increased LDL size, which was accounted for by a shift toward larger LDL particles. Whereas increased fish consumption was associated with higher levels of LDL particle concentration and apo B100, increased intake of dietary n‐3 fatty acids was not associated with higher levels of either measure. This finding suggests that another factor (possibly cooking oil) may underlie the association of fish consumption with increased LDL particles and apo B100.
The VLDL fractions showed similar associations with both higher habitual fish consumption and higher total n‐3 fatty acid intake in the form of a reduction in all VLDL subfractions, which was most pronounced for the large subfraction. As such, total VLDL particle concentration and average VLDL particle size were also reduced. With regard to HDL particles, only n‐3 fatty acids were associated with larger HDL size and a significant increase in concentration of large HDL particles.
We know of only 1 study that examined the detailed associations of dietary intake of n‐3 fatty acids with detailed lipoprotein particle phenotypes.38 Consistent with our findings, that study found that higher intake of dietary n‐3 fatty acids was associated with lower concentrations of large VLDL particles, smaller average VLDL size, and higher concentration of large HDL. In contrast, a direct association with large LDL particle concentration was found only in men and not in women in that study. Two Finnish cohorts used serum markers of n‐3 fatty acids as a surrogate for habitual fish consumption. One cohort used the ratio of DHA to total fatty acid,39 and the other cohort used circulating n‐3 fatty acids.40 These were both found to be negatively correlated with VLDL particle size and positively correlated with HDL particle size. One of these cohorts also found that a 6‐year change in serum n‐3 was positively correlated with a 6‐year change in HDL and LDL particle size but was negatively correlated with change in VLDL particle size.40 Our findings are additionally supported by 2 small randomized placebo‐controlled feeding studies that found an increase in HDL particle size with fatty fish consumed 3 times weekly for 12 weeks41 or 4 times weekly for 8 weeks.42
Mechanistically, n‐3 fatty acids reduce VLDL production by inhibiting the hepatic synthesis of fatty acids and reducing packaging of VLDL and increase the clearance of VLDL by enhancing lipoprotein lipase activity,43 thus explaining the lower levels of all VLDL subclasses and triglycerides. The role of n‐3 fatty acids on LDL metabolism is unclear, particularly regarding clearance, although the increase in VLDL metabolism with n‐3 fatty acids may imply a high turnover toward larger LDL particles, as shown in our study. Improvement in reverse cholesterol transport with n‐3 fatty acid supplementation44 provides a biological rationale for the associated increase toward large HDL size with higher intake of fish or dietary n‐3 fatty acids. The differential association we found between fish consumption versus dietary n‐3 fatty acids on LDL particle concentration or apo B100 deserves further mention. This likely relates to their differential association with LDL‐C, similar to findings from other studies showing that fatty fish consumption increases LDL‐C,16 but n‐3 polyunsaturated fatty acids (PUFA) supplementation reduces the apo B100 concentration without reducing LDL‐C levels and subsequently increases LDL particle size.45 Accounting for other sources of fat, as in our n‐3 fatty analysis, was not possible when we examined fish consumption. This may partly explain the differential association in relation to LDL particle concentration apo B100.
To our knowledge, no prior study has examined dietary doses of various n‐3 fatty acids types with lipoprotein particles. With the exception of a few notable differences, the associations we found for n‐3 fatty acids were generally similar to those observed for dietary‐derived EPA or DHA, the major types of fish‐derived n‐3 fatty acids, and those observed for ALA, the plant‐derived n‐3 fatty acid. Unlike EPA, both DHA and ALA were associated with a redistribution of HDL particles toward larger ones. In addition, ALA was the only n‐3 associated with lower levels of LDL‐C and lower apo B100, suggesting that a reduction in total LDL particle number may partly account for its differential association on LDL‐C (by comparison, fish intake was associated with increased levels of LDL‐C, as shown by other studies14, 45, 46). The biological activity of ALA is mostly linked to its conversion to EPA and DHA, with the direct effects of ALA‐rich oils being largely unknown. Diets high in ALA have been shown to reduce hepatic cholesterol synthesis, which may account for the unique inverse association we found between ALA and LDL‐C.47 It is also plausible that residual confounding arising from the plant‐based source of ALA partly accounts for its LDL‐lowering effect.
In this study, we report for the first time that higher habitual fish consumption and higher levels of dietary‐derived n‐3 fatty acid intake were both associated with greater differences in lipoprotein subfractions than traditional lipids. This finding suggests that dietary‐derived n‐3 fatty acids may influence CVD risk through lipid and lipoprotein metabolism, perhaps more than previously appreciated. Lipoprotein particle numbers, particularly LDL and HDL, have been shown to perform better than their respective major lipid components (ie, LDL‐C and HDL‐C) in assessing CVD risk.22, 23, 24 In this regard, the direction of association we observed for the lipoprotein subfractions was consistent with a generally beneficial profile for future CVD risk, as observed in studies evaluating lipoproteins and CVD risk.23, 24, 48, 49, 50, 51 In a prior analysis of the same WHS population, we found that larger average sizes of LDL and HDL were associated with lower risk of future CVD.24 Similar results were observed in analyses of other more recent studies.48, 49 Furthermore, recent studies have shown that other components of VLDL particles, such as VLDL cholesterol and remnant cholesterol, perform better than triglycerides in assessing CVD risk.52 This also suggests that the larger magnitude of association that we found for VLDL particles compared with triglycerides may relate to dietary‐derived n‐3 fatty acid intake affecting CVD risk, occurring more through lipoproteins than through traditional lipids. Nonetheless, additional studies will be needed to confirm our findings and to directly examine the relative impact of lipids versus lipoproteins in mediating the attributable CVD risk of dietary n‐3 fatty acids.
The current study has potential limitations. First, dietary assessment was ascertained in only 1 baseline FFQ; as such, we could not account for dietary changes over time. Second, given that participants were asked to self‐report their average dietary pattern over the year before the questionnaires were administered, and it is possible that estimated dietary intake may not reflect dietary intake at the time of the blood draw; however, we expect such misclassification to be nondifferential and thus bias the observed results toward the null hypothesis. Third, the study design limits our ability to infer causality for dietary fish or n‐3 intake and the lipid phenotypes that we examined because we cannot exclude the possibility of residual confounding or reverse causation, although we adjusted for many potential confounders. Moreover, the n‐3 fatty acid intake came from food sources and/or supplements that represent different sources of n‐3 fatty acid. Fourth, our study was restricted to middle‐aged women who were healthcare professionals. As such, the generalizability of our findings to other populations may be limited, particularly for those with prevalent diabetes mellitus, who would likely have high TG as findings of other studies may not be directly applicable to this population of relatively healthy women. Multiple comparisons were performed, increasing the chance of a type I error. However, many P values were highly statistically significant, and the findings are supported by prior biological and epidemiologic studies. Nonetheless, given the multiplicity of hypotheses tested, these results should be viewed as hypothesis‐generating and require further validation in additional cohorts. Our study had notable strengths including the large sample size, comprehensive assessment of dietary factors and other cardiometabolic risk markers, and detailed assessment of lipids and lipoprotein particle measurements. A unique attribute of our study is the use of energy‐adjusted dietary measures, which provide the most robust parameterization of nutrients for assessing CVD risk, underscored by the reciprocal composition of macronutrients that comprise total caloric intake.
In sum, our study adds insights to the literature by showing that fish consumption and isocaloric dietary‐derived n‐3 fatty acids relate to a profile of lipoprotein particles consistent mostly with cardiovascular benefit, with differences noted for high intake of EPA (lower VLDL particles and VLDL triglycerides) compared with DHA and ALA (larger LDL and HDL size) and with ALA (lower LDL‐C and apo B100) that were apparent with detailed lipoprotein phenotyping. These hypothesis‐generating findings warrant further study in randomized controlled trials.
Sources of Funding
Akinkoulie was supported by National Heart, Lung, and Blood Institute (NHLBI) T32 HL007575. The Women's Health Study was funded by National Institutes of Health grants CA047988, HL043851, HL080467, HL099355, and UM1 CA182913. Additional funding was received from a charitable gift from the Molino Family Trust. Mora received funding from the NHLBI of the National Institutes of Health (R01 HL134811, R01 HL117861, and K24 HL 136852) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK112940). The funding agencies played no role in the design, conduct, data management, or analysis related to this article or its preparation.
Disclosures
Mora received research grant support from Atherotech Diagnostics and NHLBI; served as consultant to Quest Diagnostics, for work outside the current study; and is coinventor on a patent on the use of an nuclear magnetic resonance (NMR)–measured biomarker (GlycA) for predicting risk of colorectal cancer. Amigó and Correig are stock owners of Biosfer Teslab and have a patent for an NMR method for lipoprotein characterization. The remaining authors have no disclosures to report.
Supporting information
Table S1. Particle Diameter Ranges
Table S2. Baseline Characteristics of 26 034 Apparently Healthy Women According to Quintiles of Fish and Energy‐Adjusted Marine n‐3 Intake
Table S3. Baseline Characteristics of 26 034 Women According to Quintiles of Energy‐Adjusted α‐Linolenic, Docosahexaenoic, and Eicosapentaenoic Fatty Acid Intake
Table S4. LDL (Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S5. LDL (Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S6. LDL (Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S7. VLDL (Very Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S8. VLDL (Very Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S9. VLDL (Very Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S10. HDL (High‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S11. HDL Related Variables. Strategy Based on Modeling Energy Adjusted Variables
Table S12. HDL (High‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S13. Correspondence Between Absolute Values and per 1% and 5% Differences
Table S14. Adjusted Means for the Lower Exposure Quintiles (Q1) That Showed a Significant Association (P trend<0.05).
Acknowledgments
We thank Mallory Heath (Brigham and Women's Hospital, Boston, MA) for proofreading and English editing of the article.
Author contributions: Mora, Akinkuolie, Chiuve and Cook designed the research; Amigó and Akinkuolie conducted the research; Amigó, Akinkuolie, Correig and Mora analyzed the data; Amigó, Akinkuolie and Mora wrote the article; and Mora had primary responsibility for final content. All authors read and approved the final manuscript.
(J Am Heart Assoc. 2020;9:e014963 DOI: 10.1161/JAHA.119.014963.)
References
- 1. Marik PE, Varon J. Omega‐3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Wu JH. Omega‐3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. [DOI] [PubMed] [Google Scholar]
- 3. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberg ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Virani SS, Williams KA, Yeboah J, Ziaeian B. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. J Am Coll Cardiol. 2019:26029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega‐3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta‐analysis. J Am Med Assoc. 2012;308:1024–1033. [DOI] [PubMed] [Google Scholar]
- 5. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D'Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE. Marine n‐3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 7. Reiffel JA, McDonald A. Antiarrhythmic effects of omega‐3 fatty acids. Am J Cardiol. 2006;98:50–60. [DOI] [PubMed] [Google Scholar]
- 8. Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–1499. [DOI] [PubMed] [Google Scholar]
- 9. Goodfellow J, Bellamy MF, Ramsey MW, Jones CJ, Lewis MJ. Dietary supplementation with marine omega‐3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol. 2000;35:265–270. [DOI] [PubMed] [Google Scholar]
- 10. Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega‐3 fatty acids. Lancet. 2010;376:540–550. [DOI] [PubMed] [Google Scholar]
- 11. Okuda Y, Kawashima K, Sawada T, Tsurumaru K, Asano M, Suzuki S, Soma M, Nakajima T, Yamashita K. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem Biophys Res Commun. 1997;232:487–491. [DOI] [PubMed] [Google Scholar]
- 12. Leslie MA, Cohen DJ, Liddle DM, Robinson LE, Ma DW. A review of the effect of omega‐3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis. 2015;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris WS, Bulchandani D. Why do omega‐3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17:387–393. [DOI] [PubMed] [Google Scholar]
- 14. Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega‐3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. [DOI] [PubMed] [Google Scholar]
- 15. Minihane AM, Khan S, Leigh‐Firbank EC, Talmud P, Wright JW, Murphy MC, Griffin BA, Williams CM. ApoE polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arterioscler Thromb Vasc Biol. 2000;20:1990–1997. [DOI] [PubMed] [Google Scholar]
- 16. Oelrich B, Dewell A, Gardner C. Effect of fish oil supplementation on serum triglycerides, LDL cholesterol and LDL subfractions in hypertriglyceridemic adults. Nutr Metab Cardiovasc Dis. 2013;23:350–357. [DOI] [PubMed] [Google Scholar]
- 17. Kris‐Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega‐3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. [DOI] [PubMed] [Google Scholar]
- 18. Rivellese AA, Maffettone A, Vessby B, Uusitupa M, Hermansen K, Berglund L, Louheranta A, Meyer BJ, Riccardi G. Effects of dietary saturated, monounsaturated and n‐3 fatty acids on fasting lipoproteins, LDL size and post‐prandial lipid metabolism in healthy subjects. Atherosclerosis. 2003;167:149–158. [DOI] [PubMed] [Google Scholar]
- 19. Li Z, Lamon‐Fava S, Otvos J, Lichtenstein AH, Velez‐Carrasco W, McNamara JR, Ordovas JM, Schaefer EJ. Fish consumption shifts lipoprotein subfractions to a less atherogenic pattern in humans. J Nutr. 2004;134:1724–1728. [DOI] [PubMed] [Google Scholar]
- 20. Mori TA, Burke V, Puddey IB, Watts GF, O'Neal DN, Best JD, Beilin LJ. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–1094. [DOI] [PubMed] [Google Scholar]
- 21. Mostad I, Bjerve K, Lydersen S, Grill V. Effects of marine n‐3 fatty acid supplementation on lipoprotein subclasses measured by nuclear magnetic resonance in subjects with type II diabetes. Eur J Clin Nutr. 2008;62:419. [DOI] [PubMed] [Google Scholar]
- 22. Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, Wilson PW, D'Agostino RB. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study—implications for LDL management. J Clin Lipidol. 2007;1:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kontush A. HDL particle number and size as predictors of cardiovascular disease. Front Pharmacol. 2015;6:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ridker PM, Cook NR, Lee I‐M, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. [DOI] [PubMed] [Google Scholar]
- 26. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 27. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self‐administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. [DOI] [PubMed] [Google Scholar]
- 28. Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long‐chain ω‐3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003;107:1852–1857. [DOI] [PubMed] [Google Scholar]
- 29. Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE. Fish and omega‐3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. [DOI] [PubMed] [Google Scholar]
- 30. Willett W. Nutritional Epidemiology. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 31. Composition of Foods: Raw, Processed, Prepared 1 USDA National Nutrient Database for Standard Reference, Release 23. 2010.
- 32. Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Intake of fish and omega‐3 fatty acids and risk of stroke in women. JAMA. 2001;285:304–312. [DOI] [PubMed] [Google Scholar]
- 33. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 34. Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am J Cardiol. 2002;90:22–29. [DOI] [PubMed] [Google Scholar]
- 35. Mora S, Lee I‐M, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412–1419. [DOI] [PubMed] [Google Scholar]
- 36. Zhang SM, Lee I‐M, Manson JE, Cook NR, Willett WC, Buring JE. Alcohol consumption and breast cancer risk in the Women's Health Study. Am J Epidemiol. 2007;165:667–676. [DOI] [PubMed] [Google Scholar]
- 37. Chiuve SE, Rimm EB, Sandhu RK, Bernstein AM, Rexrode KM, Manson JE, Willett WC, Albert CM. Dietary fat quality and risk of sudden cardiac death in women. Am J Clin Nutr. 2012;96:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Annuzzi G, Rivellese AA, Wang H, Patti L, Vaccaro O, Riccardi G, Ebbesson SO, Comuzzie AG, Umans JG, Howard BV. Lipoprotein subfractions and dietary intake of n‐3 fatty acid: the Genetics of Coronary Artery Disease in Alaska Natives study. Am J Clin Nutr. 2012;95:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bogl L, Pietiläinen K, Rissanen A, Kangas A, Soininen P, Rose R, Ala‐Korpela M, Kaprio J. Association between habitual dietary intake and lipoprotein subclass profile in healthy young adults. Nutr Metab Cardiovasc Dis. 2013;23:1071–1078. [DOI] [PubMed] [Google Scholar]
- 40. Mäntyselkä P, Niskanen L, Kautiainen H, Saltevo J, Würtz P, Soininen P, Kangas AJ, Ala‐Korpela M, Vanhala M. Cross‐sectional and longitudinal associations of circulating omega‐3 and omega‐6 fatty acids with lipoprotein particle concentrations and sizes: population‐based cohort study with 6‐year follow‐up. Lipids Health Dis. 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lankinen M, Kolehmainen M, Jääskeläinen T, Paananen J, Joukamo L, Kangas AJ, Soininen P, Poutanen K, Mykkänen H, Gylling H. Effects of whole grain, fish and bilberries on serum metabolic profile and lipid transfer protein activities: a randomized trial (Sysdimet). PLoS One. 2014;9:e90352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Erkkilä AT, Schwab US, Lehto S, de Mello VD, Kangas AJ, Soininen P, Ala‐Korpela M, Uusitupa MI. Effect of fatty and lean fish intake on lipoprotein subclasses in subjects with coronary heart disease: a controlled trial. J Clin Lipidol. 2014;8:126–133. [DOI] [PubMed] [Google Scholar]
- 43. Harris WS, Connor WE, Alam N, Illingworth D. Reduction of postprandial triglyceridemia in humans by dietary n‐3 fatty acids. J Lipid Res. 1988;29:1451–1460. [PubMed] [Google Scholar]
- 44. Chadli FK, Nazih H, Krempf M, Nguyen P, Ouguerram K. Omega 3 fatty acids promote macrophage reverse cholesterol transport in hamster fed high fat diet. PLoS One. 2013;8:e61109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yanai H, Masui Y, Katsuyama H, Adachi H, Kawaguchi A, Hakoshima M, Waragai Y, Harigae T, Sako A. An improvement of cardiovascular risk factors by omega‐3 polyunsaturated fatty acids. J Clin Med Res. 2018;10:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jacobson TA, Glickstein SB, Rowe JD, Soni PN. Effects of eicosapentaenoic acid and docosahexaenoic acid on low‐density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol. 2012;6:5–18. [DOI] [PubMed] [Google Scholar]
- 47. Yang L, Leung KY, Cao Y, Huang Y, Ratnayake W, Chen Z‐Y. α‐Linolenic acid but not conjugated linolenic acid is hypocholesterolaemic in hamsters. Br J Nutr. 2005;93:433–438. [DOI] [PubMed] [Google Scholar]
- 48. Mora S, Glynn RJ, Ridker PM. High‐density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mackey RH, Greenland P, Goff DC, Lloyd‐Jones D, Sibley CT, Mora S. High‐density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pirillo A, Norata GD, Catapano AL. High‐density lipoprotein subfractions‐what the clinicians need to know. Cardiology. 2013;124:116–125. [DOI] [PubMed] [Google Scholar]
- 51. Amigó N, Mallol R, Heras M, Martínez‐Hervás S, Blanco‐Vaca F, Escolà‐Gil JC, Plana N, Yanes O, Masana L, Correig X. Lipoprotein hydrophobic core lipids are partially extruded to surface in smaller HDL: “Herniated” HDL, a common feature in diabetes. Sci Rep. 2016;6:19249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lawler PR, Akinkuolie A, Glynn R, Ridker P, Mora S. Atherogenic lipoprotein particle subclasses and residual cardiovascular risk: an analysis of the Jupiter trial. J Am Coll Cardiol. 2015;65:A1362. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Particle Diameter Ranges
Table S2. Baseline Characteristics of 26 034 Apparently Healthy Women According to Quintiles of Fish and Energy‐Adjusted Marine n‐3 Intake
Table S3. Baseline Characteristics of 26 034 Women According to Quintiles of Energy‐Adjusted α‐Linolenic, Docosahexaenoic, and Eicosapentaenoic Fatty Acid Intake
Table S4. LDL (Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S5. LDL (Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S6. LDL (Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S7. VLDL (Very Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S8. VLDL (Very Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S9. VLDL (Very Low‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S10. HDL (High‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S11. HDL Related Variables. Strategy Based on Modeling Energy Adjusted Variables
Table S12. HDL (High‐Density Lipoprotein)–Related Variables, Strategy Based on Modeling Energy‐Adjusted Variables
Table S13. Correspondence Between Absolute Values and per 1% and 5% Differences
Table S14. Adjusted Means for the Lower Exposure Quintiles (Q1) That Showed a Significant Association (P trend<0.05).


